Abstract
Purpose
Fetal mice require light exposure in utero during early gestation for normal vascular development in the eye. Because angiogenic abnormalities in retinopathy of prematurity (ROP) are manifested in preterm infants, we investigated whether day length during early gestation was associated with severe ROP (SROP).
Design
Single-center, retrospective cohort study.
Participants
We included a total of 343 premature infants (401–1250 g birth weight [BW], from 1998–2002): 684 eyes (1 eye each of 2 patients excluded) with 76 eyes developing SROP, defined as (1) classic threshold ROP in zone I or II, (2) type 1 ROP in zone I, or (3) in a few eyes, type 1 ROP in posterior zone II that was treated.
Methods
For each infant, average day length (ADL) was calculated during different cumulative time periods and time windows after the estimated date of conception (EDC). Multiple logistic regression analysis (with generalized estimating equations to account for inter-eye correlation) was performed.
Main Outcome Measures
Association of ADL during early gestation with SROP.
Results
In a model evaluating all 684 eyes with 76 eyes developing SROP, BW, gestational age, multiple births, race, per capita income in the mother's residence ZIP code, and ADL during the first 90 days after the EDC were factors associated with the development of SROP. Each additional hour of ADL (90 days) decreased the likelihood of SROP by 28% (P = 0.015; odds ratio [OR], 0.72; 95% confidence interval [CI], 0.55–0.94). In a model evaluating the subset of 146 prethreshold ROP eyes with 76 eyes developing SROP, each additional hour of ADL during the first 105 days after the EDC decreased the likelihood of SROP by 46% (P = 0.001; OR, 0.54; 95% CI, 0.37–0.78). Time windows when ADL was most closely associated with SROP were 31 to 60 days and 61 to 90 days after the EDC for the all eyes and the prethreshold ROP eyes models, respectively.
Conclusions
Higher ADL during early gestation was associated with a lower risk for SROP and may imply a role for prophylactic light treatment during early gestation to decrease the risk of SROP.
Financial Disclosure(s)
Proprietary or commercial disclosure may be found after the references.
Retinopathy of prematurity (ROP) is a potentially blinding condition that affects the developing retinal vasculature of premature infants. Previous studies have addressed whether light exposure after birth influences the progression of ROP. The Light Reduction in Retinopathy of Prematurity (LIGHT-ROP) randomized study (ClinicalTrials.gov number, NCT00000156) evaluated the use of light-reducing goggles in premature infants after birth and found no change in the incidence or severity of ROP.1 Likewise, there was no effect of postpartum light exposure (equivalent to late gestation in humans) in animal models of oxygen-induced retinopathy.2,3
We recently identified in the mouse model a fetal light-response pathway that requires melanopsin to regulate retinal neuron number and angiogenesis. This pathway is active in the fetal mouse during a gestational period that approximates the first trimester in humans.4 It normally suppresses retinal neuron number, limits retinal hypoxia, and consequently controls the local expression of vascular endothelial growth factor (VEGF). By contrast, dark-rearing from late gestation (embryonic days 16 to 17; equivalent to the 58-day-old human fetus)5–7 resulted in increases (at postpartum day 8) in the (1) retinal neuron number, (2) density of retinal vessels, and (3) retinal hypoxia associated with a corresponding increase in VEGF expression. By using mice that have a mutation in the melanopsin-encoding gene (Opn4), we were able to replicate the vascular anomalies associated with dark-rearing. Measurements of photon flux in the visceral cavity of mice suggested that there was sufficient light penetration at 480 nm, which is required to activate melanopsin-expressing, intrinsically photosensitive retinal ganglion cells. When extrapolated to human fetal development, these findings suggested that insufficient exposure to light in early gestation could result in the subsequent development of severe ROP (SROP) by rendering the abnormal retinal vessels susceptible to pathologic neovascularization during phase 2 of ROP when there is relative hypoxia and increased VEGF levels.8 The purpose of this study was to investigate this hypothesis by evaluating whether average day length (ADL) during early gestation was a prognostic factor for the development of SROP in a cohort of premature infants.
Methods
Study Participants
This study was approved by the institutional review board and conforms to the requirements of the US Health Insurance Portability and Accountability Act. We retrospectively analyzed only complete years (1998–2002) of a previously described cohort of premature infants with a birth weight (BW) of 401 to 1250 g who were admitted to the neonatal intensive care unit of the University of Cincinnati Medical Center.9,10 Some infants required additional care and were transferred to Cincinnati Children's Hospital Medical Center. Infants discharged from the hospital typically received eye examinations as outpatients at Cincinnati Children's Hospital Medical Center. Eye examination results, the traditional prognostic variables (i.e., BW, gestational age [GA] at birth, multiple births, birth location inside the study hospital [inborn status], and race) from the Cryotherapy for ROP (CRYO-ROP) randomized trial (ClinicalTrials.gov number, NCT00000133),11 gender, ZIP code of mother's residence, and presence or absence of prenatal care were abstracted by chart review. The race of the infant was defined as the mother's race. Exclusion criteria for infants were ambiguous genitalia and an inadequate sequence of eye examinations to determine eye disease progression and outcome, which may be due to death, inadequate records, or loss of follow-up (Fig 1).10 The eye findings were documented with fundus drawings by fellowship-trained pediatric ophthalmologists and occasionally a retinal surgeon using the International Classification for Retinopathy of Prematurity.12 Definitions of threshold and prethreshold ROP (Fig 2) were as described in the CRYO-ROP study and the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial (ClinicalTrials.gov number, NCT00027222).13,14 The outcome variable, SROP, was defined as (1) classic threshold ROP in zones I or II; (2) in a few eyes, type 1 prethreshold ROP in zone I or posterior zone II that examiners chose to treat because of very high risk for poor outcome; or (3) type 1 prethreshold ROP in zone I in any remaining eyes. The first 2 components in the definition of SROP were described in a previous study as ROP warranting surgery.10 The decision to treat only some eyes with type 1 prethreshold ROP was because the time period of care for these premature infants preceded the adoption of the recommendations of the ETROP study.14
Figure 1.
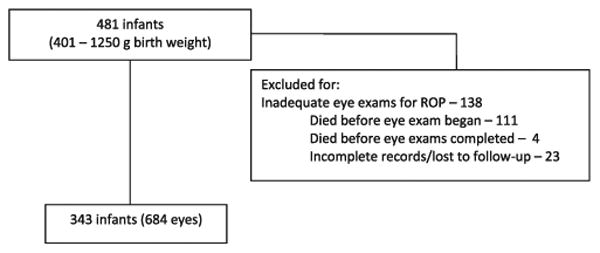
Inclusion/exclusion criteria. The number of eyes is not exactly twice that of infants because 2 infants each had only 1 eye that met criteria for an adequate sequence of eye examinations. ROP = retinopathy of prematurity.
Figure 2.
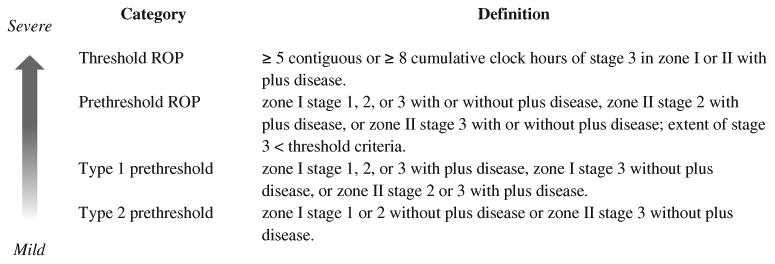
Retinopathy of prematurity (ROP); plus disease is a standardized degree of dilation and tortuosity of vessels in the posterior pole around the optic nerve.11,12
Average Day Length
Day length from June 1997 to December 31, 2002 in the Cincinnati, Ohio, area was obtained from http://www.timeanddate.com/worldclock/sunrise.html (accessed June 18, 2012) (Fig 3). From this seasonal variation in day length, ADLs after each date for cumulative periods of 30, 45, 60, 75, 90, 105, 120, and 150 days and for nonoverlapping, consecutive time windows of 30 days duration (days 1–30, 31–60, 61–90, 91–120, and 121–150) were calculated. For each infant, an estimated date of conception (EDC) was obtained by calculating the date of the last menstrual period using the estimated GA at birth and adding 2 weeks. Then the ADLs for different cumulative days or time windows corresponding to the infant's EDC were obtained. The sum of day lengths during gestation was calculated for each infant. With the exception of 2 infants, the mothers of all infants lived within 0.75 degrees latitude or 51.75 miles of Cincinnati, Ohio, a distance that results in differences in day lengths of less than 4 minutes per day throughout the year. Separate day length, ADL, and sum of day length calculations were performed for the 2 outlier infants. Per capita income in the mother's residence ZIP code was calculated for the year 2000 using MapPoint (version 2002, Microsoft Corp, Redmond, WA).
Figure 3.
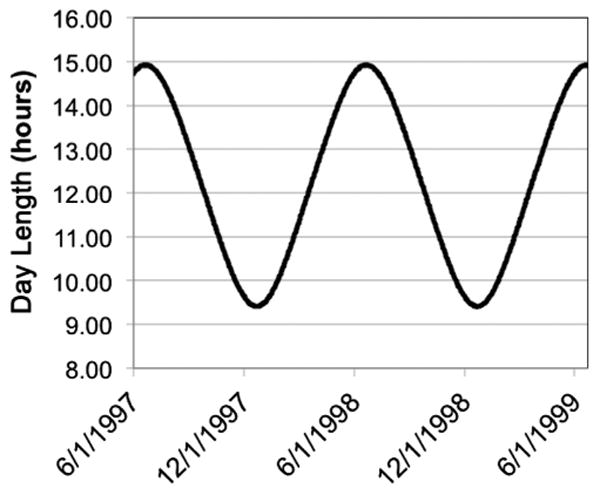
Seasonal variation in day length from June 1997 to June 1999 in Cincinnati, Ohio. The cycle continues similarly for subsequent years.
Statistical Analysis
Predictive models for an eye outcome of SROP were developed using multiple logistic regression (SPSS 21.0 Advanced Models; IBM Inc, Armonk, NY). Generalized estimating equations accounted for correlation between eyes of each infant. Birth weight, GA, inborn status, multiple births, race, gender, per capita income in the mother's residence ZIP code, ADL, and presence or absence of prenatal care were the prognostic variables analyzed in 3 risk models patterned after those described by Hardy et al15 for threshold ROP. Model 1 included all 684 eyes in the cohort, model 2 included the subset of 468 eyes that developed “any” ROP, and model 3 included the subset of 146 prethreshold ROP eyes. All 3 models evaluated risk factors for the development of an outcome of SROP found in 76 eyes. Variations of each model were analyzed using different ADL variables, that is, ADL (x), where x represents cumulative days after the EDC or nonoverlapping consecutive 30-day time windows after the EDC, and correspondingly designated as model 1 (x), model 2 (x), or model 3 (x). Model predictive capacities were calculated as areas under the receiver operating characteristic curves (AUCs) and compared (MedCalc 12.4, MedCalc Software, Ostend, Belgium).16
Results
A total of 343 infants (684 eyes) met the inclusion criteria (Fig 1). The baseline characteristics of the infants in our study were similar to those in the CRYO-ROP study, except in CRYO-ROP the percentage of infants born in the study hospital was lower (81.8%) and the percentage of singleton births was higher (81.4%) compared with our population (Table 1). The per capita income of the mother's residence ZIP code and the number of eyes or infants reaching threshold ROP, ROP warranting surgery, and SROP are also shown. Two infants each had only 1 eye that met the inclusion criteria, thus, 684 instead of 686 eyes.
Table 1. Baseline Characteristics of Infants.
| Characteristics | Results |
|---|---|
| No. of infants/eyes | 343/684 |
| BW (g) | 928±192 |
| GA (wks) | 27.2±2.1 |
| Born in the study hospital (%) | 96.8 |
| Singleton births (%) | 74.9 |
| Non-black race (%) | 60.1 |
| Male (%) | 48.4 |
| Per capita income ($, thousands) | 20.4±8.0 |
| Threshold ROP (infants/eyes) | 33/63 |
| ROP warranting surgery (infants/eyes) | 36/68 |
| SROP (infants/eyes) | 40/76 |
BW = birth weight; GA = gestational age; ROP = retinopathy of prematurity; SROP = severe retinopathy of prematurity.
Plus-minus values are means ± standard deviations. Eyes with ROP warranting surgery included those with classic threshold ROP in zone I or II and a few eyes with type 1 prethreshold ROP in zone I or posterior zone II that examiners chose to treat because of high risk of poor outcome. SROP was defined as ROP warranting surgery or type 1 prethreshold ROP in zone I because some eyes with type 1 prethreshold ROP in zone I were not treated at that level of disease and spontaneously regressed or progressed to threshold ROP and were then treated.
The seasonal distribution of the months of conception for all premature infants in the cohort showed higher numbers of infants conceived in the summer and winter compared with spring and fall (Fig 4A). For the 40 infants who developed SROP, the number of infants conceived was highest in winter followed by fall, summer, and spring (Fig 4B). We performed multiple logistic regression analysis for 3 models of SROP outcome. Models 1, 2, and 3 evaluated all 684 eyes, the subset of 468 “any” ROP eyes, and the subset of 146 prethreshold ROP eyes for the development of SROP, respectively. Table 2 shows the results for models in which ADL was 90 days cumulative (model 1) or 105 days cumulative (models 2 and 3) after the EDC. These cumulative time periods were chosen because they corresponded with peak model predictive capacity as described later in this article. Neither inborn status nor prenatal care was a significant predictive factor of SROP outcome, and these were excluded from the final models.
Figure 4.
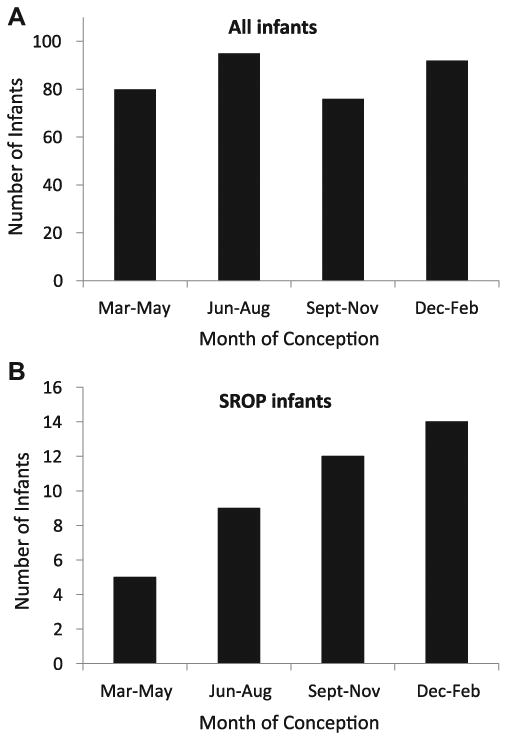
Seasonal distribution based on the month of conception of (A) all 343 premature infants in the cohort and (B) the 40 infants who developed severe retinopathy of prematurity (SROP).
Table 2. Multivariate Logistic Regression for Evaluation of Risk Factors for Severe Retinopathy of Prematurity.
| Model 1 (90 Days) All 684 Eyes with 76 Eyes Developing SROP |
Model 2 (105 Days) 468 ROP Eyes with 76 Eyes Developing SROP |
Model 3 (105 Days) 146 Prethreshold ROP Eyes with 76 Eyes Developing SROP |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Beta | Odds Ratio (95% CI) | P Value | Beta | Odds Ratio (95% CI) | P Value | Beta | Odds Ratio (95% CI) | P Value | |
| BW (each 100 g increase) | −0.475 | 0.62 (0.42–0.93) | 0.021 | −0.416 | 0.66 (0.44–0.98) | 0.039 | −0.136 | 0.87 (0.58–1.32) | 0.52 |
| GA (wks) | − 0.643 | 0.53 (0.37–0.74) | <0.0005 | −0.566 | 0.57 (0.40–0.81) | 0.002 | −0.317 | 0.73 (0.52–1.02) | 0.067 |
| Multiple birth (yes = 1, no = 0) | 1.889 | 6.6 (2.3–19.3) | 0.001 | 1.866 | 6.5 (2.2–18.9) | 0.001 | 2.247 | 9.5 (2.4–36.7) | 0.001 |
| Race (black = 1, non-black = 0) | −1.612 | 0.20 (0.075–0.53) | 0.001 | −1.533 | 0.22 (0.079–0.59) | 0.003 | −0.774 | 0.32 (0.099–2.14) | 0.323 |
| Gender (male = 1, female = 0) | 0.569 | 1.8 (0.78–3.99) | 0.171 | 0.546 | 1.7 (0.75–3.98) | 0.199 | 1.230 | 3.4 (1.12–10.5) | 0.031 |
| Per capita income (each $10 000) | −0.056 | 0.95 (0.90–0.999) | 0.044 | −0.057 | 0.94 (0.90–0.995) | 0.033 | −0.042 | 0.96 (0.89–1.03) | 0.277 |
| ADL (hours; cumulative 90 days after EDC) | −0.334 | 0.72 (0.55–0.94) | 0.015 | −0. | −0. | – | – | – | – |
| ADL (hours; cumulative 105 days after EDC) | – | – | – | −0.363 | 0.70 (0.52–0.93) | 0.013 | −0.624 | 0.54 (0.37–0.78) | 0.001 |
ADL = average day length; BW = birth weight; CI = confidence interval; EDC = estimated date of conception; GA = gestational age; ROP = retinopathy of prematurity; SROP = severe retinopathy of prematurity.
For model 1 (90 days), several factors or covariates were found to increase or decrease the likelihood of an eye developing SROP. Each 100 g increase in BW and each additional week of GA resulted in a 38% and 47% reduction in the likelihood of an eye developing SROP, respectively. Twin or greater birth increased the odds of developing SROP by 6.6-fold, whereas black race resulted in an 80% reduction in the likelihood of developing SROP. These findings paralleled those of the CRYO-ROP study.11 Gender was not a significant predictor. For each additional $10000 increase in per capita income in the mother's residence ZIP code, there was a 5% reduction in the likelihood of SROP. Each additional hour of ADL during the first 90 days after conception was associated with a 28% reduction in the likelihood of an eye developing SROP.
For model 2 (105 days), the odds ratios for each of the prognostic factors and their statistical significance were similar to those found in model 1 (90 days), and each additional hour of ADL during the first 105 days after conception was associated with a 30% reduction in the likelihood of an eye developing SROP. For model 3 (105 days), each additional week of GA showed a trend association with a 27% reduction in the likelihood of developing SROP. However, in contrast with models 1 and 2, BW, per capita income, and black race were not significant predictors of SROP. The eyes of infants who were products of twin or greater birth had a 9.5-fold increase in the odds of developing SROP, and male gender increased the odds of developing SROP by 3.4-fold. For each additional hour of ADL during the first 105 days after the EDC, there was a 46% reduction in the likelihood of an eye with prethreshold ROP developing SROP.
To determine whether ADL contributed significantly to the overall predictive capacity of the model, we compared the AUC between full models with all prognostic factors, including ADL, and “base” models without ADL. Model 1 (90 days) fell short of showing a statistically significant improvement in model predictive capacity (Fig 5A, P = 0.07), whereas model 2 (105 days) and model 3 (105 days) showed statistically significant improvement compared with the base models (Fig 5B, P = 0.019; Fig 5C, P = 0.006).
Figure 5.
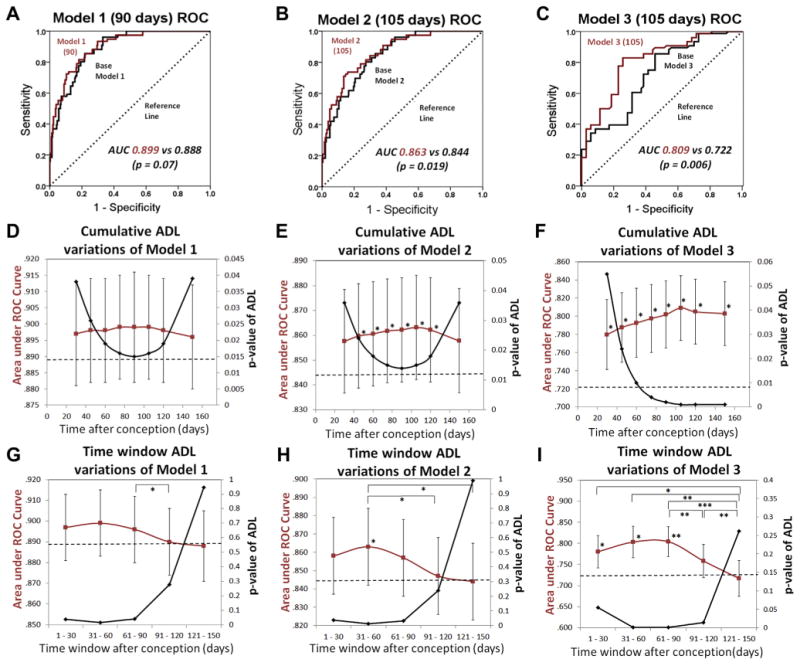
Performance of models of risk for severe retinopathy of prematurity (SROP). The receiver operating characteristic (ROC) curves for (A) the multiple logistic regression model 1, which evaluates the risk of all eyes developing SROP, with (red) and without (black) the average day length (ADL) (90 days) variable calculated for the first 90 days after the estimated date of conception (EDC). (B) Model 2, which evaluates risk of “any” retinopathy of prematurity (ROP) eyes developing SROP, with (red) and without (black) the ADL (105 days) variable, calculated for the first 105 days after the EDC. (C) Model 3, which evaluates risk of prethreshold ROP eyes developing SROP, with (red) and without (black) the ADL (105 days) variable, calculated for the first 105 days after the EDC. The areas under the ROC curves (AUCs) were compared; the diagonal reference line (dashed) is due to a random coin toss model and has an area of 0.5 (A–C). Variations of models 1, 2, and 3 that use different ADLs calculated for different cumulative time periods from the EDC to the number of days listed on the x-axis (D–F) and for different consecutive, nonoverlapping time window periods of 30 days duration (G–I). Model AUC (red) and the corresponding P values (black) for ADLs of different cumulative days or 30-day time window periods (D–I). The horizontal dashed line represents the AUC for the base model with all prognostic variables except ADL included (D–I). Model AUCs for each cumulative day or time window variation of models 1, 2, or 3 were compared with the respective base model AUCs (horizontal dashed lines) and with each other (D–I). Error bars, standard error. *P < 0.05. **P< 0.01. ***P < 0.001.
We initially chose 90 days after EDC as the cumulative time period in which to evaluate the association of ADL with SROP in humans because it included the time period after conception (58 days for humans) that is equivalent to the stage in mouse gestation (embryonic day 16) in which the mouse fetus was light responsive.4 Subsequently, in systematic analyses of various cumulative time periods, 90 and 105 days (when used for ADL calculations in model 1 and models 2 and 3, respectively) were noted to provide the best model fit based on the peak in the AUC and corresponded to the lowest P values for ADL(x), as x (the cumulative time period in days after the EDC) varied from 30 to 150 days (Fig 5D–F).
For model 1, the overall trend showed an initial increase and then a decrease in the model AUC (with opposite directional changes in the P value of the ADL variable) as the cumulative time periods after the EDC for ADL calculations were increased to 90 days and then beyond. This finding suggested that day lengths subsequent to 90 days after the EDC reduced the association of the ADL variable with SROP (Fig 5D). These results were corroborated by using different nonoverlapping consecutive time windows of 30 days during the first 150 days after EDC for the ADL variable (Fig 5G). The peak model AUC occurred when a time window of 31 to 60 days was used for ADL. Time windows after 90 days resulted in a decrease in model AUC with a corresponding increase in the P value of ADL to >0.05. These findings suggested that the first 90 days after the EDC was the important time period for the association of ADL with SROP. Model 2 results were similar to those of model 1 except that the peak AUC occurred at 105 cumulative days after the EDC, whereas the trough in the P value of ADL occurred at 90 cumulative days after the EDC (Fig 5E). Time window analysis for model 2 showed a pattern similar to model 1, again suggesting that the first 90 days (and possibly the more specific time window of 31–60 days) after the EDC was the important time period for the association of ADL with SROP (Fig 5H). In model 3, the drop-off in the AUC and the corresponding plateau in the P value of ADL variables after 105 cumulative days after the EDC indicated a lower contribution of day lengths after 105 days to the predictive value of the ADL variable (Fig 5F). In contrast with models 1 and 2, time window analysis for model 3 showed that peak model AUC occurred slightly later at 61 to 90 days. Beyond 90 days, the model AUCs decreased with a correspondingly higher P value for ADL that became nonsignificant for the final time window of 121 to 150 days (Fig 5I). These results suggested that for eyes that developed prethreshold ROP, the first 105 days after the EDC and possibly slightly later was the important time period for the association of ADL with SROP.
When a postnatal illness factor (number of days of oxygen use during the first 6 weeks of life) was included, we found that both duration of oxygen use and ADL were associated with SROP outcome in models 2 and 3, but only ADL was associated with SROP outcome in model 1 (Table 3, available at http://aaojournal.org). However, oxygen information was not available for a small percentage (<7%) of premature infants. When the sum of day lengths during the total length of gestation of each infant was used instead of ADL calculated for equal cumulative days or time windows, there was no association between the sum of day lengths and SROP in any of the 3 models (data not shown).
Discussion
The present study showed that higher ADL during early gestation was associated with lower risk for the subsequent postnatal development of SROP in pre-term infants. These results seem to be consistent with experiments in mice that suggest that external light penetrating through the body wall of the dam can activate (in the fetus) melanopsin-expressing, intrinsically photosensitive retinal ganglion cells, which regulate retinal vascular development.4 In addition, the time windows when the association of ADL with SROP outcome was most significant (31–60 days after conception for models 1 and 2 and 61–90 days after conception for model 3) seemed well correlated with the time of development in humans (day 58 after conception) when light response for vascular development in the eye would have been expected on the basis of observations in mice.4 Thus, the causal element for the seasonal variation in SROP may be natural light. However, it is possible that ADL is simply a surrogate for seasonal variation in general, which is a nonspecific factor that has many potential confounders, including family planning, vitamin D deficiency, activity of the mother, immunity, temperature, nutrition, and disease.17–20 Although it is not possible to exclude all of these seasonal change variables entirely, 2 of these possibilities, family planning and vitamin D deficiency, may be considered.
In their report of an association between perinatal light exposure and severe myopia, Mandel and colleagues17 suggested (and eliminated) family planning as an alternative explanation for the seasonal variation in eye outcome that they observed. Better-educated parents (who are generally more myopic whether due to genetic or environmental factors) may plan to have their babies born in the summer by conceiving in late fall to early winter, thus increasing the apparent number of subjects with myopia born in the summer. However, it seems unlikely that family planning could explain the higher risk of SROP associated with ADL because higher levels of maternal education and socioeconomic status are generally associated with a lower risk of premature birth.21 We did not detect an association between the development of SROP and the presence or absence of prenatal care, but the quality of prenatal care cannot be ascertained given the retrospective nature of our study. However, our findings are supported by those of Higgins et al,22 who previously reported no difference in the risk of ROP between premature infants with poor prenatal care (0–2 visits) and those with prenatal care. We did find that the development of SROP was associated with a decrease in the per capita income of the mother's residence ZIP code. However, this is an imperfect surrogate for the mother's actual educational level or socioeconomic status, because the actual educational level and socioeconomic status of the mothers were incomplete or could not be accessed. Nevertheless, these findings, along with the lack of a statistical correlation among prenatal care, per capita income, or ADL, seem to argue against family planning as an explanation for the observed association between ADL and SROP.
Another potential explanation for seasonally related SROP outcome is a seasonal variation in vitamin D deficiency. However, the effect of vitamin D deficiency on pregnancy and perinatal outcomes is controversial with many contradictory reports that do not adequately control for confounders.23 Of note, there have been no reports of vitamin D deficiency being associated with ROP. However, through its potential associations with BW and race, vitamin D deficiency may theoretically affect ROP outcome. A seasonal variation in BW has been reported among African Americans but not among Caucasians.24 In one study, African American mothers and neonates had a higher prevalence of vitamin D deficiency than Caucasians, although with less seasonal variation.25 Because we effectively controlled for BW and race through multiple logistic regression, our models should have already accounted for the potential interaction of vitamin D deficiency with SROP via BW or race. That ADL was an independent prognostic factor for SROP in our models leaves open the possibilities that vitamin D deficiency has an effect (if any) on ROP that is independent of its effect on BW, light has an effect on ROP that is independent of vitamin D status, or both.
In considering the hypothesis that seasonal variation in light is the causal element in SROP, we acknowledge that we did not directly measure the light levels in the mother's visceral cavity during early gestation but assumed them to be correlated with her exposure to light, which was also not measured directly but was indirectly associated with the length of day. The light that actually reached the mother's abdomen may be affected by individual and seasonal variations in clothing, in the amount of light indoors, and in the amount of time spent outdoors. During winter compared with summer, thicker layers of clothing are generally worn and less time is spent outdoors. However, these natural tendencies would likely accentuate, not reduce, the association of the seasonal variation in ADL with SROP. Thus, it will be valuable to measure the seasonal variation in light exposure of pregnant women using wearable light sensors.
Additional studies may be performed to support the association of day length with SROP. The seasonal variation in day length increases in amplitude as one travels away from the equator toward the poles, and it changes its phase by 6 months in the Southern Hemisphere. Thus, the association of ADL with SROP may be more readily observed in a city north of Cincinnati; and in the Southern Hemisphere, the corresponding winter months when premature infants with SROP would more likely be conceived would shift to June to August.
Although we found that ADL during early gestation remained a significant prognostic factor when duration of oxygen use was included in our models, it will be important to evaluate other measures of postnatal illness severity in future studies. We did not evaluate ADL beyond 150 days after conception in our models, because this would extend beyond the birthdate of the youngest premature infants (mid-23 weeks estimated GA). However, we did evaluate the sum of day lengths during the entire period of gestation for each infant (as a potential measure of total exposure to daylight during the course of gestation) and found no association with SROP. This finding seems to strengthen the case for early gestation as the important time of light exposure associated with SROP outcome.
Previous experiments in mice also demonstrated greater attenuation of external light across the body wall of darkly pigmented compared with lightly pigmented mice.4 Thus, premature infants born to darker pigmented mothers may theoretically be more susceptible to the effects of dark-rearing during early gestation and exhibit greater risk for ROP. However, in studies from the United States, black race has been shown to be associated with a lower likelihood for the development of SROP, a finding that is supported by the current study.9–11,26 This observation initially seemed to contradict our hypothesis that lower light exposure in utero increases the risk of SROP. However, in model 1, which evaluated the risk of all eyes in the cohort developing SROP, black race and ADL were independent predictors. One possible explanation is that there are protective factors associated with black race that are not necessarily dependent on skin color. For example, a genetic polymorphism in G protein–coupled receptor kinases provides a form of endogenous beta-blockade that may lower the risk for infantile capillary hemangiomas and ROP.27–29 Second, although darker skin may attenuate light transmission, the amount of light reaching the visceral cavity in experiments using pigmented mice appears to be sufficient to stimulate the light response that is involved in retinal vascular development.4 The ROP outcome in black premature infants may therefore be a balance between the putative protective factors associated with race and the seasonal variation in light.
Study Limitations
Our study is limited by its single-center, retrospective design. Examinations were performed by several ophthalmologists and subjected to inter-examiner variability. However, the tendency for examiners to treat at classic threshold ROP during the pre-ETROP era may have had the benefit of increasing the likelihood that only the most severe cases of ROP were included as SROP.
In conclusion, we found that an increase in ADL during early gestation was associated with a lower likelihood of developing SROP. This finding may be plausibly related to a seasonal variation in light exposure to the fetus.4 Further research is needed to understand whether there is a role for higher ADLs during early gestation and the influence of other potentially confounding variables. If supported by additional prospective multicenter studies, these findings may have implications for the use of light during early gestation as a prophylactic treatment to decrease the risk of SROP.
Acknowledgments
The authors thank Sheela Masifi, MS, and Patricia Cobb, BS, for assistance in tabulating length of day and per capita income data.
M.B.Y. was supported by departmental funds from the Abrahamson Pediatric Eye Institute, Division of Pediatric Ophthalmology, Cincinnati Children's Hospital Medical Center. S.R., D.R.C., and R.A.L. were supported by the National Institutes of Health for related basic science research. The sponsors or funding organizations had no role in the design or conduct of this research.
Footnotes
Presented in part at: the Annual Meetings of the American Association for Pediatric Ophthalmology and Strabismus, April 6, 2013, Boston, Massachusetts, and the Association for Research in Vision and Ophthalmology, May 8, 2013, Seattle, Washington.
Financial Disclosure(s): The author(s) have made the following disclosure(s): R.A.L. has a patent application on a light-emitting diode device.
References
- 1.Reynolds JD, Hardy RJ, Kennedy KA, et al. Light Reduction in Retinopathy of Prematurity (LIGHT-ROP) Cooperative Group. Lack of efficacy of light reduction in preventing retinopathy of prematurity. N Engl J Med. 1998;338:1572–6. doi: 10.1056/NEJM199805283382202. [DOI] [PubMed] [Google Scholar]
- 2.Wesolowski E, Smith LE. Effect of light on oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:112–9. [PubMed] [Google Scholar]
- 3.Kremer I, Levitt A, Goldberg G, et al. The effect of light on oxygen-induced vasoproliferative retinopathy in newborn kittens. Invest Ophthalmol Vis Sci. 1992;33:1595–8. [PubMed] [Google Scholar]
- 4.Rao S, Chun C, Fan J, et al. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature. 2013;494:243–6. doi: 10.1038/nature11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNSW Embryology. Carnegie Stage Comparison. [Accessed March 11, 2013]; Available at: http://php.med.unsw.edu.au/embryology/index.php?title=Carnegie_Stage_Comparison.
- 6.O'Rahilly R. Early human development and the chief sources of information on staged human embryos. Eur J Obstet Gynecol Reprod Biol. 1979;9:273–80. doi: 10.1016/0028-2243(79)90068-6. [DOI] [PubMed] [Google Scholar]
- 7.Theiler K. The House Mouse: Atlas of Mouse Development. New York: Springer-Verlag; 1989. pp. 113–21. [Google Scholar]
- 8.Hellstrom A, Perruzzi C, Ju M, et al. Low IGF-I suppresses VEGF-survival signalling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci U S A. 2001;98:5804–8. doi: 10.1073/pnas.101113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang MB, Donovan EF. Risk analysis and an alternative protocol for reduction of screening for retinopathy of prematurity. J AAPOS. 2009;13:539–45. doi: 10.1016/j.jaapos.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Yang MB, Donovan EF, Wagge JR. Race, gender, and Clinical Risk Index for Babies (CRIB) score as predictors of severe retinopathy of prematurity. J AAPOS. 2006;10:253–61. doi: 10.1016/j.jaapos.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Schaffer DB, Palmer EA, Plotsky DF, et al. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Prognostic factors in the natural course of retinopathy of prematurity. Ophthalmology. 1993;100:230–7. doi: 10.1016/s0161-6420(93)31665-9. [DOI] [PubMed] [Google Scholar]
- 12.Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Arch Ophthalmol. 1984;102:1130–4. doi: 10.1001/archopht.1984.01040030908011. [DOI] [PubMed] [Google Scholar]
- 13.Palmer EA, Flynn JT, Hardy RJ, et al. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Incidence and early course of retinopathy of prematurity. Ophthalmology. 1991;98:1628–40. doi: 10.1016/s0161-6420(91)32074-8. [DOI] [PubMed] [Google Scholar]
- 14.Early Treatment of Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the Early Treatment for Retinopathy of Prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–96. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 15.Hardy RJ, Palmer EA, Schaffer DB, et al. Multicenter Trial of Cryotherapy for Retinopathy of Prematurity Cooperative Group. Outcome–based management of retinopathy of prematurity. J AAPOS. 1997;1:46–54. doi: 10.1016/s1091-8531(97)90023-9. [DOI] [PubMed] [Google Scholar]
- 16.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–43. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 17.Mandel Y, Grotto I, El-Yaniv R, et al. Season of birth, natural light, and myopia. Ophthalmology. 2008;115:686–92. doi: 10.1016/j.ophtha.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 18.Chodick G, Flash S, Deoitch Y, Shalev V. Seasonality in birth weight: review of global patterns and potential causes. Hum Biol. 2009;81:463–77. doi: 10.3378/027.081.0405. [DOI] [PubMed] [Google Scholar]
- 19.Tam WH, Sahota DS, Lau TK, et al. Seasonal variation in preeclamptic rate and its association with the ambient temperature and humidity in early pregnancy. Gynecol Obstet Invest. 2008;66:22–6. doi: 10.1159/000114252. [DOI] [PubMed] [Google Scholar]
- 20.Darrow LA, Strickland MJ, Klein M, et al. Seasonality of birth and implications for temporal studies of preterm birth. Epidemiology. 2009;20:699–706. doi: 10.1097/EDE.0b013e3181a66e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansen PW, Tiemeier H, Jaddoe VW, et al. Explaining educational inequalities in preterm birth: the Generation R study. Arch Dis Child Fetal Neonatal Ed. 2009;94:F28–34. doi: 10.1136/adc.2007.136945. [DOI] [PubMed] [Google Scholar]
- 22.Higgins RD, Mendelsohn AL, DeFeo MJ, et al. Retinopathy of prematurity: lack of association with prenatal care. J AAPOS. 1999;3:114–6. doi: 10.1016/s1091-8531(99)70081-9. [DOI] [PubMed] [Google Scholar]
- 23.Urrutia RP, Thorp JM. Vitamin D in pregnancy: current concepts. Curr Opin Obstet Gynecol. 2012;24:57–64. doi: 10.1097/GCO.0b013e3283505ab3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Hanswijck de Jonge L, Waller G, Stettler N. Ethnicity modifies seasonal variations in birth weight and weight gain of infants. J Nutr. 2003;133:1415–8. doi: 10.1093/jn/133.5.1415. [DOI] [PubMed] [Google Scholar]
- 25.Bodnar LM, Simhan HN, Powers RW, et al. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007;137:447–52. doi: 10.1093/jn/137.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saunders RA, Donahue ML, Christmann LM, et al. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Racial variation in retinopathy of prematurity. Arch Ophthalmol. 1997;115:604–8. doi: 10.1001/archopht.1997.01100150606005. [DOI] [PubMed] [Google Scholar]
- 27.Good WV, Hardy RJ, Wallace DK, et al. Beta-blocking and racial variation in the severity of retinopathy of prematurity. Arch Ophthalmol. 2012;130:117–8. doi: 10.1001/archopht.130.1.117. [DOI] [PubMed] [Google Scholar]
- 28.Frieden IJ, Drolet BA. Propranolol for infantile hemangiomas: promise, peril, pathogenesis. Pediatr Dermatol. 2009;26:642–4. doi: 10.1111/j.1525-1470.2009.00977.x. [DOI] [PubMed] [Google Scholar]
- 29.Liggett SB, Cresci S, Kelly RJ, et al. A GRK5 polymorphism that inhibits beta-adrenergic receptor signaling is protective in heart failure. Nat Med. 2008;14:510–7. doi: 10.1038/nm1750. [DOI] [PMC free article] [PubMed] [Google Scholar]


