Abstract
Background
The prevalence of chronic heart failure is increasing, and increases with increasing age. Major symptoms include breathlessness and restricted activities of daily living due to reduced functional capacity, which in turn affects quality of life. Exercise training has been shown to be effective in patients with coronary heart disease and has been proposed as an intervention to improve exercise tolerance in patients with heart failure.
Objectives
To determine the effectiveness of exercise based interventions compared with usual medical care on the mortality, morbidity, exercise capacity and health related quality of life, of patients with heart failure.
Search strategy
We searched the Cochrane Controlled Trials Register (The Cochrane Library Issue 2, 2001), MEDLINE (2000 to March 2001), EMBASE (1998 to March 2001), CINAHL (1984 to March 2001) and reference lists of articles. We also sought advice from experts.
Selection criteria
RCTs of exercise based interventions. The comparison group was usual medical care as defined by the study, or placebo. Adults of all ages with chronic heart failure. Only those studies with criteria for diagnosis of heart failure (based on clinical findings or objective indices) have been included.
Data collection and analysis
Studies were selected, and data were abstracted, independently by two reviewers. Authors were contacted where possible to obtain missing information.
Main results
Twenty-nine studies met the inclusion criteria, with 1126 patients randomised. The majority of studies included both patients with primary and secondary heart failure, NYHA class II or III. Only one study specifically examined the effect of exercise training on mortality and morbidity. Exercise training significantly increased VO2 max by (WMD random effects model) 2.16 ml/kg/min (95% CI 2.82 to 1.49), exercise duration increased by 2.38 minutes (95% CI 2.85 to 1.9), work capacity by 15.1 Watts (95% CI 17.7 to 12.6) and distance on the six minute walk by 40.9 metres (95% CI 64.7 to 17.1). Improvements in VO2 max were greater for training programmes of greater intensity and duration. HRQoL improved in the seven of nine trials that measured this outcome.
Authors’ conclusions
Exercise training improves exercise capacity and quality of life in patients mild to moderate heart failure in the short term. One study found beneficial effects of exercise on cardiac mortality and hospital readmissions over 3 years of follow-up, the remaining included studies did not aim to measure clinical outcomes and were of short duration. The findings of the review are based on small-scale trials in patients who are unrepresentative of the total population of patients with heart failure. Other groups (more severe patients, the elderly, women) may also benefit. Large-scale pragmatic trials of exercise training of longer duration, recruiting a wider spectrum of patients are needed to address these issues.
Medical Subject Headings (MeSH): *Exercise Therapy, Cardiac Output, Low [*rehabilitation; therapy], Chronic Disease, Heart Failure [*rehabilitation; therapy], Quality of Life, Randomized Controlled Trials as Topic
MeSH check words: Humans
BACKGROUND
The prevalence and incidence of chronic heart failure (CHF) is steadily increasing with approximately 550,000 new cases annually in the United States (AHA). Whilst improved management of hypertension has reduced this condition as an aetiological factor in the development of heart failure, the increased survival rate from myocardial infarction has lead to a subsequent increase in the number of cases of chronic heart failure (Kostis 1997), as has increasing longevity in developed countries. In the developing world the occurrence of heart failure can often be attributed to valvular heart disease and nutritional cardiac disease (Lip 2000). Estimates of the prevalence of heart failure in Europe range from 0.4 to 2% in middle aged adults (Cowie 1997), but over 65 years of age the prevalence of CHF is in the region of 6 to 10%. (Kannel 1991). Hospital admission rates for heart failure are rising in all industrialised countries, particularly among the elderly (McMurray 2000).
Patients with heart failure present with a variety of symptoms most of which are often non specific (Watson 2000). The most frequently presenting symptom is exertional breathlessness. Additional important symptoms may be fatigue and lethargy, in addition to swelling of the feet and ankles. Symptoms and functional exercise capacity are used to classify the severity of heart failure (using the New York Heart Association (NYHA) classification) and judge the response to treatment. Whilst the classification of severity is based upon symptoms, the diagnosis is secured with objective measures. The European Task Force report (CHF Taskforce 2001) proposes that the definition of heart failure should rely on two criteria: symptoms of heart failure at rest or during exercise (typically breathlessness and fatigue) and objective evidence of cardiac dysfunction at rest. Where the diagnosis is in doubt, a response to treatment directed towards heart failure may also be used in addition to the above criteria. However, like many chronic diseases there is a poor correlation between symptoms and the degree of cardiac impairment, and also between symptoms and disease prognosis (Hülsmann 2002; Opasich 2001; van den Brock 1992).
Coupled with the pathological changes of heart failure and associated symptoms is a reduction in exercise tolerance due to impaired skeletal muscle function. Muscle function depends upon perfusion, muscle mass, fibre composition and energy metabolism - alteration of any of these will influence physical performance. Muscle performance can be defined as either strength or endurance and both can be reduced in chronic heart failure. For heart failure there is reduced capillary fibre ratio, reduced peripheral blood flow and an alteration in mitochondrial density, leading to a reduced oxidative capacity of the peripheral muscle (Drexler 1992; Mancini 1992; Sullivan 1989). Inactivity contributes to these changes. Decreased exercise capacity restricts activities of daily living, this in turn influences an individual’s independence and quality of life.
The management of chronic heart failure is characterised by a combination of drugs and lifestyle changes. Drug therapy aims to control symptoms by controlling fluid balance and blocking neurohormonal activation. Lifestyle management is important in chronic heart failure and includes diet and exercise. Decreased exercise capacity is the main factor restricting daily activity for this patient group. Patients become trapped in a vicious circle of inactivity and decreasing functional capacity. Historically patients with heart failure were advised to avoid exertion for fear of worsening cardiac function due to myocardial stress. In the late 1970s and early 1980s it was reported that exercise training was safe in patients with impaired ventricular function. These studies reported a significant improvement in work capacity after training (Conn 1982; Lee 1979). In 1990 Coats and colleagues demonstrated a beneficial effect of exercise training on exercise tolerance, peak oxygen consumption and symptoms in a cross over trial of patients with heart failure, and concluded that rest as the mainstay of treatment for chronic heart failure should no longer be accepted (Coats 1990). This has been confirmed in a number of trials subsequently (e.g. Keteyian 1996; Kiilavuori 1996,). More recently studies have focused on the central and peripheral physiological changes after precisely prescribed exercise regimens. The positive effects of exercise training are thought to occur mainly peripherally in skeletal muscle. These include reduced lactate production (delayed onset of anaerobic metabolism), improved aerobic capacity, reduced sympathetic drive and increased vagal tone (Belardinelli 1992; Coats 1992; Hambrecht 1995; Tyni-Lenne/Gordon 96). Ventilatory parameters also improve. Changes in peak oxygen consumption are consistently documented as are improved anaerobic threshold and decreases in the ventilatory equivalent for carbon dioxide (Tyni-Lenne 2001; Wielenga 1999 CHANGE). Some central cardio-vascular changes have been reported such as improved myocardial perfusion (Belardinelli 1999), improved cardiac output at sub-maximal work rates (Coats 1992), associated reduction in peak heart rates (Keteyian 1996) and improved peripheral blood flow (Dziekan 1998; Sullivan 1988).
The effectiveness of exercise based rehabilitation in patients with coronary heart disease (CHD) has been demonstrated in two early meta-analyses (Oldridge 1988; O’Connor 1989), and in an updated recent Cochrane systematic review (Jolliffe 2001). These systematic reviews specifically excluded patients with heart failure. Exercise based rehabilitation significantly reduces all cause and cardiac mortality, cholesterol and cigarette smoking in patients with CHD (Jolliffe 2001). The provision of cardiac rehabilitation for patients with CHD in the UK is required by recent policy (National Service Framework for Coronary Heart disease - Standard 12, DOH 2000). It is suggested that cardiac rehabilitation should also be considered as an option for patients with heart failure who may potentially benefit (Standard 11, DOH 2000). Currently, patients with heart failure are underrepresented in cardiac rehabilitation programmes (NHS 1998).
The current review in a series on cardiac rehabilitation (Jolliffe 2001; Rees 2004) will concentrate on exercise based interventions for heart failure. The effectiveness of exercise based rehabilitation for patients with heart failure has been examined in a recent qualitative overview where the authors found beneficial effects on physical performance in 27 of 31 studies identified, on mortality in 1 of 31 studies, and of quality of life in 1 of 16 studies (Lloyd-Williams 2002). Whilst this review was comprehensive in its search strategy in that many data sources were searched, it did exclude non-English language publications, it included non randomised and before and after studies (only 22 of 31 studies were randomised controlled trials), and made no attempt to pool data statistically from those trials which were identified. Thus no estimate of effect size for exercise interventions for CHF patients was obtained for any outcome examined. Furthermore, several randomised controlled trials have been published which were not included in the above review.
OBJECTIVES
To determine the effectiveness of exercise based interventions compared with usual medical care on the mortality, morbidity, exercise capacity and health related quality of life, of patients with heart failure. A secondary objective was to examine any adverse events associated with exercise in these patients.
METHODS
Criteria for considering studies for this review
Types of studies
Randomised controlled trials either a parallel group or cross-over design.
Types of participants
All adults with chronic heart failure. Only those studies with criteria for diagnosis of heart failure (based on clinical findings or objective indices) have been included. Where possible we have distinguished between patients with primary heart failure (dilated cardiomyopathy - DCM), and those with heart failure secondary to coronary heart disease (CHD). Studies including patients who have previously been offered cardiac rehabilitation for either myocardial infarction or heart failure have been excluded.
Types of interventions
Exercise based interventions, either alone or as a component of comprehensive cardiac rehabilitation (defined as programmes including also other components such as health education and psychological interventions, in addition to exercise interventions). The comparison group was usual medical care as defined by the study, or an “attention placebo”.
Types of outcome measures
All cause mortality;
Morbidity - non-fatal myocardial infarction, revascularisation;
Hospital admissions/re-hospitalisation;
Exercise capacity;
Physical activity levels;
Validated measures of health related quality of life (HRQoL).
Search methods for identification of studies
We searched the Cochrane Controlled Trials Register (The Cochrane Library Issue 2, 2001) using the strategy outlined below. This was updated by searching MEDLINE (2000 to March 2001) on Ovid using a standard RCT filter (Dickersin 1994) and EMBASE (1998 to March 2001) using an EMBASE RCT filter (Lefebvre 1996) and searching CINAHL (1984 to March 2001). In addition, we searched reference lists of articles and sought expert advice. No language restrictions were applied.
CCTR Search Strategy
HEART-FAILURE-CONGESTIVE*:ME
(HEART and FAILURE)
(CARDIAC and FAILURE)
((#1 or #2) or #3)
REHABILITATION*:ME
EXERCISE*:ME
EXERCISE-THERAPY*:ME
SPORTS*:ME
PHYSICAL-EDUCATION-AND-TRAINING*:ME
EXERTION*:ME
REHABILITAT*
(PHYSICAL* near FIT)
(PHYSICAL* near FITNESS)
(PHYSICAL near TRAIN*)
(PHYSICAL* near ACTIVIT*)
(TRAIN* near STRENGTH*)
(TRAIN* near AEROBIC*)
(AEROBIC* near EXERCISE*)
KINESIOTHERAP*
(EXERCISE* near TRAIN*)
(((((((((((((((#5 or #6) or #7) or #8) or #9) or #10) or #11) or #12) or #13) or #14) or #15) or #16) or #17) or #18) or #19) or #20)
(#4 and #21)
Data collection and analysis
Study selection
From the searches, the title and abstract of each paper was reviewed by one reviewer (KR) and potentially relevant references retrieved. Following this initial screening, two reviewers (KR, RT) independently selected trials to be included in this review using predetermined inclusion criteria. In all cases disagreements about any study inclusions were resolved by consensus. The general agreement for two raters over all categories (study included, excluded or pending) was assessed by Cohen’s kappa (weighted). Observed agreement was 88.3%, expected agreement 54%, Kappa = 0.75 (95% confidence interval = 0.57 to 0.93). The quality of trials was assessed in terms of concealment of allocation, losses to follow up, and blind assessment of outcomes. Quality was also assessed by using the Jadad Scale (Jadad 1996).
Data extraction
Study outcome data were extracted by one reviewer (KR) and chief investigators were contacted to provide additional relevant information. Data extraction was checked by a second reviewer (RT) in a random sample of 10 studies.
Data analysis
Dichotomous outcomes were expressed as odds ratios, and 95% confidence intervals (CI) were calculated for each study. For continuous variables net changes were compared (i.e. control group minus intervention group differences) and a weighted mean difference (WMD) or standardised mean difference (SMD) and 95% CI have been calculated for each study. Where standard deviation differences were not reported in the source papers, allowance has been made for within patient correlation from baseline to follow up measurements by using the correlation coefficient between the two (Cochrane Heart Group; Follmann 1992).
For cross-over trials, data from the first arm only has been included. Where data for the two arms is combined then this has been accepted only if the authors state there are no carryover effects, or there is evidence of washout.
For each outcome, a test of heterogeneity was carried out. In the situation of no heterogeneity, fixed effects meta-analysis was used. If substantial heterogeneity (p<0.1) was detected, the reviewers looked for possible explanations (e.g. participants and intervention) for this. If the heterogeneity could not be explained, the reviewers considered the following options: not to aggregate the studies at all, or use a random effects model with appropriate cautious interpretation. Where a random effects model has been used this is indicated in parentheses.
Stratified analyses and meta-regression were used to examine the effect of intensity of the intervention, age, duration of follow up and trial quality on the outcome VO2 max, the outcome most frequently reported in the included studies. These subgroups were defined in advance. Sensitivity analysis was carried out to examine the effect of excluding the few studies which did not include an aerobic training component.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
From the searching, 1162 studies were found, the title and abstracts of these were screened and 79 went forward for formal inclusion or exclusion. Thirty three separate trials met the inclusion criteria. Of these, 4 were later excluded; 3 because of insufficient data despite contacting the authors to obtain information, and one because of overlap in the participants recruited in another included study. Data from the remaining 29 studies with 1126 patients randomised were used in the analyses. Of these 29 trials, 23 evaluate an aerobic intervention and 6 report resistance training of peripheral muscle groups. Twenty three of the trials are parallel group design, the remaining 6 are cross over trials. The majority of trials include both patients with primary (dilated cardiomyopathy) and secondary (ischemic heart disease) heart failure, with only 4 trials reporting these patient groups separately. All patients included met the New York Heart Association (NYHA) criteria for symptoms (class II or III) and all had a left ventricular ejection fraction (LVEF) < 40%. The mean age of participants across the included studies ranged from 51 to 77 years. Most studies recruited predominantly male patients with the exception of two studies that recruited only women (Pu 2001; Tyni-Lenne 1997). Mean follow up was 20 (SD 14) weeks (range 4 to 60 weeks) for exercise variables and quality of life. One study reported clinical data at 3.3 years of follow-up (Belardinelli 1999).
Details of the studies included in the review are shown in the table ‘Characteristics of included studies’. Reasons for exclusion are presented in the table ‘Characteristics of excluded studies’.
Risk of bias in included studies
The methodological quality of the included studies are presented in Table 01. The methodological quality of trials in terms of the method of randomisation, allocation concealment, blinding of outcome assessors and losses to follow up, is as was reported in the papers. For the majority of studies, both the method of randomisation, and the method of allocation concealment were unclear. Most of the trials were short term studies and so therefore the loss to follow up was low, with the exception of five trials where this was greater than 20%. The median Jadad score for the 34 trials was 2 (interquartile range 2 to 3, range 1 to 4), out of a possible score of 5 (Jadad 1996). The Jadad score has been used as an overall indicator of trial quality in the stratified analyses to examine its impact on outcome (see results section).
Effects of interventions
Clinical events
Only one study with long term follow-up aimed to examine the effect of exercise on mortality and morbidity (Belardinelli 1999). This study showed that cardiac mortality was statistically significantly reduced with the exercise intervention over 3.3 years of follow-up (99 patients randomised, odds ratio 0.32 (95% CI 0.13, 0.8)), as were hospital readmissions for heart failure (odds ratio 0.28 (95% CI 0.09, 0.85)). There was no statistically significant effect of the intervention on non-fatal myocardial infarction (odds ratio 0.48 (95% CI 0.04, 5.47)).
Several studies report deaths as dropouts during the study period, which the authors state were unrelated to the intervention (Gottlieb 1999; Hambrecht 1995; Hambrecht 1998; Hambrecht 2000; Owen 2000; Ponikowski 1997; Teo 1995 EXERT; Wielenga 1999 CHANGE). Nonetheless, if we pool this data for all cause mortality there is no significant difference between the intervention and control groups (odds ratio 1.12 (95% CI 0.58 to 2.15)). The follow-up period for these trials ranged from 16 to 52 weeks. One study reported a non-fatal MI in the control group (Ponikowski 1997), none of the studies report other clinical events.
Adverse events
Whilst not a primary objective of the review, all studies were examined for reports of any adverse events during exercise training. Adverse events were those as defined by the authors, and/or clinical events (including deaths, myocardial infarction, arrhythmias) reported to be associated with exercise training. The majority of studies state that there were no adverse events associated with the intervention (17 of 29 studies), although these studies were not designed to examine safety, nor did they state in their objectives that they would do so. Only one trial reported complications associated with training, but these were confined to the most severe patients with ejection fractions below 30% (Jette 1991).
Exercise variables
For all variables concerned with exercise capacity, an increase in value from baseline to follow-up indicates improvement with the exercise intervention. For this reason, the sign of the mean change in these variables for both the intervention and control groups has been changed for the pooled analysis so the direction of effect is in the appropriate direction on the Metaview plots.
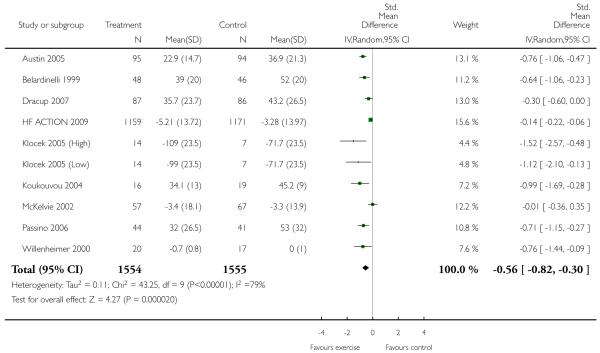
VO2 max was measured in 24 studies (848 participants randomised) and improved markedly with exercise training by 2.16 ml/kg/min (WMD random effects model, 95% CI 2.82 to 1.49). Similar significant improvements were seen for exercise duration measured in 15 studies (510 participants randomised) which increased by 2.38 minutes (WMD 95% CI 2.85 to 1.92), and maximum work capacity measured in six studies (219 participants randomised) which increased by 15.1 Watts (WMD 95% CI 17.7 to 12.6) in the training group. Distance on the six minute walk was measured in eight studies (282 participants randomised) which increased by 40.9 metres (WMD random effects model 95% CI 64.7 to 17.1). Heterogeneity between studies for this outcome is due to one trial (Teo 1995 EXERT) that showed no effect of intervention, whilst the remaining seven showed large positive effects. This trial measured outcomes over the short (3 months) and longer term (12 months) and results were similar for both time periods.
Subgroup analyses
Marked heterogeneity was seen for the outcome VO2 max (chi square 61.27, p < 0.00001), and to explore this further we carried out a number of prespecified subgroup analyses. The cut-off points used for each explanatory variable were the median values for the studies reporting this outcome.
The variables thought to have an impact on heterogeneity between studies included
the intensity of the intervention (“Dose” calculated as the number of weeks multiplied by the number of sessions per week, multiplied by the duration of sessions in hours);
duration of the intervention;
trial quality assessed by the Jadad score (determined from what was reported in the papers);
duration of follow-up and
mean age.
The “dose” of the intervention varied from 3 to 112 units (mean 40.3 SD 30.7), and the duration of the intervention varied from 3 to 52 weeks (mean 16 SD 11.5) across the studies.
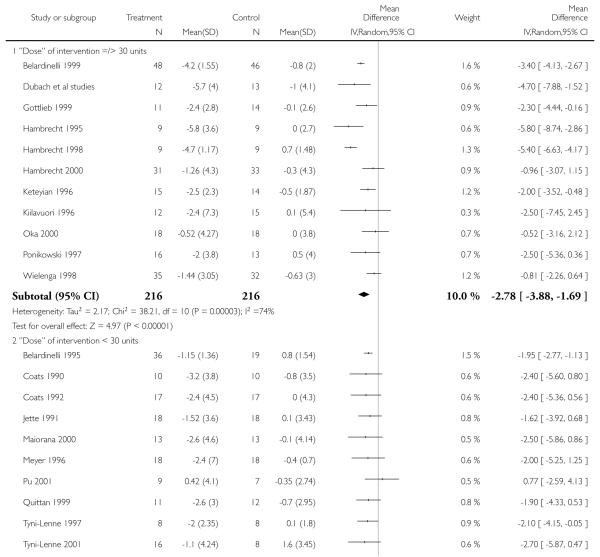
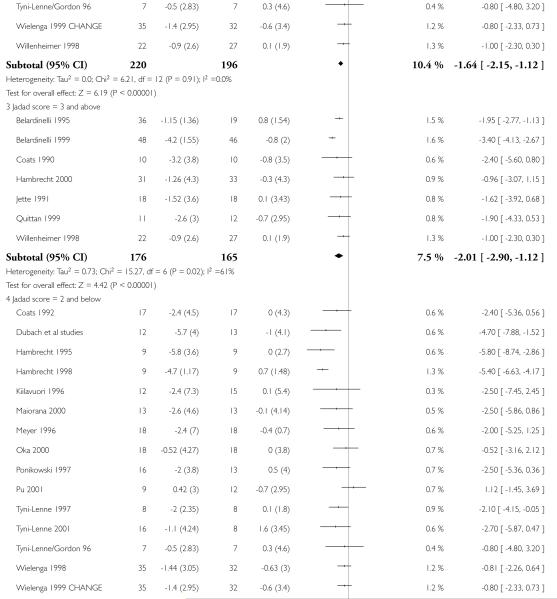
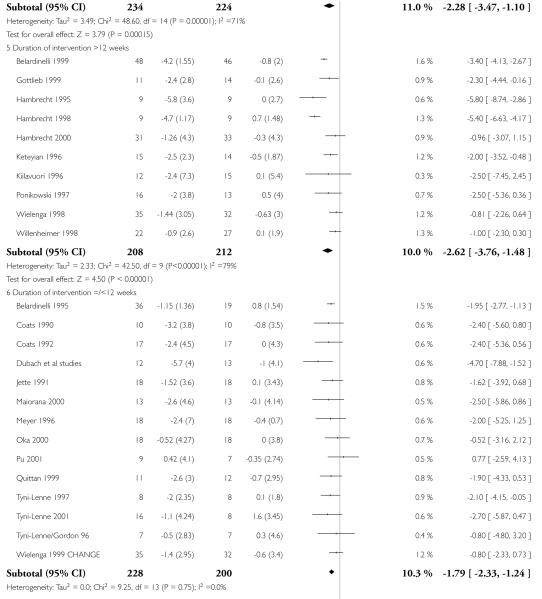
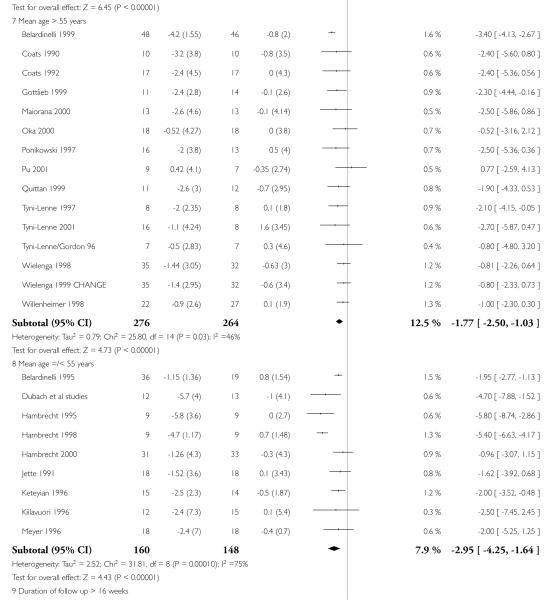
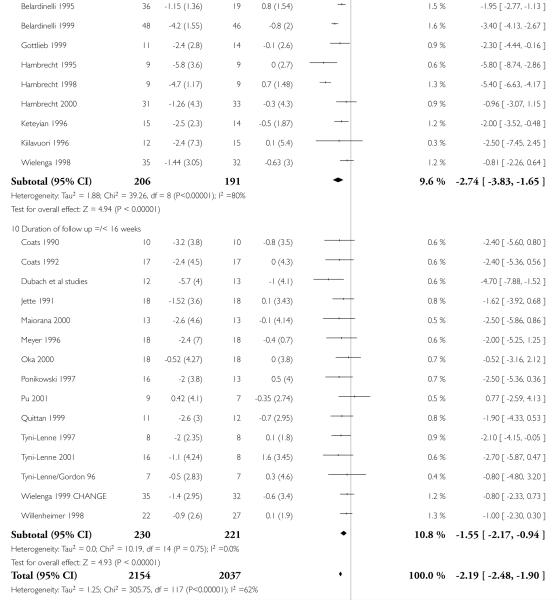
Interventions of greater intensity had a larger effect on the improvements seen in VO2 max but the 95% CI’s overlapped with lower doses suggesting that the difference between the two was not statistically significant (Dose > 30 units WMD, random effects model 2.78 (95% CI 3.88 to 1.69)), Dose < 30 units WMD 1.64 (95% CI 2.2 to 1.1). Slightly larger effects on VO2 max were seen with studies of poorer methodological quality (Jadad score 2 and below), but this was not statistically significant. The improvement in VO2 max was greater in patients aged less than 55 years WMD 2.95 (95% CI 4.25 to 1.64) versus greater than 55 years WMD 1.77 (95% CI 2.5 to 1.03), but again this does not reach statistical significance. Age is reported in all studies as an aggregate measure and thus comparison between these two age groups may be misleading as individual patient data are not available to examine effects across the whole age range. The effect size increased with increasing duration of follow-up, but this is a marker also for the duration of the intervention since most studies were short term with follow-up assessment at or shortly following the end of the intervention.
Meta regression
Heterogeneity for the outcome VO2 max was also examined with meta-regression. Covariates defined a priori included trial quality, dose of intervention, duration of intervention and length of follow-up. In univariate analyses, the Jadad score had no effect on the level of VO2 max achieved at the end of exercise training. Statistically significant associations were seen with dose of intervention, duration of intervention and duration of follow-up. In multivariate analyses both the dose of the intervention and the duration of follow-up contributed to the heterogeneity of VO2 max, where the weighted mean difference (WMD) for VO2 max is multiplied by a factor of 1.01 per unit increase in dose (coefficient log WMD 0.0137 95% CI 0.0019 to 0.025 p=0.023), and the weighted mean difference for VO2 max is multiplied by a factor of 1.03 per week increase in follow-up duration (coefficient log WMD 0.0302 95% CI 0.013, 0.046 p<0.001). Thus the level of VO2 max at the end of exercise training increases with increasing dose of the intervention, and increasing follow-up period. As noted above, since most studies were short term studies, the follow-up period is a marker for exercise duration, which is also included in the estimate of dose.
Sensitivity analyses
The majority of studies include an aerobic exercise component. To determine the impact of aerobic exercise alone, sensitivity analyses were carried out excluding studies which focused exclusively on resistance training. This had no significant effect on the pooled analysis for the outcomes VO2 max or distance on the 6 minute walk.
Health related quality of life
The number of different scales used to assess HRQoL, and the different intervention groups (both aerobic and resistance training), as well as the relatively few trials that reported this as an outcome, meant that a pooled analysis was inappropriate. Data has been presented qualitatively in Table 02. Nine of 29 trials reported HRQoL as an outcome. Seven out of nine studies found improvements in HRQoL in the intervention group compared to control. Five studies used the disease specific measure the Minnesota Living with Heart Failure questionnaire (Belardinelli 1999; Parnell 2002; Ponikowski 1997; Teo 1995 EXERT; Tyni-Lenne 2001) where significant improvements were seen for 4/5 studies over the short term. Two of these 5 studies measured quality of life over the longer term of 12 months, one showed improvement was maintained over the long term (Belardinelli 1999), the other showed no difference between intervention and control groups, as for the shorter term assessment (Teo 1995 EXERT). Other scales used included the MOS Short Form (SF) 36, CHF questionnaire, Sickness Impact Profile (SIP) and Nottingham Health Profile (NHP) parts 1 and 2.
DISCUSSION
This is the most comprehensive systematic review to date to examine the effectiveness of exercise training for heart failure. However, the trials identified for inclusion were mostly small and of relatively poor methodological quality, recruiting mostly men, and patients with stable chronic heart failure. Patients with severe disease and co-morbidities were often excluded. The findings therefore are confined to this particular group of patients and may not be generalisable to all patients with heart failure.
Highly statistically significant improvements in exercise capacity were found as assessed by maximum oxygen uptake, exercise duration, distance on the 6 minute walk and physical work capacity. These improvements in intermediate outcomes confirm previous findings (Lloyd-Williams 2002). As most trials are of relatively short duration, it is unclear whether these improvements are sustained. Indeed, conflicting results were found for the two largest trials monitoring the effects of exercise training over 12 months where sustained improvement in functional capacity was found in one trial (Belardinelli 1999), and no effect of exercise training was found in the other (Teo 1995 EXERT).
One of our principal objectives was to determine the effect of exercise training on clinical outcomes. Only one trial with a long follow-up period examined the effect of exercise training on mortality and morbidity, where favorable outcomes were seen for cardiac mortality and hospital readmissions (Belardinelli 1999). The authors of this trial state that the patients who died had a higher resting heart rate, end-diastolic diameter and wall thickening score index, and lower systolic blood pressure and VO2 max at peak exercise than those patients who survived. Other studies did report clinical events as reasons for loss to follow-up, but the majority of these (7/8 trials) were short term studies. Pooling this data for the 8 of 29 trials reporting all cause mortality showed no evidence of effect of exercise training on this outcome. Further longer term trials are needed to examine the effect of exercise training on clinical outcomes. Several trials are currently underway and we await the results with interest. The Exercise Intervention Strategies in Heart Failure Trial (EXIST) is a multicentre trial designed to examine the effect of exercise training on morbidity and mortality in heart failure patients (NRR ongoing trial 1). The trial was due to be completed in December 2002. Another trial, due to complete in January 2004 looks specifically at elderly patients with heart failure and whether long term provision of exercise training can improve mortality and morbidity (NRR ongoing trial 2).
Whilst it was not our primary aim to examine adverse events, it is noteworthy that only one of 29 trials reported complications associated with training, and in the most severe patients (Jette 1991). As most of the trials are conducted in patients with stable chronic heart failure with NYHA functional class II or III, perhaps this is not unexpected. Concerns about safety have historically been the reason for exclusion of heart failure patients from exercise training and cardiac rehabilitation programmes. Today, it is now generally accepted that exercise training is safe in patients with stable heart failure, but that there is as yet no trial evidence to recommend exercise training in unstable heart failure or those with NYHA class IV (CHF Guidelines 2001). We were unable to examine the effects of exercise training separately for those patients with dilated cardiomyopathy and those with heart failure secondary to coronary heart disease as most trials recruited a mix of these patients, the majority being those in the latter group. Similarly we were unable to examine the effects of gender or age on outcome. Lack of research evidence in these groups may result in exclusion of women and the elderly from training programmes.
Relatively few trials examined health related quality of life. Of those that used validated scales to assess this, many different instruments were used over different time periods which meant that pooling data statistically was inappropriate. Most of the studies that measured this outcome (7 of 9 studies) showed beneficial effects of exercise training. As for the exercise variables above, the 2 trials which measured quality of life over the long term (12 months) had conflicting results (Belardinelli 1999; Teo 1995 EXERT). More large scale trials of longer duration are needed to resolve these discrepancies. The publication of the two longer term ongoing trials cited above (NRR ongoing trial 1, NRR ongoing trial 2) will add to the existing evidence and may help resolve these issues.
The exercise intervention varied in mode, intensity and duration between studies. The current guidelines for exercise training in heart failure acknowledge the benefits of aerobic training but that the precise protocol is yet to be established and should be individually tailored to baseline clinical and functional status. Relatively little attention has been paid to resistance training, and it is not generally recommended until further data are available (CHF Guidelines 2001). Most of the studies included in this review examined aerobic training. Exclusion of the few studies which focused exclusively on resistance training had no effect on functional outcomes. The outcome VO2 max was most frequently reported and showed large heterogeneity between studies. To try to explain this and examine the impact of different levels of exercise on VO2 max, we performed metaregression. The dose of the intervention (the product of exercise duration, number of sessions and duration of training period) explained much of the variation, with improvements in VO2 max seen for increasing “doses” of exercise training. This finding is important and with the advent of longer term studies, the longer term effects of different training periods can be established.
AUTHORS’ CONCLUSIONS
Implications for practice
This review shows that exercise training improves exercise capacity and HRQoL in patients with NYHA functional status class II or III heart failure. However, further work is necessary to inform clinical guideline development. At present exercise training is only recommended in those patients with stable heart failure and NYHA functional class II or III, and only aerobic exercise training is recommended in a supervised hospital based setting, each protocol being tailored to individual needs. Whilst each patient must be treated as an individual, some broad protocols informed by research would help in clinical practice. Due to lack of research there is little evidence of benefit in certain groups (more severe patients, elderly people, women) who may then subsequently be omitted from programmes. The trial evidence to date is unlikely to represent the majority of patients with heart failure.
Implications for research
The findings are based on small trials in patients who are unrepresentative of the total population of patients with heart failure. The effectiveness of exercise training on functional capacity and quality of life is clear in the short term but is unknown in the longer term. Large, long-term pragmatic trials of exercise training are needed to determine the effectiveness of exercise training on morbidity, quality of life, and mortality. The value of continued exercise training for maintenance of benefit also requires evaluation.
PLAIN LANGUAGE SUMMARY.
Exercise training improves exercise tolerance and quality of life in people with mild to moderate heart failure
People with heart failure experience breathlessness and restricted activities of daily living because of their restricted heart capacity. This can reduce their amount of exercise, which can further reduce fitness, making their symptoms worse. The review found short-term trials of exercise training in people with mild to moderate heart failure only, which do not represent most of the people who have heart failure. The kinds of exercise programs varied greatly, but most included aerobic exercise rather than resistance training (such as working with weights). Exercise improved people’s fitness and quality of life, without causing harm.
ACKNOWLEDGEMENTS
We would like to acknowledge the following authors for providing us with additional data, A Maiorana, A Gordon.
We would like to acknowledge Dr Andrea Conti and Dr Jan Melichar for translations.
SOURCES OF SUPPORT
Internal sources
Department of Social Medicine, University of Bristol, UK.
Department of Public Health and Epidemiology, University of Birmingham, UK.
External sources
British Heart Foundation, UK.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Parallel group RCT |
| Participants | . HF diagnosis mixed, n=12 IHD, n=8 DCM. LVEF<40%. 20 patients randomised, mean age 61.5 years, 90% men |
| Interventions | Aerobic exercise in outpatient setting for 8 weeks, 3 times a week for one hour. Workload was prgressively increased from 40% VO2max to 65% in the last week. Follow-up assessment at end of intervention period of 8 weeks |
| Outcomes | Exercise duration |
| Notes | Been translated from Italian publication |
| Methods | Parallel group RCT |
| Participants | HF diagnosis mixed - n=18 IHD, n=37 DCM, NYHA class 2 or 3. 55 patients randomised, 85% men, mean age 55 years |
| Interventions | Aerobic exercise, 1 hour 3 times a week for 8 weeks on a bike ergometer, intensity altered according to individuals progression, patients monitored by telemetry. Follow-up assessment at 12 months |
| Outcomes | VO2max Peak workload |
| Notes |
| Methods | Parallel group RCT |
| Participants | HF diagnosis mixed - 85% ischaemic cardiomyopathy, 15% idiopathic DCM, LVEF <40%. 99 patients randomised, 89% men, mean age 59 years |
| Interventions | Aerobic exercise - 2 phases - 8 week programme and 12 months maintainance programme. Phase 1 - warm up stretching 15-20 minutes, 40 mins on bike ergometer for at 60% VO2max, 5 minutes cool down, 3 times a week. Phase 2 - repeat of phase 1 but 2 times a week for remaining 12 months. Compliance from attendance at sessions −89%. Follow-up measurement at 14 months (repeat exercise test), patients monitored for 3.3 years |
| Outcomes | VO2max HRQoL |
| Notes |
| Methods | Parallel group RCT |
| Participants | Mixed CHF patients, most secondary to IHD. NYHA class 2 or 3, CHF diagnosis for at least a year. 24 patients randomised, 67% men, mean age 63 years |
| Interventions | Strength/resistance training - peripheral dynamic training (circuit weight training regimen 60% 1 rep maximum), 60 minutes, 2 times a week for 5 months. Compliance 75%. Follow-up assessment at end of intervention at 5 months |
| Outcomes | HRQoL |
| Notes |
| Methods | Cross over RCT |
| Participants | All patients had diagnosis of HF secondary to MI, LVEF<40%. 11 patients randomised, all men, mean age 63 (7.6) years |
| Interventions | Aerobic exercise on bike ergometer at 70-80% max HR. 1 minute warm up, 20 minutes cycling, 1 minute cool down, 5 days a week for 8 weeks. Exercise performed at home - patients given a HR monitor. Control group asked to avoid exercise over and above their normal level, particularly if it caused breathlessness or fatigue. Following crossover from the trained group, bikes were taken from them and the same activity restriction was recommended. Data has been combined for the 2 arms, but the authors explicitly state there were no carry over effects. Compliance to exercise was monitored by bike revolutions (mean compliance 74%). Followed up at the end of the intervention at 16 weeks |
| Outcomes | VO2 max Exercise duration |
| Notes |
| Methods | Cross over RCT |
| Participants | All patients had diagnosis of HF secondary to MI, LVEF<40%, NYHA class 2 or 3. 19 patients randomised, all men, mean age 61.8 years |
| Interventions | Aerobic exercise on bike ergometer at 50 rpm for 20mins 5 times a week for 8 weeks. Bikes used at home by the patient - given HR monitor. Compliance from bike revolutions - mean 77.3%. Control group asked to avoid exercise over and above their current level, particularly if it caused dyspnoea. Data combined for the 2 arms of the trial, but the authors explicitly state there were no carry-over effects. Followed up at the end of the intervention at 16 weeks |
| Outcomes | VO2 max Exercise duration |
| Notes |
| Methods | Parallel group RCT |
| Participants | CHF secondary to CHD - all patients had had a prior MI, NYHA class 2 or 3, LVEF <40%. 25 patients randomised, all men, mean age 55 years |
| Interventions | Aerobic exercise - 8 week residential course in Swiss mountains. Comprehensive rehabilitation including exercise, education and a low fat diet. Outdoor walking for 1 hour twice a day , stationary cycling 4 times a week for 45 minutes at 70-80% HR reserve. Follow-up assessment at end of intervention period of 8 weeks |
| Outcomes | VO2max Exercise time Exercise capacity |
| Notes |
| Methods | Parallel group RCT |
| Participants | Mixed CHF - primary n=18, ischaemic n=7, NYHA 2 or 3, LVEF <40%. 33 patients randomised, 88% men, age range 64-67 years |
| Interventions | Aerobic exercise, 6 months supervised training 3 times a week, cycling and treadmill walking, exercise progressively increased to perceived exertion of 12-13 on the Borg Scale. Compliance 75%. Outcome assessment at end of intervention period of 6 months |
| Outcomes | VO2 max Exercise duration Distance on 6 minute walk |
| Notes |
| Methods | Parallel group RCT |
| Participants | Majority of patients DCM (19/22), remainder IHD. NYHA class 2 or 3, LVEF <40%. 22 patients randomised, all men, mean age 51 years |
| Interventions | Aerobic exercise, 3 weeks in hospital, remainder of 6 months at home, and group sessions. Hospital - 10 minutes 6 times a day for 3 weeks on a bike ergometer under strict supervision, workload 70% VO2max. Home - asked to exercise close to target HR (bikes and pulse rate monitoring equipment loaned to patients) twice a day for 40 minutes, plus also attend 2 group sessions per week for 1 hour each. Follow-up at end of the intervention period of 6 months |
| Outcomes | VO2 max Exercise duration |
| Notes |
| Methods | Parallel group RCT |
| Participants | Mixed CHF - DCM n=13, CHD n=7, NYHA 2 or 3, LVEF<40%. 20 patients randomised, all men, mean age 55 years |
| Interventions | Aerobic exercise, 3 weeks on an individual care ward and remaining 6 months at home. 3 weeks - bike ergometer 6 times daily for 10 minutes at 70% VO2max. Home - patient took bike ergometer home to exercise 5 times a week for 40 minutes (total of twice daily), and attend 1 group session per week. Compliance to home training estimated to be 70%. Follow-up assessment at end of intervention period of 6 months |
| Outcomes | VO2max |
| Notes |
| Methods | Parallel group RCT |
| Participants | Majority of patients DCM (61/73), remainder IHD. LVEF <40%. 73 patients randomised, all men, mean age 54.5 years |
| Interventions | Aerobic exercise, 2 weeks on an individual care ward and remaining 6 months at home. 2 weeks - bike ergometer 46 times daily for 10 minutes at 70% VO2max. Home - patient took bike ergometer home to exercise 5 times a week for 20 minutes at 70% VO2max, and attend 1 group session per week of 1 hour duration. Follow-up assessment at end of intervention period of 6 months |
| Outcomes | VO2 max Exercise duration |
| Notes |
| Methods | Parallel group RCT |
| Participants | CHF secondary to MI, NYHA 2 or 3 and LVEF<50%. 39 patients randomised, all men, mean age 50.8 years |
| Interventions | Aerobic exercise 5 days a week for 4 weeks - jogging 5 mins 3 times a day, cycling 15 mins (both at 70-80% max HR), calisthenics (30 mins) and relaxation training (20 mins). Follow-up assessment at end of intervention period at 4 weeks |
| Outcomes | VO2max Peak workload |
| Notes |
| Methods | Parallel group RCT |
| Participants | Mixed CHF - IHD (n=9) and DCM (n=20) CHF patients, NYHA 2 or 3, LVEF 35% or less. All men, mean age 54 years |
| Interventions | Aerobic exercise, 45 minutes (5 mins warm up, 35 mins 3 types aerobic activity, 5 mins cool down) 3 times a week for 24 weeks. Follow-up measurements at 24 weeks |
| Outcomes | VO2max Exercise duration |
| Notes |
| Methods | Parallel group RCT |
| Participants | Mixed CHF - CHD (n=9) and DCM (n=18), NYHA 2 or 3, LVEF<40%. 27 patients randomised, 96% men, mean age 52 years |
| Interventions | Aerobic exercise - 3 months supervised, 3 months home based. Supervised - bike ergometer 30 mins 3 times a week at 50-60% VO2max for 2-3 weeks, then increased workload depending on individual HR thereafter. Home based - walking, rowing, cycling or swimming depending on individual instruction. Control group advised not to change their previous physical activity during the 6 months. Follow-up assessment at 6 months |
| Outcomes | VO2max Exercise duration |
| Notes |
| Methods | Cross over RCT |
| Participants | Mixed CHF, n=7 CHD, n=6 DCM, NYHA 1-3, LVEF mean 26%. 13 patients randomised, all men, mean age 60 years |
| Interventions | Aerobic and resistance training. 8 weeks training, 1 hour 3 times a week. Whole body exercise concentrating on the large muscle groups. Combination of circuit training, cycle ergometry, treadmill and resistance weight training. Intensity and duration of exercise gradually increased over the 8 week period. Follow-up assessment at 16 weeks (cross over trial) |
| Outcomes | VO2max Exercise duration |
| Notes |
| Methods | Cross over RCT |
| Participants | Severe CHF, patients are hospitalised. Mix of CHF due to DCM (n=9) and IHD (n=9). 18 patients randomised, all men, mean age 52 years |
| Interventions | Aerobic and resistance training. 3 weeks in hospital training on bike ergometer and treadmill at 50% max work rate,10-15 minutes 3-5 times per week for each exercise respectively. Also muscle strength exercises, coordination and inspiratory muscle training 3 times a week for 20 minutes. Follow up after 6 week intervention period. Data presented combined for the 2 arms, but authors explicitly state that no evidence of carry-over effects was found |
| Outcomes | VO2 max Peak workload (Watts) |
| Notes |
| Methods | Parallel group RCT |
| Participants | Mixed CHF, LVEF <40%. 40 patients randomised, 77.5% men, age range 30-76 years |
| Interventions | Aerobic and resistance training at home for 3 months. Walking at home 3 times a week increasing the intensity and duration over the initial 2-3 weeks to 70% peak HR for 40-60 minutes. Total body unilateral resistance exercises 2 times a week up to 75% 1 rep max. Weekly phonecalls to answer questions and monitor adherence. Follow-up assessment at end of intervention period of 3 months |
| Outcomes | VO2max Exercise duration HRQoL |
| Notes |
| Methods | Cross over RCT |
| Participants | Mixed aetiology of CHF, 67% CHD, 33% AF, LVEF<40%. Focus on the elderly >75 years, mean age 81 years, 75% men. 22 patients randomised |
| Interventions | Resistance training - 6 station circuit with stations alternating between stamina and strengthening exercises. Patients worked in a range comfortable to them and warned not to exceed 70% max HR (showed how to monitor this). Average session 1 hour, once a week for 12 weeks. Follow-up assessment at end of intervention period at 24 weeks (cross over trial) |
| Outcomes | Distance on 6 min walk |
| Notes |
| Methods | Parallel group RCT |
| Participants | Mixed aetiology of CHF, NYHA 2 or 3. 21 patients randomised, 91% men, age range 53-57 years |
| Interventions | Aerobic exercise - 8 weeks of walking, cycling and light weights at 50-60% maximum HR, increasing exercise duration progressively from 30 mins-60 mins per day for 5-7 days per week. Follow-up assessment at end of intervention period of 8 weeks |
| Outcomes | Distance on 6 min walk HRQoL |
| Notes |
| Methods | Parallel group RCT |
| Participants | Mixed aetiology of CHF, most (88%) CHD, 12% DCM, LVEF <45%. 32 patients randomised, 81% men, mean age 57 years |
| Interventions | Aerobic exercise training - first 1-2 weeks in hospital, following modified Royal Canadian Airforce Training programme, and graded walking, under the supervision of a physiotherapist (max HR 75-80%). 35-45 mins exercise per day. For remaining 16 weeks, similar walking programme to be done individually at home for 6 days each week. Comparison group - usual care. Follow-up assessment at end of 16 weeks |
| Outcomes | VO2max Exercise duration Peak workload (Watts) Clinical outcomes. HRQoL |
| Notes | Been translated from Polish publication |
| Methods | Parallel group RCT |
| Participants | Mixed group of mild-moderate CHF, ischaemic and idiopathic, NYHA class 1-3. All women over the age of 65 years (mean 77 years), 16 patients randomised |
| Interventions | Resistance training - high intensity progressive training 3 times a week for 10 weeks, 1 hour sessions. Dynamic contraction of large upper and lower body groups, at 80% weight that could be lifted in good form (1 repetition max). Control group received “placebo” controlled stretching. Attendance at classes averaged 98% in both groups. Follow-up assessment at end of intervention period of 10 weeks |
| Outcomes | VO2max Distance on 6 min walk |
| Notes |
| Methods | Parallel group RCT |
| Participants | All DCM patients, NYHA 2 or 3, LVEF <30%. 25 patients randomised, 81.5% men, mean age 55.3 years |
| Interventions | Aerobic exercise programme - 3 months bike ergometer training and step exercises 1 hour (short warm up and cool down and 2 times 25 minutes training) 2-3 times per week, at 50% maximal functional capacity. Follow up assessment at end of intervention period of 3 months |
| Outcomes | VO2max Exercise duration HRQoL |
| Notes |
| Methods | Parallel group RCT |
| Participants | Mixed CHF, for majority of patients CHD (76%), NYHA 1-3, LVEF >40%. 181 patients randomised, 81% men, mean age 65.5 years |
| Interventions | Aerobic and resistance training in supervised rehabilitation programme for 3 months and continued home based training for a further 9 months. Aerobic training HR set at 60-70% maximum HR. Cycle, treadmill and arm ergometry exercise for 30 mins per session twice a week and walking at home once a week. Supervised resistance training twice a week at 40-60% 1 repetition maximum - arm curls, knee extensions and leg press. After 3 months patients provided with a bike and set of free weight and instructed to train 3 times a week for remaining 9 months. Follow-up assessment at 12 months |
| Outcomes | Distance on 6 min walk HRQoL |
| Notes |
| Methods | Cross over RCT |
| Participants | Aetiology of CHF both CHD (n=8) and DCM (n=8). NYHA class 2 or 3, LVEF<40%. 16 patients randomised - all women, mean age 62 years |
| Interventions | Endurance training of leg muscles 3 times a week for 8 weeks on a modified knee extensor ergometer. 15 minutes at 60 reps per minutes intensity progressively increasing from 65-75% peak work rate. Follow-up at the end of 16 weeks (crossover study) |
| Outcomes | VO2 max Distance on 6 minute walk |
| Notes | Contacted authors re: QoL data |
| Methods | Parallel group RCT |
| Participants | Aetiology of CHF both CHD and DCM. LVEF<40% and NYHA class 2 or 3. 24 patients randomised, 54% men, mean age 62.5 years. Randomisation to intervention 2:1 |
| Interventions | Strength/resistance exercise using resistance rubber bands. 8 weeks supervised group exercise 3 times a week for 1 hour. 6 minutes warm up, 45 minutes training - continuous repetitive contractions against a resistance - one muscle group at a time with 25 reps, 9 minutes cool down. Compliance measured as attendance - 95%. Follow-up assessment at the end of the 8 week intervention period |
| Outcomes | VO2 max Distance on 6 minute walk HRQoL |
| Notes |
| Methods | Parallel group RCT |
| Participants | Aetiology of CHF both CHD and DCM. NYHA class 2 or 3. 21 patients randomised, all men, mean age 60 years |
| Interventions | Endurance training with continuous knee extensor exercises at 60 reps per minute performed on a modified bike ergometer. Two intervention groups - 1 legged exercise and 2 legged exercise where the same relative quantity of muscle work per session was performed, but the quantity of muscle mass activated was different. 8 weeks of training 3 times a week for 25-40 minutes. Compliance estimated to be between 90-100%. Follow-up assessment at the end of the intervention period at 8 weeks |
| Outcomes | Distance on 6 minute walk HRQoL VO2max (Gordon 96) |
| Notes | Assuming same study as Gordon 1996. VO2max measured in that study |
| Methods | Parallel group RCT |
| Participants | Aetiology of CHF both CHD and DCM. LVEF <40% and NYHA class 2 or 3. 67 patients randomised, all men, mean age 64 years. Patients stratified and outcomes expressed in terms of those greater and less than 65 years of age |
| Interventions | Aerobic exercise training - 12 weeks training 3 times a week - 45 minute sessions of walking, cycling and ball games 10 mins each with 5 mins rest. Target HR maintained for at least 20 minutes. Follow-up assessment at end of intervention period of 12 weeks |
| Outcomes | VO2max Exercise duration |
| Notes |
| Methods | Parallel group RCT |
| Participants | Aetiology of CHF both CHD and DCM. LVEF <40% and NYHA class 2 or 3. 80 patients randomised, all men, mean age 56.6 years |
| Interventions | Aerobic exercise, 12 weeks training 3 times a week, comprising 3 exercises for 10 mins each separated by 5 mins rest (cycling, walking and ball games), at 60% max HR. Walking progressed to slow running during the course of the training period. 85.4% compliance. Follow-up assessment at end of the intervention period of 12 weeks |
| Outcomes | VO2max Exercise duration |
| Notes |
| Methods | Parallel group RCT |
| Participants | Aetiology of CHF both CHD (75%) and DCM. Boston HF criteria and LVEF <45%. 54 patients randomised, 71.5% men, mean age 64 years |
| Interventions | Aerobic exercise - 16 weeks interval training on a bike ergometer - 90 seconds exercise at 80% VO2 max, 30 seconds rest. Exercise time gradually increased from 15 minutes total 2 times a week to 45 minutes 3 times a week from week 7. Mean compliance 74.5%. Follow-up assessment at end of intervention period of 16 weeks |
| Outcomes | VO2max Peak workload (Watts) |
| Notes |
AF: atrial fibrillation
CHF: chronic heart failure
CHD: coronary heart disease
DCM : dilated cardiomyopathy
HR: heart rate
HRQoL: health related quality of life
LVEF: left ventricular ejection fraction
Mins: minutes
NYHA: New York Heart Association classification
QoL: quality of life
RCT : randomised controlled trial
VO2max: peak oxygen uptake
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adamopoulos 1995 | No relevant outcomes |
| Barnard 2000 | No relevant outcomes |
| Belardinelli2 1995 | Not an RCT |
| Cohen-Solal 1994 | Not an RCT |
| Davey 1992 | As stated in Coats 1992, there is overlap in some of the patients taking part in each trial. Efforts have been made to obtain data for those that participated only in the Davey trial but these studies were conducted over 10 years ago. To be conservative we have excluded this study from the analyses |
| Gordon 1999 | Exercise versus exercise comparison - not usual care |
| Johnson 1998 | Inspiratory muscle training, not whole body training |
| Kavanagh 1996 | Not an RCT |
| Koch 1992 | Only relevant outcome was QoL with little detail given in the paper. Written to the authors for the data available but no response |
| Metra 1998 | Not an RCT |
| Page 1994 | Exercise versus exercise comparison - not usual care |
| Taylor 1999 | No baseline data were presented in the publication for the outcomes of interest. Written to authors to try to obtain these, but no response |
| Tokmakova 1999 | No data were presented for the control group in the publication. Written to authors to try to obtain these, but no response |
| Tyni-Lenne 1998 | Not an RCT |
| Tyni-Lenne 1999 | Patients were previously trained |
DATA AND ANALYSES
Comparison 1. All exercise interventions versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiac mortality | 1 | 99 | Odds Ratio (M-H, Fixed, 95% CI) | 0.32 [0.13, 0.80] |
| 2 Non-fatal Myocardial Infarction | 1 | 99 | Odds Ratio (M-H, Fixed, 95% CI) | 0.48 [0.04, 5.47] |
| 3 Hospital readmission for Heart Failure | 1 | 99 | Odds Ratio (M-H, Fixed, 95% CI) | 0.28 [0.09, 0.85] |
| 4 Deaths not associated with training but reported reasons for loss to follow-up | 9 | 483 | Odds Ratio (M-H, Fixed, 95% CI) | 1.12 [0.58, 2.15] |
| 5 VO2 max (ml/kg/min) | 24 | 848 | Mean Difference (IV, Random, 95% CI) | −2.16 [−2.82, −1.49] |
| 6 Exercise duration (mins) | 15 | 510 | Mean Difference (IV, Fixed, 95% CI) | −2.38 [−2.85, −1.92] |
| 7 Maximum work capacity (Watts) | 6 | 219 | Mean Difference (IV, Fixed, 95% CI) | −15.13 [−17.67, −12. 59] 59] |
| 8 Distance on 6 minute walk (meters) | 8 | 282 | Mean Difference (IV, Random, 95% CI) | −40.87 [−64.65, −17. 10] |
Comparison 2. Subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 VO2 max (ml/kg/min) | 24 | 4191 | Mean Difference (IV, Random, 95% CI) | −2.19[−2.48,−1.90] |
| 1.1 “Dose” of intervention =/> 30 units | 11 | 432 | Mean Difference (IV, Random, 95% CI) | −2.78[−3.88,−1.69] |
| 1.2 “Dose” of intervention < 30 units | 13 | 416 | Mean Difference (IV, Random, 95% CI) | −1.64[−2.15,−1.12] |
| 1.3 Jadad score = 3 and above | 7 | 341 | Mean Difference (IV, Random, 95% CI) | −2.01[−2.90,−1.12] |
| 1.4 Jadad score = 2 and below | 15 | 458 | Mean Difference (IV, Random, 95% CI) | −2.28[−3.47,−1.10] |
| 1.5 Duration of intervention >12 weeks | 10 | 420 | Mean Difference (IV, Random, 95% CI) | −2.62[−3.76,−1.48] |
| 1.6 Duration of intervention =/<12 weeks | 14 | 428 | Mean Difference (IV, Random, 95% CI) | −1.79[−2.33,−1.24] |
| 1.7 Mean age > 55 years | 15 | 540 | Mean Difference (IV, Random, 95% CI) | −1.77[−2.50,−1.03] |
| 1.8 Mean age =/< 55 years | 9 | 308 | Mean Difference (IV, Random, 95% CI) | −2.95[−4.25,−1.64] |
| 1.9 Duration of follow up > 16 weeks | 9 | 397 | Mean Difference (IV, Random, 95% CI) | −2.74[−3.83,−1.65] |
| 1.10 Duration of follow up =/< 16 weeks | 15 | 451 | Mean Difference (IV, Random, 95% CI) | −1.55[−2.17,−0.94] |
Analysis 1.1. Comparison 1 All exercise interventions versus usual care, Outcome 1 Cardiac mortality.
Review: Exercise based rehabilitation for heart failure
Comparison: 1 All exercise interventions versus usual care
Outcome: 1 All cause mortality up to12 month follow up
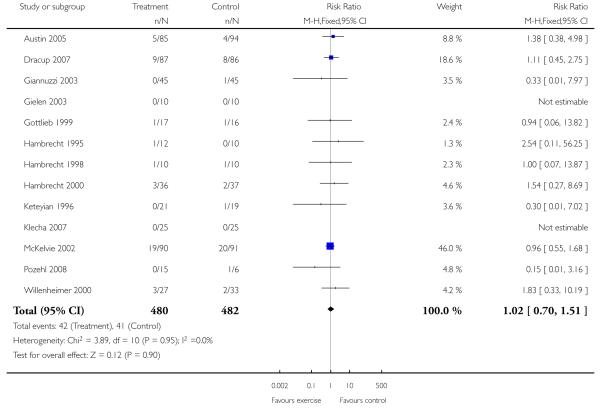 |
Analysis 1.2. Comparison 1 All exercise interventions versus usual care, Outcome 2 Non-fatal Myocardial Infarction.
Review: Exercise based rehabilitation for heart failure
Comparison: 1 All exercise interventions versus usual care
Outcome: 2 All cause mortality more than 12 months follow up
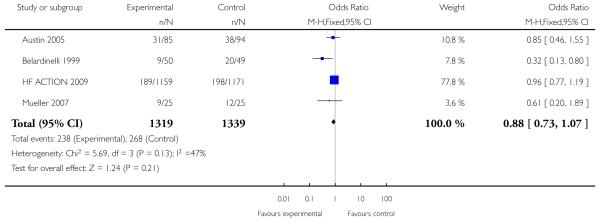 |
Analysis 1.3. Comparison 1 All exercise interventions versus usual care, Outcome 3 Hospital readmission for Heart Failure.
Review: Exercise based rehabilitation for heart failure
Comparison: 1 All exercise interventions versus usual care
Outcome: 3 Hospital admission up to 12 month follow up
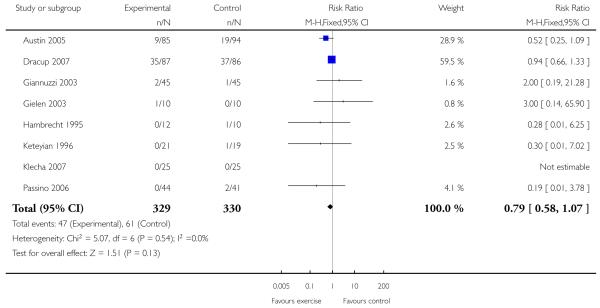 |
Analysis 1.4. Comparison 1 All exercise interventions versus usual care, Outcome 4 Deaths not associated with training but reported reasons for loss to follow-up.
Review: Exercise based rehabilitation for heart failure
Comparison: 1 All exercise interventions versus usual care
Outcome: 4 Hospital admission more than 12 months follow up
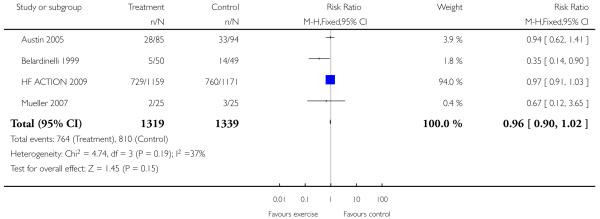 |
Analysis 1.5. Comparison 1 All exercise interventions versus usual care, Outcome 5 VO2 max (ml/kg/min).
Review: Exercise based rehabilitation for heart failure
Comparison: 1 All exercise interventions versus usual care
Outcome: 5 Hospital admission heart failure only
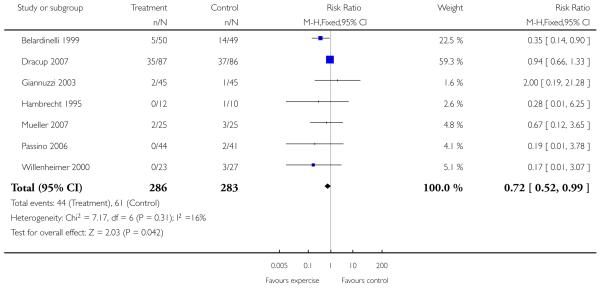 |
Analysis 1.6. Comparison 1 All exercise interventions versus usual care, Outcome 6 Exercise duration(mins).
Review: Exercise based rehabilitation for heart failure
Comparison: 1 All exercise interventions versus usual care
Outcome: 6 Health related quality of life - MLWHF
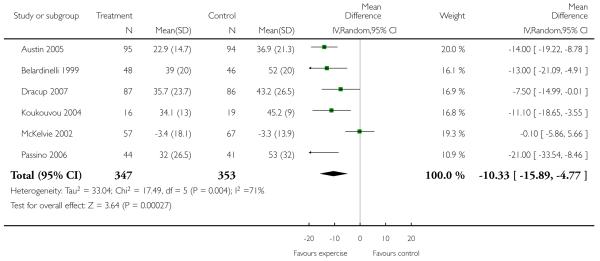 |
Analysis 1.7. Comparison 1 All exercise interventions versus usual care, Outcome 7 Maximum work capacity (Watts).
Review: Exercise based rehabilitation for heart failure
Comparison: 1 All exercise interventions versus usual care
Outcome: 7 Health related quality of life - all scales
 |
Analysis 1.8. Comparison 1 All exercise interventions versus usual care, Outcome 8 Distance on 6 minute walk (meters).
Review: Exercise based rehabilitation for heart failure
Comparison: 1 All exercise interventions versus usual care
Outcome: 8 Distance on 6 minute walk (meters)
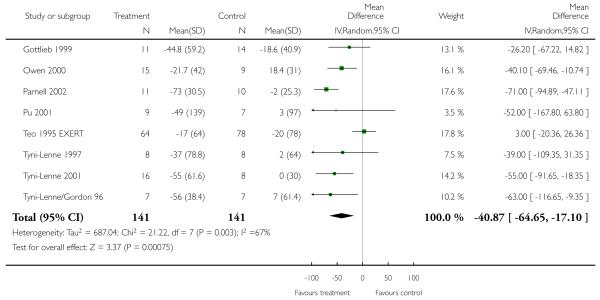 |
Analysis 2.1. Comparison 2 Subgroup analyses, Outcome 1 VO2 max (ml/kg/min).
Review: Exercise based rehabilitation for heart failure
Comparison: 2 Subgroup analyses
Outcome: 1 VO2 max (ml/kg/min)
 |
 |
 |
 |
 |
 |
WHAT’S NEW
Last assessed as up-to-date: 17 May 2004.
| Date | Event | Description |
|---|---|---|
| 8 September 2008 | Amended | Converted to new review format. |
HISTORY
Protocol first published: Issue 4, 2001
Review first published: Issue 3, 2004
| Date | Event | Description |
|---|---|---|
| 18 May 2004 | New citation required and conclusions have changed | Substantive amendment |
Footnotes
DECLARATIONS OF INTEREST
RT - former chair of BACR Scientific Sub-Committee, written a number of publications in the field of cardiac rehabilitation and currently involved in two UK RCTs of cardiac rehabilitation.
AC - author of 2 of the included trials, and a number of papers in the field of heart failure.
NOTES
The Peninsula Technology Assessment Group (PenTAG) at Peninsula Medical School, Exeter, UK and the Cochrane Heart Group have been awarded a 3-year grant from the National Institute for Health Research to update existing Cochrane systematic reviews relevant to public health, primary care and rehabilitation.
This review is scheduled to be updated in the first year of the program. Publication of the updated review is anticipated by issue 2, 2009 at the latest.
References to studies included in this review
- Belardinelli 1992 {published data only} .Belardinelli R, Scocco V, Mazzanti M, et al. Effects of aerobic training in patients with moderate chronic heart failure. Giornale italiano di cardiologia. 1992;22(8):919–30. [PubMed] [Google Scholar]
- Belardinelli 1995 {published data only} .Belardinelli R, Georgiou D, Cianci G, Berman N, Ginzton L, Purcaro A. Exercise training improves left ventricular diastolic filling in patients with dilated cardiomyopathy. Clinical and prognostic implications. Circulation. 1995;91(11):2775–84. doi: 10.1161/01.cir.91.11.2775. [DOI] [PubMed] [Google Scholar]
- Belardinelli 1999 {published data only} .Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999;99(9):1173–82. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- Cider 1997 {published data only} .Cider A, Tygesson H, Hedberg M, Seligman L, Wennerblom B, Sunnerhagen KS. Peripheral muscle training in patients with clinical signs of heart failure. Scandinavian Journal of Rehabilitation Medicine. 1997;29(2):121–7. [PubMed] [Google Scholar]
- Coats 1990 {published data only} .Coats A, Adamopoulos S. A cross-over comparison of outpatient physical training versus rest in the treatment of moderate to severe heart failure [abstract] Clinical Science. 1989;77(Suppl 21):19P. [Google Scholar]
- Coats AJ, Adamopoulos S, Meyer TE, Conway J, Sleight P. Effects of physical training in chronic heart failure. Lancet. 1990;335(8681):63–6. doi: 10.1016/0140-6736(90)90536-e. [DOI] [PubMed] [Google Scholar]
- Coats 1992 {published data only} .Coats AJ, Adamopoulos S, Radaelli A, McCance A, Meyer TE, Bernardi L, et al. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation. 1992;85(6):2119–31. doi: 10.1161/01.cir.85.6.2119. [DOI] [PubMed] [Google Scholar]
- Dubach et al. studies {published data only} .Callaerts-Vegh Z, Wenk M, Goebbels U, Dziekan G, Myers J, Dubach P, et al. Influence of intensive physical training on urinary nitrate elimination and plasma endothelin-1 levels in patients with congestive heart failure. Journal of Cardiopulmonary Rehabilitation. 1998;18(6):450–7. doi: 10.1097/00008483-199811000-00008. [DOI] [PubMed] [Google Scholar]
- Dubach P, Myers J, Dziekan G, Goebbels U, Reinhart W, Muller P, et al. Effect of high intensity exercise training on central hemodynamic responses to exercise in men with reduced left ventricular function. Journal of the American College of Cardiology. 1997;29(7):1591–8. doi: 10.1016/s0735-1097(97)82540-5. [DOI] [PubMed] [Google Scholar]
- Duru F, Candinas R, Dziekan G, Goebbels U, Myers J, Dubach P. Effect of exercise training on heart rate variability in patients with new-onset left ventricular dysfunction after myocardial infarction. American Heart Journal. 2000;140(1):157–61. doi: 10.1067/mhj.2000.106606. [DOI] [PubMed] [Google Scholar]
- Dziekan G, Myers J, Goebbels U, Muller P, Reinhart W, Ratti R, et al. Effects of exercise training on limb blood flow in patients with reduced ventricular function. American Heart Journal. 1998;136(1):22–30. doi: 10.1016/s0002-8703(98)70177-2. [DOI] [PubMed] [Google Scholar]
- Goebbels U, Myers J, Dziekan G, Muller P, Kuhn M, Ratte R, et al. A randomized comparison of exercise training in patients with normal vs reduced ventricular function. Chest. 1998;113(5):1387–93. doi: 10.1378/chest.113.5.1387. [DOI] [PubMed] [Google Scholar]
- Myers J, Gianrossi R, Schwitter J, Wagner D, Dubach P. Effect of exercise training on postexercise oxygen uptake kinetics in patients with reduced ventricular function. Chest. 2001;120(4):1206–11. doi: 10.1378/chest.120.4.1206. [DOI] [PubMed] [Google Scholar]
- Myers J, Goebbels U, Dzeikan G, Froelicher V, Bremerich J, Mueller P, et al. Exercise training and myocardial remodeling in patients with reduced ventricular function: One-year follow-up with magnetic resonance imaging. American Heart Journal. 2000;139(21):252–61. doi: 10.1067/mhj.2000.101500. [DOI] [PubMed] [Google Scholar]
- Gottlieb 1999 {published data only} .Gottlieb SS, Fisher ML, Freudenberger R, Robinson S, Zietowski G, Alves L, et al. Effects of exercise training on peak performance and quality of life in congestive heart failure patients. Journal of Cardiac Failure. 1999;5(3):188–94. doi: 10.1016/s1071-9164(99)90002-7. [DOI] [PubMed] [Google Scholar]
- Hambrecht 1995 {published data only} .Hambrecht R, Niebauer J, Fiehn E, Kalberer B, Offner B, Hauer K, et al. Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. Journal of the American College of Cardiology. 1995;25(6):1239–49. doi: 10.1016/0735-1097(94)00568-B. [DOI] [PubMed] [Google Scholar]
- Hambrecht 1998 {published data only} .Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98(24):2709–15. doi: 10.1161/01.cir.98.24.2709. [DOI] [PubMed] [Google Scholar]
- Hambrecht 2000 {published data only} .Hambrecht R, Gielen S, Linke A, Fiehn E, Yu J, Walther C, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: A randomized trial. JAMA. 2000;283(23):3095–101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- Jette 1991 {published data only} .Jette M, Heller R, Landry F, Blumchen G. Randomized 4 week exercise program in patients with impaired left ventricular function. Circulation. 1991;84(4):1561–7. doi: 10.1161/01.cir.84.4.1561. [DOI] [PubMed] [Google Scholar]
- Keteyian 1996 {published data only} .Keteyian SJ, Levine AB, Brawner CA, Kataoka T, Rogers FJ, Schairer JR, et al. Exercise training in patients with heart failure. A randomized, controlled trial. Annals of Internal Medicine. 1996;124(12):1051–7. doi: 10.7326/0003-4819-124-12-199606150-00004. [DOI] [PubMed] [Google Scholar]
- Kiilavuori 1996 {published data only} .Kiilavuori K, Sovijarvi A, Naveri H, Ikonen T, Leinonen H. Effect of physical training on exercise capacity and gas exchange in patients with chronic heart failure. Chest. 1996;110(4):985–91. doi: 10.1378/chest.110.4.985. [DOI] [PubMed] [Google Scholar]
- Maiorana 2000 {published data only} .Maiorana A, O’Driscoll G, Cheetham C, Collis J, Goodman C, Rankin S, et al. Combined aerobic and resistance exercise training improves functional capacity and strength in CHF. Journal of Applied Physiology. 2000;88(5):1565–70. doi: 10.1152/jappl.2000.88.5.1565. [DOI] [PubMed] [Google Scholar]
- Meyer 1996 {published data only} .Meyer K, Schwaibold M, Westbrook S, Beneke R, Hajric R, Gornandt L, et al. Effects of short-term exercise training and activity restriction on functional capacity in patients with severe chronic congestive heart failure. American Journal of Cardiology. 1996;78(9):1017–22. doi: 10.1016/s0002-9149(96)00527-9. [DOI] [PubMed] [Google Scholar]
- Meyer K, Schwaibold M, Westbrook S, Beneke R, Hajric R, Lehmann M, et al. Effects of exercise training and activity restriction on 6-minute walking test performance in patients with chronic heart failure. American Heart Journal. 1997;133(4):447–53. doi: 10.1016/s0002-8703(97)70187-x. [DOI] [PubMed] [Google Scholar]
- Oka 2000 {published data only} .Oka RK, De Marco T, Haskell WL, Botvinick E, Dae MW, Bolen K, et al. Impact of a home-based walking and resistance training program on quality of life in patients with heart failure. American Journal of Cardiology. 2000;85(3):365–9. doi: 10.1016/s0002-9149(99)00748-1. [DOI] [PubMed] [Google Scholar]
- Owen 2000 {published data only} .Owen A, Croucher L. Effect of an exercise programme for elderly patients with heart failure. European Journal of Heart Failure. 2000;2(1):65–70. doi: 10.1016/s1388-9842(99)00067-7. [DOI] [PubMed] [Google Scholar]
- Parnell 2002 {published data only} .Parnell MM, Holst DP, Kaye DM. Exercise training increases arterial compliance in patients with congestive heart failure. Clinical Science. 2002;102(1):1–7. [PubMed] [Google Scholar]
- Ponikowski 1997 {published data only} .Ponikowski P, Szelemej R, Kowalska-Superlak M, Kratochwil D, Sobkowicz B, Sebzda T, et al. Exercise rehabilitation in patients with moderate-severe chronic heart failure. [CZY CHORZY Z NIEWYDOLNOSCIA KRAZENIA KORZYSTAJA Z REHABILITACJI RUCHOWEJ?] Kardiologia Polska. 1997;47(10):291–300. [Google Scholar]
- Pu 2001 {published data only} .Pu CT, Johnson MT, Forman DE, Hausdorff JM, Roubenoff R, Foldvari M, et al. Randomized trial of progressive resistance training to counteract the myopathy of chronic heart failure. Journal of Applied Physiology. 2001;90(6):2341–50. doi: 10.1152/jappl.2001.90.6.2341. [DOI] [PubMed] [Google Scholar]
- Quittan 1999 {published data only} .Quittan M, Sturm B, Wiesinger GF, Pacher R, Fialka-Moser V. Quality of life in patients with chronic heart failure: a randomized controlled trial of changes induced by a regular exercise program. Scandinavian Journal of Rehabilitation Medicine. 1999;31(4):223–8. doi: 10.1080/003655099444399. [DOI] [PubMed] [Google Scholar]
- Sturm B, Quittan M, Wiesinger GF, Stanek B, Frey B, Pacher R. Moderate-intensity exercise training with elements of step aerobics in patients with severe chronic heart failure. Archives of physical medicine and rehabilitation. 1999;80:746–50. doi: 10.1016/s0003-9993(99)90221-6. [DOI] [PubMed] [Google Scholar]
- Teo 1995 EXERT {published data only} .McKelvie RS, Teo KK, Roberts R, McCartney N, Humen D, Montague T, Hendrican K, Yusuf S. Effects of exercise training in patients with heart failure: The Exercise Rehabilitation Trial (EXERT) American heart journal. 2002;144(23-30) doi: 10.1067/mhj.2002.123310. [DOI] [PubMed] [Google Scholar]
- Teo K, McKelvie R, Yusuf S, McCartney N, Guyatt G, Roberts R, et al. Randomized controlled trial of aerobic plus resistance exercise training in patients with congestive heart failure. The Exercise Rehab Trial (EXERT) Investigators [abstract] Controlled clinical trials. 1995;16(3 Suppl):99S. [Google Scholar]
- Tyni-Lenne 1997 {published data only} .Tyni-Lenne R, Gordon A, Jansson E, Bermann G, Sylven C. Skeletal muscle endurance training improves peripheral oxidative capacity, exercise tolerance, and health-related quality of life in women with chronic congestive heart failure secondary to either ischemic cardiomyopathy or idiopathic dilated cardiomyopathy. American Journal of Cardiology. 1997;80(8):1025–9. doi: 10.1016/s0002-9149(97)00597-3. [DOI] [PubMed] [Google Scholar]
- Tyni-Lenne 2001 {published data only} .Tyni-Lenne R, Dencker K, Gordon A, Jansson E, Sylven C. Comprehensive local muscle training increases aerobic working capacity and quality of life and decreases neurohormonal activation in patients with chronic heart failure. European Journal of Heart Failure. 2001;3(1):47–52. doi: 10.1016/s1388-9842(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Tyni-Lenne/Gordon 96 {published data only} .Gordon A, Tyni-Lenne R, Persson H, Kaijser L, Hultman E, Sylven C. Markedly improved skeletal muscle function with local muscle training in patients with chronic heart failure. Clinical Cardiology. 1996;19(7):568–74. doi: 10.1002/clc.4960190709. [DOI] [PubMed] [Google Scholar]
- Tyni-Lenne R, Gordon A, Sylven C. Improved quality of life in chronic heart failure patients following local endurance training with leg muscles. Journal of Cardiac Failure. 1996;2(2):111–7. doi: 10.1016/s1071-9164(96)80029-7. [DOI] [PubMed] [Google Scholar]
- Wielenga 1998 {published data only} Wielenga RP, Huisveld IA, Bol E, Dunselman PH, Erdman RA, Baselier MR, et al. Exercise training in elderly patients with chronic heart failure [published erratum appears in Coron Artery Dis. 1999;10(1):57. doi: 10.1097/00019501-199809110-00010. [DOI] [PubMed] [Google Scholar]; Coronary Artery Disease. 1998;9(11):765–70. doi: 10.1097/00019501-199809110-00010. [DOI] [PubMed] [Google Scholar]
- Wielenga 1999 CHANGE {published data only} .Wielenga RP, Huisveld IA, Bol E, Dunselman PH, Erdman RA, Baselier MR, et al. Safety and effects of physical training in chronic heart failure. Results of the Chronic Heart Failure and Graded Exercise study (CHANGE) European Heart Journal. 1999;20(12):872–9. doi: 10.1053/euhj.1999.1485. [DOI] [PubMed] [Google Scholar]
- Willenheimer 1998 {published data only} .Willenheimer R, Erhardt L, Cline C, Rydberg E, Israelsson B. Exercise training in heart failure improves quality of life and exercise capacity. European Heart Journal. 1998;19(5):774–81. doi: 10.1053/euhj.1997.0853. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Adamopoulos 1995 {published data only} .Adamopoulos S, Ponikowski P, Cerquetani E, Piepoli M, Rosano G, Sleight P, et al. Circadian pattern of heart rate variability in chronic heart failure patients. Effects of physical training. European Heart Journal. 1995;16(10):1380–6. doi: 10.1093/oxfordjournals.eurheartj.a060746. [DOI] [PubMed] [Google Scholar]
- Barnard 2000 {published data only} .Barnard KL, Adams KJ, Swank AM, Kaelin M, Kushnik MR, Denny DM. Combined high-intensity strength and aerobic training in patients with congestive heart failure. Journal of strength and conditioning research. 2000;14:383–8. [Google Scholar]
- Belardinelli2 1995 {published data only} .Belardinelli R, Georgiou D, Scocco V, Barstow TJ, Purcaro A. Low intensity exercise training in patients with chronic heart failure. Journal of the American College of Cardiology. 1995;26(4):975–82. doi: 10.1016/0735-1097(95)00267-1. [DOI] [PubMed] [Google Scholar]
- Cohen-Solal 1994 {published data only} .Cohen-Solal A, Aupetit JF, Gueret P, Kolsky H, Zannad F, The VO2 French Study Group Can anaerobic threshold be used as an end-point for therapeutic trials in heart failure? Lessons from a multicentre randomized placebo-controlled trial. European Heart Journal. 1994;15(2):236–41. doi: 10.1093/oxfordjournals.eurheartj.a060482. [DOI] [PubMed] [Google Scholar]
- Davey 1992 {published data only} .Davey P, Meyer T, Coats A, Adamopoulos S, Casadei B, Conway J, et al. Ventilation in chronic heart failure: Effects of physical training. British heart journal. 1992;68(5):473–7. doi: 10.1136/hrt.68.11.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon 1999 {published data only} .Gordon A, Tyni-Lenne R, Jansson E, Jensen-Urstad M, Kaijser L. Beneficial effects of exercise training in heart failure patients with low cardiac output response to exercise - a comparison of two training models. Journal of Internal Medicine. 1999;246(2):175–82. doi: 10.1046/j.1365-2796.1999.00555.x. [DOI] [PubMed] [Google Scholar]
- Johnson 1998 {published data only} .Johnson PH, Cowley AJ, Kinnear WJ. A randomized controlled trial of inspiratory muscle training in stable chronic heart failure. European Heart Journal. 1998;19(8):1249–53. doi: 10.1053/euhj.1998.1024. [DOI] [PubMed] [Google Scholar]
- Kavanagh 1996 {published data only} .Kavanagh T, Myers MG, Baigrie RS, Mertens DJ, Sawyer P, Shephard RJ. Quality of life and cardiorespiratory function in chronic heart failure: effects of 12 months’ aerobic training. Heart. 1996;76(1):42–9. doi: 10.1136/hrt.76.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch 1992 {published data only} .Koch M, Douard H, Broustet JP. The benefit of graded physical exercise in chronic heart failure. Chest. 1992;101(5 Suppl):231S–235S. doi: 10.1378/chest.101.5_supplement.231s. [DOI] [PubMed] [Google Scholar]
- Metra 1998 {published data only} .Metra M, Nodari S, Raccagni D, Garbellini M, Boldi E, Bontempi L, et al. Maximal and submaximal exercise testing in heart failure. Journal of Cardiovascular Pharmacology. 1998;32(Suppl 1):S36–S45. doi: 10.1097/00005344-199800003-00007. [DOI] [PubMed] [Google Scholar]
- Page 1994 {published data only} .Page E, Cohen-Solal A, Jondeau G, Douard H, Roul G, Kantelip JP, et al. Comparison of treadmill and bicycle exercise in patients with chronic heart failure. Chest. 1994;106(4):1002–6. doi: 10.1378/chest.106.4.1002. [DOI] [PubMed] [Google Scholar]
- Taylor 1999 {published data only} .Taylor A. Physiological response to a short period of exercise training in patients with chronic heart failure. Physiotherapy Research International. 1999;4(4):237–49. doi: 10.1002/pri.171. [DOI] [PubMed] [Google Scholar]
- Tokmakova 1999 {published data only} .Tokmakova M, Dobreva B, Kostianev S. Effects of short-term exercise training in patients with heart failure. Folia Medica (Plovdiv) 1999;41(1):68–71. [PubMed] [Google Scholar]
- Tyni-Lenne 1998 {published data only} .Tyni-Lenne R, Gordon A, Europe E, Jansson E, Sylven C. Exercise-based rehabilitation improves skeletal muscle capacity, exercise tolerance, and quality of life in both women and men with chronic heart failure. Journal of Cardiac Failure. 1998;4(1):9–17. doi: 10.1016/s1071-9164(98)90503-6. [DOI] [PubMed] [Google Scholar]
- Tyni-Lenne 1999 {published data only} .Tyni-Lenne R, Gordon A, Jensen-Urstad M, Dencker K, Jansson E, Sylven C. Aerobic training involving a minor muscle mass shows greater efficiency than training involving a major muscle mass in chronic heart failure patients. Journal of Cardiac Failure. 1999;5(4):300–7. doi: 10.1016/s1071-9164(99)91334-9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Kayanakis 1994 {published data only} .Kayanakis JG, Page E, Aros F, Borau F. [Rehabilitation of patients with chronic cardiac insufficiency. Immediate and midterm effects]. [French] Presse Medicale. 1994;23(3):121–6. [PubMed] [Google Scholar]
References to ongoing studies
- NRR ongoing trial 1 {published data only} .EXIST - Exercise Intervention Strategies in Heart Failure Trial. National Research Register, Publication ID N0201112792. Start date 01/07/2002, end date 31/12/2002. [Google Scholar]
- NRR ongoing trial 2 {published data only} .A randomised trial of exercise intervention for older patients with heart failure. National Research Register, Publication ID NO514100933. Start date 01/01/2001, end date 01/01/2004. [Google Scholar]
Additional references
- AHA .American Heart Association www.americanheart.org.
- CHF Guidelines 2001 .Working Group on Cardiac Rehabilitation and Exercise Physiology and Working Group on Heart Failure of the European Society of Cardiology. Recommendations for exercise training in chronic heart failure patients. European Heart Journal. 2001;22:125–35. [Google Scholar]
- CHF Taskforce 2001 .The task force for the diagnosis and treatment of chronic heart failure. Guidelines for the diagnosis and treatment of chronic heart failure. European Heart Journal. 2001;22:1527–60. doi: 10.1053/euhj.2001.2783. [DOI] [PubMed] [Google Scholar]
- Cochrane Heart Group .Cochrane Heart Group [Accessed May 2004];Preferred method for handling continuous variables. http://www.epi.bris.ac.uk/cochrane/stats3.html.
- Conn 1982 .Conn EH, Williams RS, Wallace AG. Exercise responses before and after physical conditioning in patients with severely depressed left ventricular function. The American Journal of Cardiology. 1982;49:296–300. doi: 10.1016/0002-9149(82)90504-5. [DOI] [PubMed] [Google Scholar]
- Cowie 1997 .Cowie MR, Struthers AD, Wood DA, et al. Value of natriuretic peptides in the assessment of patients with possible new hear failure in primary care. Lancet. 1997;350:1349–53. doi: 10.1016/S0140-6736(97)06031-5. [DOI] [PubMed] [Google Scholar]
- Dickersin 1994 .Dickersin K, Scherer R, Lefebve C. Identifying relevant studies for systematic reviews. BMJ. 1994;309:1286–91. doi: 10.1136/bmj.309.6964.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOH 2000 .National Service Framework for Coronary Heart Disease. http://www.doh.gov.uk/pdfs/chdnsf.pdf.
- Drexler 1992 .Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751–9. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- Dziekan 1998 .Dziekan G, Myers J, Goebbels U, Muller P, Reinhart W, Ratti R, et al. Effects of exercise training on limb blood flow in patients with reduced ventricular function. American Heart Journal. 1998;136:22–7. doi: 10.1016/s0002-8703(98)70177-2. [DOI] [PubMed] [Google Scholar]
- Follmann 1992 .Follman D, Elliot P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology. 1992;45:769–73. doi: 10.1016/0895-4356(92)90054-q. [DOI] [PubMed] [Google Scholar]
- Hülsmann 2002 .Hülsmann M, Berger R, Sturm B, Bojic A, Woloszczuk W, Bergler-Klein, et al. Prediction of outcome by neurohumoral activation, the six-minute walk test and the Minnesota living with heart failure questionnaire in an outpatient cohort with congestive heart failure. European Heart Journal. 2002;23:886–91. doi: 10.1053/euhj.2001.3115. [DOI] [PubMed] [Google Scholar]
- Jadad 1996 .Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, et al. Assessing the quality of reports of clinical trials: Is blinding necessary? Controlled Clinical Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Jolliffe 2001 .Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise based rehabilitation for coronary heart disease (Cochrane Review) The Cochrane Library. 2001;(2) doi: 10.1002/14651858.CD001800. [DOI] [PubMed] [Google Scholar]
- Kannel 1991 .Kannel WB, Belanger AJ. Epidemiology of heart failure. American Heart Journal. 1991;121:951–7. doi: 10.1016/0002-8703(91)90225-7. [DOI] [PubMed] [Google Scholar]
- Kostis 1997 .Kostis JB, Davis BR, Cutler J, et al. SHEP Cooperative Research Group Prevention of heart failure antihypertensive drug treatment in older persons with isolated systemic hypertension. JAMA. 1997;278(3):212–6. [PubMed] [Google Scholar]
- Lee 1979 .Lee AP, Ice R, Blessey R, Sanmarco ME. Long term effects of physical training on coronary patients with impaired ventricular function. Circulation. 1979;60(1519-26) doi: 10.1161/01.cir.60.7.1519. [DOI] [PubMed] [Google Scholar]
- Lefebvre 1996 .Lefebvre C, McDonald S. Development of a sensitive search strategy for reports of randomised controlled trials in EMBASE; Conference Abstracts for the Fourth International Cochrane Colloquium; Adelaide, Australia. 1996.Oct 20-24, [Google Scholar]
- Lip 2000 .Lip GYH, Gibbs CR, Beevers DG. Aetiology: ABC of heart failure. BMJ. 2000;320:104–7. doi: 10.1136/bmj.320.7227.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Williams 2002 .Lloyd-Williams F, Mair FS, Leitner M. Exercise training and heart failure: A systematic review of current evidence. British Journal of General Practice. 2002;52(474):47–55. [PMC free article] [PubMed] [Google Scholar]
- Mancini 1992 .Mancini DM, Walter G, Reichek N, et al. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85:1364–73. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- McMurray 2000 .McMurray JJ, Steward S. Heart failure: Epidemiology, aetiology, and prognosis of heart failure. Heart. 2000;83:596–602. doi: 10.1136/heart.83.5.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHS 1998 .NHS Centre for Reviews and Dissemination Cardiac Rehabilitation. Effective Health Care Bulletin. 1998;4 [Google Scholar]
- O’Connor 1989 .O’Connor GT, Buring JE, Yusuf S, Goldhaber SZ, Olmstead EM, Paffenbarger RS, et al. An overview of randomised trials of rehabilitation with exercise after myocardial infarction. Circulation. 1989;80:234–44. doi: 10.1161/01.cir.80.2.234. [DOI] [PubMed] [Google Scholar]
- Oldridge 1988 .Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction. Combined experience of randomised clinical trials. JAMA. 1988;260:945–50. [PubMed] [Google Scholar]
- Opasich 2001 .Opasich C, Pinna GD, Mazza A, Febo O, Riccardi R, Riccardi PG, et al. Six-minute walking performance in patients with moderate-to-severe heart failure; is it a useful indicator in clinical practice? European Heart Journal. 2001;22(6):445–8. doi: 10.1053/euhj.2000.2310. [DOI] [PubMed] [Google Scholar]
- Rees 2004 .Rees K, Bennett PD, West RR, Davey-Smith G, Ebrahim S. Psychological Interventions for coronary heart disease. The Cochrane Library. 2004;(2) doi: 10.1002/14651858.CD002902.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan 1988 .Sullivan MJ, Higginbotham MB, Cobb FR. Exercise training in patients with severe left ventricular dysfunction: hemodynamic and metabolic effects. Circulation. 1988;78:506–15. doi: 10.1161/01.cir.78.3.506. [DOI] [PubMed] [Google Scholar]
- Sullivan 1989 .Sullivan MJ, Knight D, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Circulation. 1989;80:769–81. doi: 10.1161/01.cir.80.4.769. [DOI] [PubMed] [Google Scholar]
- van den Brock 1992 .van den Brock SA, van Veldhuisen DJ, de Graeff PA, Landsman ML, Hillege H, Lie KI. Comparison between New York Heart Association classification and peak oxygen consumption in the assessment of functional status and prognosis in patients with mild to moderate chronic congestive heart failure secondary to either ischemic or idiopathic dilated cardiomyopathy. American Journal of Cardiology. 1992;70:359–63. doi: 10.1016/0002-9149(92)90619-a. [DOI] [PubMed] [Google Scholar]
- Watson 2000 .Watson RDS, Gibbs CR, Lip GYH. ABC of heart failure: clinical features and complications. BMJ. 2000;320:236–9. doi: 10.1136/bmj.320.7229.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Indicates the major publication for the study


