Abstract
Many genetic studies report mixed results both for the associations between COMT polymorphisms and schizophrenia and for the effects of COMT variants on common intermediate phenotypes of the disorder. Reasons for this may include small genetic effect sizes and the modulation of environmental influences. To improve our understanding of the role of COMT in the disease etiology, we investigated the effect of DNA methylation in the MB-COMT promoter on neural activity in the dorsolateral prefrontal cortex during working memory processing as measured by fMRI - an intermediate phenotype for schizophrenia. Imaging and epigenetic data were measured in 102 healthy controls and 82 schizophrenia patients of the Mind Clinical Imaging Consortium (MCIC) study of schizophrenia. Neural activity during the Sternberg Item Recognition Paradigm was acquired with either a 3T Siemens Trio or 1.5T Siemens Sonata and analyzed using the FMRIB Software Library (FSL). DNA methylation measurements were derived from cryo-conserved blood samples. We found a positive association between MB-COMT promoter methylation and neural activity in the left dorsolateral prefrontal cortex in a model using a region-of-interest approach and could confirm this finding in a whole-brain model. This effect was independent of disease status. Analyzing the effect of MB-COMT promoter DNA methylation on a neuroimaging phenotype can provide further evidence for the importance of COMT and epigenetic risk mechanisms in schizophrenia. The latter may represent trans-regulatory or environmental risk factors that can be measured using brain-based intermediate phenotypes.
Keywords: DNA methylation, COMT, schizophrenia, intermediate phenotype, fMRI
Introduction
The enzyme catechol-O-methyltransferase (COMT) degrades catecholamines such as dopamine, epinephrine, and norepinephrine in the synapse. Since schizophrenia is thought to be associated with dopamine dysfunction, COMT has become one of the most studied genes in schizophrenia research. Despite some evidence for the involvement of COMT polymorphisms in the etiology of schizophrenia,1-3 there have also been many negative genetic case-control association studies, including three meta-analyses, which all failed to support an association between COMT polymorphisms and schizophrenia.4-6 To better understand if COMT plays a significant role in disease pathophysiology, other factors that modulate COMT enzyme activity, such as epigenetic changes, should be investigated.
DNA methylation, which involves the addition of a methyl group to the cytosine residue of a C-phosphate-G (CpG) dinucleotide, is a well described epigenetic mechanism and is commonly associated with silencing gene expression.7,8 Investigating the association between COMT DNA methylation and a diagnosis of schizophrenia, previous studies have focused on the promoter regions of either one of the two COMT isoforms: the soluble isoform (S-COMT) or the membrane-bound isoform (MB-COMT). The latter is the predominant form found in brain tissue9 and has a greater affinity for dopamine.10 Whereas S-COMT promoter methylation appears to be increased in schizophrenia patients,11 MB-COMT promoter methylation has been shown to be decreased.12,13 Investigating the relationship between COMT promoter methylation and gene expression in post-mortem brain samples of schizophrenia patients, Abdolmaleky et al.12 found decreased MB-COMT promoter methylation to be associated with increased COMT gene expression. However, negative findings have also been reported for both isoforms14,15 implying that the methylation-expression association is more complex and/or that measurements were influenced by analysis methods, age differences or the exact location of the studied CpG-sites.
Unlike post-mortem studies, in vivo obtained neuroimaging measures can be used to gain insight into the functional correlates of DNA methylation. Neuroimaging-based intermediate phenotypes are disease-associated, heritable and stable traits that reduce phenotypic complexity and likely display a stronger relationship with risk factors than behavior or diagnosis, due to their greater proximity to the underlying biology.16,17 Dorsolateral prefrontal cortex (DLPFC) dysfunction during working memory (WkM) processing is a heritable marker closely related to schizophrenia.18 Compared with matched healthy controls, patients and individuals at risk for schizophrenia show prefrontal neural abnormalities, i.e., they need to recruit more neural resources than controls at low task difficulty levels and may show decreased neural activity (hypofrontality) when task difficulty increases.19-22 Studying the effect of epigenetic changes in the promoter region of the brain-linked MB-COMT isoform on brain function—rather than disease status—might provide better insight into disease-associated changes and mechanisms on a neuroscience systems level.
To date, only one study has investigated the effect of S-COMT methylation on prefrontal activity. Studying healthy controls Ursini et al.23 found an association between S-COMT methylation in exon 4 and prefrontal activity during a WkM task. The aim of the present investigation was to study the association of DNA methylation in the MB-COMT promoter with a brain-based intermediate phenotype for schizophrenia in schizophrenia patients and healthy controls.
Results
Demographics
Patients and controls did not differ in demographic variables such as age, parental socio-economic status and Annett handedness score (Table 1). There were significantly more female participants in the control group and patients had a significantly lower WRAT-IIIRT score. We found no differences in gender, age, WRAT-IIIRT, parental socio-economic status or handedness between acquisition sites with different scanner field strengths (Table 1). For information on demographics split by acquisition site and on clinical data in the patient group, see Tables S1 and S2.
Table 1. Basic demographic characteristics.
| Site | Sample | Gender | Age | WRAT-IIIRT | Parental SES | Handedness | ||
|---|---|---|---|---|---|---|---|---|
| (tesla) | (female) | (years) | ||||||
| N | N | % | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| 1.5 | SCZ | 67 | 15b | 22.4 | 33.82 (10.09) | 46.48 (6.66) | 2.79a (1.00) | 1.20 (3.04) |
| HC | 85 | 37b | 43.5 | 33.11 (11.19) | 50.78 (4.05) | 2.79a (0.71) | 0.80 (2.53) | |
| 3 | SCZ | 15 | 5 | 33.3 | 33.47 (13.10) | 44.71a (7.55) | 3.00a (1.18) | 1.67 (3.37) |
| HC | 17 | 3 | 17.6 | 30.41 (12.68) | 51.76a (3.36) | 2.00a (0.61) | 1.35 (2.69) | |
| Total | SCZ | 82 | 20b | 24.4 | 33.76 (10.61) | 46.17a (6.81) | 2.83 (1.03) | 1.29 (3.09) |
| HC | 102 | 40b | 39.2 | 32.66 (11.43) | 50.94a (3.95) | 2.66 (0.75) | 0.89 (2.55) |
A series of t tests or chi-square tests were performed to detect significant differences of gender, age, WRAT-IIIRT score, parental socio-economic status and handedness between study sites with different scanner field strength or between diagnostic groups. Abbreviations: WRAT-IIIRT, reading subtest of the Wide Range Achievement Test - III; SES, socio-economic status; handedness, Annett Handedness Scale; SCZ, schizophrenia patients; HC, healthy controls. aSignificantly different between SCZ and HC based on a t test (P < 0.05); bSignificantly different between SCZ and HC based on a chi-square test (P < 0.05).
MB-COMT promoter methylation did not differ between sites with different scanner field strength or by gender, but patients had lower MB-COMT promoter methylation values (meanpatients 0.7854 ± 0.0177; meancontrols 0.7917 ± 0.0199; tdiagnosis[182] = –2.22, P = 0.028). There were no significant correlations between MB-COMT promoter methylation and age, parental socio-economic status, WRAT-IIIRT score or handedness. MB-COMT promoter methylation correlated with Sternberg Item Recognition Paradigm (SIRP) performance at a trend level (Table 2). There were no significant effects of clinical variables such as typical and atypical cumulative lifetime or current antipsychotic drug dose, length of illness and negative or positive symptoms on MB-COMT promoter methylation (Table S3).
Table 2. Spearman correlations.
| Correlation with MB-COMT promoter methylation | ||
|---|---|---|
| Spearman's rho | P | |
| Age | 0.07 | 0.36 |
| SIRP performance | 0.14 | 0.06 |
| Parental SES | 0.01 | 0.94 |
| WRAT-IIIRT | 0.09 | 0.26 |
| Handedness | -0.11 | 0.15 |
Correlations between the MB-COMT promoter methylation and age. SIRP performance, parental socio-economic status, WRAT-IIIRT score and handedness.
Functional MRI
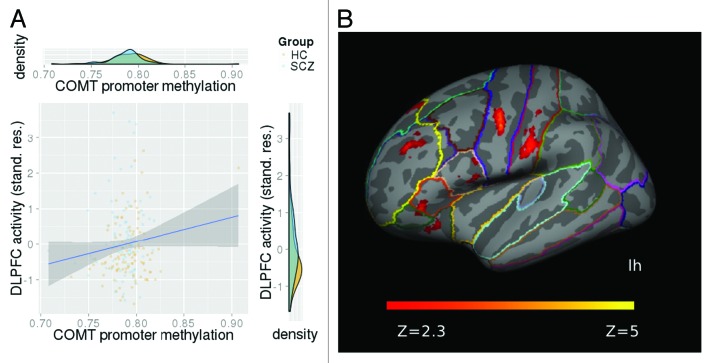
We found a positive association between MB-COMT promoter methylation and neural activity in the left DLPFC (F[1176] = 4.60, P = 0.03) covarying for the effects of diagnosis, scanner field strength, sex, age, and population stratification (Fig. 1A). We found no MB-COMT promoter methylation by diagnosis and no MB-COMT promoter methylation by sex interaction effect on DLPFC activity. Left DLPFC activity did not differ between males and females and did not correlate with current and cumulative antipsychotic drug dose.
Figure 1. (A) Increased MB-COMT promoter methylation was associated with WkM-related mean neural activity (% BOLD change, controlled for scanner field strength, gender, age and population stratification) in the left DLPFC, independent of disease status. (B) Functional map illustrating increased neural activity with MB-COMT promoter methylation in an exploratory whole-brain model. Effects were found in a cluster comprising the DLPFC (highlighted in yellow), VLPFC (BA 45), premotor and primary sensory cortex in the left hemisphere. Results were cluster-corrected and the model included the same covariates as described above. The z-values are represented according to the color code.
In an exploratory whole-brain model we were able to confirm the effect of MB-COMT promoter methylation on neural activity in the left DLPFC. The identified cluster also included the ventrolateral prefrontal cortex (VLPFC) (BA 45), premotor and primary sensory cortex in the left hemisphere (z-max [-56 -2 44] = 4.72; P = 6.66*10−9, cluster-corrected, Fig. 1B) in a model using the same covariates. We again found no interaction between MB-COMT promoter methylation and diagnosis and no effect in the right DLPFC.
Discussion
In the present study we used functional brain imaging to explore potential effects of MB-COMT promoter methylation on neural activity during a WkM task, a schizophrenia-related brain-based intermediate phenotype. We found that MB-COMT promoter methylation was lower in patients and that higher methylation was associated with greater signal change in the left DLPFC in both, schizophrenia patients and healthy controls.
Lower MB-COMT promoter methylation in patients compared with healthy controls, as found here, is in line with several other studies. Using DNA derived from saliva, Nohesara et al.13 found the MB-COMT promoter to be hypomethylated in schizophrenia patients compared with controls. However, there is much debate over the issue, whether and to what extent methylation results from peripheral tissues such as blood or saliva relate to methylation in the brain. Abdolmaleky et al.,12 analyzing post-mortem brain samples from the frontal lobe, also reported MB-COMT promoter hypomethylation in schizophrenia patients. Effects were stronger in the left hemisphere and lower methylation was related to increased COMT gene expression. These results suggest that—at least in the case of the MB-COMT promoter—methylation results may be less tissue-specific and also relate to gene expression.
Furthermore, we found that increased MB-COMT methylation correlated with WkM-related neural activity in the left DLPFC, the left VLPFC as well as the left-hemispheric premotor and primary sensory cortices. All of these regions have been previously found to be activated during WkM processing24-26 and especially the prefrontal regions were reported to be hypoactivated in schizophrenia patients and their relatives during WkM processing.27-30 So far, only one group studied the effect of COMT methylation on a brain-based phenotype. Ursini et al.23 reported that higher stress levels and lower COMT methylation in exon 4 were associated with increased WkM-related cortical activity in healthy controls. Differences with respect to the direction of the methylation effect might be due to the location of the studied CpG (MB-COMT promoter in our study vs. exon 4 in the one by Ursini et al.). Despite these differences, the study by Ursini et al. and our own results show how changes in DNA methylation—which may be influenced by non-genetic factors—are related to brain function. Studying epigenetic effects on a brain-based correlate of schizophrenia—instead of disease status—reduces symptom heterogeneity. Furthermore, it allowed us to investigate the effect of a possible risk factor on a continuously distributed brain-based trait in patients as well as in healthy controls.
Increased methylation is thought to relate to decreased gene expression in general, which has also been observed in the case of COMT.12 We therefore speculate that a potential risk mechanism may entail decreased COMT methylation, as observed in schizophrenia patients, and subsequently higher COMT expression levels. Increased COMT activity may result in lower synaptic dopamine levels following neurotransmitter release, which would ultimately decrease dopaminergic stimulation of the post-synaptic neuron, particularly in the prefrontal regions, where the dopamine transporter DAT is less abundant.31 This decreased prefrontal activity (hypofrontality) may explain decreased executive functioning as commonly observed using WkM tasks in schizophrenia patients.27-29
The findings of our study need to be seen in the light of the following limitations. First, although neither MB-COMT promoter methylation nor neural activity correlated with current or cumulative antipsychotic drug dose, we cannot distinguish between the potential effects of antipsychotic medications vs. those of the underlying disease process on measures of brain function. However, given the lack of association with methylation and given the fact that the effect of methylation on prefrontal dysfunction was also observed in healthy controls, in this study and in previous reports,23 suggests that the reported association is likely to be medication-independent. Second, the effect of MB-COMT promoter methylation was not specific to schizophrenia patients, suggesting that the observed effect describes a general effect of MB-COMT methylation on cortical functioning. Third, our sample was not matched for sex and COMT genotype by sex interaction effects have been reported for schizophrenia risk and a range of schizophrenia-associated traits (for a review see ref. 32). Although we could not identify a sex effect on MB-COMT methylation, which is in line with previous studies,12,13 or a sex by methylation effect on DLPFC activity, sex remains an important variable when studying COMT. Fourth, we measured MB-COMT methylation in blood samples and it is unclear how well these methylation profiles translate to methylation in the brain. Since we did not measure COMT expression in this study, we cannot conclude with certainty that an increase in DNA methylation relates to lower gene expression of COMT. However, analyzing MB-COMT DNA methylation in post-mortem human brain tissue from the frontal lobe, Abdolmaleky et al. also found a decrease in patients and could furthermore relate this decrease to an increase in COMT gene expression, suggesting that our observed effect might also apply to brain tissue and gene expression.
In summary, MB-COMT promoter methylation was related to cortical processing during a WkM task in a sample of schizophrenia patients and healthy controls. This provides compelling evidence not only for the importance of COMT in schizophrenia etiology, but also for epigenetic mechanisms, possibly indicative of trans-regulatory or environmental risk factors in schizophrenia pathophysiology.
Methods
Participants
Imaging, genetic, epigenetic and behavioral data from participants of the Mind Clinical Imaging Consortium (MCIC) study of schizophrenia from four participating sites (the University of New Mexico [UNM], the University of Minnesota [UMN], Massachusetts General Hospital [MGH], and the University of Iowa [UI]) were used to determine DNA methylation measurements and genetic polymorphisms in cryo-conserved blood samples and to analyze whole-brain neural activity during a WkM task. Out of a total of 328 participants, blood samples were available for 234 participants. DNA methylation and genetic data of 216 participants passed genetic and epigenetic quality control procedures (see below), resulting in a final data set of 82 schizophrenia patients and 102 healthy controls after imaging quality control steps (see below). Diagnoses were based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) and established using a Structured Clinical Interview for DSM disorders (SCID)33 and a review of case files by trained clinicians. Patients had a diagnosis of either schizophrenia (n = 79) or schizophreniform disorder (n = 3). In the initial cohort, controls were matched to the patient group for age, sex, and parental education and were excluded if they had a history of a medical or Axis I psychiatric diagnosis. All subjects gave written informed consent prior to study enrolment. The human subjects research committees at each of the four sites approved the study protocol. For additional details about the participants and clinical measures, see Supplementary Materials (SM) 1.1 and references 34 and 35.
Behavioral task
The Sternberg Item Recognition Paradigm (SIRP) is a WkM task, previously shown to consistently activate the DLPFC in healthy controls and schizophrenia patients.20 The SIRP was administered during six 46 s blocks per run for three 360 s runs. In each block a memory set, composed of one (load 1), three (load 3), or five (load 5) digits, was presented (two blocks per load condition). This Encode phase was followed by a presentation of 14 digits, one at a time (the Probe phase) and participants responded to each probe to indicate whether or not the probe digit was in the memory set. Participants were instructed to respond as quickly and accurately as possible and were given a bonus of 5 cents for each correct response. This bonus was provided after completion of the scan. For additional details about the paradigm, see SM 1.2. The stimuli and responses were presented and collected using the E-prime software (EPrime v1.1, Psychology Software Tools, Inc.). Four participants (three patients and one control) were excluded from further analysis, because they completed a block with less than a 75% accuracy rate and/or with more than 6 probes not answered within a block.
Image acquisition and preprocessing
Structural magnetic resonance imaging (MRI) data was acquired with either a 1.5T Siemens Sonata (UNM, MGH, UI) or a 3T Siemens Trio (UMN). Functional MRI data was acquired with either a 1.5T Siemens Sonata (UNM) or a 3T Siemens Trio (UMN, MGH, UI). Cortical reconstruction and volumetric segmentation based on high resolution structural MRI scans was performed with the FreeSurfer surface reconstruction software (http://surfer.nmr.mgh.harvard.edu). Functional data were analyzed using fMRIB Software Library (FSL) (http://www.fmrib.ox.ac.uk/fsl). We fit a general linear model to the fMRI time course at each voxel to estimate the average activation during the three loads of the probe condition in all trials. Equal weight was given to all loads. For a region-of-interest (ROI) analysis, we derived DLPFC ROIs from FreeSurfer cortical parcellations as described previously,36,37 and extracted indices of mean activation of the DLPFC in percent signal change (%Δ) during the three loads of the probe condition in all trials. Because there is strong evidence for a cortical dysfunction especially in the left DLPFC in schizophrenia,19,38,39 we focused on this region in our ROI analysis. We also performed an exploratory whole-brain model to investigate MB-COMT promoter methylation effects across all cortical and subcortical regions. For additional details about data acquisition and preprocessing, see SM 1.3 and reference 39.
DNA methylation sample processing and quality control
ETDA blood samples, obtained from 234 participants and stored at –80 °C, were sent to the Harvard Partners Center for Genetics and Genomics for DNA extraction. All DNA extraction, bisulphite modification, and hybridization steps were done blinded to group assignment. Bisulphite modification and hybridization on the blinded DNA was performed at the Mind Research Network Neurogenetics Core Lab on an Infinium HumanMethylation27 BeadChip using Illumina Infinium Methylation Assay covering 27 578 CpG sites. Built-in controls were used to evaluate the quality of individual arrays. No sample failed the bisulphite (BS) conversion efficiency threshold (mean BS control intensity values < 4000). For more information on DNA methylation preprocessing and intensity data extraction, see SM 1.4 and SM 1.5.
All samples and CpG sites were filtered further according to coverage. In detail, we defined all β-values with a Beadstudio P value of signal detection above 0.05 as missing and removed samples or CpG sites with a coverage of below 95%. After this first QC step, 229 samples remained in the analysis. Next, we removed five samples displaying gender mismatch (females should show high β-values on the X chromosome), resulting in a data set of 224. After excluding participants who had no imaging data or imaging/behavioral/genetic data of insufficient quality (see above and SM 1.3/1.6), MB-COMT promoter methylation β-values were extracted from a final data set of 82 schizophrenia patients and 102 healthy controls. The investigated CpG—at base pair position 18308445 on chromosome 22—is 864 base pair positions upstream from the transcription start site of the membrane-bound isoform of COMT (Fig. S2).
We performed a singular value decomposition on the genome-wide methylation data and tested all principal components for effects of potential confounding factors, including chip, BS conversion efficiency and sex. This analysis showed that the variables chip and BS conversion efficiency had significant effects on multiple components. Therefore we corrected our β-values statistically for these effects by computing residuals in a regression model accounting for the effects of chip and BS conversion efficiency using the previously derived coefficients. That is, we removed the effects of chip and BS conversion efficiency, which were not collinear with sex, age, or diagnosis. For more information, see reference 40.
Population stratification
DNA methylation has been shown to be moderately influenced by population structure, although to a much lesser degree than genetic data.41 Furthermore, through principal and independent component analyses, Liu et al.41 found that the population structure present in DNA methylation data are very well captured by the first genetic principal component. Hence, to avoid confounding effects due to population stratification, we applied principal component analysis to our genotype data using EIGENSTRAT of the EIGENSOFT 3.0 software package,42,43 and included the first principal component as covariate in our imaging models. For more details of principal components analysis, see ref. 44.
Statistical models
Basic demographic characteristics were compared across diagnostic groups and acquisition site-specific scanner field strengths using t tests for continuous variables. Chi-square statistics were used to examine differences in categorical variables. Type I error was set to 0.05 for all analyses. Sample characteristic analyses were performed with SPSS 17.0.
We performed a ROI analysis to test for an association between MB-COMT promoter methylation with neural activity in the left DLPFC using a general linear model and controlling for scanner field strength. Since age and sex effects have been reported for DNA methylation,45-49 we also covaried for these variables. To account for non-random sampling of schizophrenia patients, we explicitly modeled the effects of diagnosis in our main model and tested for a diagnosis by MB-COMT promoter methylation interaction effect. To control for population stratification, we included the first genetic principal component as a covariate.
We also performed a whole brain analysis investigating the relationship between MB-COMT promoter methylation and WkM-induced brain activity in patients and controls using mixed effects models in FSL, estimating the between-subject variability as random effects. The model was cluster-corrected according to FSL default settings with a z-value of 2.3 and a P value of 0.05 and controlled for diagnosis, field strength, sex, age, and population stratification.
Supplementary Material
Disclosure of Potential Conflicts of Interest
Veit Roessner has received lecture fees from Eli Lilly, Janssen-Cilag, Medice, Novartis and was a member of advisory boards of Eli Lilly, Novartis. Dr Calhoun has received research support from the National Institutes of Health, National Science Foundation, Department of Energy, has done some consultation, has performed grant reviews for the National Institutes of Health and other agencies; has guest-edited journal sections; has given academic lectures in various scientific venues; and has generated books or book chapters for publishers of various texts. All other authors declare no biomedical financial interests or other potential conflict of interests.
Acknowledgments
This work was supported by the National Institutes of Health (NIH/NCRR P41RR14075, K08 MH068540), the Department of Energy (DE-FG02-99ER62764), the MIND Research Network, Morphometry BIRN (1U24, RR021382A), Function BIRN (U24RR021992-01, NIH.NCRR MO1 RR025758-01), the National Institute of General Medical Sciences and the National Institute of Biomedical Imaging and Bioengineering (NIGMS P20-GM103472 and NIBIB 2R01-EB000840 to V.C.), and the National Association for Research in Schizophrenia and Affective Disorders (NARSAD award to S.E.).
Glossary
Abbreviations:
- BS
bisulphite
- COMT
catechol-O-methyltransferase
- CpG,
C-phosphate-G
- DLPFC
Dorsolateral prefrontal cortex
- DNA
deoxyribonucleic acid
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- FSL
FMRIB Software Library
- HC
healthy controls
- MB-COMT
membrane-bound catechol-O-methyltransferase
- MCIC
Mind Clinical Imaging Consortium
- MGH
Massachusetts General Hospital
- ROI
region-of-interest
- SCID,
Structured Clinical Interview for DSM disorders
- S-COMT
soluble catechol-O-methyltransferase
- SCZ,
schizophrenia patients
- SES,
socio-economic status
- SIRP
Sternberg Item Recognition Paradigm
- SM,
Supplemental Materials
- stand. res.
Standardized residuals
- UI,
University of Iowa
- UMN
University of Minnesota
- UNM
University of New Mexico
- VLPFC
Ventrolateral prefrontal cortex
- WkM
working memory
- WRAT-IIIRT
Wide Range Achievement Test – III, reading subtest
References
- 1.Shifman S, Bronstein M, Sternfeld M, Pisanté-Shalom A, Lev-Lehman E, Weizman A, Reznik I, Spivak B, Grisaru N, Karp L, et al. A highly significant association between a COMT haplotype and schizophrenia. Am J Hum Genet. 2002;71:1296–302. doi: 10.1086/344514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wonodi I, Stine OC, Mitchell BD, Buchanan RW, Thaker GK. Association between Val108/158 Met polymorphism of the COMT gene and schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2003;120B:47–50. doi: 10.1002/ajmg.b.20037. [DOI] [PubMed] [Google Scholar]
- 3.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–22. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munafò MR, Bowes L, Clark TG, Flint J. Lack of association of the COMT (Val158/108 Met) gene and schizophrenia: a meta-analysis of case-control studies. Mol Psychiatry. 2005;10:765–70. doi: 10.1038/sj.mp.4001664. [DOI] [PubMed] [Google Scholar]
- 5.Okochi T, Ikeda M, Kishi T, Kawashima K, Kinoshita Y, Kitajima T, Yamanouchi Y, Tomita M, Inada T, Ozaki N, et al. Meta-analysis of association between genetic variants in COMT and schizophrenia: an update. Schizophr Res. 2009;110:140–8. doi: 10.1016/j.schres.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Fan J-B, Zhang C-S, Gu N-F, Li X-W, Sun W-W, Wang H-Y, Feng G-Y, St Clair D, He L. Catechol-O-methyltransferase gene Val/Met functional polymorphism and risk of schizophrenia: a large-scale association study plus meta-analysis. Biol Psychiatry. 2005;57:139–44. doi: 10.1016/j.biopsych.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Newell-Price J. Proopiomelanocortin gene expression and DNA methylation: implications for Cushing’s syndrome and beyond. J Endocrinol. 2003;177:365–72. doi: 10.1677/joe.0.1770365. [DOI] [PubMed] [Google Scholar]
- 8.Keshet I, Lieman-Hurwitz J, Cedar H. DNA methylation affects the formation of active chromatin. Cell. 1986;44:535–43. doi: 10.1016/0092-8674(86)90263-1. [DOI] [PubMed] [Google Scholar]
- 9.Tenhunen J, Salminen M, Lundström K, Kiviluoto T, Savolainen R, Ulmanen I. Genomic organization of the human catechol O-methyltransferase gene and its expression from two distinct promoters. Eur J Biochem. 1994;223:1049–59. doi: 10.1111/j.1432-1033.1994.tb19083.x. [DOI] [PubMed] [Google Scholar]
- 10.Roth JA. Membrane-bound catechol-O-methyltransferase: a reevaluation of its role in the O-methylation of the catecholamine neurotransmitters. Rev Physiol Biochem Pharmacol. 1992;120:1–29. doi: 10.1007/BFb0036121. [DOI] [PubMed] [Google Scholar]
- 11.Melas PA, Rogdaki M, Ösby U, Schalling M, Lavebratt C, Ekström TJ. Epigenetic aberrations in leukocytes of patients with schizophrenia: association of global DNA methylation with antipsychotic drug treatment and disease onset. FASEB J. 2012;26:2712–8. doi: 10.1096/fj.11-202069. [DOI] [PubMed] [Google Scholar]
- 12.Abdolmaleky HM, Cheng K-H, Faraone SV, Wilcox M, Glatt SJ, Gao F, Smith CL, Shafa R, Aeali B, Carnevale J, et al. Hypomethylation of MB-COMT promoter is a major risk factor for schizophrenia and bipolar disorder. Hum Mol Genet. 2006;15:3132–45. doi: 10.1093/hmg/ddl253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nohesara S, Ghadirivasfi M, Mostafavi S, Eskandari M-R, Ahmadkhaniha H, Thiagalingam S, Abdolmaleky HM. DNA hypomethylation of MB-COMT promoter in the DNA derived from saliva in schizophrenia and bipolar disorder. J Psychiatr Res. 2011;45:1432–8. doi: 10.1016/j.jpsychires.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Mill J, Tang T, Kaminsky Z, Khare T, Yazdanpanah S, Bouchard L, Jia P, Assadzadeh A, Flanagan J, Schumacher A, et al. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet. 2008;82:696–711. doi: 10.1016/j.ajhg.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dempster EL, Mill J, Craig IW, Collier DA. The quantification of COMT mRNA in post mortem cerebellum tissue: diagnosis, genotype, methylation and expression. BMC Med Genet. 2006;7:10. doi: 10.1186/1471-2350-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 17.Walton E, Turner JA, Ehrlich S. Neuroimaging as a potential biomarker to optimize psychiatric research and treatment. Int Rev Psychiatry. 2013;25:619–31. doi: 10.3109/09540261.2013.816659. [DOI] [PubMed] [Google Scholar]
- 18.Hall MH, Smoller JW. A new role for endophenotypes in the GWAS era: functional characterization of risk variants. Harv Rev Psychiatry. 2010;18:67–74. doi: 10.3109/10673220903523532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160:2209–15. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 20.Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, Saper CB, Warach S. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry. 1999;45:1128–37. doi: 10.1016/S0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- 21.Karlsgodt KH, Glahn DC, van Erp TGM, Therman S, Huttunen M, Manninen M, Kaprio J, Cohen MS, Lönnqvist J, Cannon TD. The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control subjects. Schizophr Res. 2007;89:191–7. doi: 10.1016/j.schres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Schlösser RGM, Koch K, Wagner G, Nenadic I, Roebel M, Schachtzabel C, Axer M, Schultz C, Reichenbach JR, Sauer H. Inefficient executive cognitive control in schizophrenia is preceded by altered functional activation during information encoding: an fMRI study. Neuropsychologia. 2008;46:336–47. doi: 10.1016/j.neuropsychologia.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Ursini G, Bollati V, Fazio L, Porcelli A, Iacovelli L, Catalani A, Sinibaldi L, Gelao B, Romano R, Rampino A, et al. Stress-related methylation of the catechol-O-methyltransferase Val 158 allele predicts human prefrontal cognition and activity. J Neurosci. 2011;31:6692–8. doi: 10.1523/JNEUROSCI.6631-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton LS, Altshuler LL, Townsend J, Bookheimer SY, Phillips OR, Fischer J, Woods RP, Mazziotta JC, Toga AW, Nuechterlein KH, et al. Alterations in functional activation in euthymic bipolar disorder and schizophrenia during a working memory task. Hum Brain Mapp. 2009;30:3958–69. doi: 10.1002/hbm.20820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim MA, Tura E, Potkin SG, Fallon JH, Manoach DS, Calhoun VD, Turner JA, FBIRN Working memory circuitry in schizophrenia shows widespread cortical inefficiency and compensation. Schizophr Res. 2010;117:42–51. doi: 10.1016/j.schres.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, 3rd, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–8. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- 27.Karlsgodt KH, Sanz J, van Erp TGM, Bearden CE, Nuechterlein KH, Cannon TD. Re-evaluating dorsolateral prefrontal cortex activation during working memory in schizophrenia. Schizophr Res. 2009;108:143–50. doi: 10.1016/j.schres.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–9. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155:1285–7. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- 30.Meda SA, Bhattarai M, Morris NA, Astur RS, Calhoun VD, Mathalon DH, Kiehl KA, Pearlson GD. An fMRI study of working memory in first-degree unaffected relatives of schizophrenia patients. Schizophr Res. 2008;104:85–95. doi: 10.1016/j.schres.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Godar SC, Bortolato M. Gene-sex interactions in schizophrenia: focus on dopamine neurotransmission. Front Behav Neurosci. 2014;8:71. doi: 10.3389/fnbeh.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.First M, Spitzer A, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Nonpatient Edition. New York: New York State Psychiatric Institute; 2002. [Google Scholar]
- 34.Ehrlich S, Morrow EM, Roffman JL, Wallace SR, Naylor M, Bockholt HJ, Lundquist A, Yendiki A, Ho B-C, White T, et al. The COMT Val108/158Met polymorphism and medial temporal lobe volumetry in patients with schizophrenia and healthy adults. Neuroimage. 2010;53:992–1000. doi: 10.1016/j.neuroimage.2009.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gollub RL, Shoemaker JM, King MD, White T, Ehrlich S, Sponheim SR, Clark VP, Turner JA, Mueller BA, Magnotta V, et al. The MCIC collection: a shared repository of multi-modal, multi-site brain image data from a clinical investigation of schizophrenia. Neuroinformatics. 2013;11:367–88. doi: 10.1007/s12021-013-9184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehrlich S, Morrow EM, Roffman JL, Wallace SR, Naylor M, Bockholt HJ, Lundquist A, Yendiki A, Ho B-C, White T, et al. The COMT Val108/158Met polymorphism and medial temporal lobe volumetry in patients with schizophrenia and healthy adults. Neuroimage. 2010;53:992–1000. doi: 10.1016/j.neuroimage.2009.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yendiki A, Greve DN, Wallace S, Vangel M, Bockholt J, Mueller BA, Magnotta V, Andreasen N, Manoach DS, Gollub RL. Multi-site characterization of an fMRI working memory paradigm: reliability of activation indices. Neuroimage. 2010;53:119–31. doi: 10.1016/j.neuroimage.2010.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Potkin SG, Turner JA, Brown GG, McCarthy G, Greve DN, Glover GH, Manoach DS, Belger A, Diaz M, Wible CG, et al. FBIRN Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophr Bull. 2009;35:19–31. doi: 10.1093/schbul/sbn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walton E, Turner J, Gollub RL, Manoach DS, Yendiki A, Ho B-C, Sponheim SR, Calhoun VD, Ehrlich S. Cumulative genetic risk and prefrontal activity in patients with schizophrenia. Schizophr Bull. 2013;39:703–11. doi: 10.1093/schbul/sbr190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J, Chen J, Ehrlich S, Walton E, White T, Perrone-Bizzozero N, Bustillo J, Turner JA, Calhoun VD. Methylation Patterns in Whole Blood Correlate With Symptoms in Schizophrenia Patients. Schizophr Bull. 2013 doi: 10.1093/schbul/sbt080. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Hutchison K, Perrone-Bizzozero N, Morgan M, Sui J, Calhoun V. Identification of genetic and epigenetic marks involved in population structure. PLoS One. 2010;5:e13209. doi: 10.1371/journal.pone.0013209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2:e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 44.Hass J, Walton E, Kirsten H, Liu J, Priebe L, Wolf C, Karbalai N, Gollub R, White T, Roessner V, et al. IMAGEN Consortium A Genome-Wide Association Study Suggests Novel Loci Associated with a Schizophrenia-Related Brain-Based Phenotype. PLoS One. 2013;8:e64872. doi: 10.1371/journal.pone.0064872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boks MP, Derks EM, Weisenberger DJ, Strengman E, Janson E, Sommer IE, Kahn RS, Ophoff RA. The relationship of DNA methylation with age, gender and genotype in twins and healthy controls. PLoS One. 2009;4:e6767. doi: 10.1371/journal.pone.0006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suñer D, Cigudosa JC, Urioste M, Benitez J, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102:10604–9. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bjornsson HT, Sigurdsson MI, Fallin MD, Irizarry RA, Aspelund T, Cui H, Yu W, Rongione MA, Ekström TJ, Harris TB, et al. Intra-individual change over time in DNA methylation with familial clustering. JAMA. 2008;299:2877–83. doi: 10.1001/jama.299.24.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang FF, Cardarelli R, Carroll J, Fulda KG, Kaur M, Gonzalez K, Vishwanatha JK, Santella RM, Morabia A. Significant differences in global genomic DNA methylation by gender and race/ethnicity in peripheral blood. Epigenetics. 2011;6:623–9. doi: 10.4161/epi.6.5.15335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J, Morgan M, Hutchison K, Calhoun VD. A study of the influence of sex on genome wide methylation. PLoS One. 2010;5:e10028. doi: 10.1371/journal.pone.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.