Abstract
The neural pathways of the auditory system underlie our ability to detect sounds and to transform amplitude and frequency information into rich and meaningful perception. While it shares some organizational features with other sensory systems, the auditory system has some unique functions that impose special demands on precision in circuit assembly. In particular, the cochlear epithelium creates a frequency map rather than a space map, and specialized pathways extract information on interaural time and intensity differences to permit sound source localization. The assembly of auditory circuitry requires the coordinated function of multiple molecular cues. Eph receptors and their ephrin ligands constitute a large family of axon guidance molecules with developmentally regulated expression throughout the auditory system. Functional studies of Eph/ephrin signaling have revealed important roles at multiple levels of the auditory pathway, from the cochlea to the auditory cortex. These proteins provide graded cues used in establishing tonotopically ordered connections between auditory areas, as well as discrete cues that enable axons to form connections with appropriate postsynaptic partners within a target area. Throughout the auditory system, Eph proteins help to establish patterning in neural pathways during early development. This early targeting, which is further refined with neuronal activity, establishes the precision needed for auditory perception.
Keywords: Cochlea, brainstem, inferior colliculus, auditory cortex, Eph receptor, ephrin
I. Introduction
Mature neural circuitry in the auditory system reflects the culmination of multiple coordinated developmental processes. In early embryonic development, morphogenesis of the elaborate structures in the inner ear relies on multiple signaling pathways. Mechanisms for cell fate specification in the peripheral and central portions of the auditory system are just beginning to be understood. During late embryonic and early postnatal development, axons are guided to appropriate targets and form synapses, which in some parts of the auditory pathway exhibit highly specialized structures. The extent and mechanisms of neuronal migration remain elusive. Some of these processes likely share molecular mechanisms with other developing systems, whereas the differences are likely to shed light on the specializations needed for auditory function. In this review we focus on the mechanisms by which axons of cells in the auditory system are guided to their targets. At each level of the auditory system, axons project to appropriate target regions, which include specialized nuclei or cortical areas. Here we consider specifically the guidance of auditory axons to appropriate regions or cell types within their target nucleus or cortical area, and we highlight the role of a family of axon guidance molecules, the Eph receptors and their ephrin ligands, in this targeting.
II. Eph Receptors and Ephrins
Eph proteins, including the Eph receptors and their ligands, the ephrins, are broadly expressed in the developing auditory system (for reviews see Bianchi et al., 2002, Pickles, 2003, Cramer, 2005, Gabriele et al., 2011). They provide multiple mechanisms for signaling and for generating precise connectivity. Eph/ephrin interactions generally regulate cellular movement and adhesion, and thus these proteins play significant roles not only in axon guidance, but also in cell migration, angiogenesis, cancer, synaptogenesis, and synaptic plasticity (Lai and Ip, 2009, Pasquale, 2010, Bush and Soriano, 2012). The basis for this broad array of functions lies in their complex interactions in a variety of biological contexts.
Binding properties
Eph receptors, the largest family of receptor tyrosine kinases in vertebrates, were first identified from an Erythropoietin-Producing Hepatocellular carcinoma (Hirai et al., 1987). Eph receptors and ephrins (Eph Receptor Interacting proteins), are divided into the A and B classes on the basis of sequence homology and binding affinity (Gale et al., 1996). In mammals there are ten EphA receptors (EphA1-A10) and six EphB receptors (EphB1-B6). Ephrin-A proteins (A1-A6) bind to all of the EphA receptors and ephrin-B proteins (B1-B3) bind to all the EphB receptors. The broad binding properties within each class provide considerable redundancy, and several family members are often co-expressed in populations of cells. Exceptions to these binding restrictions provide some degree of crosstalk between the A and B families: EphA4 can bind to both ephrin-A and ephrin-B ligands, and EphB2 can bind to ephrin-A5 (Gale et al., 1996, Himanen et al., 2004, Himanen, 2012).
Ephrins are unusual as ligands for receptor tyrosine kinases in that they are attached to cell membranes, thereby facilitating cell-cell interactions. Ephrin-A proteins are tethered to the plasma membrane with a glycosyl phoshphatidyl inositol (GPI) linkage, whereas ephrin-B ligands are integral membrane proteins with a transmembrane domain. Several facets of Eph/ephrin signaling result from this property of ephrins. First, as cells must come into contact with one another, the signaling occurs at small distances and thus tends to be important for axon targeting over limited areas. In the auditory pathways discussed here, Eph protein signaling generally regulates choice of location or postsynaptic partner within a designated target area. Second, the association of both Eph receptors and ephrins with cell membranes facilitates bidirectional signaling. In addition to traditional forward signaling, reverse signaling also occurs, whereby the activation of ephrins by Eph receptor binding activates cell signaling events in the cell expressing the ephrins (Davy et al., 1999, Huai and Drescher, 2001, Cowan and Henkemeyer, 2002, Kullander and Klein, 2002, Lim et al., 2008). It was initially thought that Eph/ephrin interactions elicited only chemorepulsion in growth cones through forward signaling; however, it has now been shown that both forward and reverse signaling are active in axon guidance, and that interactions may be attractive or repulsive (for review see Xu and Henkemeyer, 2012, Klein and Kania, 2014).
Regulation of Eph/ephrin signaling
Unlike most receptor tyrosine kinases, which can be activated by a single molecule of ligand, Eph receptors are unusual in that their activation requires membrane attached or clustered ligands (Davis et al., 1994). Activated Eph receptors are arranged as a tetramer with two ephrin molecules and two Eph receptors. Moreover, this binding results in formation of large clusters of activated Eph receptors, which reside in plasma membrane microdomains known as lipid rafts (Marquardt et al., 2005, Janes et al., 2012). These clusters can contain multiple types of Eph receptors, and may serve as an additional mechanism for crosstalk between the Eph subclasses (Janes et al., 2011, Nikolov et al., 2013). Multiple Eph receptors and ephrins are often expressed within a single tissue or cell. Ephrins can bind to Eph receptors within the same cell in cis, thereby inhibiting protein interactions with molecules on other cells in trans (Arvanitis and Davy, 2008, Kao and Kania, 2011, Falivelli et al., 2013).
While ephrins are generally associated with cell membranes, the extracellular domain of the proteins can be cleaved by metalloproteases. This cleavage was originally observed for ephrin-A proteins and is seen as a mechanism to promote contact-mediated cell repulsion. Further, it has been shown that the cleaved, soluble extracellular domains of the Eph proteins can in some cases have signaling properties on their own (Ieguchi et al., 2013). To facilitate repulsion between cells expressing EphB receptors and ephrin-B ligands, it is thought that endocytosis of the bound complex is needed (Zimmer et al., 2003). However, cleavage of EphB proteins by matrix metalloproteases has also been reported (Lin et al., 2008).
Axon guidance
Eph receptors and ephrins have a significant role in axon guidance in many areas of the developing nervous system. This function was first discovered in the retinotectal pathway of the chick embryo, in which retinal ganglion cells express a gradient of EphA3 along the nasal-temporal axis and the tectum expresses an opposing gradient of ephrin-A2 and ephrin-A5 in the recipient anterior-posterior axis (Cheng and Flanagan, 1994, Cheng et al., 1995, Drescher et al., 1995). A similar pattern was seen in mammals, with EphA5 expressed in a gradient in retinal ganglion cells. Mutations in these ephrin-A genes disrupt mapping along this axis in the superior colliculus and in the lateral geniculate nucleus (LGN). The effects are more pronounced when spontaneous activity is blocked, suggesting that topography requires both fine axon guidance and subsequent activity-dependent refinement (Feldheim et al., 2004, Pfeiffenberger et al., 2006, Cang et al., 2008b, Triplett and Feldheim, 2012). Interestingly, mutations in ephrin-A proteins resulted in erroneous placement of eye-specific layers in the LGN (Pfeiffenberger et al., 2005), and overexpression of EphA5 in ferrets resulted in an alteration of eye-specific projections (Huberman et al., 2005). In addition, control of retinal ganglion cell growth at the optic chiasm is regulated by ephrin-B proteins at the midline (Petros et al., 2009, Chenaux and Henkemeyer, 2011). These studies in the visual system demonstrate roles for Eph proteins in both graded axon guidance as well as in guidance at discrete choice points.
III. Peripheral Auditory Pathways
Organization of peripheral auditory circuits
Neural processing of auditory stimuli in mammals begins in the cochlea, where sensory receptors known as hair cells in the organ of Corti encode changes in sound pressure that are transduced through movement of the basilar membrane. Deflections in hair cell stereocilia in response to this movement result in transmitter release onto distal processes of spiral ganglion neurons (SGNs). Because the basilar membrane varies systematically in its mechanical properties, there is a strong correlation between position in the organ of Corti and the frequency that elicits the largest displacement. This relationship forms the basis of tonotopy, whereby ordered representations of frequency are seen within auditory areas. Topographic connections convey this frequency map to SGNs. Central projections of spiral ganglion cells then propagate this map into the central auditory system.
The peripherally projecting axons of SGNs form radial bundles that innervate the hair cells (Rubel and Fritzsch, 2002). Mammals have two types of auditory hair cells. Along the organ of Corti, there is one row of inner hair cells and three rows of outer hair cells. These cells have distinct functions and innervation patterns (Kiang et al., 1982). Inner hair cells contact peripheral processes of numerous type I SGNs. In contrast, outer hair cells are more sparsely innervated, making contact with relatively few type II SGNs (Figure 1). Genetic fate mapping studies suggest that these subtypes of SGN are determined at early developmental ages, and that projections are correlated with their neurogenesis (Koundakjian et al., 2007). Initial projections may undergo some refinement, however, as individual SGN axons have been shown to initially contact both inner and outer hair cells (Echteler, 1992).
Figure 1.
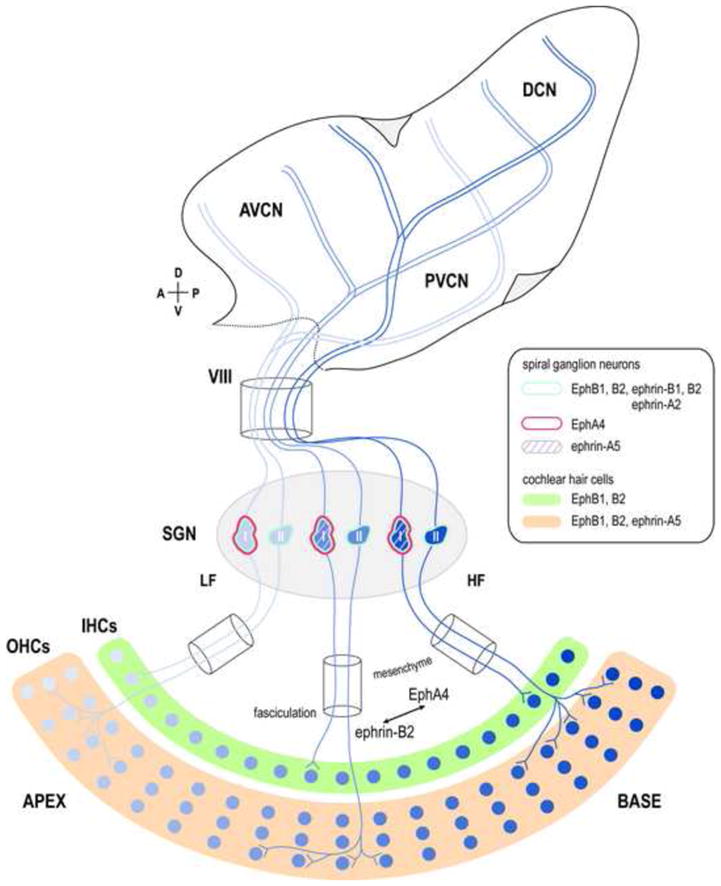
Mapping of afferents from the auditory periphery to the cochlear nucleus complex. Precise topography (indicated by shades of blue in this and all subsequent figures) preserves centrally the frequency order established in the cochlea. The organ of Corti, SGNs, and their developing connections exhibit complex complementary and overlapping Eph/ephrin expression patterns. Guidance mechanisms involving both subfamilies are responsible for accurate circuit assembly (inset key). Inner radial bundle formation and proper fasciculation require ephrin-B2/EphA4 signaling between growing SGN peripheral processes and the surrounding otic mesenchyme. Ephrin-A5-expressing outer hair cells (OHCs) repel EphA4-positive type I SGNs that preferentially map to the inner hair cell (IHCs) rank. A subpopulation of type I SGNs are ephrin-A5-positive (purple stripes). LF = low frequency, HF = high frequency.
Eph/ephrin signaling and axon guidance in peripheral auditory axons
Several studies have demonstrated that Eph receptors and ephrins are expressed in the developing inner ear in mice (Henkemeyer et al., 1994, Bianchi and Gale, 1998, Bianchi et al., 2002, Pickles et al., 2002, Zhou et al., 2011, Coate et al., 2012, Defourny et al., 2013). SGNs express ephrin-B1 and ephrin-B2, and growth of SGN processes is inhibited in vitro by EphB2 through reverse signaling (Bianchi and Gray, 2002, Zhou et al., 2011). Null mutations in EphB1-3 or ephrin-B1 lead to excessive growth of SGN axons beyond the third row of outer hair cells (Zhou et al., 2011). These studies suggest that EphB signaling delimits the region through which SGN axons grow. This view is supported by studies of SGN axon fasciculation. EphA4, which binds both ephrin-A and ephrin-B ligands, is needed for normal fasciculation of SGN axons in their peripheral trajectories (Coate et al., 2012). SGN peripheral axons in mice lacking EphA4 were more splayed and formed significantly smaller fascicles. Ephrin-B2 mutations resulted in a similar effect, suggesting that ephrin-B2/EphA4 signaling is needed for proper fasciculation.
During formation of peripheral projections, EphA4 is expressed in type I SGNs, with very limited expression in type II SGNs, and Ephrin-A5 is expressed in outer hair cells, but not inner hair cells (Defourny et al., 2013). In ephrin-A5−/− mice, outer hair cell innervation is more extensive, largely due to type I SGN axons that extend past inner hair cells into the inappropriate region. The distinct expression pattern of ephrin-A5 suggests that this protein acts on growing SGN axons via forward signaling. This study provides further evidence that Eph signaling provides instructive signals that culminate in appropriate peripheral auditory connections. An interesting possibility is that Eph molecules participate in correction of initial errors in cell contacts (Echteler, 1992).
Central projections from the cochlea toward the brainstem travel within the VIIIth cranial nerve, which contains processes from auditory and vestibular ganglia. Expression of Eph proteins in central axons has been demonstrated in chick embryos (Siddiqui and Cramer, 2005). During the formation of auditory circuitry these fibers express several Eph receptors and ephrins from both classes. Expression of EphA4 is graded in a manner consistent with the future frequency axes, but the role of the protein in forming topographic maps has not been tested. Interestingly, EphB2 and EphA4 have complementary expression patterns in the VIIIth nerve (Siddiqui and Cramer, 2005), with greater EphA4 expression in putative auditory regions of the nerve and greater EphB2 expression in putative vestibular regions. Misexpression of these genes or treatment with inhibitory fusion proteins did not result in axon mistargeting to inappropriate auditory vs. vestibular regions (Allen-Sharpley et al., 2013). Instead, these manipulations resulted in a shifting of the boundaries demarcating the auditory and vestibular projection areas of the nerve within the brainstem. In mice, EphA5 shows significantly more expression in the spiral ganglion than in vestibular ganglia (Lu et al., 2011), but the contribution of this protein to modality-specific targeting is not known.
IV. Auditory Brainstem
Topographic mapping from sensory epithelia into higher sensory areas is a feature shared with other systems, and hence tonotopy is similar to retinotopy or somatotopy. However, the auditory system is unique in that the stimulus frequency, and not the stimulus location, is mapped along the sensory receptive surface. Thus, while these systems may share common mechanisms to establish topographic projections, the central auditory system incorporates specific circuitry to obtain location information from binaural cues.
Eph proteins shape connections in avian auditory brainstem
Neuroanatomical and electrophysiological studies in birds have advanced our understanding of the role of brainstem nuclei in sound localization (Carr and Konishi, 1990, Hyson, 2005). In chicks, the central projections of the cochlear ganglion bifurcate and terminate in n. angularis and n. magnocellularis (NM) (Rubel and Fritzsch, 2002). NM axons also bifurcate, sending one branch to ipsilateral n. laminaris (NL) and the other branch to contralateral NL. Ipsilateral NM axon branches terminate on dorsal dendrites and somata of NL neurons, whereas contralateral projections terminate on ventral NL dendrites and somata (Young and Rubel, 1983). The contralateral projection creates a delay line through which signals from NM require longer time to reach lateral NL cells than to reach medial NL cells. This circuitry, together with fine coincidence detection in NL neurons, allows for the computation of interaural time differences (ITD’s) used to localize low frequency sounds (Overholt et al., 1992, Hyson, 2005, Koppl and Carr, 2008).
The NM-NL projection thus contains a graded map alongside a binary separation of ipsilateral and contralateral projections. Eph protein signaling has a demonstrated role in both aspects of this projection. During the formation of these connections, which are seen by embryonic day 10 (E10), EphA4 is more heavily expressed on dorsal dendrites of NL (Cramer et al., 2000). In this dorsal region, a concentration gradient is seen so that high frequency regions display more expression than do low frequency regions (Person et al., 2004). Disruption of EphA4 using in ovo plasmid electroporation resulted in errors in dorsal-ventral segregation of inputs, as well as broadening the topography of the NM-NL projection (Cramer et al., 2004, Huffman and Cramer, 2007).
While EphA4 has an asymmetric dorsoventral expression pattern, several other Eph proteins are expressed in both dorsal and ventral regions (Cramer et al., 2002). EphB2 is expressed similarly on both sides, and appears to work together with EphA4 in restricting NM inputs appropriately. When EphB2 signaling was blocked using either electroporation or treatment with inhibiting fusion proteins, axons made errors in dorsoventral targeting; when both EphB and EphA signaling were blocked, this effect was significantly greater (Allen-Sharpley and Cramer, 2012). Graded expression of ephrin-B2, a ligand for EphA4 and EphB2, was observed along the frequency axis in NL cell bodies, as well as in the glial cells alongside NL (Person et al., 2004). The contributions of ephrin-B2 to tonotopy and patterning in this pathway have not yet been tested. However, unlike the opposing gradients seen in central projections of the retina, the gradients seen in this pathway appear to be parallel.
Formation of brainstem pathways in the hindbrain in chick embryos requires an extended period of migration and morphogenesis (Rubel et al., 1976, Cramer and Rubel, 1998). At the earliest ages the hindbrain is divided into distinct rhombomeres. Movement across rhombomere boundaries is limited at early ages, at least in part by Eph protein-mediated repulsion at these boundaries (Xu et al., 1999). NM and NL neurons are born early in the hindbrain, from embryonic day 2 (E2) to E4 (Rubel et al., 1976). Precursors for NM and NL then coalesce to form a laterally oriented auditory anlage at about E7 (Book and Morest, 1990). The nuclei are then separate and move medially, and NL takes on its characteristic laminar appearance by E9-10, when the synapses from NM inputs form. Thus, the period of axon guidance and synaptogenesis coincides with the formation of the mature nuclei (Hendricks et al., 2006). Eph protein signaling appears to influence both processes. Disruption of EphA4 and EphB2 not only alters axon targeting, but also results in abnormally shaped auditory nuclei (Cramer et al., 2004, Allen-Sharpley and Cramer, 2012). These studies suggest that Eph proteins may coordinate the position of cells together with their arriving inputs. This dual role of Eph signaling in migration and axon targeting has been seen in several regions of the developing mammalian cerebral cortex (North et al., 2013) and suggests a potential role for coordinating cues that influence cell movement and axon outgrowth.
Sound localization circuits in mammals
In mammals, central SGN axons entering the brainstem branch and project tonotopically to three subdivisions of the cochlear nucleus, the anterior ventral cochlear nucleus (AVCN), the posterior ventral cochlear nucleus (PVCN), and the dorsal cochlear nucleus (DCN). Branches of axons from AVCN project to ipsilateral and contralateral medial superior olive (MSO), with inputs segregated onto lateral and medial dendrites, respectively, in a pathway that detects ITD’s. In addition, the globular bushy cells of the ventral cochlear nuclei (VCN) project to the medial nucleus of the trapezoid body (MNTB). These axons terminate on principal cells of the contralateral MNTB in a large synapse, the calyx of Held, which encapsulates the postsynaptic neuron (Cant, 1992). MNTB neurons provide inhibitory input to a number of auditory brainstem nuclei, including the MSO. Another target is the ipsilateral lateral superior olive (LSO), where cells receive tonotopically matched excitatory input from the spherical bushy cells of the ipsilateral ventral cochlear nucleus. This pathway allows for detection of interaural level differences (ILD’s), a cue used to determine locations of high frequency sounds.
Eph proteins shape multiple aspects of brainstem pathways
Studies of mutant mouse lines have revealed several roles for Eph proteins in the circuitry of the auditory brainstem. Evidence for a role in establishing tonotopy comes from a study in which animals were exposed to pure tones and then histologically processed to examine expression of the immediate early gene c-fos (Miko et al., 2007). Mice with reduced levels of ephrin-B2 showed increased positional spread of c-fos-positive cells in DCN in response to pure tone stimulation. Additionally, mice lacking EphA4 showed a shift in the position of c-fos-positive cells in MNTB in response to pure tones. The results suggest that ephrin-B2 and EphA4 are needed to form appropriately restricted tonotopic maps in some of the auditory brainstem nuclei. Expression studies showed gradients during development in several areas of the developing brainstem, consistent with this result. The altered frequency bands may reflect a role for Eph proteins in topographic mapping to these nuclei.
Eph proteins have an additional role in establishing VCN-MNTB projections. While this projection is normally strictly contralateral, a significant number of ipsilateral terminations are seen in ephrin-B2lacZ/+ mice and in EphB2−/−; EphB3−/− mice (Hsieh et al., 2010, Nakamura and Cramer, 2011). These ipsilateral projections have calyceal terminations that form at the same time as normal contralateral connections. Unlike the aberrant growth of vestibular inner ear efferents seen in embryonic EphB2−/−; EphB3−/− mice, this ipsilateral projection is not eliminated at later ages. The connections to ipsilateral MNTB in most cases arise from axon branches that project contralaterally, but in some cases ipsilateral calyces appear to emerge directly from VCN (Figure 2). These latter projections could reflect pruning of the contralateral branch, but could also indicate a failure of VCN axons to reach and cross the midline. The axon guidance molecules netrin-1 and DCC are needed for VCN axon growth to the midline (Howell et al., 2007), and absence of Robo3 receptor in VCN cells results in an entirely ipsilateral projection to VCN (Renier et al., 2010). In EphB mutants it appears that the majority of axons reach the midline, and that the mutations result in a more favorable environment for ipsilateral axon branches that may be stabilized in ipsilateral MNTB. These results together highlight the role of Eph signaling in determining local targets; in this case, once the axons have been instructed by other signals to cross the midline and synapse in contralateral MNTB. Consistent with this view, these mutations also lead to an enhancement of induced ipsilateral connections after unilateral cochlear removal during early postnatal development (Nakamura et al., 2012, Nakamura and Cramer, 2013).
Figure 2.
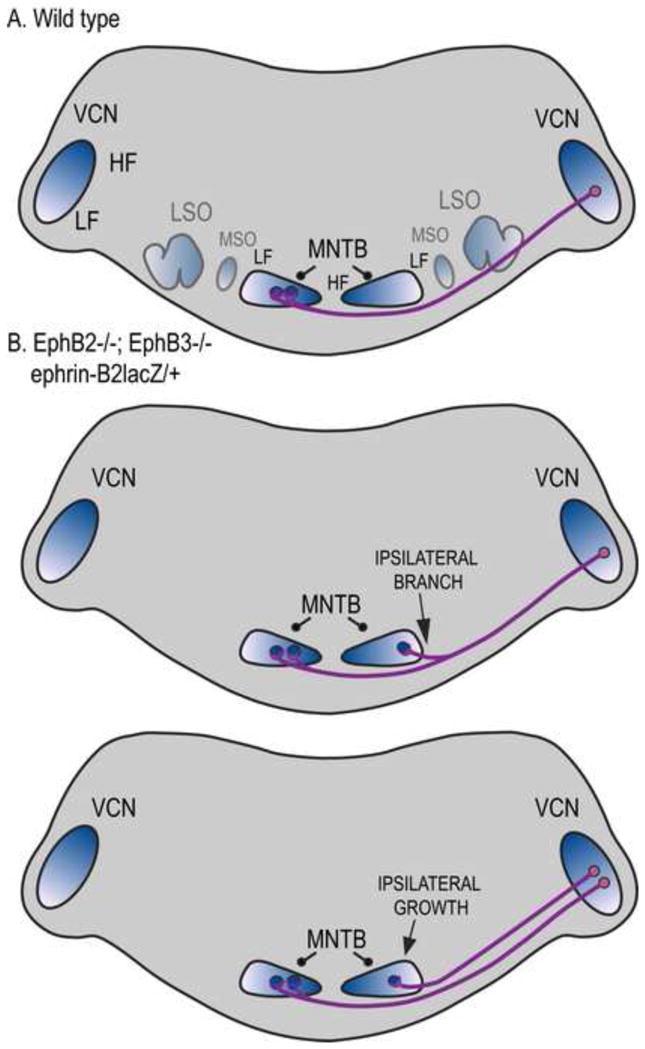
Altered brainstem projections in mice with mutations that reduce EphB signaling. A. Normal pathway from AVCN and PVCN (indicated as VCN) to MNTB in wild type mice. Nearly all of the projections are contralateral, terminating in a calyx of Held in the appropriate frequency region. Blue color gradients refer to frequency axis; dark blue represents high frequencies. LSO, lateral superior olive; MSO, medial superior olive. B. Mutations that block reverse signaling through ephrin-B receptors result in a significant number of ipsilateral calyceal projections (Hsieh et al., 2010). These ipsilateral projections in most cases appear as branches from contralaterally projecting VCN axons. In some cases, ipsilateral projections grow directly to the MNTB in the absence of a contralaterally projecting branch. For clarity, MSO and LSO are omitted; these nuclei have not been studied in the context of Eph signaling.
V. Auditory Midbrain
Continuous and compartmentalized pathways in the inferior colliculus
The inferior colliculus (IC) is a strategically located midbrain hub that integrates a multitude of converging inputs. Its position and rich multi-tiered innervation scheme emphasize its pivotal role coordinating both ascending and descending auditory circuits. In addition to its vast array of auditory afferents (Casseday et al., 2002), the IC also receives non-auditory inputs and is thereby thought to be involved in aspects of multisensory integration (Jain and Shore, 2006, Zhou and Shore, 2006). While purely auditory areas of the IC reliably preserve the tonotopic order established in the periphery (Merzenich and Reid, 1974, Schreiner and Langner, 1997, Malmierca et al., 2008), such a frequency continuum is less apparent in certain multimodal regions where topographically-mapped inputs target discrete, or discontinuous terminal fields.
The IC is functionally organized into a central nucleus (CNIC), lateral or external cortex (LCIC, ECIC: Loftus et al., 2008), and dorsal cortex (DCIC). Besides each exhibiting a unique connectivity, substantial intrinsic and commissural projections functionally link these major subdivisions (Saldaña and Merchán, 1992, Malmierca et al., 1995). The CNIC and LCIC in particular provide an intriguing model for considering guidance mechanisms as each has tonotopically-defined regions, yet differ significantly in other basic organizational features.
The CNIC is the largest subdivision and the most thoroughly studied to date, as it is the site of convergence for ascending streams that process in parallel complex spectral, temporal, and spatial signal attributes. It receives direct projections from the cochlear nuclear complex, as well as numerous pathways arising from various nuclei of the superior olivary complex (SOC) and lateral lemniscus (Winer and Schreiner, 2005). Despite its homogeneous appearance in routine cellular stains, the CNIC exhibits a layered arrangement consisting of a series of fibrodendtritic or isofrequency laminae that run perpendicular to its tonotopic axis (Morest and Oliver, 1984, Oliver and Morest, 1984). Inputs identify precise sublayers of target isofrequency laminae and terminate in characteristic patterns of alternating axonal layers. Spatial alignment of converging layered afferents is well documented (Shneiderman and Henkel, 1987, Oliver et al., 1997, Loftus et al., 2004, Malmierca et al., 2005, Loftus et al., 2010) and thought to define functional compartments or CNIC synaptic domains that receive various monaural and binaural input combinations (Oliver, 2005). Assembly of such complex circuitry requires considerable topographic precision and is necessary for the accurate assimilation of various stimulus features (Ehret and Merzenich, 1985, Schreiner and Langner, 1997) before being conveyed on to thalamus and cortex.
Far less is known concerning the topographic mapping and functionality of the LCIC. In rodent, the LCIC has a layered structure (Faye-Lund and Osen, 1985). Its deepest aspect, Layer 3, exhibits a clear frequency order with refined axonal layers similar to those in the adjacent CNIC. Layer 3 inputs are also comparable to its neighbor, arising primarily from lemniscal, intrinsic, or commissural origins (Saldaña and Merchán, 1992, Malmierca et al., 1995, Saldaña et al., 2009). In contrast, LCIC Layers 1 and 2 lack any evidence of a tonotopic organization and receive a largely unique set of connections. Despite considerable connections from the CNIC itself and modest inputs from lateral lemniscal nuclei (Rockel and Jones, 1973, Coleman and Clerici, 1987, González-Hernández et al., 1996), the heaviest influences to these more superficial areas arise primarily from auditory cortex (Herbert et al., 1991, Saldaña et al., 1996, Druga et al., 1997, Winer et al., 1998) and extramodal sources, including the dorsal column nuclei (Li and Mizuno, 1997), spinal trigeminal tract (Aitkin et al., 1981), and basal ganglia (Olazábal and Moore, 1989, Shammah-Lagnado et al., 1996). Interestingly, projection patterns to these multimodal areas are not layered, but rather appear to either preferentially target a periodic network of Layer 2 modules (Chernock et al., 2004, Zhou and Shore, 2006, Malmierca et al., 2011, Ouda and Syka, 2012), or spare these clusters of presumptive GABAergic cells and terminate in surrounding extramodular domains. Such patch-matrix-like distributions, while common in other sensory and motor systems (striatum: Gerfen and Engber, 1992, visual: Illing, 1996, somatosensory: Petersen, 2007, olfactory: Imai et al., 2010), are conspicuously absent from most auditory structures. Taken together, the CNIC and LCIC provide a promising model system for examining emergent topographic, laminar, and modular arrangements prior to experience.
Eph signaling in IC circuit assembly
Since patterns of afferent projections define functional compartments that occur within and between IC subdivisions, it is essential to understand the mechanisms that shape its early topography. Innervation of the nascent IC and the subsequent emergence of its orderly connectivity occur prior to hearing onset (postnatal day 12 in rat and mouse). In short, pioneer fibers invade appropriate subdivisions of the embryonic IC and exhibit initially diffuse projection distributions. Shortly after birth, characteristic CNIC layers become apparent as axonal arbors refine themselves, coupling selective pruning with continued elaboration within appropriate postsynaptic sublayers (Kandler and Friauf, 1993, Gabriele et al., 2000b, a, Gabriele et al., 2007, Henkel et al., 2007, Fathke and Gabriele, 2009). Bilateral LSO patterns and those arising from the contralateral DCN and dorsal nucleus of the lateral lemniscus (DNLL) all exhibit remarkable projection specificity prior to experience, targeting defined synaptic domains of frequency-band laminae (Fathke and Gabriele, 2009). Much like their layered counterparts in the CNIC, modular (Wallace et al., 2013) and extramodular (Torii et al., 2013) LCIC arrangements emerge during early postnatal development.
The precision with which IC inputs adhere to the local cytoarchitectural framework in the absence of experience suggests involvement of close-contact signaling mechanisms. An important first step in assessing whether Eph/ephrins play an instructive role in auditory midbrain map formation is the identification of protein expression patterns that correlate with the early period of projection shaping (Figure 3). Localization studies utilizing immunocytochemical and X-Gal staining approaches in lacZ mutants show a transient expression of EphA4 and ephrin-B2 in the IC leading up to the functional onset of hearing (Gabriele et al., 2011). In rat and mouse, each is expressed in a graded fashion across the tonotopic axis of the CNIC, with protein most concentrated in ventromedial, high-frequency regions. CNIC gradients are steep at birth through P4 (period of axonal sorting), before flattening to more homogeneous expression as experience ensues (Miko et al., 2007, Gabriele et al., 2011). In contrast to their continuous CNIC expression, both exhibit discrete, discontinuous patterns in the LCIC, with protein localized to presumptive modular fields that mimic those neurochemically described in the adult (Chernock et al., 2004, Malmierca et al., 2011, Ouda and Syka, 2012). This pattern remains prominent throughout the first postnatal week prior to being noticeably downregulated by P12. EphA4 and ephrin-B2 positive cells are also evident during this period in several auditory brainstem nuclei that send patterned inputs to the IC, namely the cochlear nuclei, LSO, DNLL, and ventral nucleus of the lateral lemniscus (VNLL).
Figure 3.
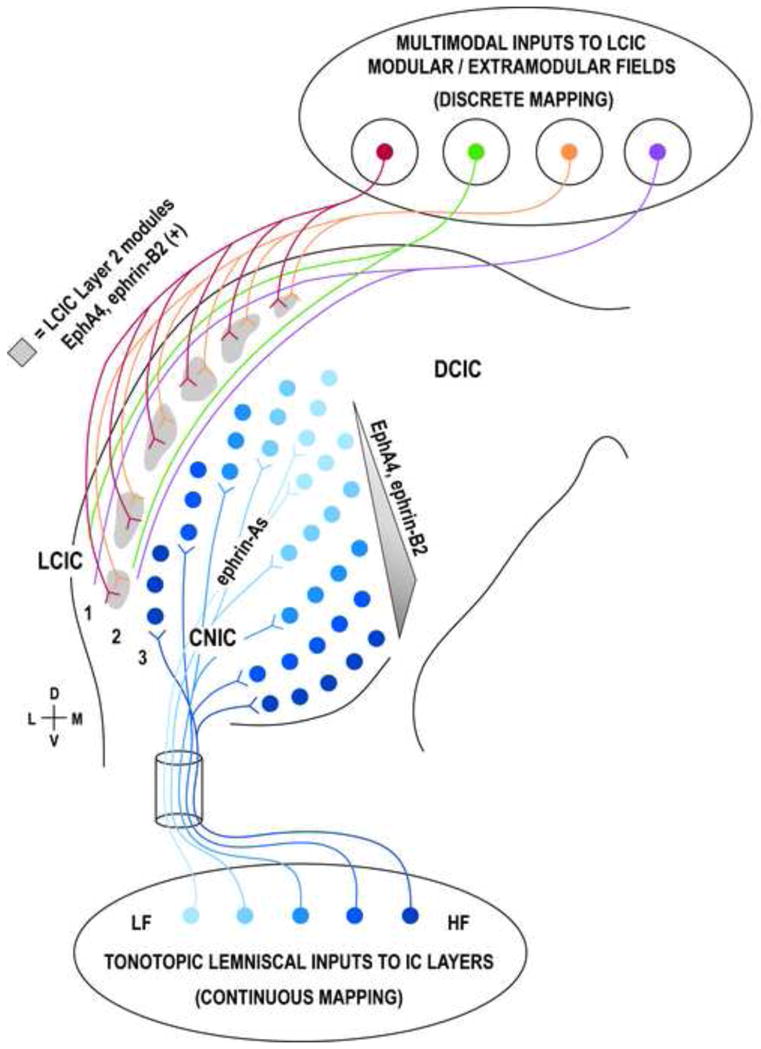
Schematic of proposed continuous vs. discrete Eph/ephrin mapping of the auditory midbrain. The CNIC and deep LCIC exhibit characteristic frequency laminae (shades of blue) that receive tonotopic inputs from multiple lemniscal sources (including the cochlear nuclei, LSO, MSO, SPN, DPO, VNLL, and DNLL). Graded expressions of certain Eph/ephrins along the ventromedial-to-dorsolateral CNIC frequency continuum appear to influence axonal targeting prior to experience. Ephrin-As are also highly expressed in the nascent CNIC, and while not yet quantified, may provide positional information necessary for mapping afferents along additional IC axes. While less characterized, inputs to more superficial aspects of the LCIC are multimodal, lack a clear frequency order, and exhibit discontinuous modular or extramodular projection distributions. Descending inputs from auditory cortex as well as intrinsic and commissural connections from the CNIC appear to spare Layer 2 modules (green, purple), while somatosensory inputs arising from the dorsal column and spinal trigeminal nuclei preferentially target these domains (red, orange). In lieu of continuous gradients, LCIC Eph/ephrin expression is conspicuously modular (gray), suggesting a role in instructing discrete maps that reflect “type” rather than “position/frequency” of inputs. SPN = superior paraolivary nucleus; DPO = dorsal periolivary group.
To assess Eph/ephrin cues in establishing continuous CNIC and discrete LCIC maps, a recent study explored the specific involvement of ephrin-B2 in developing topographic projections from the LSO to IC (Wallace et al., 2013). In contrast to the strict tonotopy observed in wild type mice, ephrin-B2lacZ/+ mutants (compromised reverse signaling) lacked any clear projection topography. Whereas focal LSO dye placements in WTs resulted in one or two frequency-matched axonal layers in the CNIC, comparably sized placements consistently yielded unrefined projection distributions encompassing a larger frequency axis extent in heterozygous mutants. Interestingly, ephrin-B2 reverse signaling was not required for aspects of pattern formation as characteristic CNIC layers still form prior to experience.
If previous findings in the retinotectal and other analogous systems are any indication (Luo and Flanagan, 2007), accurate IC map construction likely involves additional Eph/ephrin members, as well as potentially complex interactions with other signaling families. In addition to EphA4 and ephrin-B2, ephrin-A2 and ephrin-A5 mRNA is present in the embryonic IC (Zhang et al., 1996). EphA7-Fc chimeric protein binding studies reveal the presence of ephrin-As in the P4 mouse IC (Torii et al., 2013). In this same study, EphA7 overexpression in auditory cortex significantly alters targeting of corticocollicular projections to the DCIC and LCIC. Descending inputs that normally avoid Layer 2 modules exhibit a more even LCIC distribution pattern. More recently, comprehensive in situ hybridization studies implicate a host of other EphA/B proteins in the developing IC (www.brain-map.org; Allen Institute for Brain Science). Finally, preliminary studies suggest ephrin-B3 midbrain expression patterns are complementary to those for EphA4 and ephrin-B2 (Klotz et al., 2013). Ephrin-B3 is also highly expressed in the mesencephalic midline (Noftz et al., 2014), yet it remains to be determined whether it is involved in crossing decisions for intercollicular and commissural auditory fibers. Taken together, the temporal and spatial correlation of afferent shaping and Eph/ephrin expression patterns suggest their involvement in the IC circuit construction. Future experiments employing a variety of approaches are necessary to determine the precise interactions that instruct IC topography.
VI. Thalamus and Auditory Cortex
Neurons from IC project to the auditory thalamus, where they form tonotopic projections terminating in the medial geniculate body (MGB). Eph receptors and ephrins are expressed in the developing MGB as in other thalamic nuclei (Intskirveli et al., 2011, Lehigh et al., 2013, Torii et al., 2013), with graded expression of some family members. The distinct expression patterns of Eph proteins in the developing thalamus suggest a role in defining boundaries between distinct nuclei (Lehigh et al., 2013).
The role of Eph proteins in guidance of MGB axons to appropriate locations in primary auditory cortex has not been tested. Nonetheless, Eph proteins have multiple roles in every stage of cortical development (North et al., 2013), and thus an understanding of their integrated function will be of value in understanding assembly of auditory pathways. Eph proteins regulate areal specification of cortex and guidance of thalamocortical axons to the appropriate cortical areas (Dufour et al., 2003, Robichaux et al., 2014). They influence cortical cell migration (Steinecke et al., 2014), laminar specification (Mann et al., 2002), topography (Cang et al., 2008a), and columnar organization (Torii et al., 2009, Dimidschstein et al., 2013). In primary auditory cortex, null mutations in EphB2 and EphB3 result in degraded frequency selectivity (Intskirveli et al., 2011). This result could signify an effect on thalamocortical topographic mapping, but could also reflect divergent projections in lower auditory areas.
VII. Conclusions
Eph receptors and ephrins make multiple contributions to the organization of neural circuitry. Emerging evidence suggests that this protein family has a significant role in the development of auditory pathways, from the peripheral projections to the auditory cortex. Early in development, Eph signaling influences the movement of cells as nuclei form and at the same time regulates the growth of their axons to appropriate targets. Later in development, the function in axon guidance dominates, as Eph signaling seems to fine tune projections that have arrived in the correct target. Throughout this process, these molecules seem to work together with other cues, including other axon guidance signals as well as activity-dependent refinement known to occur in topographic mapping.
As a consequence of cell contact mediated binding, Eph/ephrin signaling appears to be most important for selection of regions within a target, rather than for selecting a target region. These subregions include topographic locations that rely on graded expression patterns, resulting in tonotopic maps. They also include discrete locations, such as inner vs. outer hair cells, dorsal vs. ventral NL dendrites, ipsilateral vs. contralateral nuclei, or modular vs. extramodular domains. In many cases, Eph proteins serve as an inhibitory signal that delineates compartments permitting axon growth. Nearly every stage of auditory system development exhibits expression of several ligands and receptors. These proteins have overlapping binding properties and function in many stages of neural development, including proliferation, migration, axon guidance, and synaptogenesis. Their expression and signaling capabilities help to coordinate the formation of auditory nuclei, axon growth, and precise formation of synapses throughout the auditory system.
Highlights.
Eph proteins provide complex signaling capabilities during circuit formation
Axon guidance at multiple levels of the auditory system is mediated by Eph proteins
Auditory areas receive both graded and discretely organized inputs
Eph proteins specify graded and discrete locations within auditory target areas
Acknowledgments
This work was supported by grants NIH R01 NIDCD 010796, NIH P30DC008369, NIH P30DC008369, and NIH R15 DC012421-01. The authors are grateful to Dr. Leonard Kitzes for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aitkin LM, Kenyon CE, Philpott P. The representation of the auditory and somatosensory systems in the external nucleus of the cat inferior colliculus. The Journal of comparative neurology. 1981;196:25–40. doi: 10.1002/cne.901960104. [DOI] [PubMed] [Google Scholar]
- Allen-Sharpley MR, Cramer KS. Coordinated Eph-ephrin signaling guides migration and axon targeting in the avian auditory system. Neural Dev. 2012;7:29. doi: 10.1186/1749-8104-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen-Sharpley MR, Tjia M, Cramer KS. Differential roles for EphA and EphB signaling in segregation and patterning of central vestibulocochlear nerve projections. PLoS ONE. 2013;8:e78658. doi: 10.1371/journal.pone.0078658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitis D, Davy A. Eph/ephrin signaling: networks. Genes & development. 2008;22:416–429. doi: 10.1101/gad.1630408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi LM, Dinsio K, Davoli K, Gale NW. Lac z Histochemistry and immunohistochemistry reveal ephrin-B ligand expression in the inner ear. J Histochem Cytochem. 2002;50:1641–1645. doi: 10.1177/002215540205001208. [DOI] [PubMed] [Google Scholar]
- Bianchi LM, Gale NW. Distribution of Eph-related molecules in the developing and mature cochlea. Hear Res. 1998;117:161–172. doi: 10.1016/s0378-5955(98)00010-0. [DOI] [PubMed] [Google Scholar]
- Bianchi LM, Gray NA. EphB receptors influence growth of ephrin-B1-positive statoacoustic nerve fibers. Eur J Neurosci. 2002;16:1499–1506. doi: 10.1046/j.1460-9568.2002.02248.x. [DOI] [PubMed] [Google Scholar]
- Book KJ, Morest DK. Migration of neuroblasts by perikaryal translocation: role of cellular elongation and axonal outgrowth in the acoustic nuclei of the chick embryo medulla. J Comp Neurol. 1990;297:55–76. doi: 10.1002/cne.902970105. [DOI] [PubMed] [Google Scholar]
- Bush JO, Soriano P. Eph/ephrin signaling: genetic, phosphoproteomic, and transcriptomic approaches. Semin Cell Dev Biol. 2012;23:26–34. doi: 10.1016/j.semcdb.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Niell CM, Liu X, Pfeiffenberger C, Feldheim DA, Stryker MP. Selective disruption of one Cartesian axis of cortical maps and receptive fields by deficiency in ephrin-As and structured activity. Neuron. 2008a;57:511–523. doi: 10.1016/j.neuron.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Wang L, Stryker MP, Feldheim DA. Roles of ephrin-as and structured activity in the development of functional maps in the superior colliculus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008b;28:11015–11023. doi: 10.1523/JNEUROSCI.2478-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant NB. The Mammalian Auditory Pathway: Neuroanatomy. Berlin: Springer-Verlag; 1992. The cochlear nucleus: Neuronal types and their synaptic organization. [Google Scholar]
- Carr CE, Konishi M. A circuit for detection of interaural time differences in the brain stem of the barn owl. J Neurosci. 1990;10:3227–3246. doi: 10.1523/JNEUROSCI.10-10-03227.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casseday JH, Fremouw T, Covey E. The inferior colliculus: hub of the auditory system. In: Oertel D, PA, Fay RR, editors. Springer Handbook of Auditory Research. Vol. 15. New York: Springer; 2002. pp. 238–318. [Google Scholar]
- Chenaux G, Henkemeyer M. Forward signaling by EphB1/EphB2 interacting with ephrin-B ligands at the optic chiasm is required to form the ipsilateral projection. The European journal of neuroscience. 2011;34:1620–1633. doi: 10.1111/j.1460-9568.2011.07845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HJ, Flanagan JG. Identification and cloning of ELF-1, a developmentally expressed ligand for the Mek4 and Sek receptor tyrosine kinases. Cell. 1994;79:157–168. doi: 10.1016/0092-8674(94)90408-1. [DOI] [PubMed] [Google Scholar]
- Cheng HJ, Nakamoto M, Bergemann AD, Flanagan JG. Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell. 1995;82:371–381. doi: 10.1016/0092-8674(95)90426-3. [DOI] [PubMed] [Google Scholar]
- Chernock ML, Larue DT, Winer JA. A periodic network of neurochemical modules in the inferior colliculus. Hearing research. 2004;188:12–20. doi: 10.1016/S0378-5955(03)00340-X. [DOI] [PubMed] [Google Scholar]
- Coate TM, Raft S, Zhao X, Ryan AK, Crenshaw EB, 3rd, Kelley MW. Otic mesenchyme cells regulate spiral ganglion axon fasciculation through a Pou3f4/EphA4 signaling pathway. Neuron. 2012;73:49–63. doi: 10.1016/j.neuron.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JR, Clerici WJ. Sources of projections to subdivisions of the inferior colliculus in the rat. The Journal of comparative neurology. 1987;262:215–226. doi: 10.1002/cne.902620204. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Henkemeyer M. Ephrins in reverse, park and drive. Trends Cell Biol. 2002;12:339–346. doi: 10.1016/s0962-8924(02)02317-6. [DOI] [PubMed] [Google Scholar]
- Cramer KS. Eph proteins and the assembly of auditory circuits. Hearing research. 2005;206:42–51. doi: 10.1016/j.heares.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Cramer KS, Bermingham-McDonogh O, Krull CE, Rubel EW. EphA4 signaling promotes axon segregation in the developing auditory system. Dev Biol. 2004;269:26–35. doi: 10.1016/j.ydbio.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Cramer KS, Karam SD, Bothwell M, Cerretti DP, Pasquale EB, Rubel EW. Expression of EphB receptors and EphrinB ligands in the developing chick auditory brainstem. J Comp Neurol. 2002;452:51–64. doi: 10.1002/cne.10399. [DOI] [PubMed] [Google Scholar]
- Cramer KS, Rosenberger MH, Frost DM, Cochran SL, Pasquale EB, Rubel EW. Developmental regulation of EphA4 expression in the chick auditory brainstem. J Comp Neurol. 2000;426:270–278. [PubMed] [Google Scholar]
- Cramer KS, Rubel EW. Embryonic origins of avian brainstem auditory nuclei. Soc Neurosci Abstr. 1998;24:809. [Google Scholar]
- Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, Goldfarb M, Yancopoulos GD. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266:816–819. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- Davy A, Gale NW, Murray EW, Klinghoffer RA, Soriano P, Feuerstein C, Robbins SM. Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes Dev. 1999;13:3125–3135. doi: 10.1101/gad.13.23.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defourny J, Poirrier AL, Lallemend F, Mateo Sanchez S, Neef J, Vanderhaeghen P, Soriano E, Peuckert C, Kullander K, Fritzsch B, Nguyen L, Moonen G, Moser T, Malgrange B. Ephrin-A5/EphA4 signalling controls specific afferent targeting to cochlear hair cells. Nat Commun. 2013;4:1438. doi: 10.1038/ncomms2445. [DOI] [PubMed] [Google Scholar]
- Dimidschstein J, Passante L, Dufour A, van den Ameele J, Tiberi L, Hrechdakian T, Adams R, Klein R, Lie DC, Jossin Y, Vanderhaeghen P. Ephrin-B1 controls the columnar distribution of cortical pyramidal neurons by restricting their tangential migration. Neuron. 2013;79:1123–1135. doi: 10.1016/j.neuron.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Drescher U, Kremoser C, Handwerker C, Loschinger J, Noda M, Bonhoeffer F. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82:359–370. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- Druga R, Syka J, Rajkowska G. Projections of auditory cortex onto the inferior colliculus in the rat. Physiol Res. 1997;46:215–222. [PubMed] [Google Scholar]
- Dufour A, Seibt J, Passante L, Depaepe V, Ciossek T, Frisen J, Kullander K, Flanagan JG, Polleux F, Vanderhaeghen P. Area specificity and topography of thalamocortical projections are controlled by ephrin/Eph genes. Neuron. 2003;39:453–465. doi: 10.1016/s0896-6273(03)00440-9. [DOI] [PubMed] [Google Scholar]
- Echteler SM. Developmental segregation in the afferent projections to mammalian auditory hair cells. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:6324–6327. doi: 10.1073/pnas.89.14.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G, Merzenich MM. Auditory midbrain responses parallel spectral integration phenomena. Science. 1985;227:1245–1247. doi: 10.1126/science.3975613. [DOI] [PubMed] [Google Scholar]
- Falivelli G, Lisabeth EM, Rubio de la Torre E, Perez-Tenorio G, Tosato G, Salvucci O, Pasquale EB. Attenuation of eph receptor kinase activation in cancer cells by coexpressed ephrin ligands. PLoS ONE. 2013;8:e81445. doi: 10.1371/journal.pone.0081445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathke RL, Gabriele ML. Patterning of multiple layered projections to the auditory midbrain prior to experience. Hearing research. 2009;249:36–43. doi: 10.1016/j.heares.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Faye-Lund H, Osen KK. Anatomy of the inferior colliculus in rat. Anat Embryol (Berl) 1985;171:1–20. doi: 10.1007/BF00319050. [DOI] [PubMed] [Google Scholar]
- Feldheim DA, Nakamoto M, Osterfield M, Gale NW, DeChiara TM, Rohatgi R, Yancopoulos GD, Flanagan JG. Loss-of-function analysis of EphA receptors in retinotectal mapping. J Neurosci. 2004;24:2542–2550. doi: 10.1523/JNEUROSCI.0239-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriele ML, Brubaker DQ, Chamberlain KA, Kross KM, Simpson NS, Kavianpour SM. EphA4 and ephrin-B2 expression patterns during inferior colliculus projection shaping prior to experience. Developmental neurobiology. 2011;71:182–199. doi: 10.1002/dneu.20842. [DOI] [PubMed] [Google Scholar]
- Gabriele ML, Brunso-Bechtold JK, Henkel CK. Development of afferent patterns in the inferior colliculus of the rat: projection from the dorsal nucleus of the lateral lemniscus. The Journal of comparative neurology. 2000a;416:368–382. doi: 10.1002/(sici)1096-9861(20000117)416:3<368::aid-cne8>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Gabriele ML, Brunso-Bechtold JK, Henkel CK. Plasticity in the development of afferent patterns in the inferior colliculus of the rat after unilateral cochlear ablation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000b;20:6939–6949. doi: 10.1523/JNEUROSCI.20-18-06939.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriele ML, Shahmoradian SH, French CC, Henkel CK, McHaffie JG. Early segregation of layered projections from the lateral superior olivary nucleus to the central nucleus of the inferior colliculus in the neonatal cat. Brain research. 2007;1173:66–77. doi: 10.1016/j.brainres.2007.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson DG, Pawson T, Davis S, Yancopoulos GD. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM. Molecular neuroanatomic mechanisms of Parkinson’s disease: a proposed therapeutic approach. Neurol Clin. 1992;10:435–449. [PubMed] [Google Scholar]
- González-Hernández T, Mantolán-Sarmiento B, González-González B, Pérez-González H. Sources of GABAergic input to the inferior colliculus of the rat. The Journal of comparative neurology. 1996;372:309–326. doi: 10.1002/(SICI)1096-9861(19960819)372:2<309::AID-CNE11>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Hendricks SJ, Rubel EW, Nishi R. Formation of the avian nucleus magnocellularis from the auditory anlage. J Comp Neurol. 2006;498:433–442. doi: 10.1002/cne.21031. [DOI] [PubMed] [Google Scholar]
- Henkel CK, Keiger CJ, Franklin SR, Brunso-Bechtold JK. Development of banded afferent compartments in the inferior colliculus before onset of hearing in ferrets. Neuroscience. 2007;146:225–235. doi: 10.1016/j.neuroscience.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkemeyer M, Marengere LE, McGlade J, Olivier JP, Conlon RA, Holmyard DP, Letwin K, Pawson T. Immunolocalization of the Nuk receptor tyrosine kinase suggests roles in segmental patterning of the brain and axonogenesis. Oncogene. 1994;9:1001–1014. [PubMed] [Google Scholar]
- Herbert H, Aschoff A, Ostwald J. Topography of projections from the auditory cortex to the inferior colliculus in the rat. The Journal of comparative neurology. 1991;304:103–122. doi: 10.1002/cne.903040108. [DOI] [PubMed] [Google Scholar]
- Himanen JP. Ectodomain structures of Eph receptors. Semin Cell Dev Biol. 2012;23:35–42. doi: 10.1016/j.semcdb.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, Henkemeyer M, Nikolov DB. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7:501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- Hirai H, Maru Y, Hagiwara K, Nishida J, Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 1987;238:1717–1720. doi: 10.1126/science.2825356. [DOI] [PubMed] [Google Scholar]
- Howell DM, Morgan WJ, Jarjour AA, Spirou GA, Berrebi AS, Kennedy TE, Mathers PH. Molecular guidance cues necessary for axon pathfinding from the ventral cochlear nucleus. J Comp Neurol. 2007;504:533–549. doi: 10.1002/cne.21443. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Nakamura PA, Luk SO, Miko IJ, Henkemeyer M, Cramer KS. Ephrin-B reverse signaling is required for formation of strictly contralateral auditory brainstem pathways. J Neurosci. 2010;30:9840–9849. doi: 10.1523/JNEUROSCI.0386-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huai J, Drescher U. An ephrin-A-dependent signaling pathway controls integrin function and is linked to the tyrosine phosphorylation of a 120-kDa protein. J Biol Chem. 2001;276:6689–6694. doi: 10.1074/jbc.M008127200. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Murray KD, Warland DK, Feldheim DA, Chapman B. Ephrin-As mediate targeting of eye-specific projections to the lateral geniculate nucleus. Nat Neurosci. 2005;8:1013–1021. doi: 10.1038/nn1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman KJ, Cramer KS. EphA4 misexpression alters tonotopic projections in the auditory brainstem. Dev Neurobiol. 2007;67:1655–1668. doi: 10.1002/dneu.20535. [DOI] [PubMed] [Google Scholar]
- Hyson RL. The analysis of interaural time differences in the chick brain stem. Physiol Behav. 2005;86:297–305. doi: 10.1016/j.physbeh.2005.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieguchi K, Omori T, Komatsu A, Tomita T, Deguchi A, Maru Y. Ephrin-A1 expression induced by S100A8 is mediated by the toll-like receptor 4. Biochem Biophys Res Commun. 2013;440:623–629. doi: 10.1016/j.bbrc.2013.09.119. [DOI] [PubMed] [Google Scholar]
- Illing RB. The mosaic architecture of the superior colliculus. Progress in brain research. 1996;112:17–34. doi: 10.1016/s0079-6123(08)63318-x. [DOI] [PubMed] [Google Scholar]
- Imai T, Sakano H, Vosshall LB. Topographic mapping--the olfactory system. Cold Spring Harb Perspect Biol. 2010;2:a001776. doi: 10.1101/cshperspect.a001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intskirveli I, Metherate R, Cramer KS. Null mutations in EphB receptors decrease sharpness of frequency tuning in primary auditory cortex. PLoS ONE. 2011;6:e26192. doi: 10.1371/journal.pone.0026192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R, Shore S. External inferior colliculus integrates trigeminal and acoustic information: unit responses to trigeminal nucleus and acoustic stimulation in the guinea pig. Neurosci Lett. 2006;395:71–75. doi: 10.1016/j.neulet.2005.10.077. [DOI] [PubMed] [Google Scholar]
- Janes PW, Griesshaber B, Atapattu L, Nievergall E, Hii LL, Mensinga A, Chheang C, Day BW, Boyd AW, Bastiaens PI, Jorgensen C, Pawson T, Lackmann M. Eph receptor function is modulated by heterooligomerization of A and B type Eph receptors. The Journal of cell biology. 2011;195:1033–1045. doi: 10.1083/jcb.201104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes PW, Nievergall E, Lackmann M. Concepts and consequences of Eph receptor clustering. Semin Cell Dev Biol. 2012;23:43–50. doi: 10.1016/j.semcdb.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Kandler K, Friauf E. Pre- and postnatal development of efferent connections of the cochlear nucleus in the rat. J Comp Neurol. 1993;328:161–184. doi: 10.1002/cne.903280202. [DOI] [PubMed] [Google Scholar]
- Kao TJ, Kania A. Ephrin-mediated cis-attenuation of Eph receptor signaling is essential for spinal motor axon guidance. Neuron. 2011;71:76–91. doi: 10.1016/j.neuron.2011.05.031. [DOI] [PubMed] [Google Scholar]
- Kiang NY, Rho JM, Northrop CC, Liberman MC, Ryugo DK. Hair-cell innervation by spiral ganglion cells in adult cats. Science. 1982;217:175–177. doi: 10.1126/science.7089553. [DOI] [PubMed] [Google Scholar]
- Klein R, Kania A. Ephrin signalling in the developing nervous system. Current opinion in neurobiology. 2014;27C:16–24. doi: 10.1016/j.conb.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Klotz C, Wallace MM, Harris J, Gabriele M. Countergradients and modular expression patterns of Eph-ephrin signaling proteins in the developing auditory brainstem. Assoc Res Otolaryngol Mtg 2013 [Google Scholar]
- Koppl C, Carr CE. Maps of interaural time difference in the chicken’s brainstem nucleus laminaris. Biol Cybern. 2008;98:541–559. doi: 10.1007/s00422-008-0220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koundakjian EJ, Appler JL, Goodrich LV. Auditory neurons make stereotyped wiring decisions before maturation of their targets. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:14078–14088. doi: 10.1523/JNEUROSCI.3765-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Lai KO, Ip NY. Synapse development and plasticity: roles of ephrin/Eph receptor signaling. Current opinion in neurobiology. 2009;19:275–283. doi: 10.1016/j.conb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Lehigh KM, Leonard CE, Baranoski J, Donoghue MJ. Parcellation of the thalamus into distinct nuclei reflects EphA expression and function. Gene Expression Patterns. 2013;13:454–463. doi: 10.1016/j.gep.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Mizuno N. Single neurons in the spinal trigeminal and dorsal column nuclei project to both the cochlear nucleus and the inferior colliculus by way of axon collaterals: a fluorescent retrograde double-labeling study in the rat. Neurosci Res. 1997;29:135–142. doi: 10.1016/s0168-0102(97)00082-5. [DOI] [PubMed] [Google Scholar]
- Lim YS, McLaughlin T, Sung TC, Santiago A, Lee KF, O’Leary DD. p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron. 2008;59:746–758. doi: 10.1016/j.neuron.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KT, Sloniowski S, Ethell DW, Ethell IM. Ephrin-B2-induced cleavage of EphB2 receptor is mediated by matrix metalloproteinases to trigger cell repulsion. The Journal of biological chemistry. 2008;283:28969–28979. doi: 10.1074/jbc.M804401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus WC, Bishop DC, Oliver DL. Differential patterns of inputs create functional zones in central nucleus of inferior colliculus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:13396–13408. doi: 10.1523/JNEUROSCI.0338-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus WC, Bishop DC, Saint Marie RL, Oliver DL. Organization of binaural excitatory and inhibitory inputs to the inferior colliculus from the superior olive. The Journal of comparative neurology. 2004;472:330–344. doi: 10.1002/cne.20070. [DOI] [PubMed] [Google Scholar]
- Loftus WC, Malmierca MS, Bishop DC, Oliver DL. The cytoarchitecture of the inferior colliculus revisited: a common organization of the lateral cortex in rat and cat. Neuroscience. 2008;154:196–205. doi: 10.1016/j.neuroscience.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CC, Appler JM, Houseman EA, Goodrich LV. Developmental profiling of spiral ganglion neurons reveals insights into auditory circuit assembly. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:10903–10918. doi: 10.1523/JNEUROSCI.2358-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Flanagan JG. Development of continuous and discrete neural maps. Neuron. 2007;56:284–300. doi: 10.1016/j.neuron.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Blackstad TW, Osen KK. Computer-assisted 3-D reconstructions of Golgi-impregnated neurons in the cortical regions of the inferior colliculus of rat. Hearing research. 2011;274:13–26. doi: 10.1016/j.heares.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Izquierdo MA, Cristaudo S, Hernandez O, Perez-Gonzalez D, Covey E, Oliver DL. A discontinuous tonotopic organization in the inferior colliculus of the rat. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:4767–4776. doi: 10.1523/JNEUROSCI.0238-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca MS, Rees A, Le Beau FE, Bjaalie JG. Laminar organization of frequency-defined local axons within and between the inferior colliculi of the guinea pig. The Journal of comparative neurology. 1995;357:124–144. doi: 10.1002/cne.903570112. [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Saint Marie RL, Merchan MA, Oliver DL. Laminar inputs from dorsal cochlear nucleus and ventral cochlear nucleus to the central nucleus of the inferior colliculus: two patterns of convergence. Neuroscience. 2005;136:883–894. doi: 10.1016/j.neuroscience.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Mann F, Peuckert C, Dehner F, Zhou R, Bolz J. Ephrins regulate the formation of terminal axonal arbors during the development of thalamocortical projections. Development. 2002;129:3945–3955. doi: 10.1242/dev.129.16.3945. [DOI] [PubMed] [Google Scholar]
- Marquardt T, Shirasaki R, Ghosh S, Andrews SE, Carter N, Hunter T, Pfaff SL. Coexpressed EphA receptors and ephrin-A ligands mediate opposing actions on growth cone navigation from distinct membrane domains. Cell. 2005;121:127–139. doi: 10.1016/j.cell.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Reid MD. Representation of the cochlea within the inferior colliculus of the cat. Brain research. 1974;77:397–415. doi: 10.1016/0006-8993(74)90630-1. [DOI] [PubMed] [Google Scholar]
- Miko IJ, Nakamura PA, Henkemeyer M, Cramer KS. Auditory brainstem neural activation patterns are altered in EphA4- and ephrin-B2-deficient mice. J Comp Neurol. 2007;505:669–681. doi: 10.1002/cne.21530. [DOI] [PubMed] [Google Scholar]
- Morest DK, Oliver DL. The neuronal architecture of the inferior colliculus in the cat: defining the functional anatomy of the auditory midbrain. The Journal of comparative neurology. 1984;222:209–236. doi: 10.1002/cne.902220206. [DOI] [PubMed] [Google Scholar]
- Nakamura PA, Cramer KS. Formation and maturation of the calyx of Held. Hearing research. 2011;276:70–78. doi: 10.1016/j.heares.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura PA, Cramer KS. EphB2 signaling regulates lesion-induced axon sprouting but not critical period length in the postnatal auditory brainstem. Neural Dev. 2013;8:2. doi: 10.1186/1749-8104-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura PA, Hsieh CY, Cramer KS. EphB signaling regulates target innervation in the developing and deafferented auditory brainstem. Developmental neurobiology. 2012;72:1243–1255. doi: 10.1002/dneu.20990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolov DB, Xu K, Himanen JP. Eph/ephrin recognition and the role of Eph/ephrin clusters in signaling initiation. Biochim Biophys Acta. 2013;1834:2160–2165. doi: 10.1016/j.bbapap.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noftz W, Gray L, Gabriele M. Converging midbrain afferent patterns and auditory brainstem responses in ephrin-B3 mutant mice. Assoc Res Otolaryngol Abstr 2014 [Google Scholar]
- North HA, Clifford MA, Donoghue MJ. ‘Til Eph do us part’: intercellular signaling via Eph receptors and ephrin ligands guides cerebral cortical development from birth through maturation. Cereb Cortex. 2013;23:1765–1773. doi: 10.1093/cercor/bhs183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olazábal UE, Moore JK. Nigrotectal projection to the inferior colliculus: horseradish peroxidase transport and tyrosine hydroxylase immunohistochemical studies in rats, cats, and bats. The Journal of comparative neurology. 1989;282:98–118. doi: 10.1002/cne.902820108. [DOI] [PubMed] [Google Scholar]
- Oliver DL. Neuronal organization in the inferior colliculus. In: JAW, CES, editors. The Inferior Colliculus. 69–114. New York: Springer; 2005. [Google Scholar]
- Oliver DL, Beckius GE, Bishop DC, Kuwada S. Simultaneous anterograde labeling of axonal layers from lateral superior olive and dorsal cochlear nucleus in the inferior colliculus of cat. The Journal of comparative neurology. 1997;382:215–229. doi: 10.1002/(sici)1096-9861(19970602)382:2<215::aid-cne6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Morest DK. The central nucleus of the inferior colliculus in the cat. The Journal of comparative neurology. 1984;222:237–264. doi: 10.1002/cne.902220207. [DOI] [PubMed] [Google Scholar]
- Ouda L, Syka J. Immunocytochemical profiles of inferior colliculus neurons in the rat and their changes with aging. Front Neural Circuits. 2012;6:68. doi: 10.3389/fncir.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overholt EM, Rubel EW, Hyson RL. A circuit for coding interaural time differences in the chick brainstem. J Neurosci. 1992;12:1698–1708. doi: 10.1523/JNEUROSCI.12-05-01698.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10:165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AL, Cerretti DP, Pasquale EB, Rubel EW, Cramer KS. Tonotopic gradients of Eph family proteins in the chick nucleus laminaris during synaptogenesis. J Neurobiol. 2004;60:28–39. doi: 10.1002/neu.10330. [DOI] [PubMed] [Google Scholar]
- Petersen CC. The functional organization of the barrel cortex. Neuron. 2007;56:339–355. doi: 10.1016/j.neuron.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Petros TJ, Shrestha BR, Mason C. Specificity and sufficiency of EphB1 in driving the ipsilateral retinal projection. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:3463–3474. doi: 10.1523/JNEUROSCI.5655-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffenberger C, Cutforth T, Woods G, Yamada J, Renteria RC, Copenhagen DR, Flanagan JG, Feldheim DA. Ephrin-As and neural activity are required for eye-specific patterning during retinogeniculate mapping. Nat Neurosci. 2005;8:1022–1027. doi: 10.1038/nn1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffenberger C, Yamada J, Feldheim DA. Ephrin-As and patterned retinal activity act together in the development of topographic maps in the primary visual system. J Neurosci. 2006;26:12873–12884. doi: 10.1523/JNEUROSCI.3595-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles JO. Expression of Ephs and ephrins in developing mouse inner ear. Hear Res. 2003;178:44–51. doi: 10.1016/s0378-5955(03)00029-7. [DOI] [PubMed] [Google Scholar]
- Pickles JO, Claxton C, Van Heumen WR. Complementary and layered expression of Ephs and ephrins in developing mouse inner ear. J Comp Neurol. 2002;449:207–216. doi: 10.1002/cne.10231. [DOI] [PubMed] [Google Scholar]
- Renier N, Schonewille M, Giraudet F, Badura A, Tessier-Lavigne M, Avan P, De Zeeuw CI, Chedotal A. Genetic dissection of the function of hindbrain axonal commissures. PLoS Biol. 2010;8:e1000325. doi: 10.1371/journal.pbio.1000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaux MA, Chenaux G, Ho HY, Soskis MJ, Dravis C, Kwan KY, Sestan N, Greenberg ME, Henkemeyer M, Cowan CW. EphB receptor forward signaling regulates area-specific reciprocal thalamic and cortical axon pathfinding. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2188–2193. doi: 10.1073/pnas.1324215111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockel AJ, Jones EG. The neuronal organization of the inferior colliculus of the adult cat. II. The pericentral nucleus. The Journal of comparative neurology. 1973;149:301–334. doi: 10.1002/cne.901490303. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Smith DJ, Miller LC. Organization and development of brain stem auditory nuclei of the chicken: Ontogeny of n. magnocellularis and n. laminaris. J Comp Neurol. 1976;166:469–490. doi: 10.1002/cne.901660408. [DOI] [PubMed] [Google Scholar]
- Saldaña E, Aparicio MA, Fuentes-Santamaria V, Berrebi AS. Connections of the superior paraolivary nucleus of the rat: projections to the inferior colliculus. Neuroscience. 2009;163:372–387. doi: 10.1016/j.neuroscience.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldaña E, Feliciano M, Mugnaini E. Distribution of descending projections from primary auditory neocortex to inferior colliculus mimics the topography of intracollicular projections. The Journal of comparative neurology. 1996;371:15–40. doi: 10.1002/(SICI)1096-9861(19960715)371:1<15::AID-CNE2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Saldaña E, Merchán MA. Intrinsic and commissural connections of the rat inferior colliculus. The Journal of comparative neurology. 1992;319:417–437. doi: 10.1002/cne.903190308. [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Langner G. Laminar fine structure of frequency organization in auditory midbrain. Nature. 1997;388:383–386. doi: 10.1038/41106. [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Alheid GF, Heimer L. Efferent connections of the caudal part of the globus pallidus in the rat. The Journal of comparative neurology. 1996;376:489–507. doi: 10.1002/(SICI)1096-9861(19961216)376:3<489::AID-CNE10>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Shneiderman A, Henkel CK. Banding of lateral superior olivary nucleus afferents in the inferior colliculus: a possible substrate for sensory integration. The Journal of comparative neurology. 1987;266:519–534. doi: 10.1002/cne.902660406. [DOI] [PubMed] [Google Scholar]
- Siddiqui SA, Cramer KS. Differential expression of Eph receptors and ephrins in the cochlear ganglion and eighth cranial nerve of the chick embryo. The Journal of comparative neurology. 2005;482:309–319. doi: 10.1002/cne.20396. [DOI] [PubMed] [Google Scholar]
- Steinecke A, Gampe C, Zimmer G, Rudolph J, Bolz J. EphA/ephrin A reverse signaling promotes the migration of cortical interneurons from the medial ganglionic eminence. Development. 2014;141:460–471. doi: 10.1242/dev.101691. [DOI] [PubMed] [Google Scholar]
- Torii M, Hackett TA, Rakic P, Levitt P, Polley DB. EphA signaling impacts development of topographic connectivity in auditory corticofugal systems. Cereb Cortex. 2013;23:775–785. doi: 10.1093/cercor/bhs066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii M, Hashimoto-Torii K, Levitt P, Rakic P. Integration of neuronal clones in the radial cortical columns by EphA and ephrin-A signalling. Nature. 2009;461:524–528. doi: 10.1038/nature08362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett JW, Feldheim DA. Eph and ephrin signaling in the formation of topographic maps. Semin Cell Dev Biol. 2012;23:7–15. doi: 10.1016/j.semcdb.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MM, Kavianpour SM, Gabriele ML. Ephrin-B2 reverse signaling is required for topography but not pattern formation of lateral superior olivary inputs to the inferior colliculus. The Journal of comparative neurology. 2013;521:1585–1597. doi: 10.1002/cne.23243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Larue DT, Diehl JJ, Hefti BJ. Auditory cortical projections to the cat inferior colliculus. The Journal of comparative neurology. 1998;400:147–174. [PubMed] [Google Scholar]
- Winer JA, Schreiner CE. The central auditory system: A Functional Analysis. In: Winer JA, SC, editors. The Inferior Colliculus. New York: Springer; 2005. pp. 1–68. [Google Scholar]
- Xu NJ, Henkemeyer M. Ephrin reverse signaling in axon guidance and synaptogenesis. Semin Cell Dev Biol. 2012;23:58–64. doi: 10.1016/j.semcdb.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Mellitzer G, Robinson V, Wilkinson DG. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature. 1999;399:267–271. doi: 10.1038/20452. [DOI] [PubMed] [Google Scholar]
- Young SR, Rubel EW. Frequency-specific projections of individual neurons in chick brainstem auditory nuclei. J Neurosci. 1983;3:1373–1378. doi: 10.1523/JNEUROSCI.03-07-01373.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JH, Cerretti DP, Yu T, Flanagan JG, Zhou R. Detection of ligands in regions anatomically connected to neurons expressing the Eph receptor Bsk: potential roles in neuron-target interaction. J Neurosci. 1996;16:7182–7192. doi: 10.1523/JNEUROSCI.16-22-07182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CQ, Lee J, Henkemeyer MJ, Lee KH. Disruption of ephrin B/Eph B interaction results in abnormal cochlear innervation patterns. The Laryngoscope. 2011;121:1541–1547. doi: 10.1002/lary.21861. [DOI] [PubMed] [Google Scholar]
- Zhou J, Shore S. Convergence of spinal trigeminal and cochlear nucleus projections in the inferior colliculus of the guinea pig. The Journal of comparative neurology. 2006;495:100–112. doi: 10.1002/cne.20863. [DOI] [PubMed] [Google Scholar]
- Zimmer M, Palmer A, Kohler J, Klein R. EphB-ephrinB bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat Cell Biol. 2003;5:869–878. doi: 10.1038/ncb1045. [DOI] [PubMed] [Google Scholar]


