Abstract
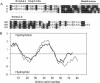
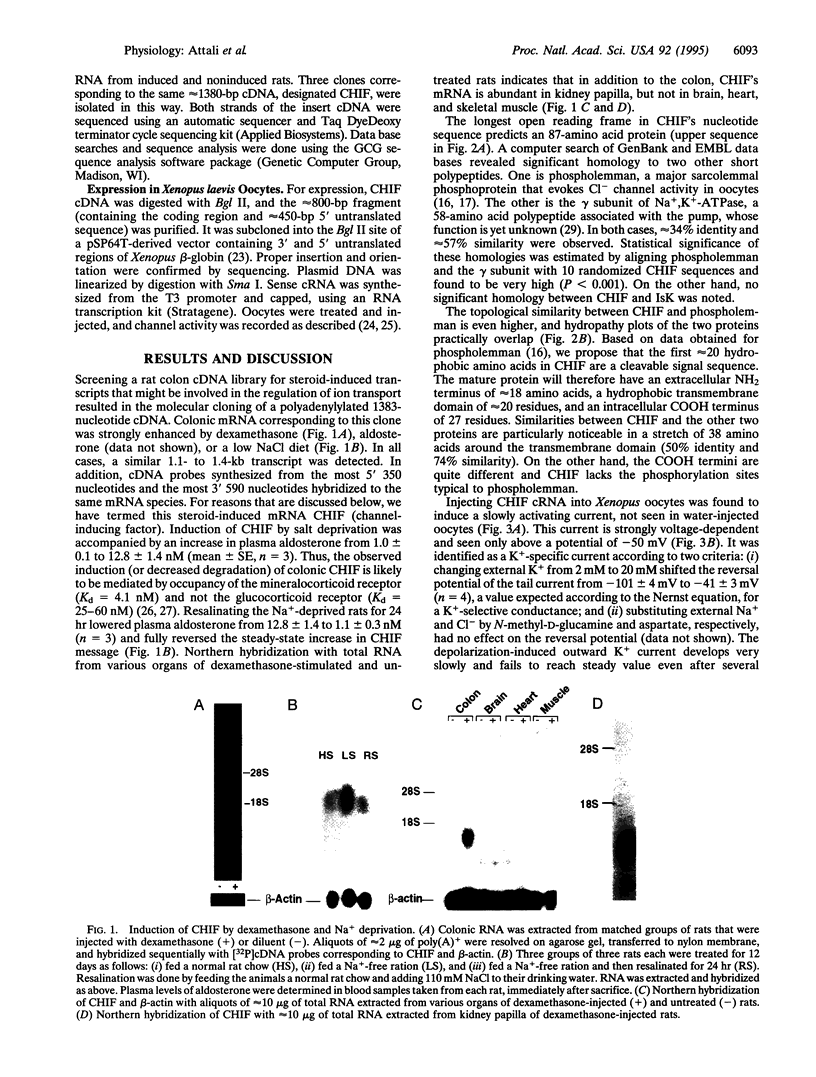
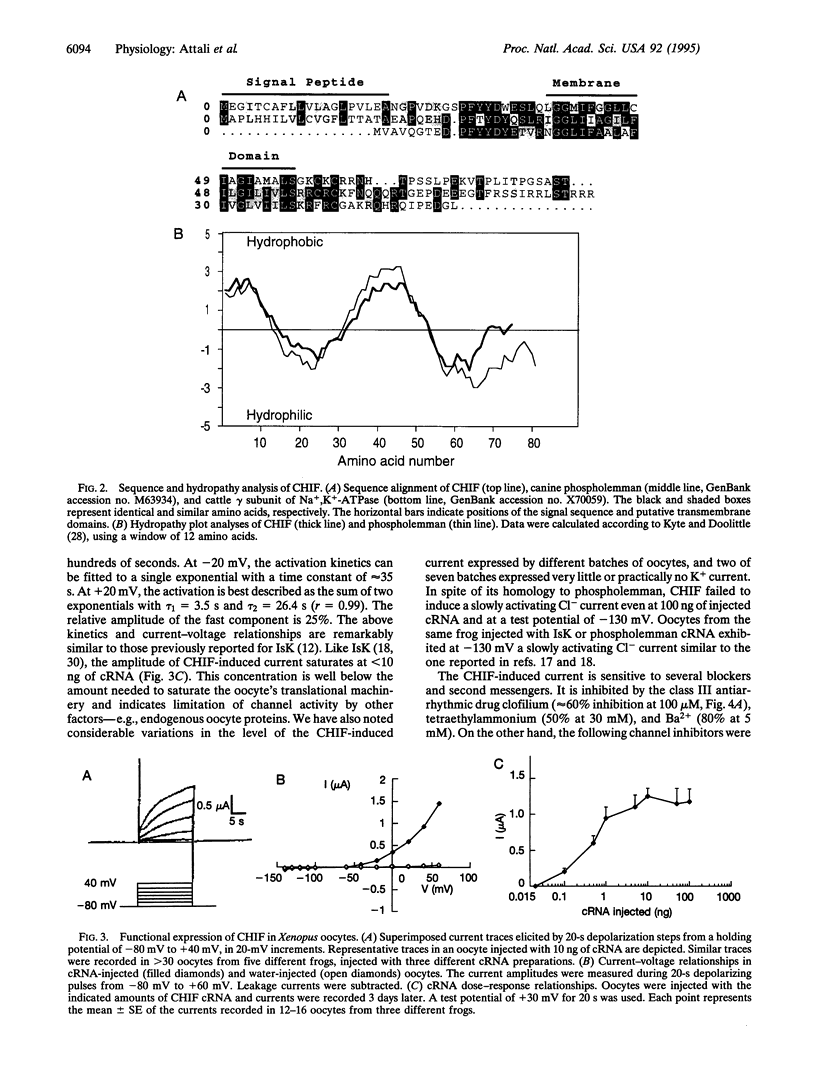
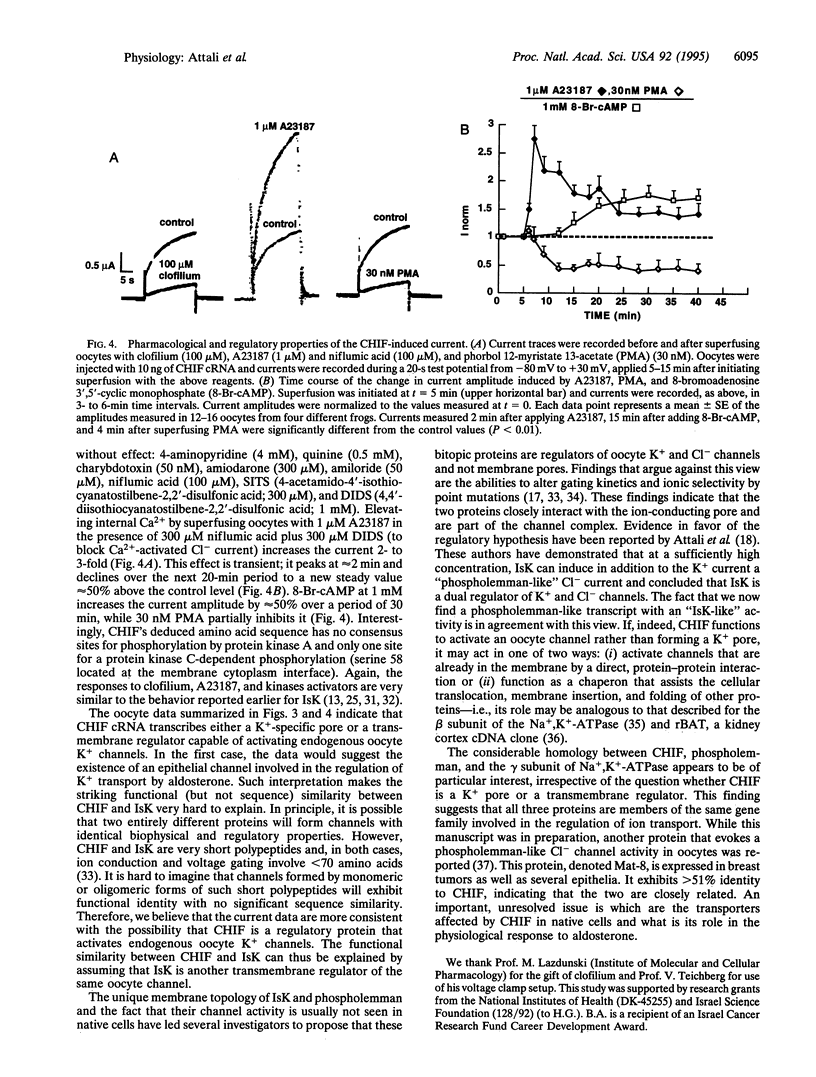
Screening a rat colon cDNA library for aldosterone-induced genes resulted in the molecular cloning of a cDNA whose corresponding mRNA is strongly induced in the colon by dexamethasone, aldosterone, and a low NaCl diet. A similar mRNA was detected in kidney papilla but not in brain, heart, or skeletal muscle. Xenopus laevis oocytes injected with cRNA synthesized from this clone, designated CHIF (channel-inducing factor), express a K(+)-specific channel activity. The biophysical, pharmacological, and regulatory characteristics of this channel are very similar to those reported before for IsK (minK). These include: slow (tau > 20 s) activation by membrane depolarization with a threshold potential above -50 mV, blockade by clofilium, inhibition by phorbol ester, and activation by 8-bromoadenosine 3',5'-cyclic monophosphate and high cytoplasmic Ca2+. The primary structure of this clone, however, shows no homology to IsK. Instead, CHIF exhibits > 50% similarity to two other short bitopic membrane proteins, phospholemman and the gamma subunit of Na+K(+)-ATPase. The data are consistent with the possibility that CHIF is a member of a family of transmembrane regulators capable of activating endogenous oocyte transport proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asher C., Garty H. Aldosterone increases the apical Na+ permeability of toad bladder by two different mechanisms. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7413–7417. doi: 10.1073/pnas.85.19.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attali B., Guillemare E., Lesage F., Honoré E., Romey G., Lazdunski M., Barhanin J. The protein IsK is a dual activator of K+ and Cl- channels. Nature. 1993 Oct 28;365(6449):850–852. doi: 10.1038/365850a0. [DOI] [PubMed] [Google Scholar]
- Bastl C. P., Hayslett J. P. The cellular action of aldosterone in target epithelia. Kidney Int. 1992 Aug;42(2):250–264. doi: 10.1038/ki.1992.284. [DOI] [PubMed] [Google Scholar]
- Bertran J., Magagnin S., Werner A., Markovich D., Biber J., Testar X., Zorzano A., Kühn L. C., Palacin M., Murer H. Stimulation of system y(+)-like amino acid transport by the heavy chain of human 4F2 surface antigen in Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5606–5610. doi: 10.1073/pnas.89.12.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal E. M., Kaczmarek L. K. Modulation by cAMP of a slowly activating potassium channel expressed in Xenopus oocytes. J Neurosci. 1992 Jan;12(1):290–296. doi: 10.1523/JNEUROSCI.12-01-00290.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal E. M., Kaczmarek L. K. The minK potassium channel exists in functional and nonfunctional forms when expressed in the plasma membrane of Xenopus oocytes. J Neurosci. 1994 May;14(5 Pt 2):3097–3105. doi: 10.1523/JNEUROSCI.14-05-03097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch A. E., Varnum M., Adelman J. P., North R. A. Hypotonic solution increases the slowly activating potassium current IsK expressed in xenopus oocytes. Biochem Biophys Res Commun. 1992 Apr 30;184(2):804–810. doi: 10.1016/0006-291x(92)90661-4. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Folander K., Smith J. S., Antanavage J., Bennett C., Stein R. B., Swanson R. Cloning and expression of the delayed-rectifier IsK channel from neonatal rat heart and diethylstilbestrol-primed rat uterus. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2975–2979. doi: 10.1073/pnas.87.8.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder J. W. Aldosterone action. Annu Rev Physiol. 1993;55:115–130. doi: 10.1146/annurev.ph.55.030193.000555. [DOI] [PubMed] [Google Scholar]
- Garty H. Molecular properties of epithelial, amiloride-blockable Na+ channels. FASEB J. 1994 May;8(8):522–528. doi: 10.1096/fasebj.8.8.8181670. [DOI] [PubMed] [Google Scholar]
- Garty H., Peterson-Yantorno K., Asher C., Civian M. M. Effects of corticoid agonists and antagonists on apical Na+ permeability of toad urinary bladder. Am J Physiol. 1994 Jan;266(1 Pt 2):F108–F116. doi: 10.1152/ajprenal.1994.266.1.F108. [DOI] [PubMed] [Google Scholar]
- Garty H. Regulation of Na+ permeability by aldosterone. Semin Nephrol. 1992 Jan;12(1):24–29. [PubMed] [Google Scholar]
- Geering K., Theulaz I., Verrey F., Häuptle M. T., Rossier B. C. A role for the beta-subunit in the expression of functional Na+-K+-ATPase in Xenopus oocytes. Am J Physiol. 1989 Nov;257(5 Pt 1):C851–C858. doi: 10.1152/ajpcell.1989.257.5.C851. [DOI] [PubMed] [Google Scholar]
- Goldstein S. A., Miller C. Site-specific mutations in a minimal voltage-dependent K+ channel alter ion selectivity and open-channel block. Neuron. 1991 Sep;7(3):403–408. doi: 10.1016/0896-6273(91)90292-8. [DOI] [PubMed] [Google Scholar]
- Guillemare E., Honoré E., Pradier L., Lesage F., Schweitz H., Attali B., Barhanin J., Lazdunski M. Effects of the level of mRNA expression on biophysical properties, sensitivity to neurotoxins, and regulation of the brain delayed-rectifier K+ channels Kv1.2. Biochemistry. 1992 Dec 15;31(49):12463–12468. doi: 10.1021/bi00164a024. [DOI] [PubMed] [Google Scholar]
- Honore E., Attali B., Lesage F., Barhanin J., Lazdunski M. Receptor-mediated regulation of IsK, a very slowly activating, voltage-dependent K+ channel in Xenopus oocytes. Biochem Biophys Res Commun. 1992 May 15;184(3):1135–1141. doi: 10.1016/s0006-291x(05)80001-4. [DOI] [PubMed] [Google Scholar]
- Honoré E., Attali B., Romey G., Heurteaux C., Ricard P., Lesage F., Lazdunski M., Barhanin J. Cloning, expression, pharmacology and regulation of a delayed rectifier K+ channel in mouse heart. EMBO J. 1991 Oct;10(10):2805–2811. doi: 10.1002/j.1460-2075.1991.tb07829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lingueglia E., Renard S., Waldmann R., Voilley N., Champigny G., Plass H., Lazdunski M., Barbry P. Different homologous subunits of the amiloride-sensitive Na+ channel are differently regulated by aldosterone. J Biol Chem. 1994 May 13;269(19):13736–13739. [PubMed] [Google Scholar]
- Marcus-Sekura C. J., Hitchcock M. J. Preparation of oocytes for microinjection of RNA and DNA. Methods Enzymol. 1987;152:284–288. doi: 10.1016/0076-6879(87)52032-8. [DOI] [PubMed] [Google Scholar]
- Mercer R. W., Biemesderfer D., Bliss D. P., Jr, Collins J. H., Forbush B., 3rd Molecular cloning and immunological characterization of the gamma polypeptide, a small protein associated with the Na,K-ATPase. J Cell Biol. 1993 May;121(3):579–586. doi: 10.1083/jcb.121.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman J. R., Palmer C. J., John J. E., 3rd, Durieux M. E., Jones L. R. Phospholemman expression induces a hyperpolarization-activated chloride current in Xenopus oocytes. J Biol Chem. 1992 Jul 25;267(21):14551–14554. [PubMed] [Google Scholar]
- Morrison B. W., Moorman J. R., Kowdley G. C., Kobayashi Y. M., Jones L. R., Leder P. Mat-8, a novel phospholemman-like protein expressed in human breast tumors, induces a chloride conductance in Xenopus oocytes. J Biol Chem. 1995 Feb 3;270(5):2176–2182. doi: 10.1074/jbc.270.5.2176. [DOI] [PubMed] [Google Scholar]
- Palmer C. J., Scott B. T., Jones L. R. Purification and complete sequence determination of the major plasma membrane substrate for cAMP-dependent protein kinase and protein kinase C in myocardium. J Biol Chem. 1991 Jun 15;266(17):11126–11130. [PubMed] [Google Scholar]
- Pácha J., Frindt G., Antonian L., Silver R. B., Palmer L. G. Regulation of Na channels of the rat cortical collecting tubule by aldosterone. J Gen Physiol. 1993 Jul;102(1):25–42. doi: 10.1085/jgp.102.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandle G. I., Hayslett J. P., Binder H. J. Effect of chronic hyperaldosteronism on the electrophysiology of rat distal colon. Pflugers Arch. 1984 May;401(1):22–26. doi: 10.1007/BF00581528. [DOI] [PubMed] [Google Scholar]
- Sargent T. D. Isolation of differentially expressed genes. Methods Enzymol. 1987;152:423–432. doi: 10.1016/0076-6879(87)52049-3. [DOI] [PubMed] [Google Scholar]
- Schulman G., Miller-Diener A., Litwack G., Bastl C. P. Characterization of the rat colonic aldosterone receptor and its activation process. J Biol Chem. 1986 Sep 15;261(26):12102–12108. [PubMed] [Google Scholar]
- Takumi T., Moriyoshi K., Aramori I., Ishii T., Oiki S., Okada Y., Ohkubo H., Nakanishi S. Alteration of channel activities and gating by mutations of slow ISK potassium channel. J Biol Chem. 1991 Nov 25;266(33):22192–22198. [PubMed] [Google Scholar]
- Takumi T., Ohkubo H., Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988 Nov 18;242(4881):1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- Varnum M. D., Busch A. E., Bond C. T., Maylie J., Adelman J. P. The min K channel underlies the cardiac potassium current IKs and mediates species-specific responses to protein kinase C. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11528–11532. doi: 10.1073/pnas.90.24.11528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrey F. Transcriptional control of sodium transport in tight epithelial by adrenal steroids. J Membr Biol. 1995 Mar;144(2):93–110. doi: 10.1007/BF00232796. [DOI] [PubMed] [Google Scholar]
- Wang W., Sackin H., Giebisch G. Renal potassium channels and their regulation. Annu Rev Physiol. 1992;54:81–96. doi: 10.1146/annurev.ph.54.030192.000501. [DOI] [PubMed] [Google Scholar]
- Zhou H., Tate S. S., Palmer L. G. Primary structure and functional properties of an epithelial K channel. Am J Physiol. 1994 Mar;266(3 Pt 1):C809–C824. doi: 10.1152/ajpcell.1994.266.3.C809. [DOI] [PubMed] [Google Scholar]