Abstract
Obesity is a global health burden and its prevalence is increasing substantially due to changing lifestyle. Chronic adiposity is associated with metabolic imbalance leading to dyslipidaemia, diabetes, hypertension and cardiovascular diseases (CVD). Adipose tissue acts as an endocrine organ releasing several adipocytokines, and is associated with increased levels of tissue and circulating inflammatory biomolecules causing vascular inflammation and atherogenesis. Further, inflammation is also associated independently with obesity as well as CVD. Keeping this in view, it is possible that a reduction in weight may lead to a decrease in inflammation, resulting in CVD risk reduction, and better management of patients with CVD. Lifestyle intervention has been endorsed by several health authorities in prevention and management of chronic diseases. A yoga-based lifestyle intervention appears to be a promising option in reducing the risk for CVD as well as management of patients with CVD as it is simple to follow and cost-effective with high compliance. The efficacy of such lifestyle intervention programmes is multifaceted, and is achieved via reduction in weight, obesity-related inflammation and stress, thereby culminating into risk reduction towards several chronic diseases including CVD. In this review, the association between obesity-related inflammation and CVD, and the role of yoga-based lifestyle intervention in prevention and management of CVD are discussed.
Keywords: Cardiovascular disease, inflammation, obesity, yoga-based lifestyle intervention
Introduction
Obesity is defined as an excess accumulation of fat due to positive energy balance, resulting from energy intake that exceeds the energy expenditure1, leading to adipocyte hypertrophy and hyperplasia, stress and inflammation within the adipose tissue. A recent study reported that the prevalence of adult overweight and obesity increased by 27.5 per cent with and number of overweight and obese individuals increasing from 857 million to 21 billion from 1980 to 20132. Obesity is an independent predictor for risk of various metabolic diseases and also a predictor of disease progression and mortality3. Excessive fat alone can contribute to several metabolic and cardiovascular diseases (CVD)4. Various studies conducted in Indian population have shown an association of obesity, dyslipidaemia, vascular inflammation, and metabolic syndrome5,6. Further, Asians, particularly South Asians have a higher prevalence of CVD, which can be attributed to an increased adipocyte size7, increased visceral adipose tissue8, higher levels of leptin9 and inflammatory mediators10. Keeping this in view, the body mass index (BMI) cut-off values for Asians were revised, and set at a lower level for obesity as compared to that for Western population (Table I). Studies done in different parts of India suggest that cumulative prevalence of obesity ranges from 10 to 50 per cent in adults (18-64 yr), however, there was a large variability in prevalence owing to different methods and cut-off points for defining obesity15. A study by Ray et al16 showed a high estimated prevalence of obesity in India, about 29.9 per cent even in young, physically active military subjects. This can be attributed to the improved socio-economic conditions, changing dietary habits and globalization of food market.
Table I.
Body mass index cut-off values (in kg/m2) for different populations

Therefore, it has been proposed that a modest weight-reduction will reduce the risk towards such chronic diseases including CVD17. For this risk reduction, a weight-loss of about 10-20 per cent of the initial body weight is recommended, which may be achieved through lifestyle interventions14 which have shown efficacy in weight-loss, resulting in CVD risk reduction15. Yoga is one such intervention that emphasizes on lifestyle modification and increased physical activity, and has been found to be efficacious in weight-loss and improvement of lipid profile in patients with coronary artery disease, diabetes and hypertension20,21,22.
Obesity: a state of inflammation
Obesity is a state of low grade inflammation23, which may later culminate in a chronic disorder if remains untreated many number of inflammatory mediators have been shown to be released by adipose tissue, which acts as an endocrine organ with autocrine regulation24. Leptin and adiponectin are primary adipocytokines which are synthesized in the adipose tissue itself25. Apart from leptin and adiponectin there are multiple adipocytokines which are upregulated in obesity such as interleukin-6 (IL-6), IL-1β, IL-10, tumour necrosis factor-α (TNF-α), monocyte chemo-attractant protein-1 (MCP-1), plasminogen activator inhibitor- 1 (PAI-1), angiotensinogen-1, endothelin-1 (ET-1), visfatin, resistin, retinol binding protein-4 (RBP-4) and serum amyloid A (SAA)26,27,28,29.
Inflammation can be both obesity- as well as disease-related. Obesity-related inflammation is a low grade inflammation associated with adipocytokines released from adipose tissue. In disease-related inflammation there is moderate to severe grade inflammation, and cytokines are site-specific30. Obesity related inflammation predisposes to a chronic inflammatory state which can culminate into various metabolic dysregulations, e.g. increased insulin resistance and endothelial dysfunction precipitating diabetes and CVD, respectively. In both obesity- as well as disease-related inflammation, cytokines are both contributors and sequelae31. The specific role of these adipocytokines in relation to pathophyisology of CVD is discussed here.
Adipokines: Leptin is a polypeptide hormone synthesized primarily in white adipose tissue and secreted in circulation. Classically, leptin is known for hypothalamic control of body weight and thermogenesis but in the last decade, its role in regulation of energy intake and energy expenditure has been well established32. Increased levels of leptin are known to be associated with elevated blood pressure and increased inflammation33. Adiponectin, another adipocytokine is exclusively expressed in adipose tissue, and the levels are negatively correlated with visceral fat34. Adiponectin levels tend to be lower in obesity along with increased levels of plasma interleukins35. Also, adiponectin is preventive not only against obesity but also against various metabolic disorders36.
Endothelin (ET-1): ET-1 is the most potent vasoconstrictor peptide released by the endothelium, which plays a key role in the regulation of vascular tone and the aetiology of atherosclerosis. Endothelial function is shown to be impaired in overweight/obese women with elevated levels of ET-135. Hyperinsulinaemia37 and oxidative stress38 stimulate ET-1 production, increasing its pathophysiological potential in obesity. Weil et al have demonstrated that overweight and obesity are associated with enhanced ET-1 levels in adiposity39.
Cytokines: Two important cytokines implicated in obesity and its metabolic consequences are IL-6 and TNF-α. It has been shown that levels of IL-6 are increased in overweight men, though circulating levels of IL-6 are shown to be associated with visceral obesity, while TNF-α levels with overall obesity40. Serum IL-6 concentration was shown to be positively correlated with the level of obesity as assessed by BMI and adipocyte size30, and total body fat percentage41. These findings suggested a possible role of adipose tissue in regulation of serum levels of IL-642, especially in individuals with central (visceral) obesity. Both IL-6 and TNF-α are known to impair adipocyte differentiation and promote inflammation43. Local production of TNF-α and IL-6 may ensue from epicardial adipose tissue44, which is indicative of cardiac and visceral obesity and related to intima media thickness and increase in vascular stiffness45. IL-6 possesses both pro-inflammatory and anti-inflammatory effects and impacts both B-cell immunoglobulin production and T-cell cytotoxic activity. IL-6 also affects platelet production and reactivity, endothelial function, and induces synthesis of acute phase proteins in liver by increasing the levels of nuclear factor kappa beta (NFĸβ) in a concentration-dependent manner46. TNF-α induces at least five different types of signals that include activation of NF-κB, apoptosis pathways, extracellular signal regulated kinase (ERK), p38 mitogen-activated protein kinase (p38MAPK), and c-Jun N-terminal kinase (JNK), playing a pivotal role in regulation of vascular functions. TNF-α along with neopterin, a biomolecule produced in monocyte/macrophages, increases expression of inducible nitric oxide synthase (iNOS), resulting in the production of cytotoxic radicals47. Neopterin has been found to be associated with cell-mediated immunity, and higher levels of neopterin have been reported in obesity48.
In summary, obesity is a state of ongoing low to moderate grade inflammation, which is largely related to visceral adipose tissue wherein adipose tissue acts as a depot for several inflammatory cytokines such as IL-6 and TNF-α.
Inflammation and cardiovascular diseases
Accumulating data suggest that inflammation contributes to the causation and progression of CVD49,50. Further, inflammatory mediators may trigger rupture of the atherosclerotic plaque which may result in coronary thrombosis, and ischaemia51. The key triggers that have recently gained recognition include IL-652, fibrinogen53, and C-reactive protein54 all of which are now identified as independent predictors of coronary heart disease55, and may serve as prospective novel biomarkers56. A practical framework for assessing the value of a novel risk marker and proposed standards with respect to critical appraisal of risk assessment methods that might be used clinically has been published by American Heart Association57. It has been shown that within the fatty streaks and atheromatous lesions, there is an overexpression of IL-6, which further strengthens its role in progression of atherosclerosis. Besides adipose tissue, IL-6 is locally produced in vascular endothelial and smooth muscle cells, and IL-6 gene is overtly expressed in human atherosclerotic lesions58. IL-6 stimulates monocytes and contributes towards deposition of fibrinogen in vessel wall and decreases lipoprotein lipase activity, which increases macrophage uptake of lipids. Additionally, circulating IL-6 stimulates the hypothalamic–pituitary–adrenal (HPA) axis, which is associated with central obesity, hypertension and insulin resistance59. In another study it has been demonstrated that plasma IL-6 levels ≥5 ng/ml are associated with a higher mortality than levels less than 5 ng/ml, suggesting that circulating IL-6 is a strong independent predictor of mortality in unstable coronary artery disease (CAD)60. TNF-α also plays an important role in endothelial dysfunction, and is implicated in heart failure61. It also causes vascular dysregulation, monocyte adhesion to endothelial cells, vascular oxidative stress, apoptosis, and atherogenic response, thereby resulting in thrombosis and coagulation through multiple signaling pathways62.
Increased levels of leptin are shown to be associated with CVD, myocardial infarction and stroke63. This association can be explained by a positive correlation of leptin with CRP64 and soluble IL-6 receptor (sIL-6R)65, which supports its role in pathophysiology of atherosclerosis. Leptin is also known to stimulate vascular remodelling by enhancing profibrotic cytokines and proatherogenic lipoprotein lipase production, platelet aggregation, PAI-1 expression, thereby development of atherosclerosis66. Adiponectin, an important regulator of endothelial nitric oxide synthase, is also a key determinant of endothelial function and angiogenesis67, and is known to oppose ET-168. It has been suggested that hypoadiponaectinaemia is an independent risk factor for hypertension69, and promotes aortic stiffness70. Prospective relationship of adiponectin to vascular disease in a case-control series selected from the Strong Heart Study, the largest cardiovascular study of American Indians, suggested a relation between low plasma adiponectin and insulin resistance in causation of CAD71. Kaplan-Meir survival analysis showed a step-wise decrease in event free survival across quartiles of adiponectin baseline concentration, which indicated that the lower level of adiponectin was associated with an adverse outcome in CAD72. Such protective effects of adiponectin may be due to several factors such as antiapoptotic and angiogenic actions on the vasculature, blocking inflammation and foam cell formation from macrophages73, and inhibiting oxidative stress66. Additionally, adiponectin also plays a protective role against cardiac ischaemic injury, hypertrophy, cardiomyopathy, and systolic dysfunction74.
Endothelins are primarily produced in the endothelium with a key role in vascular homeostasis, and are implicated in vascular diseases in various organs75. In an initial study it was observed that plasma levels of ET-1 were significantly higher in patients with symptomatic atherosclerosis as compared to control subjects, thereby suggesting that ET-1 could be a marker of arterial vascular disease76. Results from another study demonstrated that the plasma ET-1 levels were raised in patients with CAD, and possibly acted as a marker of risk of rapid stenosis progression77. A recent study has shown that plasma endothelin-1 level is a predictor of 10-year mortality in a general population78. It has been shown that adiponectin opposes endothelin-1 (ET-1)68 while leptin upregulates ET-179,80. An enhanced vascular activity of ET-1 was observed in the obese hypertensive and overweight subjects but not in lean hypertensive subjects81. Since the levels of ET-1 are increased in overweight and obese subjects and inhibit adiponectin secretion82, it is likely to cause endothelial vasodilator dysfunction and hence may play a role in the increased prevalence of hypertension with increased adiposity39.
Overall, a rise in plasma levels of these mediators induces release of various adhesion molecules, fibrinogen and PAI-1 causing hypercoagulability of blood. Apart from this, adipose tissue also releases esterified fatty acids, which in turn increase the concentration of LDL- cholesterol. Increased LDL cholesterol gets oxidized and engulfed by macrophages, which may lead to increased release of cytokines. Inflammatory cytokine signaling leads to smooth muscle proliferation and migration to sub-endothelial layer leading to initiation of atherosclerotic process83.
Obesity, inflammation and cardiovascular diseases
Obesity is among the most important causes of cardiovascular pathologies associated with endothelial dysfunction, such as arterial hypertension and atherosclerosis. Further, obesity is inadvertently associated with elevated plasma triglyceride levels, which is independently associated with an increased risk of CVD84. Adipokines directly impact triglyceride metabolism and adipocyte hypertrophy, which may lead to many changes in adipocyte function and production of anti- and pro-inflammatory cytokines (Table II). The inflammatory cytokines (adipokines) are secreted by adipose tissue, which is also located epicardially in addition to visceral location contributing to unfavourable cardio-metabolic complications85. Leptin and TNF-α are shown to diminish endothelial-dependent vasodilation when administered exogenously at pathophysiologically relevant concentrations86. On the other hand, adiponectin is associated with endothelial improvement and vascular protection87 and improves endothelial function through endothelial NO synthase (eNOS)-dependent pathways88. Therefore, lower level of adiponectin expression by epicardial adipose tissue in obesity sets the stage for coronary inflammation and endothelial dysfunction. An overview of adipocytokines in relation to CVD is presented in Fig. 1. Besides these adipocytokines, elevated CRP levels in obesity and its decrease associated with weight loss are indicative of link between CRP and obesity-associated risks for CVD89,90.
Table II.
Inflammatory biomarkers relevant to cardiovascular diseases (CVD)
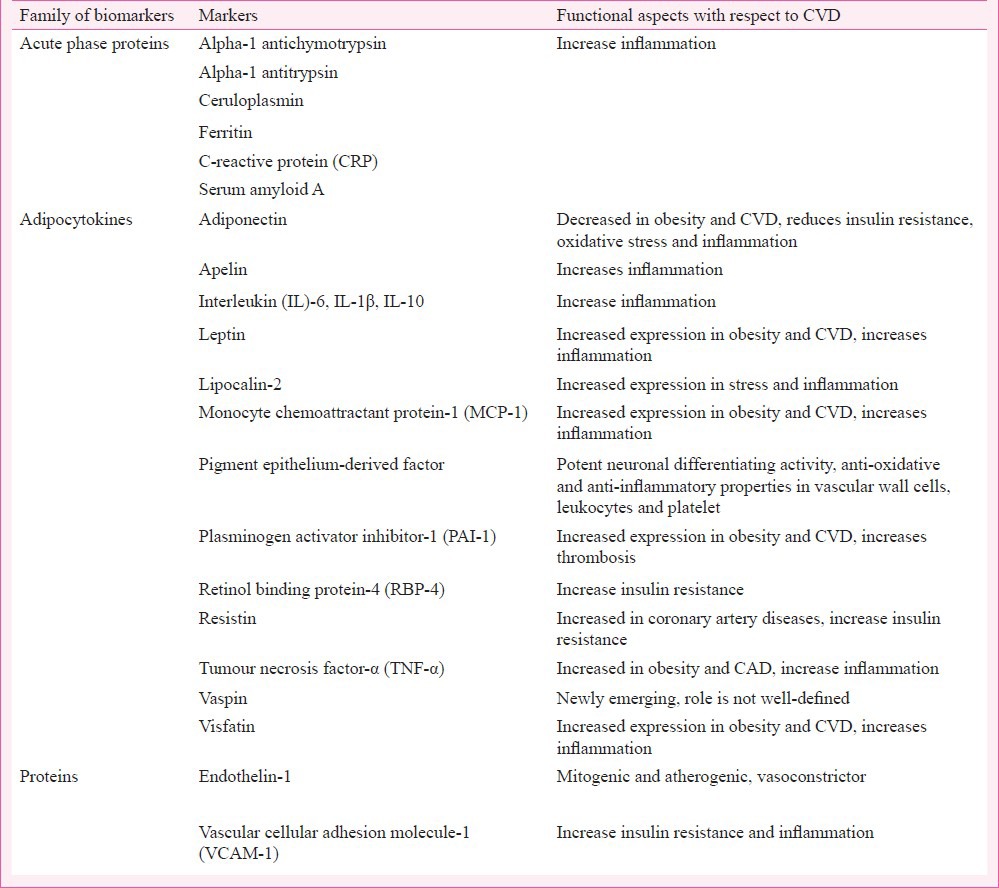
Fig. 1.
Overview of adipocytokines in relation to cardiovascular diseases. IL-6, interleukin-6; MMP, matrix metalloproteinases; NKκB, nuclear factor kappa-light-chain-enhancer of activated B cells; PAI, plasminogen activator inhibitor-1; TNF-α, tumour necrosis factor-alpha.
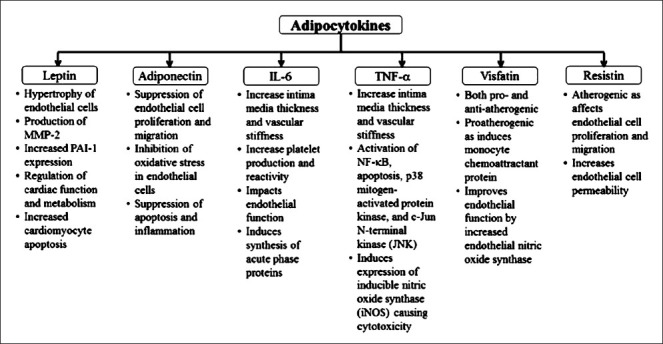
The production and release of inflammatory mediators linked to nutritional overload leads to organellar stress in obesity, with maximum stress to the endoplasmic reticulum. Endoplasmic reticulum stress is accompanied by accumulation of unfolded and misfolded proteins, which evoke unfolded protein response (UPR). UPR is associated with phosphorylation of various transcription factors and kinases, which in turn cause activation of nuclear factor-kappa beta (NF-ĸβ)91. Activation of NF-ĸβ leads to increased production of various cytokines including IL-6 and decrease in adiponectin92. Reactive oxygen species, endoplasmic reticulum stress, and ceramides are increased by adiposity, and all have also been shown to activate both JNK and NF-ĸβ93,94. Further, oxidative stress is an important link between obesity, inflammation, diabetes mellitus, and CVD95 as it has been associated independently with all of these. A model of obesity, inflammation and vascular endothelial changes is presented in Fig. 2.
Fig. 2.
Model for obesity, inflammation and vascular endothelial changes. ER, endoplasmic reticulum; NFκβ, nuclear factor kappa beta; IL-6, interleukin-6; CRP, C-receptive protein; PAI-1, plasminogen activator inhibitor-1; TNF-α, tumour necrosis factor-alpha; FFA, free fatty acids; SMC, smooth muscle cells; UPR, unfolded protein response.
These findings are important as non-traditional CVD risk factors have been identified by the American Heart Association96, and these factors may be responsible for a lowered age of CVD onset, which implicates that younger population is at an increased risk. Such premature onset of metabolic syndrome, and a subsequent risk to cardiovascular morbidity and mortality need to be addressed adequately98. Keeping these factors in view, it is important to control obesity using appropriate interventions aiming at weight loss and healthy lifestyle.
Risk factors as biomarkers: The clinical utility of these biomarkers is based on practicability, reproducibility, cost, and how well these can predict the risk vis-à-vis established biomarkers or in combination with them50. Most explored of these biomarkers in relation to CVD that have shown promising results include IL-6 and high-sensitivity CRP (hs-CRP). Of these two, CRP is a strong contender as the levels remain stable over years, and the test has high reliability, reproducibility and is cost-effective99,100,101. The IL-6 is the major initiator of acute phase response by hepatocytes and a primary determinant of hepatic CRP production102. The predictive value of IL-6 for cardiovascular ischaemic events was evaluated in a prospective cohort study and it was observed that IL-6 was associated with increased risk of future myocardial infarction in healthy middle-aged men52.
Yoga-based lifestyle intervention in obesity: Effects on inflammation and cardiovascular diseases
Despite significant progress in therapeutic modalities in CVD, an efficacious treatment remains a challenge. The treatment modalities for weight loss in the management of patients with CVD and those at an increased risk are focused on dietary interventions, increased physical activity, and pharmacotherapy103,104. Newer studies have shown that lifestyle intervention is a promising option in patients with CVD as well as those at an increased risk of CVD105,106. It has been stressed that weight loss is the key contributor towards correction of dyslipidaemia107, especially by reduction in visceral fat108. An important finding is that blood pressure can be reduced by lifestyle/behaviour modification; and though reduction may seem to be trivial, even small reduction in systolic BP (for example, 3-5 mm Hg) may produce clinically meaningful reductions109. Therefore, lifestyle modifications aiming at weight reduction by physical activity, dietary changes, breathing exercises and stress relaxation have a specific role in the management as well as prevention of chronic diseases110.
Yoga as a lifestyle intervention: Yoga combines a healthy lifestyle with mental peace111, and a modification in lifestyle and calming practices are shown to improve clinical profile of patients with various pathologies22,112. Regular practice of pranayama and meditation in healthy volunteers led to an improved cardiovascular metabolic status113,114, and lipid peroxidation even by a short term yoga based lifestyle intervention115. In a randomized controlled trial in patients with coronary atherosclerosis, a regression was observed in disease activity following a comprehensive lifestyle intervention116. In the study conducted by the same group, it has been shown that intensive lifestyle intervention may lead to regression of coronary atherosclerosis after one year and more regression of coronary atherosclerosis occurred after 5 years than after one year in the experimental group117. In a study conducted in India, the possible role of yoga-based lifestyle on retardation of coronary atherosclerosis disease was evaluated. At the end of one year, the yoga group showed significant reduction in number of angina episodes per week, an improved exercise capacity and a decrease in body weight. Serum total cholesterol, LDL cholesterol and triglyceride levels showed greater reductions as compared to control group112. Importantly, even short-term yoga based comprehensive lifestyle intervention led to notable reduction in body mass index, blood pressure, and blood glucose with a clinically meaningful improvement in lipid profile20,118. A recent study suggested that a yoga-based, residential weight loss programme may foster psychological well-being, improved nutrition behaviours, and weight loss119. Similar reduction in weight was observed in another study that included an 8-week of yoga training that resulted in an improvement in body composition and total cholesterol levels in obese adolescent boys120.
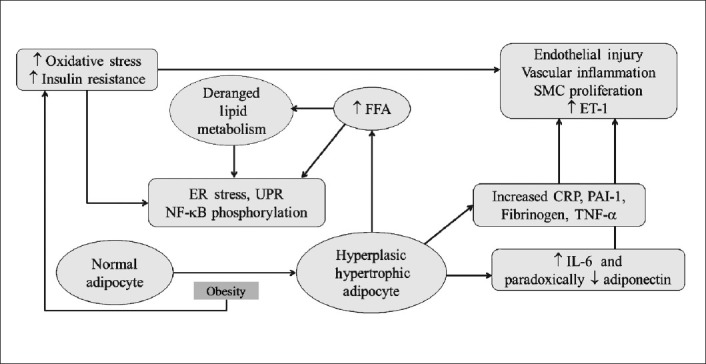
Another study showed that yoga postures (specifically suryanamaskar) resulted in improved cardiorespiratory fitness121. In a previous study in young hypertensive and pre-hypertensive patients, it was observed that there was a significant reduction in BP (SBP/DBP: 2.0/2.6 mm Hg) following yoga122. Similarly, a yoga-based lifestyle intervention resulted in a decrease in all lipid parameters except HDL. The effect started from four weeks and lasted for 14 weeks21. Together, these results indicate that a yoga-based lifestyle intervention may have an effect on some of the modifiable risk factors, which could probably explain the preventive and therapeutic beneficial effects of yoga observed in CVD. Overall, lifestyle intervention can modulate progression of the vascular inflammation at various steps of pathogenesis, thus counteracting causation/progression of CVD (Fig. 3).
Fig. 3.
Lifestyle intervention modifies various steps of vascular inflammation and pathogenesis of cardiovascular disease. ER, endoplasmic. ER, endoplasmic reticulum; NFκβ, nuclear factor kappa beta; IL-6, interleukin-6; CRP, C-receptive protein; PAI-1, plasminogen activator inhibitor-1; TNF-α, tumour necrosis factor-alpha; FFA, free fatty acids; SMC, smooth muscle cells; UPR, unfolded protein response.
A yoga-based lifestyle intervention is efficacious in weight-loss123, and it also prevents weight-gain, especially amongst those who are overweight124. Besides this lifestyle intervention also reduces inflammation as shown by a reduction in the levels of IL-6, IL-18, and CRP and increased adiponectin in obese and post-menopausal women35. Similar benefit was observed in another study where yoga improved adiponectin level, serum lipids, and metabolic syndrome risk factors in obese postmenopausal women125. A short-term yoga-based lifestyle intervention has been shown to decrease IL-6 and TNF-α in obese and normal weight individuals126, and increase adiponectin and decrease IL-6 in obese males127. IL-6, hs-CRP, extracellular superoxide dismutase levels were significantly decreased in heart failure patients after short term yogic exercises128. Also, a diet- induced weight loss led to a decrease in ET-1 and this decrease was correlated with a decrease in systolic BP129. It has been shown that an intensive lifestyle modification leads to a significant increase in plasma total antioxidants, plasma vitamin E and erythrocyte glutathione (GSH) in patients with CAD130.
Psychoneuroimmunological effects of yoga: The beneficial effects of yoga in reduction of inflammation appear to be related to reduction in stress as shown previously126. These effects of yoga can be explained using the concept of psychoneuroimmunology, which is a relatively new field of science that investigates multidirectional interactions between behaviour and immune system, mediated by nervous system and clinical implications of these linkages131. Yoga is known to induce relaxation via lowering of cortisol, and increasing the levels of beta-endorphins126. This results in lowered levels of cytokines126, as also observed in patients with hypertension132. as well as those who experienced heart failure133. A plausible reason for stress reduction by yoga is increased mindfulness134, however, there may be several other complex activities in brain that may combine to produce the relaxing effect. This is especially important in obese and overweight patients who often exhibit a low grade ongoing inflammation23 and may later culminate in a chronic disorder if goes untreated. IL-6 is a known predictor of all-cause mortality as reported in a study with a 9-year follow up in men135, and its reduction by a yoga-based lifestyle intervention may, therefore, be beneficial in reducing all-cause mortality.
Conclusion
Obesity, especially visceral adiposity, upregulates various inflammatory cytokines and other biomolecules. Chronic elevation of these inflammatory mediators leads to cardiovascular morbidity and mortality. Yoga-based lifestyle intervention can effectively prevent and retard the progression of cardiovascular and metabolic disorders. The mechanism of action of such benefit may be attributed to a reduction in weight and stress, networking at mind and body levels, thereby leading to a reduction in inflammation, and causation and progression of the disease.
References
- 1.WHO experts Committee on Obesity and overweight. Geneva: WHO; 2009. World Health Organization Global strategy on diet, physical activity and health. [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014 doi: 10.1016/S0140-6736(14)60460-8. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO expert consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 4.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–9. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 5.Kamble P, Deshmukh PR, Garg N. Metabolic syndrome in adult population of rural Wardha, central India. Indian J Med Res. 2010;132:701–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Pemminati S, Prabha Adhikari MR, Pathak R, Pai MR. Prevalence of metabolic syndrome (METS) using IDF 2005 guidelines in a semi urban south Indian (Boloor Diabetes Study) population of Mangalore. J Assoc Physicians India. 2010;58:674–7. [PubMed] [Google Scholar]
- 7.Smith J, Al-Amri M, Dorairaj P, Sniderman A. The adipocyte lifecycle hypothesis. Clin Sci (Lond) 2006;110:1–9. doi: 10.1042/CS20050110. [DOI] [PubMed] [Google Scholar]
- 8.Chandalia M, Abate N, Garg A, Stray-Gundersen J, Grundy SM. Relationship between generalized and upper body obesity to insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 1999;84:2329–35. doi: 10.1210/jcem.84.7.5817. [DOI] [PubMed] [Google Scholar]
- 9.Lilja M, Rolandsson O, Shaw JE, Pauvaday V, Cameron AJ, Tuomilehto J, et al. Higher leptin levels in Asian Indians than Creoles and Europids: a potential explanation for increased metabolic risk. Int J Obes (Lond) 2010;34:878–85. doi: 10.1038/ijo.2010.19. [DOI] [PubMed] [Google Scholar]
- 10.Chandalia M, Cabo-Chan AV, Jr, Devaraj S, Jialal I, Grundy SM, Abate N. Elevated plasma high-sensitivity C-reactive protein concentrations in Asian Indians living in the United States. J Clin Endocrinol Metab. 2003;88:3773–6. doi: 10.1210/jc.2003-030301. [DOI] [PubMed] [Google Scholar]
- 11.WHO Expert Consultation. Appropriate-body mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 12.Snehalatha C, Viswanathan V, Ramachandran A. Cutoff values for normal anthropometric variables in Asian Indian adults. Diabetes Care. 2003;26:1380–4. doi: 10.2337/diacare.26.5.1380. [DOI] [PubMed] [Google Scholar]
- 13.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Geneva: WHO; 2010. The Asia-Pacific perspective: redefining obesity and its treatment. [Google Scholar]
- 15.Mohan V. Obesity & abdominal obesity in Asian Indians. Indian J Med Res. 2006;123:593–6. [PubMed] [Google Scholar]
- 16.Ray S, Kulkarni B, Sreenivas A. Prevalence of prehypertension in young military adults & its association with overweight & dyslipidaemia. Indian J Med Res. 2011;134:162–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen T, Lau DC. The obesity epidemic and its impact on hypertension. Can J Cardiol. 2012;28:326–33. doi: 10.1016/j.cjca.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Rabasseda X. A report from the American Heart Association Scientific Sessions 2011 (November 12-16, 2011, Orlando, Florida, USA) Drugs Today (Barc) 2012;48:79–94. doi: 10.1358/dot.2012.48.1.1766315. [DOI] [PubMed] [Google Scholar]
- 19.Wing RR Look AHEAD Research Group. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–75. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bijlani RL, Vempati RP, Yadav RK, Ray RB, Gupta V, Sharma R, et al. A brief but comprehensive lifestyle education program based on yoga reduces risk factors for cardiovascular disease and diabetes mellitus. J Altern Complement Med. 2005;11:267–74. doi: 10.1089/acm.2005.11.267. [DOI] [PubMed] [Google Scholar]
- 21.Mahajan AS, Reddy KS, Sachdeva U. Lipid profile of coronary risk subjects following yogic lifestyle intervention. Indian Heart J. 1999;51:37–40. [PubMed] [Google Scholar]
- 22.Damodaran A, Malathi A, Patil N, Shah N, Suryavansihi, Marathe S. Therapeutic potential of yoga practices in modifying cardiovascular risk profile in middle aged men and women. J Assoc Physicians India. 2002;50:633–40. [PubMed] [Google Scholar]
- 23.Popko K, Gorska E, Stelmaszczyk-Emmel A, Plywaczewski R, Stoklosa A, Gorecka D, et al. Proinflammatory cytokines Il-6 and TNF-α and the development of inflammation in obese subjects. Eur J Med Res. 2010;15(Suppl 2):120–2. doi: 10.1186/2047-783X-15-S2-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leal Vde O, Mafra D. Adipokines in obesity. Clin Chim Acta. 2013;419:87–94. doi: 10.1016/j.cca.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Tilg H, Moschen AR. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 26.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release Interleukin-6. J Clin Endocrinol Metab. 1998;83:847–50. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 27.Fukuhara A, Matsuda M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, et al. Visfatin: A protein secreted by visceral fat that mimics the effect of insulin. Science. 2005;307:426–30. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 28.Shimomura I, Funahashi T, Takahashi M, Maeda K, Kotani K, Nakamura T, et al. Enhanced expression of PAI-1 in visceral fat: Possible contributor to vascular disease in obesity. Nat Med. 1996;2:800–3. doi: 10.1038/nm0796-800. [DOI] [PubMed] [Google Scholar]
- 29.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 30.Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta In press. 2014 doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Sopasakis VR, Sandqvist M, Gustafson B, Hammarstedt A, Schmelz M, Yang X, et al. High local concentrations and effects on differentiation implicate interleukin-6 as a paracrine regulator. Obes Res. 2004;12:454–60. doi: 10.1038/oby.2004.51. [DOI] [PubMed] [Google Scholar]
- 32.Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord. 2002;26:1407–33. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- 33.Bełtowski J. Role of leptin in blood pressure regulation and arterial hypertension. J Hypertens. 2006;24:789–801. doi: 10.1097/01.hjh.0000222743.06584.66. [DOI] [PubMed] [Google Scholar]
- 34.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 35.Katherine E, Alessandro P, Carmen DP, Giovanni G, Mariengela M, Raffaele M, et al. Effect of weight loss and life style changes on vascular inflammatory markers in obese women. JAMA. 2003;289:1799–804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez-Real JM, Lopez-Bermejo A, Casamitjana R, Ricart W. Novel interactions of adiponectin with the endocrine system and inflammatory parameters. J Clin Endocrinol Metab. 2003;88:2714–8. doi: 10.1210/jc.2002-021583. [DOI] [PubMed] [Google Scholar]
- 37.Ferri C, Pittoni V, Piccoli A, Laurenti O, Cassone MR, Bellini C, et al. Insulin stimulates endothelin-1 secretion from human endothelial cells and modulates its circulating levels in vivo. J Clin Endocrinol Metab. 1995;80:829–35. doi: 10.1210/jcem.80.3.7883838. [DOI] [PubMed] [Google Scholar]
- 38.Pollock DM, Pollock JS. Endothelin and oxidative stress in the vascular system. Curr Vasc Pharmacol. 2005;3:365–7. doi: 10.2174/157016105774329408. [DOI] [PubMed] [Google Scholar]
- 39.Weil BR, Westby CM, Van Guilder GP, Greiner JJ, Stauffer BL, DeSouza CA. Enhanced endothelin-1 system activity with overweight and obesity. Am J Physiol Heart Circ Physiol. 2011;301:H689–95. doi: 10.1152/ajpheart.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cartier A, Lemieux I, Alméras N, Tremblay A, Bergeron J, Després JP. Visceral obesity and plasma glucose-insulin homeostasis: contributions of interleukin-6 and tumor necrosis factor-alpha in men. J Clin Endocrinol Metab. 2008;93:1931–8. doi: 10.1210/jc.2007-2191. [DOI] [PubMed] [Google Scholar]
- 41.Bastard JP, Jardel C, Delattre J, Hainque B, Bruckert E, Oberlin F, et al. Evidence for a link between adipose tissue interleukin-6 content and serum C-reactive protein concentrations in obese subjects. Circulation. 1999;99:2219–22. [PubMed] [Google Scholar]
- 42.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–3. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 43.Gustafson B, Smith U. Cytokines promote Wnt signaling and inflammation and impair the normal differentiation and lipid accumulation in 3T3-L1 preadipocytes. J Biol Chem. 2006;281:9507–16. doi: 10.1074/jbc.M512077200. [DOI] [PubMed] [Google Scholar]
- 44.Kremen J, Dolinkova M, Krajickova J, Blaha J, Anderlova K, Lacinova Z, et al. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. J Clin Endocrinol Metab. 2006;91:4620–7. doi: 10.1210/jc.2006-1044. [DOI] [PubMed] [Google Scholar]
- 45.Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5163–8. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 46.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–82. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 47.Werner Felmeyer G, Wermer ER, Fuchs D, Hausen A, Reibnegger G, Schmidt K, et al. Pteridine biosynthesis in human endothelial cells: impact on nitric oxide mediated formation of cyclic GMP. J Biol Chem. 1993;268:1842–46. [PubMed] [Google Scholar]
- 48.Ledochowski M, Murr C, Winder B, Fuchs D. Association between insulin resistance, body mass and Neopterin concentration. Clin Chim Acta. 1999;282:115–23. doi: 10.1016/s0009-8981(99)00019-4. [DOI] [PubMed] [Google Scholar]
- 49.Taube A, Schlich R, Sell H, Eckardt K, Eckel J. Inflammation and metabolic dysfunction: links to cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2012;302:H2148–65. doi: 10.1152/ajpheart.00907.2011. [DOI] [PubMed] [Google Scholar]
- 50.Libby P, Ridker PM, Hansson GK. Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–38. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Wal AC, Becker AE, van der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994;89:36–44. doi: 10.1161/01.cir.89.1.36. [DOI] [PubMed] [Google Scholar]
- 52.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–72. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 53.Kannel WB, Wolf PA, Castelli WP, D’Agostino RB. Fibrinogen and risk of cardiovascular disease: the Framingham study. JAMA. 1987;258:1183–6. [PubMed] [Google Scholar]
- 54.Koenig W, Sund M, Frohlich M, Fischer HG, Lowel H, Doring A, et al. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort study, 1984 to 1992. Circulation. 1999;99:237–42. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 55.Luc G, Bard JM, Juhan-Vague I, Ferrieres J, Evans A, Amouyel P, et al. C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: the PRIME study. Arterioscler Thromb Vasc Biol. 2003;23:1255–61. doi: 10.1161/01.ATV.0000079512.66448.1D. [DOI] [PubMed] [Google Scholar]
- 56.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113:2335–62. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 57.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, et al. American Heart Association Expert Panel on Subclinical Atherosclerotic Diseases and Emerging Risk Factors and the Stroke Council. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–16. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szekanecz Z, Shah MR, Pearce WH, Koch AE. Human atherosclerotic abdominal aortic aneurysms produce interleukin (IL)-6 and interferon-gamma but not IL-2 and IL-4: the possible role for IL-6 and interferon-gamma in vascular inflammation. Agents Actions. 1994;42:159–62. doi: 10.1007/BF01983484. [DOI] [PubMed] [Google Scholar]
- 59.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–8. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 60.Lindmark E, Diderholm E, Wallentin L, Seigbahn A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: Effects of an early invasive or noninvasive strategy. JAMA. 2001;286:2107–13. doi: 10.1001/jama.286.17.2107. [DOI] [PubMed] [Google Scholar]
- 61.Ueland T, Yndestad A, Dahl CP, Gullestad L, Aukrust P. TNF revisited: osteoprotegerin and TNF-related molecules in heart failure. Curr Heart Fail Rep. 2012;9:92–100. doi: 10.1007/s11897-012-0088-6. [DOI] [PubMed] [Google Scholar]
- 62.Zhang H, Zhang C. Vasoprotection by dietary supplements and exercise: role of TNFα signaling. Exp Diabetes Res 2012. 2012:972679. doi: 10.1155/2012/972679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sierra-Johnson J, Romero-Corral A, Lopez-Jimenez F, Gami AS, Sert Kuniyoshi FH, Wolk R, et al. Relation of increased leptin concentrations to history of myocardial infarction and stroke in the United States population. Am J Cardiol. 2007;100:234–9. doi: 10.1016/j.amjcard.2007.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zwaka TP, Hombach V, Torzewski J. C-reactive protein-mediated low density lipoprotein uptake by macrophages: implications for atherosclerosis. Circulation. 2001;103:1194–7. doi: 10.1161/01.cir.103.9.1194. [DOI] [PubMed] [Google Scholar]
- 65.Rus HG, Vlaicu R, Niculescu F. Interleukin-6 and interleukin-8 protein and gene expression in human arterial atherosclerotic wall. Atherosclerosis. 1996;127:263–71. doi: 10.1016/s0021-9150(96)05968-0. [DOI] [PubMed] [Google Scholar]
- 66.Scotece M, Conde J, Gómez R, López V, Pino J, González A, et al. Role of adipokines in atherosclerosis: interferences with cardiovascular complications in rheumatic diseases. Mediators Inflamm 2012. 2012:125458. doi: 10.1155/2012/125458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2007;278:45021–6. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 68.Bussey CT, Kolka CM, Rattigan S, Richards SM. Adiponectin opposes endothelin-1-mediated vasoconstriction in the perfused rat hindlimb. Am J Physiol Heart Circ Physiol. 2011;301:H79–86. doi: 10.1152/ajpheart.00864.2010. [DOI] [PubMed] [Google Scholar]
- 69.Chow WS, Cheung BM, Tso AW, Xu A, Wat NM, Fong CH, et al. Hypoadiponectinemia as a predictor for the development of hypertension: a 5-year prospective study. Hypertension. 2007;49:1455–61. doi: 10.1161/HYPERTENSIONAHA.107.086835. [DOI] [PubMed] [Google Scholar]
- 70.Tsioufis C, Dimitriadis K, Selima M, Thomopoulos C, Mihas C, Skiadas I, et al. Low-grade inflammation and hypoadiponectinaemia have an additive detrimental effect on aortic stiffness in essential hypertensive patients. Eur Heart J. 2007;28:1162–9. doi: 10.1093/eurheartj/ehm089. [DOI] [PubMed] [Google Scholar]
- 71.Lindsay R, Resnick E, Zhu J, Tun ML, Howard BV, Zhang Y, et al. Adiponectin and Coronary Heart Disease: The Strong Heart Study. Arterioscler Thromb Vasc Biol. 2005;25:e15–6. doi: 10.1161/01.ATV.0000153090.21990.8c. [DOI] [PubMed] [Google Scholar]
- 72.Schnabel R, Messow MC, Lubos E, Klein CE, Rupprecht HJ, Christoph B, et al. Association of adiponectin with adverse outcome in coronary artery disease patients: results from the Athero Gene study. European Heart J. 2008;29:649–57. doi: 10.1093/eurheartj/ehn009. [DOI] [PubMed] [Google Scholar]
- 73.Hopkins TA, Ouchi N, Shibata R, Walsh K. Adiponectin actions in the cardiovascular system. Cardiovas Res. 2007;74:11–8. doi: 10.1016/j.cardiores.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nature Clin Pract Cardiovas Med. 2009;6:27–35. doi: 10.1038/ncpcardio1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schinelli S. Pharmacology and pathophysiology of brain endothelin system: an overview. Curr Med Chem. 2006;13:627–38. doi: 10.2174/092986706776055652. [DOI] [PubMed] [Google Scholar]
- 76.Lerman A, Edwards BS, Hallett JW, HeubLein DM, Sandberg SM, Burnett JC. Circulating and tissue endothelin reactivity in advanced atherosclerosis. N Engl J Med. 1991;325:997–1001. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]
- 77.Zouridakis EG, Schwartzman R, Moll XG, Cox ID, Fredericks S, Holt DW, et al. Increased plasma endothelin levels in angina patients with rapid coronary artery disease progression. Eur Heart J. 2001;22:1578–84. doi: 10.1053/euhj.2000.2588. [DOI] [PubMed] [Google Scholar]
- 78.Yokoi K, Adachi H, Hirai Y, Enomoto M, Fukami A, Ogata K, et al. Plasma endothelin-1 level is a predictor of 10-year mortality in a general population: the Tanushimaru study. Circ J. 2012;76:2779–84. doi: 10.1253/circj.cj-12-0469. [DOI] [PubMed] [Google Scholar]
- 79.Juan CC, Chuang TY, Lien CC, Lin YJ, Huang SW, Kwok CF, et al. Leptin increases endothelin type A receptor levels in vascular smooth muscle cells. Am J Physiol Endocrinol Metab. 2008;294:E481–7. doi: 10.1152/ajpendo.00103.2007. [DOI] [PubMed] [Google Scholar]
- 80.Quehenberger P, Exner M, Sunder-Plassmann R, Ruzicka K, Bieglmayer C, Endler G, et al. Leptin induces endothelin-1 in endothelial cells in vitro. Circ Res. 2002;90:711–8. doi: 10.1161/01.res.0000014226.74709.90. [DOI] [PubMed] [Google Scholar]
- 81.Cardillo C, Campia U, Lantorno M, Panza JA. Patients enhanced vascular activity of endogenous endothelin-1 in obese hypertensive patients. Hypertension. 2004;43:36–40. doi: 10.1161/01.HYP.0000103868.45064.81. [DOI] [PubMed] [Google Scholar]
- 82.Bedi D, Clarke KJ, Dennis JC, Zhong Q, Brunson BL, Morrison EE, et al. Endothelin-1 inhibits adiponectin secretion through a phosphatidylinositol 4,5-bisphosphate/actin-dependent mechanism. Biochem Biophys Res Commun. 2006;345:332–9. doi: 10.1016/j.bbrc.2006.04.098. [DOI] [PubMed] [Google Scholar]
- 83.Louis SF, Zahradka P. Vascular smooth muscle cell motility: From migration to invasion. Exp Clin Cardiol. 2010;15:e75–85. [PMC free article] [PubMed] [Google Scholar]
- 84.Firdous S. Correlation of CRP, fasting serum triglycerides and obesity as cardiovascular risk factors. J Coll Physicians Surg Pak. 2014;24:308–13. [PubMed] [Google Scholar]
- 85.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850–6. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Knudson JD, Dincer UD, Bratz IN, Sturek M, Dick GM, Tune JD. Mechanisms of coronary dysfunction in obesity and insulin resistance. Microcirculation. 2007;14:317–38. doi: 10.1080/10739680701282887. [DOI] [PubMed] [Google Scholar]
- 87.Beltowski J, Jamroz-Wisniewska A, Widomska S. Adiponectin and its role in cardiovascular diseases. Cardiovasc Hematol Disord Drug Targets. 2008;8:7–46. doi: 10.2174/187152908783884920. [DOI] [PubMed] [Google Scholar]
- 88.Deng G, Long Y, Yu YR, Li MR. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK-eNOS pathway. Int J Obes (Lond) 2010;34:165–71. doi: 10.1038/ijo.2009.205. [DOI] [PubMed] [Google Scholar]
- 89.Heilbronn LK, Noakes M, Clifton PM. Energy restriction and weight loss on very-low-fat diets reduce C-reactive protein concentrations in obese, healthy women. Arterioscler Thromb Vasc Biol. 2001;21:968–70. doi: 10.1161/01.atv.21.6.968. [DOI] [PubMed] [Google Scholar]
- 90.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. J Am Med Assoc. 2003;289:1799–804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 91.Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm 2010. 2010:ii. doi: 10.1155/2010/289645. 289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lira FS, Rosa JC, Pimentel GD, Seelaender M, Damaso AR, Oyama LM, et al. Both adiponectin and interleukin-10 inhibit LPS-induced activation of the NF-κB pathway in 3T3-L1 adipocytes. Cytokine. 2012;57:98–106. doi: 10.1016/j.cyto.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 93.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type II diabetes mellitus. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 95.Bondia-Pons I, Ryan L, Martinez JA. Oxidative stress and inflammation interactions in human obesity. J Physiol Biochem. 2012;68:701–11. doi: 10.1007/s13105-012-0154-2. [DOI] [PubMed] [Google Scholar]
- 96.Balagopal PB, de Ferranti SD, Cook S, Daniels SR, Gidding SS, Hayman LL, et al. American Heart Association Committee on Atherosclerosis Hypertension and Obesity in Youth of the Council on Cardiovascular Disease in the Young; Council on Nutrition, Physical Activity and Metabolism; Council on Epidemiology and Prevention. Nontraditional risk factors and biomarkers for cardiovascular disease: mechanistic, research, and clinical considerations for youth: a scientific statement from the American Heart Association. Circulation. 2011;123:2749–69. doi: 10.1161/CIR.0b013e31821c7c64. [DOI] [PubMed] [Google Scholar]
- 97.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Steinberger J, Daniels SR, Eckel RH, Hayman L, Lustig RH, McCrindle B, et al. American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119:628–47. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]
- 99.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 100.Glynn RJ, MacFadyen JG, Ridker PM. Tracking of high-sensitivity C-reactive protein after an initially elevated concentration: the JUPITER Study. Clin Chem. 2009;55:305–12. doi: 10.1373/clinchem.2008.120642. [DOI] [PubMed] [Google Scholar]
- 101.Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118:2243–51. doi: 10.1161/CIRCULATIONAHA.108.814251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–36. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, et al. Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr. 2003;22:331–9. doi: 10.1080/07315724.2003.10719316. [DOI] [PubMed] [Google Scholar]
- 105.Aucott L, Rothnie H, McIntyre L, Thapa M, Waweru C, Gray D. Long-term weight loss from lifestyle intervention benefits blood pressure?: a systematic review. Hypertension. 2009;54:756–62. doi: 10.1161/HYPERTENSIONAHA.109.135178. [DOI] [PubMed] [Google Scholar]
- 106.Hooper L. Primary prevention of CVD: diet and weight loss. Clin Evid (Online) 2007:ii. 0219. [PMC free article] [PubMed] [Google Scholar]
- 107.Diaz VA, Player MS, Mainous AG, 3rd, Carek PJ, Geesey ME. Competing impact of excess weight versus cardiorespiratory fitness on cardiovascular risk. Am J Cardiol. 2006;98:1468–71. doi: 10.1016/j.amjcard.2006.06.048. [DOI] [PubMed] [Google Scholar]
- 108.Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–44. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Israili ZH, Hernández-Hernández R, Valasco M. The future of antihypertensive treatment. Am J Ther. 2007;14:121–34. doi: 10.1097/01.pap.0000249915.12185.58. [DOI] [PubMed] [Google Scholar]
- 110.Goran MI, Alderete TL. Targeting adipose tissue inflammation to treat the underlying basis of the metabolic complications of obesity. Nestle Nutr Inst Workshop Ser. 2012;73:49–60. doi: 10.1159/000341287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bijlani RL. Scientific medicine shows signs of a paradigm shift. New Approaches Med Health. 2003;11:28–40. [Google Scholar]
- 112.Manchanda SC, Narang R, Reddy KS, Sachdeva U, Prabhakaran D, Dharmanand S, et al. Retardation of coronary atherosclerosis with yoga lifestyle intervention. J Assoc Physicians India. 2000;48:687–94. [PubMed] [Google Scholar]
- 113.Vyas R, Dikshit N. Effect of meditation on respiratory system, cardiovascular system and lipid profile. Indian J Physiol Pharmacol. 2002;46:487–91. [PubMed] [Google Scholar]
- 114.Prasad KVV, Sunita M, Raju PS, Reddy MV, Sahay BK, Murthy KJY. Impact of pranayama and yoga on lipid profile in normal healthy volunteers. J Exer Physiol. 2006;9:1–6. [Google Scholar]
- 115.Yadav RK, Ray RB, Vempati R, Bijlani RL. Effect of a comprehensive yoga-based lifestyle modification program on lipid peroxidation. Indian J Physiol Pharmacol. 2005;49:358–62. [PubMed] [Google Scholar]
- 116.Ornish D, Brown SE, Scherwitz LW, Billings JH, Armstrong WT, Ports TA, et al. Can lifestyle changes reverse coronary heart disease? Lancet. 1990;336:129–33. doi: 10.1016/0140-6736(90)91656-u. [DOI] [PubMed] [Google Scholar]
- 117.Ornish D, Scherwitz LW, Billings JH, Gould L, Merritt TA, Sparler S, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280:2001–7. doi: 10.1001/jama.280.23.2001. [DOI] [PubMed] [Google Scholar]
- 118.Sarvottam K, Yadav RK, Mehta N, Mahapatra SC. Effect of short term yoga on resting energy expenditure and lipid profile in overweight/obese subjects: A preliminary study. Indian J Physiol Pharmacol. 2010;54:133. [Google Scholar]
- 119.Braun TD, Park CL, Conboy LA. Psychological well-being, health behaviors, and weight loss among participants in a residential, Kripalu yoga-based weight loss program. Int J Yoga Therap. 2012;22:9–22. [PubMed] [Google Scholar]
- 120.Seo DY, Lee S, Figueroa A, Kim HK, Baek YH, Kwak YS, et al. Yoga training improves metabolic parameters in obese boys. Korean J Physiol Pharmacol. 2012;16:175–80. doi: 10.4196/kjpp.2012.16.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mody BS. Acute effects of Surya Namaskar on the cardiovascular & metabolic system. J Bodyw Mov Ther. 2011;15:343–7. doi: 10.1016/j.jbmt.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 122.Saptharishi L, Soudarssanane M, Thiruselvakumar D, Navasakthi D, Mathanraj S, Karthigeyan M, et al. Community-based randomized controlled trial of non-pharmacological interventions in prevention and control of hypertension among young adults. Indian J Community Med. 2009;34:329–34. doi: 10.4103/0970-0218.58393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sharpe PA, Blanck HM, Williams JE, Ainsworth BE, Conway JM. Use of complementary and alternative medicine for weight control in the United States. J Altern Complement Med. 2007;13:217–22. doi: 10.1089/acm.2006.6129. [DOI] [PubMed] [Google Scholar]
- 124.Kristal AR, Littman AJ, Benitez D, White E. Yoga practice is associated with attenuated weight gain in healthy, middle-aged men and women. Altern Ther Health Med. 2005;11:28–33. [PubMed] [Google Scholar]
- 125.Lee JA, Kim JW, Kim DY. Effects of yoga exercise on serum adiponectin and metabolic syndrome factors in obese postmenopausal women. Menopause. 2012;19:296–301. doi: 10.1097/gme.0b013e31822d59a2. [DOI] [PubMed] [Google Scholar]
- 126.Yadav RK, Magan D, Mehta N, Sharma R, Mahapatra SC. Efficacy of a short term yoga based lifestyle intervention in reducing stress and inflammation: Preliminary results. J Altern Complement Med. 2012;18:662–7. doi: 10.1089/acm.2011.0265. [DOI] [PubMed] [Google Scholar]
- 127.Sarvottam K, Magan D, Yadav RK, Mehta N, Mahapatra SC. Adiponectin, interleukin-6 and cardiovascular disease risk factors are modified by a short-term yoga-based lifestyle intervention in overweight/obese male subjects. J Altern Complement Med. 2013;19:397–402. doi: 10.1089/acm.2012.0086. [DOI] [PubMed] [Google Scholar]
- 128.Pullen PR, Nagamia SH, Mehta PK, Thompson WR, Benardot D, Hammoud R, et al. Effects of yoga on inflammation and exercise capacity in patients with chronic heart failure. J Card Fail. 2008;14:407–13. doi: 10.1016/j.cardfail.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 129.Maeda S, Jesmin S, Iemitsu M, Otsuki T, Matsuo T, Ohkawara K, et al. Weight loss reduces plasma endothelin-1 concentration in obese men. Exp Biol Med (Maywood) 2006;231:1044–7. [PubMed] [Google Scholar]
- 130.Jatuporn S, Sangwatanaroj S, Saengsiri AO, Rattanapruks S, Srimahachota S, Uthayachalerm W, et al. Short-term effects of an intensive lifestyle modification program on lipid peroxidation and antioxidant systems in patients with coronary artery disease. Clin Hemorheol Microcirc. 2003;29:429–36. [PubMed] [Google Scholar]
- 131.Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: New perspectives from psychoneuroimmunology. Annu Rev Psychol. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- 132.Cozzolino D, Sasso FC, Cataldo D, Gruosso D, Giammarco A, Cavalli A, et al. Acute pressor and hormonal effects of beta-endorphin at high doses in healthy and hypertensive subjects: role of opioid receptor agonism. J Clin Endocrinol Metab. 2005;90:5167–74. doi: 10.1210/jc.2004-2554. [DOI] [PubMed] [Google Scholar]
- 133.Pullen PR, Thompson WR, Benardot D, Brandon LJ, Mehta PK, Rifai L, et al. Benefits of yoga for African American heart failure patients. Med Sci Sports Exerc. 2010;42:651–7. doi: 10.1249/MSS.0b013e3181bf24c4. [DOI] [PubMed] [Google Scholar]
- 134.Dobkin PL, Zhao Q. Increased mindfulness - the active component of the mindfulness-based stress reduction program? Complement Ther Clin Pract. 2011;17:22–7. doi: 10.1016/j.ctcp.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 135.Baune BT, Rothermundt M, Ladwig KH, Meisinger C, Berger K, et al. Systemic inflammation (Interleukin 6) predicts all-cause mortality in men: results from a 9-year follow-up of the MEMO Study. Age (Dordr) 2011;33:209–17. doi: 10.1007/s11357-010-9165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


