Abstract
Viral suppression by noncytolytic CD8+ T cells, in addition to that by classic antiviral CD8+ cytotoxic T lymphocytes, has been described for human immunodeficiency virus and simian immunodeficiency virus (SIV) infections. However, the role of soluble effector molecules, especially beta-chemokines, in antiviral immunity is still controversial. In an attenuated vaccine model, approximately 60% of animals immunized with simian/human immunodeficiency virus (SHIV) 89.6 and then challenged intravaginally with SIVmac239 controlled viral replication (viral RNA level in plasma, <104 copies/ml) and were considered protected (K. Abel, L. Compton, T. Rourke, D. Montefiori, D. Lu, K. Rothaeusler, L. Fritts, K. Bost, and C. J. Miller, J. Virol. 77:3099-3118, 2003). To determine the in vivo importance of beta-chemokine secretion and CD8+-T-cell proliferation in the control of viral replication in this vaccine model, we examined the relationship between viral RNA levels in the axillary and genital lymph nodes of vaccinated, protected (n = 20) and vaccinated, unprotected (n = 11) monkeys by measuring beta-chemokine mRNA levels and protein expression, the frequency of CD8+ T cells expressing beta-chemokines, and the extent of CD8+-T-cell proliferation. Tissues from uninfected (n = 3) and unvaccinated, SIVmac239-infected (n = 9) monkeys served as controls. Axillary and genital lymph nodes from unvaccinated and vaccinated, unprotected monkeys had significantly higher beta-chemokine mRNA expression levels and increased numbers of beta-chemokine-positive cells than did vaccinated, protected animals. Furthermore, the lymph nodes of vaccinated, unprotected monkeys had significantly higher numbers of beta-chemokine+ CD8+ T cells than did vaccinated, protected monkeys. Lymph nodes from vaccinated, unprotected animals also had significantly more CD8+-T-cell proliferation and marked lymph node hyperplasia than the lymph nodes of vaccinated, protected monkeys. Thus, higher levels of virus replication were associated with increased beta-chemokine secretion and there is no evidence that beta-chemokines contributed to the SHIV89.6-mediated control of viral replication after intravaginal challenge with SIVmac239.
Despite efforts to develop an effective anti-human immunodeficiency virus (HIV) vaccine, significant obstacles remain. The difficulty in developing a vaccine lies in the basic nature of HIV infections; immune clearance of an HIV infection has never been documented. However, antiviral CD8+ T cells have been associated with the control of HIV and simian immunodeficiency virus (SIV) replication in vivo (40, 48). Viral suppression by noncytolytic CD8+ T cells, in addition to that by classic cytolytic antiviral cytotoxic T lymphocytes (CTLs), has been described for HIV and SIV infections (12, 20, 32, 48). Beta-chemokines are the natural ligands for the major coreceptor, CCR5, of HIV and SIV. Beta-chemokines are produced at sites of inflammation, where they are involved in the recruitment of T cells and macrophages (13, 33, 36, 44), but the net effect of beta-chemokine secretion by CD8+ T cells in lentiviral infections is unclear. Some studies have shown that members of the beta-chemokine family can block HIV and SIV replication (12, 20), but others have concluded that CD8+-T-cell-mediated inhibition of viral replication in vitro is due to soluble factors other than beta-chemokines (10, 11, 31, 35).
In the SIV-rhesus monkey model of HIV infection, a variety of vaccine modalities have produced various levels of protection against a challenge with pathogenic SIV or simian/human immunodeficiency virus (SHIV). However, live attenuated vaccines have consistently provided the most effective protection against a challenge with virulent SIV and they stimulate a broad range of antiviral immune responses in rhesus macaques (1, 21). Many studies have found an association between the in vitro secretion of beta-chemokines by peripheral blood mononuclear cells (PBMC) and vaccine-mediated protection from SIV or SHIV challenge (3, 5, 24, 29, 30). However, recent studies have shown that high beta-chemokine levels correlate with the disease progression of SIV and HIV infections (25, 27). It is worth noting that although SIVmac239 and SIVmac251 utilize CCR5 as their primary coreceptor, they can also use alternate coreceptors in vitro (8, 15, 17). Despite this ability to use alternate coreceptors in vitro, in vivo CCR5 seems to be the only coreceptor of consequence. Beta-chemokine expression by CD8+ T cells in vitro has been associated with protection against a SIVmac251 or SIVmac239 challenge (4, 20, 28-30). Furthermore, systemic therapy with beta-chemokine homologues decreases SIVmac251 replication in rhesus monkeys (47). Thus, CCR5 is critical for SIV replication in vivo, and a clarification of the role of beta-chemokines in HIV and SIV replication in vivo is needed.
For chronic HIV and SIV infections, CD8+-T-cell expansion in the lymphoid tissues is well documented (16, 22, 42). However, the relationship between the CD8+-T-cell expansion and the elaboration of effective antiviral immunity is not clear. Furthermore, the relationship between CD8+-T-cell proliferation, beta-chemokine expression, and viral replication is undefined.
In an earlier study, members of our laboratory showed that approximately 60% of rhesus monkeys inoculated with attenuated SHIV89.6 were protected from uncontrolled viral replication and disease after an intravaginal challenge with pathogenic SIVmac239 (1, 34). Strong antiviral CTL responses and alpha interferon expression in PBMC during the acute postchallenge period were associated with protection. However, in vitro suppression by CD8+ T cells in PBMC and beta-chemokine expression in PBMC were not associated with vaccine-mediated protection during the acute postchallenge phase (1). Analyses of beta-chemokine expression in PBMC may not reflect the conditions in the lymphoid organs, the major sites of SIV and HIV replication (19, 37, 38, 45). Thus, the analysis of beta-chemokine responses in lymphoid tissues is needed for an understanding of how beta-chemokine expression influences SIV replication in vivo.
The goal of the present study was to analyze and define the in vivo relationship between the frequency of CD8+ T cells expressing beta-chemokines, the level of CD8+-T-cell proliferation, and viral RNA (vRNA) levels in the lymph nodes of SHIV89.6-vaccinated and protected, SHIV89.6-vaccinated but unprotected, and unvaccinated animals 6 months after an SIV challenge (or initial infection) with SIVmac239. We found that the highest levels of macrophage inflammatory protein 1 alpha (MIP-1α), MIP-1β, and Ki67 expression were in the lymphoid tissues with the highest vRNA levels. Thus, vaccinated, protected animals had relatively few proliferating or beta-chemokine-secreting CD8+ T cells compared to vaccinated, unprotected monkeys. Furthermore, the difference in Ki67 and beta-chemokine expression levels by CD8+ T cells in the lymph nodes of vaccinated, protected and vaccinated, unprotected monkeys was statistically significant. Thus, there was no evidence that beta-chemokines contribute to the long-term control of virus replication in SIV infection even in a postvaccination setting.
MATERIALS AND METHODS
Animals, SHIV immunization, and SIV challenge.
The monkeys used for this study were multiparous, mature, female rhesus monkeys (Macaca mulatta) housed at the California National Primate Research Center in accordance with USDA regulations and American Association for Accreditation of Laboratory Animal Care standards. All animals were negative for antibodies to HIV type 2, SIV, type D retrovirus, and simian T-cell lymphotropic virus type 1 at the time the study was initiated. The rhesus monkeys used for this study were part of a previously published vaccine study (1). A randomly selected subgroup of 31 monkeys from the original group of 42 was included in this study. Thirty-one monkeys were inoculated with live, virulence-attenuated SHIV89.6 by one of the following routes: intravenous (n = 10), intranasal (n = 5), or intravaginal (n = 16) (1). In addition, unvaccinated monkeys were included in the study as challenge controls (n = 9). SHIV89.6-immunized or unvaccinated monkeys were challenged with at least two intravaginal inoculations of SIVmac239 (1 ml at 105 50% tissue culture infective doses/ml). This virus stock was produced in rhesus PBMC and was described previously (1).
Categorization of vaccinated monkeys by challenge outcome.
After the intravaginal SIV challenge, the efficacy of the vaccine was determined by assessing the vRNA levels in the plasma of the study animals, and vaccinated animals were categorized as either protected or unprotected (1). At all time points during the 6-month follow-up period, vaccinated, protected animals had plasma vRNA levels of <104 copies/ml, while vaccinated, unprotected animals had plasma vRNA levels of >104 copies/ml at least once (1, 2). Thus, 20 of the 31 vaccinated monkeys were considered protected and 11 of the vaccinated monkeys were considered unprotected (Table 1) (1). It is important to note that even for animals with undetectable plasma vRNA, vRNA was readily detectable in lymphoid tissues at necropsy (Table 1) (2). As previously described, the vRNA levels in the axillary and genital lymph nodes at 6 months postchallenge (p.c.) were significantly lower in the protected group than in the unprotected group (P < 0.001) (2). The highest vRNA levels were in the axillary and genital lymph nodes of the unvaccinated group, although the vRNA levels were not significantly different from those of the unprotected group. At 6 months p.c., there was a strong correlation between the vRNA levels in lymphoid tissues and the plasma vRNA level for individual monkeys (Table 1) (2). Thus, the relative tissue vRNA levels among the animals were consistent with the classification of vaccinated, protected animals versus vaccinated, unprotected animals based on plasma vRNA levels (2).
TABLE 1.
Plasma and tissue vRNA levels and lymph node histopathology in SHIV89.6-vaccinated, SIVmac239-challenged monkeys at 6 months p.c.
| Animal no. or group | Plasma vRNA level (log10 copies/ml) | Lymph node vRNA level (log10 copies/μg of RNA)b
|
Lymph node follicular hyperplasia scorea
|
||
|---|---|---|---|---|---|
| Axillary | Genital | Axillary | Genital | ||
| Protected | |||||
| 22131 | <2.6 | 3.7 | 2.4 | 1 | 1 |
| 23478 | <2.6 | 3.8 | 2.2 | 1 | 1 |
| 24251 | <2.6 | 3.9 | 3.1 | 1 | 1 |
| 25334 | <2.6 | 3.1 | 3.8 | 1 | 1 |
| 25409 | <2.6 | 3.3 | 2.5 | 1 | 1 |
| 25594 | <2.6 | 2.4 | * | 1 | 0 |
| 25759 | <2.6 | 2.3 | 4.2 | 0 | 0 |
| 25979 | <2.7 | 4.3 | 3.6 | 0 | 1 |
| 26640 | <2.7 | 2.1 | 2.1 | 1 | 1 |
| 27334 | <2.7 | 3.8 | 2.3 | 1 | 1 |
| 27608 | <2.7 | * | 2.5 | 0 | 1 |
| 27685 | 4.0 | 4.4 | 3.7 | 0 | 1 |
| 28055 | <2.7 | 1.6 | 4.7 | 2 | 1 |
| 28075 | 3.3 | 3.0 | 3.2 | 1 | 1 |
| 28229 | <2.7 | 3.1 | 4.0 | 1 | 1 |
| 28288 | <2.7 | 2.2 | 2.6 | 1 | 1 |
| 30443 | <2.6 | 3.0 | * | 1 | 1 |
| 31408 | <2.7 | 3.2 | * | 1 | 1 |
| 31420 | <2.7 | 3.7 | 2.9 | 1 | 1 |
| 31431 | <2.7 | 2.2 | 2.5 | 1 | 1 |
| Mean ± SE for group | 2.8 ± 0.07 | 3.1 ± 0.18 | 3.1 ± 0.19 | 0.85 ± 0.11 | 0.90 ± 0.07 |
| Unprotected | |||||
| 23699 | 4.6 | 4.5 | 3.2 | 2 | 2 |
| 24196 | 5.0 | 4.6 | 4.8 | 3 | 3 |
| 26154 | 5.5 | 5.4 | 5.3 | 2 | 1 |
| 28408 | 2.7 | 4.5 | 3.6 | 3 | 3 |
| 30445 | 4.0 | 5.2 | 4.9 | 1 | 1 |
| 30470 | 4.3 | 5.0 | 4.2 | 2 | 2 |
| 30474 | 6.4 | 6.3 | 6.6 | 3 | 3 |
| 31411 | 7.1 | 6.3 | 6.1 | 2 | 2 |
| 31413 | 5.4 | 6.2 | 6.1 | 3 | 3 |
| 31416 | 6.1 | 5.8 | 5.8 | 2 | 2 |
| 31434 | 3.8 | 4.2 | 4.2 | 1 | 1 |
| Mean ± SE for group | 5.0 ± 0.39 | 5.3 ± 0.24 | 5.0 ± 0.33 | 2.2 ± 0.23 | 2.1 ± 0.25 |
| Naive | |||||
| 23756 | 8.1 | 6.6 | 6.8 | 2 | 3 |
| 25301 | 6.3 | 5.6 | 5.5 | 2 | 2 |
| 25402 | 2.8 | 3.4 | * | 1 | 1 |
| 25537 | 5.9 | 6.1 | 6.1 | 3 | 3 |
| 27764 | 6.0 | 5.6 | 5.7 | 3 | 3 |
| 28048 | 4.4 | 5.2 | 5.2 | 3 | 3 |
| 28433 | 6.9 | 5.6 | 5.6 | 3 | 3 |
| 31423 | 3.4 | 4.1 | 3.7 | 3 | 3 |
| 31435 | 6.4 | 6.0 | 6.3 | 2 | 2 |
| Mean ± SE for group | 5.6 ± 0.57 | 5.4 ± 0.34 | 5.6 ± 0.33 | 2.4 ± 0.24 | 2.6 ± 0.24 |
0, normal; 1, mild; 2, moderate; 3, severe.
*, below detection.
Tissue samples.
Six months after an intravaginal challenge with SIVmac239, the monkeys were euthanized by a phenobarbitol overdose, and blood and tissues were collected as previously described (26). Briefly, blood and axillary and genital (obturator and iliac) lymph nodes were collected at the time of necropsy and divided into several fractions. A portion of each lymph node sample was placed in RNA-later (Ambion, Austin, Tex.) and stored at −20°C. Additionally, tissue samples were immersion fixed in 10% buffered formalin and 4% paraformaldehyde, and frozen samples were stored at −80°C until RNA isolation. Total RNAs were isolated as previously described (1). Briefly, total RNAs were isolated by the use of Trizol (Invitrogen, Grand Island, N.Y.) according to the manufacturer's protocol. Prior to RNA isolation, the tissue samples were homogenized with a power homogenizer (Generator [7 mm by 195 mm]; Fisher Scientific). All samples were treated with DNA-free (Ambion) DNase for 1 h at 37°C. cDNAs were prepared with random hexamer primers (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.) and Moloney murine leukemia virus reverse transcriptase (Invitrogen).
SIV vRNA quantitation.
Total RNAs isolated from lymphoid tissues were analyzed for vRNA by a quantitative branched DNA assay (14). Tissue vRNA levels are reported as viral RNA copy numbers per 1 μg of total tissue RNA. The detection limit of this assay is 500 copies.
Beta-chemokine analysis.
Previous studies have found an association between CD8+-T-cell beta-chemokine expression and vaccine-induced protection against an SIV challenge. In these studies, protection was associated with changes in the levels of all three beta-chemokines, namely RANTES, MIP-1α, and MIP-1β (4-6, 20, 28-30). Thus, to simplify the present study, we chose to focus our mRNA analysis on two of the three beta-chemokines, MIP-1α and MIP-1β.
(i) Reverse transcriptase quantitative PCR for MIP-1α and MIP-1β mRNA.
RNA isolation, cDNA preparation, and real-time PCR were performed as previously described. cDNAs were prepared with random hexamer primers (Amersham Pharmacia Biotech, Inc.) and Moloney murine leukemia virus reverse transcriptase (Invitrogen). The oligonucleotide primer and probe sequences for MIP-1α and MIP-1β were designed specifically for the TaqMan assay. The probes (Applied Biosystems) were labeled at the 3′ end with TAMRA (6-carboxytetramethylrhodamine) and at the 5′ end with FAM (6-carboxyfluorescein), except the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) probe, which was labeled with VIC at the 5′ end. The beta-chemokines MIP-1α and MIP-1β were run in multiplex assays in which the beta-chemokine gene and the GAPDH sequence were amplified in the same tube. Primer concentrations were adjusted to 60 nM for GAPDH and 900 nM for the chemokine gene. The reactions were set up in a 96-well optical plate (Applied Biosystems, Foster City, Calif.) in a 25-μl reaction volume containing 5 μl of cDNA and 20 μl of master mix (Applied Biosystems). All sequences were amplified with the model 7700 instrument's default amplification program: 2 min at 50°C and 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. The results were analyzed with SDS 7700 system software, version 1.6.3 (Applied Biosystems) on a G4 Macintosh computer (Apple Computer, Cupertino, Calif.). Beta-chemokine mRNA expression levels were calculated from delta threshold cycle (ΔCt) values and are reported as fold increases (FI) of beta-chemokine mRNA levels in SIV/SHIV-infected lymph nodes compared to those in lymph nodes from control samples (see below). Each Ct value corresponds to the cycle number at which the fluorescence due to enrichment of the PCR product reaches significant levels above the background fluorescence (threshold). For this analysis, the Ct value for the housekeeping gene (GAPDH) was subtracted from the Ct value for the target (beta-chemokine) gene. The ΔCt value for the SHIV/SIV-infected sample was then subtracted from the ΔCt value for the corresponding uninfected sample (ΔΔCt). Assuming that the target gene (beta-chemokine) and the reference gene (GAPDH) were amplified with the same efficiencies (data not shown), the FI in the mRNA level in a SHIV/SIV-infected sample compared to that in an uninfected sample is then calculated by the formula FI = 2 − ΔΔCt (ABI Prism 7700 sequence detection system user bulletin 2, Applied Biosystems).
(ii) Immunohistochemistry for MIP-1α, MIP-1β, and RANTES proteins.
For an immunohistochemical analysis of beta-chemokine expression, polyclonal antisera against MIP-1α (clone HP9010, rabbit polyclonal antiserum; HBT, Cell Sciences, Norwood, Mass.), MIP-1β (clone AF271, goat polyclonal antiserum; R&D Systems, Minneapolis, Minn.), and RANTES (clone AB278, goat polyclonal antiserum; R&D Systems) were used on formalin-fixed, paraffin-embedded tissue samples. Briefly, embedded tissues were sectioned into 4-μm-thick sections, placed on Starfrost precleaned microscope slides (Fisher), deparaffinized, and dehydrated. Antigen retrieval to expose epitopes masked by fixation was achieved by high-temperature microwave retrieval in a 1:10 dilution of AR10 solution (Biogenex, San Ramon, Calif.) followed by three washes with phosphate-buffered saline (PBS) (Sigma-Aldrich, St. Louis, Mo.). The sections were then treated with 0.3% hydrogen peroxide (EM Science, Gibbstown, N.J.) to quench endogenous peroxide activity. After a wash in PBS, nonspecific antibody binding sites were blocked with 10% normal goat or horse serum (Vector Labs, Burlingame, Calif.) at room temperature. Next, the antibody was diluted to a concentration of 4 μg of total immunoglobulin G (IgG)/ml in Hanks balanced salt solution (HBSS) (Invitrogen) supplemented with 0.1% saponin (Sigma-Aldrich), 0.01 M HEPES buffer (Roche, Indianapolis, Ind.), and 0.002% sodium azide (Sigma-Aldrich), and this was applied to tissue sections. The sections were incubated overnight at room temperature. The slides were then washed three times (for 5 min each) with an HBSS-0.1% saponin solution. Subsequently, biotinylated goat anti-rabbit IgG (Zymed, South San Francisco, Calif.) or horse anti-goat IgG (Vector Labs) and streptavidin (Zymed) were added in sequence. DAB (Vector) was used as a chromogen and Harris hematoxylin (Fisher) was used as a counterstain. Rabbit IgG (Vector), goat IgG (R&D), and the incubation of tissue sections with the omission of a primary or secondary antibody were used as negative controls.
Immunofluorescence.
To define the proportion of CD8+ T cells that expressed MIP-1α, MIP-1β, or Ki67, we performed a double-labeling immunofluorescence analysis with formalin-fixed, paraffin-embedded tissues sectioned into 4-μm-thick sections. Since the expression of Ki67 is strongly correlated to proliferating cells (39), this marker was used to assess the extent of CD8+-T-cell proliferation in this study. The first primary antibody, mouse monoclonal anti-human CD8 (clone NCL CD8 295; Vector) diluted to a concentration of 4 μg/ml in PBS (Sigma-Aldrich), was applied to tissue sections and incubated overnight at 4°C. The tissues were then washed three times (for 5 min each) in PBS. The first secondary antibody, goat anti-mouse IgG labeled with Alexa fluor 488 (Molecular Probes, Eugene, Oreg.), was diluted 1:200 in PBS and incubated for 30 min at 37°C. The tissues were then washed three times (for 10 min each) in HBSS (Invitrogen) supplemented with 0.1% saponin (Sigma-Aldrich), and the second primary antibody (anti-human MIP-1α, anti-human MIP-1β, or anti-human Ki67 [DakoCytomation Inc., Carpinteria, Calif.]) was diluted to a concentration of 4 μg of total IgG/ml in HBSS (Invitrogen) supplemented with 0.1% saponin (Sigma-Aldrich), 0.01 M HEPES buffer (Roche), and 0.002% sodium azide (Sigma-Aldrich), applied to tissue sections, and incubated overnight at room temperature. After three washes with HBSS supplemented with 0.1% saponin (Sigma-Aldrich) (for 10 min each), the second secondary antibody, goat anti-rabbit IgG or donkey anti-goat IgG labeled with Alexa fluor 568 (Molecular Probes), was diluted 1:400 in HBSS (Invitrogen) supplemented with 0.1% saponin (Sigma-Aldrich), applied to slides, and incubated for 30 min at 37°C. The tissues were then washed three times (for 10 min each) in HBSS (Invitrogen) supplemented with 0.1% saponin (Sigma-Aldrich). Finally, the slides were incubated with 4′,6-diamidino-2-phenylindole dihydrochloride hydrate (DAPI) (Molecular Probes) to label the chromosomal DNA. Mouse isotype controls or a purified, nonimmune rabbit or donkey serum was run with each staining series as the negative control.
Quantitative image analysis.
Immunofluorescent signals for fluorochrome-labeled CD8, MIP-1α, MIP-1β, and Ki67 antibodies were quantified with a Hamamatsu digital camera mounted on a Zeiss (Jena, Germany) microscope fitted with a ×40 plan neofluor objective and a polarizing filter cube with appropriate filters (Omega Optical, Brattleboro, Vt.). Digital images were captured with Openlab software (Improvision, Inc., Lexington, Mass.). One section, with representative histomorphologic components (cortex, paracortex, follicles, and medulla), per lymph node was analyzed. Five high-power (×40) microscope fields of the T-cell-rich zone (paracortex) per tissue section were randomly chosen, and images were captured digitally with the system described above. Each captured field included an area of approximately 0.04 mm2. Only cells with distinctly labeled nuclei (DAPI) and bright cytoplasmic staining covering >60% of continuous cell cytoplasm were considered positive.
Individual positive cells in the five captured high-power microscope fields of the immunohistochemical tissue sections were counted manually by a single observer by using a randomly placed square-millimeter grid system. The numbers of positive cells are presented as cells per square millimeter.
Statistical analysis.
All statistical analyses were done with InStat software (Graph Pad Software Inc., San Diego, Calif.). All data were analyzed by one-way analysis of variance with posthoc Tukey comparisons. Data from the beta-chemokine mRNA and protein analyses were log transformed prior to analysis.
RESULTS
Pathological changes and CD8+-T-cell proliferation in lymph nodes.
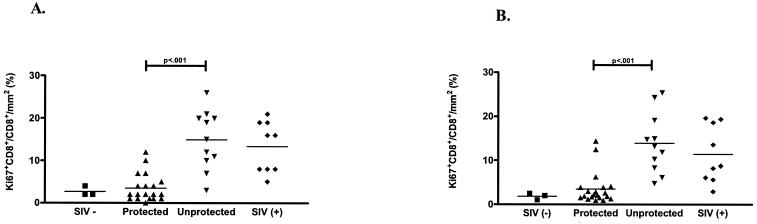
To determine if changes in lymph node morphology reflected the challenge outcome, we evaluated the axillary and genital lymph nodes for histopathologic abnormalities by using standard techniques. The lesions were subjectively graded on a scale of 0 to 3 (0, normal; 1, mild; 2, moderate; 3, severe). At 6 months post-SIV challenge, the majority of vaccinated but unprotected and unvaccinated animals (82 and 88%, respectively) had moderate to severe follicular hyperplasia and paracortical expansion in both the axillary and genital lymph nodes (Table 1). In contrast, the majority (90%) of the protected animals had normal follicles or mild follicular hyperplasia and mild to moderate paracortical expansion (Table 1). Mild to moderate lymph node medullary histiocytosis was present in both protected and unprotected animals. To determine whether proliferating CD8+ T cells contributed to this pathology, we evaluated the number of Ki67-positive CD8+ T cells in the axillary and genital lymph nodes. The vaccinated but unprotected and unvaccinated animals had significantly increased frequencies of Ki67+ CD8+ T cells compared to the vaccinated, protected animals (Fig. 1). Thus, vaccinated, protected and vaccinated, unprotected animals could be reliably distinguished based on their lymph node tissue vRNA levels, Ki67 expression in CD8+ T cells, and histopathology at 6 months post-SIV challenge.
FIG. 1.
Density of Ki67+ CD8+ T cells in the paracortex of axillary (A) and genital (B) lymph nodes of rhesus macaques 6 months after intravaginal challenge with SIVmac239. Note that the frequency of Ki67+ CD8+ T cells was higher for the vaccinated but unprotected and unvaccinated animals than for the vaccinated, protected animals. Individual monkeys from the uninfected (SIV−) (▪), vaccinated and protected (▴), vaccinated but unprotected (▾), and unvaccinated (SIV+) (♦) groups are represented. The horizontal lines represent the mean frequencies of Ki67+ CD8+ T cells for each experimental group.
Beta-chemokine mRNA levels in lymphoid tissues.
MIP-1α and MIP-1β mRNA expression levels in the lymphoid tissues were determined at 6 months postchallenge by TaqMan PCR. The MIP-1α mRNA expression levels were significantly (P < 0.05) increased in the axillary and genital lymph nodes in the vaccinated, unprotected animals compared to those in the vaccinated, protected animals (Fig. 2). While the MIP-1β mRNA levels had a similar trend, this difference did not reach the level of statistical significance (Fig. 2). It should be noted that MIP-1β mRNA levels were elevated in the genital lymph nodes for all three groups compared to those for uninfected animals. Furthermore, there was a positive linear correlation between the level of beta-chemokine mRNA expression and tissue vRNA levels (Fig. 3).
FIG. 2.
MIP-1α and MIP-1β mRNA levels in axillary (A) and genital (B) lymph nodes at 6 months p.c. Note that beta-chemokine levels were higher in the vaccinated but unprotected and unvaccinated animals than in the vaccinated, protected animals. The increase in MIP-1α and MIP-1β mRNA levels relative to those in uninfected animals (SIV−) (▪) are shown for vaccinated and protected (▴), vaccinated but unprotected (▾), and unvaccinated (SIV+) (♦) monkeys. The horizontal lines represent the mean MIP-1α and MIP-1β mRNA levels for each experimental group.
FIG. 3.
Regression analysis of beta-chemokine mRNA levels or numbers of protein-positive cells versus vRNA levels in axillary lymph nodes. Note the positive correlation between vRNA levels, both MIP-1α and MIP-1β mRNA levels, and the numbers of beta-chemokine-positive cells. Each symbol represents an individual monkey.
Localization of beta-chemokine proteins in lymphoid tissues.
To confirm the mRNA data and to determine the origin and total number of beta-chemokine-producing cells in the axillary and genital lymph nodes, we used immunohistochemistry and immunofluorescence techniques. Consistent with the mRNA levels, the numbers of cells expressing MIP-1α and MIP-1β proteins were increased in the lymph nodes of all three groups compared to those for uninfected animals (Fig. 4). Furthermore, the differences between the numbers of beta-chemokine-positive cells in the lymph nodes of the vaccinated, protected and vaccinated, unprotected animals were significant (Fig. 4). A similar trend was seen in the numbers of RANTES-positive cells. Protected monkeys had significantly fewer RANTES-positive cells than did unprotected monkeys (data not shown). The frequency of non-CD8+ T cells that expressed beta-chemokines was higher than the frequency of beta-chemokine-secreting CD8+ T cells (data not shown). Thus, antiviral CD8+ T cells were not the main source of beta-chemokine expression in lymph nodes. Furthermore, the frequencies of MIP1α+ CD8+ and MIP-1β+ CD8+ lymphocytes in the lymph nodes of the vaccinated, unprotected animals were significantly higher than those in the lymph nodes of vaccinated, protected animals (Fig. 5). Thus, the numbers of beta-chemokine-positive CD8+ T cells increased with increasing tissue vRNA levels, suggesting that viral replication drives beta-chemokine expression in CD8+ T cells. The majority of the beta-chemokine-positive CD8+ lymphocytes were located in the T-cell-rich paracortical regions of the lymph nodes (Fig. 6).
FIG. 4.
Densities of MIP-1α- and MIP-1β-positive cells in the paracortex of axillary (A) and genital (B) lymph nodes. Consistent with increased beta-chemokine mRNA levels at 6 months p.c., the vaccinated, unprotected animals had increased numbers of MIP-1α- and MIP-1β-expressing cells in axillary and genital lymph nodes than did the vaccinated, protected animals. Individual monkeys from the uninfected (SIV−) (▪), vaccinated and protected (▴), vaccinated but unprotected (▾), and unvaccinated (SIV+) (♦) groups are represented. The horizontal lines represent the mean numbers of MIP-1α- and MIP-1β-positive cells for each experimental group.
FIG. 5.
Densities of beta-chemokine-positive CD8+ T cells in the paracortex of axillary (A) and genital (B) lymph nodes at 6 months p.c. Note that the number of CD8+ T cells that coexpressed MIP-1α and MIP-1β was higher in vaccinated but unprotected and unvaccinated animals than in vaccinated, protected animals. The symbols are as described in the legend for Fig. 1.
FIG. 6.
Immunohistochemistry for CD8 and MIP-1α in the axillary and genital lymph nodes at 6 months p.c. Note that (i) the number of MIP-1α+ cells and MIP-1α+CD8+ cells was higher in the vaccinated, unprotected monkey than in the vaccinated, protected monkey and (ii) the largest proportion of these cells was present in the paracortex. Original magnification, ×40.
In summary, 6 months after an SIV challenge, beta-chemokine mRNA levels and the numbers of cells expressing MIP-1α and MIP-1β proteins were highest in the lymph nodes of vaccinated monkeys with the highest vRNA levels. Thus, beta-chemokine expression, whether by CD8+ T cells or other cell types, was not associated with vaccine-mediated protection, as beta-chemokine mRNA levels, the numbers of total beta-chemokine-positive cells, and the numbers of beta-chemokine-positive CD8+ T cells were significantly higher for the vaccinated, unprotected group than for the vaccinated, protected group.
DISCUSSION
The goal of this study was to investigate the relationship between lymph node vRNA levels, histologic abnormalities, CD8+-T-cell proliferation, and beta-chemokine expression in SHIV89.6-immunized rhesus macaques 6 months after an intravaginal challenge with pathogenic SIVmac239. Lymphoid tissue vRNA levels had a strong, linear correlation with plasma vRNA levels in these animals (2), and thus vaccinated, protected and vaccinated, unprotected animals could be distinguished by their lymph node vRNA levels. Furthermore, the level of CD8+-T-cell proliferation and the extent and nature of lymph node histopathology also correlated with lymph node vRNA levels, and these parameters were significantly different in vaccinated, protected and vaccinated, unprotected animals. High lymph node vRNA levels also corresponded with increased levels of MIP-1α and MIP-1β. Although there were CD8+ T cells expressing MIP-1α or MIP-1β, the frequency of these cells correlated negatively with the levels of protection. The frequency of proliferating CD8+ T cells was significantly higher in the lymph nodes of vaccinated but unprotected and unvaccinated monkeys than in those of vaccinated, protected monkeys. These results are consistent with the conclusion that beta-chemokine expression by cells, including CD8+ T cells and non-CD8+ T cells, is a feature of the immune activation and inflammation associated with robust SIV replication, as was previously proposed (2).
In the present study, beta-chemokine expression levels (both protein and mRNA) in the lymph nodes consistently and significantly correlated with tissue vRNA levels (Fig. 3). The axillary and genital lymph nodes of vaccinated, unprotected animals had increased MIP-1α and MIP-1β mRNA expression levels, increased numbers of beta-chemokine-positive cells, and increased numbers of beta-chemokine-positive CD8+ T cells compared to vaccinated, protected animals. Thus, the beta-chemokine-mediated control of viral replication that occurs in PBMC culture systems in vitro does not seem to play a role in vaccine outcomes. This finding contrasts with those of other studies that have reported an association between in vitro beta-chemokine secretion by CD8+ T cells in PBMC and vaccine-mediated protection against a SIV/SHIV challenge (3, 6, 7, 18, 21, 24, 29, 30). The conclusions of these studies were based on in vitro assays using mitogen stimulation. Perhaps in vitro beta-chemokine secretion in response to mitogen stimulation is a marker for a population of CD8+ T cells that produce a different set of antiviral effector molecules in vivo during SIV/HIV infections. Our results are consistent with previous studies demonstrating that increased in vivo levels of MIP-1α and MIP-1β are associated with increased viral replication and disease progression (25, 27, 43, 46). Importantly, at 6 months postchallenge, the beta-chemokine levels in PBMC did not reflect the beta-chemokine levels in lymph nodes. In PBMC at 6 months postchallenge, the beta-chemokine levels were near the baseline and there was no difference between the levels among vaccinated, protected, vaccinated but unprotected, and unvaccinated animals (1). Thus, an analysis of beta-chemokine responses in lymph nodes is important for determining the relationship of these molecules to viral replication in vivo.
The recruitment and proliferation of virus-specific CD8+ T cells in viral target tissues are critical for an effective antiviral host immune response. In chronic HIV and SIV infections, CD8+-T-cell expansion in the lymphoid tissues likely represents the recruitment and proliferation of both virus-specific T cells and bystander T cells (16). Consistent with lymph nodes of HIV-infected humans (42), the majority of the beta-chemokine-secreting cells in monkey lymph nodes were not CD8+ T cells but were associated with the areas of paracortical expansion and follicular hyperplasia. It was previously reported that the lack of protection in these same SHIV89.6-vaccinated animals was associated with an increased expression of the proinflammatory gamma interferon-inducible chemokines CXCL10/IP-10 and CXCL9/Mig in lymph nodes (2). Thus, beta-chemokine secretion is likely part of a nonspecific, proinflammatory response associated with viral replication. This inflammation leads, on balance, to increased viral replication, perhaps by promoting the accumulation of target CD4+ T cells in lymph nodes.
Relatively high lymphoid tissue vRNA levels were associated with abnormalities in lymph node architecture and CD8+-T-cell proliferation. The lymph nodes of vaccinated, unprotected animals and unvaccinated controls had marked lymphadenopathy characterized by lymphofollicular hyperplasia, dysplasia, and paracortical expansion compared to the vaccinated, protected animals. The paracortical expansion was consistent with the large increase in Ki67+ CD8+ T cells in these lymph nodes. Thus, the extent of paracortical expansion and the density of Ki67+ CD8+ T cells correlated with the challenge outcome. The lymph nodes of vaccinated, unprotected animals had a significantly higher frequency of Ki67+ CD8+ T cells than did those of vaccinated, protected animals. Although there was marked CD8+-T-cell proliferation in the lymph nodes of vaccinated, unprotected animals, there was no difference in the level of expression of caspase 3, a marker of apoptosis, between the two groups (data not shown). Thus, CD8+ T cells in the lymph nodes of vaccinated, unprotected animals proliferated but did not undergo detectable apoptosis. This conclusion is consistent with the growing body of evidence that the expansion of CD8+ T cells in the lymph nodes during HIV and SIV infections is due to a combination of proliferating virus-specific T cells and nonspecific T cells and to lymphocyte homing and recirculation abnormalities (16, 23). Taken together, our results suggest that the lymph node paracortical expansion was due to the recruitment of lymphocytes by beta-chemokines and other chemoattractants combined with increased cell proliferation but no detectable cell death.
The goal of our study was to better understand the relative contributions of beta-chemokine secretion and CD8+-T-cell proliferation to the control of viral replication in the context of vaccination. There was no evidence of CD8+-T-cell-mediated control of SIV replication by beta-chemokine expression in vivo. The marked lymphadenopathy and CD8+-T-cell proliferation found in vaccinated, unprotected monkeys were consistent with chronic reactivity due to viral replication. Furthermore, the increased levels of beta-chemokines were associated with high levels of gamma interferon mRNA expression in vaccinated, unprotected animals (2), and all of these molecules contribute to nonspecific inflammation and immune activation. Beta-chemokines in the lymph nodes of vaccinated, unprotected animals were not produced by antiviral CD8+ T cells, but rather by non-CD8+ T cells. Thus, beta-chemokine secretion by non-CD8+ T cells may contribute to the immunopathogenesis of HIV/SIV infections, as viral replication results in an increased beta-chemokine secretion that draws potential target T cells into lymphoid tissues to support further viral replication. It is probably important that the natural hosts of SIV, African green monkeys and sooty mangabeys, have high viral loads but little associated inflammation or immune activation (9, 41), and these monkeys do not develop AIDS. In fact, the lack of immune activation and cell cycle dysregulation in the sooty mangabey may explain why these animals do not develop AIDS (M. Paiardini, B. Cervasi, G. Constantino, B. Sumpter, H. McClure, S. O'Neill, M. Magnani, S. Staprans, G. Piedimonte, M. Feinberg, and G. Silvestri, Abstr. AIDS Vaccine Meet., abstr. 403, 2003). Together, these results indicate that a successful HIV vaccine must generate robust specific and innate antiviral immunity and allow the recipient to make adaptive immune responses without the immune activation and inflammation that contributes to viral replication.
Acknowledgments
We thank Don Canfield, Sona Santos, Anne Canfield, Katherine Lantz, Blia Vang, Rino Dizon, Sajjad Khan, the Immunology Care Laboratory, and the California National Primate Research Center Colony Services for their expert technical assistance.
This work was supported by Public Health Science grants RR00169 and RR14555 from the National Center for Research Resources and by grant AI44480 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Abel, K., L. Compton, T. Rourke, D. Montefiori, D. Lu, K. Rothaeusler, L. Fritts, K. Bost, and C. J. Miller. 2003. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J. Virol. 77:3099-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abel, K., L. LaFranco-Scheuch, T. Rourke, Z.-M. Ma, V. de Silva, B. Fallert, L. Beckett, T. A. Reinhart, and C. J. Miller. 2004. Gamma interferon-mediated inflammation is associated with lack of protection from intravaginal SIVmac239 challenge in simian-human immunodeficiency virus 89.6-immunized rhesus macaques. J. Virol. 78:841-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed, R. K., B. Makitalo, K. Karlen, C. Nilsson, G. Biberfeld, and R. Thorstensson. 2002. Spontaneous production of RANTES and antigen-specific IFN-gamma production in macaques vaccinated with SHIV-4 correlates with protection against SIVsm challenge. Clin. Exp. Immunol. 129:11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed, R. K., C. Nilsson, G. Biberfeld, and R. Thorstensson. 2001. Role of CD8+ cell-produced anti-viral factors in protective immunity in HIV-2-exposed but seronegative macaques resistant to intrarectal SIVsm challenge. Scand. J. Immunol. 53:245-253. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed, R. K., C. Nilsson, Y. Wang, T. Lehner, G. Biberfeld, and R. Thorstensson. 1999. β-Chemokine production in macaques vaccinated with live attenuated virus correlates with protection against simian immunodeficiency virus (SIVsm) challenge. J. Gen. Virol. 80:1569-1574. [DOI] [PubMed] [Google Scholar]
- 6.Aubertin, A., R. LeGrand, Y. Wang, C. Beyer, L. Tao, O. Neildez, F. Barre-Sinoussi, B. Hurtrel, C. Moog, T. Lehner, and M. Girard. 2000. Generation of CD8+ T cell-generated suppressor factor and β-chemokines by targeted iliac lymph node immunization in rhesus monkeys challenged with SHIV-89.6P by the rectal route. AIDS Res. Hum. Retrovir. 16:381-392. [DOI] [PubMed] [Google Scholar]
- 7.Babaahmady, K., L. A. Bergmeier, T. Whittall, M. Singh, Y. Wang and T. Lehner. 2002. A comparative investigation of CC chemokines and SIV suppressor factors generated by CD8+ and CD4+ T cells and CD14+ monocytes. J. Immunol. Methods 264:1-10. [DOI] [PubMed] [Google Scholar]
- 8.Borsetti, A., C. Parolin, B. Ridolfi, L. Sernicola, A. Geraci, B. Ensoli, and F. Titti. 2000. CD4-independent infection of two CD4− CCR5− CXCR4+ pre-T-cell lines by human and simian immunodeficiency viruses. J. Virol. 74:6689-6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broussard, S. R., S. I. Staprans, R. White, E. M. Whitehead, M. B. Feinberg, and J. S. Allan. 2001. Simian immunodeficiency virus replicates to high levels in naturally infected African green monkeys without inducing immunologic or neurologic disease. J. Virol. 75:2262-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, Y., and P. Gupta. 1996. CD8+ T-cell-mediated suppression of HIV-1 infection may not be due to chemokines RANTES, MIP-1a and MIP-1b. AIDS 10:1434-1435. [DOI] [PubMed] [Google Scholar]
- 11.Clerici, M., C. Balotta, D. Trabattoni, L. Papagno, S. Ruzzante, S. Rusconi, M. L. Fusi, M. C. Colombo, and M. Galli. 1996. Chemokine production in HIV-seropositive long-term asymptomatic individuals. AIDS 10:1432-1433. [DOI] [PubMed] [Google Scholar]
- 12.Cocchi, F., A. L. DeVico, A. Garzino-Demo, S. K. Arya, R. C. Gallo, and P. Lusso. 1995. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811-1815. [DOI] [PubMed] [Google Scholar]
- 13.Cyster, J. 1999. Chemokines and cell migration in secondary lymphoid organs. Science 286:2098-2102. [DOI] [PubMed] [Google Scholar]
- 14.Dailey, P. J., M. Zamround, R. Kelso, J. Kolberg, and M. Urdea. 1995. Quantitation of simian immunodeficiency virus (SIV) RNA in plasma of acute and chronically infected macaques using a branched DNA (bDNA) signal amplification assay. J. Med. Primatol. 24:209. [Google Scholar]
- 15.Deng, H. K., D. Unutmaz, V. N. KewalRamani, and D. R. Littman. 1997. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature 388:296-300. [DOI] [PubMed] [Google Scholar]
- 16.Douek, D. C., L. J. Picker, and R. A. Koup. 2003. T cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 21:265-304. [DOI] [PubMed] [Google Scholar]
- 17.Edinger, A. L., A. Amedee, K. Miller, B. J. Doranz, M. Endres, M. Sharron, M. Samson, Z. H. Lu, J. E. Clements, M. Murphey-Corb, S. C. Peiper, M. Parmentier, C. C. Broder, and R. W. Doms. 1997. Differential utilization of CCR5 by macrophage and T cell tropic simian immunodeficiency virus strains. Proc. Natl. Acad. Sci. USA 94:4005-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferbas, J., J. V. Giorgi, S. Aminia, K. Grovit-Ferbas, D. J. Wiley, R. Detels, and S. Plaeger. 2000. Antigen-specific production of RANTES, macrophage inflammatory protein (MIP)-1α and MIP-1β in vitro is a correlate of reduced human immunodeficiency virus burden in vivo. J. Infect. Dis. 182:1247-1250. [DOI] [PubMed] [Google Scholar]
- 19.Fox, G. H., K. Tenner-Racz, P. Racz, A. Firpo, P. A. Pizzo, and A. S. Fauci. 1991. Lymphoid germinal centers are reservoirs of human immunodeficiency virus type 1 RNA. J. Infect. Dis. 164:1051-1057. [DOI] [PubMed] [Google Scholar]
- 20.Gauduin, M., R. L. Glickman, R. Means, and R. P. Johnson. 1998. Inhibition of simian immunodeficiency virus (SIV) replication by CD8+ lymphocytes from macaques immunized with live attenuated SIV. J. Virol. 72:6315-6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gauduin, M. C., R. L. Glickman, S. Ahmad, T. Yilma, and R. P. Johnson. 1999. Immunization with live attenuated simian immunodeficiency virus induces strong type 1 T helper responses and β-chemokine production. Proc. Natl. Acad. Sci. USA 96:14031-14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grossman, Z., Z. Bentwich, and R. B. Herberman. 1993. From HIV infection to AIDS: are the manifestations of effective immune resistance misinterpreted? Clin. Immunol. Immunopathol. 69:123-135. [DOI] [PubMed] [Google Scholar]
- 23.Haase, A. 1999. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu. Rev. Immunol. 17:625-656. [DOI] [PubMed] [Google Scholar]
- 24.Heeney, J., L. Akerblom, S. Barnett, W. Bogers, D. Davis, D. Fuller, G. Koopman, T. Lehner, P. Mooij, B. Morein, C. de Giuli Morghen, B. Rosenwirth, E. Verschoor, R. Wagner, and H. Wolf. 1999. HIV-1 vaccine-induced immune responses which correlate with protection from SHIV infection: compiled preclinical efficacy data from trials with ten different HIV-1 vaccine candidates. Immunol. Lett. 66:189-195. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman-Lehman, R., A. L. Williams, R. K. Swenerton, P. L. Li, R. A. Rasmussen, A. L. Chenine, H. M. McClure, and R. M. Ruprecht. 2002. Quantitation of simian cytokine and β-chemokine mRNAs, using real-time reverse transcriptase-polymerase chain reaction: variations in expression during chronic primate lentivirus infection. AIDS Res. Hum. Retrovir. 18:627-639. [DOI] [PubMed] [Google Scholar]
- 26.Hu, J., M. Pope, C. Brown, U. O'Doherty, and C. J. Miller. 1998. Immunophenotypic characterization of simian immunodeficiency virus-infected dendritic cells in cervix, vagina, and draining lymph nodes of rhesus monkeys. Lab. Investig. 78:435-451. [PubMed] [Google Scholar]
- 27.Jennes, W., S. Sawadogo, S. Koblavi-deme, B. Vuylsteke, C. Maurice, T. H. Roels, T. Chorba, J. N. Nkengasong, and L. Kestens. 2002. Positive association between β-chemokine-producing T cells and HIV type I viral load in HIV-infected subjects in Abidjan, Cote d'Ivoire. AIDS Res. Hum. Retrovir. 18:171-177. [DOI] [PubMed] [Google Scholar]
- 28.Lehner, T., E. Mitchell, L. Bergmeier, M. Singh, R. Spallek, M. Cranage, G. Hall, M. Dennis, F. Villinger, and Y. Wang. 2000. The role of gamma delta T cells in generating antiviral factors and beta-chemokines in protection against mucosal simian immunodeficiency virus infection. Eur. J. Immunol. 30:2245-2256. [DOI] [PubMed] [Google Scholar]
- 29.Lehner, T., Y. Wang, M. Cranage, L. A. Bergmeier, E. Mitchell, L. Tao, G. Hall, M. Dennis, N. Cook, R. Brookes, L. Klavinskis, I. Jones, C. Doyle, and R. Ward. 1996. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nat. Med. 2:767-775. [DOI] [PubMed] [Google Scholar]
- 30.Lehner, T., Y. Wang, M. Cranage, L. Tao, E. Mitchell, C. Bravery, C. Doyle, K. Pratt, G. Hall, M. Dennis, L. Villinger, and L. Bergmeier. 2000. Up-regulation of beta-chemokines and down-modulation of CCR5 co-receptors inhibit simian immunodeficiency virus transmission in non-human primates. Immunology 99:569-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leno, M., L. Carter, D. J. Venzon, J. Romano, P. D. Markham, K. Limbach, J. Tartaglia, E. Paoletti, J. Benson, G. Franchini, and M. Robert-Guroff. 1999. CD8+ lymphocyte antiviral activity in monkeys immunized with SIV recombinant poxvirus vaccines: potential role in vaccine efficacy. AIDS Res. Hum. Retrovir. 15:461-470. [DOI] [PubMed] [Google Scholar]
- 32.Levy, J. A., C. E. Mackewicz, and E. Barker. 1996. Controlling HIV pathogenesis: the role of the noncytotoxic anti-HIV response of CD8+ T cells. Immunol. Today 17:217-224. [DOI] [PubMed] [Google Scholar]
- 33.Lusso, P. 2000. Chemokines and viruses: the dearest enemies. Virology 273:228-240. [DOI] [PubMed] [Google Scholar]
- 34.Miller, C. J., M. B. McChesney, X. Lu, P. J. Dailey, C. Chutkowski, D. Lu, P. Brosio, B. Roberts, and Y. Lu. 1997. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J. Virol. 71:1911-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moriuchi, H., M. Moriuchi, C. Combadiere, P. M. Murphy, and A. S. Fauci. 1996. CD8+ T-cell-derived soluble factor(s), but not β-chemokines RANTES, MIP-1α, and MIP-1β, suppress HIV-1 replication in monocyte/macrophages. Proc. Natl. Acad. Sci. USA 93:15341-15345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olson, T. S., and K. Ley. 2002. Chemokines and chemokine receptors in leukocyte trafficking. Am. J. Physiol. Regulat. Integrative Comp. Physiol. 283:R7-R28. [DOI] [PubMed] [Google Scholar]
- 37.Pantaleo, G., C. Graziosi, L. Butini, et al. 1988. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 88:9838-9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pantaleo, G., C. Graziosi, J. F. Demarest, et al. 1993. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature 362:355-358. [DOI] [PubMed] [Google Scholar]
- 39.Ribiero, R. M., H. Mohri, D. D. Ho, and A. S. Perelson. 2002. In vivo dynamics of T cell activation, proliferation, and death in HIV-1 infection: why are CD4+ but not CD8+ T cells depleted? Proc. Natl. Acad. Sci. USA 99:15572-15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 41.Silvestri, G., D. Sodora, R. A. Koup, M. Paiardini, S. P. O'Neil, H. M. McClure, S. I. Staprans, and M. B. Feinberg. 2003. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18:441-452. [DOI] [PubMed] [Google Scholar]
- 42.Tedla, N., J. Dwyer, P. Truskett, D. Taub, D. Wakefield, and A. Lloyd. 1999. Phenotypic and functional characterization of lymphocytes derived from normal and HIV-1-infected human lymph nodes. Clin. Exp. Immunol. 117:92-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tedla, N., P. Palladinetti, M. Kelly, R. K. Kumar, N. DiGirolamo, U. Chattophadhay, B. Cooke, P. Truskett, J. Dwyer, D. Wakefield, and A. Lloyd. 1996. Chemokines and T lymphocyte recruitment to lymph nodes in HIV infection. Am. J. Pathol. 148:1367. [PMC free article] [PubMed] [Google Scholar]
- 44.Tedla, N., H. W. Wang, H. P. McNeil, N. Di Girolamo, T. Hampartzoumian, D. Wakefield, and A. Lloyd. 1998. Regulation of T lymphocyte trafficking into lymph nodes during an immune response by the chemokines macrophage inflammatory protein (MIP)-1 alpha and MIP-1 beta. J. Immunol. 161:5663-5672. [PubMed] [Google Scholar]
- 45.Tenner-Racz, K., P. Racz, and H. Schmidt. 1988. Immunohistochemical, electron microscopic and in situ hybridization evidence for the involvement of lymphatics in the spread of HIV-1. AIDS 2:299-309. [DOI] [PubMed] [Google Scholar]
- 46.Trumpfheller, C., K. Tenner-Racz, P. Racz, B. Fleischer, and S. Frosch. 1998. Expression of macrophage inflammatory protein (MIP)-1α, MIP-1β, and RANTES genes in lymph nodes from HIV+ individuals: correlation with a Th1-type cytokine response. Clin. Exp. Immunol. 112:92-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veazey, R. S., P. J. Klasse, T. J. Ketas, J. D. Reeves, M. Piatak, Jr., K. Kunstman, S. E. Kuhmann, P. A. Marx, J. D. Lifson, J. Dufour, M. Mefford, I. Pandrea, S. M. Wolinsky, R. W. Doms, J. A. DeMartino, S. J. Siciliano, K. Lyons, M. S. Springer, and J. P. Moore. 2003. Use of a small molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian-human immunodeficiency virus infection. J. Exp. Med. 198:1551-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker, C. M., D. J. Moody, D. P. Stites, and J. A. Levy. 1986. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science 234:1563-1566. [DOI] [PubMed] [Google Scholar]