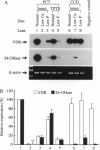
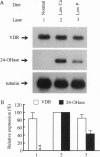
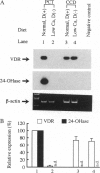
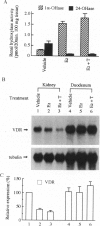
Abstract
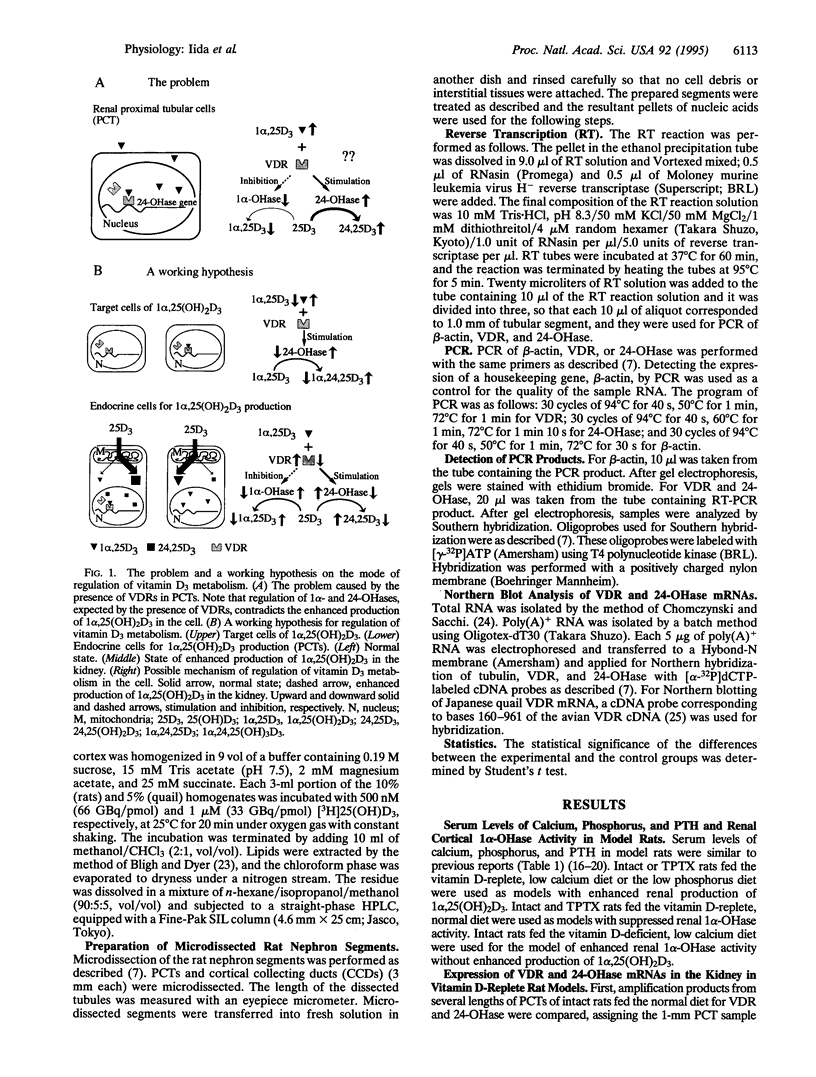
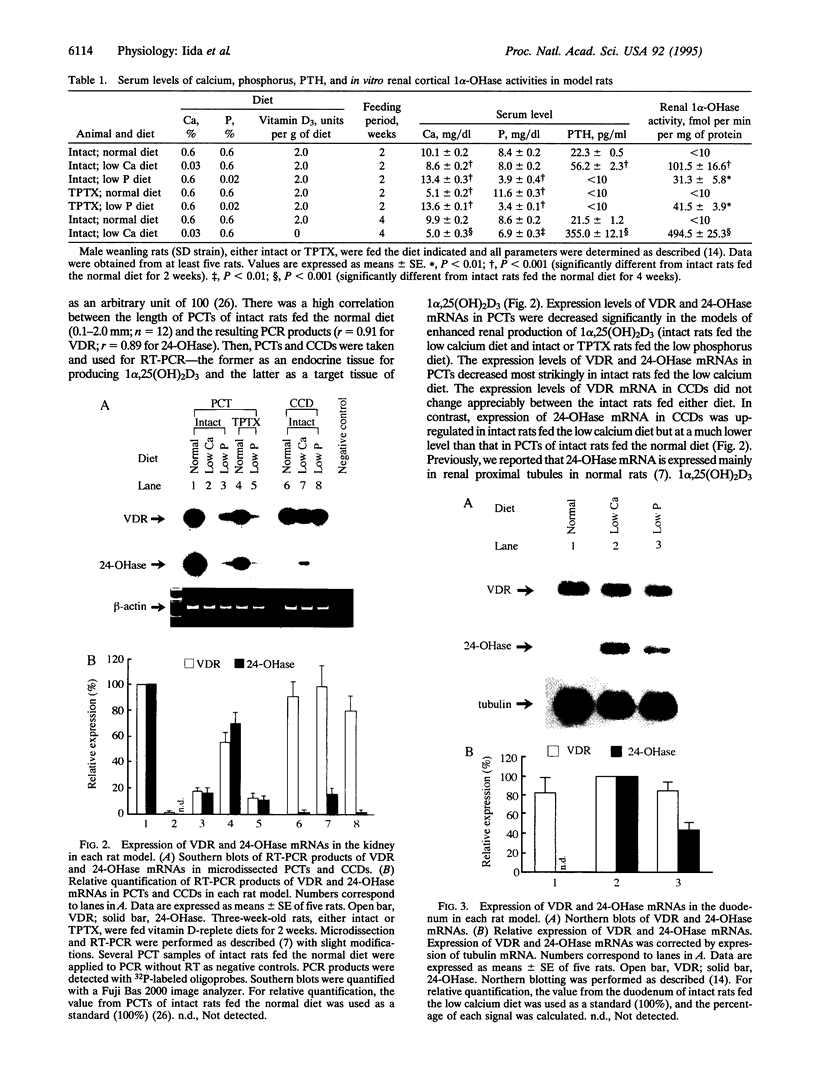
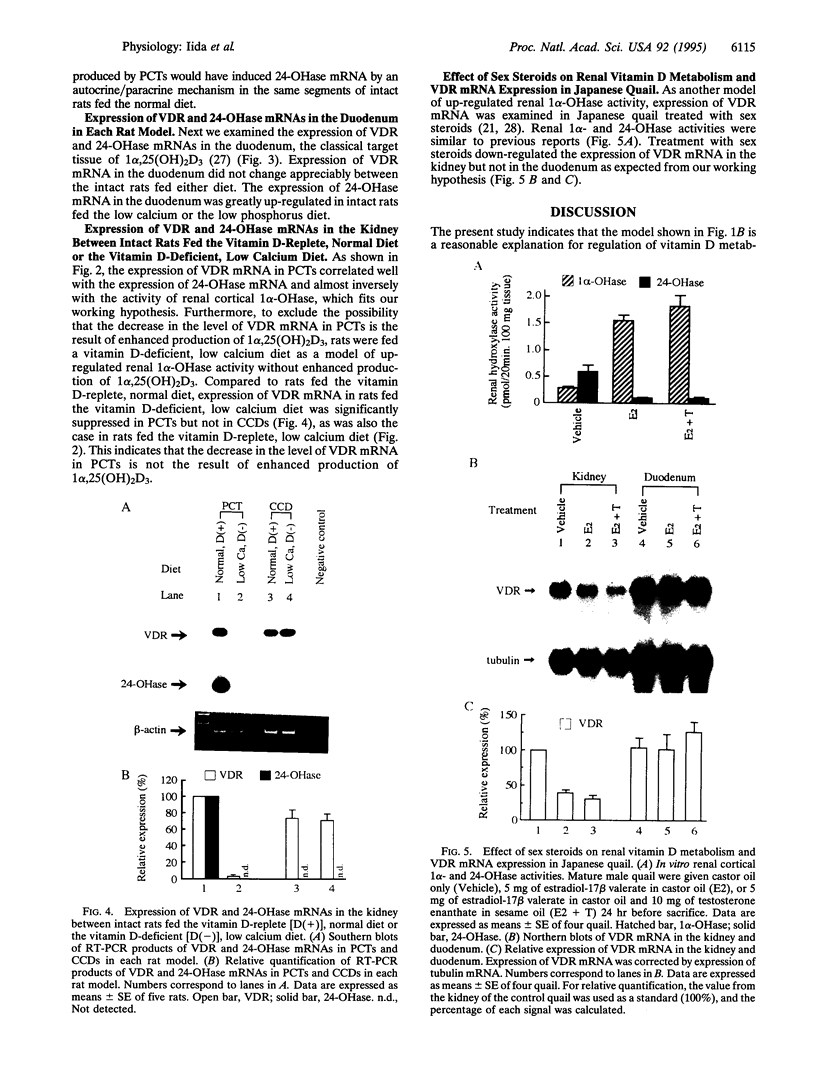
The vitamin D endocrine system is regulated reciprocally by renal 25-hydroxyvitamin D3 1 alpha- and 24-hydroxylases. Previously, we reported that renal proximal convoluted tubules, the major site of 1 alpha, 25-dihydroxyvitamin D3 production, have vitamin D receptors. In the presence of vitamin D receptors, renal proximal convoluted tubules cannot maintain the state of enhanced production of 1 alpha, 25-dihydroxyvitamin D3. To clarify this discrepancy, we proposed a working hypothesis for the reciprocal control of renal 25-hydroxyvitamin D3 1 alpha- and 24-hydroxylase activities. In rat models of enhanced renal production of 1 alpha, 25-dihydroxyvitamin D3, expression of vitamin D receptors and 25-hydroxyvitamin D3 24-hydroxylase mRNAs was strikingly suppressed in renal proximal convoluted tubules but not in the cortical collecting ducts. In vitamin D-deficient rats with up-regulated renal 25-hydroxyvitamin D3 1 alpha-hydroxylase activity, expression of vitamin D receptor mRNA in renal proximal convoluted tubules was also down-regulated, indicating that the down-regulation of vitamin D receptor mRNA is not the result of the enhanced production of 1 alpha, 25-dihydroxyvitamin D3. In Japanese quail models with up-regulated renal 25-hydroxyvitamin D3 1 alpha-hydroxylase activity by sex steroids, expression of vitamin D receptor mRNA was also down-regulated in the kidney but not in the duodenum. These results suggest that the down-regulation of vitamin D receptors plays a critical role in production of 1 alpha, 25-dihydroxyvitamin D3 in renal proximal convoluted tubules.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arabian A., Grover J., Barré M. G., Delvin E. E. Rat kidney 25-hydroxyvitamin D3 1 alpha- and 24-hydroxylases: evidence for two distinct gene products. J Steroid Biochem Mol Biol. 1993 Jun;45(6):513–516. doi: 10.1016/0960-0760(93)90167-u. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bikle D. D., Gee E. Free, and not total, 1,25-dihydroxyvitamin D regulates 25-hydroxyvitamin D metabolism by keratinocytes. Endocrinology. 1989 Feb;124(2):649–654. doi: 10.1210/endo-124-2-649. [DOI] [PubMed] [Google Scholar]
- Boyle I. T., Gray R. W., DeLuca H. F. Regulation by calcium of in vivo synthesis of 1,25-dihydroxycholecalciferol and 21,25-dihydroxycholecalciferol. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2131–2134. doi: 10.1073/pnas.68.9.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks M. H., Bell N. H., Love L., Stern P. H., Orfei E., Queener S. F., Hamstra A. J., DeLuca H. F. Vitamin-D-dependent rickets type II. Resistance of target organs to 1,25-dihydroxyvitamin D. N Engl J Med. 1978 May 4;298(18):996–999. doi: 10.1056/NEJM197805042981804. [DOI] [PubMed] [Google Scholar]
- Brunette M. G., Chan M., Ferriere C., Roberts K. D. Site of 1,25(OH)2 vitamin D3 synthesis in the kidney. Nature. 1978 Nov 16;276(5685):287–289. doi: 10.1038/276287a0. [DOI] [PubMed] [Google Scholar]
- Castillo L., Tanaka Y., DeLuca H. F., Sunde M. L. The stimulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase by estrogen. Arch Biochem Biophys. 1977 Feb;179(1):211–217. doi: 10.1016/0003-9861(77)90105-9. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F., Schnoes H. K. Vitamin D: recent advances. Annu Rev Biochem. 1983;52:411–439. doi: 10.1146/annurev.bi.52.070183.002211. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F. The vitamin D story: a collaborative effort of basic science and clinical medicine. FASEB J. 1988 Mar 1;2(3):224–236. [PubMed] [Google Scholar]
- Elaroussi M. A., Prahl J. M., DeLuca H. F. The avian vitamin D receptors: primary structures and their origins. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11596–11600. doi: 10.1073/pnas.91.24.11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D., Chen T., Cone C., Hirst M., Shani S., Benderli A., Hochberg Z. Vitamin D resistant rickets with alopecia: cultured skin fibroblasts exhibit defective cytoplasmic receptors and unresponsiveness to 1,25(OH)2D3. J Clin Endocrinol Metab. 1982 Nov;55(5):1020–1022. doi: 10.1210/jcem-55-5-1020. [DOI] [PubMed] [Google Scholar]
- Goff J. P., Reinhardt T. A., Beckman M. J., Horst R. L. Contrasting effects of exogenous 1,25-dihydroxyvitamin D [1,25-(OH)2D] versus endogenous 1,25-(OH)2D, induced by dietary calcium restriction, on vitamin D receptors. Endocrinology. 1990 Feb;126(2):1031–1035. doi: 10.1210/endo-126-2-1031. [DOI] [PubMed] [Google Scholar]
- Gray R. W., Napoli J. L. Dietary phosphate deprivation increases 1,25-dihyroxyvitamin D3 synthesis in rat kidney in vitro. J Biol Chem. 1983 Jan 25;258(2):1152–1155. [PubMed] [Google Scholar]
- Hove K., Horst R. L., Littledike E. T., Beitz D. C. Infusions of parathyroid hormone in ruminants: hypercalcemia and reduced plasma 1,25-dihydroxyvitamin D concentrations. Endocrinology. 1984 Mar;114(3):897–903. doi: 10.1210/endo-114-3-897. [DOI] [PubMed] [Google Scholar]
- Hughes M. R., Brumbaugh P. F., Hussler M. R., Wergedal J. E., Baylink D. J. Regulation of serum 1alpha,25-dihydroxyvitamin D3 by calcium and phosphate in the rat. Science. 1975 Nov 7;190(4214):578–580. doi: 10.1126/science.1188357. [DOI] [PubMed] [Google Scholar]
- Hughes M. R., Malloy P. J., Kieback D. G., Kesterson R. A., Pike J. W., Feldman D., O'Malley B. W. Point mutations in the human vitamin D receptor gene associated with hypocalcemic rickets. Science. 1988 Dec 23;242(4886):1702–1705. doi: 10.1126/science.2849209. [DOI] [PubMed] [Google Scholar]
- Iida K., Taniguchi S., Kurokawa K. Distribution of 1,25-dihydroxyvitamin D3 receptor and 25-hydroxyvitamin D3-24-hydroxylase mRNA expression along rat nephron segments. Biochem Biophys Res Commun. 1993 Jul 30;194(2):659–664. doi: 10.1006/bbrc.1993.1872. [DOI] [PubMed] [Google Scholar]
- Ishimura E., Shoji S., Koyama H., Inaba M., Nishizawa Y., Morii H. Presence of gene expression of vitamin D receptor and 24-hydroxylase in OK cells. FEBS Lett. 1994 Jan 3;337(1):48–51. doi: 10.1016/0014-5793(94)80627-6. [DOI] [PubMed] [Google Scholar]
- Kawashima H., Kurokawa K. Metabolism and sites of action of vitamin D in the kidney. Kidney Int. 1986 Jan;29(1):98–107. doi: 10.1038/ki.1986.12. [DOI] [PubMed] [Google Scholar]
- Kawashima H., Torikai S., Kurokawa K. Localization of 25-hydroxyvitamin D3 1 alpha-hydroxylase and 24-hydroxylase along the rat nephron. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1199–1203. doi: 10.1073/pnas.78.2.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny A. D. Vitamin D metabolism: physiological regulation in egg-laying Japanese quail. Am J Physiol. 1976 Jun;230(6):1609–1615. doi: 10.1152/ajplegacy.1976.230.6.1609. [DOI] [PubMed] [Google Scholar]
- Larkins R. G., MacAuley S. J., MacIntyre I. Feedback control of vitamin D metabolism by a nuclear action of 1,25-dihydroxycholecalciferol on the kidney. Nature. 1974 Nov 29;252(5482):412–414. doi: 10.1038/252412a0. [DOI] [PubMed] [Google Scholar]
- Murer H., Werner A., Reshkin S., Wuarin F., Biber J. Cellular mechanisms in proximal tubular reabsorption of inorganic phosphate. Am J Physiol. 1991 May;260(5 Pt 1):C885–C899. doi: 10.1152/ajpcell.1991.260.5.C885. [DOI] [PubMed] [Google Scholar]
- Rader J. I., Baylink D. J., Hughes M. R., Safilian E. F., Haussler M. R. Calcium and phosphorus deficiency in rats: effects on PTH and 1,25-dihydroxyvitamin D3. Am J Physiol. 1979 Feb;236(2):E118–E122. doi: 10.1152/ajpendo.1979.236.2.E118. [DOI] [PubMed] [Google Scholar]
- Reinhardt T. A., Horst R. L. Parathyroid hormone down-regulates 1,25-dihydroxyvitamin D receptors (VDR) and VDR messenger ribonucleic acid in vitro and blocks homologous up-regulation of VDR in vivo. Endocrinology. 1990 Aug;127(2):942–948. doi: 10.1210/endo-127-2-942. [DOI] [PubMed] [Google Scholar]
- Reinhardt T. A., Horst R. L. Phorbol 12-myristate 13-acetate and 1,25-dihydroxyvitamin D3 regulate 1,25-dihydroxyvitamin D3 receptors synergistically in rat osteosarcoma cells. Mol Cell Endocrinol. 1994 May;101(1-2):159–165. doi: 10.1016/0303-7207(94)90230-5. [DOI] [PubMed] [Google Scholar]
- Sandgren M. E., DeLuca H. F. Serum calcium and vitamin D regulate 1,25-dihydroxyvitamin D3 receptor concentration in rat kidney in vivo. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4312–4314. doi: 10.1073/pnas.87.11.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinki T., Jin C. H., Nishimura A., Nagai Y., Ohyama Y., Noshiro M., Okuda K., Suda T. Parathyroid hormone inhibits 25-hydroxyvitamin D3-24-hydroxylase mRNA expression stimulated by 1 alpha,25-dihydroxyvitamin D3 in rat kidney but not in intestine. J Biol Chem. 1992 Jul 5;267(19):13757–13762. [PubMed] [Google Scholar]
- Stumpf W. E., Sar M., Reid F. A., Tanaka Y., DeLuca H. F. Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary, and parathyroid. Science. 1979 Dec 7;206(4423):1188–1190. doi: 10.1126/science.505004. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Castillo L., DeLuca H. F. Control of renal vitamin D hydroxylases in birds by sex hormones. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2701–2705. doi: 10.1073/pnas.73.8.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., DeLuca H. F. Stimulation of 24,25-dihydroxyvitamin D3 production by 1,25-dihydroxyvitamin D3. Science. 1974 Mar;183(130):1198–1200. doi: 10.1126/science.183.4130.1198. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Deluca H. F. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys. 1973 Feb;154(2):566–574. doi: 10.1016/0003-9861(73)90010-6. [DOI] [PubMed] [Google Scholar]
- Terada Y., Tomita K., Nonoguchi H., Marumo F. Polymerase chain reaction localization of constitutive nitric oxide synthase and soluble guanylate cyclase messenger RNAs in microdissected rat nephron segments. J Clin Invest. 1992 Aug;90(2):659–665. doi: 10.1172/JCI115908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhland-Smith A., DeLuca H. F. The necessity for calcium for increased renal vitamin D receptor in response to 1,25-dihydroxyvitamin D. Biochim Biophys Acta. 1993 Apr 16;1176(3):321–326. doi: 10.1016/0167-4889(93)90061-s. [DOI] [PubMed] [Google Scholar]
- Wiese R. J., Uhland-Smith A., Ross T. K., Prahl J. M., DeLuca H. F. Up-regulation of the vitamin D receptor in response to 1,25-dihydroxyvitamin D3 results from ligand-induced stabilization. J Biol Chem. 1992 Oct 5;267(28):20082–20086. [PubMed] [Google Scholar]