Abstract
Epidemiological studies report that 80% of the population maintains antibodies (Ab) to wild-type (wt) adeno-associated virus type 2 (AAV2), with 30% expressing neutralizing Ab (NAb). The blood-brain barrier (BBB) provides limited immune privilege to brain parenchyma, and the immune response to recombinant AAV (rAAV) administration in the brain of a naive animal is minimal. However, central nervous system transduction in preimmunized animals remains unstudied. Vector administration may disrupt the BBB sufficiently to promote an immune response in a previously immunized animal. We tested the hypothesis that intracerebral rAAV administration and readministration would not be affected by the presence of circulating Ab to wt AAV2. Rats peripherally immunized with live wt AAV2 and naive controls were tested with single intrastriatal injections of rAAV2 encoding human glial cell line-derived neurotrophic factor (GDNF) or green fluorescent protein (GFP). Striatal readministration of rAAV2-GDNF was also tested in preimmunized and naive rats. Finally, serotype specificity of the immunization against wt AAV2 was examined by single injections of rAAV5-GFP. Preimmunization resulted in high levels of circulating NAb and prevented transduction by rAAV2 as assessed by striatal GDNF levels. rAAV2-GFP striatal transduction was also prevented by immunization, while rAAV5-GFP-mediated transduction, as assessed by stereological cell counting, was unaffected. Additionally, inflammatory markers were present in those animals that received repeated administrations of rAAV2, including markers of a cell-mediated immune response and cytotoxic damage. A live virus immunization protocol generated the circulating anti-wt-AAV Ab seen in this experiment, while human titers are commonly acquired via natural infection. Regardless, the data show that the presence of high levels of NAb against wt AAV can reduce rAAV-mediated transduction in the brain and should be accounted for in future experiments utilizing this vector.
Recombinant adeno-associated virus (rAAV) is a replication-defective human parvovirus with excellent potential as a vector for human gene therapy applications. The most commonly employed rAAV vector is based on the wild-type (wt) AAV type 2 (AAV2) serotype. The lack of human pathology associated with infection by wt AAV2 makes it attractive as a gene therapy vector; however, natural exposure to wt AAV2 is already quite common. An estimated 80% of the population ranging in age from in utero to >70 years old maintain antibodies (Ab) to the capsid proteins of wt AAV2, and 30 to 70% demonstrate the presence of neutralizing anticapsid Ab (NAb) (3, 4, 8, 12). Ab to the other known serotypes are less studied and are found with reduced frequency (8, 15). Natural infection does not necessarily prevent reinfection with wt AAV2 in its normal life cycle, as demonstrated by the simultaneous presence of immunoglobulin M (IgM) and IgG, which reflect either reactivation of latent infection or reinfection despite existing humoral immunity (3). Interestingly, however, some studies looking at repeated administration of rAAV, in the periphery, indicate that an immune response generated after the first administration may prevent further application (18, 19, 24, 31, 42, 43). Transient immunosuppression during the first administration can eliminate this effect (19, 31). However, there are discrepancies regarding the ability to readminister the same vector (2, 9, 20), and these may be due in part to methods of study and possible viral contaminants (33). Transduction failure in these studies has been attributed to the presence of NAb found in the animal's serum; however, no correlation between Ab titer and transduction efficiency has been demonstrated.
While AAV2 capsid proteins are subject to humoral immunity, wt AAV2 can presumably circumvent cell-mediated immunity (CMI) by establishing latency in the host cell. In addition, in rAAV, all of the viral DNA except the two inverted terminal repeats is replaced with the transgene of interest and its promoter, enhancer, and any expression regulators. As a result, no viral proteins are encoded, and the peptides exhibited on the major histocompatibility complex class 1 (MHC1) complex of the transduced cell are limited to transgene products and digested capsid peptides. Notwithstanding, rAAV-induced CMI (20) has been shown (5) and is ultimately dependent on the route of administration (5, 31, 42). These studies investigated peripheral tissues, and therefore CMI induction in the brain remains a critical question.
It is uncertain whether intracerebral injection of the vector itself is sufficient to stimulate the cellular arm of an immune response. Likewise, it is not known whether Ab can cross the blood-brain barrier (BBB) in sufficient quantities to threaten transduction by blocking the initial infection of target cells and/or reduce the safety of administration by promoting inflammation or activating a T-cell-mediated response. Two studies reveal reduced or absent transgene expression in the periphery in mice preimmunized against rAAV (14, 34). These studies used rAAV, not wt AAV, to induce humoral immunity and reflect transduction events in the periphery. In contrast, all rAAV studies in the brain have been performed with naive animals, and administering rAAV in the setting of a preprimed immune system remains a concern.
Data obtained by using other recombinant viral vectors indicate that preexposure to the virus can be a problem for central nervous system (CNS) delivery. For example, first-generation adenoviral vectors are highly immunogenic in the brain parenchyma, eliciting acute cellular (6) and cytokine-mediated (7) inflammation as well as a chronic T-cell inflammatory response and even neurotoxic demyelination (11). Likewise, animals first treated with adenoviral vectors in the brain and then infected peripherally with adenovirus exhibit long-term brain inflammation (6, 23, 37). Pursuant to these discoveries, alterations were made in adenoviral vector properties, creating new-generation high-capacity adenoviral vectors lacking most of the viral genome and consequently reducing the deleterious inflammatory effects (38). This concern is further supported by studies on the brain in animals with preexisting immunity to herpes simplex virus, which reveal reduced efficiency and possible deleterious inflammatory responses (21, 21, 39). Identifying these interactions with the immune system is critical to implementing and perfecting the gene therapy bridge from animals to humans.
While it is commonly assumed that preexposure to wt AAV2 will not pose significant problems with regard to the performance of rAAV vectors in the brain, no reliable data exist to support these beliefs. Investigation of the immune response to rAAV in the brain in wt AAV-primed animals may be critically important, especially in light of the advent of rAAV-based human trials for neurological disorders (22, 29). In this study, we sought to determine the effect of preexisting immunity to wt AAV2 on rAAV-mediated transgene expression, neuroinflammation, and the ability to readminister rAAV in the brain. Based on our previous experience indicating no brain inflammation in response to rAAV and the relative immune privilege of the brain, we hypothesized that (i) preimmunization would not reduce the level of brain transduction, (ii) inflammation would be similar in preimmunized animals and controls, and (iii) immunization would not sufficiently prime the immune system to reduce the ability to readminister an identical vector at a later time point. To the contrary, immunization against wt AAV2 completely blocked rAAV2 transduction in all preimmunized animals (34a) while permitting successful rAAV5 transduction. The blockade of transduction was entirely due to the presence of NAb. Moreover, neuroinflammation was observed in those animals that received two sequential rAAV2 administrations regardless of the presence of transgene expression, and it persisted in the immunized animals at both injection sites. These novel results suggest that the immunization status of potential human subjects will be a factor in the design of future clinical trials.
MATERIALS AND METHODS
Subjects and experimental design.
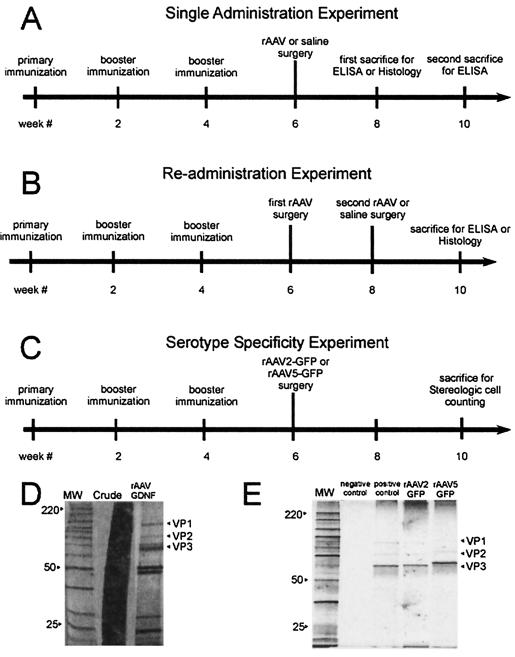
One hundred four female Sprague-Dawley rats weighing approximately 220 g were obtained from Harlan Sprague-Dawley (Indianapolis, Ind.), housed with access to food and water ad libitum on a 12-h light-dark cycle, and maintained and treated in accordance with published National Institutes of Health guidelines. The experimental groups and their chronological treatments are shown schematically in Fig. 1. The rats were divided randomly into two groups, with the first group (n = 56) receiving immunization and booster injections of wt AAV at the start of weeks 0, 2, and 4 and the other group (n = 48) remaining naive. All titer quantifications were performed following assignment of immunized rats to their respective groups, ensuring random and blinded group assignment.
FIG. 1.
(A to C) Experimental design and order of treatments. The different treatments are presented in chronological order as indicated for each experiment. Each experimental treatment group included immunized animals as well as naive controls. The multiple survival intervals shown here were necessary to control for the possibility that rAAV-mediated GDNF expression levels increase over time. Blood was drawn for NAb titer assay at each time point indicated. (A) Single-administration experimental design. All of the animals (n = 40) in this portion of the study received a single 2-μl striatal injection of rAAV-GDNF or sterile saline, as described in Materials and Methods. (B) Readministration experimental design. The animals in this portion of the study (n = 38) received a single 2-μl striatal injection of rAAV-GDNF followed by an identical injection of rAAV-GDNF or sterile saline 2 weeks later in the opposite hemisphere, as described in Materials and Methods. (C) Serotype specificity experimental design. The animals in this portion of the study (n = 26) received a single injection of 2 μl of either rAAV2-GFP or rAAV5-GFP, as described in Materials and Methods section. (D and E) Vector purification. Viral vector stocks were analyzed for the presence of cellular proteins and contaminants by PAGE analysis with silver staining. Lanes MW, molecular weight ladder (in thousands) for identification. VP1, -2, and -3 are the three AAV capsid proteins. (D) Right lane, rAAV-GDNF vector after purification; middle lane, crude stock. (E) Second lane, PBS and buffer as a negative control; third lane, standard rAAV2-CBA-GFP preparation as a positive control; fourth lane, rAAV2-GFP vector after purification; right lane, rAAV5-GFP vector after purification.
Single administration.
In the single-injection protocol, 18 immunized rats (referred to as immune-single rats) and 18 naive rats (afterwards referred to as naive-single rats) received a unilateral stereotaxic injection of 2 μl of rAAV-glial cell line-derived neurotrophic factor (rAAV-GDNF) (n = 36) in the striatum on week 6. Four immune-single rats received a single injection of 2 μl of 0.9% sterile saline as a control for our histological studies. The rats survived for 2 or 4 weeks after surgery and were then sacrificed for enzyme-linked immunosorbent assay (ELISA). Four rats from each group at the 2-week time point were perfused for histological analysis (Fig. 1A).
Repeat administration.
In the repeat-injection protocol, 22 immunized rats (referred to as immune-repeat rats) and 16 naive rats (referred to as naive-repeat rats) received primary injections of 2 μl of rAAV-GDNF in the right striatum on week 6 and after 2 weeks received either 2 μl of rAAV-GDNF, 2 μl of 0.9% sterile saline, or no injection in the contralateral striatum. The animals survived for 2 weeks after the second surgery and were then sacrificed for ELISA. Four animals from each of these groups were perfused for histological analysis (Fig. 1B).
Serotype experiment.
In the serotype specificity protocol, 26 rats (14 immunized against wt AAV2 and 12 naive) received either a unilateral stereotaxic injection of 2 μl of rAAV2-green fluorescent protein (rAAV2-GFP) (n = 13) or rAAV5-GFP (n = 13) in the striatum on week 6. The rats survived for 4 weeks after surgery and were then perfused for histological analysis and stereological cell counting (Fig. 1C). Blood was drawn for determination of Ab titer before immunization, before surgery, and at the time of sacrifice.
Virus production.
Briefly, rAAV2 virus production entailed the use of the helper-packaging plasmid pDG (16a), which supplied all of the necessary helper functions as well as rep and cap in trans. The GDNF vector plasmid consisted of the human GDNF cDNA driven by a chicken β-actin promoter with a cytomegalovirus immediate-early enhancer (CBA promoter) (45), and the GDNF was followed by the woodchuck hepatitis virus posttranscriptional regulatory element (49) flanked by AAV2 inverted terminal repeats. The GFP vector plasmids consisted of the humanized enhanced GFP (eGFP) cDNA driven by a CBA promoter (45), and flanked by AAV2 inverted terminal repeats. A 622.5-μg amount of vector plasmid was cotransfected by calcium phosphate precipitation with 1,867 μg of pDG into one cell factory of ∼70 to 95% confluent 293 cells. wt AAV2 was prepared by transfection of pSM620, a plasmid containing the wt AAV2 genome (36), into 293 cells as previously described (44). rAAV5-eGFP was a pseudotyped vector using the same transgene-containing plasmid as the rAAV2 vector described above. rAAV5 was produced similarly to the rAAV2 vector except that helper plasmid pXYZ5 was used in place of pDG (48).
rAAV2 and wt viruses were purified by iodixanol centrifugation and heparin column affinity purification, as described previously (47). rAAV5 was purified by identical iodixanol centrifugation followed by ion-exchange chromatography as described previously (48). The physical titer (genome number) was determined by dot blotting, and the infectious titer was determined by infectious-center assay, as described previously (47). The final titers of the rAAV2-CBA-human GDNF-woodchuck hepatitis virus posttranscriptional regulatory element stock and the wtAAV2 were 4.2 × 1011 and 1.0 × 1011 IU/ml, respectively. The final titers of the rAAV2-CBA-eGFP and rAAV5-CBA-eGFP stocks were 2.8 × 1012 and were 2.92 × 1013 particles/ml, respectively. Peak fractions for all vector stocks were examined by polyacrylamide gel electrophoresis (PAGE) with silver staining to characterize the protein content in each vector preparation as described previously (48) (Fig. 1D and E).
Immunization.
The immunized rats received a combination of two subcutaneous injections and one intraperitoneal injection totaling 109 infectious particles of wt AAV2 (41) in 0.5 ml of RIBI adjuvant (Sigma, St. Louis, Mo.). Identical booster immunizations were given twice successively at 2-week intervals (Fig. 1A and B). The threshold definition for positive immunization was determined as a twofold increase in the serial dilution required to neutralize transduction.
Stereotaxic injection into rat brain.
Animals were anesthetized for surgery by isoflurane inhalation and mounted in a small animal stereotaxic device (David Kopf, Tujunga, Calif.). An incision was made over the skull, and the periostium was removed. Holes were drilled according to atlas coordinates, relative to bregma, using a dissecting microscope to avoid damage to the dura mater (from dura: anterior/posterior, 0.0 mm; lateral, −3.0 mm; dorsal/ventral, −4.0 mm [for single injection or the first of two injections of rAAV or 0.9% sterile saline] and anterior/posterior, 0.0 mm; lateral, +3.0 mm; dorsal/ventral, −4.0 mm [for second injections of rAAV or 0.9% sterile saline]). The dura was cut to allow insertion of a pulled micropipette (diameter of 80 to 100 μm) to inject 2 μl of rAAV2 or 0.9% sterile saline into the striatum in both the single- and repeat-injection protocols. In the serotype experiment, 3 μl of rAAV2 or rAAV5 was injected. Each injection occurred at a rate of 0.5 μl per min and took 4 and 6 min, respectively. The micropipette rested in place for an additional minute before it was raised 1 mm, and it was then left in place for an additional 4 min. All surgical instruments were chemically sterilized for a minimum of 10 min between surgeries, using 3.4% glutaraldehyde solution (Cidex plus; Johnson and Johnson, Inc.).
GDNF ELISA.
The rats were lethally injected with sodium pentobarbital, and the brains were rapidly extracted for striatal dissection. ELISA of striatal tissue lysates was performed with the GDNF Emax ImmunoAssay System (Promega, Madison, Wis.) as per the manufacturer's instructions. Absorbances of lysates were measured at 450 nm for full-strength solutions and 1:10, 1:100, 1:500, 1:1,000, and 1:5,000 dilutions by using a Benchmark microplate reader (Bio-Rad Laboratories, Hercules, Calif.). Absorbance data were analyzed with Microplate Manager 4.0 software (Bio-Rad Laboratories). The resulting concentration of GDNF protein was adjusted for the dilution factor, and results from replicates were averaged to obtain the final concentration of GDNF protein per milligram of striatal tissue sample.
Histological processing.
The rats were lethally injected with sodium pentobarbital and transcardially perfused with sterile 0.9% saline followed by 350 ml of cold 4% paraformaldehyde in 0.01 M phosphate-buffered saline. The brains were then rapidly removed and postfixed for 3 to 12 h in the paraformaldehyde solution. The brains were then washed and transferred to a solution of 30% sucrose in 0.01 M PBS for cryoprotection.
Stereological cell counts.
Fixed brain tissue from the rats was sectioned at 40 μm, every third section was mounted on subbed slides and dehydrated, and coverslips were applied. The striatum was visualized with an Olympus BX60 fluorescence microscope, and the total number of GFP-positive cells were counted by using the Optical Fractionator probe for unbiased cell population estimates in Stereo Investigator version 5 by MicroBrightField (40). The counting frame area was maintained at 30 by 30 μm and 19 μm in thickness, for a total volume of 17,100 μm3, for all animals in the study. The sampling grid area was kept the same within groups and equaled 10,000 μm2 for those animals that received rAAV2 injections and 360,000 μm2 for those that received rAAV5. Due to differences in transduction efficiency between serotypes, these sampling grid size differences are necessary to maintain a coefficient of error of less than 0.1 in each estimate, but they in no way contribute to a counting bias.
Immunohistochemistry.
Brain tissue from rats was sectioned at 40 μm, and a series of immunohistochemical stains were performed on every sixth serial section of all of the rat brains except those processed for GDNF ELISA. Four rats in every group were evaluated histologically, and all stainings were done on all animals. The site of injection was determined by the presence of a visible needle tract running through the cortex and into the striatum. Every effort was made to photograph at the site of injection, especially in the cases of very little reaction. In the instances of high reactivity, many sections included the robust inflammation or infiltration seen.
Floating sections were washed with 0.01 M PBS and then incubated for 15 min after the addition of 0.5% H2O2 plus 10% methanol in 0.01 M PBS. The tissue was again washed and preblocked with 0.01 M PBS-0.1% Triton X-100 and 3% normal serum from the species against which the secondary Ab was raised. Primary Ab solution that contained 1% normal serum plus 0.1% Triton X-100 in 0.01 M PBS was then added and incubated overnight at room temperature. The tissue was then washed, and a secondary Ab solution of 1% normal serum plus 0.1% Triton X-100 in 0.01 M PBS that contained an appropriate Ab against the species in which the primary Ab was raised was added. After incubation for 2 h, the tissue was washed again. The color reaction for light microscopy was performed with an ABC kit (Vectastain Elite, catalog no. PK-6100). One milliliter of a solution of 2.5 mg of 3,3′-diaminobenzidine per ml in 0.01 M PBS was added and mixed with a final addition of 15 μl of 3% H2O2 in 0.01 M PBS. The tissue was then mounted on subbed slides and dehydrated, and coverslips were applied. Primary Ab used in this protocol were anti-GDNF (1:2,000; Chemicon, Temecula, Calif.), mouse anti-glial fibrillary acidic protein (anti-GFAP) (1:2,000; Chemicon), mouse anti-OX-42 (1:200; Serotec, Raleigh, N.C.), and rabbit anti-dopamine and cyclic AMP-regulated phosphoprotein (DARPP-32) (1:2,000; Chemicon). Secondary Ab used were biotinylated anti-mouse IgG and biotinylated anti-rabbit IgG (1:200; Vector Labs, Burlingame, Calif.). After the DARPP-32-stained tissue was mounted, the slides were immersed in a series of decreasing ethanol concentrations, submersed in Harris modified hematoxylin (Fischer Scientific, Fair Lawn, N.J.) for 3 min, and dehydrated, and coverslips were applied.
For the double labeling protocol, rabbit anti-CD8a (H-160) (1:100; Santa Cruz Biotechnology Santa Cruz, Calif.) and mouse anti-rat MHC1 (1:100; RT1a class I monomorphic Serotec, Raleigh, N.C.) were added simultaneously and allowed to incubate for 48 h. After washing, AlexaFlur 488 anti-mouse secondary Ab (green) (1:100; Molecular Probes, Inc., Eugene, Oreg.) and AlexaFlur 633 anti-rabbit secondary Ab (red) (1:100; Molecular Probes) were added and allowed to incubate for 2 h. The tissue was then mounted on subbed slides, coverslips were applied with Vectashield mounting medium for fluorescence, and cells were visualized with the MRC-1024 confocal laser scanning system.
Blood collection and serum sample preparation.
Blood was drawn for determination of Ab titer before immunization, before surgery, and at the time of sacrifice. Animals were anesthetized by isoflurane inhalation, and 0.3 ml of whole blood was drawn from the external jugular vein with a 23-gauge needle on a 1-ml syringe and placed in serum separator tubes (Stat Sampler MicroTubes; Fischer Scientific, Suwanee, Ga.). The blood was allowed to coagulate on ice for 30 min and then centrifuged for 10 min at a relative centrifugal force of 6,000 × g. The serum was then extracted and stored at −20°C.
Systemic NAb assay.
HeLa cells were plated in flat-bottom 96-well plates to 70% confluency. The next day, serial dilutions (with serum-free Dulbecco's modified Eagle's medium as the diluent) of individual serum samples taken at baseline, time of surgery, and time of sacrifice were prepared at a 2× final dilution. The diluted serum was then exposed to rAAV-CBA-GFP (final multiplicity of infection of 100) for 1 h at 37°C. Then, the serum-rAAV mixtures were added to the cells, followed by the addition of an equal volume of wt adenovirus (multiplicity of infection of 10) in Dulbecco's modified Eagle's medium with 20% fetal calf serum. The final volume totaled 100 μl per well, with final serum dilutions of 1:50, 1:200, 1:800, 1:3,200, 1:12,800, and 1:51200. Naive serum samples served as the animals' own controls. Each plate also contained serial dilutions of a standard concentration of a mouse monoclonal anti-intact AAV capsid Ab, A20 (American Research Products, Belmont, Mass.) and no serum as positive and negative controls. The cells were incubated in the presence of the viruses at 37°C for 28 h. GFP levels were then imaged and quantified with a Typhoon phosphorimager. Before determination of titers, the values were corrected for background by subtraction. Each increasing sample dilution was compared to its identically diluted control serum and was considered inhibited by the presence of NAb until it reached at least 50% of control fluorescence. The reciprocal of this dilution is given as the NAb titer.
Statistics.
Group differences were assessed by analysis of variance (ANOVA). The single-administration experiment was analyzed by using a one-way ANOVA. The readministration experiment was analyzed by using a two-way ANOVA. The serotype specificity experiment was analyzed by using a one-way ANOVA. Individual contrasts were performed by using simple-main effects post hoc tests. A Bonferroni correction was made such that the experiment-wise alpha level was maintained at a P value of <0.05.
RESULTS
Preimmunization.
The ultimate goal of this study was to determine whether preimmunization against wt AAV2 would affect the transduction efficiency and immunogenicity of rAAV. To accomplish this, we immunized 56 rats against wt AAV2 and tested them against 48 naive rats. Subsequently, the animals were randomly divided into treatment groups for surgical administration of rAAV and/or sterile saline as a control.
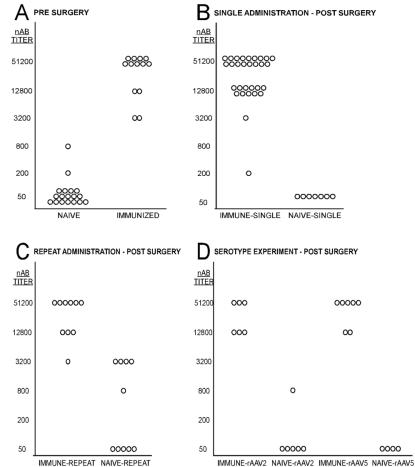
We immunized by peripherally injecting highly purified wt AAV2 (41) mixed with an adjuvant at three time points separated by 2-week intervals. Baseline blood samples were taken from all of the rats, and NAb titers were quantified and compared to those in serum samples at the time of surgery and at the end of the experiment. Figure 2A depicts the presurgical baseline NAb titers for the animals after the immunization procedure. Sera from both the naive and immunized animals were tested. The median titer for the naive animals was 50 (the lowest level of detection), while the immunized animals had a median titer of 51,200. After successful immunization, animals were randomly divided among the three surgical protocols employed in this study. NAb titers were then tested at the time of sacrifice after all surgical procedures had been completed. Postsurgical titers for the single-administration study were similar to the presurgical titers (Fig. 2B), with the median titer of the naive-single group remaining unchanged at 50 and that of the immune-single group also remaining at 51,200. Median postsurgical titers for the repeat-administration study also appeared unchanged, at 51,200 and 50 for the immune-repeat group and naive-repeat groups, respectively (Fig. 2C). However, 5 of 10 naive-repeat animals developed a significant increase in NAb titer after the second vector administration, indicating the possibility of weak immunization by sequential intracerebral injections. In the serotype specificity experiment, median postsurgical titers were similar to those in the single administration study, i.e., 50 in the naive groups and 51,200 in the immunized groups (Fig. 2D).
FIG. 2.
NAb titers. Individual animal serum samples were serially diluted and incubated with a standard amount of rAAV2 expressing GFP. GFP fluorescence intensity was quantified 28 h after transduction in HeLa cells. Pretreatment sera served as the control for each animal. NAb titers are expressed as the reciprocal of the serum dilution required to exceed 50% GFP expression of the identically diluted control serum. (A) Presurgical baseline titers for both the naive and immunized animals. Each of the animals included in the immunized groups displayed high levels of circulating NAb at the time of surgery, while the groups that were not immunized had very low titers. The median titer for the naive animals was 50 (the lowest level of detection), while the immunized animals had a median titer of 51,200. (B) The postsurgical titers for the single-administration study were similar to the presurgical titers, with the median titer of the single-naive group remaining unchanged at 50 and that the immune-single group also remaining at 51,200. (C) The median postsurgical titers for the repeat-administration study were unchanged at 51,200 and 50 for the immune-repeat group and naive-repeat groups, respectively; however, 5 of 10 naive-repeat animals developed a moderate NAb titer after the second vector administration. (D) The median postsurgical titers for the serotype specificity experiment were 50 in the naive groups and 51200 in the immunized groups.
Transgene expression after intrastriatal vector injection and readministration.
Three surgical protocols were employed to investigate the effects of immunization on both single and repeated administrations of rAAV (Fig. 1A to C). Two different transgenes were employed in the following protocols: those for GFP, a xenoprotein that remains intracellular, and for GDNF, which is a stable, secreted protein that is highly similar to its rat counterpart and can be reliably quantified by ELISA. Moreover, we and others have shown that GDNF may be therapeutic in Parkinson's disease in a gene therapy setting (16) and therefore may be a good transgene for gene therapy immunogenicity studies in the brain (13, 25, 26).
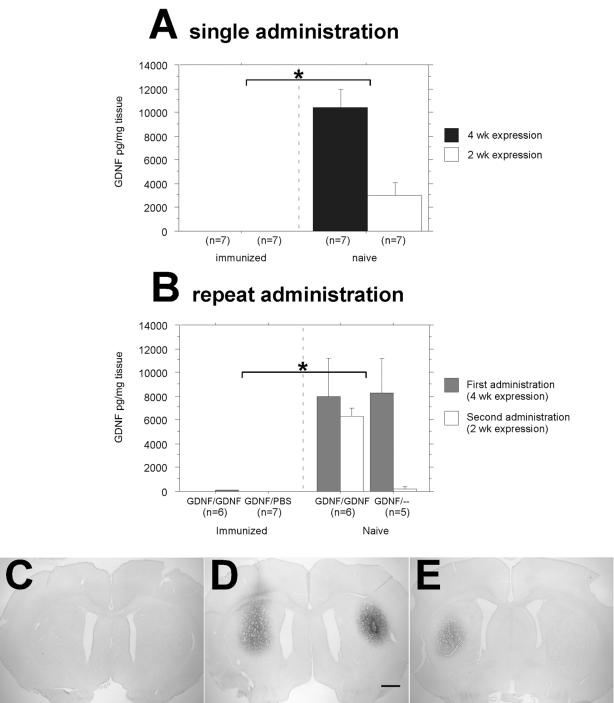
The first surgical strategy was a single-injection protocol (Fig. 1A) and employed a single injection of purified rAAV2-GDNF (Fig. 1D), or sterile saline as a control for the injection itself, in both immunized and naive animals. The groups were further divided into 2- and 4-week survival times, with 2 weeks being the optimal time to observe any adaptive immune response in the brain and 4 weeks being sufficient to observe consistently high levels of GDNF production. Striatal GDNF levels, as determined via ELISA (n = 7 per group), revealed that at both 2 and 4 weeks the naive-single groups had robust levels of GDNF expression, while expression was minimal in the immune-single animals at both time points (Fig. 3A) [F(1,24) = 53.5; P < 0.0001].
FIG. 3.
Intrastriatal GDNF expression as determined by ELISA and immunohistochemistry. (A) Single-administration experiment. Immunization with wt AAV2 completely blocked GDNF expression at both the 2- and 4-week time points after rAAV2-GDNF striatal transduction. This is demonstrated by the lack of measurable GDNF in the immunized group, compared to the robust expression seen in the naive animals (the asterisk indicates statistical significance between results for naive and immunized animals [P < 0.0001]). Error bars indicate standard errors of the mean. (B) Repeat-administration experiment. Immunization with wt AAV2 completely blocked GDNF expression in both the rAAV2-GDNF readministered and rAAV2-GDNF-saline groups. This is demonstrated by the lack of measurable GDNF in both hemispheres of the immunized group, compared to the robust expression seen in the naive animals (the asterisk indicates statistical significance between naive and immunized animals [P < 0.0001]). (C to E) Immunohistochemistry of GDNF expression in repeat-administration experiment. Representative striatal sections were immunostained for human GDNF and are oriented with the first injection side to the right. Bar, 1 mm (applies to all panels). (C) Section from immunized animal after rAAV2-GDNF injection in right striatum, followed by a second administration of rAAV2-GDNF in left striatum 2 weeks later. In agreement with the data in panel B, GDNF expression was undetectable. (D) Section from naive animal after rAAV2-GDNF injection in right striatum, followed by a second administration of rAAV2-GDNF in left striatum 2 weeks later. (E) Section from naive animal 4 weeks after rAAV2-GDNF injection in right striatum, with no further treatment. Right and left are reversed in panels D and E.
The second surgical strategy (Fig. 1B) looked at readministration in both immunized and naive animals. A unilateral injection of rAAV2-GDNF was followed 2 weeks later with a contralateral injection of rAAV2-GDNF or with an injection of sterile saline or no injection as controls (n = 9 to 11 per group). This protocol investigated the effects of reopening the BBB and its effects on new and existing administrations of rAAV2-GDNF. Striatal GDNF expression levels, as determined by ELISA (n = 5 to 7 per group), demonstrated that wt AAV immunization in the immune-repeat rats completely precluded expression of GDNF regardless of surgical treatment, thus confirming the results of the single administration study. However, identically treated naive-repeat rats expressed high levels of GDNF in both hemispheres (Fig. 3B) [F(1,40) = 31.2; P < 0.0001].
These GDNF expression data were completely substantiated by immunohistochemical staining for striatal GDNF (n = 4 per group). Thus, GDNF staining was absent at 4 weeks after transduction in immune-repeat animals (Fig. 3C). In contrast, identically treated naive-repeat rats displayed robust GDNF expression in both hemispheres (Fig. 3D). Similar to the case for the immunized rat shown in Fig. 3C, GDNF immunostaining was undetectable in immune-single rats (data not shown), while naive-single rats displayed abundant striatal GDNF expression 4 weeks after transduction (Fig. 3E).
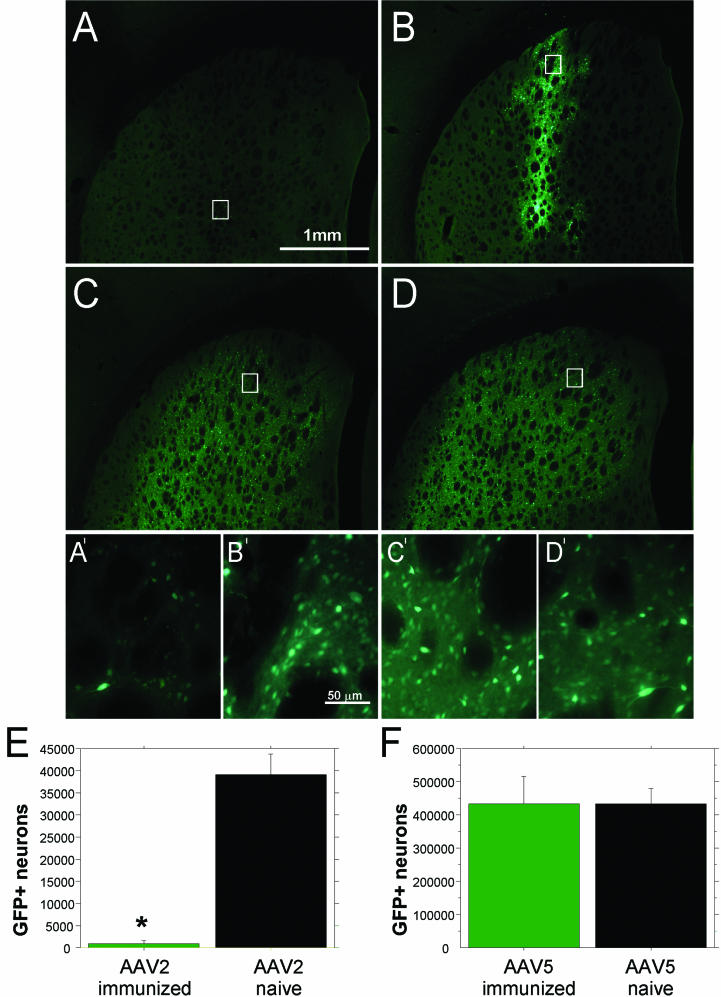
The third surgical strategy (Fig. 1C) was designed to answer questions about serotype specificity. Additionally, GFP was used as the reporter transgene to address whether the previous effects were transgene specific. Rats immunized with wt AAV2 and naive controls received unilateral injections of either purified rAAV2-GFP or purified rAAV5-GFP (Fig. 1E). After 4 weeks, stereological cell counts of GFP-expressing striatal neurons confirmed that transduction was nearly absent in immunized animals that received injections of rAAV2-GFP relative to their naive controls (Fig. 4E) [F(1,10) = 59.48; P < 0.0001]. However, animals immunized against wt AAV2 but receiving rAAV5-GFP injections had no difference in number of GFP-positive cells compared to their naive counterparts (Fig. 4F) [F(1,9) < 0.00001; P > 0.99].
FIG. 4.
GFP expressed in striatal neurons. (A to D) Native fluorescence of transduced neurons in the striatum. Forty-micrometer tissue sections were mounted on subbed slides for GFP visualization. Representative pictures from each treatment group are presented. (A) Animals that received intrastriatal rAAV2-GFP administration after peripheral immunization with wt AAV2 demonstrated very little to no GFP expression. The bar applies to panels A to D. (A′) Magnified view of the highlighted box in panel A, revealing few fluorescent neurons. (B) Naive animals that received intrastriatal rAAV2-GFP administration after no peripheral immunization demonstrated high levels of GFP expression. (B′) Magnified view of the highlighted box in panel B, showing a dense population of fluorescent neurons. The bar applies to panels A′ to D′. (C) Animals that received intrastriatal rAAV5-GFP administration after peripheral immunization with wt AAV2 also demonstrated high levels of GFP expression. (C′) The magnified view of the highlighted box in panel C is similar to that shown in panel B′, demonstrating numerous GFP-positive neurons. (D) Naive animals that received intrastriatal rAAV5-GFP administration again demonstrated high levels of GFP expression, identical to those seen in panel C. (D′) The magnified view of the highlighted box in panel D matches that shown in panels B′ and C′, with a dense population of GFP-positive neurons. (E and F) Quantification of GFP-positive striatal neurons by stereological cell counts. (E) Immunization with wt AAV2 almost completely blocked GFP expression after rAAV2-GFP striatal transduction. This is demonstrated by the significant reduction in GFP-positive neurons in the immunized group, compared to the robust expression seen in the naive animals (the asterisk indicates statistical significance between naive and immunized animals [P < 0.0001]). Error bars indicate standard errors of the means. (F) Immunization with wt AAV2 had no effect on GFP expression after rAAV5-GFP striatal transduction. This is demonstrated by equal numbers of GFP-expressing cells in the immunized group and the naive animals (P > 0.99).
Characterization of the nature of the immune response.
We also investigated the effects of immunization against wt AAV prior to striatal administration of rAAV on the induction of an immune response in the brain. Tissue sections from the 2-week single-administration and repeat-administration studies were analyzed for markers of inflammation, cellular infiltration, and tissue damage. Fixed brains were sliced into 40-μm sections, and one series (every sixth section) was stained with anti-DARPP-32, a sensitive cytoplasmic indicator of striatal tissue damage, and then counterstained with hematoxylin, a standard nuclear stain, to reveal infiltration of leukocytes into the brain parenchyma. In the single-administration study, lymphocytic cellular infiltration was minimal in all animals regardless of the immunization status (data not shown but identical to that seen in Fig. 5H and I) and remained localized to the needle tract. Similarly, we observed no diminution of DARPP-32 in these areas, indicating no damage to striatal neurons. The same pattern was seen in immunized animals that received only a saline injection (Fig. 5H and I).
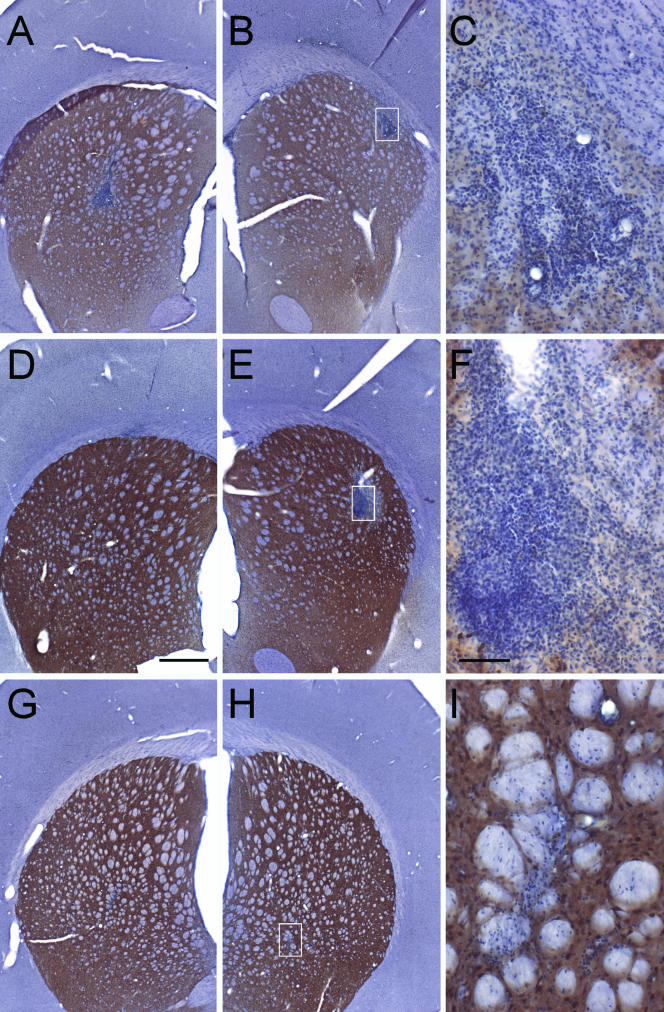
FIG. 5.
Striatal sections immunostained with DARPP-32 and counterstained with hematoxylin. The brown reaction product is DARPP-32, a specific striatal neuronal marker, which is reduced in response to striatal damage. Hematoxylin is a classical nuclear stain used to detect infiltrating leukocytes. Bar in panel D, 500 μm (also applies to panels A, B, E, G, and H). Bar in panel F, 100 μm (also applies to panels C and I). The white boxes in panels B, E, and H illustrate the areas of higher magnification shown in panels C, F, and I, respectively. (A to C) Immunized animal that received rAAV2-GDNF injection in the right striatum (A), followed by a second administration of rAAV2-GDNF in the left striatum 2 weeks later (B). Significant leukocyte infiltration is seen in both hemispheres in immunized animals. Note the association of the infiltrate with blood vessels. Likewise, the DARPP-32 staining is greatly diminished in the injection site (seen in panels A to C compared to D to F and G to I), indicating profuse tissue damage. (D to F) Section from a naive animal after rAAV2-GDNF injection in the right striatum (D), followed by a second administration of rAAV2-GDNF in the left striatum 2 weeks later (E). Leukocyte infiltration is more prevalent in the second injected hemisphere in naive animals. As in panel C, the infiltration seen in panel F is associated with a blood vessel (top of panel). Similarly, DARPP-32 staining was reduced only in the area of the second injection seen in panel F. (G to I) Section from an immunized animal after rAAV2-GDNF injection in the right striatum (G), followed by an injection of sterile saline in the left striatum 2 weeks later (H). Minimal infiltration accompanied the needle tract, and no reduction of DARPP-32 is visible. No sections from the single-injection study are depicted here, but all of the animals (both immunized and naive) had staining similar to that for the saline injection pictured in panels G and H. Left and right are reversed in panels A, B, D, E, G, and H.
In contrast, the DARPP-32-hematoxylin staining pattern observed in the repeat-administration study is indicative of both an innate inflammatory response and an adaptive immune response, even in the presence of robust transgene expression. Of the rats that received two intrastriatal injections of rAAV-GDNF, both immune-repeat and naive-repeat animals exhibited intense perivascular cuffing, lymphocytic extravasation, and lymphocytic infiltration into the striatum corresponding to the intended area of transduction. The animals in the repeat-injection groups survived 2 weeks after the second injection, and because 2 weeks is the optimal time to observe a lymphocytic response in the brain, the most significant infiltration is seen in the second injection site (Fig. 5B, C, E, and F). However, Fig. 5D shows that the infiltration in the first injection sites (4 weeks from the time of injection) of the naive animals subsides, whereas in Fig. 5A, the first injection sites of the immunized animals still contains numerous lymphocytes and evidence of their continued influx. Likewise, DARPP-32 staining was locally decreased in both the first and second injection sites of the immune-repeat animals (Fig. 5A, B, and C), and the normally dark-brown-stained cytoplasm of the striatal neurons was greatly reduced, specifically at or near the injection site (for comparison, see Fig. 5G, H, and I). In contrast, the first injection sites of the naive-repeat animals (Fig. 5D) did not exhibit any loss of DARPP-32 reactivity, indicating no tissue damage at 4 weeks, while the second injection sites of the naive-repeat animals did display loss of DARPP-32 staining, mostly in the area of inflammation (Fig. 5F).
Two additional series of brain sections were stained for the following markers of innate immunity and neuroinflammation: GFAP, which is normally expressed by astrocytes in response to activation, and OX-42, a marker that is upregulated on activated microglial cells and macrophages. In the single-administration study, GFAP staining revealed minimal active astrocytosis localized to the needle tract in both the immune-single and naive-single groups irrespective of the immunization status, and the results appeared to be identical to those for animals treated with only saline (Fig. 6H and I). However, significant differences were found in those animals in the repeat-administration study. Astrocytosis was pronounced in the second injection sites of both naive-repeat and immune-repeat rats (Fig. 6B, C, E, and F), with the most severe reactions observed in the immune-repeat animals (Fig. 6B and C). The central void in the larger areas of astrogliosis corresponds exactly to the areas of lymphocytic infiltration and striatal tissue loss, demonstrated in the DARPP-32-hematoxylin staining. Unlike the case for the infiltrating lymphocytes, the first injection sites of both the naive-repeat and immune-repeat groups (Fig. 6A and D) show that the astrocytes are able to return more quickly to their resting state and do not appear to “reactivate” upon a second injection in the contralateral striatum.
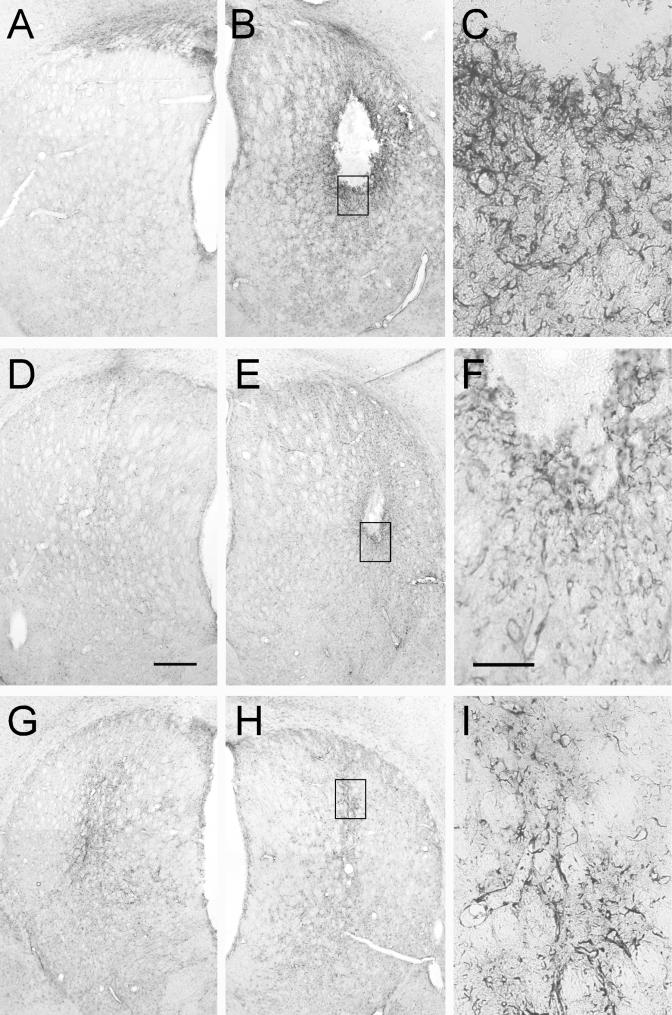
FIG. 6.
Striatal sections immunostained for GFAP. The brown reaction product is GFAP, a specific marker for reactive astrocytes that are normally activated in response to inflammation. Significant tissue reaction was observed only in the animals in the readministration experiment; therefore, all sections presented here are representative of the readministration study. Bar in panel D, 500 μm (also applies to panels A, B, E, G, and H). Bar in panel F, 100 μm (also applies to panels C and I). The black boxes in panels B, E, and H illustrate the areas of higher magnification shown in panels C, F, and I, respectively. (A to C) Immunized animal that received rAAV2-GDNF injection in the right striatum (A), followed by a second administration of rAAV2-GDNF in the left striatum 2 weeks later (B). Significant reactive astrocytosis is seen in the second injection site in the immunized animals and radiates from the area of the injection. (D to F) Section from a naive animal after rAAV2-GDNF injection in the right striatum (D), followed by a second administration of rAAV2-GDNF in the left striatum 2 weeks later (E). Significant reactive astrocytosis is again prevalent in the second injected hemisphere in naive animals. (G to I) Section from a naive animal after rAAV2-GDNF injection in the right striatum (G), followed by an injection of sterile saline in the left striatum 2 weeks later (H). Reactive astrocytosis, in this case, is limited to the needle tract. Left and right are reversed in panels A, B, D, E, G, and H.
Further examination of the tissue revealed activated microglia in a pattern similar to the observed astrocytosis. OX-42 staining in the single-administration animals displayed minimal microgliosis limited to the area of the needle tract, which resembled the results for the saline-treated animals (Fig. 7H and I). In contrast, microgliosis was prominent in the second injection sites of both naive-repeat and immune-repeat rats (Fig. 7B, C, E, and F), with the most severe reactions observed in the immune-repeat animals (Fig. 7B and C). However, unlike the case for the astrocytosis pattern, the first injection site did show the continued activation of microglia in the immune-repeat animals only (Fig. 7A). The first injection sites of the naive-repeat animals (Fig. 7D) again appeared similar to those of the animals that received only one injection (Fig. 7G).
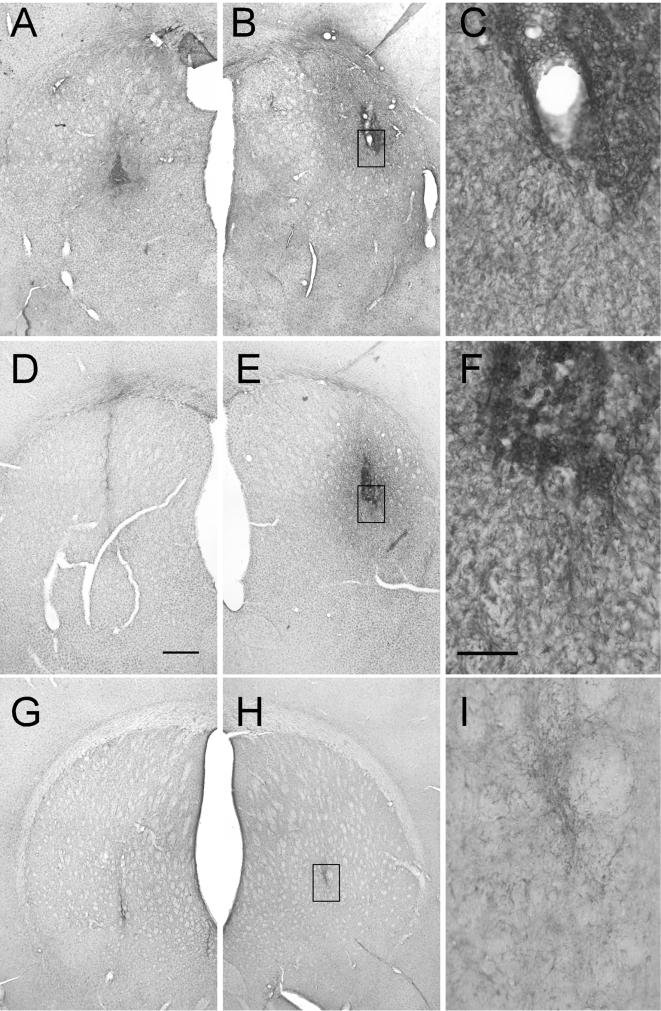
FIG. 7.
Striatal sections immunostained for activated microglia (OX-42). The brown reaction product is OX-42, a specific marker for activated microglia observed in response to inflammation. Significant tissue reaction was observed only in the animals in the readministration experiment; therefore, all sections presented here are representative of the readministration study. Bar in panel D, 500 μm (also applies to panels A, B, E, G, and H). Bar in panel F, 100 μm (also applies to panels C and I). The white boxes in panels B, E, and H illustrate the areas of higher magnification shown in panels C, F, and I, respectively. (A to C) Immunized animal that received rAAV2-GDNF injection in the right striatum (A), followed by a second administration of rAAV2-GDNF in the left striatum 2 weeks later (B). Significant reactive microgliosis is seen in both hemispheres in immunized animals and radiates from the area of the injection. (D to F) Section from a naive animal after rAAV2-GDNF injection in the right striatum (D), followed by a second administration of rAAV2-GDNF in the left striatum 2 weeks later (E). Significant reactive microgliosis is more prevalent in the second injected hemisphere in naive animals. (G to I) Section from a naive animal after rAAV2-GDNF injection in the right striatum (G), followed by an injection of sterile saline in the left striatum 2 weeks later (H). Reactive microgliosis, in this case, is limited to the needle tract. Left and right are reversed in panels A, B, D, E, G, and H.
The extent of lymphocytic infiltration seen in the DARPP-32-hematoxylin staining raised the question of a potential cell-mediated cytotoxic response. To address this, a double-staining procedure was performed, looking for the presence of upregulated MHC1 expression on the surface of virally infected neurons and the simultaneous expression of their coreceptor CD8 found specifically on activated cytotoxic T cells. In the single-administration experiment, the immunized animals exhibited a faint degree of MHC1 interaction with CD8+ cytotoxic T cells, in the area of injection (Fig. 8G). In the naive-single animals (data not shown, but equivalent to that shown in Fig. 8H), MHC1 and CD8 were virtually undetectable and were identical to those in the animals that only received a saline injection (Fig. 8H and I). However, in the repeat-administration study, both the immune-repeat and naive-repeat groups had an abundance of CD8+ T cells, juxtaposed to neurons expressing MHC1 complexes all along their surface in a large, well-defined area that corresponded directly to the infiltration seen in the DARPP-32-hematoxylin staining. This interaction was most significant in the second injection site (Fig. 8B, C, E, and F). Similar to the case for the DARPP-32-hematoxylin stain, the infiltration in the first injection sites (4 weeks survival) of the naive-repeat animals subsided (Fig. 8D), while the first injection sites of the immune-repeat animals still exhibited a profusion of CD8+ cytotoxic T lymphocytes as well as evidence of their continued influx (Fig. 8A).
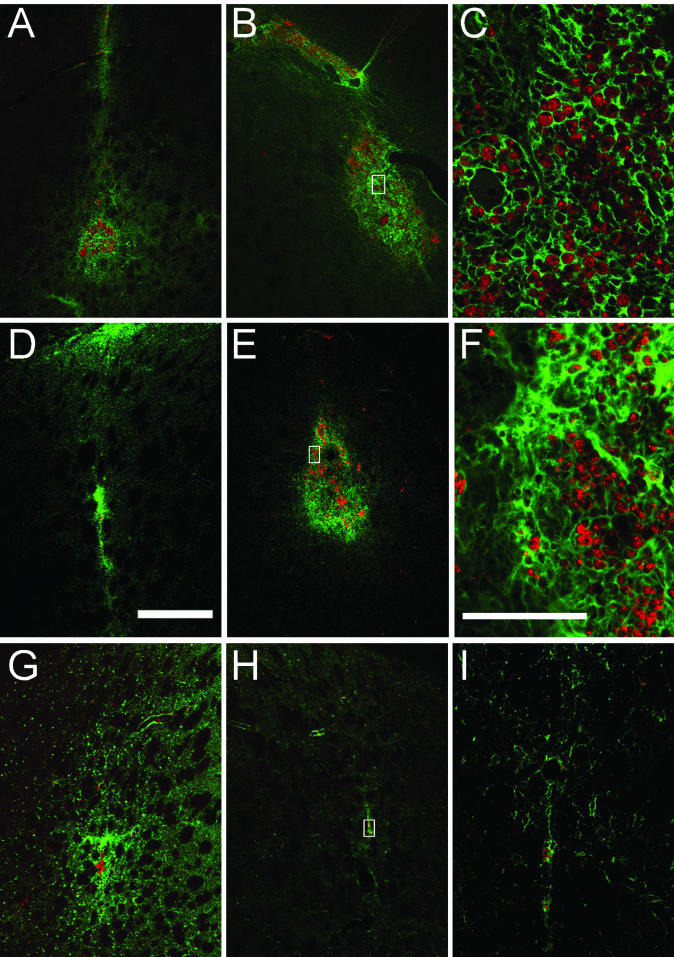
FIG. 8.
Striatal sections immunostained for MHC1, double stained for CD8α, and imaged with confocal microscopy. The green label is rt1-a, a rat-specific MHC1 marker. MHC1 is normally upregulated in virally infected cells. Activated cytotoxic T cells expressing CD8 (alpha subunit), a coreceptor for MHC1 complexes, are labeled in red. All areas injected with rAAV were positive for MHC1 in various degrees, with prominent double staining observed only in the readministration study. For consistency with other figures, all sections presented here are representative of the readministration study. Bar in panel D, 500 μm (also applies to panels A, B, E, G, and H). Bar in panel F, 100 μm (also applies to panels C and I). The white boxes in panels B, E, and H illustrate the areas of higher magnification shown in panels C, F, and I, respectively. (A to C) Immunized animal that received rAAV2-GDNF injection in the right striatum (A), followed by a second administration of rAAV2-GDNF in the left striatum 2 weeks later (B). Significant MHC1 expression and CD8+ infiltration persists in both hemispheres in immunized animals. Note the association of the infiltrate with blood vessels. (D to F) Section from a naive animal after rAAV2-GDNF injection in the right striatum (D), followed by a second administration of rAAV2-GDNF in the left striatum 2 weeks later (E). Significant MHC1 expression and CD8+ infiltration is more prevalent in the second injected hemisphere in naive animals. As in panel C, the infiltration seen in panel F is associated with a blood vessel (just to the right of the white box). (G to I) Section from a naive animal after rAAV2-GDNF injection in the right striatum (G), followed by an injection of sterile saline in the left striatum 2 weeks later (H).
DISCUSSION
The brain is distinguished by its relative immune privilege, including the presence of the BBB and lack of formal antigen-presenting cells (28). Thus, it provides a unique setting in which to study the effects of preimmunization. Like in the periphery, rAAV has been shown to achieve long-term expression in the brain with negligible inflammation in naive animals (27, 30). However, readministration studies reveal differences between the brain and the periphery. While readministration in the periphery may not be possible without immune suppression (17-19, 24, 31, 42, 43), our data and those of others show that rAAV can be readministered in the brain with no reduction of transgene expression, despite low-level production of Ab to the transgene and the capsid (27, 32; C. S. Peden and R. J. Mandel unpublished observations). Taken together, our previous experience and the relative immune privilege of the brain led us to doubt that preimmunization or readministration would pose any immunologically relevant risk to rAAV gene transfer in the brain. On the contrary, our data indicate a more significant involvement of the immune system in the brain than previously thought. In this study, immunization completely abolished transduction in all preimmunized animals, regardless of the transgene used. Quantified GDNF production levels demonstrate unilateral transduction failure in preimmunized animals relative to their naive counterparts. Immunohistochemistry verified that GDNF expression was present only in naive animals, with staining absent in immunized animals. Stereological cell counts of rAAV2-induced GFP-positive cells also revealed transduction failure in immunized animals compared to naive controls.
The immunization protocol employed here reproduced accepted immunization paradigms in the human population to induce humoral immunity in an already well-studied animal model. The use of wt virus was important to mimic the types of Ab generated in a natural infection despite the “unnatural” inoculation methods of immunization. Transduction failure occurred in response to immunization with wt virus irrespective of which transgene was present in the recombinant viruses and is therefore a likely result of an immune response, in the form of NAb to the capsid proteins and structure, which are identical in both the recombinant and the wt viruses. This conclusion is also supported by the differential blockade of two recombinant vectors, rAAV2 and rAAV5, bearing identical genomes and differing only in capsid composition. rAAV5-GFP was able to infect cells successfully despite the presence of high levels of NAb to rAAV2-GFP, while rAAV2-GFP was not. The complete blockade of transduction in the absence of active inflammation or any indication of a CMI response is thought to be due entirely to the presence of NAb, as demonstrated by a quantified NAb assay. This is entirely feasible because the intracerebral injection disrupts the BBB for 24 to 48 h, providing high levels of NAb immediate access to the virus, which completes infection in less than 3 h (1). Some studies that also report failure in peripheral gene transfer protocols allege that NAb generated by preimmunization or previous administration are responsible for the lack of transduction. To date, however, no study could correlate Ab titer with the level of transgene production. Likewise, attempts to correlate NAb titer with levels of GDNF or GFP production in this study have not proved successful, but this may be due to a threshold effect of NAb. Additionally, the circulating NAb are polyclonal and, while collectively quantifiable, may not reflect the same reactivity in individual animals. Finally, future studies will require administration in the presence of various levels of NAb to specifically determine the threshold for transduction.
In the readministration experiment, all groups that expressed GDNF had an equal probability of developing anti-GNDF Ab, but any anti-GDNF Ab produced did not reduce the level of protein in the naive readministration group, which showed equal levels of GDNF at both time points. This is in agreement with the results of Lo et al. (27), who reported that the presence of low-level Ab generated in response to the transgene protein after intracerebral injection had a minimal effect on protein expression. Additionally, the effective Ab responses for transgene and capsid can be distinguished, because GDNF expression was allowed only in naive animals and immunized animals exhibited no GDNF expression. Furthermore, the virtual homology between human and rat GDNF makes it less likely that GDNF itself would be the target of an Ab response. Similarly, in the rAAV-GFP experiment, anti-GFP Ab could have been formed in any animals that produced GFP. Nonetheless, high numbers of GFP-positive cells were found in all animals except the group that was preimmunized against wt AAV2 and then received an injection of rAAV2-GFP. Therefore, almost certainly the confirmed high levels of NAb to the capsid caused the nearly complete transduction failure of the immunized groups. Consequently, as vector persistence and gene transfer success are paramount in most gene therapy protocols, these data suggest that screening for anti-AAV capsid NAb of specific serotypes should be considered in CNS rAAV-based clinical experiments.
The efficacy issues raised in response to a preimmunization paradigm are entirely separate from the inflammation and possible safety concerns inherent in the readministration results. The lack of inflammation in the single-injection groups was in stark contrast to the significant brain inflammation observed in those animals that received two sequential rAAV administrations. Inflammation was present only in those animals with multiple injections, irrespective of GDNF expression, and similarly, those animals that have a single injection do not have inflammation regardless of GDNF expression. Therefore, inflammation does not correlate with GDNF expression in any way. While the levels of transgene expression were unaffected by readministration, there was significant inflammation at the second surgical site for both naive and immunized animals, with inflammation persisting at the first injection sites of the immunized animals. A recent study does report a significant decrease in transgene expression when readministration was performed 2 weeks later (32); however, these disparate results may be due to differences in the expression profiles of luciferase and GDNF. While luciferase turns over rapidly, GDNF has an extremely long half-life in the brain (35). The stability of GDNF may explain the failure to detect a reduction in striatal transgene expression in spite of the presence of significant inflammation as observed in this study. However, transgene expression in the presence of a robust inflammatory response in the brain has been shown repeatedly for adenoviral vectors (6, 10, 23). In addition, Mastakov et al. (32) failed to find brain inflammation 4 weeks after their second rAAV injection. Differences in the inflammation profiles in the studies could be due to the longer survival time after the second injection in their study (4 weeks) compared to the survival time after the second injection in the present study (2 weeks). Indeed, we have previously observed that inflammation induced by readministration of rAAV in immunologically naive animals was undetectable by 4 weeks after the second injection (Peden and Mandel, unpublished observations).
The present study revealed aspects of both an innate and an adaptive immune response. Staining for markers of innate immunity in the single-injection groups revealed the absence of astrocytosis and microgliosis, while identical immunohistochemical staining procedures in the repeat-injection groups revealed significant innate inflammation. This is also in agreement with a recent study that investigated innate immune responses to adenovirus and AAV, which found that a single injection of rAAV was not sufficiently immunogenic to elicit a significant, persistent innate immune response (46). Moreover, the present study shows that the adaptive arm of the immune response also plays a significant role. This is supported by the substantial infiltration of lymphocytes comprised partly of activated cytotoxic T cells, as well as tissue damage revealed by a reduction of DARPP-32 staining precisely in those areas. The juxtaposition of CD8+ T cells and neurons expressing upregulated MHC1 complexes solely in the readministration groups strongly supports a T-cell-mediated response to the rAAV injection with the potential for cytotoxic damage.
Inflammation was limited to both readministration groups irrespective of successful intrastriatal rAAV-mediated transduction. Furthermore, significant inflammation was present in both hemispheres of the immunized animals, while it was present only in the second sites of the naive readministration animals. These observations suggest that the NAb in the immune-single animals immediately bound the virus, thereby preventing transduction, but, lacking a second insult to the BBB, did not encounter enough T cells to prime a response to the future introduction of rAAV. In contrast, in the repeat-administration study, the BBB was disrupted a second time, allowing for the priming of a cellular immune response by the Ab bound to the rAAV at both injection sites in the immunized animals. Likewise, the naive-repeat animals would not have had NAb present at the first injection site. However, in accordance with previous studies (27, 32), the first rAAV administration could have induced Ab to either the transgene or capsid that would then be available at the second injection to activate T cells. The activated T cells would then be available to induce an inflammatory response in the second injection site. Confirming this, 5 of the 10 naive-repeat animals developed moderate NAb titers by the time of sacrifice, including those depicted in Fig. 5 to 8. Correspondingly, in the animals with an immune system primed by a previous intracerebral rAAV injection, astrocytosis and microgliosis appear as an innate response to cytokines secreted by the infiltrating activated T cells. Regardless, it is important to clarify that the immune response in the presence of repeated administrations is not observed in those animals that received only a single vector injection, whether preimmunized or not. Therefore, the presence of inflammation should be attributed not to prior immunization status but to the effects of repeated intrastriatal administration within a short time interval.
Despite the high prevalence of wt AAV2 infection in the human population and the predominance of people with NAb titers (3, 4, 8, 12), there are no rAAV-based CNS gene transfer studies with animals that parallel this situation. While this question has been addressed in peripheral gene transfer paradigms, nothing has been done to address this issue for the brain. We and others have shown that GDNF may be therapeutic in Parkinson's disease in a gene therapy setting (16). In light of this and other potential or existing clinical trials, this question is now of particular importance.
Addressing these questions in an animal model, while not directly comparable to the human population, is an important first step in designing future trials, including studies using naturally infected, nonhuman primates. Likewise, the human NAb titers presented in the literature to date cannot be compared to NAb levels found in the animal models in this and other studies, because previous methods of determination have not been standardized and the studies were performed by using subjective methods (3, 4, 8, 12). Moreover, there may be qualitative differences in circulating NAb derived from an immunization protocol, such as that employed here, as opposed to a naturally acquired respiratory infection in childhood.
It will be important to directly compare these titers to those seen in the human population, and by using the same automated and quantifiable NAb assay outlined in these experiments, it will be possible to do so in future studies. Currently unpublished data from our laboratory indicate that these titer levels are present in the human population, but the frequency is still unknown. In any event, we have shown in this study that immunization can radically reduce transduction efficiency but that utilization of an alternate serotype can circumvent the effect. It is presumable that in addition to utilization of alternative serotypes, capsid alterations and/or immunosuppression at the time of administration may also be helpful in permitting successful gene transfer in the brain of a preimmunized patient. Regardless, knowledge of a patient's immune status will enable treatment strategies to be customized to provide optimal safety and efficacy for the individual.
Thus, the present data unequivocally show that immune status is vitally important to brain transduction, and these novel findings suggest that the immunization status of potential human subjects should be taken into account. AAV's lack of immunogenicity in the brain has been well documented in many studies utilizing single-injection protocols. However, these studies have not attempted to model the case of an AAV-immunized patient and furthermore do not reflect the possibility of an immune response generated after repeated administrations. Therefore, with impending clinical trials it becomes necessary to utilize well-tested methods to clarify these newly identified potential complications. Additional studies specified here, including the use of various titers, transient immunosuppression, and alternate animal models, such as nonhuman primates, become warranted and even compulsory in light of these data.
Acknowledgments
This work was supported by NIH grant PO1 NS36302.
We thank the members of the Powell Gene Therapy Center Vector Core for excellent efforts in production of the rAAV used here. We gratefully acknowledge the comments of W. J. Streit. We thank Wu Xiao for providing the wt AAV used in this study and Mark Potter and Kristen Good for providing the PAGE silver stain gel purification data. We thank Izzie Williams for excellent histological assistance.
N.M. is an inventor on patents related to recombinant AAV technology and owns equity in a gene therapy company that is commercializing AAV for gene therapy applications.
REFERENCES
- 1.Bartlett, J. S., R. Wilcher, and R. J. Samulski. 2000. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J. Virol. 74:2777-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck, S. E., L. A. Jones, K. Chesnut, S. M. Walsh, T. C. Reynolds, B. J. Carter, F. B. Askin, T. R. Flotte, and W. B. Guggino. 1999. Repeated delivery of adeno-associated virus vectors to the rabbit airway. J. Virol. 73:9446-9455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blacklow, N. R., M. D. Hoggan, and W. P. Rowe. 1968. Serologic evidence for human infection with adenovirus-associated viruses. J. Natl. Cancer Inst. 40:319-327. [PubMed] [Google Scholar]
- 4.Blacklow, N. R., M. D. Hoggan, M. S. Sereno, C. D. Brandt, H. W. Kim, R. H. Parrott, and R. M. Chanock. 1971. A seroepidemiologic study of adenovirus-associated virus infection in infants and children. Am. J. Epidemiol. 94:359-366. [DOI] [PubMed] [Google Scholar]
- 5.Brockstedt, D. G., G. M. Podsakoff, L. Fong, G. Kurtzman, W. Mueller-Ruchholtz, and E. G. Engleman. 1999. Induction of immunity to antigens expressed by recombinant adeno-associated virus depends on the route of administration. Clin. Immunol. 92:67-75. [DOI] [PubMed] [Google Scholar]
- 6.Byrnes, A. P., J. E. Rusby, M. J. Wood, and H. M. Charlton. 1995. Adenovirus gene transfer causes inflammation in the brain. Neuroscience 66:1015-1024. [DOI] [PubMed] [Google Scholar]
- 7.Cartmell, T., T. Southgate, G. S. Rees, M. G. Castro, P. R. Lowenstein, and G. N. Luheshi. 1999. Interleukin-1 mediates a rapid inflammatory response after injection of adenoviral vectors into the brain. J. Neurosci. 19:1517-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chirmule, N., K. Propert, S. Magosin, Y. Qian, R. Qian, and J. Wilson. 1999. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 6:1574-1583. [DOI] [PubMed] [Google Scholar]
- 9.Chirmule, N., W. Xiao, A. Truneh, M. A. Schnell, J. V. Hughes, P. Zoltick, and J. M. Wilson. 2000. Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle. J. Virol. 74:2420-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corti, O., A. Sanchez-Capelo, P. Colin, N. Hanoun, M. Hamon, and J. Mallet. 1999. Long-term doxycycline-controlled expression of human tyrosine hydroxylase after direct adenovirus-mediated gene transfer to a rat model of Parkinson's disease. Proc. Natl. Acad. Sci. USA 96:12120-12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Agata, V., W. Zhao, and S. Cavallaro. 2000. Cloning and distribution of the rat parkin mRNA. Brain Res. Mol. Brain Res. 75:345-349. [DOI] [PubMed] [Google Scholar]
- 12.Erles, K., P. Sebokova, and J. R. Schlehofer. 1999. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV). J. Med. Virol. 59:406-411. [DOI] [PubMed] [Google Scholar]
- 13.Gash, D. M., Z. M. Zhang, A. Ovadia, W. A. Cass, A. Yi, L. Simmerman, D. Russell, D. Martin, P. A. Lapchak, F. Collins, B. J. Hoffer, and G. A. Gerhardt. 1996. Functional recovery in parkinsonian monkeys treated with GDNF. Nature 380:252-255. [DOI] [PubMed] [Google Scholar]
- 14.Ge, Y., S. Powell, M. Van Roey, and J. G. McArthur. 2001. Factors influencing the development of an anti-factor IX (fIX) immune response following administration of adeno-associated virus-fIX. Blood 97:3733-3737. [DOI] [PubMed] [Google Scholar]
- 15.Georg-Fries, B., S. Biederlack, J. Wolf, and H. zur Hausen. 1984. Analysis of proteins, helper dependence, and seroepidemiology of a new human parvovirus. Virology 134:64-71. [DOI] [PubMed] [Google Scholar]
- 16.Gill, S. S., N. K. Patel, G. R. Hotton, K. O'Sullivan, R. McCarter, M. Bunnage, D. J. Brooks, C. N. Svendsen, and P. Heywood. 2003. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 9:589-595. [DOI] [PubMed] [Google Scholar]
- 16a.Grimm, D., A. Kern, K. Rittner, and J. A. Kleinschmidt. 1998. Novel tools for production and purification of recombinant viral vectors. Hum. Gene Ther. 9:2745-2760. [DOI] [PubMed] [Google Scholar]
- 17.Halbert, C. L., E. A. Rutledge, J. M. Allen, D. W. Russell, and A. D. Miller. 2000. Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J. Virol. 74:1524-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halbert, C. L., T. A. Standaert, M. L. Aitken, I. E. Alexander, D. W. Russell, and A. D. Miller. 1997. Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J. Virol. 71:5932-5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halbert, C. L., T. A. Standaert, C. B. Wilson, and A. D. Miller. 1998. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J. Virol. 72:9795-9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernandez, Y. J., J. Wang, W. G. Kearns, S. Loiler, A. Poirier, and T. R. Flotte. 1999. Latent adeno-associated virus infection elicits humoral but not cell-mediated immune responses in a nonhuman primate model. J. Virol. 73:8549-8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrlinger, U., C. M. Kramm, K. S. Aboody-Guterman, J. S. Silver, K. Ikeda, K. M. Johnston, P. A. Pechan, R. F. Barth, D. Finkelstein, E. A. Chiocca, D. N. Louis, and X. O. Breakefield. 1998. Pre-existing herpes simplex virus 1 (HSV-1) immunity decreases, but does not abolish, gene transfer to experimental brain tumors by a HSV-1 vector. Gene Ther. 5:809-819. [DOI] [PubMed] [Google Scholar]
- 22.Janson, C., S. McPhee, L. Bilaniuk, J. Haselgrove, M. Testaiuti, A. Freese, D. J. Wang, D. Shera, P. Hurh, J. Rupin, E. Saslow, O. Goldfarb, M. Goldberg, G. Larijani, W. Sharrar, L. Liouterman, A. Camp, E. Kolodny, J. Samulski, and P. Leone. 2002. Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain. Hum. Gene Ther. 13:1391-1412. [DOI] [PubMed] [Google Scholar]
- 23.Kajiwara, K., A. P. Byrnes, H. M. Charlton, M. J. Wood, and K. J. Wood. 1997. Immune responses to adenoviral vectors during gene transfer in the brain. Hum. Gene Ther. 8:253-265. [DOI] [PubMed] [Google Scholar]
- 24.Kessler, P. D., G. M. Podsakoff, X. Chen, S. A. McQuiston, P. C. Colosi, L. A. Matelis, G. J. Kurtzman, and B. J. Byrne. 1996. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc. Natl. Acad. Sci. USA 93:14082-14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirik, D., C. Rosenblad, A. Björklund, and R. J. Mandel. 2000. Long-term rAAV mediated gene transfer of GDNF in the rat Parkinson's model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J. Neurosci. 20:4686-4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kordower, J. H., M. E. Emborg, J. Bloch, S. Y. Ma, Y. Chu, L. Leventhal, J. McBride, E. Y. Chen, S. Palfi, B. Z. Roitberg, W. D. Brown, J. E. Holden, R. Pyzalski, M. D. Taylor, P. Carvey, Z. Ling, D. Trono, P. Hantraye, N. Deglon, and P. Aebischer. 2000. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science 290:767-773. [DOI] [PubMed] [Google Scholar]
- 27.Lo, W. D., G. Qu, T. J. Sferra, R. Clark, R. Chen, and P. R. Johnson. 1999. Adeno-associated virus-mediated gene transfer to the brain: duration and modulation of expression. Hum. Gene Ther. 10:201-213. [DOI] [PubMed] [Google Scholar]
- 28.Lowenstein, P. R. 2002. Immunology of viral-vector-mediated gene transfer into the brain: an evolutionary and developmental perspective. Trends Immunol. 23:23-30. [DOI] [PubMed] [Google Scholar]
- 29.Luo, J., M. G. Kaplitt, H. L. Fitzsimons, D. S. Zuzga, Y. Liu, M. L. Oshinsky, and M. J. During. 2002. Subthalamic GAD gene therapy in a Parkinson's disease rat model. Science 298:425-429. [DOI] [PubMed] [Google Scholar]
- 30.Mandel, R. J., K. G. Rendahl, S. K. Spratt, R. O. Snyder, L. K. Cohen, and S. E. Leff. 1998. Characterization of intrastriatal recombinant adeno-associated virus mediated gene transfer of human tyrosine hydroxylase and human GTP-cyclohydroxylase I in a rat model of Parkinson's disease. J. Neurosci. 18:4271-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manning, W. C., S. Zhou, M. P. Bland, J. A. Escobedo, and V. Dwarki. 1998. Transient immunosuppression allows transgene expression following readministration of adeno-associated viral vectors. Hum. Gene Ther. 9:477-485. [DOI] [PubMed] [Google Scholar]
- 32.Mastakov, M. Y., K. Baer, C. W. Symes, C. B. Leichtlein, R. M. Kotin, and M. J. During. 2002. Immunological aspects of recombinant adeno-associated virus delivery to the mammalian brain. J. Virol. 76:8446-8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monahan, P. E., and R. J. Samulski. 2000. AAV vectors: is clinical success on the horizon? Gene Ther. 7:24-30. [DOI] [PubMed] [Google Scholar]
- 34.Moskalenko, M., L. Chen, M. van Roey, B. A. Donahue, R. O. Snyder, J. G. McArthur, and S. D. Patel. 2000. Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: implications for gene therapy and virus structure. J. Virol. 74:1761-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34a.Peden, C. S., C. Burger, N. Muzyczka, and R. J. Mandel. 2003. The effects of pre-immunization to wt AAV on single and multiple administrations of rAAV in the brain. Mol. Ther. 7:S12. [Google Scholar]
- 35.Rosenblad, C., D. Kirik, and A. Björklund. 2000. Sequential administration of GDNF into the substantia nigra and striatum promotes dopamine neuron survival and axonal sprouting but not striatal reinnervation or functional recovery in the partial 6-OHDA lesion model. Exp. Neurol. 161:503-516. [DOI] [PubMed] [Google Scholar]
- 36.Samulski, R. J., K. I. Berns, M. Tan, and N. Muzyczka. 1982. Cloning of adeno-associated virus into pBR322: rescue of intact virus from the recombinant plasmid in human cells. Proc. Natl. Acad. Sci. USA 79:2077-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas, C. E., D. Birkett, I. Anozie, M. G. Castro, and P. R. Lowenstein. 2001. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol. Ther. 3:36-46. [DOI] [PubMed] [Google Scholar]
- 38.Thomas, C. E., G. Schiedner, S. Kochanek, M. G. Castro, and P. R. Lowenstein. 2000. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc. Natl. Acad. Sci. USA 97:7482-7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas, C. E., G. Schiedner, S. Kochanek, M. G. Castro, and P. R. Lowenstein. 2001. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Hum. Gene Ther. 12:839-846. [DOI] [PubMed] [Google Scholar]
- 40.West, M. J., L. Slomianka, and H. J. Gundersen. 1991. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 231:482-497. [DOI] [PubMed] [Google Scholar]
- 41.Wu, P., W. Xiao, T. Conlon, J. Hughes, M. Agbandje-McKenna, T. Ferkol, T. Flotte, and N. Muzyczka. 2000. Mutational analysis of the adeno-associated virus type 2 (AAV2) capsid gene and construction of AAV2 vectors with altered tropism. J. Virol. 74:8635-8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao, W., N. Chirmule, M. A. Schnell, J. Tazelaar, J. V. Hughes, and J. M. Wilson. 2000. Route of administration determines induction of T-cell-independent humoral responses to adeno-associated virus vectors. Mol. Ther. 1:323-329. [DOI] [PubMed] [Google Scholar]
- 43.Xiao, X., J. Li, and R. J. Samulski. 1996. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J. Virol. 70:8098-8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao, X., J. Li, and R. J. Samulski. 1998. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 72:2224-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu, L., T. Daly, C. Gao, T. R. Flotte, S. Song, B. J. Byrne, M. S. Sands, and K. Parker Ponder. 2001. CMV-beta-actin promoter directs higher expression from an adeno-associated viral vector in the liver than the cytomegalovirus or elongation factor 1 alpha promoter and results in therapeutic levels of human factor X in mice. Hum. Gene Ther. 12:563-573. [DOI] [PubMed] [Google Scholar]
- 46.Zaiss, A. K., Q. Liu, G. P. Bowen, N. C. Wong, J. S. Bartlett, and D. A. Muruve. 2002. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J. Virol. 76:4580-4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zolotukhin, S., B. J. Byrne, E. Mason, I. Zolotukhin, M. Potter, K. Chesnut, C. Summerford, R. J. Samulski, and N. Muzyczka. 1999. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 6:973-985. [DOI] [PubMed] [Google Scholar]
- 48.Zolotukhin, S., M. Potter, I. Zolotukhin, Y. Sakai, S. Loiler, T. J. Fraites, Jr., V. A. Chiodo, T. Phillipsberg, N. Muzyczka, W. W. Hauswirth, T. R. Flotte, B. J. Byrne, and R. O. Snyder. 2002. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods 28:158-167. [DOI] [PubMed] [Google Scholar]
- 49.Zufferey, R., J. E. Donello, D. Trono, and T. J. Hope. 1999. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J. Virol. 73:2886-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]