Abstract
Background
Data suggest that the amygdala and hippocampus contribute to cocaine seeking and use, particularly following exposure to cocaine-related cues and contexts. Furthermore, indices of pre-treatment cocaine-use severity have been shown to correlate with treatment outcome in cocaine-dependent patients.
Methods
The aim of this study was to assess the relationships between amygdalar and hippocampal volumes and cocaine use before and during treatment. High-resolution magnetic-resonance brain images were obtained from 23 cocaine-dependent patients prior to treatment and 54 healthy comparison individuals. Automated segmentation of the amygdala and hippocampus images was performed in FreeSurfer. Cocaine-dependent patients subsequently received behavioral therapy alone or combined with contingency management as part of a treatment trial, and cocaine-use indices (self-report, urine toxicology) were collected.
Results
Comparison participants and cocaine-dependent patients did not show significant difference in amygdalar and hippocampal volumes at pretreatment. Within the patient group, greater hippocampal volumes were correlated with more days of cocaine use before treatment and with poorer treatment outcome as indexed by shorter durations of continuous abstinence from cocaine and lower percentages of cocaine-negative urine samples during treatment. Mediation analysis indicated that pre-treatment hippocampal volumes mediated the relationships between pre-treatment cocaine use and treatment outcomes.
Conclusions
The finding of a significant correlation between hippocampal volume and pre-treatment cocaine-use severity and treatment response suggests that hippocampal volume should be considered when developing individualized treatments for cocaine dependence.
Keywords: addiction, neuroimaging, treatment outcome, brain volume, hippocampus, cocaine, substance use disorder
1. INTRODUCTION
An important goal of clinical research involves identifying predictors of treatment response and their underlying clinical and neural mechanisms, in order to improve treatment outcomes (Donovan et al., 2013; McKay et al., 2001; Potenza et al., 2011; Reiber et al., 2002). In the context of cocaine addiction, severity of pretreatment cocaine use has been one of the measures most consistently related to cocaine use both during and after treatment (Ahmadi et al., 2006, 2009; Carroll et al., 1993; Ciraulo et al., 2003; Poling et al., 2007; Reiber et al., 2002) and to treatment attrition (Alterman et al., 1996; Kampman et al., 2001). However, the mechanism for these relationships are yet unknown, and to our knowledge, the neural mechanisms that underlie them have not been explored.
Models of addiction recognize the amygdala and hippocampus as having key roles in the development and maintenance of addiction (Volkow et al., 2004, 2011). Humans and animals form strong long-term memories of context-response-drug associations after repeated administration of cocaine or other addictive drugs (Buffalari and See, 2010; Crombag et al., 2008). The amygdala and hippocampus each play critical roles in the formation, retrieval, and reconsolidation of such long-term memories. Thus, these regions may contribute to drug-seeking and drug-using behaviors after exposure to stress, cocaine priming, or cocaine-related cues in cocaine-addicted humans and rats (Crombag et al., 2008; Fuchs et al., 2007, 2005; See, 2005; Shaham et al., 2003). For example, exposure to cocaine-related cues increases neural activity and expression of c-fos, a neuronal activity marker, in the amygdala and hippocampus in rats previously treated with cocaine (Brown et al., 1992; Carelli, 2002; Mead et al., 1999; Miller and Marshall, 2004). In addition, lesioning or pharmacological inactivation of either the amygdala or hippocampus attenuates relapse to cocaine-seeking behavior triggered by stress, cocaine priming, or cocaine-related cues (Belujon and Grace, 2011; Fuchs et al., 2007; Gardner, 2011; Grimm and See, 2000; McLaughlin and See, 2003). Findings that amygdalar or hippocampal inactivation or disconnection attenuates relapses to drug-seeking behavior following exposure to cocaine-related cues suggest impaired activation or reconsolidation of long-term memories of context-response-drug associations (Fuchs et al., 2009; Ramirez et al., 2009; Wells et al., 2011).
Consistent with these findings from animal studies, human neuroimaging studies report that stress- or drug cue-induced craving for cocaine use is associated with increased activation in the amygdala, hippocampus, and other brain regions in cocaine-dependent patients (Childress et al., 2008, 1999; Kilts, 2001; Kilts et al., 2004; Kober et al., 2008; Potenza et al., 2012; Prisciandaro et al., 2011; Wilcox et al., 2011; Yalachkov et al., 2012). Therefore, amygdalar and hippocampal function in cocaine-dependent patients may contribute importantly to long-term memories of context-response-drug associations and to cravings for cocaine use after exposure to stress or cocaine-related cues.
Further, both human and animal studies indicate that chronic cocaine use alters the structure and function of multiple brain regions. For example, chronic cocaine administration reduces neurogenesis in the hippocampus of adult rats (Dominguez-Escriba et al., 2006; Garcia-Fuster et al., 2011; Noonan et al., 2008; Sudai et al., 2011; Yamaguchi et al., 2005). Several human neuroimaging studies have found reduced gray-matter volumes in the prefrontal cortex of cocaine-dependent patients relative to healthy comparison participants (Alia-Klein et al., 2011; Ersche et al., 2011; Mackey and Paulus, 2013). However, findings regarding the volumes of the amygdala and hippocampus in cocaine-dependent patients are less consistent. One study reported increased volume in the amygdala (Ersche et al., 2012), several studies reported reduced volume in at least one of the two structures (Alia-Klein et al., 2011; Makris et al., 2004; Moreno-Lopez et al., 2012; Rando et al., 2013), and several studies did not find volumetric differences (Jacobsen et al., 2001; Narayana et al., 2010; Sim et al., 2007); reviewed in (Mackey and Paulus, 2013)).
To investigate further whether the volumes of the amygdala and hippocampus are reduced in cocaine-dependent patients, and the potential role of the two brain structures in cocaine dependence, we used an automated approach to segment the amygdala and hippocampus in cocaine-dependent patients who were imaged just prior to initiating treatment. MRI data were also collected from healthy comparison subjects. Based on extant findings, we hypothesized that: 1) pre-treatment cocaine use would be associated with treatment outcome; and 2) amygdalar and hippocampal volumes would be smaller in patients relative to comparison subjects. We additionally hypothesized (hypothesis 3) that amygdalar and hippocampal volumes would correlate with cocaine use before and during treatment, although both positive and negative correlations were considered as reasonable hypotheses. Specifically, the smaller volumes predicted for cocaine-dependent might suggest that volumes would correlate inversely with drug use. Alternatively, given animal data that lesions to the amygdala or hippocampus may attenuate relapse, a positive correlation might be predicted.
2. METHODS
2.1 Subjects
All patients met DSM-IV criteria for current cocaine dependence and received treatment as outpatients in a randomized clinical trial. Potential subjects were excluded if: (1) they did not speak English, (2) had not used cocaine within the past 28 days, (3) had an untreated psychotic or bipolar disorder which precluded outpatient treatment, or (4) were unlikely to be able to complete 12 weeks of outpatient treatment (e.g., had ongoing legal problems). Psychiatric diagnoses were obtained through structured clinical interview (SCID; First et al., 1997, 1996). Participants from the clinical trial were further screened prior to enrollment in the MRI study subcomponent. Subjects who were pregnant, breast feeding, color-blind, left-handed, or had metal in their body were excluded from participating in the MRI study component. A total of 99 patients participated in the clinical trial. Thirty-eight of them provided MRI T1 brain images. Since the clinical trial included a medication condition, the final sample included in these analyses was restricted to the 23 patients (6 females) who received cognitive behavioral therapies without receiving pharmacotherapy (i.e., disulfiram). Their demographic information and clinical characteristics are presented in Tables 1 and 2. Healthy control subjects were recruited for comparison purposes. Urine samples were assessed for recent use of cocaine, opioids, stimulants, marijuana and benzodiazepines. Control subjects were excluded if urine samples were positive for any substance. Cigarette smokers were included amongst comparison subjects. Other exclusionary criteria for control subjects included pregnancy, left-handedness, current psychiatric diagnoses, or unstable medical conditions. Demographics of comparison subjects are presented in Table 1. All participants provided written informed consent as approved by the Yale School of Medicine Human Investigation Committee.
Table 1.
Demographic information of healthy control participants and cocaine-dependent patients
| Healthy Control N=54 | Cocaine Patients N=23 | p value | |
|---|---|---|---|
| Age, years (SD) | 29.6 (10.1) | 39.7 (8.1) | < .001 |
| Education, years (SD) | 14.9 (1.7) | 12.0 (1.9) | < .001 |
| Female, N (%) | 22 (40.7%) | 6 (26.1%) | .22 |
| Cigarette Smokers, N (%) | 5 (9.3%) | 19 (82.6%) | < .01 |
| Race/Ethnicity, N (%) | < .01 | ||
| Caucasian | 34 (63%) | 7 (30.4%) | |
| African-American | 14 (25.9%) | 12 (52.2%) | |
| Hispanic | 1 (1.9%) | 2 (8.7%) | |
| Multiracial/Other | 5 (9.2%) | 2 (8.7%) |
Data are presented as mean (standard deviation) or as N (percent), as indicated.
Table 2.
Clinical characteristics of cocaine-dependent patients.
| Variable | Cocaine-Dependent Patients (n= 23) |
|---|---|
| Never Married/Living Alone, N (%) | 16 (60.5) |
| Unemployed, N (%) | 19 (82.6) |
| Total number of months incarcerated, lifetime | 32.8 (48.8) |
| Lifetime Psychiatric Diagnoses, N (%) | |
| Alcohol-use disorder | 15 (65.2) |
| Major depression | 6 (21.1) |
| Anxiety disorder | 0 (0) |
| History of Substance Use | |
| Days cocaine use in 28 days prior to treatment | 14.6 (7.2) |
| Days of alcohol use in 28 days prior to treatment | 7.1 (9.0) |
| Years of regular cocaine use, lifetime | 8.2 (5.5) |
Data are presented as mean (standard deviation) or as N (percent), as indicated.
2.2 Treatments
All cocaine-dependent participants received weekly individual cognitive-behavioral therapy (CBT). The goal of CBT is abstinence from cocaine and other substances via functional analysis of high-risk situations, development of effective coping strategies for these situations and for the regulation of craving, and altering maladaptive cognitions associated with the maintenance of cocaine use. All participants also met thrice weekly with an independent clinical evaluator who collected urine and breath samples, and monitored clinical symptoms.
In addition to CBT, 12 (52.2%) cocaine-dependent patients also received contingency management (CM). During CM, chances to draw prizes from a bowl were contingent on treatment adherence (e.g., CBT homework completion) or verified abstinence (submission of cocaine-negative urine specimens), and draws were earned in an escalating schedule using procedures described previously (Ledgerwood and Petry, 2006; Petry, 2000; Petry et al., 2000).
2.3 Clinical assessments and treatment outcome indices
Participants were assessed before treatment, weekly during treatment, and at the 12-week treatment-termination point using the Substance Abuse Calendar which uses the Timeline-Follow-Back method (Fals-Stewart et al., 2000; Hersh et al., 1999) to collect detailed day-by-day self-reports of drug and alcohol use, as described previously (Carroll et al., 2008). Substance-related problems were assessed at pretreatment and post-treatment using the Addiction Severity Index (ASI; McLellan et al., 1992). Urine samples were collected three times each week and tested for cocaine, opioids, stimulants, marijuana and benzodiazepines. Data from all samples were used in the analyses presented. The percent of negative urine samples was over all submitted urine samples, and missing samples were not included in calculation. Primary treatment outcome measures included self-reported longest duration of continuous cocaine abstinence (urine-confirmed) during the 84-day active-treatment period and percentage of cocaine-negative urine samples during treatment, as in prior studies (Brewer et al., 2008; Xu et al., 2010).
2.4 Image acquisition and processing
All MRI images were acquired within one week before treatment. High-resolution T1-weighted anatomical images were acquired using a 3-T scanner (Siemens Trio) with the following parameters: TR=1,500 ms, TE=2.83 ms, flip angle=7°, FOV=256×256 mm2, matrix=256×256, 1 mm3 isotropic voxels, 176 slices. The hippocampus and amygdala were automatically segmented using FreeSurfer version 5.1.0 (http:www.//surfer.nmr.mgh.harvard.edu; Dale et al., 1999; Fischl and Dale, 2000; Fischl et al., 2001). Automated FreeSurfer segmentation is valid and reliable (Doring et al., 2011; Morey et al., 2009). Segmentation of subcortical structures is based on a probabilistic atlas provided by FreeSurfer. The atlas is created by the Center for Morphometric Analysis from 20 unrelated, randomly selected healthy people. The detailed procedure has been described previously (Fischl et al., 2002). In brief, FreeSurfer scripts autorecon1, 2 and 3 were run in sequence on all imaging data. Processing consisted of removal of non-brain tissue, Talairach transformation, segmentation of subcortical volumetric structures including hippocampus and amygdala, intensity normalization, tessellation of the gray-matter/white-matter boundary, and labeling of each voxel based on previous probabilistic information. Intracranial volume (ICV) was generated during this processing.
2.5 Data analyses
SPSS Version 19 was used in analyses of volumetric and clinical data. General Linear Model Univariate was used to assess between-group differences in amygdalar and hippocampal volumes with diagnostic group (cocaine-dependent, healthy comparison) included as a between-subject factor and age, gender, and ICV (which did not differ between groups) included as covariates. Within the cocaine-dependent group, partial correlations were used to assess the relationships between pre-treatment and within-treatment cocaine-use indicators and amygdalar and hippocampal volumes separately for each pair, controlling for effects of ICV, age, gender, and different treatments (i.e., CBT only versus CBT + CM).
2.6 Mediation analysis
Given correlations between 1) pre-treatment cocaine use and treatment outcome, 2) pre-treatment cocaine use and hippocampal volume, and 3) hippocampal volume and treatment outcome, a mediation analysis was used to examine the extent to which hippocampal volume mediated the relationship between pre-treatment cocaine use and treatment outcome. Mediation analyses test whether the relationship between any two variables (e.g., pretreatment drug use and treatment outcome) can be explained by the values from a third variable (e.g., hippocampal volume). If hippocampal volume is a full mediator of the pre-treatment versus within-treatment cocaine use relationship, then the direct association between the two variables will become non-significant when the model controls for hippocampal volume. The mediation analysis used here applied the standard three-variable path model (Shrout and Bolger, 2002) and a bootstrapping test for the statistical significance of the model (Shrout and Bolger, 2002), following our prior work (Kober et al., 2008, 2010). This approach to mediation analysis presumes associations between all three variables under test. As such, any hypothesized relationships (e.g., mediation of pre- and within-treatment cocaine use by amygdalar volume) that did not show the necessary significant correlations were not included in formal mediation analyses. Hippocampal volume, days of cocaine use in a month prior to treatment, and treatment outcome (percent cocaine-negative urine samples during treatment) were correlated, and therefore, were subjected to mediation analyses. Given that both sides of the hippocampus were correlated with the other variables, and were highly inter-correlated, we collapsed across hemispheres to create an “average” volume.
3. RESULTS
Relative to the group receiving CBT only, the patient subgroup receiving CBT plus CM had a significantly longer duration of abstinence (t=2.3, df=21, p=.036) and greater percentage of cocaine-negative urine samples (t=3.3, df=21, p=.003). However, the two patient subgroups showed similar correlations between their pre-treatment and within-treatment indices of cocaine use, and between these indices and volumes of the amygdala and hippocampus (Figures 1 and 2). Therefore, subsequent analyses combine all cocaine-dependent patients into one group, including different treatments as a covariate. In investigating the relationship between clinical variables (hypothesis 1), we found that more days of pre-treatment cocaine use were negatively associated with abstinence within treatment (i.e., lower percentage of cocaine-negative urine samples (r=−.54, p=.02) and fewer maximum days of continuous abstinence from cocaine (r=−.46, p=.05; Fig. 1A, B)), after controlling for age, gender, and different treatments (with or without CM). These relationships were still significant after correcting for multiple (n=2) analyses using a false-discovery-rate (FDR) algorithm.
Figure 1.
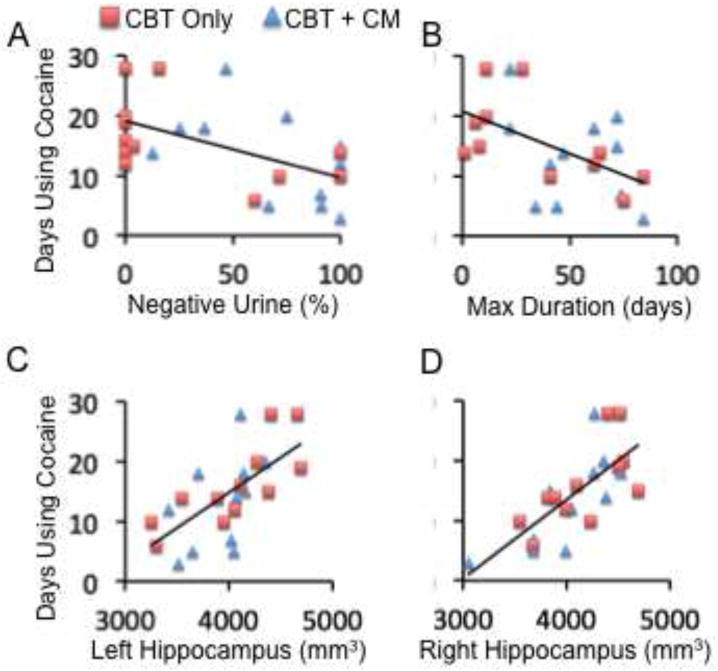
Correlations between pre-treatment cocaine use and treatment outcomes and volumes of brain structures. A and B: Correlations between number of days using cocaine within 28 days before enrollment into treatment (Y axis) and percentage of cocaine-negative urine samples and the maximum duration (days) of contiguous abstinence from cocaine during treatment, respectively. C and D: Correlations between number of days using cocaine within 28 days before enrollment into treatment (Y axis) and the volume of the left and right hippocampus (mm3), respectively. The red squares and blue triangles represent patients who received “Cognitive Behavioral Therapy (CBT) only” and “CBT + Contingency Management (CM)”, respectively.
Figure 2.
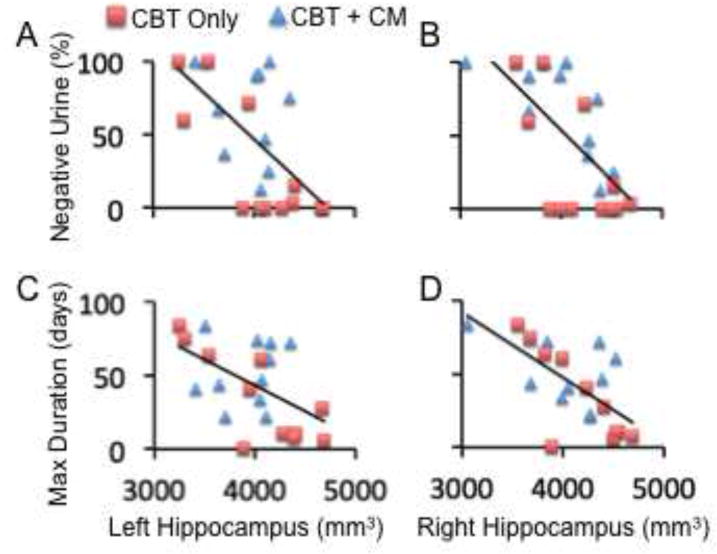
Correlations between treatment outcomes and volumes of brain structures. A and B: Correlations between percentage of cocaine-negative urine samples during treatment (Y axis) and the volume of the left and right hippocampus (mm3), respectively. C and D: Correlations between the maximum duration (days) of contiguous abstinence from cocaine during treatment (Y axis) and the volume of the left and right hippocampus (mm3), respectively. The red squares and blue triangles represent patients who received “Cognitive Behavioral Therapy (CBT) only” and “CBT + Contingency Management (CM)”, respectively.
Table 3 shows the volumes of the amygdala and hippocampus. Healthy participants and cocaine-dependent patients did not show significant differences in volumes of the amygdala and hippocampus, after controlling for age, gender, and ICV, and this finding did not support our hypothesis of reduced volumes in patients.
Table 3.
Volumes of the amygdala and hippocampus in mm3 (Mean (SD))
| Amygdala | Hippocampus | |||
|---|---|---|---|---|
| Left | Right | Left | Right | |
|
Healthy Controls (n=54) |
1725.9 (274.2) |
1832.6 (257.3) |
4048.4 (527.3) |
4188.5 (466.9) |
|
Cocaine-Dependent (n=23) |
1627.5 (223.2) |
1745.6 (226.9) |
3986.6 (402.5) |
4087.4 (399.6) |
Within the cocaine-dependent patients, we found significant positive correlations between hippocampal volumes and cocaine-use measures, consistent with hypothesis 3. Specifically, more days of self-reported cocaine use within the 28 days before enrollment into the clinical trial were associated with greater hippocampal volumes after controlling for ICV, age, and gender (Figure 1C, 1D). In turn, greater hippocampal volumes were associated with poorer treatment outcomes, measured by lower percentages of cocaine-negative urine samples and shorter durations of maximum consecutive days of cocaine-abstinence during treatment (negative correlation; Figure 2, Table 4) after controlling for ICV, age, gender, and different treatments (i.e., CBT vs. CBT + CM). These relationships remained significant after FDR correction for multiple analyses (n=12).
Table 4.
Correlations between volumes of the amygdala and hippocampus and cocaine use before and during treatment.
| Cocaine Use Indicators | Amygdala | Hippocampus | |||
|---|---|---|---|---|---|
| L | R | L | R | ||
| Mean (SD) | Correlations (r) | ||||
| Cocaine Use Days Before Treatment | 14.6 (7.2) | .419 | −.07 | .60** | .71** |
| Longest Abstinence During Treatment | 43.8 (27.4) | −.22 | .05 | −.39 | −.57* |
| % Negative Urines During Treatment | 47.6 (41.4) | .05 | .25 | −.55* | −.63** |
Cocaine Use Indicator Descriptions: Cocaine Use Days Before Treatment: number of days of cocaine use within 28 days immediately before treatment onset; Longest Abstinence During Treatment: maximum number of days of contiguous abstinence from cocaine during treatment; %Negative Urines During Treatment: percent of cocaine-negative urine samples during treatment. L= left, R=right. Correlations between cocaine use indicators and amygdalar and hippocampal volumes are presented as Pearson’s correlation coefficients (r) with significance levels indicated by
p< .05 and
p < .01 before correction for multiple analyses.
These correlations could suggest one of two possible relationships between pre-treatment cocaine use, hippocampal volumes, and treatment outcomes. The first possibility is that pre-treatment cocaine use and hippocampal volume are independently related to (and possibly contribute to) treatment outcome. The second possibility is that the relationship between pre-treatment cocaine use and treatment outcome is mediated by pre-treatment hippocampal volume. To test these possibilities, we preformed a formal mediation analysis that explicitly tested whether the relationship between pretreatment drug use and percentage of cocaine-negative urine samples during treatment was explained by participants’ pre-treatment hippocampal volume. We found that hippocampal volume fully mediated this relationship (Figure 3), meaning that including hippocampal volume in the mediation model made the relationship between pre-treatment cocaine use and percent of cocaine-negative urine samples during treatment no longer statistically significant.
Figure 3.
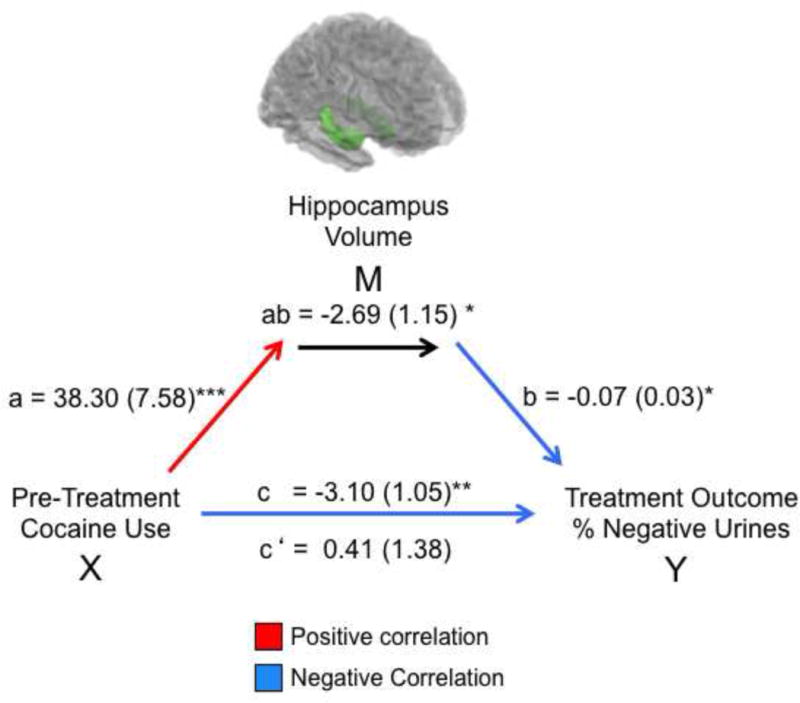
Mediation model for the association between pre-treatment cocaine use (X, in the 28 days prior to treatment), hippocampus volume at treatment onset (M, measured as the average volume of the left and right hippocampus), and treatment outcome (Y, calculated as percentage of cocaine-negative urine samples during treatment – where higher percentage represents better outcome). Path coefficients are shown next to arrows indicating each link in the analysis, with standard errors in parentheses. Path a refers to the path from X to M. Path b refers to the direct link between M and Y. Paths c and c’ refer to the association between X and Y, with and without the mediator M, respectively.* p<.05, ** p<.01, *** p<.001.
4. DISCUSSION
The main findings were that: 1) pre-treatment cocaine use negatively correlated with cocaine abstinence during treatment; and 2) pretreatment hippocampal volume positively correlated with cocaine use before and during treatment, and mediated the positive correlation between the two measures. However, cocaine-dependent and comparison subjects did not show significant differences in amygdalar and hippocampal volumes.
4.1 Hippocampal volume and cocaine use before and during treatment
As discussed in the introduction, several models of addictions implicate the amygdala and hippocampus in the maintenance of drug-taking behavior. Preclinical research indicates that the structure and function of the amygdala and hippocampus are important to cue-induced drug-seeking and drug-using behavior. It is possible that individuals with larger hippocampus may have greater hippocampal functional activities, form stronger long-term memories of context-response-drug associations after repeated administration of cocaine, experience more frequent and/or stronger craving for cocaine use in the environment with cocaine cues, and finally use cocaine more frequently. The current findings of significant correlations between hippocampal volume at treatment onset and both pre-treatment and within-treatment cocaine use are consistent with this prediction.
Another possible explanation for the positive correlation between hippocampal volume and cocaine use is gliosis in the hippocampus. Cocaine use may lead to gliosis in the hippocampus and increase its volume (Fattore et al., 2002; Narayana et al., 2010). Therefore, it is possible that more pre-treatment cocaine use may induce greater gliosis and generate larger hippocampal volumes, which may then predispose to poorer treatment outcome. Additionally, as previously proposed (Di Sclafani et al., 2002; Narayana et al., 2010), cocaine’s propensities to induce hippocampal gliosis may obscure effects of hippocampal neuronal loss. The results of the current study cannot identify which factor (e.g., greater hippocampal functional activity, greater gliosis in the hippocampus after cocaine use, or other factors) relates to more cocaine use as observed in the current study. This issue should be addressed in the future studies, because different neuropathophysiological mechanisms underlying the positive correlations between hippocampal volume and severity of cocaine use may require different treatment strategies.
The current finding that, within our mediation model, hippocampal volume statistically fully mediated the relationship between pre-treatment and within-treatment cocaine use indicates that the correlation between pre-treatment and within-treatment cocaine use becomes non-significant after accounting for individual variation in hippocampal volume. However, it does not demonstrate causality and does not preclude the possibility of additional factors (that were not included in our model) also mediating this relationship, nor does it demonstrate a change during treatment. Further investigation should directly test whether hippocampal volume changes during treatment in accordance with changes in drug use.
The current hippocampal findings also do not indicate that the hippocampus is the only brain structure that may link pre-treatment and within-treatment cocaine use. Subcortical structures such as the amygdala, hippocampus, and their output target nucleus accumbens are regulated by top-down executive control from the frontoparietal cortex (Banich et al., 2009; Corbetta et al., 2008; Hollmann et al., 2012). It has been proposed that these subcortical structures may exert a greater influence on human behavior if the top-down executive control is reduced due to the impairment of the frontoparietal cortex (Everitt and Robbins, 2005; Volkow et al., 2011). Chronic cocaine use alters multiple extensive cortical and subcortical regions including the frontoparietal cortex and striatum. Therefore, reduced top-down executive control from the frontoparietal cortex and changed subcortical functional activity might relate importantly to cocaine use. Consistent with this view, cocaine use correlated with poorer white-matter integrity in extensive regions including corpus callosum and the frontal lobes (Xu et al., 2010) and with functional activity during a cognitive-control task (i.e., Stroop task), wherein task-related activity in a subcortical network involving the hippocampus, amygdala, striatum and thalamus correlated with urine-toxicology-assessed treatment outcomes (Brewer et al., 2008; Worhunsky et al., 2013). The relationships between integrity of brain structure other than hippocampus and amygdala and severity of cocaine use should be assessed in future studies.
4.2 Amygdalar and hippocampal volumes
As mentioned in the introduction, previous findings regarding the volumes of the hippocampus and amygdala in cocaine-dependent patients are not consistent. The current finding of no significant difference between patients and healthy participants is consistent with the findings from the majority of previously published studies. Several factors could contribute to the inconsistent findings in literature, including small sample sizes, cocaine-use status (i.e., abstinent vs. non-abstinent) and other clinical characteristics, and methods for measuring amygdalar and hippocampal volumes. These factors should be evaluated in future studies.
A notable limitation of this study is the small sample size with two different treatments (47.8% of the patients received “CBT only” while the remaining patients received “CBT plus CM”). However, the correlation between hippocampal volume, pre-treatment cocaine use, and percentage of cocaine-negative urine samples during treatment remained significant after controlling for different treatments.
4.3 Implications of the present findings
This is the first study to suggest that the often-found association between pre-treatment and within-treatment cocaine use is mediated by individual volumetric differences in a brain structure: the hippocampus. The current findings that larger hippocampal volumes are not only associated with poorer treatment outcome but also mediate the relationship between pretreatment and within-treatment cocaine use have multiple implications for 1) a basic understanding of cocaine dependence, 2) the neural substrates underlying treatment effects, and 3) improving future treatments. Specifically, the findings suggest a possible neural mechanism that may underlie the relationship between pre-treatment and within-treatment cocaine use. If further studies provide additional support for this hypothesis, it would suggest that possible neuroprotective agents might be considered as an intervention to mitigate neurotoxic effects of cocaine on the hippocampus. As reviewed in the introduction, the hippocampus contributes importantly to the formation, retrieval, and reconsolidation of long-term memories of context-response-drug associations, and thus may contribute to cue-induced drug craving and drug use in cocaine-dependent patients (Crombag et al., 2008; Fuchs et al., 2007, 2005; See, 2005; Shaham et al., 2003). Taken together with our current findings, it is possible that cocaine-dependent patients with larger hippocampal volumes may experience more frequent and/or stronger drug cravings in response to drug-cue environments or to drug-cue stimuli, and thus may fare worse in treatment, although this possibility remains speculative and warrants direct investigation. If this is the case, treatments like CBT that emphasize how to avoid drug-use cues and contexts and how to regulate cue-induced craving may benefit from augmentation with psychological or pharmacological strategies for enhancing cognitive control, although this possibility too warrants direct examination. Future studies should examine both hippocampal function and volume in relationship to treatment outcome in cocaine dependence.
In summary, the findings of hippocampal volumes correlating with both pre-treatment and within-treatment cocaine use and mediating the relationships between these measures, indicate an important role for the hippocampus in the treatment of cocaine dependence and suggest possible new avenues for treatment development.
Acknowledgments
Funding:This study was funded by the following grants: National Institute on Drug Abuse (NIDA) grants K01 DA027750, P50 DA09241, R01 DA020908, P20DA027844, K12 DA00167, and R01 DA035058. EED was supported by K12 DA031050 from ORWH, NIDA, NIAAA and OD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributers: Xu, Potenza, and Carroll designed the study, Xu, Kober and Wang analyzed the data, and all authors contributed to write the paper.
Conflict of Interest: All authors declare no conflict of interest with the content of this manuscript. Dr. Potenza has consulted for Lundbeck, Ironwood and Shire pharmaceuticals; has had financial interests in Somaxon pharmaceuticals; received research support from Mohegan Sun Casino, Psyadon pharmaceuticals, the National Center for Responsible Gambling, the National Institutes of Health (NIH), Veterans Administration; has participated in surveys, mailings, or telephone consultations related to drug addiction, impulse-control disorders, or other health topics; has consulted for gambling, legal and governmental entities on issues related to addictions or impulse-control disorders; has provided clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the NIH and other agencies; has guest edited journal sections; has given academic lectures in grand rounds, Continuing Medical Education events, and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts.
References
- Ahmadi J, Kampman K, Dackis C. Outcome predictors in cocaine dependence treatment trials. Am J Addict. 2006;15:434–439. doi: 10.1080/10550490600998476. [DOI] [PubMed] [Google Scholar]
- Ahmadi J, Kampman KM, Oslin DM, Pettinati HM, Dackis C, Sparkman T. Predictors of treatment outcome in outpatient cocaine and alcohol dependence treatment. Am J Addict. 2009;18:81–86. doi: 10.1080/10550490802545174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alia-Klein N, Parvaz MA, Woicik PA, Konova AB, Maloney T, Shumay E, Wang R, Telang F, Biegon A, Wang GJ, Fowler JS, Tomasi D, Volkow ND, Goldstein RZ. Gene x disease interaction on orbitofrontal gray matter in cocaine addiction. Arch Gen Psychiatry. 2011;68:283–294. doi: 10.1001/archgenpsychiatry.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alterman AI, McKay JR, Mulvaney FD, McLellan AT. Prediction of attrition from day hospital treatment in lower socioeconomic cocaine-dependent men. Drug Alcohol Depend. 1996;40:227–233. doi: 10.1016/0376-8716(95)01212-5. [DOI] [PubMed] [Google Scholar]
- Banich MT, Mackiewicz KL, Depue BE, Whitmer AJ, Miller GA, Heller W. Cognitive control mechanisms, emotion and memory: a neural perspective with implications for psychopathology. Neurosci Biobehav Rev. 2009;33:613–630. doi: 10.1016/j.neubiorev.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Hippocampus, amygdala, and stress: interacting systems that affect susceptibility to addiction. Ann N Y Acad Sci. 2011;1216:114–121. doi: 10.1111/j.1749-6632.2010.05896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biol Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GR, Rundell JR, McManis SE, Kendall SN, Zachary R, Temoshok L. Prevalence of psychiatric disorders in early stages of HIV infection. Psychosom Med. 1992;54:588–601. doi: 10.1097/00006842-199209000-00006. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Amygdala mechanisms of Pavlovian psychostimulant conditioning and relapse. Curr Top Behav Neurosci. 2010;3:73–99. doi: 10.1007/7854_2009_18. [DOI] [PubMed] [Google Scholar]
- Carelli RM. Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. ‘natural’ reinforcement. Physiol Behav. 2002;76:379–387. doi: 10.1016/s0031-9384(02)00760-6. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Power ME, Bryant K, Rounsaville BJ. One-year follow-up status of treatment-seeking cocaine abusers. Psychopathology and dependence severity as predictors of outcome. J Nerv Ment Dis. 1993;181:71–79. doi: 10.1097/00005053-199302000-00001. [DOI] [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, Jens W, Suh J, Listerud J, Marquez K, Franklin T, Langleben D, Detre J, O’Brien CP. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS One. 2008;3:e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciraulo DA, Piechniczek-Buczek J, Iscan EN. Outcome predictors in substance use disorders. Psychiatr Clin North Am. 2003;26:381–409. doi: 10.1016/s0193-953x(02)00106-5. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Di Sclafani V, Tolou-Shams M, Price LJ, Fein G. Neuropsychological performance of individuals dependent on crack-cocaine, or crack-cocaine and alcohol, at 6 weeks and 6 months of abstinence. Drug Alcohol Depend. 2002;66:161–171. doi: 10.1016/s0376-8716(01)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Escriba L, Hernandez-Rabaza V, Soriano-Navarro M, Barcia JA, Romero FJ, Garcia-Verdugo JM, Canales JJ. Chronic cocaine exposure impairs progenitor proliferation but spares survival and maturation of neural precursors in adult rat dentate gyrus. Eur J Neurosci. 2006;24:586–594. doi: 10.1111/j.1460-9568.2006.04924.x. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Knox PC, Skytta JA, Blayney JA, DiCenzo J. Buprenorphine from detox and beyond: preliminary evaluation of a pilot program to increase heroin dependent individuals’ engagement in a full continuum of care. J Subst Abuse Treat. 2013;44:426–432. doi: 10.1016/j.jsat.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Doring TM, Kubo TT, Cruz LC, Jr, Juruena MF, Fainberg J, Domingues RC, Gasparetto EL. Evaluation of hippocampal volume based on MR imaging in patients with bipolar affective disorder applying manual and automatic segmentation techniques. J Magn Reson Imaging. 2011;33:565–572. doi: 10.1002/jmri.22473. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2011;134:2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol. 2000;68:134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- Fattore L, Puddu MC, Picciau S, Cappai A, Fratta W, Serra GP, Spiga S. Astroglial in vivo response to cocaine in mouse dentate gyrus: a quantitative and qualitative analysis by confocal microscopy. Neuroscience. 2002;110:1–6. doi: 10.1016/s0306-4522(01)00598-x. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II): User’s Guide. American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders- Patient Edition (SCID-IP, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1996. [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Bell GH, Ramirez DR, Eaddy JL, Su ZI. Basolateral amygdala involvement in memory reconsolidation processes that facilitate drug context-induced cocaine seeking. Eur J Neurosci. 2009;30:889–900. doi: 10.1111/j.1460-9568.2009.06888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Garcia-Fuster MJ, Flagel SB, Mahmood ST, Mayo LM, Thompson RC, Watson SJ, Akil H. Decreased proliferation of adult hippocampal stem cells during cocaine withdrawal: possible role of the cell fate regulator FADD. Neuropsychopharmacology. 2011;36:2303–2317. doi: 10.1038/npp.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EL. Addiction and brain reward and antireward pathways. Adv Psychosom Med. 2011;30:22–60. doi: 10.1159/000324065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, See RE. Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology. 2000;22:473–479. doi: 10.1016/S0893-133X(99)00157-8. [DOI] [PubMed] [Google Scholar]
- Hersh D, Mulgrew CL, Van Kirk J, Kranzler HR. The validity of self-reported cocaine use in two groups of cocaine abusers. J Consult Clin Psychol. 1999;67:37–42. doi: 10.1037//0022-006x.67.1.37. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hellrung L, Pleger B, Schlogl H, Kabisch S, Stumvoll M, Villringer A, Horstmann A. Neural correlates of the volitional regulation of the desire for food. Int J Obes (Lond) 2012;36:648–655. doi: 10.1038/ijo.2011.125. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Giedd JN, Kreek MJ, Gottschalk C, Kosten TR. Quantitative medial temporal lobe brain morphology and hypothalamic-pituitary-adrenal axis function in cocaine dependence: a preliminary report. Drug Alcohol Depend. 2001;62:49–56. doi: 10.1016/s0376-8716(00)00159-9. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Alterman AI, Volpicelli JR, Maany I, Muller ES, Luce DD, Mulholland EM, Jawad AF, Parikh GA, Mulvaney FD, Weinrieb RM, O’Brien CP. Cocaine withdrawal symptoms and initial urine toxicology results predict treatment attrition in outpatient cocaine dependence treatment. Psychol Addict Behav. 2001;15:52–59. doi: 10.1037/0893-164x.15.1.52. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood DM, Petry NM. What do we know about relapse in pathological gambling? Clin Psychol Rev. 2006;26:216–228. doi: 10.1016/j.cpr.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Mackey S, Paulus M. Are there volumetric brain differences associated with the use of cocaine and amphetamine-type stimulants? Neurosci Biobehav Rev. 2013;37:300–316. doi: 10.1016/j.neubiorev.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Gasic GP, Seidman LJ, Goldstein JM, Gastfriend DR, Elman I, Albaugh MD, Hodge SM, Ziegler DA, Sheahan FS, Caviness VS, Jr, Tsuang MT, Kennedy DN, Hyman SE, Rosen BR, Breiter HC. Decreased absolute amygdala volume in cocaine addicts. Neuron. 2004;44:729–740. doi: 10.1016/j.neuron.2004.10.027. [DOI] [PubMed] [Google Scholar]
- McKay JR, Merikle E, Mulvaney FD, Weiss RV, Koppenhaver JM. Factors accounting for cocaine use two years following initiation of continuing care. Addiction. 2001;96:213–225. doi: 10.1046/j.1360-0443.2001.9622134.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argerious M. The Fifth Edition Of The Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Mead AN, Vasilaki A, Spyraki C, Duka T, Stephens DN. AMPA-receptor involvement in c-fos expression in the medial prefrontal cortex and amygdala dissociates neural substrates of conditioned activity and conditioned reward. Eur J Neurosci. 1999;11:4089–4098. doi: 10.1046/j.1460-9568.1999.00828.x. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered prelimbic cortex output during cue-elicited drug seeking. J Neurosci. 2004;24:6889–6897. doi: 10.1523/JNEUROSCI.1685-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Lopez L, Catena A, Fernandez-Serrano MJ, Delgado-Rico E, Stamatakis EA, Perez-Garcia M, Verdejo-Garcia A. Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug Alcohol Depend. 2012;125:208–214. doi: 10.1016/j.drugalcdep.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, 2nd, Lewis DV, LaBar KS, Styner M, McCarthy G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45:855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayana PA, Datta S, Tao G, Steinberg JL, Moeller FG. Effect of cocaine on structural changes in brain: MRI volumetry using tensor-based morphometry. Drug Alcohol Depend. 2010;111:191–199. doi: 10.1016/j.drugalcdep.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MA, Choi KH, Self DW, Eisch AJ. Withdrawal from cocaine self-administration normalizes deficits in proliferation and enhances maturity of adult-generated hippocampal neurons. J Neurosci. 2008;28:2516–2526. doi: 10.1523/JNEUROSCI.4661-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. A comprehensive guide to the application of contingency management procedures in clinical settings. Drug Alcohol Depend. 2000;58:9–25. doi: 10.1016/s0376-8716(99)00071-x. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes, and they will come: contingency management for treatment of alcohol dependence. J Consult Clin Psychol. 2000;68:250–257. doi: 10.1037//0022-006x.68.2.250. [DOI] [PubMed] [Google Scholar]
- Poling J, Kosten TR, Sofuoglu M. Treatment outcome predictors for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33:191–206. doi: 10.1080/00952990701199416. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry. 2012;169:406–414. doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Sofuoglu M, Carroll KM, Rounsaville BJ. Neuroscience of behavioral and pharmacological treatments for addictions. Neuron. 2011;69:695–712. doi: 10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisciandaro JJ, McRae-Clark AL, Moran-Santa Maria MM, Hartwell KJ, Brady KT. Psychoticism and neuroticism predict cocaine dependence and future cocaine use via different mechanisms. Drug Alcohol Depend. 2011;116:80–85. doi: 10.1016/j.drugalcdep.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez DR, Bell GH, Lasseter HC, Xie X, Traina SA, Fuchs RA. Dorsal hippocampal regulation of memory reconsolidation processes that facilitate drug context-induced cocaine-seeking behavior in rats. Eur J Neurosci. 2009;30:901–912. doi: 10.1111/j.1460-9568.2009.06889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando K, Tuit K, Hannestad J, Guarnaccia J, Sinha R. Sex differences in decreased limbic and cortical grey matter volume in cocaine dependence: a voxel-based morphometric study. Addict Biol. 2013;18:147–160. doi: 10.1111/adb.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiber C, Ramirez A, Parent D, Rawson RA. Predicting treatment success at multiple timepoints in diverse patient populations of cocaine-dependent individuals. Drug Alcohol Depend. 2002;68:35–48. doi: 10.1016/s0376-8716(02)00103-5. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, Kim MJ, Kaufman MJ, Yurgelun-Todd DA, Renshaw PF. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;32:2229–2237. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- Sudai E, Croitoru O, Shaldubina A, Abraham L, Gispan I, Flaumenhaft Y, Roth-Deri I, Kinor N, Aharoni S, Ben-Tzion M, Yadid G. High cocaine dosage decreases neurogenesis in the hippocampus and impairs working memory. Addict Biol. 2011;16:251–260. doi: 10.1111/j.1369-1600.2010.00241.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AM, Lasseter HC, Xie X, Cowhey KE, Reittinger AM, Fuchs RA. Interaction between the basolateral amygdala and dorsal hippocampus is critical for cocaine memory reconsolidation and subsequent drug context-induced cocaine-seeking behavior in rats. Learn Mem. 2011;18:693–702. doi: 10.1101/lm.2273111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend. 2011;115:137–144. doi: 10.1016/j.drugalcdep.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worhunsky PD, Stevens MC, Carroll KM, Rounsaville BJ, Calhoun VD, Pearlson GD, Potenza MN. Functional brain networks associated with cognitive control, cocaine dependence, and treatment outcome. Psychol Addict Behav. 2013;27:477–488. doi: 10.1037/a0029092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. White matter integrity is associated with treatment outcome measures in cocaine dependence. Neuropsychopharmacology. 2010;35:1541–1549. doi: 10.1038/npp.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Naumer MJ. Functional neuroimaging studies in addiction: multisensory drug stimuli and neural cue reactivity. Neurosci Biobehav Rev. 2012;36:825–835. doi: 10.1016/j.neubiorev.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Suzuki T, Seki T, Namba T, Liu J, Arai H, Hori T, Shiga T. Decreased cell proliferation in the dentate gyrus of rats after repeated administration of cocaine. Synapse. 2005;58:63–71. doi: 10.1002/syn.20182. [DOI] [PubMed] [Google Scholar]


