Abstract
A better understanding of the biology of renal cell carcinoma (RCC) has significantly changed the treatment paradigm of the disease. Several novel VEGF and TORC inhibitors have bee recently FDA-approved. Unfortunately, the vast majority of clinical trials conducted today have been aimed to include patients with clear-cell RCC which remains the most common histologic subtype of the disease. Non-clear cell RCC represents approximately 20 to 25% of all RCC patients. Non-clear RCC is made up of multiple histologic subtypes each with a different molecular printing profile. Although VEGF and TORC inhibitors are commonly used in the management of this cohort of patients, non-clear cell histologies do not appear to be related to VHL. As such the clinical efficacy of the existing agents is quite limited. There is a need to develop more rational therapeutic approaches that specifically target the biology off each of the different subtypes of non-clear RCC. In this review, we discuss molecular and clinical characteristics of each of the non-clear cell RCC subtypes and describe ongoing efforts to develop novel agents for this subset of patients.
Introduction
Renal cell carcinoma (RCC) is not a single disease; it is made up of a number of different types of cancer, each with a different histology, a different clinical course and caused by a different gene. Clear cell RCC represents approximately 75% of renal cancers. Non-clear cell RCC is made up of a diverse group of histologic types including type 1 papillary renal cancer, TFE3 kidney cancer, type 2 papillary renal cancer, fumarate hydratase and succinate dehydrogenase associated renal cancer, chromophobe kidney cancer, collecting duct carcinoma and medullary RCC. The discovery of the VHL gene in 19931 was a seminal event in the effort to develop an effective form of therapy for clear cell kidney cancer. Although seven novel therapeutic agents that target the VHL gene pathway have been approved for treatment of patients with advanced RCC, the effectiveness of these agents in non-clear cell RCC is not well defined. While advances in genomics and large scale approaches such as The Cancer Genome Project hold great promise for identification of the genetic basis of non-clear cell RCC, much of the insights that have been gained to date about the genetic basis of non-clear cell RCC have come from the study of the inherited forms of these diseases. Figure 1
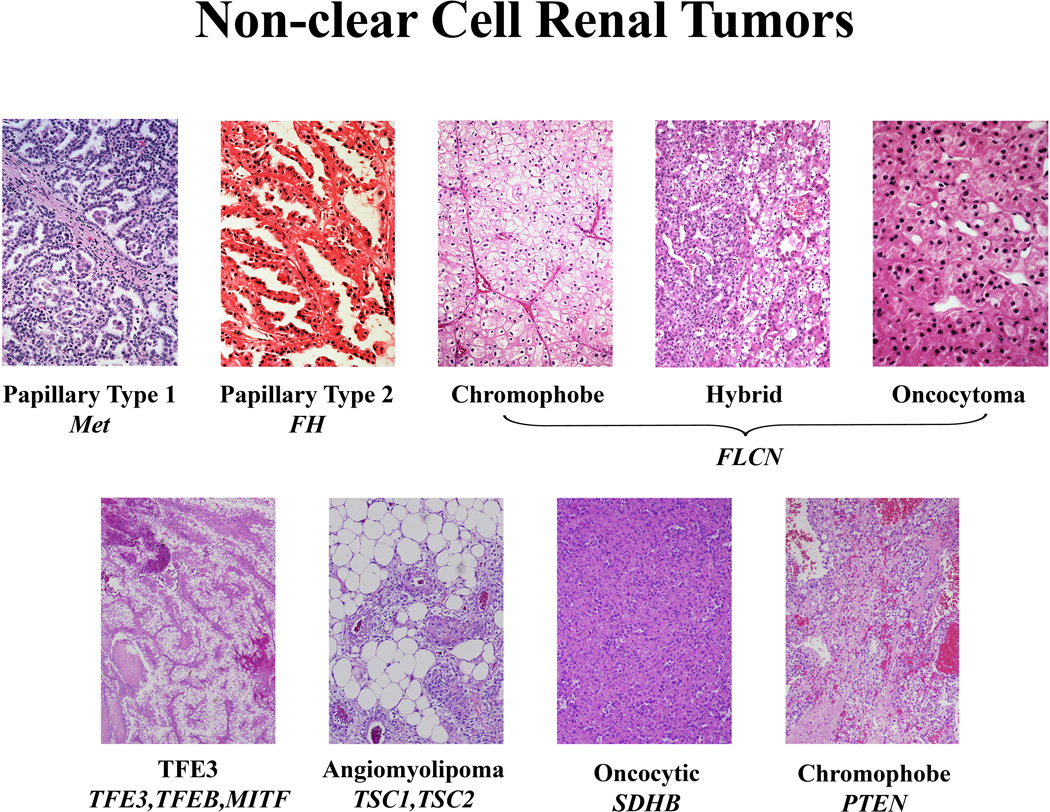
Figure 1. Non-Clear Cell Kidney Cancer.
Non-clear cell kidney cancer is not a single disease, it is made up of a number of different types of cancer, each with a different histology, a different clinical course, responding differently to therapy and caused by a different gene. Adapted from Linehan, 2012 (88)
Type 1 Papillary Renal Cancer
Papillary RCC is often divided into type 1 papillary RCC and type 2 papillary RCC. Type 1 papillary RCC occurs in both a sporadic as well as an inherited, familial form. Sporadic type 1 papillary RCC is most often multifocal, often with a single dominant mass with multiple small, “incipient” lesions (“papillary adenomas”) found in the adjacent renal parenchyma. Patients affected with type 1 papillary RCC can present with bilateral, multifocal disease. Type 1 papillary RCC tends to be hypovascular on imaging2 and may be characterized by slow growth. It is most often less likely to metastasize than clear cell RCC. Surgical resection remains the standard of care for patients with localized type 1 papillary RCC.
Hereditary Papillary Renal Carcinoma: Type 1 Papillary Kidney Cancer
Hereditary Papillary Renal Carcinoma (HPRC) is a rare hereditary cancer syndrome in which affected individuals are at risk for the development of bilateral, multifocal type 1 papillary RCC. 3(3) HPRC is highly penetrant; affected individuals have nearly a 90% chance of developing RCC by the 8th decade. 4 It is estimated that patients affected with HPRC are at risk for the development of up to 1100 tumors per kidney. 5 The management of HPRC-associated RCC cancer involves active surveillance of small renal tumors; surgical intervention is recommended when the largest tumor reaches the 3 cm threshold.6
The Genetic Basis of Type 1 Papillary Renal Cell Cancer
Genetic linkage studies performed in HPRC families localized the HPRC gene to the long arm of chromosome 7 and identified MET, the cell surface receptor for hepatic growth factor (HGF) as the gene for Hereditary Papillary RCC.7 Activating mutations in the tyrosine kinase domain of the MET gene are found in the germline of HPRC patients. Although MET is commonly amplified in type 1 papillary RCC, MET mutations have been identified in only a subset (13%) of tumors from patients with sporadic, non-hereditary papillary RCC. Although MET gene amplification is thought to play a critical role in the pathogenesis of this disease, the genetic basis of the majority of sporadic type 1 papillary RCC remains to be determined.
Targeting the MET pathway in Papillary Renal Carcinoma
There are currently no systemic agents of proven clinical benefit in patients with advanced papillary RCC (or other non-clear cell variants). Patients with unresectable disease requiring therapy usually receive either an mTOR inhibitor or a VEGF pathway antagonist, based on demonstration of modest activity in several retrospective analyses, small single arm phase 2 studies, and at least one subgroup analysis of a large randomized phase 3 study. In most studies, objective response rates following therapy with mTOR or VEGFR-targeted TKIs were low (0–36%), with a median progression free survival (PFS) of less than 6 months.8–14 Inhibitors of the epidermal growth factor receptor (EGFR) have also been evaluated in papillary RCC; in a phase 2 trial of the EGFR inhibitor erlotinib, the overall response rate in 52 patients with metastatic papillary RCC was 11%, with a 6 month PFS of only 29%.15
The identification of oncogenic MET mutations in patients with HPRC as well as in a subset of patients with sporadic papillary RCC has led to considerable interest in targeting the HGF/MET pathway in these malignancies. Foretinib (formerly XL880), an inhibitor of Met, VEGFR2, RON and AXL tyrosine kinases was one of the earliest agents targeting the Met pathway available for clinical investigation. In a recently concluded multicenter phase 2 study, two dosing schedules of foretinib were evaluated in patients with papillary RCC.16 A total of seventy-four patients were enrolled sequentially into one of two independent cohorts to receive either: A) an intermittent dosing regimen (240mg/d PO days 1–5 of every 14-day cycle, N=37) or B) a daily dosing regimen (80mg/d PO, N=37). The overall response rate in the entire cohort was 13.5%, with 10/75 patients experiencing a partial response. The median PFS was 9.3 months, considerably higher than that seen in historical controls treated with agents targeting VEGFR or mTOR pathways. The clinical activity of foretinib was most pronounced in patients with germline mutations in MET; in a preplanned subgroup analysis, 5/10 (50%) of patients with germline MET mutations experienced a PR, while only 5/57 (9%) of patients without this alteration had an objective response. Patients with papillary type 1 as well as those with type 2 tumors were enrolled on this study. Although central pathology review of all tumors was conducted to confirm papillary histology, sufficient tumor was not available in several instances to render an accurate subclassification; it was, therefore, not possible to determine if the type 1 and type 2 papillary tumors exhibited differential sensitivity to foretinib. The adverse event profile associated with this agent was reminiscent of that seen with other inhibitors of the VEGF axis. However, a higher than anticipated incidence of pulmonary thromboembolism (11%) was noted, as were alterations in dark adaptation in several patients treated on this trial. These data suggest that Met pathway antagonists are worthy of further study at least in some subgroups of papillary RCC. At least one other Met inhibitor, tivantinib (ARQ197), is currently undergoing evaluation with or without erlotinib in a randomized phase 2 trial in patients with advanced papillary RCC. (NCT01688973)
MiT family Renal Cancer: TFE3, TFEB and MITF
In 1996 a novel type of translocation RCC was described involving TFE3, a member of microphthalmia transcription factor/ transcription factor E (MITF-TFE) family of basic helix-loop helix leucine zipper (bLHL-Zip) transcription factors.17,18 In the initial report, a t(X;1)(p11.2;q21.2) translocation in papillary RCC was described involving the gene PRCC to the TFE3 transcription factor. Since the original description of TFE3 kidney cancer, a number of other TFE3 fusion partners such as NonO-TFE3, PSF-TFE3, CLTC-TFE3 and ASPL-TFE3 have been described.19–21 In 2003 Davis, et al. described the Alpha-TFEB fusion in renal tumors harboring the t(6;11)(p21;q13) chromosome translocation.22 Bertolotto et al. recently identified germline mutations in MITF, a third member of the MiT transcription factor family, in families at increased risk for the development of RCC and melanoma.23
Xp11.2 translocation/TFE3 fusion RCC occurs predominantly in children and young adults. It represents 1–1.6% of all renal tumors, 15% of kidney tumors in patients under 45 years of age and 20–45% of kidney cancers in children and young adults.24 These tumors, which are most often associated with an early age of onset, are aggressive and have a propensity towards early nodal metastasis. The treatment of localized disease is surgical and includes lymph node resection.
Treatment of Advanced TFE3 Kidney Cancer
Optimal therapy for unresectable translocation RCC remains under investigation. At least two case series have evaluated the role of VEGF-pathway antagonists in this disease. In a retrospective study of 15 patients with translocation RCC (diagnosed by either histological/imunohistochemical features or demonstrable translocation involving chromosome Xp11.2), who were treated with one of a variety of inhibitors of the VEF axis, three partial responses and seven patients with stable disease were noted. Median PFS and OS were 7.1 months and 14.3 months respectively. Investigators from the French Juvenile RCC Network reported their experience in twenty one patients with metastatic TFE3 tumors treated with systemic therapy.25 Four of eleven patients receiving sunitinib in the first line setting had an objective response, with a median PFS of 8.2 months. Responses were also seen in patients receiving this agent as well as those receiving an mTOR inhibitor as salvage therapy following failure of first line agents.
In a recent publication, Tsuda et al suggested that activation of the Met pathway was one consequence of the aberrantASPL-TFE3 fusion product, leading to the evaluation of Met antagonists in MiTF associated tumors. 26 However, in a phase 2 study of the allosteric Met inhibitor tivantinib in patients with Microphthalmia Transcription Factor-associated tumors, none of the six RCC patients treated demonstrated an objective response, with a disappointing median PFS of 1.9 months. 27 Further studies are required to validate Met as a viable therapeutic target in TFE3 tumors. Several laboratories are currently studying the molecular mechanisms underlying this RCC variant in a bid to uncover other pathways that may be amenable to pharmacologic modulation.
Type 2 Papillary Renal Cancer
Type 2 papillary kidney cancer is made up of a number of different of non-type 1 papillary RCC, including Hereditary leiomyomatosis and renal cell carcinoma (HLRCC) kidney cancer.
Hereditary Leiomyomatosis renal cell carcinoma: Type 2 papillary kidney cancer
Hereditary leiomyomatosis and renal cell carcinoma (HLRCC) is an autosomal dominant hereditary cancer syndrome in which affected individuals are at risk for the development of cutaneous and uterine leiomyomas and RCC. 28, 29 The cutaneous leiomyomas may appear as raised, reddened lesions that can occur in clusters on the trunk, limbs or face. 30 The uterine leiomyomas are highly penetrant, can be multiple and are early onset (third decade). 31 HLRCC is characterized by germline mutation of the Krebs cycle enzyme gene, fumarate hydratase. 32–34 Patients affected with HLRCC are at risk for the development of renal cysts and a very aggressive form of type 2 papillary RCC. 35, 36 HLRCC-associated kidney cancers can be bilateral and/or multifocal and can form inside renal cysts. Whereas HPRC tumors, which are type 1 papillary kidney cancer, tend to be encapsulated and slow growing, HLRCC renal tumors have a tendency to infiltrate deeply into the renal parenchyma and can spread when the tumors are very small (1/2 cm). 35 Management of localized HLRCC-associated kidney cancer involves surgical resection of localized tumors. Active surveillance is not recommended for HLRCC-associated kidney cancers and surgical treatment involves wide excision of localized tumors. Partial nephrectomy is recommended when feasible as these patients have a life-long risk for the development of bilateral, multifocal kidney tumors.
Hereditary leiomyomatosis and RCC is characterized by germline mutation of the Krebs cycle enzyme gene, fumarate hydratase (FH). 32–34 Fumarate hydratase is a loss of function, two-hit tumor suppressor gene; loss of the second FH allele is detected in HLRCC-associated kidney cancer. Fumarate hydratase-deficient RCC is prototypic example of the Warburg effect in cancer. In FH-deficient RCC oxidative phosphorylation is significantly impaired and the cancer cells undergo a metabolic shift to aerobic glycolysis for ATP production. 37, 38 Inactivation of fumarate hydratase results in an increase in fumarate which impairs prolyl hydroxylase function, leading to stabilization of HIF1α. 39 Increased glycolysis suppresses AMPK38 and FH-deficient kidney cancer cells shift to reductive glutamine dependent pathway to support the increased lipid biosynthesis required by these rapidly proliferating cells. 40
Therapeutic Approaches for Advanced HLRCC Kidney Cancer
HLRCC-associated kidney cancer is a most aggressive form of type 2 papillary RCC. These tumors can spread when they are very small and tend to progress rapidly. Therapeutic approaches are intended to target the metabolic shift to aerobic glycolysis and to take advantage of these cells’ need for increased vasculature and increased dependence on glucose for energy production in rapidly proliferating cells. Investigators from the National Cancer Institute (NCI) are evaluating the efficacy combined VEGF and EGFR blockade in HLRCC, based on the premise that this combination will impair blood flow and glucose delivery to tumor cells. A recent retrospective analysis outlined the outcomes of eighteen patients with advanced HLRCC associated kidney cancer treated with systemic therapy during a ten-year period (1998–2008). 41, 42 Seven patients were treated with combined VEGF/EGFR inhibition while 11 patients received a variety of other standard agents including cytokines, VEGFR inhibitors, and mTOR inhibitors. Patients treated with concomitant VEGF/EGFR blockade had an ORR of 71% (5/7), including one patient with a complete response (14%) and 4 patients with a partial response (57%). Responses were durable, with one patient remaining disease free 74 months after treatment initiation. Combined VEGF/EGFR blockade resulted in significantly improved overall survival compared to other treatment regimens (median 51 months vs. 14 months; P=0.0012). Based on these data, a phase 2 trial is currently underway at the NCI to evaluate the role of bevacizumab and erlotinib in patients with advanced HLRCC-associated kidney cancer (NCT01130519).
Succinate dehydrogenase kidney cancer (SDH-RCC)
Succinate dehydrogenase kidney cancer (SDH-RCC) is a hereditary cancer syndrome in which affected individuals are at risk for the development of pheochromocytomas, paragangliomas and RCC. 43, 44 These patients are characterized by germline mutations of the SDHB, SDHC or SDHD genes. 44–50 Patients affected with SDH-RCC can develop oncocytic renal tumors, clear cell or papillary RCC. SDH-RCC can be an aggressive form of RCC which has the potential to metastasize when the tumors are small. Management of localized SDH-RCC associated kidney tumors involves surgical resection when the kidney tumor is detected. Active surveillance of small SDH-RCC renal tumors is not recommended. 41
Succinate dehydrogenase is a mitochondrial multimeric complex, comprised of the SDHA, SDHB, SDHC and SDHD subunits that serves a dual role as a Krebs (TCA) cycle enzyme as well as forming a critical function in the electron transport chain (complex 2). 51 SDH-RCC is an example of the Warburg effect in cancer; mutation of succinate dehydrogenase impairs oxidative phosphorylation and the kidney cancer cells undergo a metabolic shift to aerobic glycolysis. When succinate dehydrogenase is deficient, succinate accumulates and the increased succinate inhibits cytosolic HIF-α prolyl hydroxylase, resulting in accumulation of HIF1α. 52 The resulting increase in HIF1α leads to increased transcription of vascular endothelial growth factor (VEGF) the glucose transport gene, GLUT1, which would provide increased vascularity and glucose to the rapidly growing, glucose dependent SDH-deficient kidney cancer. Therapeutic trials involving approaches targeting the vascularity and glucose dependence of SDH-RCC will be initiated in the near future.
Chromophobe Renal Cell Carcinoma
Chromophobe RCC, which was described by Thoenes, et al. in 1985,53 occurs in 4% of all cases of kidney cancer. While TNM stage groupings and sarcomatoid differentiation are significantly associated with cancer specific survival,(54) chromophobe RCC is most often detected when it is localized to the kidney. Chromophobe RCC is slow growing and tends to be less likely to metastasize than are, for example clear cell and type 2 papillary RCC, with fewer than 5% of patients presenting with advanced disease. 54, 55 The genetic basis of sporadic chromophobe RCC is not known, however, study of the inherited from of chromophobe RCC associated with Birt-Hogg-Dubé has provided insights into potential approaches for therapy of this disease.
Hereditary Chromophobe Renal Cell Carcinoma: Birt-Hogg-Dubé
Birt-Hogg-Dubé (BHD) is an autosomal dominant hereditary cancer syndrome in which affected individuals are at risk for the development of cutaneous fibrofolliculomas,56 pulmonary cysts57 and RCC. 58 Patients affected with BHD are at risk for the development of bilateral, multifocal renal tumors. BHD tumors may be chromophobe, hybrid oncocytic or clear cell RCC. 59 Surgical management of localized BHD-associated RCC involves active surveillance of renal tumors until the largest tumor reaches the 3 cm threshold, at which time surgical intervention is recommended.
Therapeutic Approaches for BHD Associated Kidney Cancer: Targeting the FLCN Gene Pathway
The BHD gene, FLCN, is located on the short arm of chromosome 17. 60,61 Inactivating mutations of the FLCN gene have been detected in the germline of over 95% of BHD families. 61,62 FLCN is a two-hit, loss of function tumor suppressor gene. Mutation or loss of the somatic FLCN allele is detected in 70% of BHD-associated kidney tumors. 62(62) FLCN is in the AMPK/TSC/mTOR pathway. (Figure 2) The product of the FLCN gene, folliculin, binds two novel proteins, FNIP1 and FNIP2, which bind the γ-subunit of the energy sensing protein complex, AMPK.63,64 Inactivation of FLCN results in activation of mTORC1 and mTORC2.63, 65 In order to evaluate the role of agents targeting the FLCN pathway, a murine model in which FLCN was specifically knocked out in the kidney was developed. In the kidney targeted FLCN knockout mouse model the affected mice develop cystic kidneys and renal insufficiency. Treatment of the FLCN knockout mice with an agent which targets the mTORC1 pathway (rapamycin) had a significant effect on the renal phenotype and doubled the life expectancy of the animals. 66 Understanding the FLCN gene pathway has provided the foundation for the development of a targeted therapeutic approach for the treatment of BHD-associated kidney cancer.
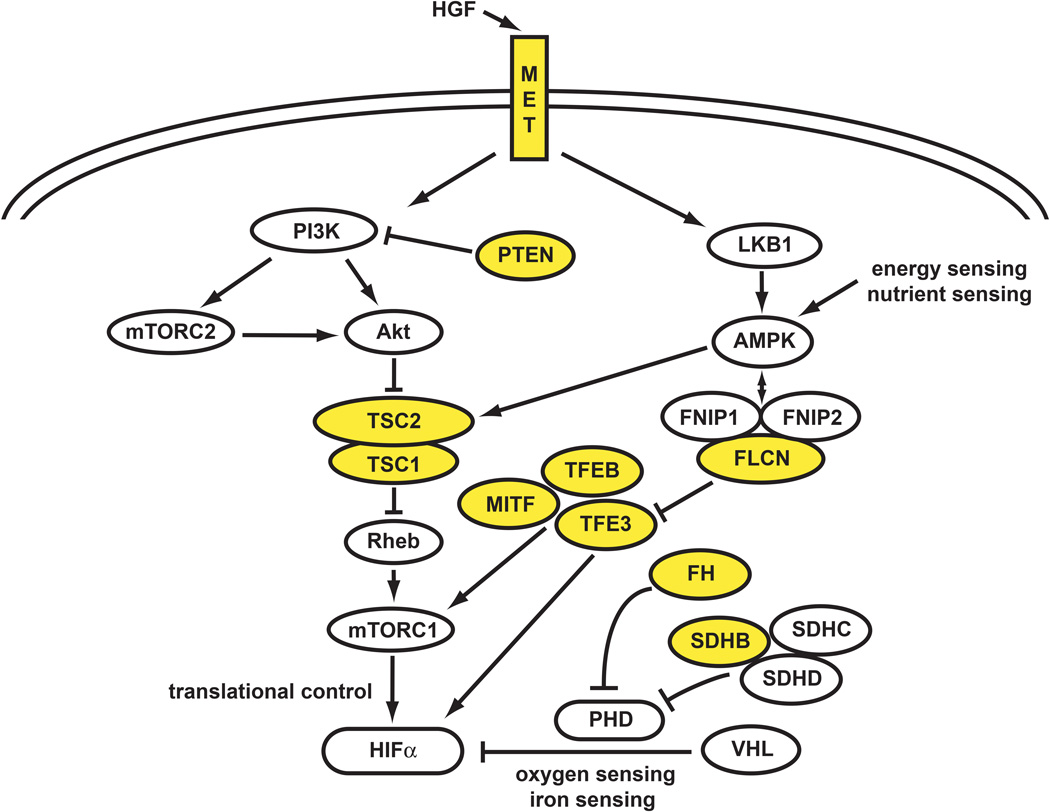
Figure 2. Non-Clear Cell Kidney Cancer Pathways.
Non-clear cell kidney cancer is a metabolic disease. The genes that are associated with non-clear cell kidney cancer, MET, FLCN, fumarate hydratase, succinate dehydrogenase, TFE3, TFEB, MITF, TSC1, TSC2, and PTEN are all associated with abnormalities of the cell’s ability to sense oxygen, iron, nutrients or energy. Adapted from Linehan, 2012 (88)
Tuberous sclerosis complex: TSC1/2
Tuberous sclerosis complex (TSC) is a hereditary hamartoma syndrome in which affected individuals are at risk for the development of multisystem manifestations, including neurologic dysfunction and CNS tumors, cutaneous angiofibromas, pulmonary lymphangiomyomatosis and renal tumors. 67 TSC-associated renal tumors are predominantly angiomyolipomas; however, TSC patients are also at risk for the development of clear cell, papillary and chromophobe RCC. 67, 68
The knowledge that TSC1/2 genes are in the LKB1/AMPK/TSC/mTORC1 pathway has provided the foundation for the development of rational approaches for TSC-associated renal tumors. 69 In a first step in the development of a therapeutic approach for TSC-associated renal tumors Bissler, et al. reported shrinkage of TSC renal tumors with treatment with rapamycin. 70
Collecting Duct and Medullary Renal Cell Carcinoma
Collecting Duct
Collecting duct RCC (CDRCC) is a rare pathological entity that comprises less than 2% of cases of kidney cancer. The prognosis of patients with CDRCC is quite poor. As such, early histological identification is important. 71, 72 This type of RCC is thought to arise from the collecting ducts in the renal medulla. Typically, CDRCC has a tubular or tubulo-papillary growth pattern that infiltrates the renal parenchyma and is associated with a desmoplastic stroma. CDRCC usually display high grade (Fuhrman 3 and 4) nuclear features. 73, 74 Most of the existing data about systemic treatment of this histologic RCC variant comes from case reports, retrospective studies and small subset analysis of RCC clinical trials where histology was not restricted to clear-cell only. Recently Wright and colleagues75 evaluated the effect of collecting duct histology on RCC outcome using the Surveillance, Epidemiology and End Results (SEER) data based program from 2001 to 2005. Only 160 CDRCC cases were identified during that period of time. Similar to previous reports, high rates of bulky disease (pT3 or greater) and nodal involvement were noted. 76, 77 Although the rates of nephrectomy appeared to be similar to those with clear cell RCC (84% and 78%, p=0.06), the 3-year disease-specific survival rates were greater for patients with clear cell histology. In fact, there was an increase in mortality for those patients with CDRCC histology (HR 2.42, 95% CI 1.72–3.39, p=0.001).(75) Previous reports by Motzer and colleagues also demonstrate that regardless of treatment received less than 5% of patients with CDRCC, survive past 2 years. 78
Most patients with localized or locally advanced disease CDRCC are found at the time of nephrectomy. The optimal systemic management for patients with advanced disease remains unknown. Multiple case reports have indicated modest response when cytokines are used. 79 The role of VEGF or mTOR inhibitor in the management of this disease is not defined. Several reports however indicate the lack of robust clinical activity of any of the available agents. 14, 80 Despite of its histological heterogeneity, CDRCC appears to overlap with urothelial carcinomas. Thus, many clinicians have adopted cisplatin-based chemotherapy as a routine regimen in the management of these complex patients. 81, 82 Recently, Oudard and colleagues from the GETUG (Groupe d’Etudes des Tumeurs Uro-Génitales) reported the first prospective multicenter phase II study evaluating cisplatin-based chemotherapy in patients with advanced CCDRCC. 83 Although this study was closed early secondary to poor accrual, all 23 patients enrolled on trial had central pathologic review. Patients received cisplatin (70 mg/m2) on D1 and Gemcitabine (1,250 mg/m2) on D1 and D8 of each 3-week cycle. Cisplatin was replaced by Carboplatin (AUC 5) in patients with impaired renal function (estimated creatinine clearance 30–60ml per minute per m2). Patients received a median of 6 cycles of therapy (range 1–8). Carboplatin was given to 5 (22%) patients. Patients experienced adverse events (AEs) typically seen with this regimen. ORR was 26% (95%CI 8 to 44) with one RECIST-defined CR and five PRs; 44% of patients achieved stable disease (SD). The median PFS and OS were 7.1 (95% CI 3 to 11.3) and 10.5 months (95% CI 3.8 to 17.1), respectively.
Medullary Carcinoma
In 1996 Davis, et al. described the presence of a rare form of kidney cancer, renal medullary carcinoma (RMC), in thirty three patients, all of whom were affected with sickle cell trait or hemoglobin SC disease. 84 These patients were of African descent and tended to be young (between the ages of 11 and 39 years). RMC is an extremely lethal disease, with a mean survival of only 15 weeks (range 2 to 52) after surgery. 84, 85 While this disease may be treated successfully by surgical resection if detected early,86 advanced disease remains resistant to treatment, including cytokine therapy or chemotherapy. 87 Unfortunately up to 95% of patients have metastatic disease at the time of diagnosis. In 2006 Ronnen, et al. reported a single patient with renal medullary carcinoma who was disease free twenty-seven months after a 7 month course of treatment with bortezomib. 87, 88 Others have evaluated the role of cytotoxic therapy with methotrexate, vinblastine, Adriamycin and cisplatin (MVAC). Despite of these encouraging reports, the prognosis of patients with advanced RMC is quite poor. 89 Multiple studies evaluating the gene expression profiling of medullary RCC have failed to demonstrate an association with clear cell or papillary histologies and suggest a unique and independent biology distinct from other forms of RCC.90 Of interest, however is the overexpression of DNA topo II α often found in medullary RCC. This finding if often used to support the clinical use of Topo II inhibitors such as doxorubicin and etoposide in the management of this disease. 91 Although standard of care for this disease has not been clearly defined, the vast majority of advanced RMC receive cytotoxic chemotherapy. There is no data to support the use of VEGF or mTOR inhibitors in this histologic variant of RCC.
Ongoing Clinical Trials for Non-Clear Cell RCC
Several clinical trials are currently evaluating a wide range of therapeutic approaches in patients with non-clear cell RCCs. Since many of these RCC variants are relatively uncommon entities, most trials are not subtype specific. The activity of everolimus monotherapy in advanced papillary RCC is currently being investigated in an ongoing phase II, single arm, multicenter, European trial (NCT00688753). Two randomized phase 2 trials comparing everolimus with sunitinib in previously untreated patients with advanced non clear cell RCC are currently accruing patients (NCT 01185366 and NCT 01108445), while a Korean phase II study evalauting single agent pazopanib in this population (NCT01538238) is expected to commence accrual soon. Lastly, combinations of bevacizumab with erlotinib (NCT01130519) or with everolimus (NCT01399918) are also being evaluated in papillary RCC patients. It is hoped that these relatively large phase 2 studies will shed further light on the activity of VEGF and mTOR pathway antagonists in pRCC.
Conclusion
Non-clear cell RCC is a heterogeneous group of cancers that encompasses multiple histologies with different molecular features. Although inhibition of the mTOR and VEGF signaling pathways might lead to some clinical benefit, this is often marginal and prognosis remains poor. Surgery for those with localized or locally advanced disease remains the initial and more important approach. For patients with advanced disease, a clinical trial should be considered when available. With the limitations in sample size and slow accrual, the aim of future studies should be based on the biology of the disease.
Acknowledgement
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The authors thank Georgia Flaten Shaw for the outstanding editorial and graphics support.
References
- 1.Latif F, Tory K, Gnarra JR, Yao M, Duh F-M, Orcutt ML, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 2.Choyke PL, Walther MM, Glenn GM, Wagner JR, Venzon DJ, Lubensky IA, et al. Imaging features of hereditary papillary renal cancers. J Comput Assist Tomogr. 1997;21:737–741. doi: 10.1097/00004728-199709000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Zbar B, Tory K, Merino MJ, Schmidt LS, Glenn GM, Choyke P, et al. Hereditary papillary renal cell carcinoma. J Urol. 1994;151:561–566. doi: 10.1016/s0022-5347(17)35015-2. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt LS, Junker K, Weirich G, Glenn G, Choyke P, Lubensky I, et al. Two North American families with hereditary papillary renal carcinoma and identical novel mutations in the MET proto-oncogene. Cancer Res. 1998;58:1719–1722. [PubMed] [Google Scholar]
- 5.Ornstein DK, Lubensky IA, Venzon D, Zbar B, Linehan WM, Walther MM. Prevalence of microscopic tumors in normal appearing renal parenchyma of patients with hereditary papillary renal cancer. J Urol. 2000;163:431–433. [PubMed] [Google Scholar]
- 6.Herring JC, Enquist EG, Chernoff A, Linehan WM, Choyke PL, Walther MM. Parenchymal sparing surgery in patients with hereditary renal cell carcinoma: 10-year experience. J Urol. 2001;165:777–781. [PubMed] [Google Scholar]
- 7.Schmidt LS, Duh FM, Chen F, Kishida T, Glenn GM, Choyke P, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nature Genetics. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 8.Choueiri TK, Plantade A, Elson P, Negrier S, Ravaud A, Oudard S, et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol. 2008;26:127–131. doi: 10.1200/JCO.2007.13.3223. [DOI] [PubMed] [Google Scholar]
- 9.Molina AM, Feldman DR, Ginsberg MS, Kroog G, Tickoo SK, Jia X, et al. Phase II trial of sunitinib in patients with metastatic non-clear cell renal cell carcinoma. Invest New Drugs. 2012;30:335–340. doi: 10.1007/s10637-010-9491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravaud A, Oudard S, Fromont DR. First-line sunitinib in type I and papillary renal cell carcinoma (PRCC): SUPAP, a phase II study of the French Genito-Urinary Group (GETUG) and the Group of Early Phase trials (GEP) Ann Oncol. 2012;23 Abstr 797PD. [Google Scholar]
- 11.Tannir NM, Plimack E, Ng C, Tamboli P, Bekele NB, Xiao L, et al. A phase 2 trial of sunitinib in patients with advanced non-clear cell renal cell carcinoma. Eur Urol. 2012;62:1013–1019. doi: 10.1016/j.eururo.2012.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 13.Dutcher JP, de SP, McDermott D, Figlin RA, Berkenblit A, Thiele A, et al. Effect of temsirolimus versus interferon-alpha on outcome of patients with advanced renal cell carcinoma of different tumor histologies. Med Oncol. 2009;26:202–209. doi: 10.1007/s12032-009-9177-0. [DOI] [PubMed] [Google Scholar]
- 14.Lee JL, Ahn JH, Lim HY, Park SH, Lee SH, Kim TM, et al. Multicenter phase II study of sunitinib in patients with non-clear cell renal cell carcinoma. Ann Oncol. 2012;23:2108–2114. doi: 10.1093/annonc/mdr586. [DOI] [PubMed] [Google Scholar]
- 15.Gordon MS, Hussey M, Nagle RB, Lara PN, Jr, Mack PC, Dutcher J, et al. Phase II study of erlotinib in patients with locally advanced or metastatic papillary histology renal cell cancer: SWOG S0317. J Clin Oncol. 2009;27:5788–5793. doi: 10.1200/JCO.2008.18.8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choueiri TK, Vaishampayan U, Rosenberg JE, Logan TF, Harzstark AL, Bukowski RM, et al. Phase II and Biomarker Study of the Dual MET/VEGFR2 Inhibitor Foretinib in Patients With Papillary Renal Cell Carcinoma. J Clin Oncol. 2012;10 doi: 10.1200/JCO.2012.43.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sidhar SK, Clark J, Gill S, Hamoudi R, Crew J, Gwilliam R, et al. The t(X;1)(p11.2;q21.2) translocation in papillary renal cell carcinoma fuses anovel gene PRCC to the TFE3 transcription factor gene. Hum Mol Genet. 1996;5:1333–1338. doi: 10.1093/hmg/5.9.1333. [DOI] [PubMed] [Google Scholar]
- 18.Weterman MA, Wilbrink M, Geurts van KA. Fusion of the transcription factor TFE3 gene to a novel gene, PRCC, in t(X;1)(p11;q21)-positive papillary renal cell carcinomas. Proc Natl Acad Sci U S A. 1996;93:15294–15298. doi: 10.1073/pnas.93.26.15294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark J, Lu YJ, Sidhar SK, Parker C, Gill S, Smedley D, et al. Fusion of splicing factor genes PSF and NonO (p54nrb) to the TFE3 gene in papillary renal cell carcinoma. Oncogene. 1997;15:2233–2239. doi: 10.1038/sj.onc.1201394. [DOI] [PubMed] [Google Scholar]
- 20.Argani P, Lui MY, Couturier J, Bouvier R, Fournet JC, Ladanyi M. A novel CLTC-TFE3 gene fusion in pediatric renal adenocarcinoma with t(X;17)(p11.2;q23) Oncogene. 2003;22:5374–5378. doi: 10.1038/sj.onc.1206686. [DOI] [PubMed] [Google Scholar]
- 21.Malouf GG, Camparo P, Molinie V, Dedet G, Oudard S, Schleiermacher G, et al. Transcription factor E3 and transcription factor EB renal cell carcinomas: clinical features, biological behavior and prognostic factors. J Urol. 2011;185:24–29. doi: 10.1016/j.juro.2010.08.092. [DOI] [PubMed] [Google Scholar]
- 22.Davis IJ, Hsi BL, Arroyo JD, Vargas SO, Yeh YA, Motyckova G, et al. Cloning of an Alpha-TFEB fusion in renal tumors harboring the t(6;11)(p21;q13) chromosome translocation. Proc Natl Acad Sci U S A. 2003;100:6051–6056. doi: 10.1073/pnas.0931430100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertolotto C, Lesueur F, Giuliano S, Strub T, de LM, Bille K, et al. A SUMOylation-defective MITF germline mutation predisposes to melanoma and renal carcinoma. Nature. 2011;480:94–98. doi: 10.1038/nature10539. [DOI] [PubMed] [Google Scholar]
- 24.Zhong M, De AP, Osborne L, Keane-Tarchichi M, Goldfischer M, Edelmann L, et al. Dual-color, break-apart FISH assay on paraffin-embedded tissues as an adjunct to diagnosis of Xp11 translocation renal cell carcinoma and alveolar soft part sarcoma. Am J Surg Pathol. 2010;34:757–766. doi: 10.1097/PAS.0b013e3181dd577e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malouf GG, Camparo P, Oudard S, Schleiermacher G, Theodore C, Rustine A, et al. Targeted agents in metastatic Xp11 translocation/TFE3 gene fusion renal cell carcinoma (RCC): a report from the Juvenile RCC Network. Ann Oncol. 2010;21:1834–1838. doi: 10.1093/annonc/mdq029. [DOI] [PubMed] [Google Scholar]
- 26.Tsuda M, Davis IJ, Argani P, Shukla N, McGill GG, Nagai M, et al. TFE3 fusions activate MET signaling by transcriptional up-regulation, defining another class of tumors as candidates for therapeutic MET inhibition. Cancer Res. 2007;67:919–929. doi: 10.1158/0008-5472.CAN-06-2855. [DOI] [PubMed] [Google Scholar]
- 27.Wagner AJ, Goldberg JM, Dubois SG, Choy E, Rosen L, Pappo A, et al. Tivantinib (ARQ 197), a selective inhibitor of MET, in patients with microphthalmia transcription factor-associated tumors: Results of a multicenter phase 2 trial. Cancer. 2012 doi: 10.1002/cncr.27582. [DOI] [PubMed] [Google Scholar]
- 28.Zbar B, Glenn GM, Lubensky IA, Choyke P, Magnusson G, Bergerheim U, et al. Hereditary papillary renal cell carcinoma: Clinical studies in 10 families. J Urol. 1995;153:907–912. [PubMed] [Google Scholar]
- 29.Launonen V, Vierimaa O, Kiuru M, Isola J, Roth S, Pukkala E, et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci U S A. 2001;98:3387–3382. doi: 10.1073/pnas.051633798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothman A, Glenn GM, Choyke L, Srinivasan R, Linehan WM, Cowen EW. Multiple painful cutaneous nodules and renal mass. J Am Acad Dermatol. 2006;55:683–686. doi: 10.1016/j.jaad.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 31.Stewart L, Glenn GM, Stratton P, Goldstein AM, Merino MJ, Tucker MA, et al. Association of germline mutations in the fumarate hydratase gene and uterine fibroids in women with hereditary leiomyomatosis and renal cell cancer. Arch Dermatol. 2008;144:1584–1592. doi: 10.1001/archdermatol.2008.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei MH, Toure O, Glenn GM, Pithukpakorn M, Neckers L, Stolle C, et al. Novel mutations in FH and expansion of the spectrum of phenotypes expressed in families with hereditary leiomyomatosis and renal cell cancer. J Med Genet. 2006;43:18–27. doi: 10.1136/jmg.2005.033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toro JR, Nickerson ML, Wei MH, Warren MB, Glenn GM, Turner ML, et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet. 2003;73:95–106. doi: 10.1086/376435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 35.Grubb RL, III, Franks ME, Toro J, Middelton L, Choyke L, Fowler S, et al. Hereditary leiomyomatosis and renal cell cancer: a syndrome associated with an aggressive form of inherited renal cancer. J Urol. 2007;177:2074–2080. doi: 10.1016/j.juro.2007.01.155. [DOI] [PubMed] [Google Scholar]
- 36.Merino MJ, Torres-Cabala C, Pinto PA, Linehan WM. The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am J Surg Pathol. 2007;31:1578–1585. doi: 10.1097/PAS.0b013e31804375b8. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Valera VA, Padilla-Nash HM, Sourbier C, Vocke CD, Vira MA, et al. UOK 262 cell line, fumarate hydratase deficient (FH-/FH-) hereditary leiomyomatosis renal cell carcinoma: in vitro and in vivo model of an aberrant energy metabolic pathway in human cancer. Cancer Genet Cytogenet. 2010;196:45–55. doi: 10.1016/j.cancergencyto.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tong WH, Sourbier C, Kovtunovych G, Jeong SY, Vira M, Ghosh M, et al. The glycolytic shift in fumarate-hydratase-deficient kidney cancer lowers AMPK levels, increases anabolic propensities and lowers cellular iron levels. Cancer Cell. 2011;20:315–327. doi: 10.1016/j.ccr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–153. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T, et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2011;481:385–388. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linehan WM, Pinto PA, Bratslavsky G, Pfaffenroth E, Merino M, Vocke CD, et al. Hereditary kidney cancer: unique opportunity for disease-based therapy. Cancer. 2009;115:2252–2261. doi: 10.1002/cncr.24230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singer EA, Marchalik D, Friend JC. Efficacy of combined vegf and egfr inhibtion in metastatic papillary rencal cell carcinoma associated with hereditary leiomyomatosis and renal cell cancer. J Canadian Urol. 2012;19:6510. [Google Scholar]
- 43.Vanharanta S, Buchta M, McWhinney SR, Virta SK, Peczkowska M, Morrison CD, et al. Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am J Hum Genet. 2004;74:153–159. doi: 10.1086/381054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ricketts CJ, Shuch B, Vocke CD, Metwalli AR, Bratslavsky G, Middelton L, et al. Succinate dehydrogenase kidney cancer: an aggressive example of the warburg effect in cancer. J Urol. 2012;188:2063–2071. doi: 10.1016/j.juro.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neumann HP, Pawlu C, Peczkowska M, Bausch B, McWhinney SR, Muresan M, et al. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA. 2004;292:943–951. doi: 10.1001/jama.292.8.943. [DOI] [PubMed] [Google Scholar]
- 46.Ricketts C, Woodward ER, Killick P, Morris MR, Astuti D, Latif F, et al. Germline SDHB mutations and familial renal cell carcinoma. J Natl Cancer Inst. 2008;100:1260–1262. doi: 10.1093/jnci/djn254. [DOI] [PubMed] [Google Scholar]
- 47.Srirangalingam U, Walker L, Khoo B, MacDonald F, Gardner D, Wilkin TJ, et al. Clinical manifestations of familial paraganglioma and phaeochromocytomas in succinate dehydrogenase B (SDH-B) gene mutation carriers. Clin Endocrinol (Oxf) 2008;69:587–596. doi: 10.1111/j.1365-2265.2008.03274.x. [DOI] [PubMed] [Google Scholar]
- 48.Henderson A, Douglas F, Perros P, Morgan C, Maher ER. SDHB-associated renal oncocytoma suggests a broadening of the renal phenotype in hereditary paragangliomatosis. Fam Cancer. 2009;8:257–260. doi: 10.1007/s10689-009-9234-z. [DOI] [PubMed] [Google Scholar]
- 49.Solis DC, Burnichon N, Timmers HJ, Raygada MJ, Kozupa A, Merino MJ, et al. Penetrance and clinical consequences of a gross SDHB deletion in a large family. Clin Genet. 2009;75:354–363. doi: 10.1111/j.1399-0004.2009.01157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malinoc A, Sullivan M, Wiech T, Schmid KW, Jilg C, Straeter J, et al. Biallelic inactivation of the SDHC gene in renal carcinoma associated with paraganglioma syndrome type 3. Endocr Relat Cancer. 2012;19:283–290. doi: 10.1530/ERC-11-0324. [DOI] [PubMed] [Google Scholar]
- 51.Oyedotun KS, Lemire BD. The quaternary structure of the Saccharomyces cerevisiae succinate dehydrogenase. Homology modeling, cofactor docking, and molecular dynamics simulation studies. J Biol Chem. 2004;279:9424–9431. doi: 10.1074/jbc.M311876200. [DOI] [PubMed] [Google Scholar]
- 52.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 53.Thoenes W, Storkel S, Rumpelt HJ. Human chromophobe renal cell carcinoma. Virchows Arch B Cell Pathology. 1985;48:207–211. doi: 10.1007/BF02890129. [DOI] [PubMed] [Google Scholar]
- 54.Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27:612–624. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Singer EA, Bratslavsky G, Linehan WM, Srinivasan R. Targeted therapies for non-clear renal cell carcinoma. Target Oncol. 2010;5:119–129. doi: 10.1007/s11523-010-0148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Birt AR, Hogg GR, Dube WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol. 1977;113:1674–1677. [PubMed] [Google Scholar]
- 57.Toro JR, Pautler SE, Stewart L, Glenn GM, Weinreich M, Toure O, et al. Lung Cysts, Spontaneous Pneumothrorax and Genetic Associations in 89 Families with Birt-Hogg-Dubé Syndrome. Am J Respir Crit Care Med. 2007;175:1044–1053. doi: 10.1164/rccm.200610-1483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pavlovich CP, Grubb RL, Hurley K, Glenn GM, Toro J, Schmidt LS, et al. Evaluation and Management of Renal Tumors in the Birt-Hogg-Dube Syndrome. J Urol. 2005;173:1482–1486. doi: 10.1097/01.ju.0000154629.45832.30. [DOI] [PubMed] [Google Scholar]
- 59.Pavlovich CP, Walther MM, Eyler RA, Hewitt SM, Zbar B, Linehan WM, et al. Renal tumors in the Birt-Hogg-Dubé syndrome. Am J Surg Pathol. 2002;26:1542–1552. doi: 10.1097/00000478-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 60.Nickerson ML, Warren MB, Toro JR, Matrosova V, Glenn GM, Turner ML, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dube syndrome. Cancer Cell. 2002;2:157–164. doi: 10.1016/s1535-6108(02)00104-6. [DOI] [PubMed] [Google Scholar]
- 61.Schmidt LS, Nickerson ML, Warren MB, Glenn GM, Toro JR, Merino MJ, et al. Germline BHD-mutation spectrum and phenotype analysis of a large cohort of families with Birt-Hogg-Dubé syndrome. Am J Hum Genet. 2005;76:1023–1033. doi: 10.1086/430842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toro JR, Wei MH, Glenn GM, Weinreich M, Toure O, Vocke CD, et al. BHD mutations, clinical and molecular genetic investigations of Birt-Hogg-Dube syndrome: a new series of 50 families and a review of published reports. J Med Genet. 2008;45:321–331. doi: 10.1136/jmg.2007.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baba M, Hong SB, Sharma N, Warren MB, Nickerson ML, Iwamatsu A, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci U S A. 2006;103:15552–15557. doi: 10.1073/pnas.0603781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hasumi H, Baba M, Hong SB, Hasumi Y, Huang Y, Yao M, et al. Identification and characterization of a novel folliculin-interacting protein FNIP2. Gene. 2008;415:60–67. doi: 10.1016/j.gene.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hasumi Y, Baba M, Ajima R, Hasumi H, Valera VA, Klein ME, et al. Homozygous loss of BHD causes early embryonic lethality and kidney tumor development with activation of mTORC1 and mTORC2. Proc Natl Acad Sci U S A. 2009;106:18722–18727. doi: 10.1073/pnas.0908853106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baba M, Furihata M, Hong SB, Tessarollo L, Haines DC, Southon E, et al. Kidney-targeted Birt-Hogg-Dubé gene inactivation in a mouse model: Erk1/2 and Akt-mTOR activation, cell hyperproliferation, and polycystic kidneys. J Natl Cancer Inst. 2008;100:140–154. doi: 10.1093/jnci/djm288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 68.Seyam RM, Bissada NK, Kattan SA, Mokhtar AA, Aslam M, Fahmy WE, et al. Changing trends in presentation, diagnosis and management of renal angiomyolipoma: comparison of sporadic and tuberous sclerosis complex-associated forms. Urol. 2008;72:1077–1082. doi: 10.1016/j.urology.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 69.van VW, Korsse SE, van de Laar L, Peppelenbosch MP. The long and winding road to rational treatment of cancer associated with LKB1/AMPK/TSC/mTORC1 signaling. Oncogene. 2011;30:2289–2303. doi: 10.1038/onc.2010.630. [DOI] [PubMed] [Google Scholar]
- 70.Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fleming S, Lewi HJE. Collecting duct carcinoma of the kidney. Histopath. 1986;10:1131–1141. doi: 10.1111/j.1365-2559.1986.tb02553.x. [DOI] [PubMed] [Google Scholar]
- 72.Kennedy SM, Merino MJ, Linehan WM, Roberts JR, Robertson CN, Neumann RD. Collecting duct carcinoma of the kidney. Hum Pathol. 1990;21:449–456. doi: 10.1016/0046-8177(90)90209-n. [DOI] [PubMed] [Google Scholar]
- 73.Srigley JR, Moch H. World Health Organization Classification of Tumors Pathology & Genetics: Tumors of the Urinary System and Male Genital Organs. Lyons: IARC; 2004. Carcinoma of the collecting ducts of Bellini; pp. 33–34. [Google Scholar]
- 74.Amin MB, Varma VD, Tickoo SK. Collecting duct carcinoma of the kidney. Adv Anat Pathol. 1997;4:85–94. [Google Scholar]
- 75.Wright JL, Risk MC, Hotaling J, Lin DW. Effect of collecting duct histology on renal cell cancer outcome. J Urol. 2009;182:2595–2599. doi: 10.1016/j.juro.2009.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tokuda N, Naito S, Matsuzaki O, Nagashima Y, Ozono S, Igarashi T. Collecting duct (Bellini duct) renal cell carcinoma: a nationwide survey in Japan. J Urol. 2006;176:40–43. doi: 10.1016/S0022-5347(06)00502-7. [DOI] [PubMed] [Google Scholar]
- 77.Pepek JM, Johnstone PA, Jani AB. Influence of demographic factors on outcome of collecting duct carcinoma: a Surveillance, Epidemiology, and End Results (SEER) database analysis. Clin Genitourin Cancer. 2009;7:E24–E27. doi: 10.3816/CGC.2009.n.017. [DOI] [PubMed] [Google Scholar]
- 78.Motzer RJ, Bacik J, Mariani T, Russo P, Mazumdar M, Reuter V. Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol. 2002;20:2376–2381. doi: 10.1200/JCO.2002.11.123. [DOI] [PubMed] [Google Scholar]
- 79.Matz LR, Latham BI, Fabian VA, Vivian JB. Collecting duct carcinoma of the kidney: a report of three cases and review of the literature. Pathology (Phila) 1997;29:354–359. doi: 10.1080/00313029700169305. [DOI] [PubMed] [Google Scholar]
- 80.Procopio G, Verzoni E, Iacovelli R, Colecchia M, Torelli T, Mariani L. Is there a role for targeted therapies in the collecting ducts of Bellini carcinoma? Efficacy data from a retrospective analysis of 7 cases. Clin Exp Nephrol. 2012;16:464–467. doi: 10.1007/s10157-012-0589-3. [DOI] [PubMed] [Google Scholar]
- 81.Gollob JA, Upton MP, DeWolf WC, Atkins MB. Long-term remission in a patient with metastatic collecting duct carcinoma treated with taxol/carboplatin and surgery. Urol. 2001;58:1058. doi: 10.1016/s0090-4295(01)01411-x. [DOI] [PubMed] [Google Scholar]
- 82.Milowsky MI, Rosmarin A, Tickoo SK, Papanicolaou N, Nanus DM. Active chemotherapy for collecting duct carcinoma of the kidney: a case report and review of the literature. Cancer. 2002;94:111–116. doi: 10.1002/cncr.10204. [DOI] [PubMed] [Google Scholar]
- 83.Oudard S, Banu E, Vieillefond A, Fournier L, Priou F, Medioni J, et al. Prospective multicenter phase II study of gemcitabine plus platinum salt for metastatic collecting duct carcinoma: results of a GETUG (Groupe d'Etudes des Tumeurs Uro-Genitales) study. J Urol. 2007;177:1698–1702. doi: 10.1016/j.juro.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 84.Davis CJ, Jr, Mostofi FK, Sesterhenn IA. Renal medullary carcinoma. The seventh sickle cell nephropathy. Am J Surg Pathol. 1995;19:1–11. doi: 10.1097/00000478-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 85.Figenshau RS, Basler JW, Simon JA, Dierks SM. Renal medullary carcinoma. J Urol. 1998;159:711–713. [PubMed] [Google Scholar]
- 86.Selby DM, Simon C, Foley JP, Thompson IM, Baddour RT. Renal medullary carcinoma: can early diagnosis lead to long-term survival? J Urol. 2000;163:1238. doi: 10.1016/s0022-5347(05)67733-6. [DOI] [PubMed] [Google Scholar]
- 87.Ronnen EA, Kondagunta GV, Motzer RJ. Medullary renal cell carcinoma and response to therapy with bortezomib. J Clin Oncol. 2006;24:e14. doi: 10.1200/JCO.2005.05.0344. [DOI] [PubMed] [Google Scholar]
- 88.Linehan WM. Genetic basis of kidney cancer: role of genomics for the development of disease-based therapeutics. Genome Res. 2012 doi: 10.1101/gr.131110.111. doi:genome.cship.org 10-15-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rathmell WK, Monk JP. High-dose-intensity MVAC for Advanced Renal Medullary Carcinoma: Report of Three Cases and Literature Review. Urol. 2008;72:659–663. doi: 10.1016/j.urology.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 90.Takahashi M, Sugimura J, Yang X, Vogelzang N, Teh BS, Furge K, et al. Gene expression profiling of renal cell carcinoma and its implications in diagnosis, prognosis, and therapeutics. Adv Cancer Res. 2003;89:157–181. doi: 10.1016/s0065-230x(03)01005-4. [DOI] [PubMed] [Google Scholar]
- 91.Schaeffer EM, Guzzo TJ, Furge KA, Netto G, Westphal M, Dykema K, et al. Renal medullary carcinoma: molecular, pathological and clinical evidence for treatment with topoisomerase-inhibiting therapy. BJU Int. 2010;106:62–65. doi: 10.1111/j.1464-410X.2009.09139.x. [DOI] [PubMed] [Google Scholar]