Abstract
To determine the effects of age and sex on in vivo mitochondrial function of distinct locomotory muscles, the tibialis anterior (TA) and medial gastrocnemius (MG), of young (Y; 24 ± 3 years) and older (O; 69 ± 4) men (M) and women (W) of similar overall physical activity (PA) was compared. In vivo mitochondrial function was measured using phosphorus magnetic resonance spectroscopy, and PA and physical function were measured in all subjects. Overall PA was similar among the groups, although O (n = 17) had fewer daily minutes of moderate-to-vigorous PA (p = 0.001), and slowed physical function (p < 0.05 for all variables), compared with Y (n = 17). In TA, oxidative capacity (Vmax; mM s−1) was higher in O than Y (p < 0.001; Y = 0.90 ± 0.12; O = 1.12 ± 0.18). There was no effect of age in MG (p = 0.5; Y = 0.91 ± 0.17; O = 0.96 ± 0.24), but women had higher oxidative capacity than men (p = 0.007; M = 0.84 ± 0.18; W = 1.03 ± 0.18). In vivo mitochondrial function was preserved in healthy O men and women, despite lower intensity PA and physical function in this group. The extent to which compensatory changes in gait may be responsible for this preservation warrants further investigation. Furthermore, women had higher oxidative capacity in the MG, but not the TA.
Keywords: Oxidative phosphorylation, Bioenergetics, Mobility, Physical function
Introduction
Despite intensive investigation in recent years, the impact of old age on skeletal muscle mitochondrial function remains unclear. For example, oxidative capacity, which is one measure of mitochondrial function, has been reported by some to decline in older (O) adults (Conley et al. 2000; Short et al. 2005; Coggan et al. 1992; McCully et al. 1991; Pastoris et al. 2000; Karakelides et al. 2010), while others report no change with aging (Lanza et al. 2005; Larsen et al. 2012b; Hutter et al. 2007; Rasmussen et al. 2003; Tevald et al. 2010; Chilibeck et al. 1998). A number of factors, including the means of assessing mitochondrial function, the health and physical activity (PA) status of the study participants, and the muscle group studied, apparently contribute to these conflicting findings (Larsen et al. 2012b).
Various in vitro (i.e., enzyme activities, isolated mitochondria assays, and permeabilized fiber assays) and in vivo (phosphorus magnetic resonance spectroscopy) approaches have been employed to assess the impact of age on the capacity of skeletal muscle mitochondria to produce ATP (Johnson et al. 2013). Although there is evidence of a correlation between the results of certain in vitro and in vivo approaches (McCully et al. 1993; Lanza et al. 2011), these approaches likely assess different aspects of mitochondrial function, which may contribute to the discrepancies in the literature and should be considered when comparing results of individual studies. Furthermore, the methods used to isolate mitochondria, which is necessary for some in vitro approaches, appear to exaggerate age-related differences in mitochondrial function (Picard et al. 2010). Although in vitro methods provide powerful mechanistic information, in vivo approaches, which assess the function of the intact system, have an important role for investigating the impact of aging on overall mitochondrial function.
Several studies using a variety of approaches have shown that skeletal muscle mitochondrial function is sensitive both to increases (Forbes et al. 2008; Gollnick et al. 1973; Jacobs and Lundby 2013; Short et al. 2003; Konopka et al. 2014; Larsen et al. 2012a) and decreases (Hikida et al. 1989) in PA. Recent studies report a direct, if modest, association between habitual PA and several markers of skeletal muscle mitochondrial function (den Hoed et al. 2008), including in vivo oxidative capacity (Larsen et al. 2009; Tartaglia et al. 2000). Although PA generally declines across the lifespan (Troiano et al. 2008), many studies lack specific, objective measures of this important lifestyle variable. It has been proposed that differences in PA, rather than aging per se, are responsible for losses in skeletal muscle oxidative capacity with aging (Brierley et al. 1996, 1997; Russ and Kent-Braun 2004). Furthermore, several studies (Kent-Braun and Ng 2000; Lanza et al. 2005, 2007; Larsen et al. 2012b) have shown preserved in vivo oxidative capacity in the tibialis anterior (TA) muscle of O adults compared to young (Y) adults with similar, sedentary PA.
In contrast with studies of the TA, other studies have shown age-related declines in oxidative capacity of the plantarflexor muscles using both in vivo (McCully et al. 1991) and in vitro approaches (e.g., enzyme activities; Coggan et al. 1992; Houmard et al. 1998). In the quadriceps muscles, which are most commonly studied, in vivo (Conley et al. 2000; Larsen et al. 2012b) and in vitro (Short et al. 2005) oxidative capacities are both lower in O adults. Additionally, diminished mitochondrial content (Conley et al. 2000), mitochondrial protein content (Short et al. 2005) and synthesis (Rooyackers et al. 1996), and diminished markers of mitochondrial biogenesis and quality control (Konopka et al. 2014), have all been reported (see Konopka and Nair 2013; Johnson et al. 2013 for recent reviews).
While the finding of reduced mitochondrial function in the quadriceps with age is not universal (Rasmussen et al. 2003), these results raise the possibility that the effects of aging on oxidative capacity may vary depending on the muscle under investigation, as has been shown with other aspects of muscle function. For example, several studies have documented greater age-related declines in the strength (Hasson and Caldwell 2012; Winegard et al. 1996; Simoneau et al. 2005) and size (Hasson et al. 2011) of the plantarflexors than the ankle dorsiflexors. The mechanisms for these muscle-specific effects are still under investigation but may include age-related changes in the patterns of use of the muscles (Schmitz et al. 2009; Benjuya et al. 2004), as well as different susceptibilities of the different fiber types to age-related changes in mitochondrial function (Jacobs et al. 2013; Proctor et al. 1995). The previously reported selective atrophy of type II fibers with age (Proctor et al. 1995) may also contribute to intermuscular variation in the effects of aging.
Studies of the oxidative capacity of multiple muscle groups in the same individual, which are ideal for investigating intermuscular variation in the effects of aging, are rare. However, the studies that we are aware of all show evidence of intermuscle variation in the impact of aging on oxidative capacity. Pastoris et al. (2000) reported lower citrate synthase activity, an in vitro marker of oxidative capacity, of the vastus lateralis, but not the rectus abdominus or gluteus medius, in O sedentary subjects. By contrast, Houmard et al. (1998) found an age-related decline in the gastrocnemius, but not the vastus lateralis, using the same technique. Finally, Larsen et al. (2012b) found that oxidative capacity was preserved in O TA, but not VL, muscles.
The results of these studies prevent simple generalizations about the impact of age on muscle oxidative capacity and make clear that further study of additional muscle groups is necessary to fully understand the impact of aging on human muscle. Therefore, the primary purpose of this study was to investigate the effect of aging on skeletal muscle in vivo oxidative capacity in two leg muscles in the same individuals. Y and O men and women with similar overall PA, determined using accelerometry, were studied. Oxidative capacity was measured by phosphorus magnetic resonance spectroscopy (31P-MRS). The TA and medial gastrocnemius (MG) muscles were selected for study because, while both are critical to physical function, gait, and fall prevention in O adults (Hasson et al. 2014; Wolfson et al. 1995), they exhibit a variety of differences. Functionally, the TA operates largely eccentrically during the stance phase of the gait cycle, while concentric activation of the MG is critical to provide forward propulsion. The TA is largely composed of type I fibers (75 % type I; Gregory et al. 2001), while the MG is mixed in fiber-type composition (50 % type I; Edgerton et al. 1975). Furthermore, while most studies of the TA do not report lower oxidative capacity in O adults (Kent-Braun and Ng 2000; Lanza et al. 2005, 2007; Larsen et al. 2012b; Tevald et al. 2010), reports on the MG differ, with studies reporting either lower (McCully et al. 1991; Coggan et al. 1992; Houmard et al. 1998) or similar (Chilibeck et al. 1998; Wray et al. 2009) oxidative capacity in O compared to Y adults. Based on the previously discussed evidence that age-related changes in several aspects of muscle performance are smaller in the TA than the MG, we hypothesized that oxidative capacity would be preserved in the TA, but not the MG, of O subjects. The results of this study will advance our understanding of the variation in age-related effects on muscle oxidative capacity.
Methods
Subjects
Thirty-four Y (21–29 years) and O (65–77 years) men (M) and women (W) participated in this study (see Table 1 for distribution). All participants gave written informed consent prior to participation. All procedures were approved by the appropriate institutional review boards at Yale University School of Medicine and the University of Massachusetts, Amherst, and conformed to the standards set by the Declaration of Helsinki.
Table 1.
Subject characteristics
| Y (17) | O (17) | p value | |
|---|---|---|---|
| Female (n (%)) | 8 (47 %) | 9 (53 %) | – |
| Age (years) | 24 ± 3 | 69 ± 3 | – |
| Height (cm) | 167.8 ± 76 | 165.9 ± 90 | 0.515 |
| Body mass (kg) | 66.9 ± 12.7 | 79.7 ± 15.5 | 0.013 |
| BMI | 23.7 ± 3.9 | 28.8 ± 4.3 | 0.001 |
Data presented as mean ± SD, except where indicated
All participants were nonsmokers, generally healthy, and sedentary (<60 min of structured PA per week, by self-report). They were free from neurological or neuromuscular disease, stroke, diabetes, or known coronary artery disease and had ankle-brachial index ≥1, suggesting the absence of peripheral vascular disease. Medications used by subjects included ace inhibitors (two OM and one OW), angiotensin receptor blockers (one OW), and diuretics (two OW) for the control of hypertension and statins (two OM and two OW) for the treatment of hypercholesterolemia. One OW was on estrogen replacement therapy. Participants were instructed to take their medications as prescribed during the course of the study.
Procedures and activity assessment
The study consisted of two testing sessions, separated by at least 48 h. The first session was conducted in the Muscle Physiology Lab at the University of Massachusetts, Amherst, and consisted of physical function testing. The second session was the metabolic testing session, which was conducted at the Magnetic Resonance Research Center at the Yale University School of Medicine. In addition, all subjects wore a uniaxial accelerometer (Actigraph GT1M; Pensacola FL) during waking hours for 7–10 days to quantify PA. Total PA (sum of all counts over the day), as well as the time spent in low-intensity PA (LPA; 1–1,951 counts min−1) and moderate-to-vigorous intensity PA (MVPA; >1,952− counts min−1), were calculated based on a modification of established thresholds for Actigraph accelerometers (Freedson et al. 1998; Matthews et al. 2008). Accelerometer data were verified against an activity diary maintained by the subjects. Days during which the participant’s activity patterns could not be reconciled with the diary, or the subject did not wear the monitor for at least 10 h, were excluded (Matthews et al. 2008). Each participant’s daily average for all variables was used to determine group averages.
Physical function testing
Physical function testing was performed to more fully characterize the mobility status of the participants. A battery of commonly used tests was selected to provide a measure of the general functional status of the individuals. The tests including the following: seated foot-tap speed over 10 s (Kent-Braun and Ng 1999; Kent-Braun et al. 1998), ten timed chair rises, timed climb, and descent of eight steps, and a 400-m walk (Simonsick et al. 2001). Foot tap speed, chair rises, and stair climb and descent were each performed twice, and the fastest time was used for analysis. The 400-m walk was performed by laps around a straight 20-m track, separated by cones. Participants were instructed to walk at the quickest pace that they could maintain for the entire walk.
Muscle metabolic testing
Participants were instructed to avoid caffeine for 6 h and strenuous activity for 24 h prior to testing due to their potential effects on neuromuscular function. All testing was performed while participants lay supine in a 4.0-Tesla whole-body superconducting magnet (Bruker Biospin, Rheinstetten, Germany). The TA and the MG muscles were tested in separate sessions on the same day, with a minimum of 30 min between sessions. For all participants, the TA was tested first, followed by the MG. The apparatus and protocol used to test the TA muscles has been described previously (Tevald et al. 2010). Procedures for the MG were similar, although the knee was positioned in approximately 15° of flexion, and the foot position at 90° with respect to the tibia. Velcro straps were used to secure the foot, ankle, and shank, and shoulder straps were used to minimize upper body movement during contractions. To verify that the source of our 31P signal was from MG only, we measured the distance from the surface of the skin to the border between MG and soleus. This distance was 2.8 ± 0.4 and 2.6 ± 0.4 cm in O and Y groups, respectively. Because the maximum depth for signal detection by a surface coil corresponds to its smallest radius (1.5 cm), signal contamination from soleus was not an issue.
The contraction protocols were initiated with two “warm-up” maximal voluntary contractions (MVC), each lasting 3–4 s. Following 2 min of rest, 31P-MRS data acquisition was initiated. After an additional 1 min of rest, the subject performed a 16-s (TA) or 20-s (MG) MVC. 31P-MRS data acquisition continued during and for 10 min following each contraction. Contraction durations were chosen, based on pilot data, to deplete PCr by ∼40 %, without intracellular acidosis.
Phosphorus spectral analysis and metabolic calculations
Spectral analysis and metabolite concentrations were performed as previously described (Tevald et al. 2010). To calculate metabolite concentrations from 31P-MRS spectra, peak areas were corrected for partial saturation effects. Correction factors for partial saturation effects for the MG were obtained in a subset of subjects (n = 8; PCr = 1.69, Pi = 2.16, ATP = 1.62), as previously described (Tevald et al. 2010), while values from a previous study (Tevald et al. 2010) were used for TA (PCr = 1.97, Pi = 1.89, ATP = 1.83). Resting and end-exercise metabolite concentrations were calculated by assuming that resting (ATP) = 8.2 mM (23), and that (PCr) + (Pi) = 42.5 mM, which is based on the assumptions that (PCr) + (creatine) = 42.5 (Harris et al. 1974), and Pi and creatine exhibit a 1:1 stoichiometry (Kemp and Radda 1994). Intramuscular pH was calculated from the chemical shift of Pi relative to PCr (Taylor et al. 1986).
Assessment of in vivo oxidative capacity
To quantify in vivo oxidative capacity, the recovery of PCr following the 16-s and 20-s MVCs was fit with a monoexponential function, and oxidative capacity (Vmax; mM ATP · s−1) was calculated as the product of the rate constant (kPCr) and resting (PCr) (Lanza et al. 2005; Meyer 1989). This analysis provides a robust measure of oxidative capacity in the intact system (Lanza et al. 2011).
Statistical analysis
Subject characteristics (body height, mass, body mass index (BMI), PA, and physical function variables) and metabolic variables (Vmax, kPCr, and metabolites at rest and the end of contraction) were compared between the Y and O groups using separate two-tailed t tests. To evaluate the potential effects of the use of medications (blood pressure medications and statins, respectively), oxidative capacity among O adults who did and did not take these drugs was compared with separate nonparametric tests (Mann–Whitney U). Because the effects of age may vary by sex, our secondary purpose regarding the influence of sex on oxidative capacity was evaluated with a two-factor (age and sex) ANOVA and post-hoc testing using Tukey’s HSD. Additional analysis included a two-tailed paired t test to determine if there were differences in oxidative capacity between the TA and MG, as well as Pearson and partial correlation to evaluate the strength of the relationships between oxidative capacity, BMI, and PA. Statistical analyses were performed using IBM SPSS 19 (IBM Corp, Armonk, NY), and α was set at 0.05. All data are presented as mean ± SD.
Results
Descriptive data for the groups are summarized in Table 1. There was no age difference in height, but the O adults were heavier and had higher BMI. The PA data are summarized in Table 2. There were no differences in the total time the subjects wore the monitors each day. Daily accelerometer counts were not different between the age groups, indicating that overall PA was not different. The total amount of time that the participants engaged in PA did not differ between the groups nor did the amount of time spent in LPA. However, the O adults spent less time in MVPA than the younger participants. Data from one O subject is missing due to technical issues.
Table 2.
Physical activity and physical function
| Y | O | p value | |
|---|---|---|---|
| Physical activity | n = 17 | n = 16 | |
| Wear timea (min day−1) | 865 ± 185 | 858 ± 72 | 0.887 |
| Activeb (min day−1) | 464 ± 128 | 209 ± 88 | 0.257 |
| PA (ct day−1,000) | 267 ± 114 | 220 ± 63 | 0.156 |
| LPA (min day−1) | 422 ± 114 | 487 ± 90 | 0.085 |
| MVPA (min day−1) | 42 ± 24 | 21 ± 15 | 0.001 |
| Physical function | |||
| Foot tapc (no. in 10 s) | 56 ± 7 | 45 ± 7 | <0.001 |
| Stair climbd (s) | 2.8 ± 0.4 | 3.8 ± 0.8 | <0.001 |
| Stair descendd (s) | 2.6 ± 0.4 | 3.6 ± 1.1 | 0.001 |
| Chair rise × 10e (s) | 11.8 ± 3.1 | 14.9 ± 4.1 | 0.022 |
| 400-m walkf (s) | 222 ± 20 | 274 ± 30 | <0.001 |
Data presented as mean ± SD. Physical activity data from one older subject missing for technical reasons; 400-m walk data missing from one older and one younger subject
PA total physical activity by accelerometer, LPA time spent in sedentary-to-low PA, MVPA time spent in moderate-to-vigorous PA
aTime spent wearing accelerometer
bTime spent in nonzero PA
cNumber of foot taps in 10 s
dTime to climb and descend, respectively, eight steps
eTime to rise from a chair ten times
fTime to walk 400 m
Performance on physical function tests by each of the groups is also summarized in Table 2. Despite the fact that the participants were reasonably healthy and of similar overall PA level, O subjects were slower on all tests of physical function. Foot tap data from one younger subject and 400 m walk time from one O subject are missing due to investigator error.
Age and oxidative capacity
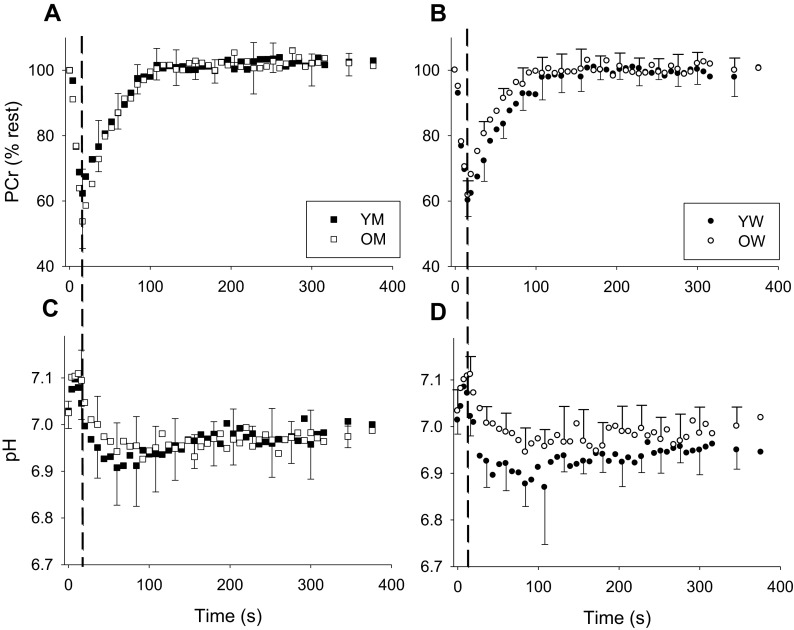
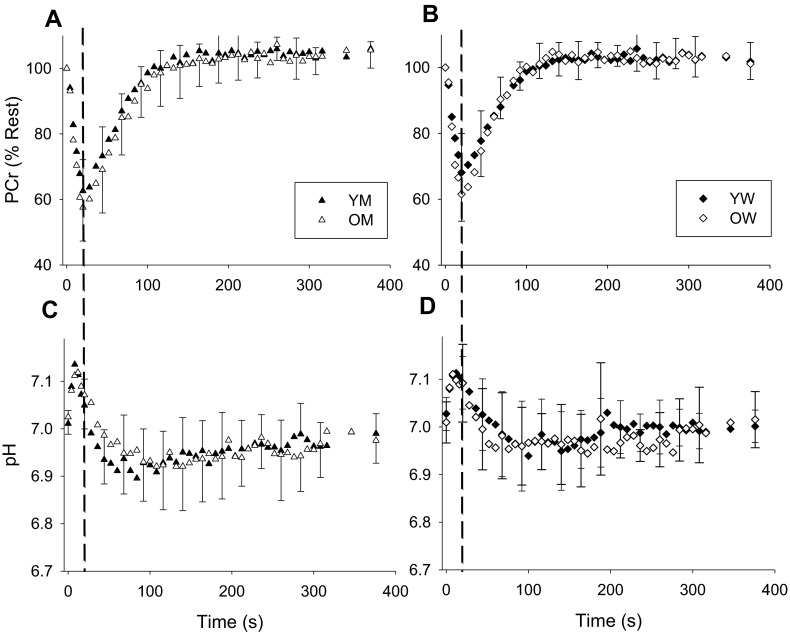
Metabolic variables for the TA are summarized in Table 3 and Fig. 1, while those for the MG are summarized in Table 4 and Fig. 2. Metabolic data from the MG of one Y and two O subjects was not available for technical reasons. At rest, no differences in (PCr) or pH were apparent in either muscle. During contraction, there were no group differences in the extent of the decline in PCr. As a result of the consumption of protons by the CK reaction, intracellular pH increased briefly at the onset of contraction, as expected (Fig. 1). In the TA, but not the MG, pH at the end of contraction was higher in O than Y, but the minimum pH observed during recovery was not significantly different for either muscle. During recovery, both kPCr and Vmax were higher in O than Y in the TA, but there were no differences in the MG.
Table 3.
Metabolic variables from the tibialis anterior muscle
| Y (n = 17) | O (n = 17) | p value | |
|---|---|---|---|
| Rest | |||
| PCr (mM) | 38.1 ± 1.3 | 38.6 ± 1.5 | 0.234 |
| pH | 7.02 ± 0.03 | 7.03 ± 0.04 | 0.519 |
| Contraction | |||
| End PCra (% rest) | 61.3 ± 6.2 | 58.0 ± 7.5 | 0.170 |
| End pHa | 7.03 ± 0.04 | 7.10 ± 0.05 | <0.001 |
| Min pHb | 6.86 ± 0.06 | 6.89 ± 0.04 | 0.066 |
| k PCr (s−1) | 0.024 ± 0.003 | 0.029 ± 0.004 | <0.001 |
| V max (mM ATP s−1) | 0.90 ± 0.12 | 1.12 ± 0.18 | <0.001 |
k PCr rate constant of PCr recovery, V max oxidative capacity
aPCr and pH at the end of contraction
bLowest pH achieved during recovery
Fig. 1.
Metabolic changes in tibialis anterior PCr (a, b) and pH (c, d) during contraction and recovery in young and older men (a, c) and women (b, d). PCr is expressed relative to resting values. The end of the 16-s contraction is indicated by the dashed vertical line on each plot. Following contraction, the recovery of PCr was well approximated by a monoexponential function in all groups (r 2 = 0.90 ± 0.06, 0.90 ± 0.04, 0.91 ± 0.03, and 0.87 ± 0.04 for YM, OM, YW, and OW, respectively). Error bars indicating SD are provided every 24 s during recovery, for clarity
Table 4.
Metabolic variables from the medial gastrocnemius muscle
| Y (n = 16) | O (n = 15) | p value | |
|---|---|---|---|
| Rest | |||
| PCr (mM) | 37.9 ± 1.2 | 38.1 ± 1.2 | 0.593 |
| pH | 7.02 ± 0.03 | 7.02 ± 0.04 | 0.707 |
| Contraction | |||
| End PCra (% rest) | 65.4 ± 10.7 | 59.4 ± 9.3 | 0.112 |
| End pHa | 7.07 ± 0.06 | 7.08 ± 0.04 | 0.605 |
| Min pHb | 6.88 ± 0.07 | 6.89 ± 0.08 | 0.965 |
| k PCr (s−1) | 0.022 ± 0.004 | 0.025 ± 0.006 | 0.551 |
| V max (mM ATP s−1) | 0.91 ± 0.17 | 0.96 ± 0.24 | 0.500 |
kPCr rate constant of PCr recovery, V max oxidative capacity
aPCr and pH at the end of contraction
aLowest pH achieved during recovery
Fig. 2.
Metabolic changes in medial gastrocnemius PCr (a, b) and pH (c, d) during contraction and recovery in young and older men (a, c) and women (b, d). PCr is expressed relative to resting values. The end of the 20-s contraction is indicated by the dashed vertical line. Recovery of PCr following contraction in MG was well approximated by a monoexponential function (r 2 = 0.89 ± 0.09, 0.92 ± 0.02, 0.86 ± 0.09, and 0.89 ± 0.06 for YM, OM, YW, OW, respectively). Error bars indicating SD are provided every 24 s during recovery, for clarity
Vmax was not different in O adults who took statins and those who did not, for either the TA (n = 4; 1.07 ± 0.17 μM/s vs. n = 13; 1.01 ± 0.17; p = 0.102) or for the MG (n = 3; 1.01 ± 0.17 μM/s vs. n = 12; 0.76 ± 0.42; p = 0.365). Similarly, Vmax was not different among O adults who took blood pressure medications and those who did not for the TA (n = 5; 1.18 ± 0.21 μM/s vs. n = 12; 1.09 ± 0.19 μM/s; p = 0.57) or MG (n = 4; 0.85 ± 0.39 μM/s vs. n = 11; 0.88 ± 0.17 μM/s; p = 0.57).
Sex and oxidative capacity
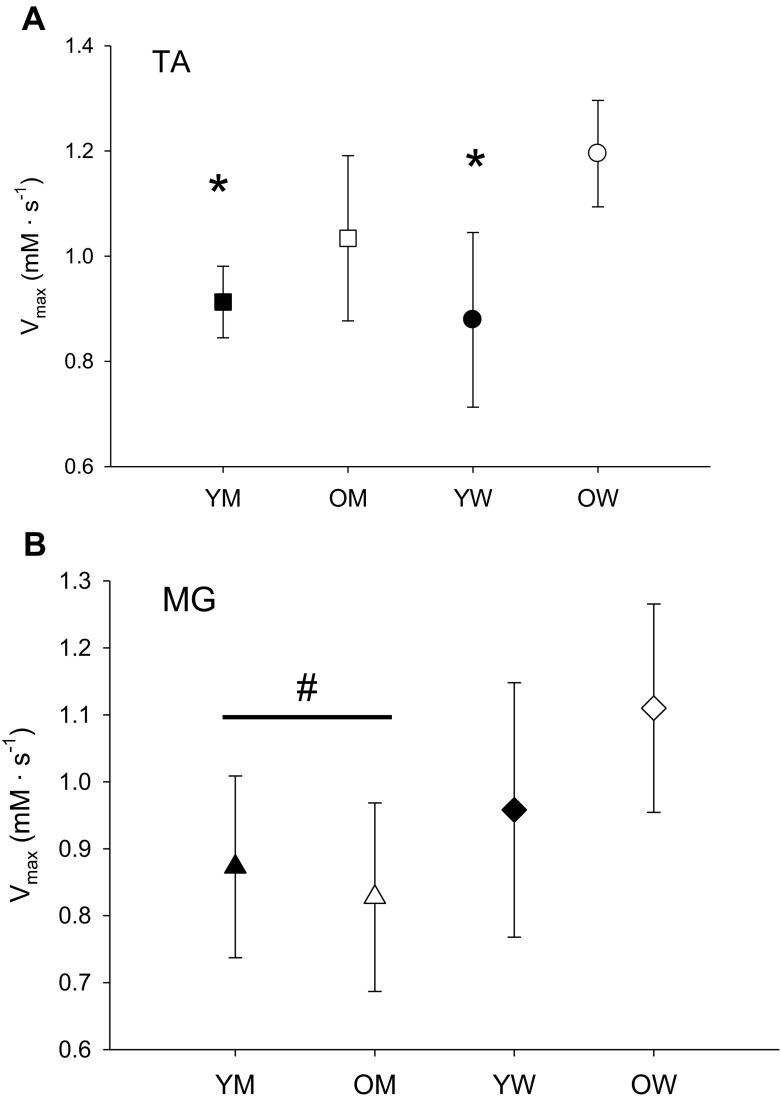
A secondary analysis was used to investigate the combined effects of age and sex on in vivo oxidative capacity. The results are displayed in Fig. 3. For the TA, there was a trend (p = 0.066) for an age by sex interaction, along with a significant effect of age. Post-hoc analysis revealed that Vmax in the OW (n = 9; 1.27 ± 0.11 mM ATP s−1) was significantly higher than in the YM (n = 9; 0.83 ± 0.11) and YW (n = 8; 0.86 ± 0.18) but not the OM (n = 8; 1.09 ± 0.28). In the MG, there was no interaction (p = 0.171) or effect of age (p = 0.367), but Vmax for women (n = 15; 1.03 ± 0.18) was significantly higher than men (n = 16; 0.84 ± 0.18; p = 0.007).
Fig. 3.
Muscle oxidative capacity V max (mM s−1) for tibialis anterior (TA, a) and medial gastrocnemius (MG, b) muscles; asterisk, significantly lower than OW; number sign, main effect of sex
Vmax of the TA (n = 31; 0.99 ± 0.03) was not significantly different from the MG (n = 31; 0.93 ± 0.04; p = 0.23) overall, or within the O (n = 15; 1.10 ± 0.17 vs. 0.96 ± 0.062; p = 0.118) or Y (n = 16; 0.89 ± 0.13 vs. 0.91 ± 0.17; p = 0.791) age groups.
Correlations
Neither kPCr nor Vmax in the TA or MG were associated with any PA variables (PA, LPA, or MVPA, r = −0.28 to 0.23; p > 0.12 for all comparisons). Vmax of the TA (r = 0.376; p = 0.029) but not the MG (−0.255; p = 0.167), was correlated with BMI. BMI was also associated with age (r = 0.549; p = 0.001), and removing the influence of age eliminated the relationship between BMI and Vmax in the TA (r = 0.095; p = 0.6).
Discussion
The results of this study provide evidence of well-preserved in vivo mitochondrial function in both the TA and MG of healthy O men and women, even though the O adults showed signs of diminished physical function. Thus, while previous studies have shown that the MG appears to be more sensitive than the TA to age-related changes in size (Hasson et al. 2011) and strength (Hasson and Caldwell 2012; Winegard et al. 1996; Simoneau et al. 2005), the same does not appear to be true for oxidative capacity when overall PA is similar among groups. We also found higher oxidative capacity in women than men for the MG muscle, and no difference in oxidative capacity between the MG and TA muscles. These results support the concept that PA may be a more important determinant of in vivo muscle mitochondrial function than aging, per se.
Age and oxidative capacity
The finding of preserved oxidative capacity in this study would appear to be counter to a number of studies that have identified age-related deficits in a variety of markers of mitochondrial function, content, and biogenesis in human muscle (reviewed in Konopka and Nair 2013; Johnson et al. 2013). However, they are consistent with the concept that the effects of age on oxidative capacity depend on the muscle being investigated and on the level of habitual PA of the individual. Several studies from this lab have reported well-preserved in vivo oxidative capacity in the TA (Kent-Braun and Ng 2000; Lanza et al. 2005; Tevald et al. 2010), including one recent study showing higher oxidative capacity in the TA of O adults but lower oxidative capacity in the quadriceps (Larsen et al. 2012b). We are not aware of studies that have used in vitro techniques to examine the effect of age on oxidative capacity of the TA.
While some of the O subjects in the current study took statin or blood pressure medications, these did not have a significant effect on oxidative capacity in either muscle. Although the small number of individuals taking these medications preclude us from drawing strong conclusions about the impact of these drugs on oxidative capacity, they did not appear to influence the results of this study. In this context, our results are consistent with previous reports.
With regard to in vivo oxidative capacity of the MG, the present results are consistent with two prior studies (Chilibeck et al. 1998; Wray et al. 2009). They are not consistent with the results of one in vivo study (McCully et al. 1991) and two in vitro (enzyme activities) studies (Coggan et al. 1992; Houmard et al. 1998), all of which showed lower oxidative capacity in the O subjects. In the first study (McCully et al. 1991), the lack of control for PA and health status of the O subjects likely contributed to lower oxidative capacity in that group. The discrepancy between the present results and those of the two in vitro studies is less clear, as both only studies included only people who reported that they were sedentary. However, self-reported means of assessing PA typically overestimate actual PA (Tucker et al. 2011) and may not have been sensitive enough to detect a difference in PA among the age groups. Additionally, the differences in the technique used to assess oxidative capacity (in vivo vs. in vitro) may contribute to the different results. Although in vitro approaches provide powerful mechanistic information, in vivo approaches assess the integrated function of the entire intact system in vivo, and therefore play a critical role in the investigation of the effects of age and disease on human physiology.
Indeed, although overall PA was similar among the groups in the current study, the O adults spent less time in moderate-to-vigorous PA (Table 2). Given the sensitivity of muscle oxidative capacity to PA, one might expect this to lead to lower oxidative capacity in the O subjects. However, Larsen et al. (2009, 2012b) found that time spent in MVPA was more strongly associated with oxidative capacity of the quadriceps (r = 0.64) than the TA (r = 0.38), in a sample that included a wide range of PA levels. Thus, it appears that age-related difference in the intensity or pattern of PA has greater effects on some muscles than others, and it may be that both the TA and MG are less affected than the quadriceps by these differences in PA. There was no association between PA and oxidative capacity in either the TA or MG in the current study, although the narrow range of PA levels (all sedentary) limits our ability to assess this potential relationship, and further study is needed to evaluate this hypothesis.
Additionally, age-related changes in muscle activation during daily activities may lead O adults to use certain muscles more than younger adults while performing the same task. O adults exhibit greater co-contraction during standing (Benjuya et al. 2004) and walking (Schmitz et al. 2009; Hortobagyi et al. 2011), leading to greater activation of the ankle dorsiflexors and plantarflexors during these tasks. These differences in muscle activation contribute to the reduced mechanical efficiency of gait in O adults (Hortobagyi et al. 2011). They also provide a potential explanation for preserved, or even enhanced, oxidative capacity in certain muscles, such as that observed here. Together, these results suggest that, unlike the quadriceps, age-related changes in the pattern of PA do not have detrimental effects on the oxidative capacity of the TA and MG when the overall amount of PA is similar in Y and O subjects.
Sex and mitochondrial function
In the current study, we found that oxidative capacity of the MG was higher in women than men. Furthermore, in the TA, the O women exhibited the highest oxidative capacity of all the groups. These results are consistent with one prior study from our lab (Kent-Braun and Ng 2000) and those of Essen-Gustavsson and Borges (1986), who found higher citrate synthase activity in the vastus lateralis of women in their sixties than age-matched men. However, other studies have shown similar (Grimby et al. 1982; Lanza et al. 2007; Russ et al. 2005) or lower (Coggan et al. 1992) oxidative capacity activity in women compared with men. The explanations for these discrepant results are not clear, although they are broadly consistent with studies of the effects of sex on fatigue (Hunter et al. 2004) and energetics (Kent-Braun et al. 2002; Ruby and Robergs 1994; Russ et al. 2005). Specifically, women are less reliant on nonoxidative glycolysis during whole body (Ruby and Robergs 1994) and small muscle mass (Russ and Kent-Braun 2003) exercise. During muscle contraction, women experience less fatigue (Hunter et al. 2004), less acidosis (Russ et al. 2005), and smaller changes in (PCr) and (Pi) (Kent-Braun et al. 2002).
Muscle differences
Overall, we found that oxidative capacity in the TA was not different from oxidative capacity in the MG in our sedentary subjects. This contrasts with two recent studies of active Y individuals, which found higher oxidative capacity in the MG than in the TA (Forbes et al. 2009b; Gregory et al. 2001), despite the higher percentage of type I fibers in the TA. The authors of both previous studies reasoned that the athletic events the active subjects participated in likely involved frequent concentric contractions of the gastrocnemius (Gottschall and Kram 2003), which are more energetically costly than the largely eccentric contractions of the TA (Ryschon et al. 1997). Regular participation in these activities may be a strong stimulus for increasing oxidative capacity in the MG. By contrast, the largely eccentric action of the TA during these activities may be a less potent stimulus for adaptation, leading to comparatively modest increases in the TA. The discrepancy between these two studies and the current one may also be explained by the fact that the sedentary subjects in the present study may not have had sufficient exposure to these types of activities to produce high oxidative capacities in the MG. Indeed, the similar overall PA in Y and O here suggests that the TA was activated during walking to a similar extent in both age groups, thus maintaining its activation overall.
Methodological considerations
The use of PCr recovery kinetics to investigate mitochondrial function is widespread and based on a concept that, after a brief contraction, PCr recovers along a monoexponential time course due to aerobic processes (Meyer 1988, 1989). Recent studies have demonstrated that, during high intensity contractions such as those used here, nonoxidative processes can contribute to the recovery of PCr in some people (Forbes et al. 2009a; Lanza et al. 2006). However, the fast, nonoxidative component of recovery does not appear to affect the accuracy of the estimate of the recovery kinetics from a monoexponential fit (Forbes et al. 2009a). Furthermore, Forbes et al. (2009a) found no evidence for higher-order recovery kinetics in the human plantar flexors under conditions that are similar to this study (e.g., approximately 40 % PCr depletion, pH >6.8). Therefore, the rate constant from a monoexponential fit are still valid indices of mitochondrial function under these conditions. Additional studies have shown that the recovery of PCr is slowed by acidosis. Although we found age- and sex-related differences in end-exercise pH, pH during recovery stayed above 6.8, suggesting that any effect of pH on the recovery of PCr was small. Finally, a recent comparison between in vivo estimates of oxidative capacity, similar to those used here, and in vitro measures of mitochondrial function demonstrated the validity and robustness of these measures (Lanza et al. 2011).
The in vivo approach used here assesses the integrated function of multiple systems that contribute to muscle oxidative capacity but provides limited information about mechanisms. Furthermore, it cannot assess the relative impacts of other physiological changes that are associated with aging. For example, O muscle can exhibit preferential atrophy of type II fibers (Proctor et al. 1995) and express a greater number of hybrid fiber types (Purves-Smith et al. 2014), which may influence the metabolic profile of the muscle. While this may be considered a limitation, the integrated nature of the approach used here provides information that is highly relevant to physiological functioning and plays an important role in the investigation of aging and disease on muscle energetics. Furthermore, the recent findings of Larsen et al. (2012b) showing higher oxidative capacity in the VL than TA in Y (but not O) adults, indicate that oxidative capacity may be a function more of usage than of fiber-type composition, per se.
We used PA monitors, worn during waking hours, to objectively quantify PA. Based on previous studies (Matthews et al. 2008), we considered a day valid if at least 10 h of data was recorded. However, some activity, including nighttime activity, was not included in our estimates of PA. If systematic differences between the groups in nighttime activity were present, these would not be reflected in our data. However, the magnitudes of these differences, if present, are likely small relative to activity during waking hours, and thus are unlikely to change the interpretation of our results.
It is well accepted that whole-body aerobic capacity declines with age (Proctor and Joyner 1997), even when overall PA level is similar (Kent-Braun and Ng 2000). For this reason, as well as the risks and costs associated with maximal exercise testing, we did not measure maximal aerobic capacity in the current study. This prevents the use of aerobic capacity as a means to compare our sample to those in other studies. However, we chose to characterize physical function with a battery of tests that are commonly used in large-scale studies of O adults (Simonsick et al. 2001) to allow comparison to those studies.
Conclusions
We found no evidence for age-related declines in in vivo oxidative capacity in two distinct locomotory muscles in sedentary, relatively healthy individuals. Our results are consistent with the concept that differences in PA, rather than aging per se, play a substantial role in the oxidative function of muscles in O adults. The extent to which age-related alterations in gait pattern may contribute to this preservation of energetics in the face of declining physical function remains to be determined.
Acknowledgments
We thank Douglas Befroy, DPhil, for his expert technical assistance, John Buonaccorsi, PhD for his statistical advice, the members of the Muscle Physiology Lab for their insightful comments, and the study participants for their cheerful assistance with this project. Funding was provided by the National Institute on Aging (R01 AG21094 and K02 AG023582 to JKB), the New Investigator Fellowship Initiative from the Foundation for Physical Therapy (MAT), and the Keck Foundation.
References
- Benjuya N, Melzer I, Kaplanski J. Aging-induced shifts from a reliance on sensory input to muscle cocontraction during balanced standing. J Gerontol A Biol Sci Med Sci. 2004;59(2):166–171. doi: 10.1093/gerona/59.2.M166. [DOI] [PubMed] [Google Scholar]
- Brierley EJ, Johnson MA, James OF, Turnbull DM. Effects of physical activity and age on mitochondrial function. QJM. 1996;89(4):251–258. doi: 10.1093/qjmed/89.4.251. [DOI] [PubMed] [Google Scholar]
- Brierley EJ, Johnson MA, James OF, Turnbull DM. Mitochondrial involvement in the ageing process. Facts and controversies. Mol Cell Biochem. 1997;174(1–2):325–328. doi: 10.1023/A:1006847319162. [DOI] [PubMed] [Google Scholar]
- Chilibeck PD, Paterson DH, McCreary CR, Marsh GD, Cunningham DA, Thompson RT. The effects of age on kinetics of oxygen uptake and phosphocreatine in humans during exercise. Exp Physiol. 1998;83(1):107–117. doi: 10.1113/expphysiol.1998.sp004087. [DOI] [PubMed] [Google Scholar]
- Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol. 1992;47(3):B71–76. doi: 10.1093/geronj/47.3.B71. [DOI] [PubMed] [Google Scholar]
- Conley KE, Jubrias SA, Esselman PC (2000) Oxidative capacity and ageing in human muscle. J Physiol 526 Pt 1:203–210 [DOI] [PMC free article] [PubMed]
- den Hoed M, Hesselink MK, van Kranenburg GP, Westerterp KR. Habitual physical activity in daily life correlates positively with markers for mitochondrial capacity. J Appl Physiol. 2008;105(2):561–568. doi: 10.1152/japplphysiol.00091.2008. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Smith JL, Simpson DR. Muscle fibre type populations of human leg muscles. Histochem J. 1975;7(3):259–266. doi: 10.1007/BF01003594. [DOI] [PubMed] [Google Scholar]
- Essen–Gustavsson B, Borges O. Histochemical and metabolic characteristics of human skeletal muscle in relation to age. Acta Physiol Scand. 1986;126(1):107–114. doi: 10.1111/j.1748-1716.1986.tb07793.x. [DOI] [PubMed] [Google Scholar]
- Forbes SC, Paganini AT, Slade JM, Towse TF, Meyer RA, Forbes SC, Paganini AT, Slade JM, Towse TF, Meyer RA. Phosphocreatine recovery kinetics following low- and high-intensity exercise in human triceps surae and rat posterior hindlimb muscles. Am J Physiol Regul Integr Comp Physiol. 2009;296(1):R161–170. doi: 10.1152/ajpregu.90704.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes SC, Slade JM, Francis RM, Meyer RA. Comparison of oxidative capacity among leg muscles in humans using gated 31P 2-D chemical shift imaging. NMR Biomed. 2009;22(10):1063–1071. doi: 10.1002/nbm.1413. [DOI] [PubMed] [Google Scholar]
- Forbes SC, Slade JM, Meyer RA. Short-term high-intensity interval training improves phosphocreatine recovery kinetics following moderate-intensity exercise in humans. Appl Physiol Nutr Metab. 2008;33(6):1124–1131. doi: 10.1139/H08-099. [DOI] [PubMed] [Google Scholar]
- Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Armstrong RB, Saltin B, Saubert CW, Sembrowich WL, Shepherd RE. Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol. 1973;34(1):107–111. doi: 10.1152/jappl.1973.34.1.107. [DOI] [PubMed] [Google Scholar]
- Gottschall JS, Kram R. Energy cost and muscular activity required for propulsion during walking. J Appl Physiol. 2003;94(5):1766–1772. doi: 10.1152/japplphysiol.00670.2002. [DOI] [PubMed] [Google Scholar]
- Gregory CM, Vandenborne K, Dudley GA (2001) Metabolic enzymes and phenotypic expression among human locomotor muscles. Muscle Nerve 24 (3):387–393. doi:10.1002/1097%E2%80%934598(200103)24:3%3C387::AID-MUS1010%3E3.0.CO;2-M [pii] [DOI] [PubMed]
- Grimby G, Danneskiold-Samsoe B, Hvid K, Saltin B. Morphology and enzymatic capacity in arm and leg muscles in 78–81 year old men and women. Acta Physiol Scand. 1982;115(1):125–134. doi: 10.1111/j.1748-1716.1982.tb07054.x. [DOI] [PubMed] [Google Scholar]
- Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest. 1974;33(2):109–120. doi: 10.3109/00365517409082477. [DOI] [PubMed] [Google Scholar]
- Hasson CJ, Caldwell GE. Effects of age on mechanical properties of dorsiflexor and plantarflexor muscles. Ann Biomed Eng. 2012;40(5):1088–1101. doi: 10.1007/s10439-011-0481-4. [DOI] [PubMed] [Google Scholar]
- Hasson CJ, Kent-Braun JA, Caldwell GE. Contractile and non-contractile tissue volume and distribution in ankle muscles of young and older adults. J Biomech. 2011;44(12):2299–2306. doi: 10.1016/j.jbiomech.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson CJ, Van Emmerik RE, Caldwell GE. Balance decrements are associated with age-related muscle property changes. J Appl Biomech. 2014 doi: 10.1123/jab.2013-0294. [DOI] [PubMed] [Google Scholar]
- Hikida RS, Gollnick PD, Dudley GA, Convertino VA, Buchanan P. Structural and metabolic characteristics of human skeletal muscle following 30 days of simulated microgravity. Aviat Space Environ Med. 1989;60(7):664–670. [PubMed] [Google Scholar]
- Hortobagyi T, Finch A, Solnik S, Rider P, DeVita P. Association between muscle activation and metabolic cost of walking in young and old adults. J Gerontol A Biol Sci Med Sci. 2011;66(5):541–547. doi: 10.1093/gerona/glr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmard JA, Weidner ML, Gavigan KE, Tyndall GL, Hickey MS, Alshami A. Fiber type and citrate synthase activity in the human gastrocnemius and vastus lateralis with aging. J Appl Physiol. 1998;85(4):1337–1341. doi: 10.1152/jappl.1998.85.4.1337. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Critchlow A, Shin IS, Enoka RM. Men are more fatigable than strength-matched women when performing intermittent submaximal contractions. J Appl Physiol. 2004;96(6):2125–2132. doi: 10.1152/japplphysiol.01342.2003. [DOI] [PubMed] [Google Scholar]
- Hutter E, Skovbro M, Lener B, Prats C, Rabol R, Dela F, Jansen-Durr P. Oxidative stress and mitochondrial impairment can be separated from lipofuscin accumulation in aged human skeletal muscle. Aging Cell. 2007;6(2):245–256. doi: 10.1111/j.1474-9726.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- Jacobs RA, Diaz V, Soldini L, Haider T, Thomassen M, Nordsborg NB, Gassmann M, Lundby C. Fast-twitch glycolytic skeletal muscle is predisposed to age-induced impairments in mitochondrial function. J Gerontol a-Biol. 2013;68(9):1010–1022. doi: 10.1093/gerona/gls335. [DOI] [PubMed] [Google Scholar]
- Jacobs RA, Lundby C. Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. J Appl Physiol (1985) 2013;114(3):344–350. doi: 10.1152/japplphysiol.01081.2012. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Robinson MM, Nair KS. Skeletal muscle aging and the mitochondrion. Trends Endocrin Met. 2013;24(5):247–256. doi: 10.1016/j.tem.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakelides H, Irving BA, Short KR, O’Brien P, Nair KS, Karakelides H, Irving BA, Short KR, O’Brien P, Nair KS. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes. 2010;59(1):89–97. doi: 10.2337/db09-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp GJ, Radda GK. Quantitative interpretation of bioenergetic data from 31P and 1H magnetic resonance spectroscopic studies of skeletal muscle: an analytical review. Magn Reson Q. 1994;10(1):43–63. [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV. Specific strength and voluntary muscle activation in young and elderly women and men. J Appl Physiol. 1999;87(1):22–29. doi: 10.1152/jappl.1999.87.1.22. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV. Skeletal muscle oxidative capacity in young and older women and men. J Appl Physiol. 2000;89(3):1072–1078. doi: 10.1152/jappl.2000.89.3.1072. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV, Doyle JW, Towse TF. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol. 2002;93(5):1813–1823. doi: 10.1152/japplphysiol.00091.2002. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Walker CH, Weiner MW, Miller RG. Functional significance of upper and lower motor neuron impairment in amyotrophic lateral sclerosis. Muscle Nerve. 1998;21(6):762–768. doi: 10.1002/(SICI)1097-4598(199806)21:6<762::AID-MUS8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Konopka AR, Nair KS. Mitochondrial and skeletal muscle health with advancing age. Mol Cell Endocrinol. 2013;379(1–2):19–29. doi: 10.1016/j.mce.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka AR, Suer MK, Wolff CA, Harber MP. Markers of human skeletal muscle mitochondrial biogenesis and quality control: effects of age and aerobic exercise training. J Gerontol A Biol Sci Med Sci. 2014;69(4):371–378. doi: 10.1093/gerona/glt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Befroy DE, Kent-Braun JA. Age-related changes in ATP-producing pathways in human skeletal muscle in vivo. J Appl Physiol. 2005;99(5):1736–1744. doi: 10.1152/japplphysiol.00566.2005. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Bhagra S, Nair KS, Port JD. Measurement of human skeletal muscle oxidative capacity by 31P-MR spectroscopy: a cross-validation with in vitro measurements. J Magn Reson Imaging. 2011;34(5):1143–1150. doi: 10.1002/jmri.22733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Larsen RG, Kent-Braun JA. Effects of old age on human skeletal muscle energetics during fatiguing contractions with and without blood flow. J Physiol. 2007;583(Pt 3):1093–1105. doi: 10.1113/jphysiol.2007.138362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Wigmore DM, Befroy DE, Kent-Braun JA. In vivo ATP production during free-flow and ischaemic muscle contractions in humans. J Physiol. 2006;577(Pt 1):353–367. doi: 10.1113/jphysiol.2006.114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen RG, Befroy DE, Kent-Braun JA. High-intensity interval training increases in vivo oxidative capacity with no effect on Pi→ATP rate in resting human muscle. Am J Physiol Regul Integr Comp Physiol. 2012 doi: 10.1152/ajpregu.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen RG, Callahan DM, Foulis SA, Kent-Braun JA. In vivo oxidative capacity varies with muscle and training status in young adults. J Appl Physiol. 2009;107(3):873–879. doi: 10.1152/japplphysiol.00260.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen RG, Callahan DM, Foulis SA, Kent-Braun JA. Age-related changes in oxidative capacity differ between locomotory muscles and are associated with physical activity behavior. Appl Physiol Nutr Metab. 2012;37(1):88–99. doi: 10.1139/h11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167(7):875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully KK, Fielding RA, Evans WJ, Leigh JS, Jr, Posner JD. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol. 1993;75(2):813–819. doi: 10.1152/jappl.1993.75.2.813. [DOI] [PubMed] [Google Scholar]
- McCully KK, Forciea MA, Hack LM, Donlon E, Wheatley RW, Oatis CA, Goldberg T, Chance B. Muscle metabolism in older subjects using 31P magnetic resonance spectroscopy. Can J Physiol Pharmacol. 1991;69(5):576–580. doi: 10.1139/y91-084. [DOI] [PubMed] [Google Scholar]
- Meyer RA. A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol. 1988;254(4 Pt 1):C548–553. doi: 10.1152/ajpcell.1988.254.4.C548. [DOI] [PubMed] [Google Scholar]
- Meyer RA. Linear dependence of muscle phosphocreatine kinetics on total creatine content. Am J Physiol. 1989;257(6 Pt 1):C1149–1157. doi: 10.1152/ajpcell.1989.257.6.C1149. [DOI] [PubMed] [Google Scholar]
- Pastoris O, Boschi F, Verri M, Baiardi P, Felzani G, Vecchiet J, Dossena M, Catapano M. The effects of aging on enzyme activities and metabolite concentrations in skeletal muscle from sedentary male and female subjects. Exp Gerontol. 2000;35(1):95–104. doi: 10.1016/S0531-5565(99)00077-7. [DOI] [PubMed] [Google Scholar]
- Picard M, Ritchie D, Wright KJ, Romestaing C, Thomas MM, Rowan SL, Taivassalo T, Hepple RT. Mitochondrial functional impairment with aging is exaggerated in isolated mitochondria compared to permeabilized myofibers. Aging Cell. 2010;9(6):1032–1046. doi: 10.1111/j.1474-9726.2010.00628.x. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Joyner MJ. Skeletal muscle mass and the reduction of VO2max in trained older subjects. J Appl Physiol. 1997;82(5):1411–1415. doi: 10.1152/jappl.1997.82.5.1411. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Sinning WE, Walro JM, Sieck GC, Lemon PW. Oxidative capacity of human muscle fiber types: effects of age and training status. J Appl Physiol. 1995;78(6):2033–2038. doi: 10.1152/jappl.1995.78.6.2033. [DOI] [PubMed] [Google Scholar]
- Purves-Smith FM, Sgarioto N, Hepple RT. Fiber typing in aging muscle. Exerc Sport Sci Rev. 2014;42(2):45–52. doi: 10.1249/JES.0000000000000012. [DOI] [PubMed] [Google Scholar]
- Rasmussen UF, Krustrup P, Kjaer M, Rasmussen HN. Human skeletal muscle mitochondrial metabolism in youth and senescence: no signs of functional changes in ATP formation and mitochondrial oxidative capacity. Pflugers Arch. 2003;446(2):270–278. doi: 10.1007/s00424-003-1022-2. [DOI] [PubMed] [Google Scholar]
- Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A. 1996;93(26):15364–15369. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby BC, Robergs RA. Gender differences in substrate utilisation during exercise. Sports Med. 1994;17(6):393–410. doi: 10.2165/00007256-199417060-00005. [DOI] [PubMed] [Google Scholar]
- Russ DW, Kent-Braun JA. Sex differences in human skeletal muscle fatigue are eliminated under ischemic conditions. J Appl Physiol. 2003;94(6):2414–2422. doi: 10.1152/japplphysiol.01145.2002. [DOI] [PubMed] [Google Scholar]
- Russ DW, Kent-Braun JA. Is skeletal muscle oxidative capacity decreased in old age? Sports Med. 2004;34(4):221–229. doi: 10.2165/00007256-200434040-00002. [DOI] [PubMed] [Google Scholar]
- Russ DW, Lanza IR, Rothman D, Kent-Braun JA. Sex differences in glycolysis during brief, intense isometric contractions. Muscle Nerve. 2005;32(5):647–655. doi: 10.1002/mus.20396. [DOI] [PubMed] [Google Scholar]
- Ryschon TW, Fowler MD, Wysong RE, Anthony A, Balaban RS. Efficiency of human skeletal muscle in vivo: comparison of isometric, concentric, and eccentric muscle action. J Appl Physiol. 1997;83(3):867–874. doi: 10.1152/jappl.1997.83.3.867. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Silder A, Heiderscheit B, Mahoney J, Thelen DG. Differences in lower-extremity muscular activation during walking between healthy older and young adults. J Electromyogr Kinesiol. 2009;19(6):1085–1091. doi: 10.1016/j.jelekin.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102(15):5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52(8):1888–1896. doi: 10.2337/diabetes.52.8.1888. [DOI] [PubMed] [Google Scholar]
- Simoneau E, Martin A, Van Hoecke J. Muscular performances at the ankle joint in young and elderly men. J Gerontol A Biol Sci Med Sci. 2005;60(4):439–447. doi: 10.1093/gerona/60.4.439. [DOI] [PubMed] [Google Scholar]
- Simonsick EM, Newman AB, Nevitt MC, Kritchevsky SB, Ferrucci L, Guralnik JM, Harris T, Health ABCSG. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56(10):M644–649. doi: 10.1093/gerona/56.10.M644. [DOI] [PubMed] [Google Scholar]
- Tartaglia MC, Chen JT, Caramanos Z, Taivassalo T, Arnold DL, Argov Z. Muscle phosphorus magnetic resonance spectroscopy oxidative indices correlate with physical activity. Muscle Nerve. 2000;23(2):175–181. doi: 10.1002/(SICI)1097-4598(200002)23:2<175::AID-MUS5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Styles P, Matthews PM, Arnold DA, Gadian DG, Bore P, Radda GK. Energetics of human muscle: exercise-induced ATP depletion. Magn Reson Med. 1986;3(1):44–54. doi: 10.1002/mrm.1910030107. [DOI] [PubMed] [Google Scholar]
- Tevald MA, Foulis SA, Lanza IR, Kent-Braun JA. Lower energy cost of skeletal muscle contractions in older humans. Am J Physiol Regul Integr Comp Physiol. 2010;298(3):R729–739. doi: 10.1152/ajpregu.00713.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- Tucker JM, Welk GJ, Beyler NK. Physical activity in U.S.: adults compliance with the Physical Activity Guidelines for Americans. Am J Prev Med. 2011;40(4):454–461. doi: 10.1016/j.amepre.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Winegard KJ, Hicks AL, Sale DG, Vandervoort AA. A 12-year follow-up study of ankle muscle function in older adults. J Gerontol A Biol Sci Med Sci. 1996;51(3):B202–207. doi: 10.1093/gerona/51A.3.B202. [DOI] [PubMed] [Google Scholar]
- Wolfson L, Judge J, Whipple R, King M (1995) Strength is a major factor in balance, gait, and the occurrence of falls. J Gerontol A Biol Sci Med Sci 50 Spec No:64–67 [DOI] [PubMed]
- Wray DW, Nishiyama SK, Monnet A, Wary C, Duteil SS, Carlier PG, Richardson RS. Antioxidants and aging: NMR-based evidence of improved skeletal muscle perfusion and energetics. Am J Physiol Heart Circ Physiol. 2009;297(5):H1870–1875. doi: 10.1152/ajpheart.00709.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]