Abstract
Although T cells bearing gamma delta T-cell receptors have long been known to be present in the epithelial lining of many organs, their specificity and function remain elusive. In the present study, we examined the intestinal epithelia of T-cell-receptor mutant mice, which were deficient in either gamma delta T cells or alpha beta T cells, and of normal littermates. The absence of gamma delta T cells was associated with a reduction in epithelial cell turnover and a downregulation of the expression of major histocompatibility complex class II molecules. No such effects were observed in alpha beta T-cell-deficient mice. These findings indicate that intraepithelial gamma delta T cells regulate the generation and differentiation of intestinal epithelial cells.
Full text
PDF

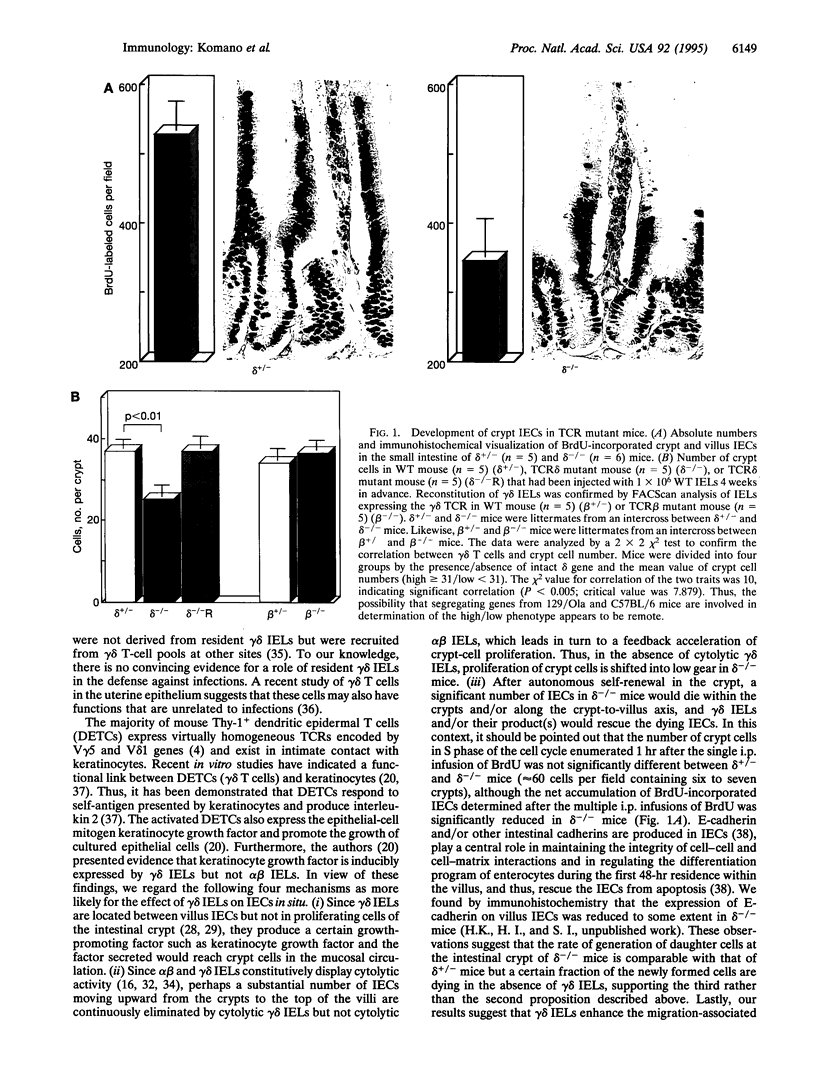
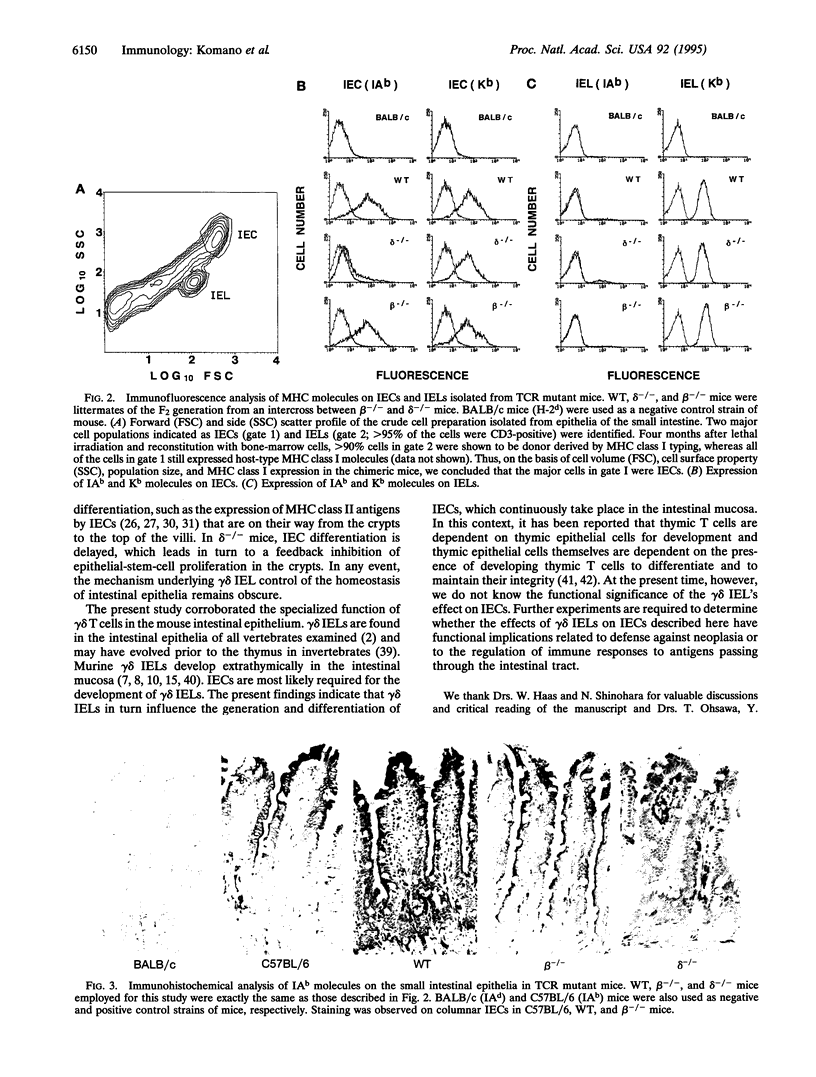
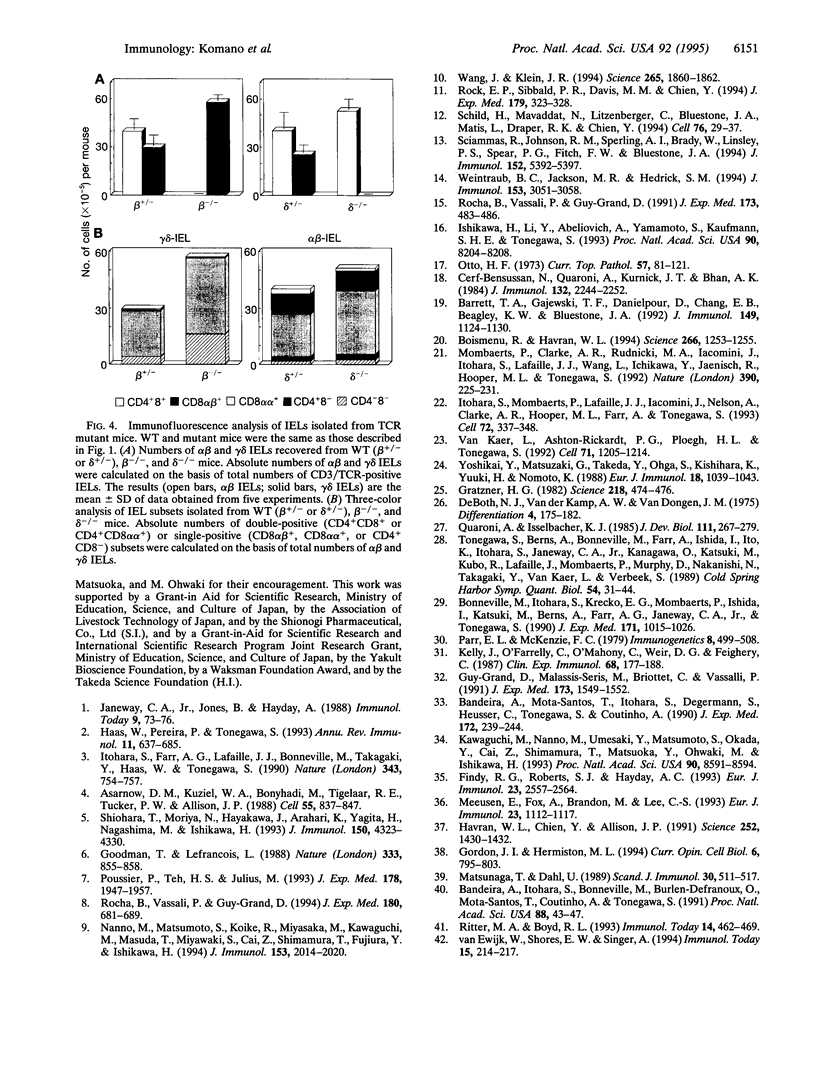
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asarnow D. M., Kuziel W. A., Bonyhadi M., Tigelaar R. E., Tucker P. W., Allison J. P. Limited diversity of gamma delta antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988 Dec 2;55(5):837–847. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- Bandeira A., Itohara S., Bonneville M., Burlen-Defranoux O., Mota-Santos T., Coutinho A., Tonegawa S. Extrathymic origin of intestinal intraepithelial lymphocytes bearing T-cell antigen receptor gamma delta. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):43–47. doi: 10.1073/pnas.88.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira A., Mota-Santos T., Itohara S., Degermann S., Heusser C., Tonegawa S., Coutinho A. Localization of gamma/delta T cells to the intestinal epithelium is independent of normal microbial colonization. J Exp Med. 1990 Jul 1;172(1):239–244. doi: 10.1084/jem.172.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T. A., Gajewski T. F., Danielpour D., Chang E. B., Beagley K. W., Bluestone J. A. Differential function of intestinal intraepithelial lymphocyte subsets. J Immunol. 1992 Aug 15;149(4):1124–1130. [PubMed] [Google Scholar]
- Boismenu R., Havran W. L. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science. 1994 Nov 18;266(5188):1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- Bonneville M., Itohara S., Krecko E. G., Mombaerts P., Ishida I., Katsuki M., Berns A., Farr A. G., Janeway C. A., Jr, Tonegawa S. Transgenic mice demonstrate that epithelial homing of gamma/delta T cells is determined by cell lineages independent of T cell receptor specificity. J Exp Med. 1990 Apr 1;171(4):1015–1026. doi: 10.1084/jem.171.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Quaroni A., Kurnick J. T., Bhan A. K. Intraepithelial lymphocytes modulate Ia expression by intestinal epithelial cells. J Immunol. 1984 May;132(5):2244–2252. [PubMed] [Google Scholar]
- Findly R. C., Roberts S. J., Hayday A. C. Dynamic response of murine gut intraepithelial T cells after infection by the coccidian parasite Eimeria. Eur J Immunol. 1993 Oct;23(10):2557–2564. doi: 10.1002/eji.1830231027. [DOI] [PubMed] [Google Scholar]
- Goodman T., Lefrançois L. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988 Jun 30;333(6176):855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- Gordon J. I., Hermiston M. L. Differentiation and self-renewal in the mouse gastrointestinal epithelium. Curr Opin Cell Biol. 1994 Dec;6(6):795–803. doi: 10.1016/0955-0674(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Gratzner H. G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982 Oct 29;218(4571):474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Malassis-Seris M., Briottet C., Vassalli P. Cytotoxic differentiation of mouse gut thymodependent and independent intraepithelial T lymphocytes is induced locally. Correlation between functional assays, presence of perforin and granzyme transcripts, and cytoplasmic granules. J Exp Med. 1991 Jun 1;173(6):1549–1552. doi: 10.1084/jem.173.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas W., Pereira P., Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- Havran W. L., Chien Y. H., Allison J. P. Recognition of self antigens by skin-derived T cells with invariant gamma delta antigen receptors. Science. 1991 Jun 7;252(5011):1430–1432. doi: 10.1126/science.1828619. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Li Y., Abeliovich A., Yamamoto S., Kaufmann S. H., Tonegawa S. Cytotoxic and interferon gamma-producing activities of gamma delta T cells in the mouse intestinal epithelium are strain dependent. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8204–8208. doi: 10.1073/pnas.90.17.8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itohara S., Farr A. G., Lafaille J. J., Bonneville M., Takagaki Y., Haas W., Tonegawa S. Homing of a gamma delta thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature. 1990 Feb 22;343(6260):754–757. doi: 10.1038/343754a0. [DOI] [PubMed] [Google Scholar]
- Itohara S., Mombaerts P., Lafaille J., Iacomini J., Nelson A., Clarke A. R., Hooper M. L., Farr A., Tonegawa S. T cell receptor delta gene mutant mice: independent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell. 1993 Feb 12;72(3):337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Jones B., Hayday A. Specificity and function of T cells bearing gamma delta receptors. Immunol Today. 1988 Mar;9(3):73–76. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- Kawaguchi M., Nanno M., Umesaki Y., Matsumoto S., Okada Y., Cai Z., Shimamura T., Matsuoka Y., Ohwaki M., Ishikawa H. Cytolytic activity of intestinal intraepithelial lymphocytes in germ-free mice is strain dependent and determined by T cells expressing gamma delta T-cell antigen receptors. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8591–8594. doi: 10.1073/pnas.90.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J., O'Farrelly C., O'Mahony C., Weir D. G., Feighery C. Immunoperoxidase demonstration of the cellular composition of the normal and coeliac small bowel. Clin Exp Immunol. 1987 Apr;68(1):177–188. [PMC free article] [PubMed] [Google Scholar]
- Matsunaga T., Dahl U. What was wrong with the T-cell receptor gamma/delta heterodimer? Divergence of the T-cell receptor alpha/beta and gamma/delta heterodimers. Scand J Immunol. 1989 Nov;30(5):511–517. doi: 10.1111/j.1365-3083.1989.tb02458.x. [DOI] [PubMed] [Google Scholar]
- Meeusen E., Fox A., Brandon M., Lee C. S. Activation of uterine intraepithelial gamma delta T cell receptor-positive lymphocytes during pregnancy. Eur J Immunol. 1993 May;23(5):1112–1117. doi: 10.1002/eji.1830230520. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Clarke A. R., Rudnicki M. A., Iacomini J., Itohara S., Lafaille J. J., Wang L., Ichikawa Y., Jaenisch R., Hooper M. L. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992 Nov 19;360(6401):225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- Nanno M., Matsumoto S., Koike R., Miyasaka M., Kawaguchi M., Masuda T., Miyawaki S., Cai Z., Shimamura T., Fujiura Y. Development of intestinal intraepithelial T lymphocytes is independent of Peyer's patches and lymph nodes in aly mutant mice. J Immunol. 1994 Sep 1;153(5):2014–2020. [PubMed] [Google Scholar]
- Otto H. F. The interepithelial lymphocytes of the intestinum. Morphological observations and immunologic aspects of intestinal enteropathy. Curr Top Pathol. 1973;57:81–121. doi: 10.1007/978-3-642-65465-7_3. [DOI] [PubMed] [Google Scholar]
- Poussier P., Teh H. S., Julius M. Thymus-independent positive and negative selection of T cells expressing a major histocompatibility complex class I restricted transgenic T cell receptor alpha/beta in the intestinal epithelium. J Exp Med. 1993 Dec 1;178(6):1947–1957. doi: 10.1084/jem.178.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A., Isselbacher K. J. Study of intestinal cell differentiation with monoclonal antibodies to intestinal cell surface components. Dev Biol. 1985 Oct;111(2):267–279. doi: 10.1016/0012-1606(85)90482-8. [DOI] [PubMed] [Google Scholar]
- Ritter M. A., Boyd R. L. Development in the thymus: it takes two to tango. Immunol Today. 1993 Sep;14(9):462–469. doi: 10.1016/0167-5699(93)90250-O. [DOI] [PubMed] [Google Scholar]
- Rocha B., Vassalli P., Guy-Grand D. The V beta repertoire of mouse gut homodimeric alpha CD8+ intraepithelial T cell receptor alpha/beta + lymphocytes reveals a major extrathymic pathway of T cell differentiation. J Exp Med. 1991 Feb 1;173(2):483–486. doi: 10.1084/jem.173.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha B., Vassalli P., Guy-Grand D. Thymic and extrathymic origins of gut intraepithelial lymphocyte populations in mice. J Exp Med. 1994 Aug 1;180(2):681–686. doi: 10.1084/jem.180.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock E. P., Sibbald P. R., Davis M. M., Chien Y. H. CDR3 length in antigen-specific immune receptors. J Exp Med. 1994 Jan 1;179(1):323–328. doi: 10.1084/jem.179.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild H., Mavaddat N., Litzenberger C., Ehrich E. W., Davis M. M., Bluestone J. A., Matis L., Draper R. K., Chien Y. H. The nature of major histocompatibility complex recognition by gamma delta T cells. Cell. 1994 Jan 14;76(1):29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Sciammas R., Johnson R. M., Sperling A. I., Brady W., Linsley P. S., Spear P. G., Fitch F. W., Bluestone J. A. Unique antigen recognition by a herpesvirus-specific TCR-gamma delta cell. J Immunol. 1994 Jun 1;152(11):5392–5397. [PubMed] [Google Scholar]
- Shiohara T., Moriya N., Hayakawa J., Arahari K., Yagita H., Nagashima M., Ishikawa H. Bone marrow-derived dendritic epidermal T cells express T cell receptor-alpha beta/CD3 and CD8. Evidence for their extrathymic maturation. J Immunol. 1993 May 15;150(10):4323–4330. [PubMed] [Google Scholar]
- Tonegawa S., Berns A., Bonneville M., Farr A., Ishida I., Ito K., Itohara S., Janeway C. A., Jr, Kanagawa O., Katsuki M. Diversity, development, ligands, and probable functions of gamma delta T cells. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):31–44. doi: 10.1101/sqb.1989.054.01.005. [DOI] [PubMed] [Google Scholar]
- Van Kaer L., Ashton-Rickardt P. G., Ploegh H. L., Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4-8+ T cells. Cell. 1992 Dec 24;71(7):1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- Wang J., Klein J. R. Thymus-neuroendocrine interactions in extrathymic T cell development. Science. 1994 Sep 23;265(5180):1860–1862. doi: 10.1126/science.8091211. [DOI] [PubMed] [Google Scholar]
- Weintraub B. C., Jackson M. R., Hedrick S. M. Gamma delta T cells can recognize nonclassical MHC in the absence of conventional antigenic peptides. J Immunol. 1994 Oct 1;153(7):3051–3058. [PubMed] [Google Scholar]
- Yoshikai Y., Matsuzaki G., Takeda Y., Ohga S., Kishihara K., Yuuki H., Nomoto K. Functional T cell receptor delta chain gene messages in athymic nude mice. Eur J Immunol. 1988 Jul;18(7):1039–1043. doi: 10.1002/eji.1830180711. [DOI] [PubMed] [Google Scholar]
- van Ewijk W., Shores E. W., Singer A. Crosstalk in the mouse thymus. Immunol Today. 1994 May;15(5):214–217. doi: 10.1016/0167-5699(94)90246-1. [DOI] [PubMed] [Google Scholar]