Abstract
Purpose
To explore the efficacy and define mechanisms of action of co-administration of the PI3K/mTOR inhibitor BEZ235 and pan-HDAC inhibitor panobinostat in DLBCL cells.
Experimental Design
Various DLBCL cells were exposed to panobinostat and BEZ235 alone or together after which apoptosis and signaling/survival pathway perturbations were monitored by flow cytometry and Western blot analysis. Genetic strategies defined the functional significance of such changes, and xenograft mouse models were used to assess tumor growth and animal survival.
Results
Panobinostat and BEZ235 interacted synergistically in ABC-, GC-, and double-hit DLBCL cells, and MCL cells, but not normal CD34+ cells. Synergism was associated with pronounced AKT dephosphorylation, GSK3 dephosphorylation/activation, Mcl-1 downregulation, Bim up-regulation and increased Bcl-2/Bcl-xL binding, diminished Bax/Bak binding to Bcl-2/Bcl-xL/Mcl-1, increased γH2A.X phosphorylation and histone H3/H4 acetylation, and abrogation of p21CIP1 induction. BEZ235/panobinostat lethality was not susceptible to stromal/microenvironmental forms of resistance. Genetic strategies confirmed significant functional roles for AKT inactivation, Mcl-1 down-regulation, Bim up-regulation, and Bax/Bak in synergism. Finally, co-administration of BEZ235 with panobinostat in immunocompromised mice bearing SU-DHL4-derived tumors significantly reduced tumor growth in association with similar signaling changes observed in vitro, and increased animal survival compared to single agents.
Conclusions
BEZ235/panobinostat exhibits potent anti-DLBCL activity, including in poor-prognosis ABC- and double-hit sub-types, but not in normal CD34+ cells. Synergism is most likely multi-factorial, involving AKT inactivation/GSK3 activation, Bim up-regulation, Mcl-1 down-regulation, enhanced DNA damage, and is operative in vivo. Combined PI3K/mTOR and HDAC inhibition warrants further attention in DLBCL.
Keywords: PI3K/AKT/mTOR, HDACIs, GSK3, Bim, Mcl-1
INTRODUCTION
Recent evidence indicates that epigenetic changes affecting chromatin remodeling and gene expression e.g., histone acetylation or methylation, and DNA methylation play critical roles in tumorigenesis [1]. Genes implicated in these processes are frequently mutated in various cancers, including non-Hodgkin lymphoma (NHL) [2, 3], providing a rationale for targeting epigenetic aberrations in cancer therapy. In this context, histone deacetylase inhibitors (HDACIs) have been used to reverse aberrant epigenetic changes and to restore normal gene expression programs, culminating in the approval of the HDACIs vorinostat and romidepsin for cutaneous T-cell lymphoma and peripheral T-cell lymphoma respectively [4, 5]. Moreover, a number of other novel HDACIs, particularly panobinostat (LBH-589), are currently under evaluation in NHL with promising preliminary results [6, 7] HDACIs exert anti-tumor activity through multiple mechanisms. In addition to their histone hyper-acetylation effects, they also modulate activity of various non-histone proteins (e.g., p53, STAT, Bcl-6, and Hsp90) [8-10], induce reactive oxygen species and ceramide [11], death receptors [12], and modulate expression of Bcl-2 family members e.g., up-regulation of the pro-apoptotic Bim through a mechanism involving E2F1 [13].
PI3K/AKT/mTOR is one of the most frequently dysregulated survival signaling pathways in cancer [14]. In NHL, aberrant activation of this pathway involves diverse mechanisms including, pTEN loss, decreased expression, or mutation, PI3Kα mutations, PI3Kδ overexpression/activation, and BCR receptor activation [15-17]. PI3K activation leads to activation of multiple downstream effectors, among which AKT/mTOR axis plays a critical role in diverse cell processes, including growth, survival, metabolism, and autophagy [18]. Other important PI3K downstream signaling pathways involve PDK1, GSK3, Mcl-1, Bim, Bad, and p53, among others [18]. In this regard, we have recently shown in a leukemia model that PI3K/AKT inhibition leads to Mcl-1 down-regulation which, in conjunction with Bim, plays critical roles in cell death mediated by regimen incorporating BH3-mimetics [19, 20]. Recently, multiple inhibitors of PI3K/AKT/mTOR pathway have been developed [21], of which several (e.g., CAL-101, BEZ235, SF1126) are currently undergoing clinical evaluation in diverse tumor types including NHL [22, 23].
We have previously reported that combined treatment with PI3K/AKT and HDAC inhibitors exhibits potent anti-leukemic activity [11, 24]. Similar findings were subsequently described in diverse solid tumors [25, 26]. However, little is known about whether this approach could be effective in NHL, particularly in diffuse large B-cell lymphoma (DLBCL), including the poor prognosis ABC and MYC/Bcl-2 double-hit sub-types, or mantle cell lymphoma. These considerations, in conjunction with recent evidence indicating frequent mutations in histone modifying proteins [2, 3], and dysregulation of the PI3K pathway [15-17] in DLBCL prompted us to investigate whether this strategy would be effective in these diseases and to elucidate mechanism of anti-tumor actions. Notably, co-administration of clinically achievable concentrations of the HDACIs panobinostat and the dual PI3K/mTOR inhibitor BEZ235 [6, 22], interacted synergistically to induce apoptosis, reduce growth and viability, and circumvent resistance mediated by stromal cells in various NHL cell lines, including the poor-prognosis ABC and MYC/Bcl-2 double-hit sub-types, while exhibiting little toxicity toward normal CD34+ cells. Furthermore, in a subcutaneous xenograft mouse model, combined treatment was well tolerated, and effectively reduced tumor growth and enhanced animal survival.
METHODS
Cells
Human non-Hodgkin lymphoma SU-DHL4 and SU-DHL16 (DLBCL GC subtype), HBL-1 and TMD8 (DLBCL ABC subtype), OCI-LY18 and CARNAVAL (DLBCL MYC/Bcl-2 double-hit), Jeko-1 (Mantle cells lymphoma) cell lines and genetically modified lines are described in details in Supplementary Methods. SU-DHL4, SU-DHL16, OCI-LY18, CARNAVAL, and Jeko-1 cells were authenticated by ATCC (Basic STR Profiling).
Stromal cells
Human bone marrow stromal HS-5 cells were purchased from American Type Culture Collection (ATCC) and cultured as above. HS-5 conditioned media was prepared by culturing HS-5 cells to 70% confluence, after which media was removed and replaced with fresh media. After 24 hr of incubation HS-5-conditionned media was collected and debris removed by centrifugation. Lymphoma cells were incubated in HS-5-conditioned media for 24 hr before treatment. For co-culture studies, lymphoma cells were incubated with HS-5 cells for 24 hours, then treated for 24 hr, after which non-adherent cells were collected and subjected to Annexin V/PI assay.
Normal CD34+ cells
Normal bone marrow CD34+ cells were obtained with informed consent from patients undergoing routine diagnostic procedures for non-myeloid hematopoietic disorders as before [20]. These studies have been sanctioned by Virginia Commonwealth University Investigational Review Board.
Reagents
The dual PI3K/mTOR inhibitor BEZ235 and the HDACI panobinostat were provided by Novartis. Idelalisib (CAL-101) and IPI-145 were purchased from ChemieTeck. The GSK3 inhibitor IX (2′Z,3′E)-6-bromoindirubin-3′-oxime (BIO), its inactive analogue MeBIO, and SBHA were purchased from Calbiochem. CHIR-98014 was purchased from Sellek chemicals.
Assessment of apoptosis
Apoptosis was routinely assessed by Annexin V/PI analysis as previously described [20].
Cell growth and viability
Cell growth and viability were assessed by CellTiter-Glo Luminescent Assay (Promega) [20].
Clonogenicity
Colony-formation assays were performed in methylcellulose as previously described [27].
Immunoprecipitation and immunoblotting
Immunoprecipitation and immunoblotting were performed as previously described [27]. Primary antibodies used in these studies are described in Supplementary Methods.
Subcellular fractionation
Cytosolic and membrane fractions were separated as previously described [11].
Bax and Bak conformational change
Bax and Bak conformational change was assessed as previously described [27].
In vivo studies
Animal studies were conducted under an approved protocol by the Virginia Commonwealth University Institutional Animal Care and Use Committee. Female beige nude mice (Charles River laboratories) were inoculated subcutaneously in the flank with 10 × 106 luciferase-expressing SU-DHL4 cells. Once tumors became apparent, mice were randomly separated into 4 groups and treated with 50 mg/kg BEZ235 (intraperitoneally), and 15 mg/kg panobinostat (by oral gavage) alone or in combination, or vehicle (controls) once daily 5 days per week. Panobinostat was dissolved in D5W at a concentration of 2 mg/mL; BEZ235 was dissolved in NMP 10% (1-methyl-2-pyrrolidone)/PEG300 90%. Tumor volumes were calculated using the formula (length × width2)/2, and when tumor length reached 1.7 cm, mice were euthanized. In some cases, mice were monitored for tumor growth using the IVIS 200 imaging system (Xenogen Corporation, Alameda, CA) as previously described [20]. For tumor analysis, mice were treated twice over a 24-hr interval (at 0 hr and at 18 hr), after which tumors were excised, lysed, and subjected to Western blot analysis.
Statistical analysis
The significance of differences between experimental conditions was determined using the Student’s t test for unpaired observations. Survival rates were analyzed by Kaplan–Meyer and comparisons of survival curves and median survival were analyzed by logrank test.
RESULTS
AKT activation opposes panobinostat lethality
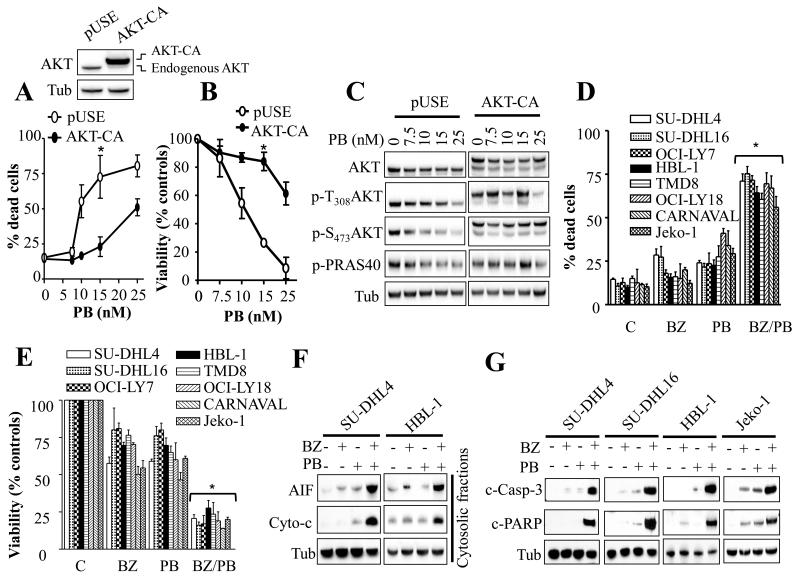
To determine whether AKT activation status had an impact on the activity of the clinically relevant HDAC inhibitor panobinostat in DLBCL, stable ectopic expression of constitutively active AKT (AKT-CA) was performed in SU-DHL16 cell line. Dose response studies revealed that AKT-CA-expressing cells exhibited significant resistance to panobinostat-mediated cell death compared to empty vector cells (Fig. 1A). These cells were also less sensitive to panobinostat-mediated growth inhibition and viability reduction (Fig. 1B). Similar results were observed in SU-DHL4 cells (Supplementary Fig. 1). Panobinostat induced dose-dependent dephosphorylation of AKT at both residues threonine 308 and serine 473 in parental cells, in association with a clear dephosphorylation of the AKT substrate PRAS40 (Fig. 1C). Notably these effects were attenuated by ectopic expression of AKT-CA. These findings indicate that PI3K/AKT activation status represents an important factor determining panobinostat activity in DLBCL and raise the possibility that PI3K/AKT pathway inhibition might potentiate panobinostat activity in NHL cells.
Fig. 1. Disruption of PI3K/AKT/mTOR pathway markedly potentiates panobinostat lethality in various NH lymphoma cell lines.
(A-C) SU-DHL16 cells ectopically expressing a constitutively active AKT construct (A, inset) were exposed to increasing panobinostat concentrations for 24 hr, after which the extent of cell death was assessed using Annexin V/PI staining assay (A) and cell growth and viability were evaluated using a CellTiter-Glo Luminescent assay (B). Alternatively, Western blot analysis was performed following 4 hr treatment (C). (D-E) Cells were exposed to BEZ235 (25 nM for SU-DHL16 and 200 nM for the remaining cell lines) and panobinostat (7.5 nM for SU-DHL16; 10 nM for HBL-1, TMD8, OCI-LY18, CARNAVAL; and Jeko-1; and 15 nM for SU-DHL4, and OCI-LY7) alone or together for 24 hr for all cell lines with the exception of CARNAVAL cells (48 hr), after which cell death or cell growth and viability were assessed as in (A-B). (F-G) Western blot analysis on cytosolic fractions (F) or whole cell lysates (G) in various NHL cell lines following 24 hr treatment with BEZ235 and panobinostat as in D. For A, B, D, E, error bars = S.D for 3 independent experiments; * P < 0.02. C-PARP = cleaved PARP.
Co-administration of panobinostat and BEZ235 markedly inhibits cell growth and viability and induces apoptosis in NHL cells
Effects of combined treatment with panobinostat and the dual PI3K/mTOR inhibitor BEZ235 were examined in diverse DLBCL subtypes including GC (SU-DHL4, SU-DHL16, and OCI-LY7) and ABC (HBL-1 and TMD8), MYC/Bcl-2 double-hit (OCI-LY18 and CARNAVAL) as well as MCL (Jeko-1) cell lines. Notably, combined treatment with very low, clinically relevant concentrations [6, 22] of panobinostat (7.5-15 nM) and BEZ235 (25-200 nM) resulted in a marked induction of cell death (Fig. 1D) in association with a sharp decline in cell growth and viability (Fig. 1E) in each cell line tested. In contrast agents administered individually had only minimal effects. Co-administration of the histone deacetylase inhibitor SBHA and the PI3Kδ inhibitor CAL-101 or the PI3Kδ/γ inhibitor IPI-145 also led to enhanced lethality in multiple DLBCL lines, although effects were somewhat less pronounced than those observed with BEZ235/Panobinostat (Supplementary Figure 2A). Significantly, median dose effect analysis performed in several cell lines including SU-DHL4, SU-DHL16, HBL-1, OCI-LY18, and Jeko-1 demonstrated highly synergistic interactions between BEZ235 and panobinostat (Supplementary Fig.2B-F). Sub-cellular localization analysis in SU-DHL4 and HBL-1 cells revealed a pronounced release of cytochrome c and AIF into the cytosol following combined, but not individual, treatment (Fig. 1F). These effects were associated with pronounced increases in caspase-3 and PARP cleavage in SU-DHL4, SU-DHL16, Jeko-1, and HBL-1 cells (Fig. 1G). Similar results were obtained in OCI-LY18 cells (data not shown). In sharp contrast, combined treatment with BEZ235 and panobinostat only minimally induced apoptosis in or reduced the colony-forming capacity of normal CD34+ progenitor cells (Figs 2A and 2B respectively).
Fig. 2. Treatment with BEZ235/panobinostat is not toxic to normal human CD34+ cells, and is associated with a marked increase in histone H3 and H4 acetylation, induction of DNA damage, and abrogation of p21CIP1 induction.
Annexin V/PI analysis at 24 hr (A), or colony formation assay (B) on normal human CD34+ cells. C) Western blot analysis following 4-8 hr cell exposure to BEZ235 ± panobinostat.
Together, these findings indicate that co-administration of low concentrations of the HDACI panobinostat and the dual PI3K/mTOR inhibitor BEZ235 sharply induces cell death in various NHL cells, including ABC-, GC-, and double-hit DLBCL and MCL cells, but not in normal CD34+ progenitor cells, raising the possibility of therapeutic selectivity.
Treatment with BEZ235/panobinostat is associated with a profound increase in histone H3 and H4 acetylation, marked DNA damage induction, and abrogation of p21CIP1 upregulation in DLBCL cells
As a primary action of panobinostat, like other HDACIs, is to increase acetylation of histones and other proteins, we sought to determine whether this effect might be potentiated by PI3K inhibition. As shown in Figure 2C and Supplementary Fig. 3, combined treatment with panobinostat and BEZ235 resulted in a pronounced increase in acetylation of histones H3 and H4 in SU-DHL4, SU-DHL16, HBL-1, OCI-LY18, as well as OCI-LY7 cells. Similarly, a marked induction of γH2A.X phosphorylation, an indicator of DNA double-strand breaks [28], was observed with combined treatment (Fig. 2C and Supplementary Fig. 3). Notably, agents administered individually had only minor effects. In studies involving human leukemia cells [24], PI3K/AKT inhibition abrogated HDACI-mediated p21CIP1/WAF1 up-regulation, which we and others have shown to promote cell death [24, 29, 30]. Interestingly, BEZ235 completely abrogated panobinostat-induced p21CIP1 up-regulation in various lymphoma cell lines, including SU-DHL4, SU-DHL16, HBL-1, and OCI-LY18 (Fig. 2C and Supplementary Fig. 3). These findings were associated with a pronounced de-phosphorylation of AKT by BEZ235, particularly when combined with panobinostat, whereas AKT protein levels were largely unaffected. Of note, panobinostat also induced a modest but discernible reduction in AKT phosphorylation without affecting AKT protein levels. Together, these findings indicate that combined HDAC and PI3K/mTOR inhibition triggers enhanced histone acetylation, DNA damage, and abrogation of p21CIP1 induction in diverse DLBCL cells.
BEZ235/panobinostat lethality involves Mcl-1 down-regulation
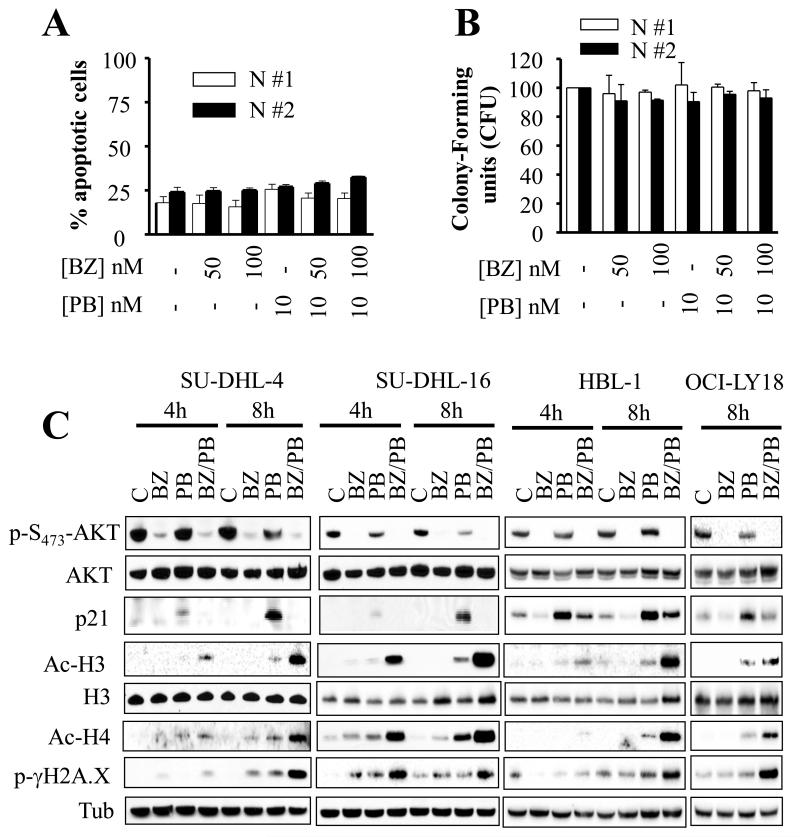
Consistent with previous reports by our and other groups involving other tumor types [20, 27, 31], BEZ235 significantly reduced Mcl-1 protein levels in SU-DHL4, HBL-1, and OCI-LY18, effects that persisted following combined treatment (Fig. 3A). Identical results were obtained in SU-DHL16 cells (Supplementary Fig. 4A). Combined treatment also induced modest but discernible increases in Bim protein levels. In contrast, no major changes in expression of other anti-apoptotic family members (e.g., Bcl-2 or Bcl-xL) or the pro-apoptotic proteins Bax or Bak, were observed (Fig. 3A). However, robust changes in Bax and Bak conformation were observed with combined treatment, whereas individual agents had minimal effects (Fig. 3B). To test whether Mcl-1 down-regulation plays a functional role in BEZ235/panobinostat-mediated lethality, studies employing siRNA against Mcl-1 were performed. Down-regulation of Mcl-1 with siRNA significantly increased sensitivity to panobinostat in SU-DHL4 and in SU-DHL16 (Fig 3C) arguing that Mcl-1 down-regulation plays a functional role in BEZ235/panobinostat-mediated cell death.
Fig. 3. BEZ235/panobinostat lethality involves Mcl-1 down-regulation, and perturbations in Bim, Bax, and Bak.
(A) Western blot analysis on cells treated for 8 hr with BEZ235 (200 nM) ± panobinostat (10 nM for HBL-1 and OCI-LY18; 15 nM for SU-DHL4). (B) Bax and Bak conformational changes in SU-DHL4 cells following 16 hr treatment as in A. C) cell growth and viability in SU-DHL4 and SU-DHL16 transiently transfected with siRNA against Mcl-1 (si-Mcl-1), or non-silencing siRNA (si-NS), and exposed to panobinostat for 16 hr. Error Bars: S.D of 3 independent experiments; * P < 0.05. D) SU-DHL4 and SU-DHL16 cells in which Bim was knocked down using lentiviral particles carrying shRNA constructs against Bim or non-silencing shRNA (sh-NS) were exposed to BEZ235 (200 nM for SUDHL4 and 25 nM for SU-DHL16) ± panobinostat (15 nM for SU-DHL4 and 7.5 nM for SU-DHL16) for 24 hr after which the extent of cell death was determined using Annexin V/PI assay. Error Bars: S.D of 3 separate experiments; * P < 0.01 for SU-DHL16 and P < 0.02 for SU-DHL4. E) Annexin V/PI assay in SU-DHL4 cells stably transfected with shRNA constructs against Bax (shBax), Bak (shBak), or non-silencing shRNA (sh-NS) following 24 hr treatment with 200 nM BEZ235 ± 15 nM panobinostat. Inset: Western blot on non-treated cells. Each sample was run twice. Error Bars: S.D of 3 separate experiments; * P < 0.05.
Bim, Bax, and Bak play important functional roles in BEZ235/panobinostat-mediated anti-DLBCL activity
To determine whether Bim plays a functional role in BEZ235/panobinostat-mediated lethality, transduction of SU-DHL4 and SU-DHL16 with lentiviruses carrying a shRNA construct against Bim was performed. As shown in Supplementary Figures 4B-C, dose-response studies revealed that Bim knockdown rendered cells significantly more resistant to either BEZ235 or panobinostat. Notably, Bim knockdown also rendered cells significantly less susceptible to the lethal action of BEZ235/panobinostat co-administration (Fig. 3D and Supplementary Fig. 4D). These effects were associated with pronounced diminution in caspase-3 and PARP cleavage (Supplementary Fig. 4E). Parallel studies involving immunoprecipitation analysis revealed that exposure to BEZ235 alone, or in combination with panobinostat, markedly increased Bim binding to Bcl-2 and Bcl-xL, an effect that correlates with significant reductions in Bak binding to all major anti-apoptotic bcl-2 members (e.g., Mcl-1, Bcl-2, and Bcl-xL) as well as a decrease in Bax binding to Mcl-1 and Bcl-2 (Supplementary Figure 5). Of note, reductions in Bak or Bax binding to Mcl-1/Bcl-2/Bcl-xL were also observed with panobinostat or BEZ235 alone.
To examine whether Bax and Bak play a functional role in BEZ235/panobinostat anti-DLBCL activity, stable knockdown experiments using shRNA against Bax or Bak were conducted. Dose-response studies revealed that knockdown of Bax or Bak rendered cells significantly more resistant to either BEZ235 or panobinostat (Supplementary Figures 6A-D). In addition, these cells were significantly less susceptible to BEZ235/panobinostat lethality compared to the control cells (Fig. 3E). Together, these findings demonstrate that co-administration of BEZ235 and panobinostat leads to increased binding of Bim to Bcl-2 and Bcl-xL, accompanied by release of Bak and Bax from the major neutralizing molecules Bcl-2, Bcl-xL. They also indicate that perturbations in Mcl-1, Bim, Bax and Bak play functional roles in BEZ235/panobinostat anti-DLBCL activity.
AKT and GSK3 contribute functionally to the anti-DLBCL activity of the BEZ235/panobinostat regimen
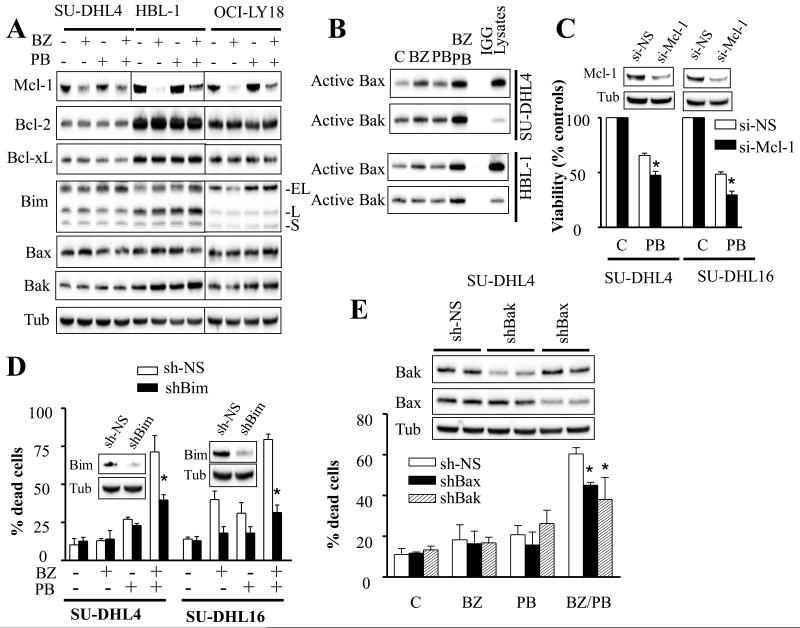
To assess the role of AKT inactivation in BEZ235/panobinostat lethality, AKT-CA-expressing SU-DHL16 cells were employed. Notably, AKT-CA-expressing cells displayed significant resistance to BEZ235/panobinostat lethality, reflected by decreased caspase-3 and PARP cleavage (Fig. 4A), diminished Annexin V/PI positivity (Fig. 4B), and marked reduction in the decline in cell growth and viability (Fig. 4C). Significantly, ectopic expression of AKT-CA also markedly decreased γH2A.X phosphorylation mediated by BEZ235/panobinostat (Fig. 4A), suggesting that AKT inactivation is required for BEZ235/panobinostat-induced DNA damage.
Fig. 4. Ectopic expression of constitutively active AKT or inhibition of GSK3 diminishes BEZ235/panobinostat lethality.
A) Western blot on constitutively active Myc-tagged AKT-expressing SU-DHL16 cells following 24 hr exposure to 25 nM BEZ235 ± 7.5 nM panobinostat. Alternatively, the extent of cell death (B) or cell growth and viability (C) were assessed at 24 hr as in Fig. 1A-B. Error Bars: S.D of 3 independent experiments; * P < 0.02. D) SU-DHL4, SU-DHL16, HBL-1, and OCI-LY18 cells were treated with BEZ235 (200 nM for SU-DHL4, HBL-1, and OCI-LY18; 25 nM for SU-DHL16) ± panobinostat (7.5 nM for SU-DHL16; 10 nM for HBL-1, and OCI-LY18; 15 nM for SU-DHL4) for 8 hr, then cells were lysed and Western blot performed using the indicated antibodies. E) SU-DHL4 and HBL-1 cells were exposed to combined treatment with BEZ235 and panobinostat as in (D) in the presence or the absence of BIO, MeBIO, or CHIR-98014 (2 μM each). Twenty-four hr after treatment, cell growth and viability were assessed using CellTiter-Glo Luminescent assay. * = significantly greater than values obtained in the absence of BIO or CHIR-98014 (P < 0.05).
In view of the important role that GSK3 plays in AKT signaling, additional studies were conducted to determine whether this kinase plays a role in cell death mediated by co-exposure to BEZ235 and panobinostat. Western blot analysis revealed that exposure to BEZ235, alone as in combination, led to diminished GSK3 phosphorylation at inhibitory serine 9/21 sites in multiple cell lines including SU-DHL4, SU-DHL16, HBL-1, and OCI-LY18 (Fig. 4D). This was accompanied by a significant decline in β-catenin protein levels, a well-established GSK3 substrate, indicating GSK3 activation. In fact, phosphorylation of β-Catenin by GSK3 leads to its ubiquitination and proteasomal degradation. Of note panobinostat alone also triggered a decrease in GSK3 phosphorylation in SU-DHL4 and SU-DHL16, but not in HBL-1 or OCI-LY18 cells. Notably, c-Myc, another GSK3 target which we and others have shown to be down-regulated by BEZ235 or panobinostat [19, 32], was also markedly down-regulated by these agents alone as well as together (Fig. 4D). Additional studies revealed that pretreatment with the GSK3α/β inhibitors BIO or CHIR-98014 diminished the inhibitory effects of BEZ235/panobinostat on cell growth and viability (Fig. 4E). In contrast, the Bio inactive analogue MeBIO had no effect on BEZ235/panobinostat activities. These findings argue that GSK3 activation contributes functionally to the anti-DLBCL activity of the BEZ235/panobinostat regimen.
BEZ235/panobinostat circumvents environmental forms of resistance mediated by stromal cells
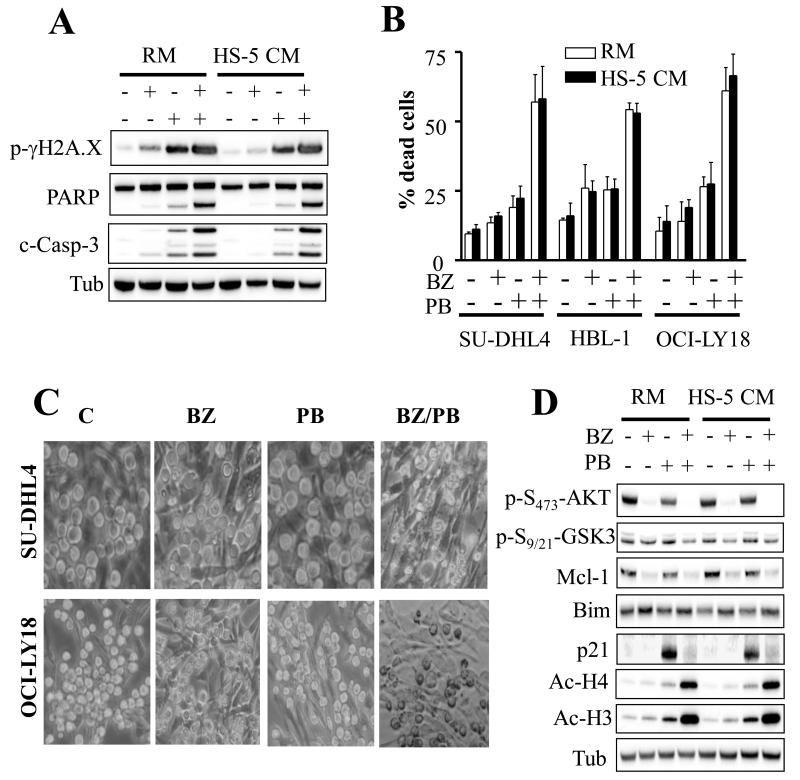
Stromal cells have been increasingly associated with lymphomagenesis and resistance to chemotherapy [33-35]. To test whether stromal cells confer resistance to BEZ235/panobinostat in SU-DHL4, HBL-1, or OCI-LY18, studies were performed in the presence of human bone marrow stromal cell HS-5-conditioned media. Notably, the BEZ235/panobinostat regimen was equally effective in inducing caspase-3 and PARP cleavage, increasing γ-H2A.X phosphorylation (Fig. 5A), promoting cell death (Fig. 5B) and reducing growth and viability (Supplementary Fig. 7A) in HS-5-conditioned media compared to regular media. In contrast, cell susceptibility to doxorubicin was significantly attenuated in the presence of HS-5-conditioned media (Supplementary Fig. 7B). Co-culture with HS-5 cells also failed to protect SU-DHL4 or OCI-LY18 cells from BEZ235/panobinostat lethality (Fig. 5C). Notably, Western blot analysis revealed a discernible increase in Mcl-1 and decrease in Bim protein levels in cells cultured in the presence of HS-5-conditioned media compared to controls (Fig. 5D). However, treatment with BEZ235 or BEZ235/panobinostat abrogated AKT phosphorylation and p21CIP1 induction, attenuated GSK3 phosphorylation, down-regulated Mcl-1, up-regulated Bim, and increased H3 and H4 acetylation in the presence of HS-5-conditionned media, similar to effects in regular media (Fig. 5D).
Fig. 5. BEZ235/panobinostat treatment is effective in the presence of a protective stromal microenvironment.
A) SU-DHL4 cells were treated with 200 nM BEZ235 ± 15 nM panobinostat in the presence of HS-5-conditioned media (HS-5 CM) or regular media (RM) for 24 hr after which cells were lysed and subjected to Western blot analysis. B) Annexin V/PI assays in SU-DHL4, HBL-1, and OCI-LY18 cells following 24 hr treatment with BEZ235 (200 nM) ± panobinostat (10 nM for HBL-1 and OCI-LY18; 15 nM for SU-DHL4). C) SU-DHL4 and OCI-LY18 cells were co-cultured with HS-5 cells for 3 hr, and then exposed to BEZ235 ± panobinostat for 24 hr as in (B) after which cells were photographed using an Olympus microscope. D) Western blot analysis of SU-DHL4 cells treated as in (A) for 8 hr.
Co-administration of BEZ235 and panobinostat significantly inhibits, tumor growth, and prolongs survival in an in vivo lymphoma xenograft model
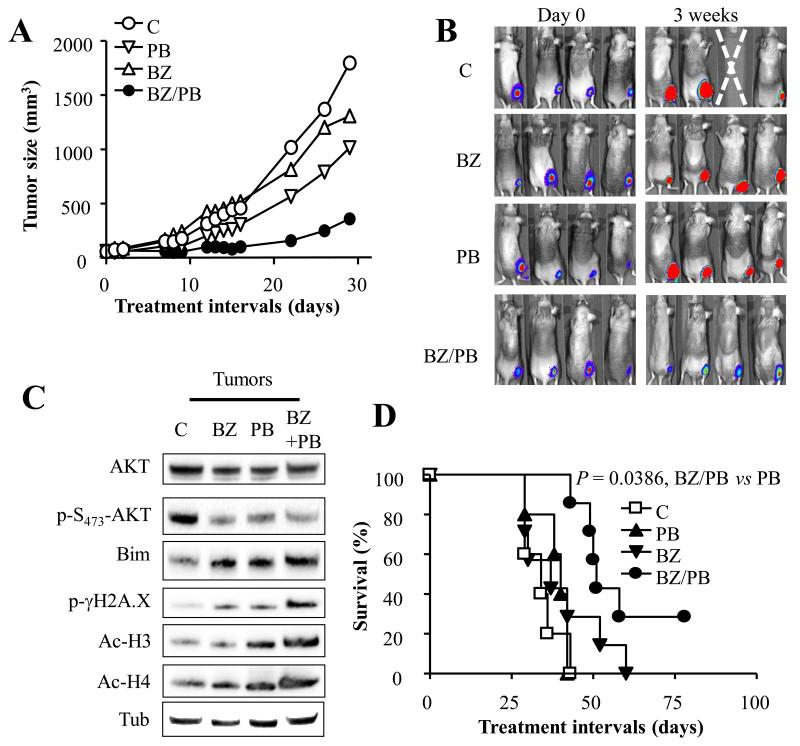
To determine whether inhibition of PI3K/mTOR enhances panobinostat lethality in vivo, a subcutaneous xenograft mouse model employing SU-DHL4 cells was employed. Interestingly, combined treatment with panobinostat and BEZ235 significantly reduced tumor growth. In contrast, individual agents had only modest effects (Figs. 6A-B). Furthermore, Western blot analysis performed on tumor tissue excised from animals treated twice over a 24 hr interval revealed identical results to those observed in vitro. Specifically, combined treatment led to a pronounced increase in Bim protein level, histone H3 and H4 acetylation, and γH2A.X phosphorylation, as well as a decrease in AKT phosphorylation (Fig. 6C). Consistent with tumor growth inhibition, Kaplan-Meier analysis (Fig. 6D) revealed that combined treatment significantly prolonged mouse survival compared to either agent alone (P = 0.001, and P = 0.0386 for combined treatment vs panobinostat or BEZ235 respectively; logrank test). Of note, while BEZ235 led to a modest prolongation in survival, panobinostat was ineffective in this regard. Significantly, combined treatment, like the agents alone, did not induce major changes in mouse weights (e.g., > 10%; Supplementary Fig. 8). Together, these findings indicate that combined treatment with BEZ235 and panobinostat significantly inhibits tumor growth in vivo and prolongs survival of mice bearing lymphoma xenografts.
Fig. 6. BEZ235/panobinostat significantly inhibits tumor growth and prolongs survival in a xenograft mouse model.
Tumor growth in beige nude mice (7 mice/condition) bearing luciferase-expressing SU-DHL4-derived tumors treated 5 days a week with BEZ235 (50 mg/kg) and panobinostat (15 mg/kg) alone or in combination. Mice were also imaged prior to and 3 weeks after treatment using an IVIS 200 imager (B). C) Xenograft-bearing mice were treated as in (A) twice over a 24 h interval after which tumors were excised, lysed, and subjected to Western blot analysis. D) Kaplan-Meyer plot involving 7 mice/condition treated as in (A). The median survival was prolonged from 34 to 51 days for mice exposed to the combined treatment. The survival curves differed significantly between combined treatment and other treatments (P = 0.001 for BEZ235/panobinostat vs panobinostat; P = 0.0386 for BEZ235/panobinostat vs BEZ235; logrank test).
DISCUSSION
Components of the PI3K/AKT/mTOR pathway as well as chromatin modifier-related proteins are frequently mutated in various cancer types, particularly those of lymphoid origin [2, 3, 15, 16]. Such considerations provided a strong rational for targeting these protein classes in cancer. In fact, preclinical and clinical studies have demonstrated promising activity of PI3K inhibitors such as BEZ235 or HDACis such as panobinostat in diverse tumor types including those of lymphoid origin [6, 7, 21]. We previously reported that non-clinically relevant PI3K inhibitors (e.g. LY294002) interacted synergistically with HDACIs in myeloid leukemia cells [11, 24], and subsequent studies demonstrated analogous interactions in epithelial tumor cells [25, 26]. The present report demonstrates that DLBCL cells may be particularly vulnerable to this strategy.
The findings that ectopic expression of constitutively active AKT renders lymphoma cells resistant to panobinostat lethality indicate that PI3K/AKT pathway activation status plays a critical role in determining panobinostat activity, and suggest that PI3K/AKT inhibition might potentiate panobinostat toxicity in lymphoma cells. Indeed, co-administration of very low (nanomolar) concentrations of panobinostat and the dual PI3K/mTOR inhibitor BEZ235 synergistically induced apoptosis in diverse DLBCL cell lines including both GC-, ABC-, poor prognosis MYC/Bcl-2 double-hit DLBCL, as well as mantle cell lymphoma cells but not in normal hematopoietic CD34+ cells, highlighting the potential for therapeutic selectivity. This selectivity may reflect the frequent addiction of DLBCL cells to PI3K/AKT activation [36] and the well-established preferential toxicity of HDACIs toward malignant vs normal cells [12, 37, 38]. Importantly, the BEZ235 and panobinostat concentrations employed were well below clinically achievable maximum plasma concentration (C-max; [6, 22]). It should be noted that we did not observe a clear correlation between AKT phosphorylation level and panobinostat sensitivity in multiple lymphoma cell lines (data not shown). These findings could reflect the fact that AKT phosphorylation is not the sole determinant of lymphoma cell responsiveness to panobinostat, and/or the possibility that high basal AKT activation may reflect enhanced reliance on this pathway for survival.
Recently, it has become apparent that Mcl-1 plays a critical role in the survival of many tumor cell types, including malignant hematopoietic cells [20]. Significantly, and consistent with recent studies in acute myeloid leukemia [20], BEZ235 effectively decreased Mcl-1 protein level in DLBCL cells, arguing for a functional role for Mcl-1 downregulation in BEZ235/panobinostat lethality in DLBCL. However, it is important to note that despite down-regulating Mcl-1, BEZ235 alone was not a potent inducer of cell death at the concentrations employed. Nevertheless, the finding that Mcl-1 knock-down significantly enhanced panobinostat lethality suggest that Mcl-1 down-regulation cooperates with other panobinostat actions to trigger cell death. It is also likely that other Mcl-1-independent BEZ235 actions contribute to synergistic interactions with panobinostat. For example, PI3K inhibition induces activation of FOXO3, a transcription factor that generally plays a pro-apoptotic role [18]. While the mechanism by which BEZ235/panobinostat exerts its anti-lymphoma activities is likely to be multi-factorial, the present observations support a model in which BEZ235/panobinostat down-regulates Mcl-1, increases Bim protein levels and binding to Bcl-2 and Bcl-xL, leading to Bax and Bak release from these anti-apoptotic proteins and promotion of cell death. This notion is supported by the marked changes in Bax and Bak conformation/activation and the ability of Bim, Bax, or Bak knock-down to render cells resistant to BEZ235/panobinostat lethality.
Previous studies have shown that HDACIs, among numerous actions, can inactivate the AKT pathway [39]. While panobinostat also decreased AKT phosphorylation in DLBCL cells in a dose-dependent manner, these effects were modest at low concentrations, and may not have been sufficient to lower the apoptotic threshold for other panobinostat actions. It is also possible that other PI3K/mTOR-dependent AKT-independent effects of BEZ235 contributed to increased panobinostat-mediated lethality. Other potential downstream PI3K targets that may contribute to BEZ235/panobinostat interactions include PDK1, which promotes cell survival in an AKT-dependent as well as an AKT-independent manner [40]. In addition, one of the mechanisms by which AKT increases cell survival is by phosphorylating the serine/threonine protein kinase GSK3 at serine 9/21, an event that leads to its cytoplasmic sequestration and inhibition [41]. The observations that GSK3 was dephosphorylated in cells treated with BEZ235/panobinostat along with down-regulation of the GSK3 downstream target β-Catenin and c-Myc proteins level indicate GSK3 inactivation. Moreover the findings that GSK3 inhibitors BIO or CHIR-98014 significantly diminished BEZ235/panobinostat lethality argue for a functional role for GSK3 in cell death mediated by this regimen. GSK3 pro-apoptotic activity involves multiple mechanisms, including perturbations in Mcl-1, Bim, FOXO3, β-catenin, c-Myc, and Cyclin D1 among others [42, 43]. However, the mechanism(s) involved in the present studies remains to be determined.
Induction of DNA damage and inhibition of DNA repair have been implicated in the anti-tumor activity of both HDACs as well as PI3K inhibitors [28, 37, 44]. The observation that co-treatment with BEZ235/panobinostat led to a sharp increase in γ-H2A.X phosphorylation, and the early onset of this phenomenon (4-8 hr) i.e., before significant cell death was observed, suggest that DNA damage contributed to combined treatment toxicity. Moreover, in view of the role of p21CIP1 in the DNA damage response [45], abrogation of panobinostat-mediated p21CIP1 induction by BEZ235 may have enhanced DNA damage and ultimately potentiated cell death. Indeed, studies by our group and others groups have shown that p21CIP1 plays an anti-apoptotic role in malignant cells exposed to HDACIs [24, 29, 30]. The mechanism by which PI3K inhibitors diminish p21CIP1 protein levels is unclear; however Rössig L et al [46] have shown that GSK3 phosphorylates the p21CIP1 protein at threonine 57, leading to its destabilization. Whether GSK3 activation by BEZ235 was responsible for p21CIP1 abrogation in lymphoma cells remains to be determined.
Another notable observation was the pronounced increase in H3 and H4 acetylation, which may reflect combined inhibition of histone deacetylation (by panobinostat) and increased histone acetylation by histone acetyl transferase CBP (by BEZ235). In this regard, Liu et al have shown that AKT inhibits CBP activity [47]. It is recognized that the mechanism and the functional significance of this finding are uncertain, and efforts to address these issues are underway.
Microenvironmental factors e.g., stroma mediate resistance to chemotherapy in multiple systems, including lymphoid malignancies [33-35]. Significantly, the ability of BEZ235/panobinostat to kill DLBCL cells in the presence of HS-5 cells or HS-5-conditioned media argues that this regimen can circumvent certain forms of microenvironmental resistance. Stromal factor-related resistance has been attributed to diverse mechanisms including, AKT activation, Bim down-regulation, and Mcl-1 up-regulation, among others[48]. Significantly, HS-5-conditioned media did not reverse BEZ235/panobinostat-mediated AKT inactivation or Mcl-1 down-regulation, although Mcl-1 protein levels in non-treated cells were slightly higher in the presence of HS-5-conditioned media compared to regular media. Notably, Bim was down-regulated in the presence of HS-5-conditioned media, in agreement with previous studies [48]. However, exposure to BEZ235 alone or in combination, restored Bim protein levels similar to those observed in cells cultured in regular media, suggesting that this action may have contributed to restoration of sensitivity.
Finally, in vivo studies employing a murine xenograft model demonstrated that co-administration of BEZ235 and panobinostat exhibited potent anti-DLBCL activity. Specifically, BEZ235/panobinostat, administered at well-tolerated doses, significantly inhibited tumor growth and enhanced murine survival. Notably, several of the changes observed in vitro e.g., AKT dephosphorylation/inactivation, Mcl-1 down-regulation, Bim upregulation, enhanced histone H3 and H4 acetylation and γ-H2A.X phosphorylation were recapitulated in tumor tissues following in vivo drug treatment, suggesting that similar mechanisms of anti-tumor efficacy may be operative in intact animals. Such findings raise the possibility that these changes might serve as pharmacodynamic response determinants in future trials.
In summary, the present studies indicate that the BEZ235/panobinostat regimen effectively induces cell death in various DLBCL lines, including poor-prognosis sub-types (e.g., ABC- and double-hit DLBCL). Importantly, it is also active in the presence of a protective microenvironment conferred by bone marrow-derived HS-5 stromal cells, and in the in vivo setting. The present findings also suggest that underlying mechanisms are likely to be multi-factorial e.g., down-regulation of Mcl-1, up-regulation of Bim, increased binding of Bim to Bcl-2 and Bcl-xL, and activation of GSK3. Of note, Mcl-1 and Bim may also play important roles in determining lymphoma cell sensitivity to chemotherapeutic agents e.g., doxorubicin or dexamethasone [49, 50]. In conjunction with evidence implicating aberrations in histone acetylation and the PI3K pathway in lymphoma [2, 3, 15, 16], these findings raise the possibility that combining PI3K/AKT/mTOR inhibitors such as BEZ235 and HDACIs like panobinostat may represent a novel and effective strategy against various NHL subtypes including poor prognosis ABC-DLBCL, MYC/Bcl-2 double-hit, as well as MCL, and possibly other hematologic malignancies. Accordingly, plans to test this concept are currently underway.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Despite recent advances in the treatment of non-Hodgkin’s lymphoma (NHL), certain subtypes e.g., diffuse large B-cell lymphoma (DLBCL)-ABC sub-type and double-hit DLBCL continue to exhibit poor prognoses. The present studies demonstrate that combined treatment with the dual PI3K/mTOR inhibitor BEZ235 and the histone deacetylase inhibitor panobinostat exhibits potent preclinical activity both in vitro and in vivo in DLBCL, including subtypes associated with poor outcomes such as ABC-DLBCL and double-hit DLBCL with overexpression of Bcl-2 and c-MYC. This strategy was also highly effective in other NHL lymphoma sub-types. Significantly, interactions were observed at extremely low drug concentrations substantially lower than clinically achievable maximum plasma levels, highlighting the therapeutic potential of this approach. The present studies also provide a molecular framework for identifying pharmacodynamic markers of patient responses.
Financial support
These studies were supported by awards CA093738, CA167708, P50CA142509, P50CA130805, R21CA137823, and P30 CA16059 from the NIH, and award 6181-10 from the Leukemia and Lymphoma Society of America.
Footnotes
Conflict of interest: The authors have no conflict of interest to disclose.
Reference List
- [1].Ellis L, Atadja PW, Johnstone RW. Epigenetics in cancer: targeting chromatin modifications. Mol Cancer Ther. 2009;8:1409–20. doi: 10.1158/1535-7163.MCT-08-0860. [DOI] [PubMed] [Google Scholar]
- [2].Morin RD, Mendez-Lago M, Mungall AJ, Goya R, Mungall KL, Corbett RD, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476:298–303. doi: 10.1038/nature10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pasqualucci L, Dominguez-Sola D, Chiarenza A, Fabbri G, Grunn A, Trifonov V, et al. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature. 2011;471:189–95. doi: 10.1038/nature09730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lemoine M, Younes A. Histone deacetylase inhibitors in the treatment of lymphoma. Discov Med. 2010;10:462–70. [PubMed] [Google Scholar]
- [5].Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5:981–9. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- [6].DeAngelo DJ, Spencer A, Bhalla KN, Prince HM, Fischer T, Kindler T, et al. Phase Ia/II, two-arm, open-label, dose-escalation study of oral panobinostat administered via two dosing schedules in patients with advanced hematologic malignancies. Leukemia. 2013;27:1628–36. doi: 10.1038/leu.2013.38. [DOI] [PubMed] [Google Scholar]
- [7].Duvic M, Dummer R, Becker JC, Poulalhon N, Ortiz Romero P, Grazia Bernengo M, et al. Panobinostat activity in both bexarotene-exposed and -naive patients with refractory cutaneous T-cell lymphoma: results of a phase II trial. Eur J Cancer. 2013;49:386–94. doi: 10.1016/j.ejca.2012.08.017. [DOI] [PubMed] [Google Scholar]
- [8].Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst. 2000;92:1210–6. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- [9].Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005;280:26729–34. doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- [10].Bereshchenko OR, Gu W, Dalla-Favera R. Acetylation inactivates the transcriptional repressor BCL6. Nat Genet. 2002;32:606–13. doi: 10.1038/ng1018. [DOI] [PubMed] [Google Scholar]
- [11].Rahmani M, Reese E, Dai Y, Bauer C, Payne SG, Dent P, et al. Coadministration of histone deacetylase inhibitors and perifosine synergistically induces apoptosis in human leukemia cells through Akt and ERK1/2 inactivation and the generation of ceramide and reactive oxygen species. Cancer Res. 2005;65:2422–32. doi: 10.1158/0008-5472.CAN-04-2440. [DOI] [PubMed] [Google Scholar]
- [12].Insinga A, Monestiroli S, Ronzoni S, Gelmetti V, Marchesi F, Viale A, et al. Inhibitors of histone deacetylases induce tumor-selective apoptosis through activation of the death receptor pathway. Nat Med. 2005;11:71–6. doi: 10.1038/nm1160. [DOI] [PubMed] [Google Scholar]
- [13].Zhao Y, Tan J, Zhuang L, Jiang X, Liu ET, Yu Q. Inhibitors of histone deacetylases target the Rb-E2F1 pathway for apoptosis induction through activation of proapoptotic protein Bim. Proc Natl Acad Sci U S A. 2005;102:16090–5. doi: 10.1073/pnas.0505585102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Abubaker J, Bavi PP, Al-Harbi S, Siraj AK, Al-Dayel F, Uddin S, et al. PIK3CA mutations are mutually exclusive with PTEN loss in diffuse large B-cell lymphoma. Leukemia. 2007;21:2368–70. doi: 10.1038/sj.leu.2404873. [DOI] [PubMed] [Google Scholar]
- [16].Sakai A, Thieblemont C, Wellmann A, Jaffe ES, Raffeld M. PTEN gene alterations in lymphoid neoplasms. Blood. 1998;92:3410–5. [PubMed] [Google Scholar]
- [17].Rickert RC. New insights into pre-BCR and BCR signalling with relevance to B cell malignancies. Nat Rev Immunol. 2013;13:578–91. doi: 10.1038/nri3487. [DOI] [PubMed] [Google Scholar]
- [18].Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rahmani M, Aust MM, Attkisson E, Williams DC, Jr., Ferreira-Gonzalez A, Grant S. Inhibition of Bcl-2 anti-apoptotic members by obatoclax potently enhances sorafenib-induced apoptosis in human myeloid leukemia cells through a Bim-dependent process. Blood. 2012;119:6089–98. doi: 10.1182/blood-2011-09-378141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rahmani M, Aust MM, Attkisson E, Williams DC, Jr., Ferreira-Gonzalez A, Grant S. Dual inhibition of Bcl-2 and Bcl-xL strikingly enhances PI3K inhibition-induced apoptosis in human myeloid leukemia cells through a. Cancer Res. 2013;73:1340–51. doi: 10.1158/0008-5472.CAN-12-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Martini M, Ciraolo E, Gulluni F, Hirsch E. Targeting PI3K in Cancer: Any Good News? Front Oncol. 2013;3:108. doi: 10.3389/fonc.2013.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Peyton JD, Ahnert JR, Burris H, Britten C, Chen LC, Tabernero J, et al. A dose-escalation study with the novel formulation of the oral pan-class I PI3K inhibitor BEZ235, solid dispersion system (SDS) sachet, in patients with advanced solid tumors. J Clin Oncol. 2011;29 2011(Suppl; abstr 3066) [Google Scholar]
- [23].Mahadevan D, Chiorean EG, Harris WB, Von Hoff DD, Stejskal-Barnett A, Qi W, et al. Phase I pharmacokinetic and pharmacodynamic study of the pan-PI3K/mTORC vascular targeted pro-drug SF1126 in patients with advanced solid tumours and B-cell malignancies. Eur J Cancer. 2012;48:3319–27. doi: 10.1016/j.ejca.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rahmani M, Yu C, Reese E, Ahmed W, Hirsch K, Dent P, et al. Inhibition of PI-3 kinase sensitizes human leukemic cells to histone deacetylase inhibitor-mediated apoptosis through p44/42 MAP kinase inactivation and abrogation of p21(CIP1/WAF1) induction rather than AKT inhibition. Oncogene. 2003;22:6231–42. doi: 10.1038/sj.onc.1206646. [DOI] [PubMed] [Google Scholar]
- [25].Denlinger CE, Rundall BK, Jones DR. Inhibition of phosphatidylinositol 3-kinase/Akt and histone deacetylase activity induces apoptosis in non-small cell lung cancer in vitro and in vivo. J Thorac Cardiovasc Surg. 2005;130:1422–9. doi: 10.1016/j.jtcvs.2005.06.051. [DOI] [PubMed] [Google Scholar]
- [26].Ellis L, Ku SY, Ramakrishnan S, Lasorsa E, Azabdaftari G, Godoy A, et al. Combinatorial antitumor effect of HDAC and the PI3K-Akt-mTOR pathway inhibition in a Pten defecient model of prostate cancer. Oncotarget. 2013;4:2225–36. doi: 10.18632/oncotarget.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rahmani M, Anderson A, Habibi JR, Crabtree TR, Mayo M, Harada H, et al. The BH3-only protein Bim plays a critical role in leukemia cell death triggered by concomitant inhibition of the PI3K/Akt and MEK/ERK1/2 pathways. Blood. 2009;114:4507–16. doi: 10.1182/blood-2008-09-177881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lee JH, Choy ML, Ngo L, Foster SS, Marks PA. Histone deacetylase inhibitor induces DNA damage, which normal but not transformed cells can repair. Proc Natl Acad Sci U S A. 2010;107:14639–44. doi: 10.1073/pnas.1008522107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Burgess AJ, Pavey S, Warrener R, Hunter LJ, Piva TJ, Musgrove EA, et al. Upregulation of p21(WAF1/CIP1) by histone deacetylase inhibitors reduces their cytotoxicity. Mol Pharmacol. 2001;60:828–37. [PubMed] [Google Scholar]
- [30].Rahmani M, Yu C, Dai Y, Reese E, Ahmed W, Dent P, et al. Coadministration of the heat shock protein 90 antagonist 17-allylamino-17-demethoxygeldanamycin with suberoylanilide hydroxamic acid or sodium butyrate synergistically induces apoptosis in human leukemia cells. Cancer Res. 2003;63:8420–7. [PubMed] [Google Scholar]
- [31].Bender A, Opel D, Naumann I, Kappler R, Friedman L, von Schweinitz D, et al. PI3K inhibitors prime neuroblastoma cells for chemotherapy by shifting the balance towards pro-apoptotic Bcl-2 proteins and enhanced mitochondrial apoptosis. Oncogene. 2011;30:494–503. doi: 10.1038/onc.2010.429. [DOI] [PubMed] [Google Scholar]
- [32].Scuto A, Kirschbaum M, Kowolik C, Kretzner L, Juhasz A, Atadja P, et al. The novel histone deacetylase inhibitor, LBH589, induces expression of DNA damage response genes and apoptosis in Phacute lymphoblastic leukemia cells. Blood. 2008;111:5093–100. doi: 10.1182/blood-2007-10-117762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Medina DJ, Goodell L, Glod J, Gelinas C, Rabson AB, Strair RK. Mesenchymal stromal cells protect mantle cell lymphoma cells from spontaneous and drug-induced apoptosis through secretion of B-cell activating factor and activation of the canonical and non-canonical nuclear factor kappaB pathways. Haematologica. 2012;97:1255–63. doi: 10.3324/haematol.2011.040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res. 2008;14:2519–26. doi: 10.1158/1078-0432.CCR-07-2223. [DOI] [PubMed] [Google Scholar]
- [35].Mraz M, Zent CS, Church AK, Jelinek DF, Wu X, Pospisilova S, et al. Bone marrow stromal cells protect lymphoma B-cells from rituximab-induced apoptosis and targeting integrin alpha-4-beta-1 (VLA-4) with natalizumab can overcome this resistance. Br J Haematol. 2011;155:53–64. doi: 10.1111/j.1365-2141.2011.08794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pfeifer M, Grau M, Lenze D, Wenzel SS, Wolf A, Wollert-Wulf B, et al. PTEN loss defines a PI3K/AKT pathway-dependent germinal center subtype of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A. 2013;110:12420–5. doi: 10.1073/pnas.1305656110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Namdar M, Perez G, Ngo L, Marks PA. Selective inhibition of histone deacetylase 6 (HDAC6) induces DNA damage and sensitizes transformed cells to anticancer agents. Proc Natl Acad Sci U S A. 2010;107:20003–8. doi: 10.1073/pnas.1013754107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bolden JE, Shi W, Jankowski K, Kan CY, Cluse L, Martin BP, et al. HDAC inhibitors induce tumor-cell-selective pro-apoptotic transcriptional responses. Cell Death Dis. 2013;4:e519. doi: 10.1038/cddis.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dudakovic A, Evans JM, Li Y, Middha S, McGee-Lawrence ME, van Wijnen AJ, et al. Histone deacetylase inhibition promotes osteoblast maturation by altering the histone H4 epigenome and reduces Akt phosphorylation. J Biol Chem. 2013;288:28783–91. doi: 10.1074/jbc.M113.489732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ, et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009;16:21–32. doi: 10.1016/j.ccr.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Martelli AM, Evangelisti C, Chiarini F, McCubrey JA. The phosphatidylinositol 3-kinase/Akt/mTOR signaling network as a therapeutic target in acute myelogenous leukemia patients. Oncotarget. 2010;1:89–103. doi: 10.18632/oncotarget.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Spokoini R, Kfir-Erenfeld S, Yefenof E, Sionov RV. Glycogen synthase kinase-3 plays a central role in mediating glucocorticoid-induced apoptosis. Mol Endocrinol. 2010;24:1136–50. doi: 10.1210/me.2009-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kim L, Kimmel AR. GSK3, a master switch regulating cell-fate specification and tumorigenesis. Curr Opin Genet Dev. 2000;10:508–14. doi: 10.1016/s0959-437x(00)00120-9. [DOI] [PubMed] [Google Scholar]
- [44].Juvekar A, Burga LN, Hu H, Lunsford EP, Ibrahim YH, Balmañà J, et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov. 2012;2:1048–63. doi: 10.1158/2159-8290.CD-11-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cazzalini O, Scovassi AI, Savio M, Stivala LA, Prosperi E. Multiple roles of the cell cycle inhibitor p21(CDKN1A) in the DNA damage response. Mutat Res. 2010;704:12–20. doi: 10.1016/j.mrrev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- [46].Rossig L, Badorff C, Holzmann Y, Zeiher AM, Dimmeler S. Glycogen synthase kinase-3 couples AKT-dependent signaling to the regulation of p21Cip1 degradation. J Biol Chem. 2002;277:9684–9. doi: 10.1074/jbc.M106157200. [DOI] [PubMed] [Google Scholar]
- [47].Liu Y, Xing ZB, Zhang JH, Fang Y. Akt kinase targets the association of CBP with histone H3 to regulate the acetylation of lysine K18. FEBS Lett. 2013;587:847–53. doi: 10.1016/j.febslet.2013.02.023. [DOI] [PubMed] [Google Scholar]
- [48].Rosich L, Saborit-Villarroya I, Lopez-Guerra M, López-Guerra M, Xargay-Torrent S, Montraveta A, et al. The phosphatidylinositol-3-kinase inhibitor NVP-BKM120 overcomes resistance signals derived from microenvironment by regulating the Akt/FoxO3a/Bim axis in chronic lymphocytic leukemia cells. Haematologica. 2013 doi: 10.3324/haematol.2013.088849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wenzel SS, Grau M, Mavis C, Hailfinger S, Wolf A, Madle H, et al. MCL1 is deregulated in subgroups of diffuse large B-cell lymphoma. Leukemia. 2013;27:1381–90. doi: 10.1038/leu.2012.367. [DOI] [PubMed] [Google Scholar]
- [50].Bachmann PS, Piazza RG, Janes ME, Wong NC, Davies C, Mogavero A, et al. Epigenetic silencing of BIM in glucocorticoid poor-responsive pediatric acute lymphoblastic leukemia, and its reversal by histone deacetylase inhibition. Blood. 2010;116:3013–22. doi: 10.1182/blood-2010-05-284968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.