Abstract
Atopic, obese asthmatics exhibit airway obstruction with variable degrees of eosinophilic airway inflammation. We previously reported that mice obese as a result of a genetic deficiency in either leptin (ob/ob mice) or the long isoform of the leptin receptor (db/db mice) exhibit enhanced airway obstruction in the presence of decreased numbers of bronchoalveolar lavage fluid (BALF) eosinophils compared with lean, wild-type mice following antigen (ovalbumin; OVA) sensitization and challenge. To determine whether the genetic modality of obesity induction influences the development of OVA-induced airway obstruction and OVA-induced pulmonary inflammation, we examined indices of these sequelae in mice obese as a result of a genetic deficiency in carboxypeptidase E, an enzyme that processes prohormones and proneuropeptides involved in satiety and energy expenditure (Cpefat mice). Accordingly, Cpefat and lean, wild-type (C57BL/6) mice were sensitized to OVA and then challenged with either aerosolized PBS or OVA. Compared with genotype-matched, OVA-sensitized and PBS-challenged mice, OVA sensitization and challenge elicited airway obstruction and increased BALF eosinophils, macrophages, neutrophils, IL-4, IL-13, IL-18, and chemerin. However, OVA challenge enhanced airway obstruction and pulmonary inflammation in Cpefat compared with wild-type mice. These results demonstrate that OVA sensitization and challenge enhance airway obstruction in obese mice regardless of the genetic basis of obesity, whereas the degree of OVA-induced pulmonary inflammation is dependent on the genetic modality of obesity induction. These results have important implications for animal models of asthma, as modeling the pulmonary phenotypes for subpopulations of atopic, obese asthmatics critically depends on selecting the appropriate mouse model.
Keywords: asthma, atopic, eosinophil, interleukin-13, ovalbumin
obesity is an epidemic among children, adolescents, and adults and is a clearly recognized determinant of breast and colon cancer, cardiovascular disease, nonalcoholic steatohepatitis, and Type 2 diabetes (24, 53, 58, 62). Furthermore, a number of investigators have reported an increased prevalence and incidence of asthma in obese children, adolescents, and adults (8, 50, 68). Obesity also increases asthma severity, decreases asthma control, and decreases the efficacy of standard asthma medications (40). As the prevalence of obesity is high in both developed and developing countries (80), a significant number of individuals are at risk for developing asthma. To understand and ultimately prevent the negative health effects of asthma caused by obesity, elucidating the mechanistic relationships between obesity and asthma is urgently needed.
Obesity is common in individuals with severe or refractory asthma (3, 33, 34, 46, 57). Data from Holguin and colleagues (33) demonstrate at least two distinct phenotypes of severe asthma in obesity: early-onset and late-onset asthma, which are categorized by the age of onset of asthma, airway responsiveness to methacholine, and atopic status. Specifically, obese subjects with early-onset asthma have significantly greater airway responsiveness to methacholine and a significantly higher prevalence of atopy compared with obese subjects with late-onset asthma (33). Dixon et al. (20) also reported that atopic status can be used to differentiate obese human asthmatic subjects into subpopulations. Specifically, surgically induced weight loss significantly decreased airway responsiveness to methacholine in obese nonatopic, but not obese atopic, asthmatic subjects (20). On the basis of these results, Dixon et al. (20) suggested that there are at least two distinct phenotypes of asthma in obesity: late-onset, nonatopic asthma due to obesity and early-onset, atopic asthma that is complicated by the development of obesity. However, the mechanisms underlying the development of these distinct phenotypes in obese asthmatics are not well understood.
We have been using obese mice to characterize the mechanisms underlying the relationship between obesity and asthma, and we previously reported that obese mice exhibit innate airway hyperresponsiveness (AHR) to nonspecific bronchoconstrictors, such as methacholine and serotonin (36–39, 52, 64, 70). We have used mice that are obese as a result of a genetic deficiency in leptin, a satiety hormone, (ob/ob mice), as a model for determining the effects of obesity on atopic asthma. Specifically, we have previously reported that ob/ob mice develop airway obstruction in the presence of decreased numbers of bronchoalveolar lavage fluid (BALF) eosinophils, lymphocytes, and macrophages compared with lean wild-type C57BL/6 mice following antigen (ovalbumin; OVA) sensitization and challenge (39). OVA sensitization and challenge lead to a pulmonary phenotype in mice that mimics many of the characteristic features of atopic asthma in humans (44). We also obtained similar results with mice that are obese because of a genetic deficiency in the long isoform of the leptin receptor (db/db mice) (39).
Consistent with our observations in mice, data from human asthmatic subjects demonstrate that indices of atopic pulmonary inflammation, and, in particular, sputum eosinophils, decrease with increasing body mass index (20, 42, 73, 76). In contrast, recent data from Desai et al. (19) demonstrate that select indices of atopic pulmonary inflammation, including IL-5 and submucosal eosinophils, increase with increasing body mass index. Taken together, these data suggest that even among atopic asthmatics, obesity has different effects on the development of pulmonary inflammation. Similarly, the genetic modality of obesity induction in mice may result in different phenotypic responses to OVA sensitization and challenge.
In this context, the major objective of this study was to determine the effect of OVA sensitization and challenge on the oscillatory mechanics of the lung and pulmonary inflammation in mice that are obese because of a genetic deficiency in carboxypeptidase E (Cpefat mice). Carboxypeptidase E, a zinc-dependent exopeptidase, is expressed in the central nervous system and in endocrine cells and processes propeptides, such as proinsulin, procholecystokinin, and proopiomelanocortin, into biologically active peptides (14). Many of these biologically active peptides generated from carboxypeptidase E-induced proteolytic processing of propeptides are intimately involved in satiety and energy expenditure (14). Because of a missense mutation in the gene encoding carboxypeptidase E in Cpefat mice, carboxypeptidase E enzymatic activity is severely reduced in these animals (60), which prevents the processing of propeptides into their biologically active peptide configuration (48). Consequently, because of disrupted satiety and energy expenditure signaling pathways, Cpefat mice exhibit increased body mass by 7 wk of age and extreme obesity by 14–16 wk of age (37, 38).
In humans, a single nucleotide polymorphism in the gene encoding carboxypeptidase E is positively associated with obesity (51). Furthermore, Cpefat mice, similar to db/db and ob/ob mice, exhibit a number of obesity-related sequelae, including hypercholesterolemia (54), hyperglycemia (26, 49, 66), insulin resistance (5, 35, 75), and tachypnea (52, 68, 70). Collectively, these data demonstrate that Cpefat mice are a relevant preclinical model of human obesity that can be used to enhance our understanding of the mechanisms by which obesity influences the development of atopic pulmonary inflammation in asthmatics.
In this current study, we report that Cpefat mice exhibit enhanced airway obstruction compared with lean wild-type (C57BL/6) mice following OVA sensitization and challenge, which is similar to our previous observations in db/db and ob/ob mice (39). However, in contrast to db/db and ob/ob mice, OVA-induced pulmonary inflammation is significantly greater in Cpefat compared with wild-type mice. These results demonstrate that OVA sensitization and challenge enhance airway obstruction in obese mice regardless of the genetic basis of obesity, whereas the degree of OVA-induced pulmonary inflammation is dependent upon the genetic modality of obesity induction. Thus, when modeling the pulmonary phenotypes that exist in subpopulations of atopic, obese asthmatics, it is critically important to select the appropriate mouse model.
MATERIALS AND METHODS
Animals.
Female Cpefat mice were purchased from The Jackson Laboratory (Bar Harbor, ME) at 3−7 wk of age. Because Cpefat mice were backcrossed into a C57BL/6J genetic background for at least 10 generations, age-matched, female C57BL/6J mice were used as wild-type controls and purchased from The Jackson Laboratory at the same time as the Cpefat mice. All mice were housed in individually ventilated, microisolator cages (Tecniplast S.p.a., Buguggiate, Varese, Italy), containing no more than five animals per cage, within a multi-species-housed, modified barrier animal care facility, where they were given irradiated food (PicoLab Rodent Diet 20, LabDiet, Brentwood, MO) and autoclaved water ad libitum, exposed to a 12:12-h light-dark cycle, and acclimated to their new environment for at least 3 days prior to entering the experimental protocol at 4−9 wk of age. All of the experimental protocols used in this study were approved by The University of Texas Health Science Center at Houston Animal Welfare Committee.
Antigen sensitization and challenge.
On day 0 of the experimental protocol, when the mice were 4−9 wk of age, each animal was initially sensitized to albumin from chicken egg white (ovalbumin, OVA, Grade V; Sigma-Aldrich, St. Louis, MO) via an intraperitoneal injection of 20 μg of OVA and 2 mg of adjuvant, aluminum hydroxide powder (Mallinckrodt Baker, Phillipsburg, NJ), dispersed in 0.2 ml of PBS. On days 14 and 56 of the experimental protocol, each animal was given an intraperitoneal injection of the same reagents that were administered on day 0. A 1-ml insulin syringe with a 28-gauge hypodermic needle (Becton Dickinson, Franklin Lakes, NJ) was used for all intraperitoneal injections. On days 70–76 of the experimental protocol, the animals were challenged for 25 min once per day with either an aerosol of PBS containing 4% OVA (wt/vol) or an aerosol of PBS alone. The aerosol was generated by driving air from a PRONEB Ultra II air compressor (PARI Respiratory Equipment, Midlothian, VA) into a PARI LC Sprint nebulizer (PARI Respiratory Equipment), which contained 8 ml of either PBS containing 4% OVA or PBS alone. To challenge the mice with an aerosol, the animals were individually placed into 1 of 12 ventilated chambers of a circular pie cage (Braintree Scientific, Braintree, MA). On day 77 of the experimental protocol, which was 24 h following the cessation of the final aerosol challenge, the animals, which were 15−20 wk of age at this time, were subjected to one or more of the subsequently described experimental procedures.
Protocol.
Three separate cohorts of wild-type and Cpefat mice were used in this study. In the first cohort, airway and lung parenchymal oscillation mechanics were assessed in anesthetized mice 24 h following the cessation of the final PBS or OVA aerosol challenge. In the second cohort, blood was collected from each animal, and a bronchoalveolar lavage (BAL) was performed on mice that were euthanized 24 h following the cessation of the final PBS or OVA aerosol challenge. In the third cohort, blood was collected, and the lungs were fixed in situ in mice that were euthanized 24 h following the cessation of the final PBS or OVA aerosol challenge.
Measurement of airway and lung parenchymal oscillation mechanics.
Mice in the first cohort were anesthetized with pentobarbital sodium (50 mg/kg ip; Hospira, Lake Forest, IL) and xylazine hydrochloride (7 mg/kg ip; Akorn, Decatur, IL) 24 h following the cessation of the final PBS or OVA aerosol challenge. Once the mouse was acceptably anesthetized, as determined by unresponsiveness to a hind paw pinch, a tracheostomy was performed, and an 18-gauge tubing adaptor (Becton Dickinson) was inserted into the trachea. Subsequently, wild-type and Cpefat mice were artificially ventilated at 150 or 180 breaths/min, respectively, with a tidal volume (VT) of 0.3 ml and a positive end-expiratory pressure of 3 cm H2O using a specialized ventilator (flexiVent, SCIREQ Scientific Respiratory Equipment, Montréal, Québec, Canada), as we have previously described (2, 64, 70). The higher breathing frequency used for the Cpefat mice was chosen to conform to the spontaneous breathing frequency of these animals, yet VT was slightly, but not significantly, reduced in Cpefat compared with wild-type mice during spontaneous breathing (68). Consequently, spontaneous minute ventilation was not different between Cpefat and wild-type mice (68). Once ventilation was established, a wide incision in the chest wall was made bilaterally, and any adipose tissue overlying the lungs was carefully removed to expose the lungs to atmospheric pressure.
Total lung impedance (ZL) at baseline was determined by the forced oscillation technique, as described in detail by others (30, 32, 63). Specifically, ZL was measured by perturbing ventilation for 3 s and then simultaneously delivering 13 sinusoidal forcing functions, which ranged in frequency from 1 to 20.5 Hz, to the animal. The flexiVent was used both to ventilate the lungs and to deliver the sinusoidal forcing functions. The constant-phase model, as described by Hantos et al. (30), was used to partition ZL into components representing airway resistance (Raw), the coefficient of lung tissue damping (G), the coefficient of lung tissue elastance (H), and lung tissue hysteresivity (η = G/H). Measurements of the real and imaginary components of ZL, which were obtained from OVA-sensitized and PBS-challenged wild-type and Cpefat mice, as a function of breathing frequency, conformed to the constant phase model (data not shown).
The following protocol was executed to facilitate measurements of ZL at baseline in our mice. First, ventilation was paused for 6 s, and a pressure of 30 cm H2O was applied to the system to inflate the lungs to capacity to standardize lung volume history. Next, ventilation was allowed to resume for at least 6 s, and then a 2.5-Hz sinusoidal forcing function was applied, while ventilation was perturbed for 1.25 s, to measure total lung resistance (RL), as described by Bates et al. (4). RL was then measured at least five more times using this method to ensure that RL was stable. Once RL was stable, a sinusoidal forcing function, as described above, perturbed ventilation for 1.25 s to measure RL. Subsequently, ZL was determined by simultaneously delivering 13 sinusoidal forcing functions to the animal, ranging in frequency from 1 to 20.5 Hz. At this time, ZL was also partitioned into Raw, G, H, and η. The measurement of RL and ZL was repeated two more times for a total of three measurements. Once all measurements were complete, the perturbations ceased, and ventilation resumed uninterrupted. The three measurements of Raw, G, and H, which were partitioned from the three distinct measurements of ZL, were averaged to determine a mean value of Raw, G, and H for each animal. To establish the goodness of fit of the constant-phase model to our data, the flexiVent software (version 5.3) calculates a coefficient of determination (COD). Any measurement with a COD less than 0.9 was excluded from our study.
Blood collection and isolation of serum.
Twenty-four hours following the cessation of the final PBS or OVA aerosol challenge, each mouse in the second cohort was given an intraperitoneal injection of pentobarbital sodium (200 mg/kg ip; Vortech Pharmaceuticals, Dearborn, MI). Once each mouse was deeply anesthetized and unresponsive to any stimuli, it was placed in the supine position, and a median sternotomy was performed to expose the heart and lungs in situ. To collect blood from the animal, the heart was punctured with a 25-gauge hypodermic needle attached to a 1-ml syringe (Becton Dickinson). After the blood was collected, it was placed into Microtainer serum separator tubes (Becton Dickinson). The blood was then allowed to clot at room temperature for at least 30 min prior to being centrifuged at 15,000 g for 2 min at 4°C so as to isolate serum. The serum was then stored at −20°C until needed.
BAL.
After blood was collected from each animal, the animal was prepared for and then subjected to a BAL. First, the trachea and larynx were exposed in situ by removing any overlying fur, skin, and fascia covering these tissues. Next, a small incision was made on the ventral surface of the trachea directly distal to the larynx with microscissors. A 20-gauge fluorinated ethylene propylene polymer catheter (Becton Dickinson) attached to a 1-ml syringe containing 0.6 ml of lavage buffer (PBS containing 0.6 mM of ethylenediaminetetraacetic acid) was then inserted into the tracheal incision. Subsequently, the lungs were gently lavaged four times. The first lavage was with 0.6 ml of lavage buffer, and the second lavage was with the resulting lavagate instilled into the lungs again, subsequently retrieved a second time, and then stored on ice. This process was repeated again with a separate aliquot (0.6 ml) of lavage buffer, and the resulting lavagate from the second retrieval in this instance was pooled with the first lavagate, which was already stored on ice. The pooled lavagate was centrifuged at 600 g for 10 min at 4°C. Once centrifugation was complete, the supernatant was isolated and stored at −80°C until needed. The remaining cell pellet was resuspended in 1 ml of Hanks' balanced salt solution (HyClone Laboratories, Logan, UT), and the total number of cells within this cell suspension was enumerated with a hemacytometer. To perform a differential cell analysis on the cells recovered in the BALF, an aliquot of the cell suspension containing 25,000 cells was spun at 800 rpm for 10 min at room temperature using a Cytospin 3 cytocentrifuge (Thermo Shandon Limited, Runcorn, UK). The slides were then air dried and stained with a Hema 3 stain set (Fisher Diagnostics, Middletown, VA). Cells were classified as eosinophils, lymphocytes, macrophages, neutrophils, or respiratory epithelial cells, according to standard morphological characteristics (25, 27). Specifically, respiratory epithelial cells were identified by the presence of cilia. Cells that could not be clearly identified as one of the aforementioned cell types were categorized as indeterminates. At least 300 cells per slide were counted during the differential cell analysis.
Enzyme-linked immunosorbent assays.
The concentrations of adiponectin, chemerin, eotaxin, IL-4, IL-5, IL-13, IL-18, leptin, OVA-specific IgE and IgG1, and total IgE in the BALF and/or serum were measured using ELISAs. The OVA-specific and total IgE assays were purchased from BioLegend, (San Diego, CA), while the OVA-specific IgG1 assay was purchased from Cayman Chemical (Ann Arbor, MI). All other assays were purchased from R&D Systems (Minneapolis, MN). The assays were performed according to the manufacturer's instructions.
Lung histology.
Twenty-four hours following the cessation of the final PBS or OVA aerosol challenge, wild-type and Cpefat mice in the third cohort were administered an overdose of pentobarbital sodium (200 mg/kg ip), and once unresponsive to a hind paw pinch, a median sternotomy was performed to expose the heart and lungs in situ. Next, as described above, blood was collected from the heart, serum was isolated from the blood, and the isolated serum was stored at −20°C until needed. Afterward, the abdominal aorta was severed, and the right ventricle of the heart was punctured with a 25-gauge hypodermic needle (Smiths Medical ASD, Keene, NH) that was attached to a syringe containing 10 ml of ice-cold PBS. Subsequently, the heart and the circulatory system of the animal were perfused with 10 ml of ice-cold PBS. Then, we performed a tracheostomy on the animal and inserted a 19-gauge blunt needle (BRICO Medical Supplies, Dayton, NJ) into the trachea, which we secured by tying it in place. The lungs were then fixed in situ with 10% phosphate-buffered formalin (Fisher Scientific, Fair Lawn, NJ) at a pressure of 25 cm H2O and removed en bloc. The lungs were then placed in 10% phosphate-buffered formalin at 4°C, where they remained for at least 24 h. Afterward, the lungs were dehydrated in ethanol gradients, cleared with xylene, and infiltrated with paraffin. The lungs were then embedded in paraffin blocks and 4-μm-thick coronal sections of the right and left lung were cut with a microtome. The resulting sections were mounted onto a microscope slide. Sections were stained with hematoxylin and eosin or subjected to the periodic acid Schiff (PAS) reaction, dried overnight, covered with mounting medium, and overlaid with a cover slip.
Inflated portions of hematoxylin-and eosin-stained lung sections were blindly examined under light microscopy to assess the inflammation score of each section. We determined the inflammation score, which is the product of the severity and prevalence of inflammation, in a manner similar to Hamada et al. (28). Severity was assigned a numerical value based on the thickness of the inflammatory cell infiltrates surrounding the airways and blood vessels in the lung (0 = no cells; 1 = 1−3 cells thick; 2 = 4−6 cells thick; 3 = 7−9 cells thick; 4 = greater than or equal to 10 cells thick). Prevalence was assigned a numerical value, according to the percentage of airways and blood vessels in each section encompassed by inflammatory cells (0 = no airways or blood vessels; 1 = <25%; 2 = 25−50%; 3 = 51−75%; 4 = >75%).
Using light microscopy, mucin-containing goblet cells, which are those cells that responded positively to the PAS reaction, were counted on a blinded basis in lung sections obtained from our animals. Specifically, the number of PAS-positive goblet cells along an inflated, nonoblique 100-μm section of the respiratory mucosa from three different secondary or tertiary airways was counted. The three different enumerations of PAS-positive goblet cells were averaged to create a mean value for each mouse.
Statistical analysis of data.
The effect of genotype and aerosol challenge on indices of airway and lung parenchymal oscillation mechanics, BALF and histological indices of inflammation, the number of mucin-containing goblet cells, and serum immunoglobulins were assessed by a two-way ANOVA for normally distributed data or by a Kruskal-Wallis one-way ANOVA for nonnormally distributed data. In all of these analyses, genotype (wild-type and Cpefat) and aerosol challenge (PBS and OVA) were the main effects. Depending on whether the data were normally or nonnormally distributed, the Fisher's least significant difference test or the Wilcoxon signed-rank test, respectively, were used as a follow-up to determine the significance of differences between the various experimental groups. Body masses and serum levels of adiponectin, chemerin, eotaxin, IL-18, and leptin were analyzed using an unpaired Student's t-test or the Wilcoxon signed-rank test as appropriate. Stata 12 (StataCorp LP, College Station, TX) was used for all statistical analyses. The results are expressed as means ± SE. A P value equal to or less than 0.05 was considered significant.
RESULTS
Effect of carboxypeptidase E deficiency on body mass.
On the final day of the experimental protocol (day 77) when the mice were 15−20 wk of age, the body mass of each animal in this study was measured prior to initiating the experimental procedures described in materials and methods. Consistent with our previous observations (37, 38, 69), Cpefat mice weighed significantly more than wild-type C57BL/6 mice (46.0 ± 0.7 and 21.5 ± 0.2 g, respectively).
Effect of obesity and OVA sensitization and challenge on airway and lung parenchymal oscillation mechanics.
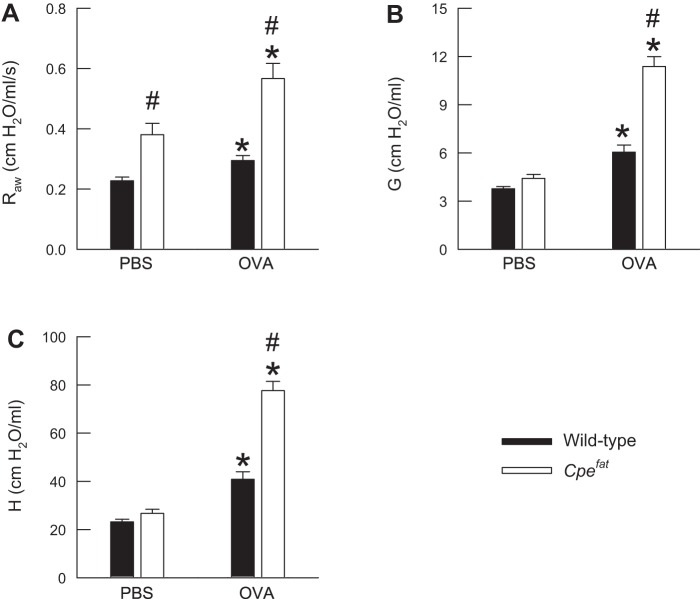
Figure 1 illustrates the baseline values of airway (Raw) and lung parenchymal (G and H) oscillation mechanics in OVA-sensitized wild-type and Cpefat mice 24 h following the cessation of the final PBS or OVA aerosol challenge. Raw, G, and H were greater in PBS-challenged Cpefat compared with PBS-challenged wild-type mice. However, statistical significance was only achieved for Raw (P = 0.002). OVA challenge significantly increased Raw, G, and H in wild-type and Cpefat mice, and there were genotype-related differences in these indices following OVA challenge. Specifically, Raw, G, and H were significantly greater in Cpefat compared with wild-type mice following OVA challenge. Finally, because indices of airway and lung parenchymal oscillation mechanics were extremely elevated in Cpefat mice following OVA challenge, airway responsiveness to methacholine was not assessed in any of our mice, since any further increases in these indices induced by methacholine could not be accurately measured.
Fig. 1.
Measurements of airway resistance (Raw; A), the coefficient of lung tissue damping (G; B), and the coefficient of lung tissue elastance (H; C) obtained from ovalbumin (OVA)-sensitized wild-type (C57BL/6) and carboxypeptidase E-deficient (Cpefat) mice challenged with aerosols of either PBS or PBS containing 4% OVA (wt/vol) once per day for seven consecutive days. Measurements were made 24 h following the cessation of the final aerosol challenge. Each value is expressed as the means ± SE. n = 7−9 mice for each group. *P < 0.05 compared with genotype-matched mice challenged with PBS. #P < 0.05 compared with wild-type (C57BL/6) mice with an identical series of aerosol challenges.
Effect of obesity and OVA sensitization and challenge on BALF cell differentials.
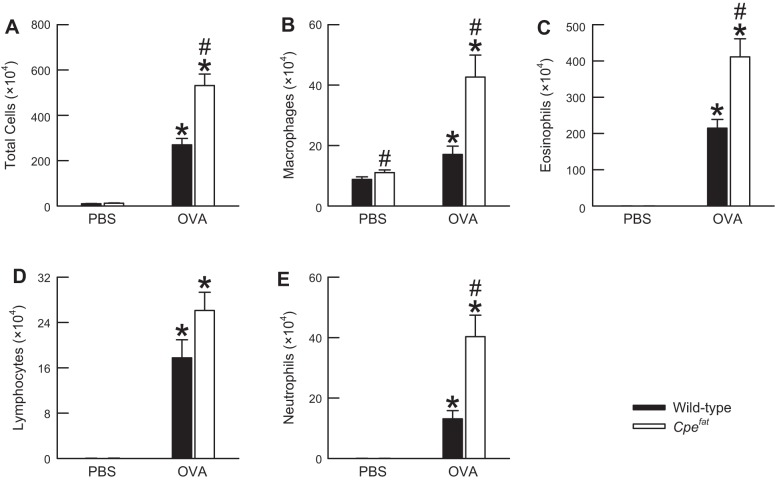
For each of the cell types enumerated in the BALF 24 h following the cessation of the final PBS or OVA aerosol challenge, a Kruskal-Wallis one-way ANOVA by ranks revealed a significant difference between at least two of the four experimental groups (P = 0.0001 in each instance; Fig. 2). The number of total cells retrieved from the BALF of PBS-challenged Cpefat mice was greater than that of PBS-challenged wild-type mice. However, this difference was not statistically significant (P = 0.07; Fig. 2A). The greater number of total cells in the BALF of PBS-challenged Cpefat mice was driven by a significant increase in the number of BALF macrophages (P = 0.04; Fig. 2B). There were no genotype-related differences in the number of BALF eosinophils, lymphocytes, or neutrophils in PBS-challenged mice (Fig. 2, C, D, E). In fact, these cell types were rarely observed in the BALF of PBS-challenged wild-type and Cpefat mice. OVA challenge caused a very robust and significant increase in the number of total cells, macrophages, eosinophils, lymphocytes, and neutrophils in the BALF of wild-type and Cpefat mice (Fig. 2). However, the number of BALF total cells, macrophages, eosinophils, and neutrophils were significantly greater in OVA-challenged Cpefat mice compared with OVA-challenged wild-type mice.
Fig. 2.
The number of total cells (A), macrophages (B), eosinophils (C), lymphocytes (D), and neutrophils (E) in the bronchoalveolar lavage fluid (BALF) obtained from OVA-sensitized C57BL/6 and Cpefat mice challenged with aerosols of either PBS or PBS containing 4% OVA (wt/vol) once per day for seven consecutive days. BALF was obtained from the mice 24 h following the cessation of the final aerosol challenge. Each value is expressed as the means ± SE. n = 9−13 mice for each group. *P < 0.05 compared with genotype-matched mice challenged with PBS. #P < 0.05 compared with wild-type (C57BL/6) mice with an identical series of aerosol challenges.
Effect of obesity and OVA sensitization and challenge on lung histopathology.
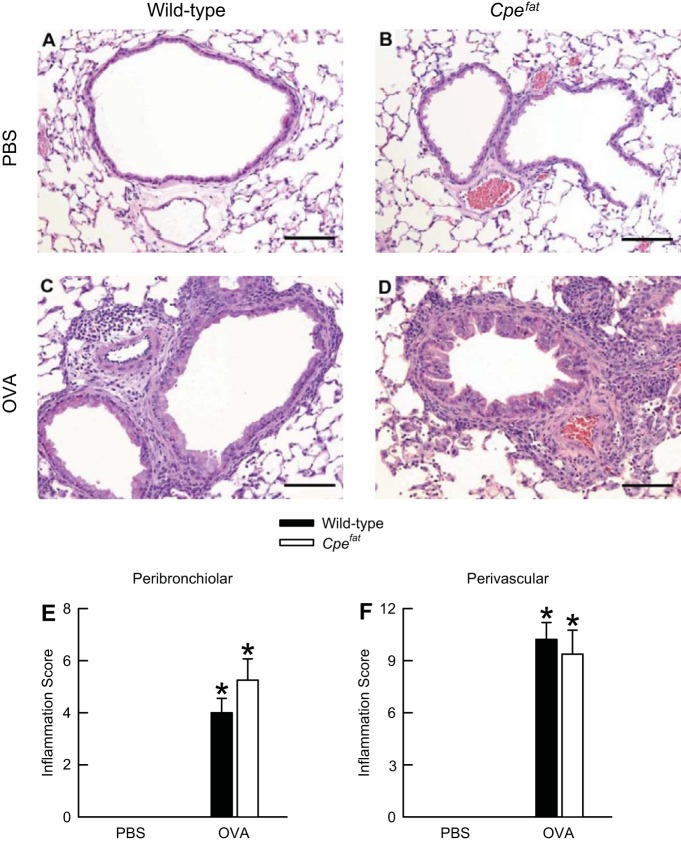
Because the number of inflammatory cells in the air spaces of Cpefat mice was significantly greater than those of wild-type mice following OVA sensitization and challenge (Fig. 2), we semiquantitatively determined the degree of peribronchiolar and perivascular inflammation in hematoxylin-and-eosin-stained sections that were prepared from formalin-fixed lungs. The lungs were obtained from OVA-sensitized wild-type and Cpefat mice 24 h following the cessation of the final PBS or OVA aerosol challenge. No inflammatory lesions were observed in PBS-challenged wild-type and Cpefat mice (Fig. 3, A, B, E and F). In both wild-type and Cpefat mice, OVA challenge elicited significant peribronchiolar and perivascular inflammation, which was primarily characterized by mononuclear inflammatory cells (macrophages and lymphocytes) with moderate to heavy infiltrates of eosinophils (Fig. 3, C and D). The inflammation was primarily directed toward blood vessels with collateral involvement of the airways (Fig. 3, E and F). We observed no differences in peribronchiolar and perivascular inflammation scores between wild-type and Cpefat mice following OVA challenge (Fig. 3, E and F).
Fig. 3.
A−D: representative light micrographs of hematoxylin-and-eosin-stained histological sections as well as peribronchiolar (E) and perivascular (F) inflammation scores from the lungs of OVA-sensitized C57BL/6 and Cpefat mice challenged with aerosols of either PBS or PBS containing 4% OVA (wt/vol) once per day for seven consecutive days. A and B: lung sections from PBS-challenged wild-type and Cpefat mice, respectively. C and D: lung sections from OVA-challenged wild-type and Cpefat mice, respectively. The lungs were fixed in situ with 10% phosphate-buffered formalin 24 h following the cessation of the final aerosol challenge. In A−D, the images have been magnified with a 20× objective lens, while each of the scale bars in A−D represents 100 μm. E and F: each value is expressed as the mean ± SE. n = 8−9 mice for each group. *P < 0.05 compared with genotype-matched mice challenged with PBS.
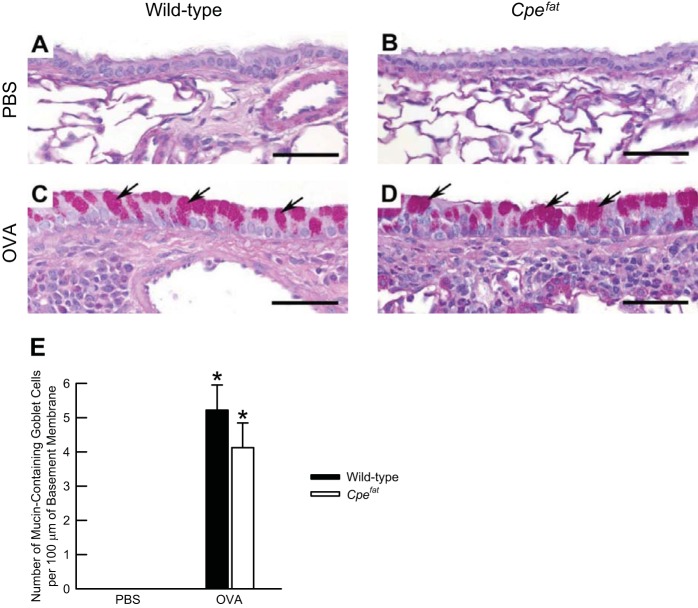
There were no PAS-positive, mucin-containing goblet cells present in the lung sections of OVA-sensitized and PBS-challenged wild-type and Cpefat mice (Fig. 4, A and B). Although OVA challenge significantly increased the number of PAS-positive, mucin-containing goblet cells in the lungs of wild-type and Cpefat mice, no genotype-related differences existed following OVA challenge (Fig. 4, C–E).
Fig. 4.
A−D: representative light micrographs of histological sections demonstrating the prevalence of the periodic-acid Schiff (PAS) reaction, as well as the number of mucin-containing goblet cells (E) in the lungs of OVA-sensitized C57BL/6 and Cpefat mice challenged with aerosols of either PBS or PBS containing 4% OVA (wt/vol) once per day for seven consecutive days. A and B: lung sections from PBS-challenged wild-type and Cpefat mice, respectively. C and D: lung sections from OVA-challenged wild-type and Cpefat mice, respectively. The arrows in C and D are directed at PAS-positive, mucin-containing goblet cells, which were found in the respiratory mucosa of secondary or tertiary bronchi. The lungs were fixed in situ with 10% phosphate-buffered formalin 24 h following the cessation of the final aerosol challenge. A−D: images have been magnified with a 40× objective lens, while each of the scale bars in A−D represent 50 μm. E: each value is expressed as the means ± SE. n = 8−9 mice for each group. *P < 0.05 compared with genotype-matched mice challenged with PBS.
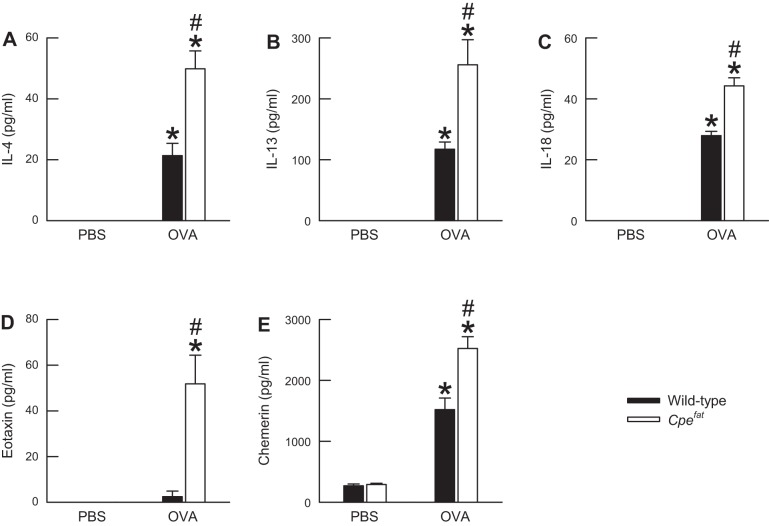
Effect of obesity and OVA sensitization and challenge on BALF cytokines.
Twenty-four hours following the cessation of the final PBS aerosol challenge, there were no differences in the levels of BALF IL-4, IL-13, IL-18, eotaxin, and chemerin between wild-type and Cpefat mice (Fig. 5). OVA challenge significantly increased the levels of BALF IL-4, IL-13, IL-18, and chemerin in both wild-type and Cpefat mice (Fig. 5). However, the levels of these cytokines were significantly greater in Cpefat compared with wild-type mice following OVA challenge. Eotaxin was significantly increased by OVA challenge in Cpefat but not wild-type mice (Fig. 5D). In addition, eotaxin levels were significantly higher in OVA-challenged Cpefat compared with OVA-challenged wild-type mice. Finally, we analyzed the BALF for IL-5. However, in both wild-type and Cpefat mice, IL-5 was below the detection limit of our assay regardless of the aerosol challenge (data not shown).
Fig. 5.
The concentration of IL-4 (A), IL-13 (B), IL-18 (C), eotaxin (D), and chemerin (E) in the BALF obtained from OVA-sensitized C57BL/6 and Cpefat mice challenged with aerosols of either PBS or PBS containing 4% OVA (wt/vol) once per day for seven consecutive days. BALF was obtained from the mice 24 h following the cessation of the final aerosol challenge. Each value is expressed as the mean ± SE. n = 4−11 mice for each group. *P < 0.05 compared with genotype-matched mice challenged with PBS. #P < 0.05 compared with C57BL/6 mice with an identical series of aerosol challenges.
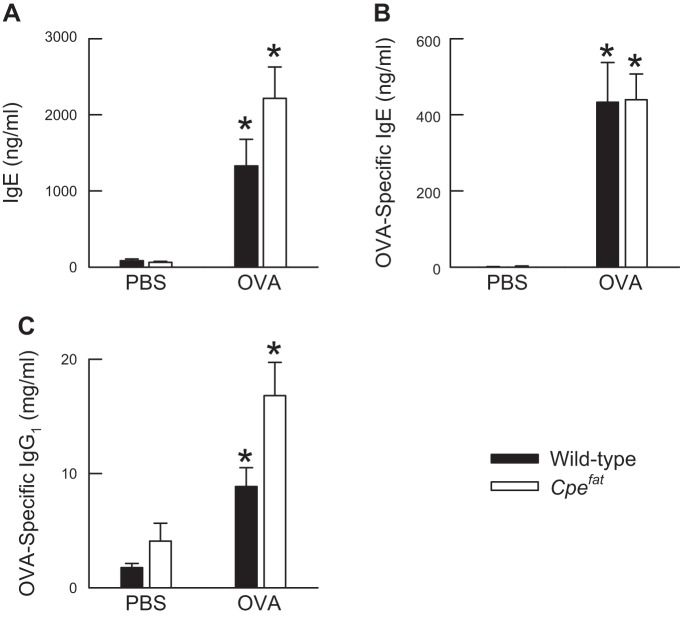
Effect of obesity and OVA sensitization and challenge on total IgE and OVA-specific immunoglobulins in the serum.
There were no genotype-related differences in the serum IgE levels of OVA-sensitized wild-type and Cpefat mice challenged with PBS (Fig. 6A). OVA challenge significantly increased IgE levels in the serum of both wild-type and Cpefat mice. However, no genotype-related differences in IgE existed following OVA challenge. Similar results were obtained for OVA-specific IgE and IgG1 (Fig. 6, B and C).
Fig. 6.
The concentration of immunoglobulin (Ig) E (A), OVA-specific IgE (B), and OVA-specific IgG1 (C) in serum isolated from the blood of OVA-sensitized, C57BL/6 and Cpefat mice challenged with aerosols of either PBS or PBS containing 4% OVA (wt/vol) once per day for seven consecutive days. Blood was obtained from the mice and serum isolated from the blood 24 h following the cessation of the final aerosol challenge. Each value is expressed as the mean ± SE. n = 8−13 mice for each group. *P < 0.05 compared with genotype-matched mice challenged with PBS.
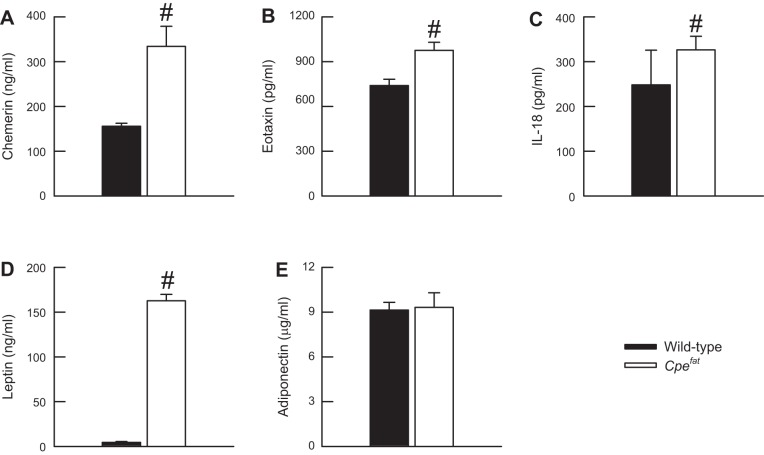
Effect of obesity on indices of chronic systemic inflammation.
A number of obesity-related sequelae, including asthma, cardiovascular disease, and Type 2 diabetes, have been mechanistically linked to obesity-induced, chronic systemic inflammation, which is characterized by elevated blood levels of cytokines, chemokines, hormones, and soluble cytokine receptors (7, 9, 10, 13, 37–39, 59, 73, 79, 84). The majority of these moieties, collectively termed adipokines, are proinflammatory in nature. Thus, to confirm previous observations of the existence of chronic systemic inflammation in Cpefat mice (37, 38, 79, 84), and perhaps provide a mechanistic basis for our current observations in Cpefat mice, we measured a number of indices of chronic systemic inflammation in the serum of OVA-sensitized and PBS-challenged wild-type and Cpefat mice (Fig. 7). Twenty-four hours following the cessation of the final PBS aerosol challenge, the serum levels of chemerin, eotaxin, IL-18, and leptin were significantly greater in Cpefat compared with wild-type mice. There were no genotype-related differences in serum adiponectin levels following PBS challenge (Fig. 7E).
Fig. 7.
The concentration of chemerin (A), eotaxin (B), IL-18 (C), leptin (D), and adiponectin (E) in the serum of OVA-sensitized C57BL/6 and Cpefat mice challenged with an aerosol of PBS once per day for seven consecutive days. Serum was isolated from the blood that was obtained from the mice 24 h following the cessation of the final PBS challenge. Each value is expressed as the mean ± SE. n = 5−9 mice for each group. #P < 0.05 compared with C57BL/6 mice.
DISCUSSION
In comparison to wild-type mice sensitized to and challenged with OVA, our data demonstrate that OVA-sensitized and challenged Cpefat mice exhibit greater airway obstruction and increased pulmonary inflammation. The greater airway obstruction observed in Cpefat mice following OVA sensitization and challenge (Fig. 1) is consistent with our previous observations in obese db/db and obese ob/ob mice (39). However, in contrast to db/db and ob/ob mice (39), Cpefat mice exhibit increased pulmonary inflammation in response to OVA sensitization and challenge (Figs. 2 and 5). Taken together, these results demonstrate that enhanced airway obstruction is consistently observed in genetically obese mice following OVA sensitization and challenge, whereas the development of pulmonary inflammation in obese mice in response to OVA sensitization and challenge is dependent upon the genetic modality of obesity induction.
Raw, which is a measure of airflow obstruction within the conducting airways (74), was significantly greater in OVA-sensitized Cpefat compared with OVA-sensitized wild-type mice following PBS challenge (Fig. 1A). A linear relationship exists between airway conductance, the inverse of Raw, and lung volume (83), which is reduced in obesity due to a decrease in lung and chest wall compliance from the mass-loading effects of obesity on the chest wall and abdomen (61). However, it is doubtful that a reduction in lung volume in Cpefat mice as a consequence of decreased chest wall compliance accounts for the observed increase in Raw, since all of our mice were studied with an open chest and a fixed-positive end-expiratory pressure. Following PBS challenge, we also observed no genotype-related differences in H (Fig. 1C), the inverse of compliance. Accordingly, differences in lung compliance between Cpefat and wild-type mice can also be excluded as a potential mechanism by which Raw is elevated in OVA-sensitized and PBS-challenged Cpefat mice. However, our data and those of other investigators suggest that chronic systemic inflammation, which is exhibited by OVA-sensitized and PBS-challenged Cpefat mice (Fig. 7 and Refs. 37, 38, 79, 84) contributes to the increase in Raw that we observed in these animals (Fig. 1A). For example, IL-17A and TNF-α, which are two proinflammatory adipokines elevated in the serum of Cpefat mice (79, 84), enhance airway smooth muscle contractility in response to bronchoconstrictors (1, 43). Thus, the increase in Raw that we observed in OVA-sensitized and PBS-challenged Cpefat mice may be a result of these adipokines enhancing the effects of endogenous ACh on basal airway smooth muscle contraction. Our observation that proinflammatory adipokines are elevated in the serum of OVA-sensitized and PBS-challenged Cpefat mice supports this hypothesis (Fig. 7).
OVA sensitization and challenge caused significant airway obstruction in wild-type and Cpefat mice (Fig. 1A). Furthermore, airway obstruction induced by OVA sensitization and challenge was exacerbated in Cpefat mice. OVA sensitization and challenge can enhance methacholine-induced airway obstruction through a number of diverse inflammatory moieties, including IL-4, IL-13, IL-18, major basic protein, and neutrophil elastase (23, 29, 41, 47, 81). Because the aforementioned cytokines, eosinophils (the source of major basic protein), and neutrophils (the source of neutrophil elastase) are increased in the lungs of OVA-sensitized and -challenged wild-type and Cpefat mice (Figs. 2 and 5), it is probable that the increased airway obstruction observed in OVA-sensitized and -challenged mice of both genotypes is mechanistically linked to the pulmonary inflammation seen in these animals. For example, the aforementioned inflammatory moieties may be acting directly on the airway smooth muscle or affecting neural innervation to potentiate the contractile effects of endogenous ACh on the airway smooth muscle. Because inflammatory moieties are increased in the BALF of Cpefat compared with wild-type mice following OVA sensitization and challenge, any effect of these moieties on airway obstruction is likely enhanced in Cpefat mice. Thus, increased pulmonary inflammation is a potential mechanism by which airway obstruction is exacerbated in OVA-challenged Cpefat mice. Taken together with our prior observations in db/db and ob/ob mice (39), our results demonstrate that OVA sensitization and challenge enhance airway obstruction in obese mice regardless of the modality of obesity induction.
As Fig. 1, B and C illustrates, OVA sensitization and challenge significantly increased G and H, which are indices of lung parenchymal resistance and elastance, respectively (31). Increases in G and H could result from enhanced closure of the small airways due to greater constriction of the conducting airways and/or surfactant dysfunction (77, 78). Similar to Raw, G and H were increased to a greater extent in Cpefat compared with wild-type mice following OVA sensitization and challenge. Such increases in G and H in Cpefat mice could be a result of greater constriction of the conducting airways, which is exhibited by these animals. Alternatively, OVA sensitization and challenge lead to surfactant dysfunction (82), which may be exacerbated in Cpefat mice due to greater levels of BALF IL-13, which inhibits surfactant function (85). Thus, obesity has deleterious effects on the oscillation mechanics of both the conducting airways and lung parenchyma following OVA sensitization and challenge.
Because airway obstruction is exacerbated in genetically obese mice following OVA sensitization and challenge, this suggests that a common mechanism may exist among Cpefat, db/db, and ob/ob mice to account for this phenomenon. However, as discussed in detail below, potential shared mechanisms such as increased pulmonary inflammation, decreased serum adiponectin levels, and increased serum IgE levels are unlikely. If a common mechanism does exist, our data and those from others suggest that it may be chronic systemic inflammation. First, increased pulmonary inflammation can be excluded as a common mechanism, as indices of pulmonary inflammation, such as IL-4, IL-13, eosinophils, and neutrophils, were either not different or were reduced in db/db and/or ob/ob compared with wild-type mice following OVA sensitization and challenge (39). Second, although we have previously reported that leptin can enhance methacholine-induced airway obstruction in OVA-challenged, wild-type mice (71), we can also exclude leptin as a common mechanism by which airway obstruction is enhanced in obese mice following OVA sensitization and challenge since leptin signaling is impaired in db/db and ob/ob mice (48). Third, compared with age- and sex-matched, wild-type mice, db/db and ob/ob mice have significantly lower circulating levels of adiponectin, an anti-inflammatory adipokine (18, 52). Shore et al. (72) have previously demonstrated that elevating serum levels of adiponectin by administering exogenous adiponectin reduces OVA-induced AHR and inflammation. However, it is very doubtful that that the enhanced airway obstruction in obese mice following OVA sensitization and challenge is due to a decrease in serum adiponectin since there were no differences in serum adiponectin between wild-type and Cpefat mice (Fig. 7E). Fourth, the role of IgE in the development of OVA-induced airway obstruction and pulmonary inflammation in mice is controversial (17, 29, 45, 55). However, there are studies that demonstrate that IgE is important to the development of OVA-induced AHR (17, 45). We previously reported that the serum levels of IgE are elevated in OVA-sensitized and -challenged ob/ob mice (39). However, it is doubtful that IgE is the common mechanism that enhances airway obstruction in obese mice following OVA sensitization and challenge, since we observed no differences in total or OVA-specific IgE between Cpefat and wild-type mice following OVA challenge (Fig. 6). Finally, if a common mechanism does account for the enhanced airway obstruction observed in genetically obese mice following OVA sensitization and challenge, then chronic systemic inflammation is a plausible consideration. Cpefat, db/db, and ob/ob mice develop chronic systemic inflammation (Fig. 7 and Refs. 37–39, 52, 79, 84), and Sideleva and colleagues (73) have previously reported that obesity-induced asthma is more strongly associated with systemic and adipose tissue inflammation, as opposed to pulmonary inflammation. Dixon et al. (20) demonstrated that obese asthmatics who lose weight via bariatric surgery become less reactive to methacholine despite the fact that pulmonary inflammation actually increases. Taken together, these data demonstrate that increased pulmonary inflammation does not account for the increased airway responsiveness to methacholine observed in obese human asthmatic subjects. Consequently, despite the fact that Cpefat mice exhibit increased pulmonary inflammation, the primary mechanism for enhanced airway obstruction in these animals may be systemic in nature.
The marked differences in the development of pulmonary inflammation among Cpefat, db/db, and ob/ob mice in response to OVA sensitization and challenge are intriguing, given that we previously reported that pulmonary inflammation is enhanced in Cpefat, db/db, and ob/ob mice following exposure to ozone, a common environmental pollutant (37, 38, 52, 70). The mechanism underlying the divergent inflammatory responses to OVA sensitization and challenge among these obese mice is not known. However, we have considered two potential possibilities for this observation. First, in contrast to db/db and ob/ob mice, leptin signaling is intact in Cpefat mice (48). Furthermore, leptin is elevated in the serum of Cpefat mice, and leptin is a proinflammatory cytokine (Fig. 7 and Refs. 37, 38, 70, 84). We previously reported that exogenous leptin administration had no effect on OVA-induced pulmonary inflammation in wild-type mice following OVA sensitization and challenge (71) Furthermore, mice with diet-induced obesity, which also have elevated leptin (36), have reduced BALF eosinophils following OVA sensitization and challenge (12). Taken together, it is doubtful that leptin enhances OVA-induced pulmonary inflammation in Cpefat mice. Secondly, because of a missense mutation in the gene encoding carboxypeptidase E, Cpefat mice cannot process a number of prohormones and proneuropeptides into biologically active peptides (14, 48, 60). For example, proopiomelanocortin and procholecystokinin are processed by carboxypeptidase E into their biologically active forms: α-melanocyte-stimulating hormone (α-MSH) and CCK. Both α-MSH and CCK have anti-inflammatory effects and are reduced in mice with impaired carboxypeptidase E activity (6, 11, 15, 21, 56, 65). Of particular interest to this study, α-MSH has been shown to decrease antigen-induced skin inflammation (6, 21). Furthermore, proopiomelanocortin can be processed by carboxypeptidase E into adrenocorticotropin (14), which is also reduced in Cpefat mice (67). Adrenocorticotropin stimulates the release of corticosteroids, which have been previously shown to reduce OVA-induced pulmonary inflammation (22). Interestingly, corticosterone levels are elevated in db/db and ob/ob mice (48), which exhibit decreased OVA-induced pulmonary inflammation, whereas corticosterone levels are not elevated in Cpefat mice (49), which exhibit enhanced pulmonary inflammation in response to OVA sensitization and challenge. Taken together, the inability of Cpefat mice to produce sufficient quantities of endogenous anti-inflammatory mediators due to improper processing of their precursors would be expected to exacerbate systemic and pulmonary inflammation.
In humans, obesity appears to be a stronger risk factor for nonatopic compared with atopic asthma (16), yet the development of obesity appears to worsen preexisting atopic asthma (20). Given that the degree of pulmonary inflammation among obese individuals with atopic asthma varies substantially, even though all exhibit airway obstruction (19, 20, 42, 73, 76), suggests that different obese mice may be necessary to model the different phenotypes observed among the various human subpopulations of atopic, obese asthmatics. To that end, we propose that db/db and ob/ob mice are better suited to study mechanisms underlying airway obstruction in atopic, obese asthmatics with minimal eosinophilic pulmonary inflammation, whereas Cpefat mice are more suited to study the mechanisms of airway obstruction in atopic, obese asthmatics with substantial pulmonary inflammation, such as those described in the study by Desai et al. (19).
Perspectives and Significance
In conclusion, our results demonstrate that OVA sensitization and challenge enhances airway obstruction in obese mice regardless of the genetic basis of obesity, whereas the development of OVA-induced pulmonary inflammation is dependent upon the genetic modality of obesity induction. Given that pulmonary inflammation in response to OVA sensitization and challenge develops according to the genetic modality of obesity induction, future studies are warranted to investigate the molecular basis of this phenomenon. However, the results of this study have important implications for animal models of asthma, as modeling the pulmonary phenotypes for subpopulations of atopic, obese asthmatics critically depends on selecting the appropriate mouse model. Therefore, obese mice will be useful tools to test the preclinical efficacy of medications aimed at preventing or alleviating airway obstruction in various subpopulations of atopic, obese asthmatics with variable degrees of pulmonary inflammation.
GRANTS
The research reported in this publication was supported, in part, by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) under award number R03AI107432 and the National Institute of Environmental Health Sciences of the NIH under award number R03ES022378. Furthermore, the content contained within this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.H.D., H.K.-Q., R.E.P., F.M., C.Y.S., R.X.B., and R.A.J. performed experiments; P.H.D., K.R.C., S.S., R.E.P., S.S.H., M.R.B., I.U.H., and R.A.J. analyzed data; P.H.D., J.B.R., K.R.C., S.S., R.E.P., S.S.H., M.R.B., I.U.H., and R.A.J. interpreted results of experiments; P.H.D., R.E.P., and R.A.J. prepared figures; P.H.D., J.B.R., I.U.H., and R.A.J. drafted manuscript; P.H.D., J.B.R., H.K.-Q., K.R.C., S.S., R.E.P., F.M., C.Y.S., R.X.B., S.S.H., M.R.B., I.U.H., and R.A.J. edited and revised manuscript; P.H.D., J.B.R., H.K.-Q., K.R.C., S.S., R.E.P., F.M., C.Y.S., R.X.B., S.S.H., M.R.B., I.U.H., and R.A.J. approved final version of manuscript; J.B.R., S.S., M.R.B., I.U.H., and R.A.J. conception and design of research.
REFERENCES
- 1.Amrani Y, Martinet N, Bronner C. Potentiation by tumour necrosis factor-α of calcium signals induced by bradykinin and carbachol in human tracheal smooth muscle cells. Br J Pharmacol 114: 4–5, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barreno RX, Richards JB, Schneider DJ, Cromar KR, Nadas AJ, Hernandez CB, Hallberg LM, Price RE, Hashmi SS, Blackburn MR, Haque IU, Johnston RA. Endogenous osteopontin promotes ozone-induced neutrophil recruitment to the lungs and airway hyperresponsiveness to methacholine. Am J Physiol Lung Cell Mol Physiol 305: L118–L129, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barros LL, Souza-Machado A, Correa LB, Santos JS, Cruz C, Leite M, Castro L, Coelho AC, Almeida P, Cruz AA. Obesity and poor asthma control in patients with severe asthma. J Asthma 48: 171–176, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Bates JH, Shardonofsky F, Stewart DE. The low-frequency dependence of respiratory system resistance and elastance in normal dogs. Respir Physiol 78: 369–382, 1989 [DOI] [PubMed] [Google Scholar]
- 5.Belke DD, Severson DL. Diabetes in mice with monogenic obesity: The db/db mouse and its use in the study of cardiac consequences. Methods Mol Biol 933: 47–57, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Bohm M, Apel M, Sugawara K, Brehler R, Jurk K, Luger TA, Haas H, Paus R, Eiz-Vesper B, Walls AF, Ponimaskin E, Gehring M, Kapp A, Raap U. Modulation of basophil activity: A novel function of the neuropeptide α-melanocyte-stimulating hormone. J Allergy Clin Immunol 129: 1085–1093, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394: 894–897, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Brumpton B, Langhammer A, Romundstad P, Chen Y, Mai XM. General and abdominal obesity and incident asthma in adults: the HUNT study. Eur Respir J 41: 323–329, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Bruun JM, Stallknecht B, Helge JW, Richelsen B. Interleukin-18 in plasma and adipose tissue: effects of obesity, insulin resistance, and weight loss. Eur J Endocrinol 157: 465–471, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Bruun JM, Verdich C, Toubro S, Astrup A, Richelsen B. Association between measures of insulin sensitivity and circulating levels of interleukin-8, interleukin-6 and tumor necrosis factor-alpha. Effect of weight loss in obese men. Eur J Endocrinol 148: 535–542, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Cain BM, Wang W, Beinfeld MC. Cholecystokinin (CCK) levels are greatly reduced in the brains but not the duodenums of Cpefat/Cpefat mice: a regional difference in the involvement of carboxypeptidase E (Cpe) in pro-CCK processing. Endocrinology 138: 4034–4037, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Calixto MC, Lintomen L, Andre DM, Leiria LO, Ferreira D, Lellis-Santos C, Anhe GF, Bordin S, Landgraf RG, Antunes E. Metformin attenuates the exacerbation of the allergic eosinophilic inflammation in high fat-diet-induced obesity in mice. PLoS One 8: e76786, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catalan V, Gomez-Ambrosi J, Rodriguez A, Ramirez B, Rotellar F, Valenti V, Silva C, Gil MJ, Salvador J, Fruhbeck G. Increased levels of chemerin and its receptor, chemokine-like receptor-1, in obesity are related to inflammation: tumor necrosis factor-alpha stimulates mRNA levels of chemerin in visceral adipocytes from obese patients. Surg Obes Relat Dis 9: 306–314, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Cawley NX, Wetsel WC, Murthy SR, Park JJ, Pacak K, Loh YP. New roles of carboxypeptidase E in endocrine and neural function and cancer. Endocr Rev 33: 216–253, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cawley NX, Yanik T, Woronowicz A, Chang W, Marini JC, Loh YP. Obese carboxypeptidase E knockout mice exhibit multiple defects in peptide hormone processing contributing to low bone mineral density. Am J Physiol Endocrinol Metab 299: E189–E197, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Rennie D, Cormier Y, Dosman J. Atopy, obesity, and asthma in adults: the Humboldt study. J Agromedicine 14: 222–227, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Coyle AJ, Wagner K, Bertrand C, Tsuyuki S, Bews J, Heusser C. Central role of immunoglobulin (Ig) E in the induction of lung eosinophil infiltration and T helper 2 cell cytokine production: Inhibition by a non-anaphylactogenic anti-IgE antibody. J Exp Med 183: 1303–1310, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delporte ML, El Mkadem SA, Quisquater M, Brichard SM. Leptin treatment markedly increased plasma adiponectin but barely decreased plasma resistin of ob/ob mice. Am J Physiol Endocrinol Metab 287: E446–E453, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Desai D, Newby C, Symon FA, Haldar P, Shah S, Gupta S, Bafadhel M, Singapuri A, Siddiqui S, Woods J, Herath A, Anderson IK, Bradding P, Green R, Kulkarni N, Pavord I, Marshall RP, Sousa AR, May RD, Wardlaw AJ, Brightling CE. Elevated sputum interleukin-5 and submucosal eosinophilia in obese individuals with severe asthma. Am J Respir Crit Care Med 188: 657–663, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, Garudathri J, Raymond D, Poynter ME, Bunn JY, Irvin CG. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol 128: 508–515, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Etori M, Yonekubo K, Sato E, Mizukami K, Hirahara K, Karasuyama H, Maeda H, Yamashita M. Melanocortin receptors 1 and 5 might mediate inhibitory effects of α-melanocyte-stimulating hormone on antigen-induced chronic allergic skin inflammation in IgE transgenic mice. J Invest Dermatol 132: 1925–1927, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Eum SY, Maghni K, Hamid Q, Eidelman DH, Campbell H, Isogai S, Martin JG. Inhibition of allergic airways inflammation and airway hyperresponsiveness in mice by dexamethasone: Role of eosinophils, IL-5, eotaxin, and IL-13. J Allergy Clin Immunol 111: 1049–1061, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Eum SY, Maghni K, Tolloczko B, Eidelman DH, Martin JG. IL-13 may mediate allergen-induced hyperresponsiveness independently of IL-5 or eotaxin by effects on airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 288: L576–L584, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med 161: 1581–1586, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Fredrickson TN, Harris AW. Atlas of Mouse Hematopathology. Singapore: Harwood Academic Publishers, 2000 [Google Scholar]
- 26.Garthwaite TL, Martinson DR, Tseng LF, Hagen TC, Menahan LA. A longitudinal hormonal profile of the genetically obese mouse. Endocrinology 107: 671–676, 1980 [DOI] [PubMed] [Google Scholar]
- 27.Griesenbach U, Munkonge FM, Sumner-Jones S, Holder E, Smith SN, Boyd AC, Gill DR, Hyde SC, Porteous D, Alton EW, UK Cystic Fibrosis Gene Therapy Consortium. Assessment of CFTR function after gene transfer in vitro and in vivo. Methods Mol Biol 433: 229–242, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Hamada K, Suzaki Y, Goldman A, Ning YY, Goldsmith C, Palecanda A, Coull B, Hubeau C, Kobzik L. Allergen-independent maternal transmission of asthma susceptibility. J Immunol 170: 1683–1689, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Hamelmann E, Takeda K, Haczku A, Cieslewicz G, Shultz L, Hamid Q, Xing Z, Gauldie J, Gelfand EW. Interleukin (IL)-5 but not immunoglobulin E reconstitutes airway inflammation and airway hyperresponsiveness in IL-4-deficient mice. Am J Respir Cell Mol Biol 23: 327–334, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 72: 168–178, 1992 [DOI] [PubMed] [Google Scholar]
- 31.Hartney JM, Robichaud A. Assessment of airway hyperresponsiveness in mouse models of allergic lung disease using detailed measurements of respiratory mechanics. Methods Mol Biol 1032: 205–217, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Hirai T, McKeown KA, Gomes RF, Bates JH. Effects of lung volume on lung and chest wall mechanics in rats. J Appl Physiol 86: 16–21, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, Fitzpatrick AM, Gaston B, Israel E, Jarjour NN, Moore WC, Peters SP, Yonas M, Teague WG, Wenzel SE. Obesity and asthma: An association modified by age of asthma onset. J Allergy Clin Immunol 127: 1486–1493, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holguin F, Comhair SA, Hazen SL, Powers RW, Khatri SS, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Fitzpatrick AM, Gaston B, Israel E, Jarjour NN, Moore WC, Peters SP, Teague WG, Chung KF, Erzurum SC, Wenzel SE. An association between l-arginine/asymmetric dimethyl arginine balance, obesity, and the age of asthma onset phenotype. Am J Respir Crit Care Med 187: 153–159, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.JAX Research Systems. New Cpe fat mouse model for diabetes and obesity research. In: JAX Notes. Bar Harbor, ME: The Jackson Laboratory, 2002 [Google Scholar]
- 36.Johnston RA, Theman TA, Lu FL, Terry RD, Williams ES, Shore SA. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J Appl Physiol 104: 1727–1735, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Johnston RA, Theman TA, Shore SA. Augmented responses to ozone in obese carboxypeptidase E-deficient mice. Am J Physiol Regul Integr Comp Physiol 290: R126–R133, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Johnston RA, Zhu M, Hernandez CB, Williams ES, Shore SA. Onset of obesity in carboxypeptidase E-deficient mice and effect on airway responsiveness and pulmonary responses to ozone. J Appl Physiol 108: 1812–1819, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston RA, Zhu M, Rivera-Sanchez YM, Lu FL, Theman TA, Flynt L, Shore SA. Allergic airway responses in obese mice. Am J Respir Crit Care Med 176: 650–658, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juel CT, Ulrik CS. Obesity and asthma: Impact on severity, asthma control, and response to therapy. Respir Care 58: 867–873, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Koga H, Miyahara N, Fuchimoto Y, Ikeda G, Waseda K, Ono K, Tanimoto Y, Kataoka M, Gelfand EW, Tanimoto M, Kanehiro A. Inhibition of neutrophil elastase attenuates airway hyperresponsiveness and inflammation in a mouse model of secondary allergen challenge: neutrophil elastase inhibition attenuates allergic airway responses. Respir Res 14: 8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Komakula S, Khatri S, Mermis J, Savill S, Haque S, Rojas M, Brown L, Teague GW, Holguin F. Body mass index is associated with reduced exhaled nitric oxide and higher exhaled 8-isoprostanes in asthmatics. Respir Res 8: 32, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, Wang Y, Bernstein X, Li JT, Atabai K, Huang X, Sheppard D. IL-17A produced by αβ T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med 18: 547–554, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar RK, Herbert C, Foster PS. The “classical” ovalbumin challenge model of asthma in mice. Curr Drug Targets 9: 485–494, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Lack G, Oshiba A, Bradley KL, Loader JE, Amran D, Larsen GL, Gelfand EW. Transfer of immediate hypersensitivity and airway hyperresponsiveness by IgE-positive B cells. Am J Respir Crit Care Med 152: 1765–1773, 1995 [DOI] [PubMed] [Google Scholar]
- 46.Lang JE, Hossain J, Smith K, Lima JJ. Asthma severity, exacerbation risk, and controller treatment burden in underweight and obese children. J Asthma 49: 456–463, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Lefort J, Nahori MA, Ruffie C, Vargaftig BB, Pretolani M. In vivo neutralization of eosinophil-derived major basic protein inhibits antigen-induced bronchial hyperreactivity in sensitized guinea pigs. J Clin Invest 97: 1117–1121, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leibel RL, Chung WK, Chua SC., Jr The molecular genetics of rodent single gene obesities. J Biol Chem 272: 31937–31940, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Leiter EH, Kintner J, Flurkey K, Beamer WG, Naggert JK. Physiologic and endocrinologic characterization of male sex-biased diabetes in C57BLKS/J mice congenic for the fat mutation at the carboxypeptidease E locus. Endocrine 10: 57–66, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Leone N, Courbon D, Berr C, Barberger-Gateau P, Tzourio C, Alperovitch A, Zureik M. Abdominal obesity and late-onset asthma: cross-sectional and longitudinal results: the 3C study. Obesity (Silver Spring) 20: 628–635, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Li P, Tiwari HK, Lin WY, Allison DB, Chung WK, Leibel RL, Yi N, Liu N. Genetic association analysis of 30 genes related to obesity in a European American population. Int J Obes 38: 724–729, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu FL, Johnston RA, Flynt L, Theman TA, Terry RD, Schwartzman IN, Lee A, Shore SA. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am J Physiol Lung Cell Mol Physiol 290: L856–L865, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Marchesini G, Moscatiello S, Di Domizio S, Forlani G. Obesity-associated liver disease. J Clin Endocrinol Metab 93: S74–S80, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Martins IJ, Tran JM, Redgrave TG. Food restriction normalizes chylomicron remnant metabolism in murine models of obesity as assessed by a novel stable isotope breath test. J Nutr 132: 176–181, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Mehlhop PD, van de Rijn M, Goldberg AB, Brewer JP, Kurup VP, Martin TR, Oettgen HC. Allergen-induced bronchial hyperreactivity and eosinophilic inflammation occur in the absence of IgE in a mouse model of asthma. Proc Natl Acad Sci USA 94: 1344–1349, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miyamoto S, Shikata K, Miyasaka K, Okada S, Sasaki M, Kodera R, Hirota D, Kajitani N, Takatsuka T, Kataoka HU, Nishishita S, Sato C, Funakoshi A, Nishimori H, Uchida HA, Ogawa D, Makino H. Cholecystokinin plays a novel protective role in diabetic kidney through anti-inflammatory actions on macrophage: Anti-inflammatory effect of cholecystokinin. Diabetes 61: 897–907, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D'Agostino R, Jr, Castro M, Curran-Everett D, Fitzpatrick AM, Gaston B, Jarjour NN, Sorkness R, Calhoun WJ, Chung KF, Comhair SA, Dweik RA, Israel E, Peters SP, Busse WW, Erzurum SC, Bleecker ER, National Heart, Lung, and Blood Institute's Severe Asthma Research Program. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med 181: 315–323, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morimoto LM, White E, Chen Z, Chlebowski RT, Hays J, Kuller L, Lopez AM, Manson J, Margolis KL, Muti PC, Stefanick ML, McTiernan A. Obesity, body size, and risk of postmenopausal breast cancer: the Women's Health Initiative (United States). Cancer Causes Control 13: 741–751, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Musaad S, Haynes EN. Biomarkers of obesity and subsequent cardiovascular events. Epidemiol Rev 29: 98–114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naggert JK, Fricker LD, Varlamov O, Nishina PM, Rouille Y, Steiner DF, Carroll RJ, Paigen BJ, Leiter EH. Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat Genet 10: 135–142, 1995 [DOI] [PubMed] [Google Scholar]
- 61.O'Donnell DE, O'Donnell CD, Webb KA, Guenette JA. Respiratory consequences of mild-to-moderate obesity: Impact on exercise performance in health and in chronic obstructive pulmonary disease. Pulm Med 2012: 818925, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief 1–8, 2012 [PubMed] [Google Scholar]
- 63.Pillow JJ, Korfhagen TR, Ikegami M, Sly PD. Overexpression of TGF-α increases lung tissue hysteresivity in transgenic mice. J Appl Physiol 91: 2730–2734, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Rivera-Sanchez YM, Johnston RA, Schwartzman IN, Valone J, Silverman ES, Fredberg JJ, Shore SA. Differential effects of ozone on airway and tissue mechanics in obese mice. J Appl Physiol 96: 2200–2206, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Saia RS, Bertozi G, Mestriner FL, Antunes-Rodrigues J, Queiroz Cunha F, Carnio EC. Cardiovascular and inflammatory response to cholecystokinin during endotoxemic shock. Shock 39: 104–113, 2013 [DOI] [PubMed] [Google Scholar]
- 66.Shao J, Yamashita H, Qiao L, Friedman JE. Decreased Akt kinase activity and insulin resistance in C57BL/KsJ-Leprdb/db mice. J Endocrinol 167: 107–115, 2000 [DOI] [PubMed] [Google Scholar]
- 67.Shen FS, Loh YP. Intracellular misrouting and abnormal secretion of adrenocorticotropin and growth hormone in Cpefat mice associated with a carboxypeptidase E mutation. Proc Natl Acad Sci USA 94: 5314–5319, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shore SA, Johnston RA. Obesity and asthma. Pharmacol Ther 110: 83–102, 2006 [DOI] [PubMed] [Google Scholar]
- 69.Shore SA, Lang JE, Kasahara DI, Lu FL, Verbout NG, Si H, Williams ES, Terry RD, Lee A, Johnston RA. Pulmonary responses to subacute ozone exposure in obese vs. lean mice. J Appl Physiol 107: 1445–1452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shore SA, Rivera-Sanchez YM, Schwartzman IN, Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol 95: 938–945, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol 115: 103–109, 2005 [DOI] [PubMed] [Google Scholar]
- 72.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol 118: 389–395, 2006 [DOI] [PubMed] [Google Scholar]
- 73.Sideleva O, Suratt BT, Black KE, Tharp WG, Pratley RE, Forgione P, Dienz O, Irvin CG, Dixon AE. Obesity and asthma: An inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med 186: 598–605, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tomioka S, Bates JH, Irvin CG. Airway and tissue mechanics in a murine model of asthma: alveolar capsule vs. forced oscillations. J Appl Physiol 93: 263–270, 2002 [DOI] [PubMed] [Google Scholar]
- 75.Tomita T, Doull V, Pollock HG, Krizsan D. Pancreatic islets of obese hyperglycemic mice (ob/ob). Pancreas 7: 367–375, 1992 [DOI] [PubMed] [Google Scholar]
- 76.van Veen IH, Ten Brinke A, Sterk PJ, Rabe KF, Bel EH. Airway inflammation in obese and nonobese patients with difficult-to-treat asthma. Allergy 63: 570–574, 2008 [DOI] [PubMed] [Google Scholar]
- 77.Wagers S, Lundblad LK, Ekman M, Irvin CG, Bates JH. The allergic mouse model of asthma: normal smooth muscle in an abnormal lung? J Appl Physiol 96: 2019–2027, 2004 [DOI] [PubMed] [Google Scholar]
- 78.Wagers SS, Haverkamp HC, Bates JH, Norton RJ, Thompson-Figueroa JA, Sullivan MJ, Irvin CG. Intrinsic and antigen-induced airway hyperresponsiveness are the result of diverse physiological mechanisms. J Appl Physiol 102: 221–230, 2007 [DOI] [PubMed] [Google Scholar]
- 79.Williams AS, Chen L, Kasahara DI, Si H, Wurmbrand AP, Shore SA. Obesity and airway responsiveness: Role of TNFR2. Pulm Pharmacol Ther 26: 444–454, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.World Health Organization. World Health Statistics 2013. Geneva, Switzerland: WHO Press, 2013 [Google Scholar]
- 81.Yamagata S, Tomita K, Sato R, Niwa A, Higashino H, Tohda Y. Interleukin-18-deficient mice exhibit diminished chronic inflammation and airway remodelling in ovalbumin-induced asthma model. Clin Exp Immunol 154: 295–304, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu ZW, Zhang JH. Effect of inhaled budesonide on surfactant protein expression in asthmatic mice. Allergy Asthma Proc 29: 486–492, 2008 [DOI] [PubMed] [Google Scholar]
- 83.Zerah F, Harf A, Perlemuter L, Lorino H, Lorino AM, Atlan G. Effects of obesity on respiratory resistance. Chest 103: 1470–1476, 1993 [DOI] [PubMed] [Google Scholar]
- 84.Zhu M, Williams AS, Chen L, Wurmbrand AP, Williams ES, Shore SA. Role of TNFR1 in the innate airway hyperresponsiveness of obese mice. J Appl Physiol 113: 1476–1485, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu Z, Enhorning G, Zheng T, Chen Q, Chen NY, Homer R, Elias JA. Interleukin-13 induces surfactant function abnormality in the murine lung. Chest 123: 375S-376S, 2003 [PubMed] [Google Scholar]