Abstract
The gut-brain axis plays a key role in the control of energy balance and glucose homeostasis. In response to luminal stimulation of macronutrients and microbiota-derived metabolites (secondary bile acids and short chain fatty acids), glucagon-like peptides (GLP-1 and -2) are cosecreted from endocrine L cells in the gut and coreleased from preproglucagonergic neurons in the brain stem. Glucagon-like peptides are proposed as key mediators for bariatric surgery-improved glycemic control and energy balance. Little is known about the GLP-2 receptor (Glp2r)-mediated physiological roles in the control of food intake and glucose homeostasis, yet Glp1r has been studied extensively. This review will highlight the physiological relevance of the central nervous system (CNS) Glp2r in the control of energy balance and glucose homeostasis and focuses on cellular mechanisms underlying the CNS Glp2r-mediated neural circuitry and intracellular PI3K signaling pathway. New evidence (obtained from Glp2r tissue-specific KO mice) indicates that the Glp2r in POMC neurons is essential for suppressing feeding behavior, gastrointestinal motility, and hepatic glucose production. Mice with Glp2r deletion selectively in POMC neurons exhibit hyperphagic behavior, accelerated gastric emptying, glucose intolerance, and hepatic insulin resistance. GLP-2 differentially modulates postsynaptic membrane excitability of hypothalamic POMC neurons in Glp2r- and PI3K-dependent manners. GLP-2 activates the PI3K-Akt-FoxO1 signaling pathway in POMC neurons by Glp2r-p85α interaction. Intracerebroventricular GLP-2 augments glucose tolerance, suppresses glucose production, and enhances insulin sensitivity, which require PI3K (p110α) activation in POMC neurons. Thus, the CNS Glp2r plays a physiological role in the control of food intake and glucose homeostasis. This review will also discuss key questions for future studies.
Keywords: glucagon-like peptides, glucose homeostasis, food intake, gastric emptying, gut-brain axis, insulin sensitivity, central nervous system
among the greatest threats to public health are increasing rates of obesity and diabetes. Obesity promotes the development of more than 50 chronic disorders and costs ∼9% of all U.S. health care expenditures (22). Thus, it is imperative to identify novel therapeutic targets and develop effective approaches to prevent and treat obesity and diabetes. Bariatric surgery (particularly, Roux-en-Y gastric bypass, RYGB) has proven to be the most effective therapy for morbid obesity and diabetes (19, 26, 111), in which the gut-brain axis is highly involved in mediating beneficial effects on food intake, glycemic control, and weight loss.
The gut-brain axis (i.e., the bidirectional communication between the gut and the brain) plays key roles in the regulation of energy and glucose homeostasis. Gut-derived signals (for nutritional, neural, immune, and endocrine information) are input from the gut to the brain; meanwhile, autonomic and neuroendocrine messengers are output from the brain to the gut. The hypothalamus, a primary region of central integration for homeostatic signals, plays crucial roles in the regulation of energy balance and glucose homeostasis (7, 104, 114). In the arcuate nucleus (ARC) of the hypothalamus, neurons expressing proopiomelanocortin (POMC), a key component of the central melanocortin system, are positioned to integrate hormonal/nutritional signals reflecting energy availability (25). Activation of POMC neurons enhances energy expenditure and suppresses food intake, yet neuroendocrine mechanisms are not fully established. The gastrointestinal (GI) tract plays a critical role in digestion, absorption, and utilization of nutrients (32), thus, controlling energy intake at the first pass. Through the gut-brain axis, gut hormones play a crucial role in the regulation of energy balance (including food intake) and glucose homeostasis (27, 50). Recently, it has been shown that infusion of glucose or lipid into the rat jejunum improves glucose homeostasis in uncontrolled diabetes, suggesting that nutrient sensing by the gut-brain axis may play a key role in the control of glucose production (14, 31), yet little is known about the nutrient sensor identity and intracellular signaling network.
In response to luminal stimulation of macronutrients and microbiota-derived metabolites (secondary bile acids and short-chain fatty acids), glucagon-like peptides (GLP-1 and GLP-2) are cosecreted from endocrine L cells in the gut (59, 100, 102, 106, 108, 123, 124) and are presumably coreleased from preproglucagonergic (PPG) neurons in the brain stem as well. GLP-1 and GLP-2 are key neuroendocrine signals for the gut-brain axis to control food intake and glucose homeostasis (92) and have been proposed as important mediators for bariatric surgery-improved glycemic control and insulin sensitivity (37, 47, 73, 74, 99, 121). In fact, GLP-1 and -2 receptor agonists are used clinically for the prevention and treatment of diabetes and digestive diseases (92). By means of neural and endocrine pathways, gut-derived GLP-1 and -2 signals are transduced to the brain to coordinate food intake and glucose homeostasis (14, 130), although their physiological roles are largely unknown. Thus, elucidation of GLP-1 and -2 intracellular signaling and metabolic action in the control of energy balance may reveal novel targets for the treatment of intestinal dysfunctions, obesity, and diabetes (92).
Through acting on a specific G protein-coupled receptor (Glp2r), GLP-2 promotes intestinal epithelial homeostasis and function, enhances intestinal nutrient absorption and blood flow, assists gut immune defense, and improves outcomes of short bowel syndrome (9, 47, 57). Except for its enterotrophic role, GLP-2-mediated metabolic action has not been fully recognized. Without the incretin effect, GLP-2 was considered insignificant in glucose homeostasis. Recent studies reveal that central infusion of GLP-2 suppresses food intake and increases glucose tolerance (118, 121, 122). Importantly, GLP-2 has been proposed as a neurotransmitter in the control of feeding behavior (121, 122) and may mediate PPG neuron-induced synaptic transmission linking the hypothalamus and the brain stem (121). The GLP-2 receptor (Glp2r) is not only expressed in nutrient-sensing endocrine cells (such as enteroendocrine cells and pancreatic α-cells), but also neurons (such as enteric neurons, vagal sensory neurons, and central neurons) (30, 45, 91, 95, 131, 138), suggesting complex modes for GLP-2 action via endocrine, paracrine, or neuroendocrine mechanisms. Thus, GLP-2 may act as a crucial neuroendocrine signal (for temporary energy availability) to the hypothalamus in the control of feeding behavior (food intake) and glucose homeostasis. It seems that bariatric surgery-improved glucose tolerance is independent of Glp1r activation in rodents (85, 133, 136). Notably, bariatric surgery not only improves glucose tolerance, but also restores insulin sensitivity. Glp2r/Glp1r (two G protein-coupled receptors) play distinct roles, yet GLP-1 and GLP-2 are cosecreted. Glp2r and Glp1r in postprandial glycemic control have not been determined. Thus, it is still crucial to determine whether Glp2r is a key contributor to improving glycemic control and insulin sensitivity after bariatric surgery.
To decode the GLP-2 physiological role and signaling pathway, my laboratory has generated a Glp2r floxed mouse line that has provided a powerful, genetic tool to dissect Glp2r metabolic and mitogenic actions in a tissue (or cell)-specific manner. Using Glp2r tissue-specific knockout mouse models, Guan et al. show that Glp2r is a key satiety signal for the physiological control of food intake and gastric emptying, and this may contribute to the homeostatic control of energy balance and body weight (46). Moreover, Glp2r in hypothalamic POMC neurons is required for promoting hepatic insulin sensitivity and glycemic control (118). Through the gut-brain-stomach (or -liver) axis, GLP-2 plays key physiological roles in the control of gastric emptying and hepatic glucose production (HGP) (46, 118). In this review, we will focus on the physiological significance and cellular action of the central nervous system (CNS) GLP-2 in the control of feeding behavior and glucose homeostasis and propose that GLP-2 as an endocrine and neural signal may transmit intestinal nutrient sensing to the hypothalamus-brain stem to suppress feeding behavior and glucose production in a negative-feedback manner.
GLP-2R Is Expressed in the Hypothalamus and Brain Stem
The rodent Glp2r mRNA is not only expressed in the hypothalamus [dorsomedial nucleus of the hypothalamus (DMH) and ARC], but also localized in the hippocampus and the brain stem (DMV and PBN) (81, 121, 131). Using fluorescence in situ hybridization and immunohistochemistry [using validated Glp2r antibody(45)], we further confirmed that Glp2r mRNA and protein are expressed in neurons in the ARC and DMH, as reported previously (118, 121). Moreover, the Glp2r protein is expressed in the POMC neurons (118). Intracerebroventricular infusion of GLP-2 increases POMC mRNA expression in the mouse ARC region (46). These data suggest that Glp2r in POMC neurons may play an important, physiological role in the control of feeding behavior and glucose homeostasis.
CNS Glp2r Suppresses Feeding Behavior
Is the CNS GLP-2 a key satiety signal for the physiological short-term control of feeding behavior?
It was debated that intracerebroventricular GLP-2 suppresses food intake, but peripheral GLP-2 does not (80, 121, 122). The hypothalamus plays pivotal roles in the regulation of energy balance and glucose homeostasis (7, 46, 104, 105, 114). It was unknown whether the CNS GLP-2 plays any physiological role in the control of energy homeostasis (e.g., feeding behavior). A key experiment was warranted to address whether GLP-2 in targeted neurons of the hypothalamus modulates feeding behavior and gastric emptying. Two groups of neurons in the arcuate nucleus of the hypothalamus control feeding: Satiety POMCARC neurons are activated by PACAPVMH neurons, while hunger AgRPARC neurons are activated by SIM1PVH neurons (69). Since POMC neurons play a critical role in the homeostatic control of energy balance (36), we hypothesized that Glp2r in POMC neurons plays an important, physiological role in the control of energy balance, specifically feeding behavior. Using the Cre-LoxP system, we generated POMC-Glp2r KO mice with Glp2r deletion selectively in POMC neurons (46). The POMC-Glp2r KO mice display hyperphagic behavior (indicated by increased food intake) and late-onset obesity, suggesting a physiological role of the CNS GLP-2 in the control of feeding behavior (46).
Melanocortin receptor 4 (Mc4r, a G protein-coupled receptor) binds α-melanocyte stimulating hormone (α-MSH) and play a central role in the regulation of body weight, glucose homeostasis, energy intake, and expenditure. The brain stem dorsal vagal complex (DVC) is a critical site of Mc4r action for POMC-derived α-MSH input and gut signals to inhibit food intake (10, 25, 71, 110, 125). GLP-2 is produced from PPG neurons in the brain stem nucleus tractus solitarius (NTS), while Glp2r is expressed in the brain stem DMV and PBN (unpublished data). We speculated that the brain stem may be an additional site of GLP-2 action on feeding behavior. Interestingly, GLP-2 infusion (into the 4th ventricle) suppressed food intake and gastric emptying, while GLP-2-mediated effects were abolished in the Mc4r KO mice (46).
This study indicates that Glp2r in POMC neurons plays an important, physiological role in the control of feeding behavior, and this is mediated through the melanocortin system (46). Thus, the CNS Glp2r is a physiological, satiety signal.
CNS Glp2r Decelerates In Vivo Gastric Emptying
Gastrointestinal motility is fine-tuned by metabolic, neuronal, and hormonal signals and plays a crucial role in the control of digestion and absorption, influencing the intake rate of net energy. Specifically, gastric emptying is a critical process for the short-term control of food intake and perhaps contributes to the long-term maintenance of energy homeostasis (46, 86, 134, 141). Gastric emptying is enhanced with overeating (34) and obesity (18, 134). The overall impact of faster gastric emptying results in shorter satiety period, increasing meal frequency, and energy intake (56). Thus, gastric emptying may be a target for controlling appetite (34, 56).
Peripheral GLP-2 induced the gastric relaxation in vitro in rodents (3) but showed inconsistent gastric emptying in humans (93). Glp2r is expressed in both excitatory and inhibitory enteric neurons (24, 45). Thus, peripheral GLP-2 inhibits the gastrointestinal motility by decreasing cholinergic neurotransmission and/or increasing nitric oxide or vasoactive intestinal peptide release (2, 4). Little was known about the physiological relevance of the CNS GLP-2 in the regulation of gastric emptying. To determine whether Glp2r in POMC neurons modulates gastric motility to control intake of net energy, we quantified the mouse gastric emptying under the conscious condition using 13C-octanoic acid breath test (46). Both half-excretion time (T1/2) and lag phase (Tlag) for liquid meal were dramatically shorter, while gastric emptying coefficient was higher in the POMC-Glp2r KO mice than those in the WT mice. Thus, the new evidence that Glp2r deletion in POMC neurons accelerated rates of gastric emptying not only explains the hyperphagic behavior, but also supports the notion of ileal break that GLP-1/2 secreted from the endocrine L cells in the ileum slow down gastric emptying to suppress food intake, at least in part, via the gut-brain-stomach axis. Moreover, the Mc4r signaling is required for intracerebroventricular GLP-2-mediated suppression of both food intake and gastric emptying (46). Inhibition of Mc4r-positive cholinergic neurons in the dorsal motor nucleus of the vagus (DMV) would alleviate excitatory input to the gastrointestinal tract, decreasing gastric emptying (42, 110). The CNS Mc4r signaling is important for the tonic control of gastric emptying. Further studies are warranted to determine whether GLP-2 acts on Mc4r-positive neurons in the brain stem through presynaptic input (from POMC-neurons in the NTS) and whether Glp2r deletion in POMC neurons impairs nutrient absorption by the gut per se.
CNS Glp2r Promotes Postprandial Glycemic Control and Hepatic Insulin Sensitivity
Increased hepatic glucose production (HGP; mainly gluconeogenesis) is a major factor in the pathogenesis of hyperglycemia in Type 2 diabetes. GLP-1/2 signals are proposed to contribute to improving metabolic outcomes (including glycemic control and insulin sensitivity) after gastric bypass surgery (37, 73, 74), which may be mediated via a neuroendocrine mechanism instead of incretin action. Except for pancreatic islets, the brain regulates glucose homeostasis (115). Direct action of insulin and leptin on POMC neurons is required to maintain glucose homeostasis via the control of HGP (52, 76, 96, 135). Except for insulin action in the liver, HGP is also suppressed by enhanced hepatic vagal input (43, 67, 71, 98, 105). Moreover, leptin signaling in POMC neurons is sufficient to correct diabetes in Leprdb/db mice (58), while insulin receptor (InsR) activation in POMC neurons promotes HGP (76, 96), pointing to the central control of POMC neurons in glucose homeostasis.
Peripheral GLP-2 regulates glucose homeostasis through promoting intestinal absorption of glucose and pancreatic secretion of glucagon (47, 83). Because of its enterotrophic action, GLP-2 is highlighted as a key signal to drive intestinal reprogramming of glucose metabolism to contribute to glycemic improvement after RYGB (113). The CNS GLP-2, like insulin and leptin, may play a crucial role in the control of glucose homeostasis and insulin sensitivity (i.e., suppressing HGP) via enhanced hepatic vagal input (43, 67, 98, 105). Glp2r global deficiency shows severer hyperglycemia and impaired glucose tolerance in Lepob/ob mice (8), suggesting that Glp2r activation is required for glucose homeostasis in obese mice (8). Leptin can stimulate GLP-1 and -2 secretion from L cells (5). Leptin directly depolarizes PPG neurons in the brain stem (55), suggesting that the CNS GLP may act as key downstream neurotransmitters of the brain stem leptin receptor (LepR) in the control of food intake and glucose homeostasis. PPG neurons innervate primarily autonomic regions (including hypothalamic ARC, DMH, and PVN; and brain stem DMV) where Glp2r is expressed (46, 77, 118, 121). Our recent data indicate that Glp2r is required for postprandial glycemic control. It is difficult to interpret these data since Glp2r is expressed not only in endocrine cells (such as enteroendocrine cells and pancreatic α-cells), but also in neurons (such as enteric neurons, vagal sensory neurons, and the CNS neurons) (45, 131). To dissect the physiological relevance of tissue-specific Glp2r in glycemic control, a site-specific inducible Glp2r knockout mouse model is greatly needed. As Shi et al. have reported (118), mice lacking Glp2r selectively in POMC neurons display impaired postprandial glucose tolerance and hepatic insulin resistance (by increased gluconeogenesis). Intracerebroventricular infusion of GLP-2 increases glucose tolerance and insulin sensitivity, and this requires Glp2r activation in POMC neurons (118), pointing to the physiological significance of GLP-2 neural action in glycemic control. Considering 50% of glycemic control through insulin-independent mechanisms (115), it will be important to quantify the extent to which GLP-2 can distinctly normalize glycemia under insulin and leptin resistance.
Glp2r deletion in POMC neurons impairs postprandial glycemic control and hepatic insulin sensitivity.
POMC-Glp2r KO mice display postprandial hyperglycemia and glucose intolerance after intravenous glucose tolerance test (118). Using hyperinsulinemic euglycemic clamp coupled with stable isotopic tracers (2H2O and 6,6-2H2-glucose), the author's laboratory has demonstrated that POMC-Glp2r KO mice had lower glucose infusion rate (representing whole body insulin sensitivity), which was attributed mainly to higher HGP and gluconeogenesis since glucose disappearance rate (representing glucose disposal) was not altered. Thus, Glp2r deletion in POMC neurons leads to hepatic insulin resistance, suggesting that Glp2r in POMC neurons is required for insulin-mediated suppression of HGP (i.e., gluconeogenesis). It is noted that glucose tolerance and insulin sensitivity are reduced in obesity. To avoid any confounding effects of body fat mass, glucose tolerance test, and hyperinsulinemic euglycemic clamp were performed in mice at the age of 12 wk (i.e., prior to the onset of obesity). Thus, the metabolic phenotype of hepatic insulin resistance (characterized in POMC-Glp2r KO mice) cannot be attributed to hyperphagic obesity, indicating a distinct role of Glp2r in POMC neurons in glycemic control.
Intracerebroventricular GLP-2 promotes glycemic control and insulin sensitivity in POMC Glp2r- and PI3K-dependent manners.
As reported, Glp2r activation in POMC neurons is required for both intracerebroventricular and intravenous GLP-2 to enhance insulin-mediated suppression of HGP (via gluconeogenesis) (118). PI3K signaling in POMC neurons is essential for leptin-induced activation and insulin-induced inhibition of POMC neurons (53). Increased PI3K activity in POMC neurons improves insulin sensitivity, whereas decreased PI3K signaling impairs glucose homeostasis. Thus, the PI3K signaling in POMC neurons is important for both energy balance and glucose homeostasis (54, 118). Importantly, intracerebroventricular infusion of GLP-2 augments glucose tolerance, suppresses basal HGP, and enhances insulin sensitivity, which are dependent on PI3K (p110α) signaling in POMC neurons (118). These data indicate that the CNS GLP-2 promotes glycemic control and insulin sensitivity via Glp2r-PI3K-mediated central melanocortin system, which is independent of incretin-mediated insulin action (118).
GLP-2 Differentially Modulates the Excitability of POMC Neurons in Glp2r- and PI3K-Dependent Manners
GLP-2 modulates the excitability of two subgroups of POMC neurons differentially.
GLP-2 acutely potentiates L-type, voltage-gated Ca2+ (Cav) channel activity in primary hippocampal neurons (131). Although Glp2r is expressed in POMC neurons (46), it was unknown whether GLP-2 can directly modulate their excitability. To determine the GLP-2-induced action on POMC neuronal excitability, we performed whole cell patch-clamp recording on green fluorescent protein (GFP)-labeled POMC neurons in brain slices from POMC-Glp2r KO and WT mice (118). There are two subgroups of hypothalamic POMC neurons in response to acute GLP-2 (118), i.e., GLP-2 (100 nM) acutely depolarized ∼50% of POMC neurons with increased spontaneous firing rate; in contrast, GLP-2 (100 nM) acutely hyperpolarized ∼50% of POMC neurons with decreased spontaneous firing rate. Of note, leptin-responsive POMC neurons (i.e., acutely activated by leptin) are segregated from insulin-responsive POMC neurons (i.e., acutely inhibited by insulin) (132). In agreement with this new notion, we further identified that GLP-2-excited POMC neurons expressed leptin receptor (LepR), while GLP-2-inhibited POMC neurons expressed insulin receptor (InsR) by single-cell RT-PCR (118). Currently, little is known about segregated subgroups of hypothalamic POMC neurons (such as neuronal signature, spatial projection, and synaptic transmission, and distinct neural function and metabolic phenotype) in the control of energy balance and glucose homeostasis. It will be important to characterize GLP-2 differentially activated subgroups of POMC neurons, which will ultimately determine their distinct neuroendocrine actions.
GLP-2 modulates the excitability of POMC neurons in Glp2r- and PI3K-dependent manners.
Moreover, activation of Glp2r or PI3K is required to modulate the membrane excitability of POMC neurons (118). As shown, GLP-2 neither depolarized nor hyperpolarized POMC neurons with Glp2r deletion in the brain slices, indicating GLP-2 direct action on membrane potential (not attributable to synaptic transmission). Activation of PI3K signaling in POMC neurons is crucial for energy and glucose homeostasis (1, 52, 54, 67, 88, 103) and is required for leptin and insulin to activate transient receptor potential canonical (TRPC) and KATP channels, respectively, resulting in depolarization and hyperpolarization of POMC neurons to suppress food intake and HGP (11, 53, 68, 103, 109, 119). The PI3K-Akt signaling pathway (as a conserved central node) may underlie GLP-2-modulated membrane excitability. In the presence of PI3K inhibitor, POMC neurons do not respond to GLP-2 anymore. Interestingly, both leptin and insulin activate PI3K signaling in POMC neurons, resulting in depolarization (leptin) or hyperpolarization (insulin). In summary, GLP-2 directly modulates postsynaptic membrane excitability of POMC neurons in Glp2r- and PI3K-dependent manners, and this may be synchronized with leptin and insulin signaling in segregated subgroups of POMC neurons.
Putative ion channels underlying GLP-2 neural action.
It is unknown through which ion channels GLP-2 modulates membrane excitability of POMC neurons. Activation of PI3K-Akt signaling pathway potentiates L-type, voltage-dependent calcium channel (Cav) activation and enhances Cav currents in neurons (13, 21, 126). In addition, leptin (via the PI3K signaling) evokes L-type Ca2+ currents (129) and TRPC currents in POMC neurons (107), exciting them. As discussed below, GLP-2 activates the PI3K signaling in POMC neurons (118). Moreover, Glp2r interacts in vitro with calmodulin (82), a protein partner with TRPC channels (96). Using the whole cell patch clamp, Wang and Guan (131) showed that GLP-2 acutely induces inward Ca2+ currents (i.e., increases intracellular Ca2+ level) through enhancing L-type, Cav channel activity in primary hippocampal neurons. It will be important to identify whether putative ion channels (Cav and TRPC) are required for GLP-2 to excite LepR-expressing POMC neurons. ATP-sensitive K+ (KATP) channels in the hypothalamus are essential for glucose homeostasis (84). Activation of KATP channels in hypothalamic neurons is required for insulin- and leptin-mediated suppression of HGP (97, 98, 104, 120). Insulin-activated PI3K signaling (through PIP3) activates the Sur1/Kir6.2 KATP channels in POMC neurons to decrease HGP (1, 60, 70, 98, 103, 104). Thus, we hypothesized that GLP-2 acutely enhances KATP channel activity, inhibiting InsR-expressing POMC neurons. A pharmacological study indicates that KATP channel activation is responsible for GLP-2-induced inhibition of POMC neurons (118).
In summary, GLP-2 directly modulates postsynaptic membrane excitability of POMC neurons in Glp2r- and PI3K-dependent manners. GLP-2 differentially depolarizes or hyperpolarizes POMC neurons in the hypothalamus, and this paradoxical action may be synchronized with leptin and insulin signaling in segregated subgroups of POMC neurons. Moreover, KATP channel activation is responsible for GLP-2-induced inhibition of POMC neurons expressing InsR (118). Studies are warranted to characterize neural and metabolic phenotypes of functionally segregated POMC neurons and to identify putative ion channels underlying GLP-2R-mediated excitation of POMC neurons expressing LepR.
GLP-2R-Activated Intracellular Signaling Pathways in the Brain
How does GLP-2 act on ARC POMC neurons intracellularly?
Little is known about how Glp2r activates intracellular signaling pathways in neurons or endocrine cells. In Glp2r-expressing cells, GLP-2 activates PI3K and PKA signaling pathways (17, 33, 75, 117, 118, 131, 137). The PI3K-Akt signaling pathway in hypothalamic neurons plays a critical role in the control of energy balance and glucose homeostasis (88, 103). Increased PI3K activity in POMC neurons improves insulin sensitivity, whereas decreased PI3K activity impairs HGP (54). As the PI3K major catalytic subunit (38, 61, 66), p110α is required for leptin- and insulin-induced PI3K activity in the hypothalamus (38, 67). Specifically, PI3K signaling in POMC neurons is essential for leptin-induced activation and insulin-induced inhibition of POMC neurons (1, 53). PI3K signaling is required for leptin and insulin to activate TRPC and KATP channels, respectively, resulting in depolarization and hyperpolarization of segregated POMC neurons to suppress food intake and glucose production. We proposed that GLP-2-activated intracellular signaling pathways are cell-specific, i.e., PI3K-dependent Glp2r action in POMC neurons.
GLP-2 activates PI3K signaling by initiating Glp2r-p85α binding.
Through Glp2r primary sequence analysis, we noticed a putative p85α-binding motif in the Glp2r amino acid sequence. It has been shown using immunoprecipitation that Glp2r interacts with the PI3K regulatory subunit (p85α) in primary hippocampal neurons (118). This Glp2r-p85α interaction was further confirmed in HEK293 cells transfected with Glp2r-c-myc and p85α-GFP using antibodies against c-myc and GFP. Moreover, Glp2r is required for the basal level of Akt phosphorylation in POMC neurons (118).
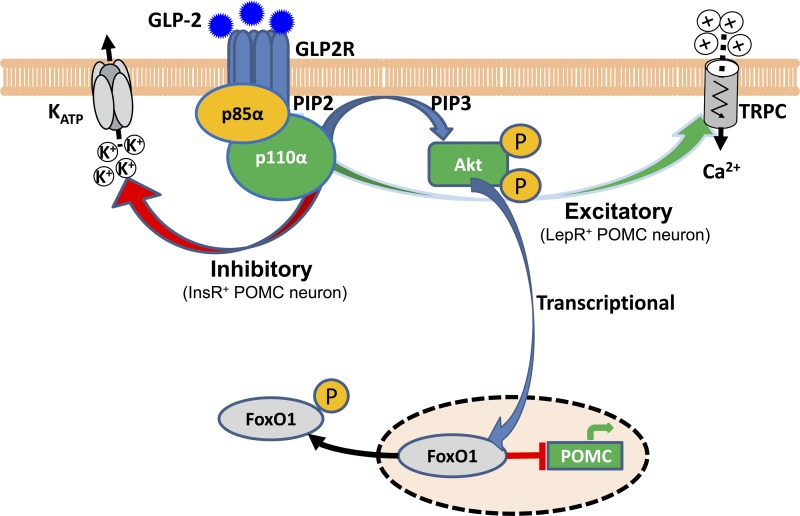
GLP-2 activates PI3K-Akt-FoxO1 signaling in POMC neurons.
FoxO1 is a downstream target of the PI3K/Akt signaling pathway and regulates energy balance (62, 65). The Cre-dependent expression and nuclear exclusion of FoxO1-GFP not only indicates transcriptional repression of FoxO1, but also reports signaling activation of PI3K-Akt pathway (39). The PI3K-Akt-FoxO1 pathway is considered a key universal pathway in hormonal, nutritional, and neural actions. GLP-2 rapidly phosphorylates Akt in primary hippocampal neurons in a PI3K-dependent pathway (117). Therefore, we proposed that GLP-2-activated PI3K-Akt signaling can be indicated by FoxO1 nuclear exclusion. As shown, GLP-2 enhances PI3K-dependent p-Akt signaling, facilitating FoxO1 nuclear exclusion in hypothalamic POMC neurons (118). It should be noted that PI3K deletion (i.e., p110α KO) or inhibition (e.g., LY294002 application) negates GLP-2-mediated effects, which would block the entire PI3K-PIP3-Akt signaling pathway (118). Importantly, PI3K does not directly phosphorylate Akt. PI3K activation phosphorylates membrane-bound PIP2 to generate PIP3. Through the binding of its PH domain to PIP3, Akt is translocated to the plasma membrane and phosphorylated at Thr-308 by PDK1 and at Ser-473 by mTORC2, respectively. It has been shown that GLP-2 facilitates Akt phosphorylation at the plasma membrane and induces Akt phosphorylation at Thr-308 (which requires the intact p85α-binding site on Glp2r) (118). Moreover, GLP-2 increases nuclear exclusion of phosphorylated FoxO1 in a p110α (a catalytic subunit of PI3K)-dependent manner. However, these results do not necessarily point to GLP-2-mediated, direct phosphorylation on Akt or FoxO1 per se. Interestingly, brain stem Glp1r-mediated suppression of food intake requires a PI3K-dependent decrease in phosphorylation of membrane-bound Akt (112). Therefore, GLP-2 activates the PI3K-Akt-FoxO1 signaling pathway via initiating an interaction of Glp2r and p85α. However, it is still unknown whether GLP-2 induces PI3K-independent phosphorylation of Akt or FoxO1.
PI3K in POMC neurons is required for GLP-2 to promote glucose homeostasis and insulin sensitivity.
It appears that GLP-2 acts on POMC neurons through activating the PI3K-Akt-FoxO1 signaling pathway. Is the PI3K activation required for the CNS GLP-2 metabolic action? As a major catalytic subunit of PI3K, p110α is required for leptin- and insulin-induced PI3K activity in the hypothalamus (38, 61, 66). Thus, we generated POMC-p110α KO mice to address this question. Intracerebroventricular GLP-2 promotes insulin sensitivity and glycemic control (indicated by increased glucose tolerance and suppressed basal HGP), and this was abolished in mice with p110α deletion selectively in POMC neurons.
In summary, three lines of evidence indicate that GLP-2 activates the PI3K-Akt-FoxO1 signaling in POMC neurons: 1) Glp2r physically binds to p85α (a PI3K regulator subunit), enhances Akt phosphorylation, and activates PI3K-Akt-dependent nuclear exclusion of FoxO1 in POMC neurons (118). Moreover, GLP-2 rapidly activates the PI3K-Akt-mTOR pathway in primary hippocampal neurons (117). 2) PI3K signaling is required for GLP-2-mediated membrane excitation of POMC neurons (as discussed above). Of note, leptin excites POMC neurons via activation of TRPC channels in a PI3K-dependent manner (53, 88, 89). 3) p110α (a PI3K catalytic subunit) in POMC neurons is required for GLP-2-mediated glycemic control and insulin sensitivity (118). Thus, the CNS GLP-2 plays a key role in the control of HGP via PI3K-dependent activation and nuclear transcription of POMC neurons. We speculate that Glp2r activation → POMC neuronal excitation (via PI3K-dependent activation of TRPC channels) → α-MSH release (as a slow-acting neurotransmitter) on postsynaptic Mc4r-expressing autonomic neurons →→ glycemic control. Glp2r-induced intracellular signaling in hypothalamic POMC neurons is summarized in Fig. 1.
Fig. 1.
Glucagon-like peptide 2 receptor (Glp2r)-induced intracellular signaling in hypothalamic proopiomelanocortin (POMC) neurons. In hypothalamic POMC neurons, GLP-2 induces Glp2r-p85α protein interaction, activating the PI3K-Akt-FoxO1 signaling pathway to derepress POMC transcription. GLP-2-activated PI3K signaling depolarizes one subgroup of POMC neurons (expressing leptin receptor) via activating putative transient receptor potential canonical (TRPC) channels; and hyperpolarizes another subgroup of POMC neurons (expressing insulin receptor) via activating KATP channels. This paradox action on membrane excitability may be synchronized with leptin and insulin signaling in segregated POMC neurons. GLP-2-activated POMC neurons are integrated with central autonomic control by enhancing vagal outflows: decelerating gastric emptying or suppressing hepatic glucose production. [Modified from Cell Metabolism, 18(1), Shi X, Zhou F, Li X, Chang B, Li D, Wang Y, Tong Q, Xu Y, Fukuda M, Zhao JJ, Li D, Burrin DJ, Chan L, and Guan X. Central GLP-2 enhances hepatic insulin sensitivity via activating PI3K signaling in POMC neuron, p. 86–98, 2013, with permission from Elsevier (118)].
Key Questions Need To Be Addressed in Future Studies
Are the NTS/DMV direct targets for GLP-2-mediated control of autonomic outflow?
Selective activation of distinct neurons in the DVC decreases HGP (mainly gluconeogenesis) (105, 142). As an integrative center from ascending and descending signals, the NTS via postsynaptic GABA/glutamate modulates the DMV that fine-tunes the autonomic outflow to discrete visceral areas (6, 29, 41). Importantly, NTS PPG neurons project widely to key central autonomic sites, including the brain stem and hypothalamus (77, 78, 127), suggesting a potential role of downstream GLP-1/2 receptor signaling in the control of autonomic outputs (79). Specifically, PPG neurons innervate brain stem autonomic neurons, including the dorsal motor nucleus of the vagus (DMV), where Glp2r is expressed (unpublished data) (77). Leptin directly excites PPG neurons (55), suggesting that GLP-1 and -2 may serve as downstream neurotransmitters for brain leptin action and relay vagal afferent signals. GLP-2 indirectly activates the NTS neurons through vagal afferents (95). We speculate that GLP-2 secretion (from enteroendocrine L cells) → Glp2r activation on vagal sensory neurons → presynaptic input to PPG neurons in the brain NTS → neuronal GLP-2 release → Glp2r activation in the hypothalamus and brain stem →→ autonomic control of peripheral glucose metabolism. Regardless of GLP-2 secreted from L cells in the gut or released from PPG neurons in the brain stem, Glp2r activation on DMV neurons in the brain stem may modulate vagal outflow, reprogramming peripheral glucose metabolism.
How does GLP-2 act in brain stem DMV neurons?
Studies show that the plasticity at the NTS-DMV synapse depends on cAMP-PKA pathway (15). Little is known about Glp2r-activated intracellular signaling pathways in the brain stem, yet the fourth intracerebroventricular GLP-2 suppresses food intake (46), and GLP-2 directly excites liver-projecting DMV neurons (unpublished data). GLP-2 activates cAMP-PKA signaling pathway (16, 81, 128, 137). As GLP-2 regulates mRNA expression of hypothalamic genes in a PKA-dependent manner (28), GLP-2 potentiates L-type Ca2+ channel activity in hippocampal neurons (131). Brain stem Glp1r activation suppresses food intake and body weight through PKA-mediated suppression of AMPK and activation of MAPK (48, 49). We speculate that GLP-2R activation → DMV neuronal excitation (via PKA-dependent long-term potentiation) → ACh release (as a rapid-acting neurotransmitter) on postganglionic neurons →→ glycemic control. Thus, we favor a model that the DMV Glp2r activation initiates the PKA-dependent Ca2+-triggered depolarization, enhancing the synaptic transmission in the DMV →→ glycemic control. However, this model for Glp2r action in the brain stem waits for experimental support.
Do GLP-2-sensitive ARC POMC neurons signal to brain stem DVC via α-MSH-Mc4r pathway?
ARC POMC neurons project to selected target sites in the hypothalamus and brain stem (35, 63). A brain stem DVC (including NTS and DMV) integrates hypothalamic inputs and vagal afferent inputs to regulate the autonomic control of feeding behavior and glucose homeostasis (71, 87, 116, 139, 140). As suggested, ARC DVC-projecting POMC neurons may represent an anatomically and functionally distinct subgroup of POMC neurons (63, 140). As the major α-MSH receptor, Mc4r plays a pivotal role in energy homeostasis and is a key target for the treatment of obesity and diabetes (143). Mc4r is heavily expressed in the brain stem DVC (42, 64, 90). The brain stem DVC is a critical site for ARC POMC-derived α-MSH input to suppress food intake and HGP (10, 25, 36, 44, 79, 110), yet little is known about how the NTS-DMV plasticity is modulated to fine-tune hepatic vagal output, influencing HGP (125). Interestingly, local infusion of GLP-2 (2.5 nmol into the 4th ventricle) suppresses food intake and gastric emptying, but these effects are abolished in Mc4r KO mice (46). Activation of Mc4r-positive cholinergic neurons in the DMV would enhance excitatory input to the liver, suppressing HPG (42, 110). Thus, it is conceivable that GLP-2 activates POMC neurons to release α-MSH, activating Mc4r-positive neurons (expressing choline acetyltransferase) in the DMV that enhances hepatic vagal output to suppress HGP.
Does GLP-2 directly modulate vagal afferents to improve glycemic control?
Vagal afferent neurons convey primarily information from the viscera to the CNS. Vagal afferents may be a key neural pathway that regulates food intake, gastrointestinal motility, and glucose homeostasis (23, 42, 42, 130). Activation of N-methyl-D-aspartate receptors in the DVC is sufficient to trigger a brain-liver axis to lower HGP (71, 72, 105). Moreover, gastrointestinal hormones excite neurons in the rodent nodose ganglia by closure of K+ channels (40, 51). GLP-2 activates vagal afferent pathway where Glp2r is expressed (95). Interestingly, GLP-2 mediates the gut-brain communication via activating vagal afferents (94, 95). We speculate that Glp2r activation on vagal afferents may influence neural inputs to 1) preproglucagonergic (PPG) neurons in the brain stem NTS → GLP-2 release → Glp2r activation in the hypothalamic ARC/DMH and the brain stem DMV →→ autonomic outputs; and 2) POMC neurons in the brain stem NTS → α-MSH release → Mc4r activation in the hypothalamic ARC/DMH and the brain stem DMV →→ autonomic outputs. However, GLP-2-modulated neural circuitries (including distinct neurons on vagal afferents) have not been identified. Of note, chemical and/or surgical vagotomy does not selectively inactivate distinct types of neurons (12). It will be important to reassess the physiological relevance of targeted neurons in the vagus using the Cre-dependent pharmacogenetic modulation and determine whether GLP-2-induced activation of vagal afferent neurons impacts glucose homeostasis and insulin sensitivity.
Does augmented GLP-2 signaling contribute to improving metabolic outcomes after bariatric surgery?
Bariatric surgery (particularly RYGB) is the most effective therapy for morbid obesity and diabetes (19, 26, 111), in which the gut-brain axis is highly involved in mediating beneficial effects on food intake and glycemic control. A recent report indicates that metabolic reprogramming of glucose to support intestinal hypertrophy renders the gut itself a major tissue for glucose disposal, contributing to improved metabolic outcomes after RYGB (113). In fact, RYGB increases blood GLP-2 (by 200%) at postprandial status (73) and enhances proliferation rates of the intestinal crypt epithelial cells, suggesting that GLP-2 (as a potent enterotrohic factor) may be an important signal to drive intestinal adaptation. However, it is still unclear whether GLP-2 is a key neuroendocrine signal via the gut-brain axis to enhance insulin sensitivity and glucose homeostasis following RYGB.
Limitations are not addressed in current GLP-2 studies.
There are two major limitations in current studies. One is how to define the physiological significance of Glp2r in site-specific populations of POMC neurons. The POMC-Cre-mediated recombination of floxed Glp2r allele will result in Glp2r deletion in POMC neurons, including POMC neurons in the hypothalamus, brain stem, and pituitary gland. Thus, Glp2r metabolic action in extrahypothalamic POMC neurons should be considered as well. However, the metabolic phenotype of POMC-Glp2r knockout mice may be largely attributed to the Glp2r deletion in POMC neurons in the hypothalamic ARC (118). There are three lines of evidence to support this speculation: 1) The POMC Cre mouse line had little Cre expression in the brain stem NTS when crossed with a Cre reporter mouse line (such as Rosa26-tdTomatto). 2) The Glp2r has not been shown to express in the brain stem NTS or pituitary gland. 3) POMC neurons in the ARC play important roles in energy/glucose homeostasis (101). To further dissect the physiological role of Glp2r in site-specific populations of POMC neurons, one should manipulate Glp2r deficiency in distinct sites by nucleus-specific injection of Cre-dependent, lentiviral mediated Glp2r shRNA (to knock down Glp2r expression).
Another one is how to distinguish the physiological significance of GLP-2 from distinct sources in the control of energy and glucose homeostasis. GLP-2 can be originated from endocrine L cells in the gut and PPG neurons in the brain stem NTS, while Glp2r is expressed in endocrine and neural cells. Thus, GLP-2 action can be mediated via endocrine and neural manners. In a majority of pharmacological studies, administration routes are used to distinguish so-called peripheral vs. central action of neuropeptides. This is not appropriate specifically for neuropeptides that cross the blood-brain barrier via passive permeability or active transport. We speculate that peripheral GLP-2 can be transported into the hypothalamic ARC, where the blood-brain barrier is semipermeable or taken up through the median eminence. In addition, it is very challenging to quantify metabolic effects of endocrine or neural GLP-2 (i.e., originated from endocrine L cells or PPG neurons). As a fact, there are no genetic approaches available to distinguish the physiological significance of GLP-2 from distinct sources. Instead, we propose to define the functional relevance of CNS GLP-2 by deleting its receptor only in central distinct neurons. It is demonstrated using tissue-specific Glp2r KO mouse model that Glp2r activation in POMC neurons is required for intravenous and intracerebroventricular GLP-2 to promote glucose homeostasis and insulin sensitivity, and for GLP-2 to modulate the excitability of hypothalamic POMC neurons ex vivo (118), pointing to the functional relevance of Glp2r action on POMC neurons regardless of the source of GLP-2.
Innovative Approaches Need To Define the CNS Control of Feeding and Metabolism
One major interest is to determine the CNS control of glucose homeostasis and feeding behavior. Increasing evidence indicates that the CNS neural circuitry is selective for glucose homeostasis or feeding behavior, in which subgroups of distinct neurons are segregated. To remotely modulate activation or inactivation of targeted neurons in conscious mice, one innovative pharmacogenetic approach, namely designer receptors exclusively activated by designer drugs (DREADDs), has been employed to elucidate the CNS neural circuits for feeding behavior (69). It has been very challenging to genetically dissect metabolic actions of targeted neurons on glycemic control or insulin sensitivity, which are confounded per se by developmental, behavioral, or body-adiposity factors. The DREADDs can remotely, acutely, and reversibly control circuit activity in vivo and, thus, will provide a powerful approach to dissect metabolic phenotypes of targeted neurons in defined spatial and temporal manners. Another state-of-the-art approach is in vivo stable isotopic tracer methodologies (in conjunction with oral glucose tolerance test and hyperinsulinemic euglycemic clamp) to quantify dynamic glycemic kinetics at postprandial and postabsorptive states and assess tissue-specific insulin sensitivity in conscious mice (46). Thus, it will be instrumental to combine in vivo stable isotopic tracer methodologies (including MS-based targeted metabolomics) with the remote control of targeted neuron approaches (such as DREADDs) to address the CNS control of glucose metabolism independent of body adiposity.
Another major interest is to map distinct hypothalamus-brain stem neural circuits underlying glycemic control. Optogenetic activation and inactivation of targeted neural circuitry have been used to establish how the neural circuits are wired functionally in the brain in vivo. Specifically, optogenetics has been successfully employed to define the hypothalamic neural circuitry controlling feeding behavior in rodents (20). Future studies are warranted to integrate optogenetic, electrophysiological, and metabolic approaches to define the functioning neural circuits responsible for glucose homeostasis.
Perspectives and Significance
GLP-1 and -2 are coproduced from enteroendocrine L cells in the gut and preproglucagonergic neurons in the brain stem. Dietary macronutrients and gut microbiota-derived metabolites (secondary bile acids and short-chain fatty acids) function as effective secretagogues of GLP-1 and-2. Because of Glp2r expression in nutrient-sensing endocrine cells and neurons, GLP-2 acts as a key endocrine and neural signal to regulate energy balance and glucose homeostasis. In the gastrointestinal tract, GLP-2 promotes intestinal epithelial homeostasis and function, enhances intestinal nutrient absorption and blood flow, and inhibits gastrointestinal motility, thus controlling energy intake in the first place. In the hypothalamus and brain stem, Glp2r plays physiological roles in regulating food intake and glucose homeostasis, which are mediated through fine-tuning vagal outputs via the proposed neural circuits (Fig. 2). New evidence underscores the CNS GLP-2-mediated promotion of glycemic control and insulin sensitivity via the PI3K-dependent, central melanocortin system. Importantly, the GLP-2 gut-brain axis may be physiologically modulated by nutrition, gut microbiota, and bariatric surgery. Using Glp2r-floxed mouse line in combination of state-of-the-art approaches (such as stable isotopic tracer methodologies and the remote control of targeted neurons), one will further dissect the GLP-2 gut-brain axis and identify novel targets for the treatment of gastrointestinal diseases, obesity, and diabetes.
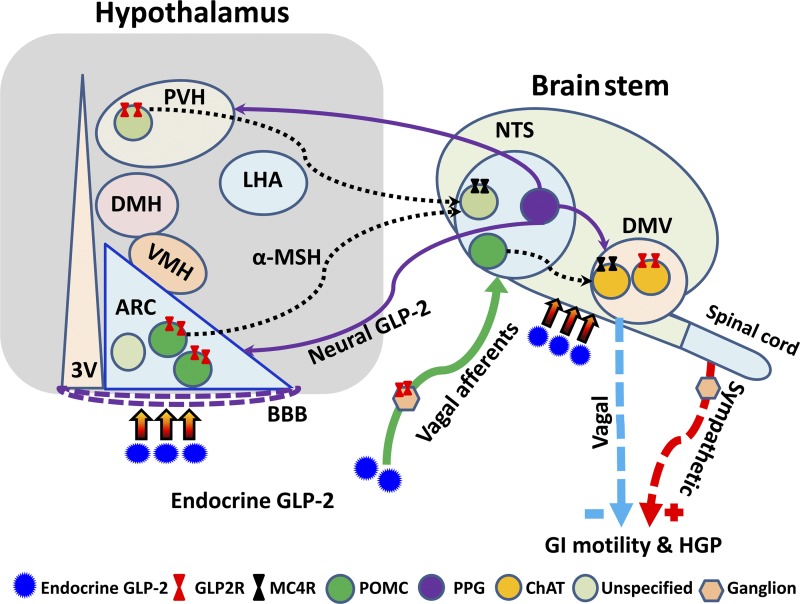
Fig. 2.
Glucagon-like peptide-2 receptor (Glp2r)-activated neural circuits in the hypothalamus and brain stem. GLP-2 [secreted from endocrine L cells and preproglucagonergic (PPG) neurons] may fine-tune autonomic outputs via the proposed neural circuits. In addition to sympathetic outflow, Glp2r activation in proopiomelanocortin (POMC) neurons in the hypothalamus and brain stem → α-melanocyte stimulating hormone (α-MSH) release → melanocortin receptor 4 (Mc4r) activation in the brain stem dorsal vagal complex (DVC) → vagal outflow. Moreover, Glp2r activation on vagal afferents may influence neural inputs to 1) PPG neurons in the brain stem nucleus of the solitary tract (NTS) → GLP-2 release → Glp2r activation in the hypothalamic arcuate nucleus (ARC)/paraventricular nucleus of the hypothalamus (PVH) and the brain stem dorsal motor nucleus of the vagus (DMV) →→ vagal outflows; and 2) POMC neurons in the brain stem NTS → α-MSH release → melanocortin 4 receptor (Mc4r) activation in the hypothalamic ARC/ventromedial hypothalamus (VMH) and the brain stem DMV →→ vagal outflows. However, GLP-2-modulated neural circuitries have not been fully defined. BBB, blood-brain barrier; GI, gastrointestinal; HGP, hepatic glucose production; 3V, third ventricle; DMH, dorsomedial hypothalamus; ChAT, choline acetyltransferase; LHA, lateral hypothalamic area.
GRANTS
This work is supported by the USDA/ARS under Cooperative Agreement No. 6250-51000-049-02S-2 and 6250-51000-054-00D-3 (to X. Guan); National Institutes of Health (NIH) Grants DK-075489 and DK-084125 (XG); and NIH grant DK-56338 (which supports the Texas Medical Center Digestive Diseases Center).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
Author contributions: X.G. conception and design of research; X.G. performed experiments; X.G. analyzed data; X.G. interpreted results of experiments; X.G. prepared figures; X.G. drafted manuscript; X.G. edited and revised manuscript; X.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The author thanks Dr. Xuemei Shi at Baylor College of Medicine for scientific and technical support. This work is a publication of the United States Department of Agriculture, Agricultural Research Service (USDA/ARS) Children's Nutrition Research Center, Departments of Pediatrics and Medicine, Baylor College of Medicine, and Texas Children's Hospital, Houston, TX. The contents of this publication do not necessarily reflect the views or policies of the USDA, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
REFERENCES
- 1.Al-Qassab H, Smith MA, Irvine EE, Guillermet-Guibert J, Claret M, Choudhury AI, Selman C, Piipari K, Clements M, Lingard S, Chandarana K, Bell JD, Barsh GS, Smith AJ, Batterham RL, Ashford ML, Vanhaesebroeck B, Withers DJ. Dominant role of the p110beta isoform of PI3K over p110alpha in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab 10: 343–354, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amato A, Baldassano S, Serio R, Mule F. Glucagon-like peptide-2 relaxes mouse stomach through vasoactive intestinal peptide release. Am J Physiol Gastrointest Liver Physiol 296: G678–G684, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Amato A, Baldassano S, Serio R, Mule F. Glucagon-like peptide-2 relaxes mouse stomach through vasoactive intestinal peptide release. Am J Physiol Gastrointest Liver Physiol 296: G678–G684, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Amato A, Rotondo A, Cinci L, Baldassano S, Vannucchi MG, Mule F. Role of cholinergic neurons in the motor effects of glucagon-like peptide-2 in mouse colon. Am J Physiol Gastrointest Liver Physiol 299: G1038–G1044, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes 52: 252–259, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Babic T, Browning KN, Travagli RA. Differential organization of excitatory and inhibitory synapses within the rat dorsal vagal complex. Am J Physiol Gastrointest Liver Physiol 300: G21–G32, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science 307: 1909–1914, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Bahrami J, Longuet C, Baggio LL, Li K, Drucker DJ. Glucagon-like peptide-2 receptor modulates islet adaptation to metabolic stress in the ob/ob mouse. Gastroenterology 139: 857–868, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Bahrami J, Yusta B, Drucker DJ. ErbB activity links the glucagon-like peptide-2 receptor to refeeding-induced adaptation in the murine small bowel. Gastroenterology 138: 2447–2456, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123: 493–505, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Belgardt BF, Husch A, Rother E, Ernst MB, Wunderlich FT, Hampel B, Klockener T, Alessi D, Kloppenburg P, Bruning JC. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab 7: 291–301, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Berthoud HR, Shin AC, Zheng H. Obesity surgery and gut-brain communication. Physiol Behav 105: 106–119, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blair LA, Bence-Hanulec KK, Mehta S, Franke T, Kaplan D, Marshall J. Akt-dependent potentiation of L channels by insulin-like growth factor-1 is required for neuronal survival. J Neurosci 19: 1940–1951, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breen DM, Rasmussen BA, Kokorovic A, Wang R, Cheung GW, Lam TK. Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat Med 18: 950–955, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Browning KN, Travagli RA. Plasticity of vagal brainstem circuits in the control of gastrointestinal function. Auton Neurosci 161: 6–13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burrin DG, Stoll B, Guan X, Cui L, Chang X, Hadsell D. GLP-2 rapidly activates divergent intracellular signaling pathways involved in intestinal cell survival and proliferation in neonatal piglets. Am J Physiol Endocrinol Metab 292: E281–E291, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Burrin DG, Stoll B, Guan X, Cui L, Chang X, Holst JJ. Glucagon-like peptide 2 dose-dependently activates intestinal cell survival and proliferation in neonatal piglets. Endocrinology 146: 22–32, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Cardoso-Junior A, Coelho LG, Savassi-Rocha PR, Vignolo MC, Abrantes MM, de Almeida AM, Dias EE, Vieira JG, de Castro MM, Lemos YV. Gastric emptying of solids and semi-solids in morbidly obese and non-obese subjects: an assessment using the 13C-octanoic acid and 13C-acetic acid breath tests. Obes Surg 17: 236–241, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Carlsson LM, Peltonen M, Ahlin S, Anveden A, Bouchard C, Carlsson B, Jacobson P, Lonroth H, Maglio C, Naslund I, Pirazzi C, Romeo S, Sjoholm K, Sjostrom E, Wedel H, Svensson PA, Sjostrom L. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 367: 695–704, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature 503: 111–114, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catalucci D, Zhang DH, DeSantiago J, Aimond F, Barbara G, Chemin J, Bonci D, Picht E, Rusconi F, Dalton ND, Peterson KL, Richard S, Bers DM, Brown JH, Condorelli G. Akt regulates L-type Ca2+ channel activity by modulating Cavalpha1 protein stability. J Cell Biol 184: 923–933, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. CDC overweight and obesity: Health & Economic consequences. Centers for Disease Control and Prevention. http://www.cdc.gov/obesity/causes/health.html, 2013 [Google Scholar]
- 23.Cheung GW, Kokorovic A, Lam CK, Chari M, Lam TK. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab 10: 99–109, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Cinci L, Faussone-Pellegrini MS, Rotondo A, Mule F, Vannucchi MG. GLP-2 receptor expression in excitatory and inhibitory enteric neurons and its role in mouse duodenum contractility. Neurogastroenterol Motil 23: e383–e392, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci 8: 571–578, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Cummings DE. Metabolic surgery for type 2 diabetes. Nat Med 18: 656–658, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest 117: 13–23, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalvi PS, Belsham DD. Glucagon-like peptide-2 directly regulates hypothalamic neurons expressing neuropeptides linked to appetite control in vivo and in vitro. Endocrinology 153: 2385–2397, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Davis SF, Derbenev AV, Williams KW, Glatzer NR, Smith BN. Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res 1017: 208–217, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Heer J, Pedersen J, Orskov C, Holst JJ. The alpha cell expresses glucagon-like peptide-2 receptors and glucagon-like peptide-2 stimulates glucagon secretion from the rat pancreas. Diabetologia 50: 2135–2142, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Diakogiannaki E, Gribble FM, Reimann F. Nutrient detection by incretin hormone secreting cells. Physiol Behav 106: 387–393, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest 117: 24–32, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dube PE, Rowland KJ, Brubaker PL. Glucagon-like peptide-2 activates β-catenin signaling in the mouse intestinal crypt: role of insulin-like growth factor-I. Endocrinology 149: 291–301, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Duggan JP, Booth DA. Obesity, overeating, and rapid gastric emptying in rats with ventromedial hypothalamic lesions. Science 231: 609–611, 1986 [DOI] [PubMed] [Google Scholar]
- 35.Ellacott KL, Cone RD. The central melanocortin system and the integration of short- and long-term regulators of energy homeostasis. Recent Prog Horm Res 59: 395–408, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci 7: 335–336, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Field BC, Chaudhri OB, Bloom SR. Bowels control brain: gut hormones and obesity. Nat Rev Endocrinol 6: 444–453, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Foukas LC, Claret M, Pearce W, Okkenhaug K, Meek S, Peskett E, Sancho S, Smith AJ, Withers DJ, Vanhaesebroeck B. Critical role for the p110α phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature 441: 366–370, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Fukuda M, Jones JE, Olson D, Hill J, Lee CE, Gautron L, Choi M, Zigman JM, Lowell BB, Elmquist JK. Monitoring FoxO1 localization in chemically identified neurons. J Neurosci 28: 13640–13648, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaisano GG, Park SJ, Daly DM, Beyak MJ. Glucagon-like peptide-1 inhibits voltage-gated potassium currents in mouse nodose ganglion neurons. Neurogastroenterol Motil 22: 470–479, e111, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Gao H, Smith BN. Tonic GABAA receptor-mediated inhibition in the rat dorsal motor nucleus of the vagus. J Neurophysiol 103: 904–914, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gautron L, Lee C, Funahashi H, Friedman J, Lee S, Elmquist J. Melanocortin-4 receptor expression in a vago-vagal circuitry involved in postprandial functions. J Comp Neurol 518: 6–24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.German J, Kim F, Schwartz GJ, Havel PJ, Rhodes CJ, Schwartz MW, Morton GJ. Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology 150: 4502–4511, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab 16: 296–309, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guan X, Karpen HE, Stephens J, Bukowski JT, Niu S, Zhang G, Stoll B, Finegold MJ, Holst JJ, Hadsell D, Nichols BL, Burrin DG. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology 130: 150–164, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Guan X, Shi X, Li X, Chang B, Wang Y, Li DP, Chan L. GLP-2 receptor in POMC neurons suppresses feeding behavior and gastric motility. Am J Physiol Endocrinol Metab 303: E853–E864, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guan X, Stoll B, Lu X, Tappenden KA, Holst JJ, Hartmann B, Burrin DG. GLP-2-mediated up-regulation of intestinal blood flow and glucose uptake is nitric oxide-dependent in TPN-fed piglets 1. Gastroenterology 125: 136–147, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 150: 2654–2659, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, De Jonghe BC, Kanoski SE, Grill HJ, Bence KK. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab 13: 320–330, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heijboer AC, Pijl H, Van den Hoek AM, Havekes LM, Romijn JA, Corssmit EP. Gut-brain axis: regulation of glucose metabolism. J Neuroendocrinol 18: 883–894, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Heldsinger A, Grabauskas G, Song I, Owyang C. Synergistic interaction between leptin and cholecystokinin in the rat nodose ganglia is mediated by PI3K and STAT3 signaling pathways: implications for leptin as a regulator of short term satiety. J Biol Chem 286: 11707–11715, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, Lauzon D, Lee CE, Coppari R, Richardson JA, Zigman JM, Chua S, Scherer PE, Lowell BB, Bruning JC, Elmquist JK. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab 11: 286–297, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest 118: 1796–1805, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hill JW, Xu Y, Preitner F, Fukuda M, Cho YR, Luo J, Balthasar N, Coppari R, Cantley LC, Kahn BB, Zhao JJ, Elmquist JK. Phosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis. Endocrinology 150: 4874–4882, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hisadome K, Reimann F, Gribble FM, Trapp S. Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: electrical properties of glucagon-like peptide 1 neurons. Diabetes 59: 1890–1898, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horner KM, Byrne NM, Cleghorn GJ, Naslund E, King NA. The effects of weight loss strategies on gastric emptying and appetite control. Obes Rev 12: 935–951, 2011 [DOI] [PubMed] [Google Scholar]
- 57.Hsieh J, Longuet C, Maida A, Bahrami J, Xu E, Baker CL, Brubaker PL, Drucker DJ, Adeli K. Glucagon-like peptide-2 increases intestinal lipid absorption and chylomicron production via CD36. Gastroenterology 137: 997–1005, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Huo L, Gamber K, Greeley S, Silva J, Huntoon N, Leng XH, Bjorbaek C. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab 9: 537–547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iakoubov R, Ahmed A, Lauffer LM, Bazinet RP, Brubaker PL. Essential role for protein kinase Czeta in oleic acid-induced glucagon-like peptide-1 secretion in vivo in the rat. Endocrinology 152: 1244–1252, 2011 [DOI] [PubMed] [Google Scholar]
- 60.Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Ronnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology 144: 1331–1340, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, Zhang J, Signoretti S, Loda M, Roberts TM, Zhao JJ. Essential roles of PI(3)K-p110β in cell growth, metabolism and tumorigenesis. Nature 454: 776–779, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim MS, Pak YK, Jang PG, Namkoong C, Choi YS, Won JC, Kim KS, Kim SW, Kim HS, Park JY, Kim YB, Lee KU. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci 9: 901–906, 2006 [DOI] [PubMed] [Google Scholar]
- 63.King CM, Hentges ST. Relative number and distribution of murine hypothalamic proopiomelanocortin neurons innervating distinct target sites. PLoS One 6: e25864, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol 457: 213–235, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Kitamura T, Feng Y, Kitamura YI, Chua SC, Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med 12: 534–540, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, Balla T, Weiss WA, Williams RL, Shokat KM. A pharmacological map of the PI3-K family defines a role for p110α in insulin signaling. Cell 125: 733–747, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koch C, Augustine RA, Steger J, Ganjam GK, Benzler J, Pracht C, Lowe C, Schwartz MW, Shepherd PR, Anderson GM, Grattan DR, Tups A. Leptin rapidly improves glucose homeostasis in obese mice by increasing hypothalamic insulin sensitivity. J Neurosci 30: 16180–16187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Bruning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 5: 438–449, 2007 [DOI] [PubMed] [Google Scholar]
- 69.Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, Liberles SD, Lowell BB. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507: 238–242, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lam CK, Chari M, Lam TK. CNS regulation of glucose homeostasis. Physiology 24: 159–170, 2009 [DOI] [PubMed] [Google Scholar]
- 71.Lam CK, Chari M, Rutter GA, Lam TK. Hypothalamic nutrient sensing activates a forebrain-hindbrain neuronal circuit to regulate glucose production in vivo. Diabetes 60: 107–113, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lam CK, Chari M, Su BB, Cheung GW, Kokorovic A, Yang CS, Wang PY, Lai TY, Lam TK. Activation of N-methyl-d-aspartate (NMDA) receptors in the dorsal vagal complex lowers glucose production. J Biol Chem 285: 21913–21921, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.le Roux CW, Borg C, Wallis K, Vincent RP, Bueter M, Goodlad R, Ghatei MA, Patel A, Bloom SR, Aylwin SJ. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann Surg 252: 50–56, 2010 [DOI] [PubMed] [Google Scholar]
- 74.le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lonroth H, Fandriks L, Ghatei MA, Bloom SR, Olbers T. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 246: 780–785, 2007 [DOI] [PubMed] [Google Scholar]
- 75.Leen JL, Izzo A, Upadhyay C, Rowland KJ, Dube PE, Gu S, Heximer SP, Rhodes CJ, Storm DR, Lund PK, Brubaker PL. Mechanism of action of glucagon-like peptide-2 to increase IGF-I mRNA in intestinal subepithelial fibroblasts. Endocrinology 152: 436–446, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin HV, Plum L, Ono H, Gutierrez-Juarez R, Shanabrough M, Borok E, Horvath TL, Rossetti L, Accili D. Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in Agouti-related protein and POMC neurons. Diabetes 59: 337–346, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Llewellyn-Smith IJ, Gnanamanickam GJ, Reimann F, Gribble FM, Trapp S. Preproglucagon (PPG) neurons innervate neurochemically identified autonomic neurons in the mouse brainstem. Neuroscience 229: 130–143, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Llewellyn-Smith IJ, Gnanamanickam GJ, Reimann F, Gribble FM, Trapp S. Preproglucagon (Ppg) neurons innervate neurochemically identified autonomic neurons in the mouse brainstem. Neuroscience 229: 130–143, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience 180: 111–121, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lovshin J, Estall J, Yusta B, Brown TJ, Drucker DJ. Glucagon-like peptide (GLP)-2 action in the murine central nervous system is enhanced by elimination of GLP-1 receptor signaling. J Biol Chem 276: 21489–21499, 2001 [DOI] [PubMed] [Google Scholar]
- 81.Lovshin JA, Huang Q, Seaberg R, Brubaker PL, Drucker DJ. Extrahypothalamic expression of the glucagon-like peptide-2 receptor is coupled to reduction of glutamate-induced cell death in cultured hippocampal cells. Endocrinology 145: 3495–3506, 2004 [DOI] [PubMed] [Google Scholar]
- 82.Mahon MJ, Shimada M. Calmodulin interacts with the cytoplasmic tails of the parathyroid hormone 1 receptor and a sub-set of class b G protein-coupled receptors. FEBS Lett 579: 803–807, 2005 [DOI] [PubMed] [Google Scholar]
- 83.Meier JJ, Nauck MA, Pott A, Heinze K, Goetze O, Bulut K, Schmidt WE, Gallwitz B, Holst JJ. Glucagon-like peptide 2 stimulates glucagon secretion, enhances lipid absorption, and inhibits gastric acid secretion in humans. Gastroenterology 130: 44–54, 2006 [DOI] [PubMed] [Google Scholar]
- 84.Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, Horiuchi M, Ashcroft F, Minokoshi Y, Roeper J, Seino S. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci 4: 507–512, 2001 [DOI] [PubMed] [Google Scholar]
- 85.Mokadem M, Zechner JF, Margolskee RF, Drucker DJ, Aguirre V. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol Metab 3: 191–201, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moran TH, McHugh PR. Cholecystokinin suppresses food intake by inhibiting gastric emptying. Am J Physiol Regul Integr Comp Physiol 242: R491–R497, 1982 [DOI] [PubMed] [Google Scholar]
- 87.Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Baskin DG, Schwartz MW. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest 115: 703–710, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab 2: 411–420, 2005 [DOI] [PubMed] [Google Scholar]
- 89.Morton GJ, Schwartz MW. Leptin and the central nervous system control of glucose metabolism. Physiol Rev 91: 389–411, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol 8: 1298–1308, 1994 [DOI] [PubMed] [Google Scholar]
- 91.Munroe DG, Gupta AK, Kooshesh F, Vyas TB, Rizkalla G, Wang H, Demchyshyn L, Yang ZJ, Kamboj RK, Chen H, McCallum K, Sumner-Smith M, Drucker DJ, Crivici A. Prototypic G protein-coupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc Natl Acad Sci USA 96: 1569–1573, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature 444: 854–859, 2006 [DOI] [PubMed] [Google Scholar]
- 93.Nagell CF, Wettergren A, Pedersen JF, Mortensen D, Holst JJ. Glucagon-like peptide-2 inhibits antral emptying in man, but is not as potent as glucagon-like peptide-1. Scand J Gastroenterol 39: 353–358, 2004 [DOI] [PubMed] [Google Scholar]
- 94.Nelson DW, Liu X, Holst JJ, Raybould HE, Ney DM. Vagal afferents are essential for maximal resection-induced intestinal adaptive growth in orally fed rats. Am J Physiol Regul Integr Comp Physiol 291: R1256–R1264, 2006 [DOI] [PubMed] [Google Scholar]
- 95.Nelson DW, Sharp JW, Brownfield MS, Raybould HE, Ney DM. Localization and activation of glucagon-like peptide-2 receptors on vagal afferents in the rat. Endocrinology 148: 1954–1962, 2007 [DOI] [PubMed] [Google Scholar]
- 96.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev 87: 165–217, 2007 [DOI] [PubMed] [Google Scholar]
- 97.Ning K, Miller LC, Laidlaw HA, Watterson KR, Gallagher J, Sutherland C, Ashford ML. Leptin-dependent phosphorylation of PTEN mediates actin restructuring and activation of ATP-sensitive K+ channels. J Biol Chem 284: 9331–9340, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8: 1376–1382, 2002 [DOI] [PubMed] [Google Scholar]
- 99.Ochner CN, Gibson C, Shanik M, Goel V, Geliebter A. Changes in neurohormonal gut peptides following bariatric surgery. Int J Obes (Lond) 35: 153–166, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Parker HE, Reimann F, Gribble FM. Molecular mechanisms underlying nutrient-stimulated incretin secretion. Expert Rev Mol Med 12: e1, 2010 [DOI] [PubMed] [Google Scholar]
- 101.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 449: 228–232, 2007 [DOI] [PubMed] [Google Scholar]
- 102.Petersen N, Reimann F, Bartfeld S, Farin HF, Ringnalda FC, Vries RG, van den BS, Clevers H, Gribble FM, de Koning EJ. Generation of L cells in mouse and human small intestine organoids. Diabetes 63: 410–420, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Munzberg H, Shanabrough M, Burdakov D, Rother E, Janoschek R, Alber J, Belgardt BF, Koch L, Seibler J, Schwenk F, Fekete C, Suzuki A, Mak TW, Krone W, Horvath TL, Ashcroft FM, Bruning JC. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest 116: 1886–1901, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, guilar-Bryan L, Rossetti L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature 434: 1026–1031, 2005 [DOI] [PubMed] [Google Scholar]
- 105.Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab 1: 53–61, 2005 [DOI] [PubMed] [Google Scholar]
- 106.Poreba MA, Dong CX, Li SK, Stahl A, Miner JH, Brubaker PL. Role of fatty acid transport protein 4 in oleic acid-induced glucagon-like peptide-1 secretion from murine intestinal L cells. Am J Physiol Endocrinol Metab 303: E899–E907, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qiu J, Fang Y, Ronnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci 30: 1560–1565, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab 8: 532–539, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rosse C, Linch M, Kermorgant S, Cameron AJ, Boeckeler K, Parker PJ. PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol 11: 103–112, 2010 [DOI] [PubMed] [Google Scholar]
- 110.Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab 13: 195–204, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rubino F, Cummings DE. Surgery: The coming of age of metabolic surgery. Nat Rev Endocrinol 8: 702–704, 2012 [DOI] [PubMed] [Google Scholar]
- 112.Rupprecht LE, Mietlicki-Baase EG, Zimmer DJ, McGrath LE, Olivos DR, Hayes MR. Hindbrain GLP-1 receptor-mediated suppression of food intake requires a PI3K-dependent decrease in phosphorylation of membrane-bound Akt. Am J Physiol Endocrinol Metab 305: E751–E759, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saeidi N, Meoli L, Nestoridi E, Gupta NK, Kvas S, Kucharczyk J, Bonab AA, Fischman AJ, Yarmush ML, Stylopoulos N. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science 341: 406–410, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science 307: 375–379, 2005 [DOI] [PubMed] [Google Scholar]
- 115.Schwartz MW, Seeley RJ, Tschop MH, Woods SC, Morton GJ, Myers MG, D'Alessio D. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature 503: 59–66, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000 [DOI] [PubMed] [Google Scholar]
- 117.Shi X, Li X, Wang Y, Zhang K, Zhou F, Chan L, Li D, Guan X. Glucagon-like peptide-2-stimulated protein synthesis through the PI 3-kinase-dependent Akt-mTOR signaling pathway. Am J Physiol Endocrinol Metab 300: E554–E563, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shi X, Zhou F, Li X, Chang B, Li D, Wang Y, Tong Q, Xu Y, Fukuda M, Zhao JJ, Li D, Burrin DG, Chan L, Guan X. Central GLP-2 enhances hepatic insulin sensitivity via activating PI3K signaling in POMC neurons. Cell Metab 18: 86–98, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sohn JW, Xu Y, Jones JE, Wickman K, Williams KW, Elmquist JK. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron 71: 488–497, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci 3: 757–758, 2000 [DOI] [PubMed] [Google Scholar]
- 121.Tang-Christensen M, Larsen PJ, Thulesen J, Romer J, Vrang N. The proglucagon-derived peptide, glucagon-like peptide-2, is a neurotransmitter involved in the regulation of food intake. Nat Med 6: 802–807, 2000 [DOI] [PubMed] [Google Scholar]
- 122.Tang-Christensen M, Vrang N, Larsen PJ. Glucagon-like peptide containing pathways in the regulation of feeding behaviour. Int J Obes Relat Metab Disord 25 Suppl 5: S42–S47, 2001 [DOI] [PubMed] [Google Scholar]
- 123.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61: 364–371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tolhurst G, Zheng Y, Parker HE, Habib AM, Reimann F, Gribble FM. Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology 152: 405–413, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol 68: 279–305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Viard P, Butcher AJ, Halet G, Davies A, Nurnberg B, Heblich F, Dolphin AC. PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nat Neurosci 7: 939–946, 2004 [DOI] [PubMed] [Google Scholar]
- 127.Vrang N, Hansen M, Larsen PJ, Tang-Christensen M. Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Res 1149: 118–126, 2007 [DOI] [PubMed] [Google Scholar]
- 128.Walsh NA, Yusta B, DaCambra MP, Anini Y, Drucker DJ, Brubaker PL. Glucagon-like peptide-2 receptor activation in the rat intestinal mucosa. Endocrinology 144: 4385–4392, 2003 [DOI] [PubMed] [Google Scholar]
- 129.Wang JH, Wang F, Yang MJ, Yu DF, Wu WN, Liu J, Ma LQ, Cai F, Chen JG. Leptin regulated calcium channels of neuropeptide Y and proopiomelanocortin neurons by activation of different signal pathways. Neuroscience 156: 89–98, 2008 [DOI] [PubMed] [Google Scholar]
- 130.Wang PY, Caspi L, Lam CK, Chari M, Li X, Light PE, Gutierrez-Juarez R, Ang M, Schwartz GJ, Lam TK. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature 452: 1012–1016, 2008 [DOI] [PubMed] [Google Scholar]
- 131.Wang Y, Guan X. GLP-2 potentiates L-type Ca2+ channel activity associated with stimulated glucose uptake in hippocampal neurons. Am J Physiol Endocrinol Metab 298: E156–E166, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci 30: 2472–2479, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wilson-Perez HE, Chambers AP, Ryan KK, Li B, Sandoval DA, Stoffers D, Drucker DJ, Perez-Tilve D, Seeley RJ. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide 1 receptor deficiency. Diabetes 62: 2380–2385, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wright RA, Krinsky S, Fleeman C, Trujillo J, Teague E. Gastric emptying and obesity. Gastroenterology 84: 747–751, 1983 [PubMed] [Google Scholar]
- 135.Yadav VK, Oury F, Tanaka KF, Thomas T, Wang Y, Cremers S, Hen R, Krust A, Chambon P, Karsenty G. Leptin-dependent serotonin control of appetite: temporal specificity, transcriptional regulation, and therapeutic implications. J Exp Med 208: 41–52, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ye J, Hao Z, Mumphrey MB, Townsend RL, Patterson LM, Stylopoulos N, Munzberg H, Morrison CD, Drucker DJ, Berthoud HR. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am J Physiol Regul Integr Comp Physiol 306: R352–R362, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yusta B, Estall J, Drucker DJ. Glucagon-like peptide-2 receptor activation engages bad and glycogen synthase kinase-3 in a protein kinase A-dependent manner and prevents apoptosis following inhibition of phosphatidylinositol 3-kinase. J Biol Chem 277: 24896–24906, 2002 [DOI] [PubMed] [Google Scholar]
- 138.Yusta B, Huang L, Munroe D, Wolff G, Fantaske R, Sharma S, Demchyshyn L, Asa SL, Drucker DJ. Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology 119: 744–755, 2000 [DOI] [PubMed] [Google Scholar]
- 139.Zheng H, Patterson LM, Phifer CB, Berthoud HR. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol 289: R247–R258, 2005 [DOI] [PubMed] [Google Scholar]
- 140.Zheng H, Patterson LM, Rhodes CJ, Louis GW, Skibicka KP, Grill HJ, Myers MG, Jr., Berthoud HR. A potential role for hypothalamomedullary POMC projections in leptin-induced suppression of food intake. Am J Physiol Regul Integr Comp Physiol 298: R720–R728, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zipfel S, Sammet I, Rapps N, Herzog W, Herpertz S, Martens U. Gastrointestinal disturbances in eating disorders: clinical and neurobiological aspects. Auton Neurosci 129: 99–106, 2006 [DOI] [PubMed] [Google Scholar]
- 142.Zsombok A, Bhaskaran MD, Gao H, Derbenev AV, Smith BN. Functional plasticity of central TRPV1 receptors in brainstem dorsal vagal complex circuits of streptozotocin-treated hyperglycemic mice. J Neurosci 31: 14024–14031, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zsombok A, Smith BN. Plasticity of central autonomic neural circuits in diabetes. Biochim Biophys Acta 1792: 423–431, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]