Abstract
Parasite cultivation techniques constitute a substantial segment of present-day study of parasites, especially of protozoa. Success in establishing in vitro and in vivo culture of parasites not only allows their physiology, behavior and metabolism to be studied dynamically, but also allows the nature of the antigenic molecules in the excretory and secretory products to be vigorously pursued and analyzed. The complex life-cycles of various parasites having different stages and host species requirements, particularly in the case of parasitic helminths, often make parasite cultivation an uphill assignment. Culturing of parasites depends on the combined expertise of all types of microbiological cultures. Different parasites require different cultivation conditions such as nutrients, temperature and even incubation conditions. Cultivation is an important method for diagnosis of many clinically important parasites, for example, Entamoeba histolytica, Trichomonas vaginalis, Leishmania spp., Strongyloides stercoralis and free-living amoebae. Many commercial systems like InPouch TV for T. vaginalis, microaerophilous stationary phase culture for Babesia bovis and Harada-Mori culture technique for larval-stage nematodes have been developed for the rapid diagnosis of the parasitic infections. Cultivation also has immense utility in the production of vaccines, testing vaccine efficacy, and antigen - production for obtaining serological reagents, detection of drug-resistance, screening of potential therapeutic agents and conducting epidemiological studies. Though in vitro cultivation techniques are used more often compared with in vivo techniques, the in vivo techniques are sometimes used for diagnosing some parasitic infections such as trypanosomiasis and toxoplasmosis. Parasite cultivation continues to be a challenging diagnostic option. This review provides an overview of intricacies of parasitic culture and update on popular methods used for cultivating parasites.
KEY WORDS: Cultivation, in vitro, in vivo, parasites
INTRODUCTION
Pathogenic microbes can be cultured to aid in identification and this is normally used as the gold standard for the majority of bacterial infections. For many other infections such as viral, rickettsial and amoebic, they represent a good reference method. In most of the parasitic infections, culture is not a routine identification technique. However, culture is useful for clinching the diagnosis in some protozoan parasitic infections, e.g. in case of Central Nervous System infections by free living amoebae; and also culture has immense role in research related to pathogenic parasites. Parasite cultivation is a tricky task, which requires expertise and knowledge of all kinds of microbiological cultures. The earliest attempt in this field was the Novy, Mc Neal and Nicole (NNN) medium for Trypanosoma and Leishmania, made by Novy and McNeal in 1904[1] and modified by Nicolle in 1908.[2] Success in establishing in vitro and in vivo cultures of parasites has allowed their morphology, physiology, behavior and metabolism to be studied dynamically; this understanding has helped tremendously in diagnosis, management, control and prevention of human parasitic diseases.
USES OF CULTIVATION OF PARASITES
Cultivation of parasites is invaluable for a number of reasons, which may be broadly divided as follows:
Patient care
Cultivation is an important adjunct to diagnosis of many clinically important parasites, for example, Entamoeba histolytica, Trichomonas vaginalis, Leishmania spp., free-living amoebae (FLA) and Trypanosoma spp. Live parasitic cultures are used for detection of drug-resistance and for screening of potential therapeutic agents.
Research
Parasite cultivation may be used to study the biochemistry, physiology, and metabolism of the parasites, determine their nutritional requirements, understand their ultra-structural organization, elucidate their patho-physiology, life-cycle and host-parasite relationship, as well as assess functional antibodies and cell-mediated protective systems against the parasites. It is also of use for producing antigens used to prepare monoclonal and polyclonal antibodies against the organisms for use in immunological tests, for providing the inoculums used for experimental animals and for obtaining serological reagents. Identifying specific proteins that enhance the invasiveness of parasites may help in developing monoclonal antibodies to neutralize parasitic invasion. In vitro cultivation also provides a system to assess vaccine efficacy, since that can be done only by using intact parasites, obtained in large quantities and without the contaminating influences of host components.
Epidemiology
Cultivating parasites may help in differentiating clinical isolates. The techniques such as iso-enzyme electrophoresis, monoclonal antibody techniques, and/or DNA probe techniques can be easily applied on cultures; to explicate isolate and strain differences which are useful for epidemiological studies.
Teaching
For demonstrating the characteristics of pathogenic parasites to medical students to facilitate understanding the morphology and physiology; while keeping a stock of cultured parasites is of enormous worth.
DIFFICULTIES IN CULTIVATION OF PARASITES
Parasite cultivation techniques are complex procedures involving a number of issues, some of which are known while some are still undefined Most of the parasites have complex life-cycles with different morphological stages and may have both cold-blooded and warm-blooded animals as hosts within the life-cycle. These stages involve number of variables including parasitic form, host site, host temperature, host immune responses, parasite species and/or strain, and parasite-protective mechanisms. To simulate the host environment, especially in an in vitro culture system can be extremely demanding, assuming one can actually determine all the relevant variables.[3,4,5]
Parasites are often fastidious and require medium components that may be toxic. Filter sterilization may be required in some cases. Human or animal sera, which are expensive and highly variable are usually required for successful culturing of parasites. In some cases, growth factors have been identified and substituted for serum. Much research has been devoted to the development of defined media, although, even with the elimination of serum, other components may not have been totally defined.[6]
TERMINOLOGY
Three types of culture media may be used for cultivating parasites:
Xenic culture - It refers to culture of parasites grown in association with unknown microbiota, for example stool specimens cultured for E. histolytica in National Institute of Health medium. It is used for primary growth of parasites.[7]
Monoxenic culture - If the parasites are grown with a single known bacterium, the culture is referred to as monoxenic, for example corneal biopsy specimens cultured with Escherichia coli as a means of recovering species of Acanthamoeba. It can be used for primary growth as well as a transitional phase in isolation.[7]
Axenic culture - It is a pure culture without any bacterial associate or any other metabolizing cells. It is mainly used as isolation medium for the parasites, but can be used for primary growth also, for example TYI-S-33 medium in case of T. vaginalis.[7]
GENERAL PRINCIPLES
Although the province of parasitic cultivation is very diverse, there are certain principles which are applicable at large to the subject:
Parasitic helminths are more difficult to cultivate than protozoa. The complexity of helminth body configuration and metabolism, and inability to meet essential environmental conditions account for failure to complete their life-cycles under artificial conditions.[8]
Cell cultures are used for the obligate intracellular parasites, for example Plasmodium spp. and coccidia.[9,10]
Various kinds of nutrients such as blood, serum, haem, egg, peptone, minerals and carbohydrates are used in the culture media.[6,9]
Temperature required for optimum growth is usually 37°C though lower temperatures may be required in few cases, e.g. 25°C for Leishmania promastigotes.[11]
Incubation condition is aerobic with some exceptions like microaerophilic conditions for amoebae and Giardia and 5% CO2 for Plasmodium spp.[9,12]
Identification tools include parasite's characteristic morphology, direct fluorescent antibody assay, polymerase chain reaction, enzyme immunoassay, etc.[6,13]
Positive controls need to be run in parallel to keep a check on the medium and the method used.[6]
IN VITRO CULTIVATION OF DIFFERENT PARASITES
Luminal parasitic protists
Luminal protists are first grown in xenic cultures, gradually weaned, then isolated in axenic cultures. While T. vaginalis and Giardia intestinalis can be established directly into axenic cultures, E. histolytica and Blastocystis hominis have never been grown axenically without first being established in xenic cultures. Dientamoeba fragilis and Balantidium coli have never been grown successfully in axenic culture to the best our knowledge.[12,14,15] Some of the important media used for cultivating luminal parasitic protists are described in Table 1. Figure 1 shows the flowchart of culturing luminal protists.
Table 1.
Media for cultivation of luminal parasitic protists
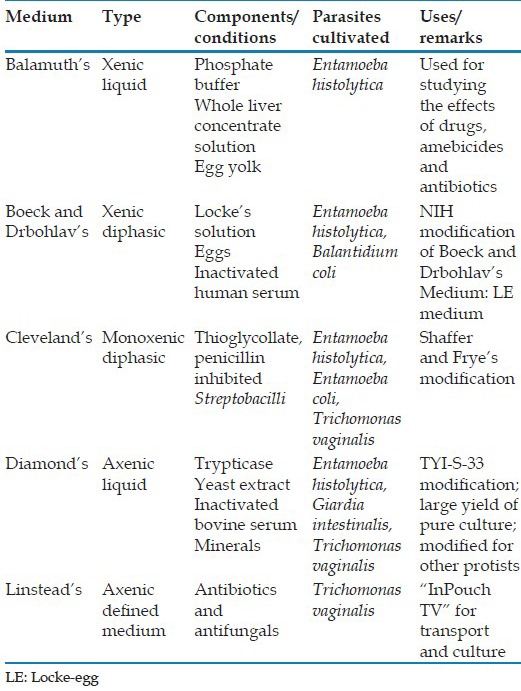
Figure 1.
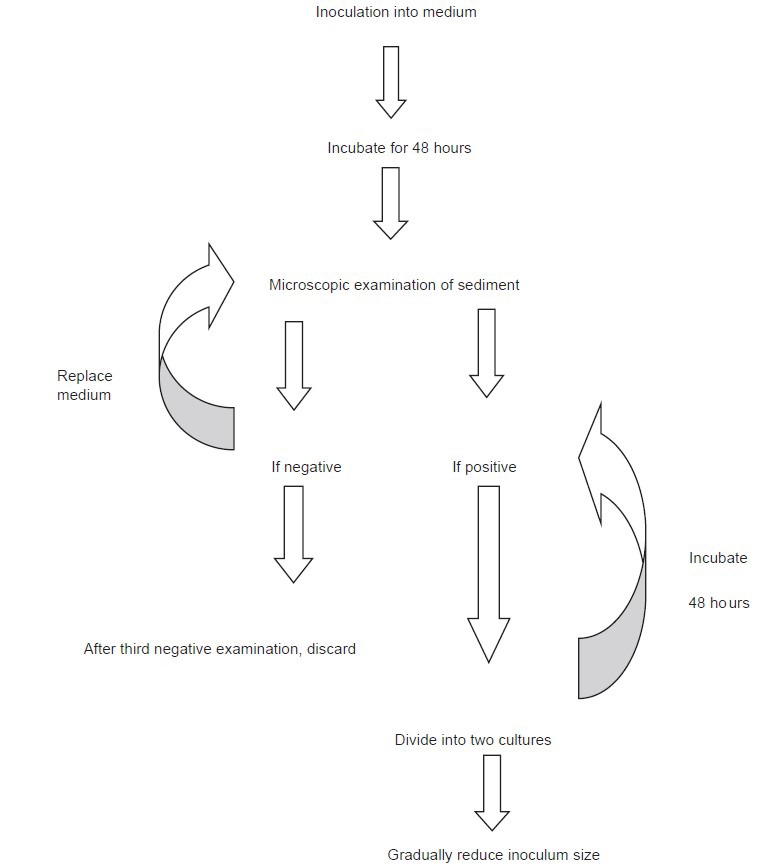
Flow diagram illustrating the stages in establishing luminal protists in culture
Culture is a very sensitive (95%) procedure for the diagnosis of trichomoniasis.[16] It is recommended when microscopy is negative. T. vaginalis grows best at 35°C-37°C under anaerobic conditions. Trussell and Johnson's medium and simplified trypticase serum medium with pH 5.5-6.0 are suitable for isolation of T. vaginalis. A commercial and proprietary product with a reasonable shelf life called InPouch TV (Biomed Diagnostics Inc. White City-OR, USA) gives comparable results and has the advantage of being self-contained.[17]
Maintenance of cultures
Established cultures of all parasites are handled largely in the same way. Xenic cultures of E. histolytica, D. Fragilis, B. hominis and B. coli are routinely passaged at 48–72 h intervals. More inoculum should be used when incubating for longer duration than that for shorter incubation periods. Xenic cultures should be passaged using two or more inoculum sizes to ensure a successful subculture. Established axenic cultures of T. vaginalis, E. histolytica, G. intestinalis, and B. hominis are passaged at 72 and 96 h intervals. For doing subculture, cultures are chilled in an ice-water bath for 5 min (xenic and axenic cultures of E. histolytica) or 10 min (G. intestinalis) to release trophozoites attached to the glass culture tube. In T. vaginalis, B. coli, and B. hominis cultures, most organisms will be nonadherent and the tubes need not be chilled unless an accurate count is desired. Tubes are inverted several times to disperse the cells and a measured inoculum is passed aseptically to a culture tube containing fresh medium. The tubes are capped tightly and incubated at 37°C, either vertically (xenic cultures of T. vaginalis, and axenic cultures of B. hominis) or at 5° to the horizontal (established axenic cultures of E. histolytica and G. intestinalis). For axenic B. hominis cultures the medium must be pre-reduced for 48 h before inoculation in an anaerobic jar.[12]
Free-living amoebae
Culture is often the most readily available procedure for diagnosing infection by FLA.[18] Naegleria and Acanthamoeba are easily isolated from environmental samples; However, the isolation of Balamuthia is a complicated task due to the parasite's slow growth, apparent inability to feed on bacteria, along with the presence of highly competitive soil fauna (fungi, bacteria, other protozoa, and metazoa).[19]
The important media used for cultivating FLA are summarized in Table 2. Naegleria spp. and Acanthamoeba spp. grow readily on nonnutrient agar or agar media containing low concentrations of nutrients in the presence of living or killed bacteria. Nutrients enhance bacterial overgrowth and inhibit the proliferation of amebae. The bacteria of choice include nonmucoid strains of Klebsiella pneumoniae, Enterobacter spp. and E. coli, the presence of a mucoid capsule impedes phagocytosis by amebae and leads to bacterial overgrowth.[20,21] In Acanthamoeba keratitis significantly higher sensitivity of parasite detection can be obtained with samples processed by filtration compared with centrifugation.[20] Balamuthia does not grow with bacteria as a food source, but has been grown from brain tissue by providing tissue culture cells as a feeder layer.[22]
Table 2.
Media used for cultivating free living amoebae
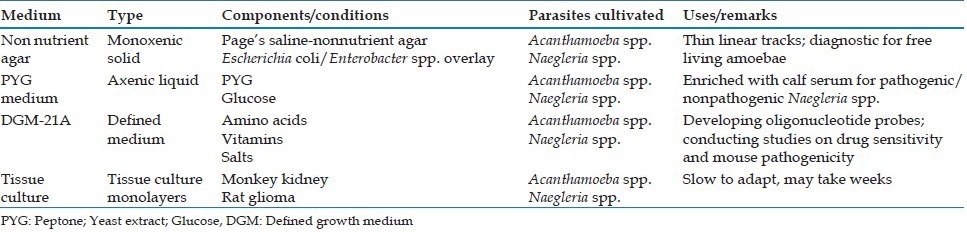
Free-living amoebae are first grown in xenic cultures, followed by gradual axenization. To various degrees, these parasites can be established in axenic culture from initially bacterized cultures by providing an enriched nutrient medium with antibiotics (penicillin-streptomycin and gentamicin) added to inhibit growth of contaminating bacteria.[18,19] All three genera can be grown axenically in the presence of tissue culture monolayers, where the tissue culture cells provide a feeder layer for the actively phagocytic amebae.[18]
Hemoflagellates
Culture is useful for diagnosing difficult cases of leishmaniasis, particularly post kala-azar dermal leishmaniasis. The commonly used media used for cultivation of hemoflagellates are summarized in Table 3.
Table 3.
Media for cultivation of haemoflagellates
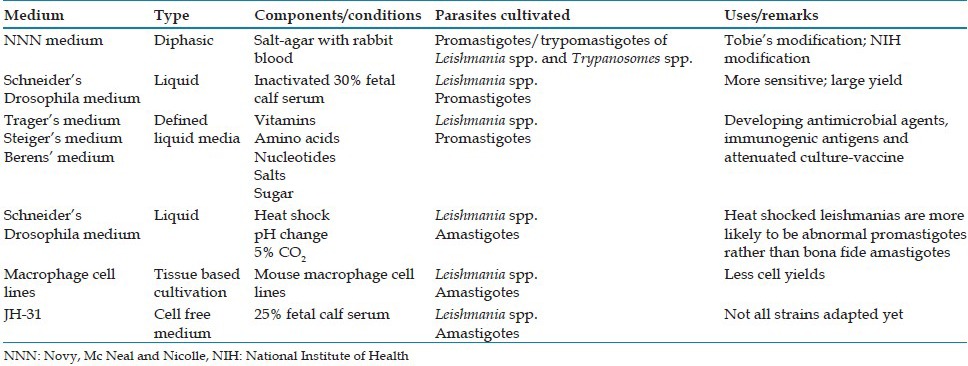
The nutritional requirements of these parasites has been determined using cultivation techniques. Haemoflagellate culture has not only provided a basis for selecting or designing antimicrobial agents that can be tailored to specific pathways, but has also helped in knowing mechanism of antigenic variation, defining immunogenic antigens, and developing attenuated strains that can be used for protecting humans and cattle from hemoflagellate-caused diseases.[11]
Leishmania promastigotes are cultured at temperatures below 28°C.[23,24] NNN medium, first used for isolation of the agent of oriental sore by Nicolle,[2] and other biphasic-type media are routinely used for maintenance, producing yields of 107–108 cells/ml.[25,26,27] The original formulation of NNN medium remains useful for establishment of strains in culture.[4] A number of media developed for trypanosomes, either directly or with modifications, have also been used successfully to culture Leishmania promastigotes. Blood, preferably anticoagulated and heat inactivated rabbit blood - is an essential component of these media.[4,25,27] Axenic growth of amastigotes in cell-free media has been recently achieved but the yield is less. All Leishmania strains are not well adapted to grow in cell-free media.[11]
In cases of leishmaniasis, blood samples, needle aspirates, and punch or deep organ biopsies can be used for culture. In a study done by López-Vélez et al. the hemoculture positivity was found to be 67%, with average growing time of 10 days.[28] More useful samples are spleen aspirations (94% positivity), liver biopsies (76-90% positivity), and bone-marrow biopsies (76-90% positivity).[29] However, as retrieval of liver and spleen biopsy specimens can result in severe complications, most preferred is the use of hemoculture. Sensitivity of the culture reported in the course of cutaneous leishmaniasis is comparable to that of visceral leishmaniasis; however, contamination concerns much more cutaneous samples than blood or bone-marrow ones.[30]
Bello et al. have demonstrated interaction of Leishmania chagasi with cells derived from Lutzomyia longipalpis (the main vector for this parasite), as well as the development, biological cycle and intracellular replication of the parasite; suggesting that these cells and in future - other phlebotomine cell cultures could be considered for studying in vitro interaction between Leishmania parasites and sand fly vectors.[31]
Plasmodium spp.
Out of the five species of genus Plasmodium, which are pathogenic to humans, four - Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, and Plasmodium malariae, have been cultured or maintained in vitro. P. falciparum, however, is the only species for which all life-cycle stages have been established in culture.[32,33,34]
The important media used for cultivating malarial parasite are shown in Table 4. While in vitro growth of plasmodia is significant, growth under a completely cell-free condition is more important for research.[9] Toward this end, successful development of P. falciparum from merozoites to ring stage under cell-free conditions has been demonstrated.[35] The studies done have suggested that factors present in the erythrocyte but not necessarily the intracytoplasmic location of the parasite were needed for development.[35,36] These extracellular forms react to the same monoclonal antibodies that the intracellular forms respond to, suggesting a similar pattern of molecular differentiation.[9,37]
Table 4.
Media for cultivation of Plasmodium spp.
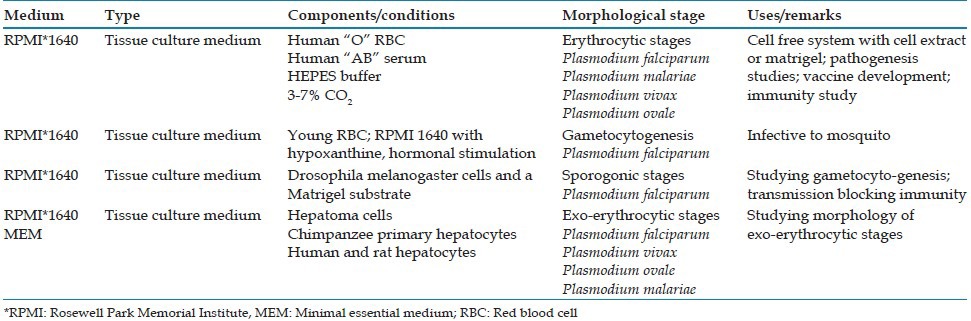
Further refinement of technique is the use of a system with merozoites embedded in a Matrigel substrate in a well containing a fluid medium overlay,[38,39] which could be changed with minimal disturbance to the parasites developing within the Matrigel layer. Ring-stage parasites, gametocytes and shizonts; all have been demonstrated by different workers in this system.[38,40]
Cell suspension (using a shaking-incubator) - significantly increases culture growth, prolongs culture synchrony, and reduces rate of multiple infections.[41] Despite the advances, the practice of culturing the parasite under static conditions inside a candle-jar remains widespread, as this can be used in laboratories almost anywhere in the world where there is an incubator, a candle and a desiccator.[42] Differences exist between strains of Plasmodium,[9] and isolates also undergo change once in culture, perhaps due to selection. In P. falciparum, for example, gametocyte formation is typical of recently cultured strains but is lost with prolonged cultivation. In this regard, it is important to note that cryopreservation of isolates can maintain those characteristics in a strain that may be lost on prolonged cultivation.[43]
Babesia spp.
Techniques for culturing Babesia have been developed only for animal species as yet.[44] Given the similar niches of the malaria parasite and Babesia, one might suppose that the methods for cultivation of Plasmodium would work for Babesia; however - this has not been the case.[44,45,46]
Cattle parasite Babesia bovis was the first one to be established in continuous cultures using an agitated suspension culture technique and later on using a stationary layer of erythrocytes called the microaerophilous stationary phase (MASP) culture, where the parasites proliferate in a settled layer of blood cells.[46,47,48] The MASP system is considered more convenient for parasite growth.[46,49] Working with B. bovis, James et al. utilized the MASP culture technique to demonstrate exo-antigens released by the parasite.[44,50] Another study revealed that supernatants derived from Babesia antigen-treated monocyte cultures were inhibitory for B. bovis cultures.[44,51] Culture - attenuated live babesias and exo-antigens have been used to immunize cattle, with the former being more protective than the latter.[44,52]
Recently a novel method for the culture of Babesia canis involving use of Ham's medium F10 or F12 incubated first under higher temperature of 34°C-38°C followed by lower temperature of 0°C-10°C, has been developed by Nathalie Laurent under US patent 4777036. This method is useful for large scale production of B. canis antigens, which may be used for producing vaccine against canine piroplasmosis.[53]
Coccidia and microsporidia
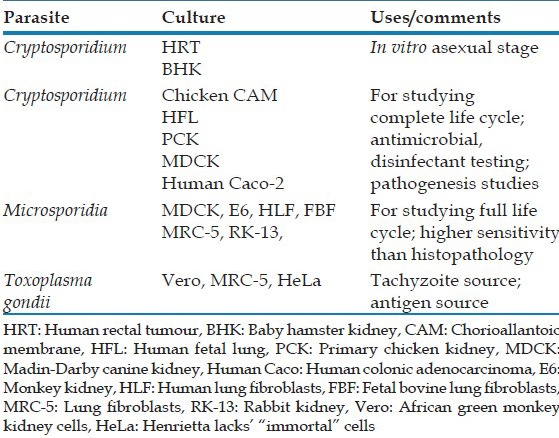
The cell lines/culture environments which have been found useful for cultivating coccidian and microspoidia are summarized in Table 5.[54] The earliest attempts were done by Current and Long, who described the development of Cryptosporidium spp. in the endoderm cells of the chorioallantoic membrane (CAM) of chicken embryos maintained at 37°C.[55] Despite this initially reported success, later workers could not completely replicate the results. Afterwards, some success was achieved in human fetal lung, primary chicken kidney, and porcine kidney-10 cells.[10,13] Human colonic tumour-8 cell lines have by far performed best in cultivating Crptosporidia, when done under 5% CO2 at 37°C.[13,56,57,58,59,60,61] It is worth mentioning here that the most human-relevant species of Microsporidia- Enterocytozoon bieneusi cannot be cultured.
Table 5.
Media for cultivation of coccidia and microsporidia
Helminths
Attempts have been made to culture parasitic helminths in nutrient media, and in some instances partial success has been achieved. Some common media used for helminth cultivation are shown in Table 6. Aseptic techniques in bacteria-free media have prolonged the life of the worms, and in some species have resulted in development from larva to a more mature sexual stage or to sexual maturity. The complexity of helminth metabolism and inability to meet essential environmental conditions account for failure to complete the life-cycles of many of these organisms under artificial conditions.[8]
Table 6.
Media for cultivation of helminths
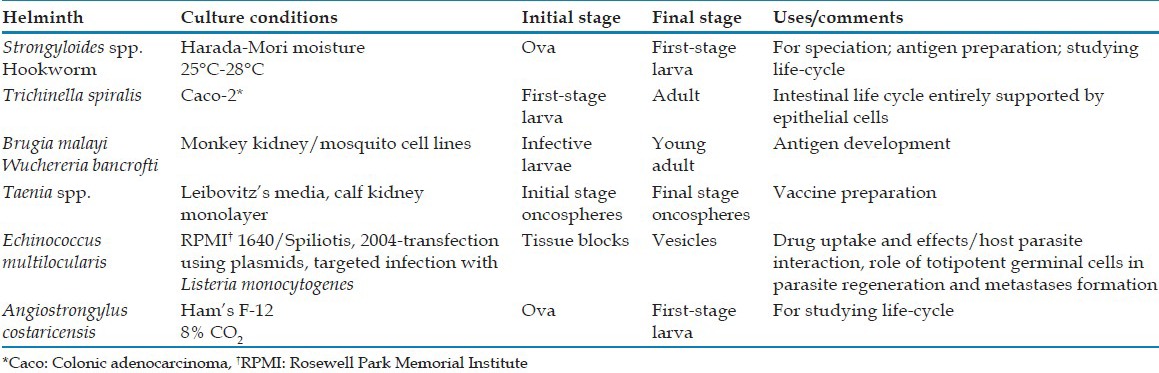
Considerable success has been achieved in efforts to develop parasitic nematodes in axenic liquid media through free-living larval-stages and in some instances the more mature (3rd and 4th) parasitic larval-stages.[62]
One of the few helminth systems for which in vitro cultivation has been relatively successfully carried out is the larval-stage of Echinococcus multilocularis. Spiliotis et al. have introduced the first truly axenic system for long-term in vitro maintenance of meta-cestode vesicles.[63]
It is relatively easy to obtain the free-living stages of Strongyloides spp. exhibiting indirect development. Rhabditiform larvae of these strains, recovered from the stool of natural hosts, provide the inoculums. If conditions of moisture, pH and nutrition are properly maintained, development may be continued through several free-living generations, and theoretically for an indefinite period before the organism produces filariform larvae.[64]
Out of the various cultivation techniques used for Strongyloides infection, Baermann technique is the most popular.[65] Agar plate culture is more sensitive in which stool is placed onto agar plates and visible tracks are created as the larvae crawl carrying bacteria with them. The plates are examined under the microscope for confirmation of the presence of larvae.[65]
Larval-stage nematodes may also be cultured using Harada-Mori technique, which employs a filter paper to which fecal material is added and is inserted into a test tube. Moisture is provided by water in the tube, and incubation under suitable conditions favors hatching of ova and/or development of larvae. Fecal specimens to be cultured should not be refrigerated as some parasites (especially Necator americanus) may fail to develop after refrigeration.[66,67,68]
MAINTENANCE OF STOCK CULTURES
There are various methods for maintaining stock cultures of protozoan parasites, for example repeated subcultures, freezing at −20°C and ultra-low temperature freezing at −70°C to −196°C using cryoprotectants such as 10-30% glycerol, 5% dimethyl sulfoxide and sucrose.[69] They can also be stored as dried filter paper strips at room temperature.[70]
IN VIVO CULTIVATION OF PARASITES
Some animal parasites which have thus far been refractory to in vitro cultivation may be developed in appropriate animal tissues.[71,72] Some important examples of animal inoculation being used an in vivo diagnostic method are summarized in Table 7.
Table 7.
Animals used for in vivo cultivation of parasites

XENODIAGNOSIS
Triatomid bugs have been employed for diagnosing Trypanosoma cruzi infections where it has not been possible to demonstrate the organisms in blood films. Laboratory reared bugs are fed upon patient's blood, and the intestinal contents of the bugs are examined for flagellates 10-30 days after the blood meal. These may then be inoculated into mice for further confirmation.[73]
Davis and Mavros have employed the xeno-diagnostic principle to identify Borrelia hispanica using ticks and guinea pigs,[74] while Beck fed human diaphragms to clean white rats to detect Trichinella spiralis larvae that otherwise would have escaped discovery.[75]
SUMMARY
In 1904, Novy, Mc Neal and Nicole initiated culture of parasites by their success in cultivating Trypanosoma and Leishmania sp. In conjunction with the rapid advancement in microbiological culture techniques, great strides were also made in culture of parasites. Individuals working in this field have indicated that successful parasitic culture is a science as well as art; therefore, clinical laboratories have been somewhat hesitant to undertake these types of diagnostic procedures on a routine basis. However, with proper culture controls in addition to patient specimen cultures, clinically relevant information can be obtained from cultures when other diagnostic methods may be unsuccessful. Though both in vitro and in vivo cultivation techniques are significant in their own rights, the in vitro techniques are easier to standardize than that of in vivo, hence it is easier to compare the data generated by various researchers. Cultivation of parasites that cause human diseases is invaluable, as it provides information on the development and natural history of the parasite and opens avenues for new approaches to the containment and/or eradication of the parasite.
Footnotes
Source and Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Novy FG, McNeal WJ. On the cultivation of Trypanosoma brucei. J Infect Dis B. 1904;1:1–30. [Google Scholar]
- 2.Nicolle CH. Culture du parasite du Bouton d’Orient. C R Acad Sci (Paris) 1908;146:842. [Google Scholar]
- 3.Diamond LS. Entamoeba, Giardia and Trichomonas. In: Taylor AE, Baker JR, editors. In Vitro Methods for Parasite Cultivation. Orlando, Fla: Academic Press; 1987. pp. 1–28. [Google Scholar]
- 4.Evans DA. Leishmania. In: Taylor AE, Baker JR, editors. In vitro methods for parasite cultivation. Orlando, Fla: Academic Press; 1987. pp. 52–75. [Google Scholar]
- 5.Visvesvara GS. Pathogenic and opportunistic free-living amebae. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of Clinical Microbiology. 7th ed. Washington, D.C: ASM Press; 1999. pp. 1383–90. [Google Scholar]
- 6.Visvesvara GS, Garcia LS. Culture of protozoan parasites. Clin Microbiol Rev. 2002;15:327–8. doi: 10.1128/CMR.15.3.327-328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia LS. 4th ed. Washington, D.C: ASM Press; 2001. Diagnostic Medical Parasitology; pp. 850–72. [Google Scholar]
- 8.Yasuraoka K, Hata H. In Vitro cultivation of parasitic helminths. Prog Med Parasitol Jpn. 2003;7:211–26. [Google Scholar]
- 9.Schuster FL. Cultivation of plasmodium spp. Clin Microbiol Rev. 2002;15:355–64. doi: 10.1128/CMR.15.3.355-364.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Current WL, Haynes TB. Complete development of Cryptosporidium in cell culture. Science. 1984;224:603–5. doi: 10.1126/science.6710159. [DOI] [PubMed] [Google Scholar]
- 11.Schuster FL, Sullivan JJ. Cultivation of clinically significant hemoflagellates. Clin Microbiol Rev. 2002;15:374–89. doi: 10.1128/CMR.15.3.374-389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark CG, Diamond LS. Methods for cultivation of luminal parasitic protists of clinical importance. Clin Microbiol Rev. 2002;15:329–41. doi: 10.1128/CMR.15.3.329-341.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arrowood MJ. In vitro cultivation of Cryptosporidium species. Clin Microbiol Rev. 2002;15:390–400. doi: 10.1128/CMR.15.3.390-400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia LS. Parasitology. In: Isenberg HD, Garcia LS, editors. Clinical Microbiology Procedures Handbook. Sec. 7. Washington, D.C: American Society for Microbiology; 1992. p. 7. 0.1.7.10.8.2. [Google Scholar]
- 15.Taylor AE, Baker JR. Oxford, United Kingdom: Blackwell Scientific Publications, Ltd; 1968. The Cultivation of Parasites In Vitro. [Google Scholar]
- 16.Arora DR, Arora BB. Flagellates. In: Arora DR, Arora BB, editors. Medical Parasitology. 3rd edition. Delhi-India: CBS Publishers and Distributers Private Limited; 2010. p. 46. [Google Scholar]
- 17.Borchardt KA, Smith RF. An evaluation of an InPouch TV culture method for diagnosing Trichomonas vaginalis infection. Genitourin Med. 1991;67:149–52. doi: 10.1136/sti.67.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuster FL. Cultivation of pathogenic and opportunistic free-living amebas. Clin Microbiol Rev. 2002;15:342–54. doi: 10.1128/CMR.15.3.342-354.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuster FL, Visvesvara GS. Axenic growth and drug sensitivity studies of Balamuthia mandrillaris, an agent of amebic meningoencephalitis in humans and other animals. J Clin Microbiol. 1996;34:385–8. doi: 10.1128/jcm.34.2.385-388.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winiecka-Krusnell J, Linder E. Acanthamoeba keratitis: Increased sensitivity of the detection of parasites by modified cultivation procedure. Scand J Infect Dis. 1998;30:639–41. doi: 10.1080/00365549850161340. [DOI] [PubMed] [Google Scholar]
- 21.Weekers PH, Bodelier PL, Wijen JP, Vogels GD. Effects of grazing by the free-living soil Amoebae Acanthamoeba castellanii, Acanthamoeba polyphaga, and Hartmannella vermiformis on various bacteria. Appl Environ Microbiol. 1993;59:2317–9. doi: 10.1128/aem.59.7.2317-2319.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Visvesvara GS, Martinez AJ, Schuster FL, Leitch GJ, Wallace SV, Sawyer TK, et al. Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J Clin Microbiol. 1990;28:2750–6. doi: 10.1128/jcm.28.12.2750-2756.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balanco JM, Pral EM, da Silva S, Bijovsky AT, Mortara RA, Alfieri SC. Axenic cultivation and partial characterization of Leishmania braziliensis amastigote-like stages. Parasitology. 1998;116:103–13. doi: 10.1017/s003118209700214x. [DOI] [PubMed] [Google Scholar]
- 24.Pan AA. Leishmania mexicana: Serial cultivation of intracellular stages in a cell-free medium. Exp Parasitol. 1984;58:72–80. doi: 10.1016/0014-4894(84)90022-5. [DOI] [PubMed] [Google Scholar]
- 25.Chang KP, Fish WR. Leishmania. In: Jensen JB, editor. In Vitro Cultivation of Protozoan Parasites. Boca Raton, Fla: CRC Press, Inc; 1983. pp. 111–53. [Google Scholar]
- 26.Taylor AR, Baker JR, editors. London: Academic Press; 1978. Methods of Cultivating Parasites In Vitro; pp. 55–88. [Google Scholar]
- 27.Collee JG, Fraser AG, Marmion BP, Simmons A, editors. 14th edition. London- United Kingdom: Churchill Livingstone (Imprint of Elsevier); 1996. Mackie and McCartney, Practical Medical Microbiology. [Google Scholar]
- 28.López-Vélez R, Laguna F, Alvar J, Pérez-Molina JA, Molina R, Martinez P, et al. Parasitic culture of buffy coat for diagnosis of visceral leishmaniasis in human immunodeficiency virus-infected patients. J Clin Microbiol. 1995;33:937–9. doi: 10.1128/jcm.33.4.937-939.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zijlstra EE, Ali MS, el-Hassan AM, el-Toum IA, Satti M, Ghalib HW, et al. Kala-azar: A comparative study of parasitological methods and the direct agglutination test in diagnosis. Trans R Soc Trop Med Hyg. 1992;86:505–7. doi: 10.1016/0035-9203(92)90086-r. [DOI] [PubMed] [Google Scholar]
- 30.Chouihi E, Amri F, Bouslimi N, Siala E, Selmi K, Zallagua N, et al. Cultures on NNN medium for the diagnosis of leishmaniasis. Pathol Biol (Paris) 2009;57:219–24. doi: 10.1016/j.patbio.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Bello FJ, Mejía AJ, Corena Mdel P, Ayala M, Sarmiento L, Zuñiga C, et al. Experimental infection of Leishmania (L.) chagasi in a cell line derived from Lutzomyia longipalpis (Diptera: Psychodidae) Mem Inst Oswaldo Cruz. 2005;100:519–25. doi: 10.1590/s0074-02762005000600004. [DOI] [PubMed] [Google Scholar]
- 32.Hollingdale MR. Is culture of the entire plasmodium cycle, in vitro, now a reality? Parasitol Today. 1992;8:223. doi: 10.1016/0169-4758(92)90114-h. [DOI] [PubMed] [Google Scholar]
- 33.Beaudoin RL. Should cultivated exoerythrocytic parasites be considered as a source of antigen for a malaria vaccine? Bull World Health Organ. 1977;55:373–6. [PMC free article] [PubMed] [Google Scholar]
- 34.Geiman QM, Anfinsen CB, McKee RW, Ormsbee RA, Ball EG. Studies on malarial parasites: VII. Methods and techniques for cultivation. J Exp Med. 1946;84:583–606. doi: 10.1084/jem.84.6.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trager W, Lanners HN. Initial extracellular development in vitro of merozoites of Plasmodium falciparum. J Protozool. 1984;31:562–7. doi: 10.1111/j.1550-7408.1984.tb05503.x. [DOI] [PubMed] [Google Scholar]
- 36.Trager W, Zung J, Tershakovec M. Initial extracellular development in vitro of erythrocytic stages of malaria parasites (Plasmodium falciparum) Proc Natl Acad Sci U S A. 1990;87:5618–22. doi: 10.1073/pnas.87.15.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zung JM, Trager W, Gubert E. Initial extracellular forms of Plasmodium falciparum: Their ultrastructure and their definition with monoclonal antibodies. Proc Natl Acad Sci U S A. 1991;88:89–92. doi: 10.1073/pnas.88.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trager W, Williams J. Extracellular (axenic) development in vitro of the erythrocytic cycle of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1992;89:5351–5. doi: 10.1073/pnas.89.12.5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trager W, Williams J, Gill GS. Extracellular development, in vitro, of the erythrocytic cycle of Plasmodium falciparum. Parasitol Today. 1992;8:384–7. doi: 10.1016/0169-4758(92)90177-4. [DOI] [PubMed] [Google Scholar]
- 40.Williams JH, Gill GS, Trager W. Effect of erythrocyte membrane on extracellular development of the erythrocytic cycle of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1995;92:566–8. doi: 10.1073/pnas.92.2.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allen RJ, Kirk K. Plasmodium falciparum culture: The benefits of shaking. Mol Biochem Parasitol. 2010;169:63–5. doi: 10.1016/j.molbiopara.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Sherman I. Washington, D.C: ASM Press; 2010. Magic Bullets to Conquer Malaria: From Quinine to Qinghaos. [Google Scholar]
- 43.Ponnudurai T. Plasmodiidae: Erythrocytic stages. In: Taylor AE, Baker JR, editors. In Vitro Methods for Parasite Cultivation. New York: Academic Press; 1987. pp. 153–79. [Google Scholar]
- 44.Schuster FL. Cultivation of Babesia and Babesia-like blood parasites: Agents of an emerging zoonotic disease. Clin Microbiol Rev. 2002;15:365–73. doi: 10.1128/CMR.15.3.365-373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erp EE, Gravely SM, Smith RD, Ristic M, Osorno BM, Carson CA. Growth of Babesia bovis in bovine erythrocyte cultures. Am J Trop Med Hyg. 1978;27:1061–4. doi: 10.4269/ajtmh.1978.27.1061. [DOI] [PubMed] [Google Scholar]
- 46.Levy MG, Ristic M. Babesia bovis: Continuous cultivation in a microaerophilous stationary phase culture. Science. 1980;207:1218–20. doi: 10.1126/science.7355284. [DOI] [PubMed] [Google Scholar]
- 47.Erp EE, Smith RD, Ristic M, Osorno BM. Continuous in vitro cultivation of Babesia bovis. Am J Vet Res. 1980;41:1141–2. [PubMed] [Google Scholar]
- 48.Goff WL, Yunker CE. Effects of PH, buffers and medium-storage on the growth of Babesia bovis in vitro. Int J Parasitol. 1988;18:775–8. doi: 10.1016/0020-7519(88)90118-x. [DOI] [PubMed] [Google Scholar]
- 49.Canning EU, Winger CM. Babesiidae. In: Taylor AE, Baker JR, editors. In Vitro Methods for Parasite Cultivation. New York: Academic Press; 1987. pp. 199–229. [Google Scholar]
- 50.James MA, Levy MG, Ristic M. Isolation and partial characterization of culture-derived soluble Babesia bovis antigens. Infect Immun. 1981;31:358–61. doi: 10.1128/iai.31.1.358-361.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montealegre F, Levy MG, Ristic M, James MA. Growth inhibition of Babesia bovis in culture by secretions from bovine mononuclear phagocytes. Infect Immun. 1985;50:523–6. doi: 10.1128/iai.50.2.523-526.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shkap V, Pipano E. Culture-derived parasites in vaccination of cattle against tick-borne diseases. Ann N Y Acad Sci. 2000;916:154–71. doi: 10.1111/j.1749-6632.2000.tb05286.x. [DOI] [PubMed] [Google Scholar]
- 53.Laurent N, l’Etoile M. Process for the culture of Babesia canis. Application to the preparation of antigens and vaccine against pyroplasmosis. Patent No. US 4,777,036. 1988 [Google Scholar]
- 54.Visvesvara GS. In vitro cultivation of microsporidia of clinical importance. Clin Microbiol Rev. 2002;15:401–13. doi: 10.1128/CMR.15.3.401-413.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Current WL, Long PL. Development of human and calf Cryptosporidium in chicken embryos. J Infect Dis. 1983;148:1108–13. doi: 10.1093/infdis/148.6.1108. [DOI] [PubMed] [Google Scholar]
- 56.Upton SJ. In vitro culture. In: Fayer R, editor. Cryptosporidium and Cryptosporidiosis. Boca Raton, Fla: CRC Press; 1997. pp. 43–64. [Google Scholar]
- 57.Upton SJ, Tilley M, Brillhart DB. Comparative development of Cryptosporidium parvum (Apicomplexa) in 11 continuous host cell lines. FEMS Microbiol Lett. 1994;118:233–6. doi: 10.1111/j.1574-6968.1994.tb06833.x. [DOI] [PubMed] [Google Scholar]
- 58.Upton SJ, Tilley M, Brillhart DB. Comparative development of Cryptosporidium parvum in MDBK and HCT-8 cells under select atmospheres. Biomed Lett. 1994;49:265–71. [Google Scholar]
- 59.Upton SJ, Tilley M, Brillhart DB. Effects of select medium supplements on in vitro development of Cryptosporidium parvum in HCT-8 cells. J Clin Microbiol. 1995;33:371–5. doi: 10.1128/jcm.33.2.371-375.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Upton SJ, Tilley M, Mitschler RR, Oppert BS. Incorporation of exogenous uracil by Cryptosporidium parvum in vitro. J Clin Microbiol. 1991;29:1062–5. doi: 10.1128/jcm.29.5.1062-1065.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Upton SJ, Tilley M, Nesterenko MV, Brillhart DB. A simple and reliable method of producing in vitro infections of Cryptosporidium parvum (Apicomplexa) FEMS Microbiol Lett. 1994;118:45–9. doi: 10.1111/j.1574-6968.1994.tb06801.x. [DOI] [PubMed] [Google Scholar]
- 62.Faust EC. Culture techniques. In: Faust EC, Russell PF, Jung RC, editors. Clinical Parasitology. 8th ed. Philadelphia: Lea and Febiger; 1970. pp. 800–5. [Google Scholar]
- 63.Spiliotis M, Tappe D, Sesterhenn L, Brehm K. Long-term in vitro cultivation of Echinococcus multilocularis metacestodes under axenic conditions. Parasitol Res. 2004;92:430–2. doi: 10.1007/s00436-003-1046-8. [DOI] [PubMed] [Google Scholar]
- 64.Beaver PC. Culture methods. In: Beaver PC, Jung RC, Wayne E, editors. Clinical Parasitology. 9th ed. Philadelphia: Lea and Febiger; 1984. p. 763. [Google Scholar]
- 65.Arora DR, Arora BB. Nematodes. In: Arora DR, Arora BB, editors. Medical Parasitology. 3rd edition. Delhi-India: CBS Publishers and Distributers Private Limited; 2010. p. 183. [Google Scholar]
- 66.Harada U, Mori O. A new method for culturing hookworm. Yonada Acta Med. 1955;1:177–9. [Google Scholar]
- 67.Hsieh HC. A Test-tube Filter Paper Method for the Diagnosis of Ancylostoma duodenale, Necator americanus and Strongyloides stercoralis. World Health Organ Tech Rep Ser. 1962;255:27–30. [Google Scholar]
- 68.Hayashi S, Tanaka H, Shirasaka R. Application of test-tube cultivation method on the survey of bookworm and related human nematodes infection. Jpn J Exp Med. 1958;28:129–37. [PubMed] [Google Scholar]
- 69.Miyake Y, Karanis P, Uga S. Cryopreservation of protozoan parasites. Cryobiology. 2004;48:1–7. doi: 10.1016/j.cryobiol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 70.Pens CJ, Rott MB. Acanthamoeba spp. cysts storage in filter paper. Parasitol Res. 2008;103:1229–30. doi: 10.1007/s00436-008-1093-2. [DOI] [PubMed] [Google Scholar]
- 71.Peyron F, Lobry JR, Musset K, Ferrandiz J, Gomez-Marin JE, Petersen E, et al. Serotyping of Toxoplasma gondii in chronically infected pregnant women: Predominance of type II in Europe and types I and III in Colombia (South America) Microbes Infect. 2006;8:2333–40. doi: 10.1016/j.micinf.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 72.Sibley LD, Mordue DG, Su C, Robben PM, Howe DK. Genetic approaches to studying virulence and pathogenesis in Toxoplasma gondii. Philos Trans R Soc Lond B Biol Sci. 2002;357:81–8. doi: 10.1098/rstb.2001.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maekelt GA. A modified procedure of xenodiagnosis for chagas disease. Am J Trop Med Hyg. 1964;13:11–5. doi: 10.4269/ajtmh.1964.13.11. [DOI] [PubMed] [Google Scholar]
- 74.Davis GE, Mavros AJ. The long survival of Borrelia hispanica (de Buen) in the argasid tick Ornithodorus nicollei Mooser. J Parasitol. 1955;4:277–81. doi: 10.1016/0014-4894(55)90031-x. [DOI] [PubMed] [Google Scholar]
- 75.Beck JW. Xenodiagnostic technic as an aid in diagnosis of trichinosis. Am J Trop Med Hyg. 1953;2:97–101. doi: 10.4269/ajtmh.1953.2.97. [DOI] [PubMed] [Google Scholar]


