Abstract
Leptin is secreted by adipose tissue and regulates energy homeostasis, glucose and lipid metabolism, immune function, and other systems. The binding of leptin to its specific receptor activates various intracellular signaling pathways, including Janus kinase 2 (JAK2)/ signal transducer and activator of transcription 3 (STAT3), insulin receptor substrate (IRS)/phosphatidylinositol 3 kinase (PI3K), SH2-containing protein tyrosine phosphatase 2 (SHP2)/mitogen-activated protein kinase (MAPK), and 5' adenosine monophosphate-activated protein kinase (AMPK)/ acetyl-CoA carboxylase (ACC), in the central nervous system and peripheral tissues. Understanding of leptin signaling provides insights into its roles in health and disease.
Introduction
Leptin is primarily synthesized and secreted by white adipose tissue and acts in the brain and several peripheral tissues. Leptin plays important roles in the regulation of food intake, energy expenditure, metabolism, neuroendocrine axis, and immune function. The binding of leptin to its specific receptor in the brain leads to activation of multiple signal transduction pathways. A number of intracellular signaling pathways are involved in modulating leptin's central and peripheral effects. In this review, we will focus mainly on leptin signaling under normal physiological conditions.
Physiology of leptin
Leptin is mainly synthesized and secreted by white adipose tissue and acts in the brain to regulate energy homeostasis. Leptin is also expressed in a variety of tissues, including placenta, ovary, skeletal muscle, pituitary gland, and lymphoid tissue [1]. Circulating leptin levels are directly proportional to the amount of body fat, thereby reflecting the status of long-term energy stores. Leptin levels fluctuate according to changes in calorie intake, with a marked decrease during starvation and an increase in overfed and obese states [2]. Leptin is secreted in a pulsatile fashion and also displays a circadian rhythm. Leptin levels exhibit a sexual dimorphism. Women have higher leptin levels than men because of an increase in leptin expression in subcutaneous adipose tissue, stimulation of leptin synthesis by estrogen, and inhibition of leptin synthesis by testosterone [3]. Leptin levels are increased by insulin, glucocorticoids, and pro-inflammatory cytokines and decreased by catecholamines [4].
Leptin mediates its effects by binding to leptin receptors (LepRs) expressed in the brain and peripheral tissues. Various alternatively spliced isoforms of LepR have been described, but the long isoform of leptin receptor (LepRb) is primarily responsible for leptin signaling. LepRb is strongly expressed in the hypothalamus and other areas of the brain, where it regulates energy homeostasis, hedonic regulation of feeding, neuroendocrine function, and memory and learning. Leptin-deficient ob/ob mice and LepRb-deficient db/db mice develop hyperphagia, morbid obesity, infertility, and reduced linear growth [5]. Congenital leptin deficiency or loss-of-function mutations of the leptin receptor in humans also result in hyperphagia and morbid obesity.
The binding of leptin to LepRb activates a number of signaling pathways, JAK2/STAT 3 and STAT5, IRS/PI3K, SHP2/MAPK, and AMPK/ACC (Figure 1). The leptin signaling cascade is terminated by the induction of a suppressor of cytokine signaling 3 (SOCS3). SOCS3 inhibits JAK2/STAT3 signaling, providing a negative feedback mechanism. In addition, protein tyrosine phosphatase 1B (PTP1B) is implicated in the negative regulation of leptin signaling (Figure 1) [2,3].
Figure 1. Multiple leptin signaling pathways.
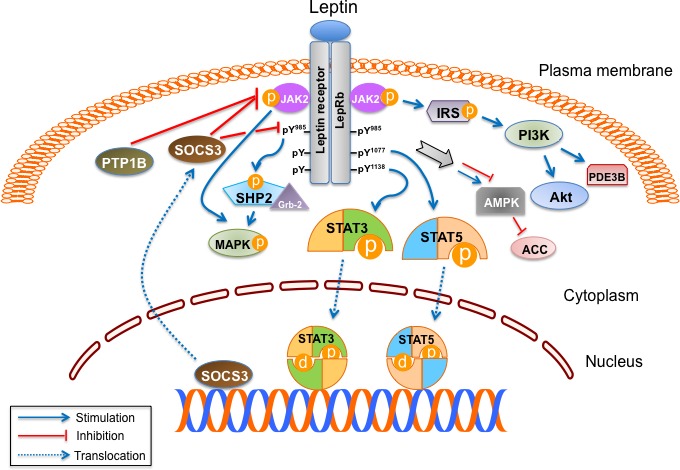
The binding of leptin to the long isoform of leptin receptor (LepRb) results in its dimerization, leading to the formation of the LepRb/Janus kinase 2 (JAK2) complex. The activated JAK2 phosphorylates itself and also Tyr985, Tyr1077, and Tyr1138 in LepRb. Signal transducer and activator of transcription (STAT) 3 and STAT5 bind to phospho-Tyr1138 and phospho-Tyr1077 in LepRb and are subsequently phosphorylated. Active STAT3 and STAT5 dimers then translocate to the nucleus and activate the transcription of their target genes, which mediate leptin's anorexigenic effect. Suppressor of cytokine signaling 3 (SOCS3), a target gene of STAT3, inhibits the JAK2/STAT3 pathway by interacting with phospho-Tyr985 or JAK2 and acting as a feedback inhibitor of leptin signaling. Protein tyrosine phosphatase 1B (PTP1B) also inhibits leptin signaling through dephosphorylation of JAK2. After JAK2 activation, SH2-containing protein tyrosine phosphatase 2 (SHP2) binds to phospho-Tyr985 in the LepRb and recruits the adaptor protein growth factor receptor-bound protein 2 (Grb2), leading to activation of the mitogen-activated protein kinase (MAPK) signaling cascade. Leptin activates MAPK independent of SHP2 and also regulates phosphatidylinositol 3 kinase (PI3K) signaling through insulin receptor substrate (IRS) phosphorylation. Forkhead box O1 (FoxO1), mammalian target of rapamycin (mTOR), and phosphodiesterase 3B (PDE3B) are important downstream targets of PI3K in the leptin signaling pathway. Leptin regulates feeding and metabolism through 5' adenosine monophosphate-activated protein kinase (AMPK) and acetyl-CoA carboxylase (ACC) in the brain and peripheral organs.
Obese individuals have elevated adipose leptin expression and plasma leptin levels, and these high leptin levels fail to reduce excess adiposity, indicating a state of leptin resistance. On the basis of studies conducted mainly in rodent models, leptin resistance has been attributed to multiple factors, including impaired leptin transport across the blood-brain barrier, disruption of leptin signaling in the hypothalamus by SOCS3 and PTP1B, inflammation, endoplasmic reticulum stress, and autophagy [3,6,7].
Leptin signaling and energy homeostasis
Leptin is highly expressed in the hypothalamus, particularly in the arcuate nucleus (ARC) and ventromedial hypothalamus (VMH). Leptin directly targets two neuronal populations in the ARC co-expressing pro-opiomelanocortin (POMC)/cocaine- and amphetamine-regulated transcript (CART), and agouti-related peptide (AgRP) and neuropeptide Y (NPY) [8]. Leptin stimulates POMC/CART expression and inhibits AgRP/NPY expression, thereby reducing food intake, increasing energy expenditure, and decreasing body weight. In addition, leptin inhibits feeding by reducing the expression of melanin-concentrating hormone (MCH) and orexins in the lateral hypothalamic area (LHA). Leptin has also been shown to stimulate the expression of brain-derived neurotrophic factor and steroidogenic factor-1 (SF-1) neurons in the VMH, leading to inhibition of feeding [9,10].
The binding of leptin to LepRb activates JAK2, and the activated JAK2 phosphorylates Tyr985, Tyr1077, and Tyr1138 in the cytoplasmic domain of LepRb. Mutation of these three tyrosines in LepRb induces obesity in mice but to a lesser degree than in LepRb-deficient db/db mice, indicating that LepRb affects energy homeostasis through both tyrosine-dependent and -independent mechanisms [11]. Leptin's activation of JAK2/STAT3 signaling appears to play a major role in energy homeostasis and neuroendocrine function. Once phosphorylated, STAT3 is translocated from the cytoplasm into the nucleus, where it binds to pomc and agrp promoters, stimulating POMC expression and inhibiting AgRP. Indeed, deletion of STAT3 in neurons decreases POMC and increases AgRP and NPY levels, culminating in hyperphagia, obesity, infertility, and thermal dysregulation [12]. Furthermore, a specific deletion of Tyr1138 in LepRb or STAT3 in LepRb-expressing neurons results in hyperphagia and morbid obesity [13,14]. Mice with a STAT5 deletion in the brain develop hyperphagia and obesity but less severely than in mice with a STAT3 deletion, indicating that JAK2/STAT5 plays a minor role in leptin's regulation of feeding and body weight [15].
Leptin activates PI3K and Akt through IRS phosphorylation. Interestingly, leptin and insulin signaling have similar intracellular pathways in hypothalamic neurons (Figure 2) [16-18]. The PI3K pathway stimulates POMC-expressing neurons through ATP-sensitive potassium channels and voltage-gated calcium channels [1,19]. Inhibition of PI3K in the brain prevents leptin-induced anorexia [20]. Mice with targeted PI3K disruption in POMC neurons show blunted responses to leptin-mediated suppression of feeding but exhibit normal long-term body weight regulation [21]. Forkhead box O1 (FoxO1), a transcription factor inactivated by Akt, appears to be an important downstream mediator of PI3K signaling. FoxO1 stimulates expression of NPY and AgRP, inhibits POMC, and blocks STAT3 action in AgRP and POMC neurons. Inactivation of FoxO1 via leptin or insulin signaling allows STAT3 to bind to pomc and agrp promoters [9,22,23]. A constitutively active FoxO1 in ARC blunts leptin's ability to inhibit food intake in mice [24]. In contrast, deletion of FoxO1 in either POMC or AgRP neurons decreases food intake in mice [25,26]. Another pathway recruited by leptin is the mammalian target of rapamycin (mTOR), a downstream target of PI3K/Akt. Leptin stimulates phosphorylation of p70 S6 kinase (S6K) via mTOR in the hypothalamus. Inhibition of mTOR attenuates the anorexigenic effect of leptin [27]. Systemic deletion of S6K1 or selective inhibition of S6K in the ARC abolishes the anorexigenic action of leptin [28,29]. Leptin also induces phosphodiesterase 3B (PDE3B) activity, which results in a decrease in cyclic adenosine monophosphate (cAMP) levels via the PI3K pathway in the hypothalamus. Inhibition of PDE3B activity reverses leptin's effects on food intake and body weight, suggesting that PDE3B plays an important role in mediating leptin signaling in the hypothalamus [30,31]. Furthermore, it has been shown that leptin administered centrally in mice increases renal sympathetic outflow via activation of PI3K [32].
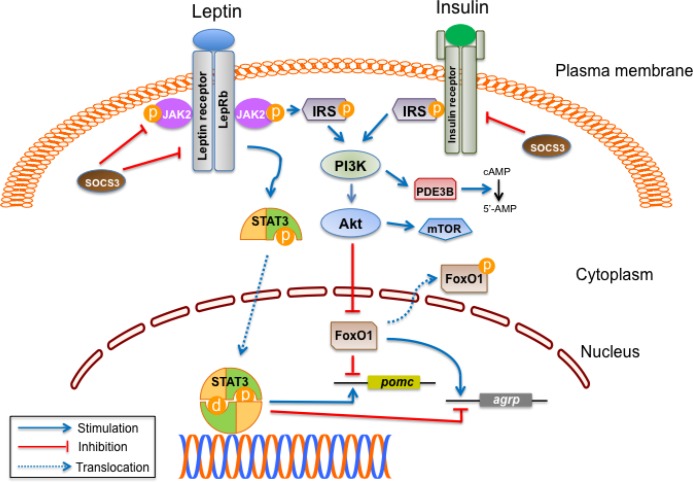
Figure 2. Interactions of leptin and insulin signaling in hypothalamus.
Leptin activates signal transducer and activator of transcription (STAT) 3 when it binds to the long isoform of leptin receptor (LepRb) in the arcuate nucleus, ventromedial hypothalamus, and other hypothalamic neurons. Activated STAT3 stimulates pomc expression and inhibits agrp expression in the hypothalamus. Leptin and insulin signaling pathways converge on phosphatidylinositol 3 kinase (PI3K). Activation of PI3K by either leptin or insulin leads to phosphorylation of Forkhead box O1 (FoxO1) through Akt, which can also activate the mammalian target of rapamycin (mTOR). Inactivation of FoxO1 by phosphorylation results in its export from the nucleus and allows for the binding of STAT3 to pomc or agrp promoter. In addition, the leptin-mediated activation of PI3K recruits phosphodiesterase 3B (PDE3B), thereby reducing cAMP (cyclic adenosine monophosphate) levels. STAT3 increases the expression of suppressor of cytokine signaling 3 (SOCS3), which leads to feedback inhibition of leptin and insulin signaling.
Abbreviation: JAK2, Janus kinase 2.
Extracellular signal-related kinase (ERK), a member of the MAPK family, acts downstream of LepRb. Activation of ERK1/2 appears to be mediated by either SHP2 from Tyr985 of LepRb or directly from JAK2. Neuron-specific deletion of SHP2 results in obesity and leptin resistance in mice [33]. Pharmacological blockade of hypothalamic ERK1/2 abrogates the anorectic and weight-reducing effects of leptin in rats. Inhibition of ERK also prevents leptin-induced sympathetic activation of brown adipose tissue, indicating that SHP2/MAPK signaling is involved in leptin's regulation of food intake and energy expenditure [34].
Leptin exerts an inhibitory effect on AMPK in the hypothalamus, thereby stimulating ACC and subsequently suppressing food intake. Constitutive activation of hypothalamic AMPK blocks leptin's anorexigenic effect. In addition, inhibition of hypothalamic ACC attenuates leptin-mediated decrease in food intake and body weight [35,36]. Recently, it was shown that mTOR/S6K regulates feeding through leptin-induced inhibition of AMPK in the hypothalamus [37]. Together, these data demonstrate diverse interactions of leptin and signaling molecules in energy homeostasis.
Leptin signaling and glucose and lipid metabolism
Leptin has rapid effects on glucose and lipid metabolism independent of body weight regulation [38]. Restoration of LepRb expression in the ARC in obese LepR-deficient mice normalizes blood glucose levels. Moreover, selective expression of LepRb in the POMC neurons dramatically improves glucose levels in db/db mice [39,40]. Deletion of SOCS3 in POMC-expressing neurons improves glucose tolerance and insulin sensitivity without affecting body weight. Mice with a POMC-specific deletion of PTP1B exhibit improved insulin sensitivity and increased energy expenditure [41,42]. POMC neurons have glucose-sensing capabilities and are likely to mediate leptin's effects on glucose homeostasis [38,43]. Mice lacking FoxO1 in AgRP neurons or FoxO1 in SF-1 neurons show improved glucose tolerance and increased insulin sensitivity, and these pathways may contribute to the central action of leptin [26,44].
Interestingly, mice with a mutation of STAT3-binding sites in LepRb are less hyperglycemic than db/db mice, suggesting that pathways other than tyrosine- or STAT3-mediated signaling are involved in leptin's effect on glucose homeostasis [8,11,13]. It has been shown that mouse models with the alteration of PI3K activity in POMC neurons have disrupted hepatic insulin sensitivity, suggesting that the PI3K pathway in POMC neurons is involved in glucose homeostasis [45]. Leptin inhibits de novo lipogenesis and stimulates lipolysis, but these effects are lost when hypothalamic PI3K signaling is disrupted [46,47]. Central leptin's effect on improving insulin sensitivity in skeletal muscle is dependent on PI3K/Akt modulating AMPK/ACC signaling in skeletal muscle [48,49].
Although leptin acts mainly in the brain, evidence suggests a direct action in peripheral tissues. Leptin directly stimulates fatty acid oxidation and glucose uptake through activation of AMPK in skeletal muscle. In contrast to its inhibitory effect in the hypothalamus, leptin activates AMPK, thereby inhibiting ACC in skeletal muscle, preventing steatosis and lipotoxicity [50]. In addition to AMPK, p38 MAPK may contribute to leptin's effect on fatty acid oxidation [1]. Peripheral leptin administration also stimulates fatty acid oxidation by a p38 MAPK-dependent mechanism in isolated rat cardiomyocytes [51].
Leptin suppresses hepatic glucose production by ameliorating hyperglucagonemia and stimulating peripheral glucose uptake through central mechanisms, possibly involving POMC and AgRP neurons in the ARC, and the modulation of the autonomic nervous system [38,52,53]. Leptin directly regulates glucose metabolism in isolated hepatocytes via PI3K-dependent activation of PDE3B [54]. Leptin also inhibits glucose-stimulated insulin secretion by acting directly on pancreatic β cells. Although the precise mechanisms are unknown, it is possible that leptin-mediated PI3K-dependent activation of PDE3B is involved in β-cell regulation [55]. Leptin inhibits insulin synthesis via JAK2/STAT/SOCS3 signaling, while an induction of SOCS3 by leptin inhibits STAT3/5b-dependent regulation of preproinsulin 1 gene promoter [56,57].
Leptin signaling and immune function
Leptin has important roles in modulating both innate and adaptive immunity. Leptin binds to its receptor in monocytes and macrophages, stimulating neutrophil chemotaxis and promoting macrophage phagocytosis. Leptin increases the production of pro-inflammatory cytokines such as interleukin-6 (IL-6), IL-12, and tumor necrosis factor-alpha (TNF-α) [58,59]. Leptin also protects neutrophils from apoptosis through PI3K- and MAPK-dependent signaling. Leptin regulates the maturation of dendritic cells through Akt activation, and induces CD40 expression in murine dendritic cells [60,61]. In addition, leptin promotes natural killer cell activation and cytotoxicity through STAT3 activation [62,63].
Leptin regulates the adaptive immune response in normal and pathological conditions, and it promotes the proliferation of native T cells and the switch toward T helper 1 cell immune responses by increasing interferon-γ and TNF-α in memory T cells [64]. Recently, it was reported that leptin acts as a negative signal for the proliferation of human regulatory T (Treg) cells that are involved in the prevention of autoimmune diseases [65]. Leptin has been shown to activate the mTOR pathway in Treg cells [66]. Moreover, leptin activates human B cells to secrete cytokines, such as pro-inflammatory IL-6 and TNF-α and anti-inflammatory IL-10, via the activation of JAK2/STAT3 and p38 MAPK signaling pathways, indicating a role of leptin in inflammation and immunoregulation [64,67].
Conclusions
The discovery of leptin 20 years ago enhanced our understanding of nutritional physiology and provided molecular tools for studying mechanisms underlying feeding behavior, energy homeostasis, neuroendocrine regulation, and other systems. Here, we have discussed how central and peripheral leptin signaling interacts with various intracellular molecules to affect energy homeostasis, glucose and lipid metabolism, and the immune system. Further studies are needed to determine whether leptin signaling can lead to novel diagnostic and therapeutic strategies for obesity, diabetes, and related diseases.
Acknowledgments
Rexford S. Ahima is supported by National Institutes of Health grants P01-DK049210 and P30-DK19525.
Abbreviations
- ACC
acetyl-CoA carboxylase
- AgRP
agouti-related peptide
- AMPK
5' adenosine monophosphate-activated protein kinase
- ARC
arcuate nucleus
- cAMP
cyclic adenosine monophosphate
- CART
cocaine- and amphetamine-regulated transcript
- ERK
extracellular signal-related kinase
- FoxO1
Forkhead box O1
- IL
interleukin
- IRS
insulin receptor substrate
- JAK2
Janus kinase 2
- LepR
leptin receptor
- LepRb
long isoform of leptin receptor
- MAPK
mitogen-activated protein kinase
- mTOR
mammalian target of rapamycin
- NPY
neuropeptide Y
- PDE3B
phosphodiesterase 3B
- PI3K
phosphatidylinositol 3 kinase
- POMC
pro-opiomelanocortin
- PTP1B
protein tyrosine phosphatase 1B
- S6K
p70 S6 kinase
- SF-1
steroidogenic factor-1
- SHP2
SH2-containing protein tyrosine phosphatase 2
- SOCS3
suppressor of cytokine signaling 3
- STAT
signal transducer and activator of transcription
- TNF-α
tumor necrosis factor-alpha
- Treg
regulatory T
- VMH
ventromedial hypothalamus
Disclosures
The authors declare that they have no disclosures.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/b/6/73
References
- 1.Dardeno TA, Chou SH, Moon H, Chamberland JP, Fiorenza CG, Mantzoros CS. Leptin in human physiology and therapeutics. Front Neuroendocrinol. 2010;31:377–93. doi: 10.1016/j.yfrne.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalamaga M, Chou SH, Shields K, Papageorgiou P, Polyzos SA, Mantzoros CS. Leptin at the intersection of neuroendocrinology and metabolism: current evidence and therapeutic perspectives. Cell Metab. 2013;18:29–42. doi: 10.1016/j.cmet.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Moon H, Dalamaga M, Kim S, Polyzos SA, Hamnvik O, Magkos F, Paruthi J, Mantzoros CS. Leptin's role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr Rev. 2013;34:377–412. doi: 10.1210/er.2012-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahima RS, Osei SY. Leptin signaling. Physiol Behav. 2004;81:223–41. doi: 10.1016/j.physbeh.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med. 2010;152:93–100. doi: 10.7326/0003-4819-152-2-201001190-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myers MG, Heymsfield SB, Haft C, Kahn BB, Laughlin M, Leibel RL, Tschöp MH, Yanovski JA. Challenges and opportunities of defining clinical leptin resistance. Cell Metab. 2012;15:150–6. doi: 10.1016/j.cmet.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung CH, Kim M. Molecular mechanisms of central leptin resistance in obesity. Arch Pharm Res. 2013;36:201–7. doi: 10.1007/s12272-013-0020-y. [DOI] [PubMed] [Google Scholar]
- 8.Xu Y, Elmquist JK, Fukuda M. Central nervous control of energy and glucose balance: focus on the central melanocortin system. Ann N Y Acad Sci. 2011;1243:1–14. doi: 10.1111/j.1749-6632.2011.06248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297:E1247–59. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim KW, Sohn J, Kohno D, Xu Y, Williams K, Elmquist JK. SF-1 in the ventral medial hypothalamic nucleus: a key regulator of homeostasis. Mol Cell Endocrinol. 2011;336:219–23. doi: 10.1016/j.mce.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718529397
- 11.Jiang L, You J, Yu X, Gonzalez L, Yu Y, Wang Q, Yang G, Li W, Li C, Liu Y. Tyrosine-dependent and -independent actions of leptin receptor in control of energy balance and glucose homeostasis. Proc Natl Acad Sci U S A. 2008;105:18619–24. doi: 10.1073/pnas.0804589105. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718529398
- 12.Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu X. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci U S A. 2004;101:4661–6. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718529399
- 13.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso Annette WK, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–9. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1012706
- 14.Piper ML, Unger EK, Myers MG, Xu AW. Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Mol Endocrinol. 2008;22:751–9. doi: 10.1210/me.2007-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J, Muenzberg H, Gavrilova O, Reed JA, Berryman D, Villanueva EC, Louis GW, Leinninger GM, Bertuzzi S, Seeley RJ, Robinson GW, Myers MG, Hennighausen L. Loss of cytokine-STAT5 signaling in the CNS and pituitary gland alters energy balance and leads to obesity. PLoS ONE. 2008;3:e1639. doi: 10.1371/journal.pone.0001639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belgardt BF, Brüning JC. CNS leptin and insulin action in the control of energy homeostasis. Ann N Y Acad Sci. 2010;1212:97–113. doi: 10.1111/j.1749-6632.2010.05799.x. [DOI] [PubMed] [Google Scholar]
- 17.Könner AC, Brüning JC. Selective insulin and leptin resistance in metabolic disorders. Cell Metab. 2012;16:144–52. doi: 10.1016/j.cmet.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Varela L, Horvath TL. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep. 2012;13:1079–86. doi: 10.1038/embor.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elias CF, Purohit D. Leptin signaling and circuits in puberty and fertility. Cell Mol Life Sci. 2013;70:841–62. doi: 10.1007/s00018-012-1095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–5. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 21.Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718529400
- 22.Kim M, Pak YK, Jang P, Namkoong C, Choi Y, Won J, Kim K, Kim S, Kim H, Park J, Kim Y, Lee K. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci. 2006;9:901–6. doi: 10.1038/nn1731. [DOI] [PubMed] [Google Scholar]
- 23.Morrison CD, Morton GJ, Niswender KD, Gelling RW, Schwartz MW. Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. Am J Physiol Endocrinol Metab. 2005;289:E1051–7. doi: 10.1152/ajpendo.00094.2005. [DOI] [PubMed] [Google Scholar]
- 24.Kitamura T, Feng Y, Kitamura YI, Chua SC, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–40. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718529401
- 25.Plum L, Lin HV, Dutia R, Tanaka J, Aizawa KS, Matsumoto M, Kim AJ, Cawley NX, Paik J, Loh YP, DePinho RA, Wardlaw SL, Accili D. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nat Med. 2009;15:1195–201. doi: 10.1038/nm.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718529402
- 26.Ren H, Orozco IJ, Su Y, Suyama S, Gutiérrez-Juárez R, Horvath TL, Wardlaw SL, Plum L, Arancio O, Accili D. FoxO1 target Gpr17 activates AgRP neurons to regulate food intake. Cell. 2012;149:1314–26. doi: 10.1016/j.cell.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718529403
- 27.Cota D, Proulx K, Smith Kathi A Blake, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–30. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/13773
- 28.Cota D, Matter EK, Woods SC, Seeley RJ. The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J Neurosci. 2008;28:7202–8. doi: 10.1523/JNEUROSCI.1389-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blouet C, Ono H, Schwartz GJ. Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metab. 2008;8:459–67. doi: 10.1016/j.cmet.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao AZ, Huan J, Gupta S, Pal R, Sahu A. A phosphatidylinositol 3-kinase phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nat Neurosci. 2002;5:727–8. doi: 10.1038/nn885. [DOI] [PubMed] [Google Scholar]
- 31.Sahu A. Intracellular leptin-signaling pathways in hypothalamic neurons: the emerging role of phosphatidylinositol-3 kinase-phosphodiesterase-3B-cAMP pathway. Neuroendocrinology. 2011;93:201–10. doi: 10.1159/000326785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension. 2003;41:763–7. doi: 10.1161/01.HYP.0000048342.54392.40. [DOI] [PubMed] [Google Scholar]
- 33.Zhang EE, Chapeau E, Hagihara K, Feng G. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc Natl Acad Sci U S A. 2004;101:16064–9. doi: 10.1073/pnas.0405041101. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718529404
- 34.Rahmouni K, Sigmund CD, Haynes WG, Mark AL. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes. 2009;58:536–42. doi: 10.2337/db08-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718529405
- 35.Minokoshi Y, Alquier T, Furukawa N, Kim Y, Lee A, Xue B, Mu J, Foufelle F, Ferré P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–74. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1003097
- 36.Gao S, Kinzig KP, Aja S, Scott KA, Keung W, Kelly S, Strynadka K, Chohnan S, Smith WW, Tamashiro Kellie LK, Ladenheim EE, Ronnett GV, Tu Y, Birnbaum MJ, Lopaschuk GD, Moran TH. Leptin activates hypothalamic acetyl-CoA carboxylase to inhibit food intake. Proc Natl Acad Sci U S A. 2007;104:17358–63. doi: 10.1073/pnas.0708385104. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718529406
- 37.Dagon Y, Hur E, Zheng B, Wellenstein K, Cantley LC, Kahn BB. p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin's effect on food intake. Cell Metab. 2012;16:104–12. doi: 10.1016/j.cmet.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717949452
- 38.Coppari R, Bjørbæk C. Leptin revisited: its mechanism of action and potential for treating diabetes. Nat Rev Drug Discov. 2012;11:692–708. doi: 10.1038/nrd3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC, Lowell BB, Elmquist JK. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Huo L, Gamber K, Greeley S, Silva J, Huntoon N, Leng X, Bjørbaek C. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab. 2009;9:537–47. doi: 10.1016/j.cmet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718529411
- 41.Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab. 2006;4:123–32. doi: 10.1016/j.cmet.2006.06.010. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718529412
- 42.Banno R, Zimmer D, De Jonghe Bart C, Atienza M, Rak K, Yang W, Bence KK. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J Clin Invest. 2010;120:720–34. doi: 10.1172/JCI39620. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/2319957
- 43.Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Rønnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology. 2003;144:1331–40. doi: 10.1210/en.2002-221033. [DOI] [PubMed] [Google Scholar]
- 44.Kim KW, Donato J, Berglund ED, Choi Y, Kohno D, Elias CF, DePinho RA, Elmquist JK. FOXO1 in the ventromedial hypothalamus regulates energy balance. J Clin Invest. 2012;122:2578–89. doi: 10.1172/JCI62848. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718529413
- 45.Hill JW, Xu Y, Preitner F, Fukuda M, Cho Y, Luo J, Balthasar N, Coppari R, Cantley LC, Kahn BB, Zhao JJ, Elmquist JK. Phosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis. Endocrinology. 2009;150:4874–82. doi: 10.1210/en.2009-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718529414
- 46.Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, Su K, Cheng B, Li X, Harvey-White J, Schwartz GJ, Kunos G, Rossetti L. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14:667–75. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scherer T, Buettner C. Yin and Yang of hypothalamic insulin and leptin signaling in regulating white adipose tissue metabolism. Rev Endocr Metab Disord. 2011;12:235–43. doi: 10.1007/s11154-011-9190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roman Erika AFR, Reis D, Romanatto T, Maimoni D, Ferreira EA, Santos GA, Torsoni AS, Velloso LA, Torsoni MA. Central leptin action improves skeletal muscle AKT, AMPK, and PGC1 alpha activation by hypothalamic PI3K-dependent mechanism. Mol Cell Endocrinol. 2010;314:62–9. doi: 10.1016/j.mce.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 49.Donato J, Frazão R, Elias CF. The PI3K signaling pathway mediates the biological effects of leptin. Arq Bras Endocrinol Metabol. 2010;54:591–602. doi: 10.1590/S0004-27302010000700002. [DOI] [PubMed] [Google Scholar]
- 50.Minokoshi Y, Kim Y, Peroni OD, Fryer Lee GD, Müller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–43. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718529415
- 51.Sharma V, Mustafa S, Patel N, Wambolt R, Allard MF, McNeill JH. Stimulation of cardiac fatty acid oxidation by leptin is mediated by a nitric oxide-p38 MAPK-dependent mechanism. Eur J Pharmacol. 2009;617:113–7. doi: 10.1016/j.ejphar.2009.06.037. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718529416
- 52.German JP, Thaler JP, Wisse BE, Oh-I S, Sarruf DA, Matsen ME, Fischer JD, Taborsky GJ, Schwartz MW, Morton GJ. Leptin activates a novel CNS mechanism for insulin-independent normalization of severe diabetic hyperglycemia. Endocrinology. 2011;152:394–404. doi: 10.1210/en.2010-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berglund ED, Vianna CR, Donato J, Kim MH, Chuang J, Lee CE, Lauzon DA, Lin P, Brule LJ, Scott MM, Coppari R, Elmquist JK. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest. 2012;122:1000–9. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao AZ, Shinohara MM, Huang D, Shimizu M, Eldar-Finkelman H, Krebs EG, Beavo JA, Bornfeldt KE. Leptin induces insulin-like signaling that antagonizes cAMP elevation by glucagon in hepatocytes. J Biol Chem. 2000;275:11348–54. doi: 10.1074/jbc.275.15.11348. [DOI] [PubMed] [Google Scholar]
- 55.Marroquí L, Gonzalez A, Ñeco P, Caballero-Garrido E, Vieira E, Ripoll C, Nadal A, Quesada I. Role of leptin in the pancreatic β-cell: effects and signaling pathways. J Mol Endocrinol. 2012;49:R9–17. doi: 10.1530/JME-12-0025. [DOI] [PubMed] [Google Scholar]
- 56.Laubner K, Kieffer TJ, Lam NT, Niu X, Jakob F, Seufert J. Inhibition of preproinsulin gene expression by leptin induction of suppressor of cytokine signaling 3 in pancreatic beta-cells. Diabetes. 2005;54:3410–7. doi: 10.2337/diabetes.54.12.3410. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718529417
- 57.Lee Y, Magkos F, Mantzoros CS, Kang ES. Effects of leptin and adiponectin on pancreatic β-cell function. Metab Clin Exp. 2011;60:1664–72. doi: 10.1016/j.metabol.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 58.Carbone F, La Rocca C, Matarese G. Immunological functions of leptin and adiponectin. Biochimie. 2012;94:2082–8. doi: 10.1016/j.biochi.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 59.Paz-Filho G, Mastronardi C, Franco CB, Wang KB, Wong M, Licinio J. Leptin: molecular mechanisms, systemic pro-inflammatory effects, and clinical implications. Arq Bras Endocrinol Metabol. 2012;56:597–607. doi: 10.1590/S0004-27302012000900001. [DOI] [PubMed] [Google Scholar]
- 60.Bruno A, Conus S, Schmid I, Simon H. Apoptotic pathways are inhibited by leptin receptor activation in neutrophils. J Immunol. 2005;174:8090–6. doi: 10.4049/jimmunol.174.12.8090. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718529418
- 61.Lam Queenie Lai Kwan, Zheng B, Jin D, Cao X, Lu L. Leptin induces CD40 expression through the activation of Akt in murine dendritic cells. J Biol Chem. 2007;282:27587–97. doi: 10.1074/jbc.M704579200. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718529419
- 62.Zhao Y, Sun R, You L, Gao C, Tian Z. Expression of leptin receptors and response to leptin stimulation of human natural killer cell lines. Biochem Biophys Res Commun. 2003;300:247–52. doi: 10.1016/S0006-291X(02)02838-3. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718529420
- 63.Iikuni N, Lam Queenie Lai Kwan, Lu L, Matarese G, La Cava A. Leptin and Inflammation. Curr Immunol Rev. 2008;4:70–9. doi: 10.2174/157339508784325046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Procaccini C, Jirillo E, Matarese G. Leptin as an immunomodulator. Mol Aspects Med. 2012;33:35–45. doi: 10.1016/j.mam.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 65.Rosa V de, Procaccini C, Calì G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–55. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1068803
- 66.Procaccini C, Rosa V de, Galgani M, Abanni L, Calì G, Porcellini A, Carbone F, Fontana S, Horvath TL, La Cava A, Matarese G. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity. 2010;33:929–41. doi: 10.1016/j.immuni.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/8262956
- 67.Agrawal S, Gollapudi S, Su H, Gupta S. Leptin activates human B cells to secrete TNF-α, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J Clin Immunol. 2011;31:472–8. doi: 10.1007/s10875-010-9507-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718529421