Abstract
Small-vessel vasculitis is a life-threatening autoimmune disease that is frequently associated with anti-neutrophil cytoplasmic antibodies (ANCAs). Conventional immunotherapy including steroids and cyclophosphamide can cause serious adverse events, limiting the efficacy and safety of treatment. Eicosapentaenoic acid (EPA), a key component of fish oil, is an omega-3 polyunsaturated fatty acid widely known to be cardioprotective and beneficial for vascular function. We report two elderly patients with systemic ANCA-associated vasculitis (AAV) in whom the administration of EPA in concert with steroids safely induced and maintained remission, without the use of additioal immunosuppressants. To explore the mechanisms by which EPA enhances the treatment of AAV, we employed SCG/Kj mice as a spontaneous murine model of AAV. Dietary enrichment with EPA significantly delayed the onset of crescentic glomerulonephritis and prolonged the overall survival. EPA-derived anti-inflammatory lipid mediators and their precursors were present in the kidney, plasma, spleen, and lungs in the EPA-treated mice. Furthermore, a decrease in ANCA production and CD4/CD8-double negative T cells, and an increase in Foxp3+ regulatory T cells in the lymph nodes of the kidney were observed in the EPA-treated mice. These clinical and experimental observations suggest that EPA can safely support and augment conventional therapy for treating autoimmune small-vessel vasculitis.
Necrotizing small-vessel vasculitis (SVV) is a life-threatening autoimmune disease that usually targets the kidneys and lungs in the form of inflammatory lesions. SVV can occur in various inflammatory diseases, but is frequently associated with autoantibodies to neutrophil cytoplasmic antigens (anti-neutrophil cytoplasmic antibodies, ANCA) such as myeloperoxidase (MPO) and proteinase 3 (PR3). The current standard treatment for ANCA-associated vasculitis (AAV) is a combination of steroids and immunosuppressants1,2,3,4. For severe cases, rituximab, a monoclonal antibody to CD20, has been shown to be as effective as cyclophosphamide in inducing remission5,6.
However, the detrimental side effects of these therapeutic agents can often lead to serious adverse events. In fact, Little et al. reported that the greatest threat to patients with AAV in the first year of therapy is from adverse events rather than active vasculitis; this problem has been shown in patients recruited to four European AAV prospective clinical trials7. Additionally, recent studies have revealed that patients with AAV are at a greater risk of cardiovascular disease, and that late mortality is mainly due to cardiovascular events8,9. As markers for AAV, an increase in circulating endothelial cells damaged by activated neutrophils has been reported10, and accumulating evidence indicates accelerated atherosclerosis11,12,13,14,15 and endothelial dysfunction16 in AAV.
Steroids or immunosuppressants are not suitable for AAV therapy, particularly in elderly patients with cardiovascular disease. In this regard, the therapeutic strategy for AAV should aim at cardiovascular protection as well as the control of inflammation and immunomodulation. A therapeutic strategy aimed at endothelial protection can provide supportive treatment that improves the prognosis and quality of life for the patients.
Eicosapentaenoic acid (EPA) is an omega-3 polyunsaturated fatty acid (PUFA) and is known to be beneficial for preventing cardiovascular disease17. Recently, we reported a patient with the renal-limited type of AAV complicated with severe ischemic heart disease. In this case, highly purified EPA successfully induced and maintained remission, without the use of steroids and immunosuppressants18. In addition, we encountered two elderly patients with AAV manifesting a highly inflammatory systemic reaction, in which EPA, in combination with steroids, efficiently and safely induced remission and succeeded in maintaining remission for more than five years, without the use of any immunosuppressants other than steroids. Of note, these patients have retained a high quality of life during the course of the remission maintenance therapy. From these clinical experiences, we hypothesize that EPA can be a supportive agent and/or an alternative to conventional therapy for AAV. The purpose of this study was to investigate the therapeutic mechanism of EPA and thereby establish a new therapeutic strategy for SVV, including AAV.
Results
We encountered two elderly patients with AAV for whom highly purified EPA in combination with steroids safely promoted the induction and maintenance of remission, without the administration of additional immunosuppressants. It appeared that EPA supported the steroid therapy to maintain remission, although we initially intended to use EPA to treat dyslipidemia following the steroid therapy.
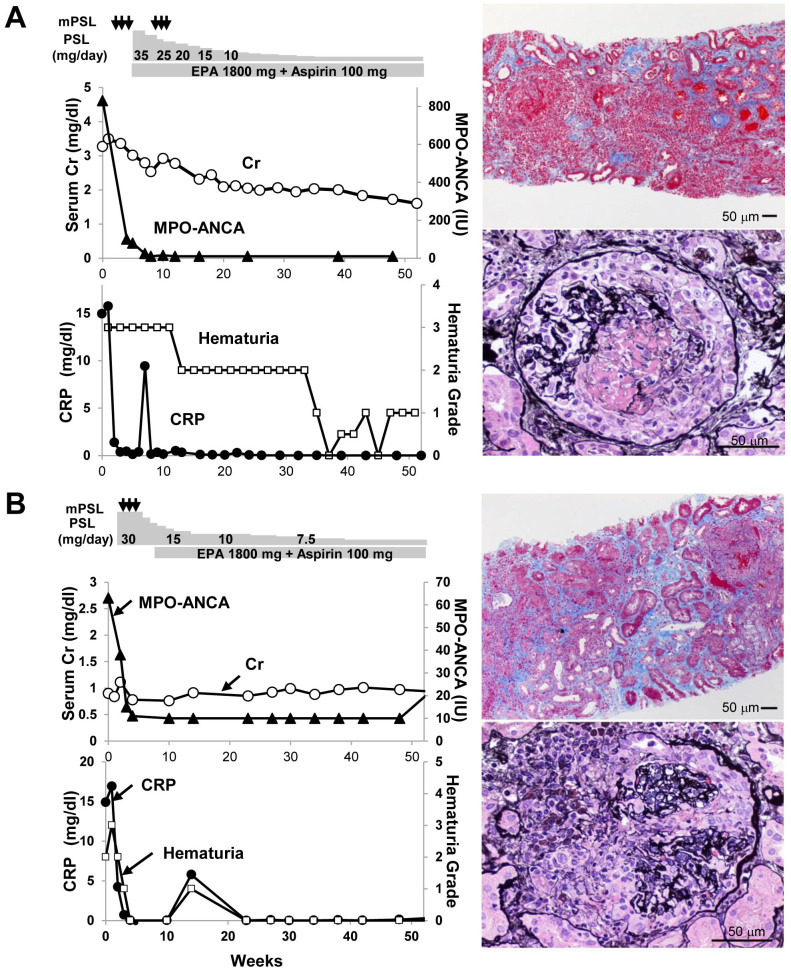
Case 1: A 73-year-old man was admitted with significant fever, cough, and gross hematuria. Laboratory tests revealed anemia and rapidly progressive kidney injury with high C-reactive protein (CRP; 15.8 mg/dL) and MPO-ANCA at a titer of 830 EU (normal, <10 EU). Urinalysis indicated hematuria (3+) and proteinuria (3+). Computed tomography showed bilateral interstitial pneumonia, and malignancies were ruled out. Renal biopsy revealed tubulointerstitial nephritis with severe neutrophil infiltration, and glomerulonephritis (GN) with cellular crescents and thrombus formation. Based on the diagnosis of microscopic polyangiitis (MPA), the patient received two rounds of methylprednisolone pulse therapy (1 g/day for three consecutive days) and eight plasma exchanges with fresh frozen plasma, followed by prednisolone (PSL) (35 mg/day) in combination with highly purified EPA (1,800 mg/day) and low-dose aspirin (100 mg/day), to treat dyslipidemia and to prevent thrombosis, respectively. This treatment led to the rapid normalization of serum CRP levels and sustained improvement in kidney function (Fig. 1A). The patient remained in remission with improved kidney function for five years after the onset of the disease, with successfully tapered oral PSL (5 mg/day) together with highly purified EPA (2,700 mg/day) and low-dose aspirin. The patient's serum MPO-ANCA titer remained very low throughout the course of the therapy. He has experienced no apparent cardiovascular events or mineral bone disease. He enjoys playing golf and maintains his quality of life because of the absence of detrimental side effects of the therapy. We obtained informed consent from the patient.
Figure 1. Clinical courses and renal biopsies in two patients with ANCA-associated systemic vasculitis who were successfully treated with eicosapentaenoic acid in combination with steroids.
Clinical course of Case 1 (A) and Case 2 (B). Medications are indicated in the top panel. Serum creatinine (Cr) and serum MPO-ANCA titers are indicated in the middle panel. Hematuria grade and serum CRP are indicated in the bottom panel. The pathology views of the renal biopsy specimens are shown on the right (original magnification 400×). mPSL, methylprednisolone; EPA, eicosapentaenoic acid. Conversion factor for units: serum creatinine in mg/dL to mol/L, × 88.4.
Case 2: A 79-year old woman presented with intermittent fever and high inflammatory reactions, including elevated CRP levels (14.9 mg/dL). MPO-ANCA, but not PR3-ANCA, was positive at a titer of 63 EU (normal, <10 EU). Kidney biopsy showed tubulointerstitial nephritis with infiltration of plasma cells and neutrophils, GN with cellular crescents and necrotizing vasculitis in the small vessels. Based on these findings, MPA due to AAV was diagnosed. To induce and maintain remission, steroid pulse therapy (methylprednisolone, 0.25 g/day for three consecutive days) was performed, which was followed by highly-purified EPA (1,800 mg/day) and low-dose aspirin (100 mg/day) to treat dyslipidemia and to prevent thrombosis, respectively, together with daily oral administration of PSL (30 mg/day) (Fig. 1B). Oral PSL was slowly tapered to 5 mg/day successfully. The patient maintained remission for more than five years, without significant recurrence or adverse events. The patient maintains her quality of life and enjoys walking every morning, because of the adjunctive therapy, without detrimental side effects. We obtained informed consent from the patient.
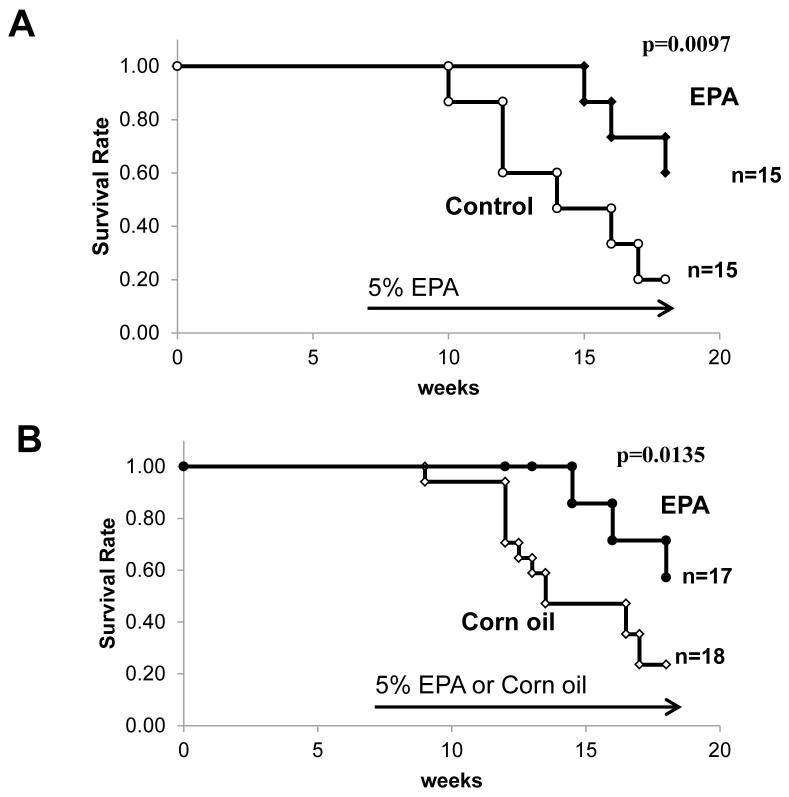
Next, we decided to explore the underlying mechanism by which EPA exerts beneficial effects on the treatment of patients with AAV using SCG/Kj mice, which have been reported to serve as a spontaneous model of AAV19,20,21. A high percentage of SCG/Kj mice develop SVV and crescentic glomerulonephritis and produce MPO-ANCA, although they do not fully recapitulate human AAV because of the presence of massive immune deposits in glomerulus and remarkable lymphadenopathy19. Their median life span is reported to be about 120 days and 135 days for female and males mice, respectively, with renal failure as the main cause of death19. Dietary enrichment with highly purified EPA (5%) markedly prolonged the survival of SCG/Kj mice (Fig. 2A). In contrast, a diet supplemented with corn oil (5%), rich in omega-6 PUFAs, as a caloric control did not change the overall survival time (Fig. 2B). These results suggest that the beneficial effect of EPA is not attributable to the caloric difference, but rather to a class-specific effect of the omega-3 PUFAs.
Figure 2. Dietary supplementation with 5% eicosapentaenoic acid prolonged survival of SCG/Kj mice.
(A) Survival of SCG/Kj mice fed a control diet (n = 15) versus those fed a 5% EPA-containing diet (n = 15) beginning at 7 weeks of age. (B) Survival of SCG/Kj mice fed a 5% corn oil (n = 17) versus those fed a 5% EPA-containing diet (n = 18) beginning at 7 weeks of age. Lifespan was calculated with the Kaplan-Meier method, and statistical analysis was conducted by the log-rank test. (*p < 0.05).
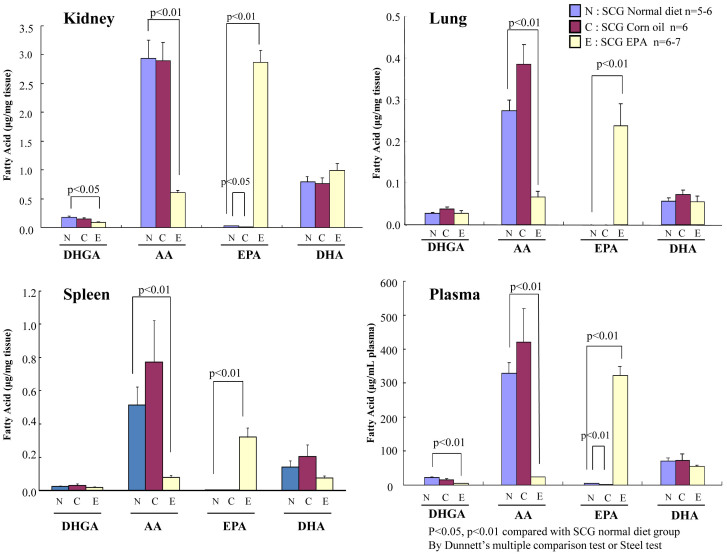
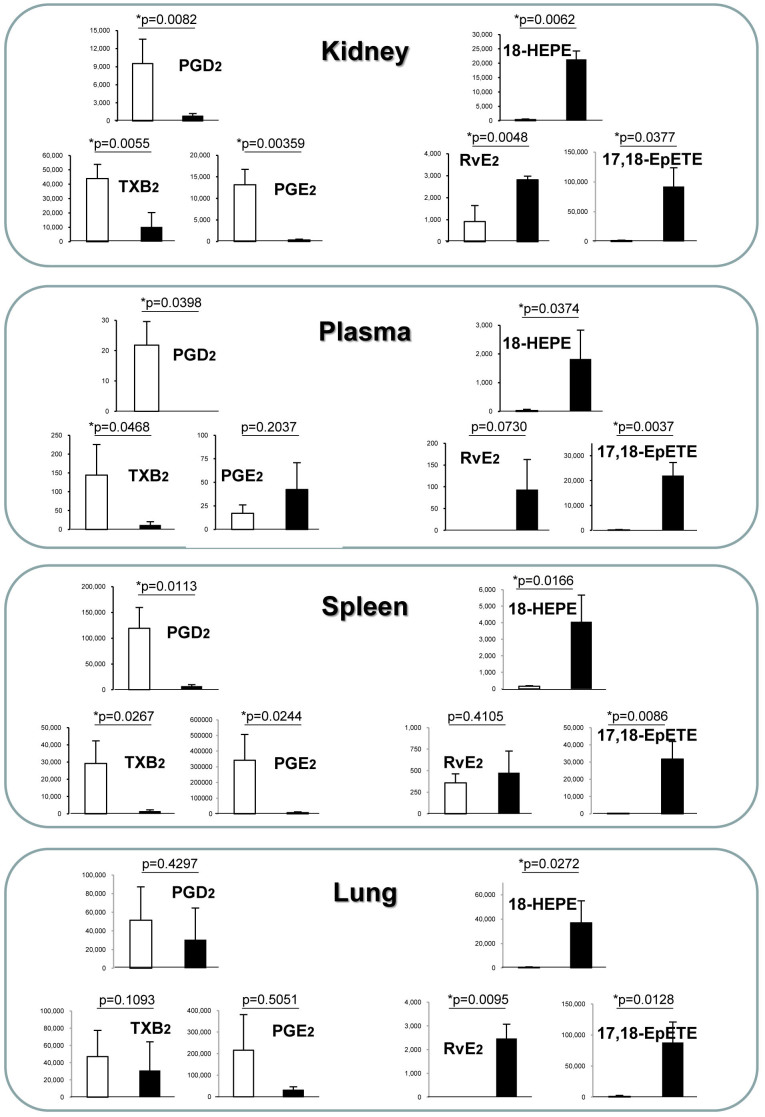
To investigate whether EPA ingested from the diet is systemically distributed to the organs, we performed lipid analysis to determine the fatty acid composition in plasma and homogenized tissues from the lungs, kidneys, and spleen. In the kidney, a primary target of inflammation in this model, EPA ingested through the diet was abundantly distributed. In particular, the proportion of omega-6 PUFAs such as arachidonic acid (AA) and dihomo-γ-linolenic acid (DHGA) was strongly decreased in the EPA-fed group, indicating that EPA functions as a competitor of omega-6 PUFAs deposition (Fig. 3). One prevailing theory accounting for the anti-inflammatory action of EPA is that it competes with AA as a substrate for cyclooxygenase and 5-lipoxygenase, blocking the production of proinflammatory eicosanoids. However, recent research on lipid mediators by Serhan et al.22,23 has revealed that the non-inflammatory state is achieved not only by the attenuation of proinflammatory signals, but also by promoting the resolution of inflammation. This is accompanied by lipid mediator class switching from the proinflammatory prostaglandins (PGs) and leukotrienes (LTs) to the anti-inflammatory AA-derived lipoxins (LXs)24 and omega-3 PUFA-derived pro-resolving mediators such as resolvins and protectins22,23,25,26,56. In fact, the production of PGE2 and TXB2 in the kidneys was suppressed in the EPA-treated group, but we detected significant production of resolvin (Rv) E227, 18-hydroxyeicosapentaenoic acid (18-HEPE) and 17,18-epoxy-eicosapentaenoic acid (17, 18-EpETE)28 which have been reported as potent anti-inflammatory and pro-resolving lipid mediators and their precursors derived from EPA (Fig. 4). Similar lipid profiles were observed in the lung, spleen, and plasma (Fig. 4 and see Supplementary Fig. S1 online). These results indicate that the biosynthesis of the EPA-derived anti-inflammatory lipid mediators is active and it possibly contributes to protection against local inflammation associated with vasculitis.
Figure 3. Comparisons of fatty acid compositions in tissue of the kidney, spleen, and lung and in the plasma of SCG/Kj mice fed with control diet, 5% EPA-containing diet, and 5% corn oil diet.
The levels of four types of fatty acids (dihomo-γ-linolenic acid, DHGA; arachidonic acid, AA; eicosapentaenoic acid, EPA; and docosahexaenoic acid, DHA) were determined using gas chromatography and normalized by tissue weight. The results are expressed as mean ± SEM. Comparisons with the control diet group were made with Dunnett's multiple comparison test and non-parametric Steel test.
Figure 4. Lipid mediator lipidomics in SCG/Kj mice that received diet supplemented with EPA.
The SCG/Kj mice produced lipid mediators derived from arachidonic acid (AA) and eicosapentaenoic acid (EPA) in the kidneys, spleen, and the plasma. AA- and EPA-derived products were quantified by LC-MS/MS and multiple reaction monitoring. Concentrations (pg/mg tissue or plasma) of AA-derived lipid mediators (PGD2, PGE2 and TXB2) and EPA-derived lipid mediators (18-HETE, RvE2, and 17, 18-EpETE) in the homogenized tissues of the kidney, spleen, and plasma are shown. Production of AA-derived inflammatory mediators, such as PGD2 and TXB2, were largely suppressed in the EPA-treated group compared with the control. On the other hand, anti-inflammatory mediators, such as AA-derived lipoxin (LXA4), EPA-derived 18-HETE, RvE2, and RvE3, were elevated in the EPA-treated group. The other products detected in this system are shown in Supplemental Fig. S2.
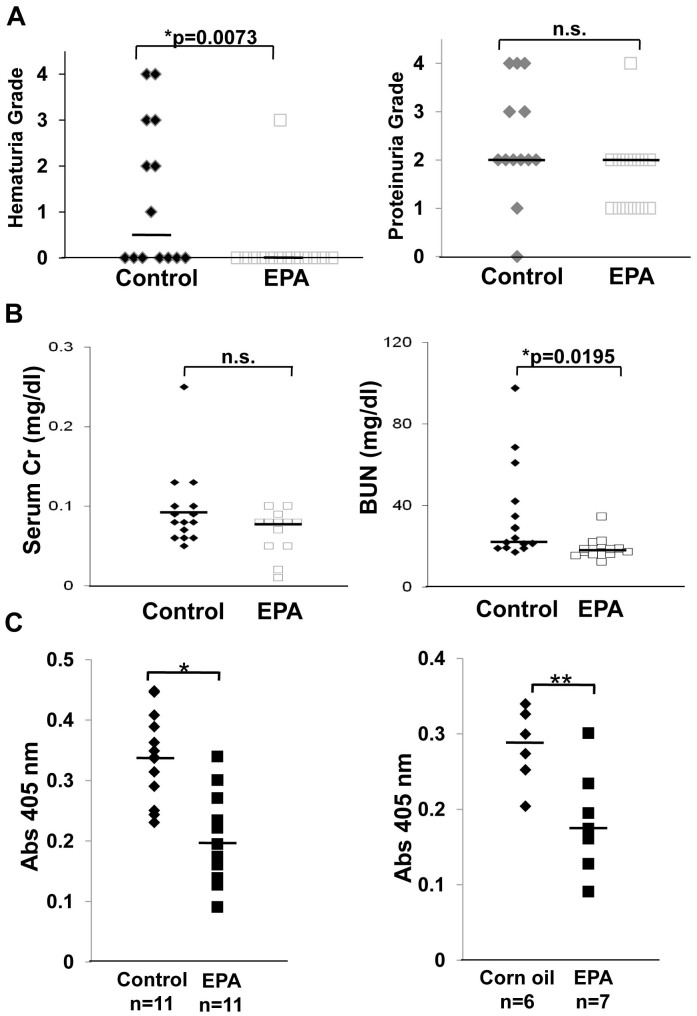
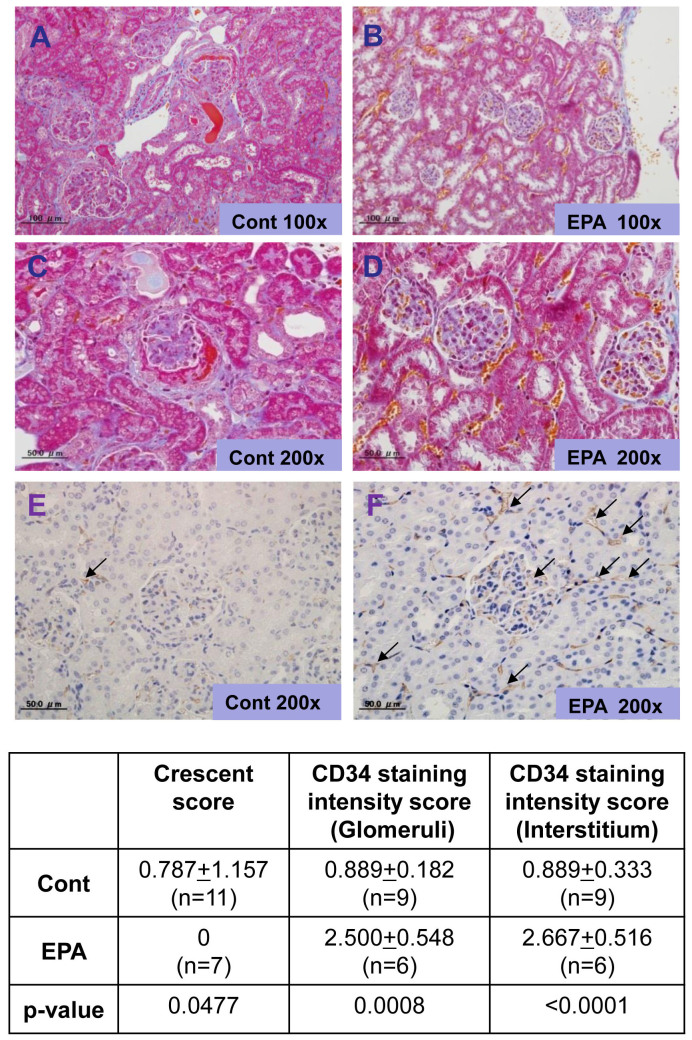
The SCG/Kj mice presented with crescentic GN after approximately 11 weeks of age, and they began dying soon after exhibiting hematuria. At 12 weeks of age, the mice were euthanized and analyzed. In the EPA-fed group, hematuria, a marker of vasculitis, was significantly suppressed relative to the control-diet group (Fig. 5A). The elevation of blood urea nitrogen observed in the control group was prevented in the EPA-treated group (Fig. 5B). The SCG/Kj mice produced MPO-ANCA and developed necrotizing GN, as previously reported19. Interestingly, dietary intake of EPA, but not corn oil, strongly inhibited MPO-ANCA production, suggesting a mechanism for the immunoregulatory function of EPA (Fig. 5C). Histological analysis revealed that severe crescentic GN (Fig. 6A, B, C, D) observed in the control group was significantly inhibited in the EPA-treated group. In addition, immunostaining with anti-CD34 antibody revealed that the endothelial cells in the glomeruli and peritubular capillaries, both of which were damaged in the control group, were clearly protected by treatment with EPA (Fig. 6G, H). Unlike the control group, abundant RBCs can be seen in peritubular capillaries in the EPA-treated group, indicating a protective role for EPA in the maintenance of the blood stream in the inflamed kidney. These results suggest that the beneficial effect of EPA in preventing the development of AAV could be attributable to its function of endothelial protection as well as its immunomodulation capability.
Figure 5. Effects of dietary enrichment with 5% eicosapentaenoic acid on renal function, urinalysis, and MPO-ANCA titers in SCG/Kj mice.
(A) Grade of hematuria (left) and proteinuria (right) in SCG/Kj mice fed a control or a 5% eicosapentaenoic acid (EPA)-containing diet. EPA supplementation significantly relieved hematuria, but not proteinuria. (B) Serum creatinine (left) and blood urea nitrogen (right) in SCG/Kj mice fed a control (n = 11) or a 5% EPA-containing (n = 11) diet. EPA supplementation prevented impairment of renal function. (C) MPO-ANCA titers in SCG/Kj mice fed a control diet or a 5% EPA-containing diet (left) and a 5% corn oil or a 5% EPA-containing diet (right). EPA supplementation strongly blocked production of MPO-ANCA. Each bold bar indicates a median value. Statistical differences were evaluated using Student's t-test. (*p < 0.05 compared with control).
Figure 6. Histological and immunohistochemical analysis of kidneys.
Representative histopathology of kidney sections harvested from mice at 14 weeks of age. SCG/Kj mice were fed a control diet (A, C, E) or a 5% EPA-containing diet (B, D, F) beginning at age 7 weeks. The diets continued for 7 weeks and the mice were euthanized at 14 weeks of age. A and B, Masson-Trichrome staining (original magnification 100×). C and D, Masson-Trichrome staining (original magnification 200×). E and F, immunostaining of endothelial cells with anti-CD34 antibody (original magnification 200×). Arrows in Figure 6E and F indicate the staining of CD34 positive endothelial cells in glomeruli and interstitium in the kidney. SCG/Kj mice fed a 5% eicosapentaenoic acid-containing diet had less crescentic glomerulonephritis, less neutrophil accumulation into the glomeruli, and restored endothelial cell damage in the peritubular capillaries.
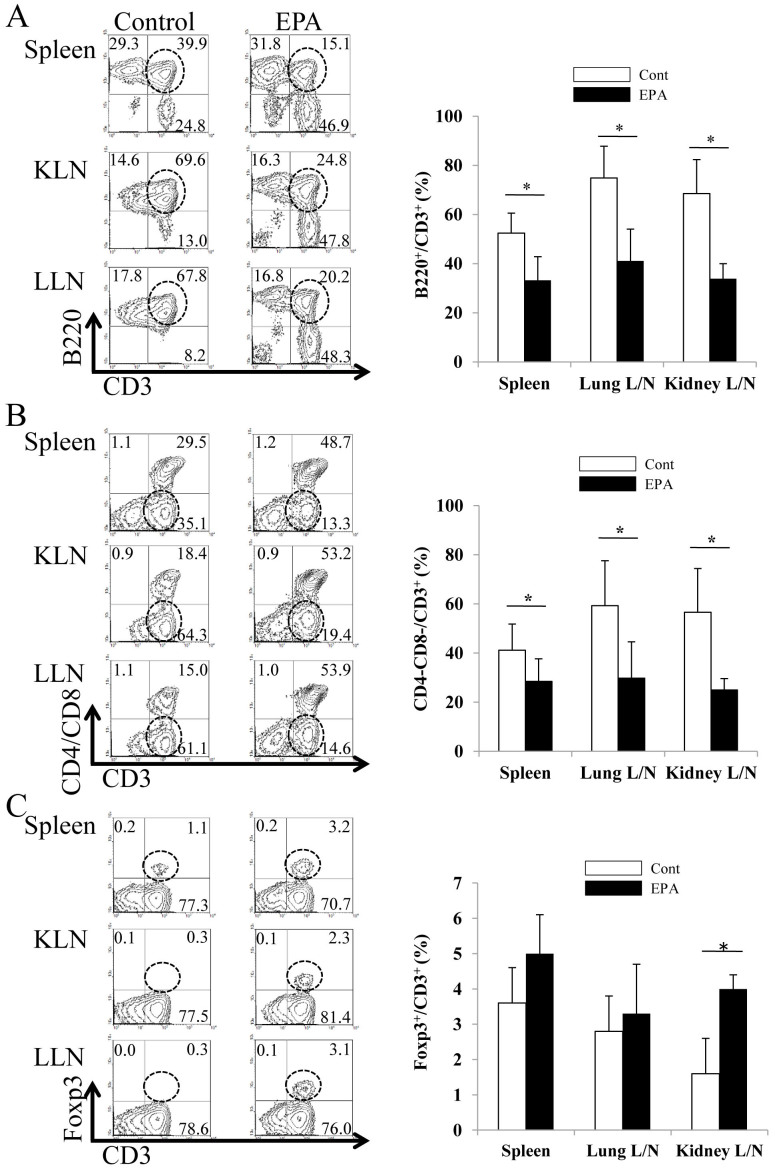
The finding that dietary enrichment with EPA suppressed MPO-ANCA autoantibody production demonstrates its autoimmunity-modulating function. To further investigate this mechanism, fluorescence-activated cell sorting (FACS) analysis using cells from the lymph nodes of the kidneys, lungs, and spleen was performed. SCG/Kj mice developed remarkable lymphoid hyperplasia similar to that in the MRL-Fas/lpr mice; this is attributed to the accumulation of CD3+ T cells that lack both CD4 and CD8 co-receptors and express B220 (a B-cell marker) molecules, the so-called B220+ double-negative (DN) T cells. We detected marked remodeling of the composition of T cell subsets in the spleen and in the lymph nodes of the kidneys and lungs (Fig. 7) of the EPA-treated group: a marked increase in the number of B220+CD3− or B220−CD3+ cells and a reduction in the number of DNT cells in the spleen and in the lymph nodes of the kidneys and lungs was observed. SCG/Kj mice develop remarkable lymphadenopathy and splenomegaly, which is associated with a Fas mutation leading to the accumulation of the double negative CD4−CD8−B220+ T cells. The precise role for these cells in the induction of glomerulonephritis and vasculitis continues to remain unclear; however, therapeutic agents such as NK02668029 and 15-deoxysperguarin30 promoted a decrease in these cells in SCG/Kj mice.
Figure 7. Fluorescence-activated cell sorting analysis of kidney and spleen T cell populations.
Lymphocyte profiling in the spleens and lymph nodes of SCG/Kj mice fed a control diet (open bar) or a 5% EPA-containing diet (closed bar). Single cell suspensions from the spleens and the lymph nodes of the kidneys (KLN) or the lungs (LLN) were stained with anti-CD3, anti-CD4, anti-CD8, or anti-B220 antibodies and anti-Foxp3 antibody. Lymphoproliferation in SCG/Kj mice is characterized by the accumulation of Thy-1+, Ly-1+, CD4/CD8 double negative and B220+ T cells. Representative dot plots are shown on the left. The percentages of CD3+B220+, CD3+Foxp3+, and CD3+CD4−CD8− cells in CD3+ cells, were determined. The expression of these cells was analyzed by flow cytometry. Results are expressed as mean ± SEM percentages of CD3+B220+, CD3+CD4−CD8−, and CD3+Foxp3+ cells in the spleen and KLN and LLN of three mice per group fed a control diet (open bar) or a 5% EPA-containing diet (closed bar). (*p < 0.05 versus control by Student's t-test).
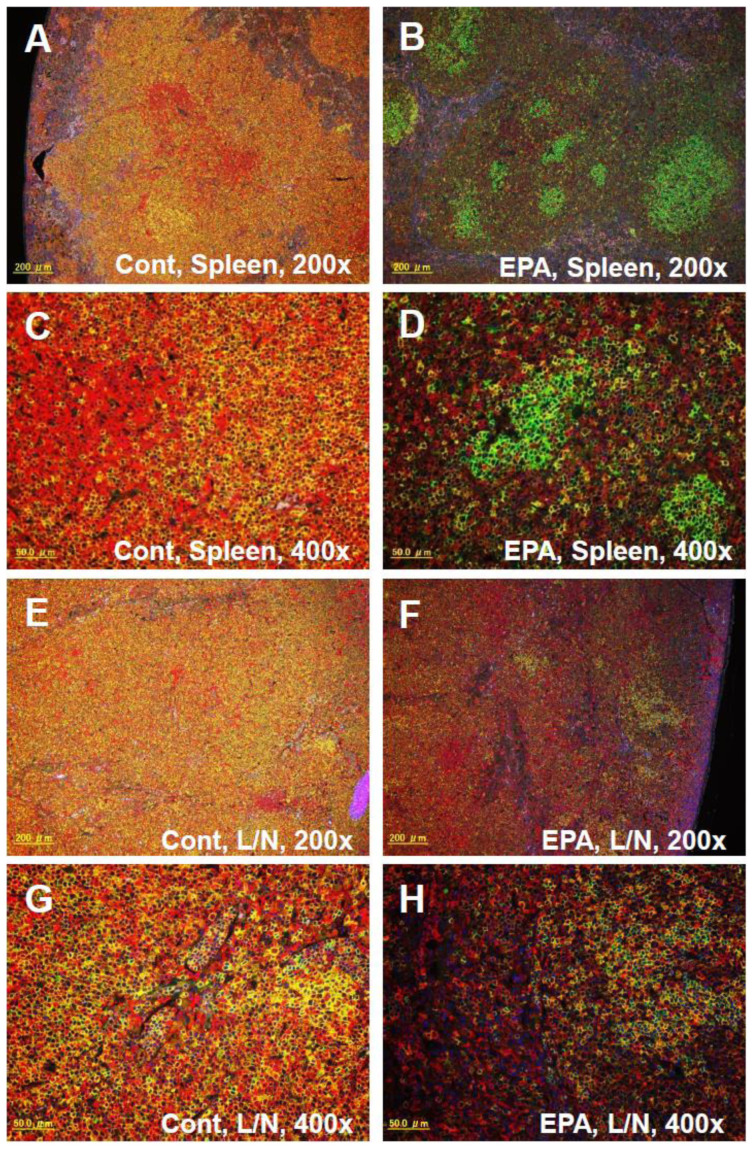
In addition, the administration of EPA significantly increased Foxp3+CD3+ cells in the lymph nodes of the kidney, which are indicative of regulatory T cells (Tregs). This suggests that EPA exerts its immunomodulatory function by inducing the production of Foxp3+ Tregs, whose ability to suppress immune responses critically requires cytotoxic T-lymphocyte antigen 4 (CTLA-4). Accordingly, using quantitative reverse transcriptase polymerase chain reaction (qRT-PCR), we detected the upregulated expressions of mRNA of the Treg markers, Foxp3 and CTLA-4, in the kidneys of SCG/Kj mice (see Supplementary Fig. S2A online). In addition, the upregulation of Foxp3 protein expression in the kidneys of the EPA-treated group was confirmed by western blot (see Supplementary Fig. S2B online). Immunofluorescent staining with anti-CD3 and anti-B220 antibodies confirmed the marked remodeling of the composition of T cell subsets in the spleen and lymph nodes of the EPA-treated group (Fig. 8), which corresponded to the results of flow cytometry in Fig. 7. These results suggest that immunomodulation with EPA, including enhancement of Tregs and suppression of DNT cells, is responsible for the beneficial effects in the treatment of AAV.
Figure 8. Immunohistochemical analysis of the spleen and lymph nodes.
Immunofluorescent staining with Alexa594 conjugated anti-CD3 (red) and Alexa488 conjugated anti-B220 antibodies (green) in the spleen (A, B, C, and D) and the lymph nodes (E, F, G, and H) of SCG/Kj mice fed a control diet (A, C, E, and G) or a 5% EPA-containing diet (B, D, F, and H) for 7 weeks. A, B, E, and F; Original magnification 200×. C, D, G, and H; Original magnification 400×.
Discussion
The current standard treatment for AAV consists of immunosuppressive therapy to control the inflammatory process. However, this strategy sometimes fails in balancing the systemic immune response with the healing of vascular damage. This is often associated with fatal infections and cardiovascular disease, leading to therapy-related mortality and morbidity.
Based on our clinical observations in inducing and maintaining remission by the administration of EPA, we conducted a basic experimental investigation and confirmed the therapeutic potential of EPA to silence autoimmunity and protect the endothelial cells in the kidney. Considering that most patients with AAV are elderly, we proposed the following strategies as required for the relief of this disease: 1. combatting active inflammation; 2. silencing the autoimmune disorder imbalance; and 3. protecting the endothelial cells and restoring their function to prevent cardiovascular events.
To combat active inflammation in AAV, we focused on neutrophil-mediated endothelial damage as an effector mechanism. In the innate immune system, endothelium–neutrophil interactions are highly regulated to allow neutrophils to adhere and migrate to the site of inflammation and infection without causing critical damage to the vascular system. In contrast, ANCA disrupts the highly regulated interactions in the vasculature of patients with AAV under the background of autoimmune disorder31,32. In the inflammatory setting of AAV, primed neutrophils firmly adhere to intercellular adhesion molecule (ICAM)-1-expressing endothelial cells, causing a massive increase in MPO- and PR3-ANCA antigen expression on the neutrophil surface, which is dependent on an adhesion molecule, Mac-1 (CD11b/CD18, CR3)33,34. Thus, the regulation of Mac-1 is an attractive therapeutic target of AAV. Indeed, we recently reported that Mac-1 is critical in the pathology of the other vasculitis and glomerulonephritis models35,36. In the present study, neutrophil recruitment into the inflamed glomeruli was prominent in the mice fed the control diet, but it was clearly prevented by dietary EPA supplementation. One mechanism explaining the results of this study is that EPA inhibits the adhesion of leukocytes to endothelial cells and their recruitment across endothelial cells by reducing the expression of endothelial adhesion molecules such as ICAM-1 and vascular cell adhesion molecule (VCAM)-137,38. Alternatively, EPA-derived lipid mediators, including resolvins, counterregulate CD18 on neutrophils25. In fact, through metabolomic analysis, we detected abundant EPA-derived lipid mediators biosynthesized in the kidney, specifically in the EPA-supplemented mice. Taken together, one of the therapeutic potential benefits of EPA in AAV is attributable to the inhibition of this leukocyte-endothelium interaction.
To silence the autoimmune disorder, recent advances in molecular biology and immunology have revealed candidates for molecular targets in the treatment of AAV, in addition to conventional immunotherapy. Based on these findings, several biological therapeutics, including monoclonal antibodies to CD20, BAFF, and Blys, are applied to the treatment of AAV; however, adverse events from these treatments remain to be determined5,6. These observations suggest that the development of an alternative strategy for silencing autoimmunity is needed. The imbalance between Th17 cells and Tregs has been recognized as an important factor in the pathogenesis of autoimmune disease39. The findings that serum IL-17 levels are elevated in patients with AAV40 and that IL-17-deficient mice were protected from autoimmune anti-MPO GN41 indicate that IL-17 is an attractive therapeutic target. In addition, impaired Treg function or reduced numbers of Tregs have been observed in autoimmune diseases, including AAV42. In patients with granulomatosis with polyangiitis, an immunosuppressive function of Tregs has been reported43 while a numerical deficiency, rather than a functional deficiency, of Tregs was demonstrated in patients with MPA44. Restoring the number and function of Tregs is a reasonable therapeutic goal.
Previous immunological research revealed that fish oil prolongs survival in animal models of autoimmune disease45,46,47 via an unknown mechanism. In a murine model of cardiac transplantation, intraperitoneal injection of highly purified EPA can markedly block allograft rejection by generating Tregs48. Thus, EPA induces tolerance in transplant immunity in addition to performing an anti-inflammatory function. In the present study, we detected a restorative effect of EPA on the number of Tregs in the lymph nodes of the kidney as a target organ of autoimmune insult; however, the assessment of functional alterations of Tregs remains to be conducted.
To protect endothelial cells and maintain their physiological functions, we further considered the possibility of cardiovascular morbidity in autoimmune disease. As demonstrated in rheumatoid arthritis and systemic lupus erythematosus, evidence revealing accelerated atherosclerosis and endothelial dysfunction in AAV is accumulating15. Because endothelial injury is a prominent consequence of vasculitis, protection and healing of the endothelium is another reasonable therapeutic goal in AAV. In the experimental animal model of AAV, we observed that the autoimmune insult led to a significant reduction of CD34 in the peritubular capillaries of the interstitium, as also shown in the human study49, but the endothelial damage was reversed by dietary enrichment with EPA.
Furthermore, we utilized metabolomic analysis to study EPA-derived anti-inflammatory lipid mediators. To our knowledge, this is the first metabolomic analysis demonstrating the production of EPA-derived anti-inflammatory lipid mediators in the kidney. Importantly, the production of EPA-derived anti-inflammatory mediators is markedly promoted by aspirin at the site of neutrophil-endothelial interactions50,51,52. Because the synthesis of the precursor 18-HEPE is triggered by aspirin-acetylated cyclooxygenase (COX)-2 in humans22, the use of aspirin possibly strengthened the anti-inflammatory potential of EPA during the treatment of AAV in our patients. Most patients recruited to the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico (GISSI) trial53, which revealed the potential of omega-3 PUFAs to protect against a second cardiovascular event, were taking low-dose aspirin in addition to omega-3 PUFA. We should consider the interaction between aspirin and EPA as a strategy to augment the effects of anti-inflammatory therapy in a clinical setting. The immunostabilizing effect of EPA at a dose of 1,800–2,700 mg/day in combination with aspirin at a dose of 100–200 mg/day in humans was evident because this treatment spared the use of immunosuppressants to maintain remission for an extended duration (more than five years). Thus, aspirin could potentiate the beneficial effects of EPA.
There are some limitations in this study. To better show the beneficial supportive effect of EPA in AAV treatment, additional animal experiments with added groups, including steroid alone, steroid + EPA, and steroid + cyclophosphamide, should be considered. This experimental design will provide the following important information: Is using EPA alone better than using steroids? Can EPA provide additional benefits in conjunction with steroids? Can EPA replace cyclophosphamide when combined with steroids?
In conclusion, the combination therapy of EPA and aspirin is an ideal treatment option that potentiates conventional immunotherapy and prevents adverse side effects. We propose that EPA should support conventional therapy because it is non-toxic, protects against cardiovascular disease, and minimizes the use of steroids and immunosuppressants. Further clinical trials should investigate whether EPA is suitable for inducing and maintaining remission in patients with AAV.
Methods
Mice
Female SCG/Kj mice aged 6 to 18 weeks (Nippon Kayaku, Tokyo, Japan) were used in the present study. The mice had free access to food and water. Mice were fed with laboratory powder chow which did not contain fish products (F1; Funabashi Farm, Chiba, Japan) or chow supplemented with 5% highly purified eicosapentaenoic acid (EPA) ethyl ester (purity: >98%, Mochida Pharmaceutical Co., Ltd., Tokyo, Japan) or 5% corn oil (LA; Sigma-Aldrich, St. Louis, MO, USA). All the mice used in this study were euthanized under anesthesia with ether. Animal care and experimental procedures were conducted according to the policies of Tokyo University. Experimental procedures were approved by the Animal Care and Use Committee of Tokyo University, Tokyo, Japan.
Functional assessment of renal injury
Peripheral blood was collected from the retro-orbital plexus of anesthetized mice with glass tubes to obtain plasma. The plasma values for creatinine (Cr) and blood urea nitrogen (BUN) were obtained with the Hitachi 7700 Automatic Analyzer at MBC (Tokyo, Japan). Hematuria grading was conducted by dipstick analysis using Uro-paper (Eiken Chemical Co., Tokyo, Japan).
Histological and immunohistochemical analysis of kidney and spleen
Crescent formation was determined by modified method of Floege et al.54 as follows: 22 glomerular cross-sections were graded by a relative area of the crescent occupied in Bowman's capsule as 0 (negative), 1 (1–25%), 2 (26–50%), 3(51–75%), 4 (76–100%); the total grade in 22 glomeruli was defined as a crescent score. Coronal sections (4-μm) of paraffin-embedded kidneys were stained with Periodic-Acid Schiff (PAS) or Masson-Trichrome reagent for analysis of glomerular and interstitial injury. Immunohistochemical staining was performed to examine endothelial injury in the kidneys by staining with rat monoclonal antibody to CD34 (GeneTex, Inc., San Antonio, TX, USA). Staining intensity was graded as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). We examined lymphocyte composition in the spleens and lymph nodes of the kidneys by staining with Alexa-rat monoclonal antibody to B220 and Alexa-CD3. Stained sections were mounted with FluorSave (Calbiochem; EMD Millipore, Billerica, MA, USA) and observed by fluorescence microscopy (Olympus IX-71, Tokyo, Japan).
Flow Cytometry
Antibodies used for flow cytometry included phycoerythrin (PE)-conjugated anti-CD3, FITC-conjugated anti-CD4, FITC-conjugated anti-CD8, or PE-Cy5-conjugated anti-B220 and anti-rat Foxp3 (eBioscience, San Diego, CA, USA). Splenocytes and cells isolated from lymph nodes of the kidney were incubated at 4°C for 20 min with FITC-or PE-conjugated antibody to CD3 or B220 (CD45RA) in the presence of Fc block. In order to identify lymphocyte populations, we first gated cells based on forward and side scatter properties. Next, we identified specific cell populations among these lymphocyte populations using fluorescence-labeled mAbs. The percentages of CD3+B220+, CD3+Foxp3+, and CD3+CD4−CD8− cells in CD3+ cells were determined. The expression was analyzed on a FACScan (Becton Dickinson, Tokyo, Japan) using the CellQuest software (version 3.3; Becton Dickinson, San Jose, CA, USA). Lymphoproliferation in SCG/Kj mice is characterized by the accumulation of Thy-1+, Ly-1+, CD4/CD8 double negative, and B220+ T cells.
Gene expression analysis by quantitative reverse transcriptase PCR (qRT-PCR)
For gene expression analysis, total RNA was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and purified using the Purelink Micro-to-Midi total RNA purification system (Invitrogen), according to the manufacturer's instructions. cDNA was generated using the SuperScript III First-Strand Synthesis System (Invitrogen). qRT-PCRs were performed using an ABI Prism 7000 or 7500 (Applied Biosystems, Foster City, CA, USA) and TaqMan gene expression assay kits (code nos. Mm00480849_ml for CTLA-4 and Mm00475152_ml for Foxp3, respectively; Applied Biosystems). Measurement of 18s rRNA was obtained using TaqMan ribosomal RNA control reagents (Applied Biosystems) as an endogenous control.
Western blot analysis
Tissues were homogenized in RIPA buffer (Themo Scientific, Rockford, IL, USA) containing a mixture of protease inhibitors that included 2 μg/mL aprotinin, 25 μg/mL calpain inhibitor I, 5 μg/mL pepstatin A, 1 mM dithiothreitol, 10 μg/mL leupeptin (Sigma Aldrich) and 0.5 mM pefabloc (Roche Diagnostics, Mannheim, Germany). Homogenates were centrifuged at 18,000 × g for 10 min at 4°C. The resulting supernatants were separated by SDS-PAGE. Western blot analysis was performed using anti-CTLA-4 antibody (sc-9094, Santa Cruz Biotechnology, Dallas, TX, USA), anti-Foxp3 antibody (12653, Cell Signaling Technology, Danvers, MA, USA) and anti-β-actin antibody (4970, Cell Signaling Technology) as an internal control. Blots were visualized with the enhanced chemiluminescent (ECL) Western Blotting Analysis System or ECL Prime Western Blotting Analysis System (GE Healthcare, Buckinghamshire, UK). The intensity of the CTLA-4 and Foxp3 bands was quantified with image analysis software (ImageQuant TL, GE Healthcare).
Measurement of fatty acid composition of plasma and tissues
To measure the fatty acid levels of plasma, kidney, lung, and spleen, lipid fractions from each specimen were extracted by the Folch procedure. The levels of four types of fatty acids (dihomo-γ-linolenic acid, arachidonic acid, eicosapentaenoic acid, and docosahexaenoic acid) were determined using the SHIMADZU GC-17A gas chromatograph (Shimadzu Corporation, Kyoto, Japan) and normalized by tissue weight.
Autoantibody determination
Antibody titer to MPO was measured as described previously55. Briefly, recombinant mouse MPO was coated onto an enzyme-linked immunosorbent assay (ELISA) plate (Toyoshima Co., Tokyo, Japan) overnight at 4°C. The plate was blocked with 1% bovine serum albumin (Sigma) and then incubated with mouse serum (50-fold dilution) for 1.5 h at room temperature. The bound MPO-ANCA was detected by 2 h incubation with alkaline phosphatase-labeled anti-mouse IgG antibody (Cappel, West Chester, PA, USA). The bound secondary antibodies were subsequently quantified by changes in the absorbance at 405 nm after incubation with 1 mg/mL p-nitrophenyl phosphate (Sigma).
Mediator lipidomics
Tissue from 12-week old mice (n = 3–5) was harvested and immediately frozen in liquid nitrogen. Frozen tissue samples were homogenized in ice-cold methanol and the supernatants were diluted with ice-cold water and adjusted to 10% (v/v) methanol. Deuterium-labeled internal standards were added to each sample; the clear supernatants were acidified to pH 4.0, and immediately applied to preconditioned solid phase extraction cartridges (Sep-Pak C18, Waters, Milford, MA, USA) to extract the lipid mediators. LC-MS/MS-based lipidomic analyses were performed using an HPLC system (Waters UPLC) with a linear ion trap quadrupole mass spectrometer (QTRAP5500; AB SCIEX, Tokyo, Japan) equipped with an Acquity UPLC BEH C18 column (Waters) as described previously56. MS/MS analyses were conducted in negative ion mode and fatty acid metabolites were identified and quantified by multiple reaction monitoring (MRM).
Statistical analysis
Results are expressed as the mean ± standard error of the mean (SEM) for data resulting from in vivo analyses of the mice. In all cases other than fatty acid composition analysis, the Student's unpaired t-test was used to compare two groups. A P value of <0.05 was considered statistically significant. For the analysis of fatty acid composition, the results are expressed as the mean ± SEM. Comparisons of the results from multiple groups were made with Dunnett's multiple comparison test, non-parametric Steel test. P values of <0.05 were considered significant.
Author Contributions
J.H. conducted experiments and wrote the main manuscript text and prepared all the figures. K.K. contributed to flow cytometry. M.A., R.I. and Mi.H. conducted mediator lipidomics and analyzed the data. M.N., KanS. and Kaz.S. conducted experiments and analyzed the data. S.K. supports the research by his grant. Y.H., M.T., M.K., K.O., A.T. and G.S. conducted clinical practice. T.F., O.T., T.M., Ma.H. and K.H. contributed to the concept of this research. All authors reviewed the manuscript.
Supplementary Material
Supplementary Figures
Acknowledgments
We gratefully thank Ms. Michiko Kamio for technical assistance on LC-MS/MS analyses. J.H. is supported by a Grant-in-aid from SENSHIN Medical Research Foundation, a Grant-in-aid from the Takeda Science Foundation, and a Grant-in-aid from the Ministry of Health, Labour and Welfare, Japan (Research on rare and intractable diseases). M.A. is supported by a Grant-in-aid from the Japan Science and Technology Agency Precursory Research for Embryonic Science and Technology (PRESTO), and a Grant-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. S.K. is supported by a Grant-in-aid from the Ministry of Health, Labour and Welfare, Japan.
Footnotes
Yes, there is potential Competing Interest. The authors have applied for patents for the use of EPA as a treatment of ANCA vasculitis.
References
- Bosch X., Guilabert A., Espinosa G. & Mirapeix E. Treatment of antineutrophil cytoplasmic antibody associated vasculitis: a systematic review. JAMA 298, 655–669 (2007). [DOI] [PubMed] [Google Scholar]
- Harper L. & Savage C. O. ANCA-associated renal vasculitis at the end of the twentieth century: a disease of older patients. Rheumatology (Oxford) 44, 495–501 (2005). [DOI] [PubMed] [Google Scholar]
- Harper L. Recent advances to achieve remission induction in antineutrophil cytoplasmic antibody-associated vasculitis. Curr. Opin. Rheumatol. 22, 37–42 (2010). [DOI] [PubMed] [Google Scholar]
- Hellmich B. Update on the management of systemic vasculitis: what did we learn in 2009? Clin. Exp. Rheumatol. 28, 98 (2010). [PubMed] [Google Scholar]
- Jones R. B. et al. European Vasculitis Study Group. Rituximab versus Cyclophosphamide in ANCA-associated Renal Vasculitis. N. Engl. J. Med. 363, 211–220 (2010). [DOI] [PubMed] [Google Scholar]
- Stone J. H. et al. Rituximab versus Cyclophosphamide for ANCA-associated Vasculitis. N. Engl. J. Med. 363, 221–232 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little M. A. et al. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis. Ann. Rheum. Dis. 69, 1036–1043 (2010). [DOI] [PubMed] [Google Scholar]
- Faurschou M. et al. Increased morbidity from ischemic heart disease in patients with Wegener's granulomatosis. Arthritis Rheum. 60, 1187–1192 (2009). [DOI] [PubMed] [Google Scholar]
- Morgan M. D. et al. Increased incidence of cardiovascular events in patients with antineutrophil cytoplasmic antibody-associated vasculitides: a matched-pair cohort study. Arthritis Rheum. 60, 3493–3500 (2009). [DOI] [PubMed] [Google Scholar]
- Woywodt A. et al. Circulating endothelial cells as markers for ANCA-associated small-vessel vasculitis. Lancet 361, 206–210 (2003). [DOI] [PubMed] [Google Scholar]
- Chironi G. et al. Increased prevalence of subclinical atherosclerosis in patients with small-vessel vasculitis. Heart 93, 96–99 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw K. et al. Accelerated atherosclerosis in patients with Wegener's granulomatosis. Ann. Rheum. Dis. 64, 753–759 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnoux C., Chironi G., Simon A. & Guillevin L. Atherosclerosis in ANCA-associated vasculitides. Ann. N. Y. Acad. Sci. 1107, 11–21 (2007). [DOI] [PubMed] [Google Scholar]
- Sherer Y. & Shoenfeld Y. Mechanisms of disease: atherosclerosis in autoimmune diseases. Nat. Clin. Pract. Rheumatol. 2, 99–106 (2006). [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y. et al. Accelerated atherosclerosis in autoimmune rheumatic diseases. Circulation 112, 3337–3347 (2005). [DOI] [PubMed] [Google Scholar]
- Filer A. D. et al. Diffuse endothelial dysfunction is common to ANCA associated systemic vasculitis and polyarteritis nodosa. Ann. Rheum. Dis. 62, 162–167 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama M. et al. Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentanoeic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 369, 1090–1098 (2007). [DOI] [PubMed] [Google Scholar]
- Hirahashi J., Jo A., Ueda K., Tojo A. & Fujita T. Successful treatment of antineutrophil cytoplasmic antibody-associated vasculitis with eicosapentaenoic acid. Ann. Intern. Med. 156, 755–756 (2012). [DOI] [PubMed] [Google Scholar]
- Kinjoh K., Kyogoku M. & Good R. A. Genetic selection for crescent formation yields mouse strain with rapidly progressive glomerulonephritis and small vessel vasculitis. Proc. Natl. Acad. Sci. U. S. A. 90, 3413–3417 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huugen D. et al. Antineutrophil cytoplasmic autoantibodies and pathophysiology: new insights from animal models. Curr Opin Rheumatol. 16, 4–8 (2004). [DOI] [PubMed] [Google Scholar]
- Neumann I. et al. SCG/Kinjoh mice: A model of ANCA-associated crescentic glomerulonephritis with immune deposits. Kidney Int. 64, 140–148 (2003). [DOI] [PubMed] [Google Scholar]
- Serhan C. N. et al. Novel functional sets of lipid-derived mediators with anti-inflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal anti-inflammatory drugs and transcellular processing. J. Exp. Med. 192, 1197–1204 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N. Resolution phases of inflammation: novel endogenous anti-inflammatory and pro-resolving lipids mediators and pathways. Annu Rev Immunol 25, 101–137 (2007). [DOI] [PubMed] [Google Scholar]
- Levy B. D., Clish C. B., Schmidt B., Gronert K. & Serhan C. N. Lipid mediator class switching during acute inflammation: signals in resolution. Nature Immunol. 2, 612–619 (2001). [DOI] [PubMed] [Google Scholar]
- Dona M. et al. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood 112, 848–855 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoubian S. & Serhan C. N. New endogenous anti-inflammatory and proresolving lipid mediators: implications for rheumatic diseases. Nat. Clin. Pract. Rheumatol. 3, 570–579 (2007). [DOI] [PubMed] [Google Scholar]
- Tjonahen E. et al. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem. Biol. 13, 1193–1202 (2006). [DOI] [PubMed] [Google Scholar]
- Morin C. et al. Relaxing effects of 17(18)-EpETE on arterial and airway smooth muscles in human lung. Am J Physiol Lung Cell Mol Physiol. 296, L130–139 (2009). [DOI] [PubMed] [Google Scholar]
- Saiga K. et al. NK026680, a novel suppressant of dendritic cell function, prevents the development of rapidly progressive glomerulonephritis and perinuclear antineutrophil cytoplasmic antibody in SCG/Kj mice. Arthritis Rheum 54, 3707–3715 (2006). [DOI] [PubMed] [Google Scholar]
- Tomizawa K. et al. Reduction of MPO-ANCA epitopes in SCG/Kj mice by 15-deoxyspergualin treatment restricted by IgG2b associated with crescentic glomerulonephritis. Rheumatology 49, 1245–1256 (2010). [DOI] [PubMed] [Google Scholar]
- Halbwachs L. & Lesavre P. Endothelium-neutrophil interactions in ANCA-associated diseases. J. Am. Soc. Nephrol. 23, 1449–1461 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan S. L. et al. Mechanisms of ANCA-mediated leukocyte-endothelial cell interactions in vivo. J. Am. Soc. Nephrol. 19, 973–984 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau D. et al. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc. Natl. Acad. Sci. U. S. A. 102, 431–436 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerke U. et al. Complement receptor Mac-1 is an adaptor for NB1 (CD177)-mediated PR3-ANCA neutrophil activation. J. Biol. Chem. 286, 7070–7081 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahashi J. et al. Mac-1 signaling via Src-family and Syk kinases results in elastase-dependent thrombohemorrhagic vasculopathy. Immunity. 25, 271–283 (2006). [DOI] [PubMed] [Google Scholar]
- Hirahashi J. et al. Mac-1 (CD11b/CD18) links inflammation and thrombosis after glomerular injury. Circulation 120, 1255–1265 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samantha P. et al. Omega-3 Fatty Acids and Inflammation: Novel Interactions Reveal a New Step in Neutrophil Recruitment. In Vivo and In Vitro Inhibition of Monocyte Adhesion to Endothelial Cells and Endothelial Adhesion Molecules by Eicosapentaenoic Acid. PLoS. Biol. 7, e1000177 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H. et al. In Vivo and In Vitro Inhibition of Monocyte Adhesion to Endothelial Cells and Endothelial Adhesion Molecules by Eicosapentaenoic Acid. Arterioscler. Thromb. Vasc. Biol. 28, 2173–2179 (2008). [DOI] [PubMed] [Google Scholar]
- Bettelli E., Korn T., Oukka M. & Kuchroo V. K. Induction and effector functions of T(H)17 cells. Nature 453, 1051–1057 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira E. et al. Serum IL-17 and IL-23 levels and autoantigen-specific Th17 cells are elevated in patients with ANCA-associated vasculitis. Nephrol. Dial. Transplant. 25, 2209–2217 (2010). [DOI] [PubMed] [Google Scholar]
- Gan P. Y. et al. Th17 cells promote autoimmune anti-myeloperoxidase glomerulonephritis. J. Am. Soc. Nephrol. 21, 925–931 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia X. & Lipsky P. E. CD4 + CD25 + FoxP3+ regulatory T cells in autoimmune diseases. Nat. Clin. Pract. Rheumatol. 13, 1193–1202 (2007). [DOI] [PubMed] [Google Scholar]
- Abdulahad W. H. et al. Functional defect of circulating regulatory CD4+ T cells in patients with Wegener's granulomatosis in remission. Arthritis Rheum. 56, 2080–2091 (2007). [DOI] [PubMed] [Google Scholar]
- Chavele K. M. Regulation of myeloperoxidase-specific T cell responses during disease remission in antineutrophil cytoplasmic antibody-associated vasculitis: the role of Treg cells and tryptophan degradation. Arthritis Rheum. 62, 1539–1548 (2010). [DOI] [PubMed] [Google Scholar]
- Kelley V. E., Ferretti A., Izui S. & Strom T. B. A fish oil diet rich in eicosapentaenoic acid reduces cyclooxygenase metabolites and suppresses lupus in MRL-lpr mice. J. Immunol. 134, 1914–1919 (1985). [PubMed] [Google Scholar]
- Halade G. V. et al. Docosahexaenoic Acid-Enriched Fish Oil Attenuates Kidney Disease and Prolongs Median and Maximal Life Span of Autoimmune Lupus-Prone Mice. J. Immunol. 184, 5280–5286 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halade G. V. et al. Docosahexaenoic acid-enriched fish oil attenuates kidney disease and prolongs median and maximal life span of autoimmune lupus-prone mice. J. Immunol. 184, 5280–5286 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwami D. et al. Purified Eicosapentaenoic Acid Induces Prolonged Survival of Cardiac Allografts and Generates Regulatory T Cells. Am J Transplant 9, 1294–1307 (2009). [DOI] [PubMed] [Google Scholar]
- Nakabayashi K. et al. Tubulointerstitial nephritis without glomerular lesions in three patients with myeloperoxidase-ANCA-associated vasculitis. Clin. Exp. Nephrol. 13, 605–613 (2009). [DOI] [PubMed] [Google Scholar]
- Arita M. et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 201, 713–722 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott S. M. & Stenson W. F. Fish oil fix. Nat. Med. 11, 596–598 (2005). [DOI] [PubMed] [Google Scholar]
- Flower R. J. & Perretti M. Controlling inflammation: a fat chance? J. Exp. Med. 201, 671–674 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. 1999. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet 354, 447–455 (1999). [PubMed] [Google Scholar]
- Floege J. et al. Increased synthesis of extracellular matrix in mesangial proliferative nephritis. Kidney Int 40, 477–478 (1991). [DOI] [PubMed] [Google Scholar]
- Ishida-Okawara A. et al. Neutrophil contribution to the crescentic glomerulonephritis in SCG/Kj mice. Nephrol. Dial. Transpl. 19,1708–1715 (2004). [DOI] [PubMed] [Google Scholar]
- Arita M. Mediator lipidomics in acute inflammation and resolution. J. Biochem. 152, 313–319 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures