Abstract
Natural killer (NK) cells are critical for innate tumor immunity due to their specialized ability to recognize and kill neoplastically transformed cells. However, NK cells require a specific set of cytokine-mediated signals to achieve optimal effector function. Th1-associated cytokines promote effector functions which are inhibited by the prototypic Th-2 cytokine IL-4 and the TGF-β superfamily members TGF-β1 and activin-A. Interestingly, the largest subgroup of the TGF-β superfamily are the bone morphogenetic proteins (BMP), but the effects of BMP signaling to NK cell effector functions have not been evaluated. Here we demonstrate that blood-circulating NK cells express type I and II BMP receptors, BMP-2 and BMP-6 ligands, and phosphorylated isoforms of Smad-1/-5/-8 which mediate BMP family member signaling. In opposition to the inhibitory effects of TGF-β1 or activin-A, autocrine BMP signaling was supportive to NK cell function. Mechanistic investigations in cytokine and TLR-L activated NK cells revealed that BMP signaling optimized IFN-γ and global cytokine and chemokine production; phenotypic activation and proliferation; autologous DC activation and target cytotoxicity. Collectively, our findings identify a novel auto-activatory pathway that is essential for optimal NK cell effector function, one which might be therapeutically manipulated to help eradicate tumors.
Keywords: NK cells, BMPs, Smads, activin-A, TGF-β, noggin
Introduction
NK cells are circulating innate immune sentinels specialised to recognise and kill tumour and virus-infected cells 1. Consisting typically of 2 - 5% of peripheral blood mononuclear cells (PBMCs) NK cells represent a significant component of the human immune repertoire. Historically, human NK cells have been subdivided into at least two subgroups based on their expression of CD16 and CD56 2. CD56 intermediate(int)/CD16+ NK cells are credited with more immediate cytotoxic capability, while the numerically smaller (in blood) CD56 hi(hi)/CD16− subset is associated with more rapid IFN-γ release and proliferation upon activation 3,4. This early IFN-γ production can act in an autocrine fashion to stimulate NK cells own effector functions and can also significantly enhance important DC functions such as tumour antigen processing, presentation and cross-presentation for the priming of tumour antigen-specific CD4+ and CD8+ T cells. Nevertheless, uncontrolled proliferation and/or release of NK cell inflammatory cytokines can result in pathology or cellular transformation 5,6. Thus, the immune system has coordinately developed mechanisms to avoid these potentially life threatening events through the release of inflammatory mediators 7.
With relation to NK cells, their effector functions are best known to be negatively regulated via engagement of inhibitory receptors with MHC class I proteins or by suppressive cytokines such as IL-4 or TGF-β1 8-10. While it has been known for some time that exogenous TGF-β1 can suppress NK cells IFN-γ production, it is only relatively recently that activin-A (a further TGF-β superfamily member) has also been described to be inhibitory 11. Considering the TGF-β superfamily consists of more than 30 members, our understanding of how members other than TGF-β1 and activin-A may potentially influence NK cells effector functions is very poor. Interestingly, the largest subset of the TGF-β superfamily are the BMPs yet aside from our recent report demonstrating a role for autocrine BMP signaling in human thymic NK cell development 12 the literature is yet to describe the consequences of BMP signaling to NK cells effector functions.
The BMPs signal via type I and II serine/threonine kinase receptor heterodimers in complex with bound ligand. Three type I receptors have been shown to bind BMP ligands i.e. BMPRIA (or ALK-3), BMPRIB (or ALK-6) and the activin receptor ActRIA (or ALK-2). There are also three type II receptors termed BMPRII, ActRIIA and ActRIIB. Whereas BMPRIA, BMPRIB and BMPRII specifically bind BMPs, ActRIA, ActRIIA and ActRIIB are also receptors for the activins 13. Activated receptors relay signals to the nucleus predominantly via the canonical Smad dependant pathway (although non-canonical Smad independent pathways exist e.g. p38 MAPK 14) where phosphorylated (p)-Smads-1/-5/-8 complex with co-Smad-4 and translocate into the nucleus to trigger target gene expression 14,15.
As their name suggests, the BMPs originally defined biological function was the capacity to induce bone formation 16. However, the biological actions of the BMPs are now known to be diverse with critical roles in numerous developmental processes 17-21. Indeed, specific BMPs influence the formation of disparate tissues in mammals such as skin, eyes, teeth, heart, kidneys and testes and dysregulated signaling can result in major muscular skeletal abnormalities 22-28. These diverse functions are accounted for in part by i) their significant number; ii) the existence of numerous antagonists; iii) their complex interactions with other TGF-β superfamily members; iv) their promiscuous receptor usage; v) the presence of co-repressors and activators; and vi) the existence of multiple inhibitors of signaling such as the pseudoreceptor BAMBI, Smads-6 and -7 and the HECT-type E3 ligases Smurf 1 and 2 13,29-38.
The BMPs (as with the TGF-βs and activins) are pleiotropic in nature and can both inhibit or promote tumorigenesis. Indeed, BMP signaling can have pro-metastatic effects on breast cancer cells 39 and antagonism of BMP type I receptors has been reported to decrease growth and induce cell death of lung cancer cell lines 40. In contrast, BMP-2 signaling has been demonstrated to inhibit the development of colon cancers 41 while BMP-4 can potently inhibit the tumour-initiating capacity of human glioblastoma precursors 42. Strikingly, this study also demonstrated that prophylactic or therapeutic in vivo delivery of BMP-4 dramatically reduced mortality in mice following intracerebral grafting of human glioblastoma cells.
Given that TGF-β1 and activin-A are potent negative regulators of NK cells effector functions, we hypothesised that BMP signaling may also have important consequences. Our studies demonstrate that NK cells express cell surface and intracellular stores of BMP receptors, mRNAs for BMP-2 and -6 and p-Smads-1/-5/-8. In sharp contrast to the suppressive effects of TGF-β1 or activin-A 11, inhibition of autocrine BMP signaling in NK cells reveals a novel autocrine activatory pathway that confers optimal IFN-γ production, global cytokine and chemokine production, phenotypic maturation, proliferation, autologous DC activation and most importantly cytotoxicity. Furthermore, we have also identified that NK cells resident in the bone marrow of acute lymphoblastic leukaemia (ALL) patients at high risk of relapse display significantly reduced levels of the high affinity type I BMPR receptor, BMPRIA, and this correlates with a phenotype indicative of a reduced activatory state.
These data have important implications for the development of new methods aimed to enhance NK cells capacity to kill tumours directly in vivo or potentially to enhance NK cells effector functions prior to adoptive immune therapy into cancer patients.
Materials and Methods
Cell culture
PBMCs from buffy coats of healthy donors (Red Cross Blood Bank, Melbourne, Australia and Centro de Transfusión de la Comunidad de Madrid, Spain) were prepared by Ficoll-Paque (GE healthcare Bio-sciences) density gradient centrifugation. NK cells were isolated by negative selection using a NK cell isolation kit and MACS (Miltenyi Biotech, Auburn). In some cases NK cells were further purified by FACS (MoFlow, Beckman Coulter). Unless otherwise stated, culture media consisted of RPMI supplemented with 10% heat inactivated foetal calf serum (FCS), 20 mM HEPES, 60 mg/L penicillin, 12.5 mg/L streptomycin and 2 mM L-glutamine. NK cells were maintained in culture media alone or culture media supplemented with IL-2 at 20 - 50 ng/ml with or without addition of 10 ng/ml IL-12 together with 10 μg/ml of poly I:C (InvivoGen). In some cases NK cells were cultured in 20 - 50 ng/ml IL-15 alone. CD1c+ myeloid DCs, CD14+ monocytes and CD4+ and CD8+ T cells were isolated by positive selection by MACS. To generate monocyte derived DCs (MoDCs), CD14+ monocytes were cultured in culture media containing 10% FCS, 20 ng/ml GM-CSF and 20 ng/ml IL-4 (Invitrogen) for 6 - 7 days. For NK cell and DC co-cultures, autologous NK cells and CD1c+ or MoDCs were co-cultured at a DC:NK cell ratio of 1:1 or 1:5 respectively for 24 hours. For the experiments shown in figure 5, NK cells were treated under the conditions shown for 12 hours then extensively washed prior to co-culture with autologous MoDCs. To assess the effects of blocking BMP signaling, Compound C/dorsomorphin an inhibitor of BMPRIA, BMPRIB, ActRIA and AMPK or the highly selective BMPRIA and ActRIA inhibitor DMH1 (both TOCRIS Bioscience) or recombinant human noggin (R&D Systems) were added to NK cell cultures for 12 - 24 hours in the absence or presence of IL-2 with or without addition of IL-12 and poly I:C.
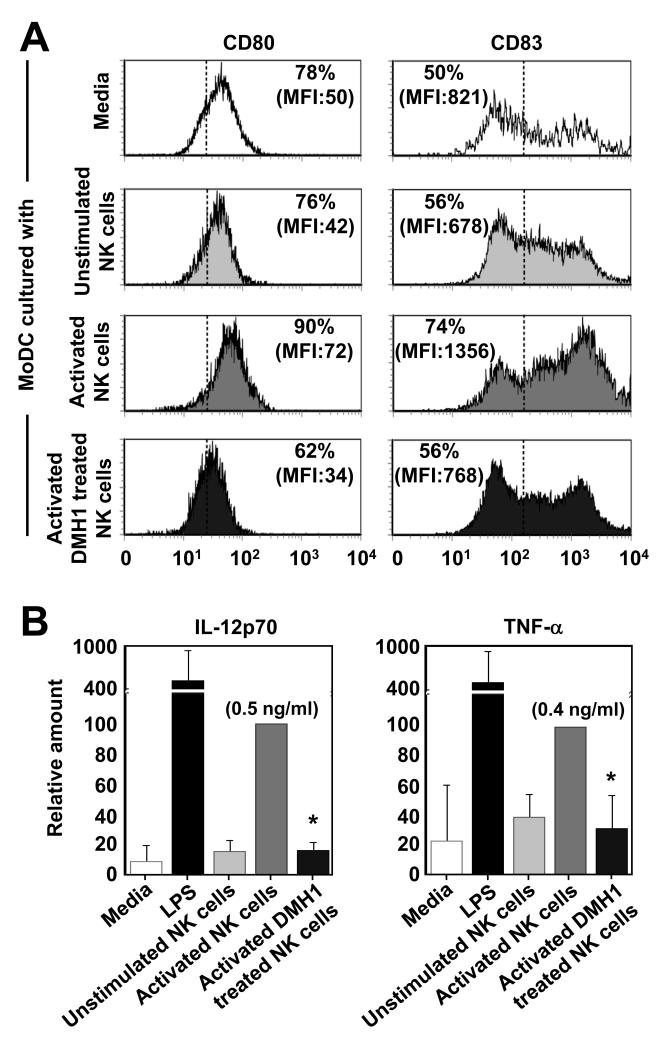
Fig. 5. DMH1-treated NK cells display a reduced capacity to induce DC maturation.
NK cells were cultured for 12 hours in culture media or IL-2 (20 ng/ml) or IL-2, -12 and poly I:C with or without addition of DMH1 (20 μM). NK cell were then washed x3 before additional culture alone or together with immature in vitro derived MoDCs at a ratio of 1:5. After 18 hours of culture the levels of CD80 and CD83 expressed by MoDCs (A) was determined by flow cytometry and in (B) the levels of IL-12p70 and TNF-α in the culture supernatants were determined by ELISA. One representative donor of three is shown in (A) and the data are the mean ± 1SD of three separate donors in (B). *p<0.01 vs. IL-2, -12 and poly I:C activated NK cells without DMH1 treatment. In (B) the addition of LPS to MoDC served as a positive control.
Quantitative real time polymerase chain reaction (qRT-PCR)
RNA was isolated using the RNeasy Mini Kit (Qiagen) and cDNA synthesised. Gene expression was quantified using a stratagene Mx3005P machine. Primers were designed for detection of BMPRIA, IB, RII, BMP-2, -4, -6 and -7 and used with probes from a Universal ProbeLibrary (Roche Applied Science). Pre-Developed TaqMan® Assay Reagents were obtained from Applied Biosystems and included: Smad-1 (Hs00195432_m1), Smad-3 (Hs00969210_m1), Smad-7 (Hs00998193_m1), BAMBI (Hs00180818_m1) and TGF-β1 (Hs00998133_m1). 18S rRNA and GNB2L1 were used for normalisation. PCR reactions were set up in 96 well plates and analysed using SDS program version v1.9. The frequency of target gene expression was calculated using the formula (1+Enorm)Ct norm / (1 +EGOI)Ct GOI where “E” = efficiency. Otherwise, the relative expression was calculated using 2(Ct18S – Ct target gene). Relative expression was then calculated using the ΔCt method and expressed relative to a calibrator i.e. ex vivo purified NK cells or relevant NK cell control cultures.
Flow cytometry
NK cells or MoDCs were co-stained with combinations of fluorochrome-labelled antibodies against human CD1c, CD3, CD14, CD16, CD56, CD69, CD80, CD83, CD94, LAMP1/CD107a, BMPRIA, BMPRIB, BMPRII, NKp30, NKp46, KIR2DL2/L3, NKG2A and D (BD Biosciences, Biolegend and R&D Systems). Intracellular staining was performed using anti-human BMPRIA (R&D Systems) and anti-human p-Smad-1/-5/-8 antibodies (Ser463/Ser465; Santa Cruz Biotechnology). The proportion of apoptotic NK cells was determined by Annexin-V (BioLegend) staining and flow cytometry and the data was analysed using FlowJo software (version 3.4).
Assessment of NK cell proliferation
Purified NK cells were labeled with 1 μM CFSE (Molecular Probes) at 37°C for 10 minutes. After washing the cells were cultured in IL-2 or IL-15 alone or IL-2 in combination with IL-12 and poly I:C with or without the addition of 100 ng/ml of noggin or 20 μM DMH1 for 5 or 6 days.
Cytokine ELISA and multiplex cytokine and chemokine arrays
Cytokine ELISA kits were used to quantify IFN-γ, IL-12p70 and TNF-α (BD Biosciences and Biolegend). Cytokine and chemokine bead arrays (LINCOplex) were used to quantify IFN-γ, IL-6, -10, -13, TNF-α, GM-CSF, CXCL8, CXCL10, CCL3 and CCL4 and samples analysed on a Luminex instrument (all Millipore). IL-6, -10, GM-CSF, CCL5, CXCL10 and CCL2 were also measured using a Cytometric Bead Array Flex Set system (BD Biosciences).
Assessment of NK cell cytotoxicity
Purified NK cells were cultured overnight in media containing IL-2 alone or IL-2 together with IL-12 and poly I:C in the absence or presence of increasing concentrations of DMH1 before being washed and then co-cultured with the erythroleukemic K562 target cell line in 96-well plates for 4 hours at the effector-to-target ratios indicated. In some case DMH1 was added during the 4 hour cytotoxicity assay. Specific lysis was determined using a nonradioactive Cytotoxicity Detection Kit (LDH, Roche Diagnostics) by measuring lactate dehydrogenase activity in culture supernatants. In some experiments LAMP-1/CD107a expression by NK cells was assessed by flow cytometry after 4 hours of culture at an NK cell/K562 ratio of 10:1.
Bone marrow samples
Samples were obtained from bone marrow aspirates at diagnosis (n=12) or at relapse (n=5) from children with ALL at Hospital Niño Jesús (Madrid, Spain). Patients were treated under the PETHEMA protocol 43. The patients were classified according to this protocol into high-risk (n=5) or low/intermediate-risk (n=7). Expression levels of different surface markers on NK cells were determined by multi-parameter flow cytometry on bone marrow samples. A CD45-positive CD3-negative CD56-positive gate was used for each sample in order to analyze the NK cell population. Samples were acquired on a FACS Canto II flow cytometer (BD Biosciences) and analysed with FlowJo software.
Statistics
Unless indicated, results are expressed as the mean ± 1SD of 3 or more donors. Data was analysed using the Student’s t test and P<0.01 (*) were considered significant.
Results
BMP receptor and ligand gene expression in human NK cells
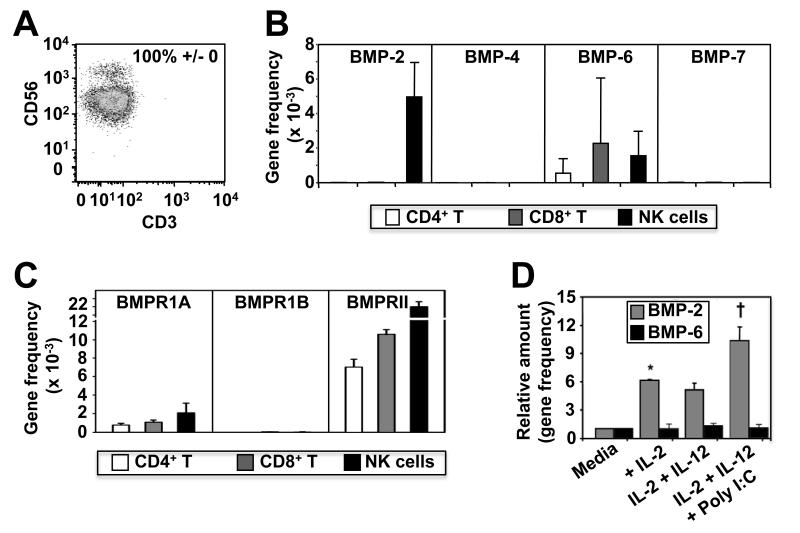
To assess the relevance of BMP signaling to NK cells we first investigated BMP receptor and ligand expression at the mRNA level. To do this, we highly purified NK cells (Figure 1A) and autologous CD4+ and CD8+ T cells from human blood and analysed the expression of mRNAs for BMP-2, -4, -6 and -7 ligands and the BMP type I (BMPRIA and BMPRIB) and type II (BMPRII) receptors by qRT-PCR. We found that NK cells exclusively expressed BMP-2 but that each cell type commonly expressed BMP-6 message (Fig 1B). Furthermore, although highly expressed on skeletal muscle controls (not shown), BMP-4 and -7 mRNAs were absent from each of the lymphocyte populations tested (Fig. 1B). The analysis further demonstrated that BMPRIA and BMPRII mRNA’s are expressed by each of these lymphocyte populations but that NK cells expressed significantly higher levels of BMPRII mRNA than either CD4+ or CD8+ T cells (Figure 1C). Interestingly, BMPRIB expression was absent from each cell type suggesting that NK cells and CD4+ and CD8+ T cells are likely to use a BMPRIA/BMPRII receptor pair for BMP ligand binding and signal transduction. Finally, NK cells express TLR-3 and the TLR-3 ligand poly I:C is currently being evaluated in experimental clinical settings to enhance NK cell mediated immunity against cancers. Furthermore, we previously have found that the addition of poly I:C to IL-2 and IL-12 stimulated NK cells significantly enhances their pro-inflammatory cytokine and chemokine production, proliferation and killing functions (Robson et al, Blood 2009). We therefore tested whether a brief exposure to IL-2 alone or IL-2 in combination with IL-12, with or without addition of poly I:C could influence NK cells mRNA expression of BMP ligands. We found that the expression of BMP-6 mRNAs by NK cells was unaffected by the addition of cytokines or poly I:C, but culture in a low concentration of IL-2 (20 ng/ml) was sufficient to induce up-regulated BMP-2 mRNA expression (Figure 1D). Interestingly, the addition of IL-12 to NK cell cultures did not synergise with IL-2 to enhance BMP-2 expression but the addition of poly I:C increased BMP-2 mRNA levels significantly above that induced by IL-2 alone (Fig. 1D). BMP-4 or BMP-7 mRNA expression however was not induced under any of the conditions tested. Furthermore, NK cells cultured in IL-2, -12 and poly I:C significantly up-regulated mRNA for BMPRIA and BMP signaling specific Smad-1, but conversely, simultaneously down regulated mRNA for inhibitory Smad-7, TGF-β1, Smad-3 and BAMBI (Supplemental Fig. 1).
Fig. 1. NK cells express mRNAs for BMP receptors and ligands.
In (A - C) NK cells and autologous CD4+ and CD8+ T cells were purified from buffy coats of healthy donors by MACS separation and FACS cell sorting. Highly purified (>99%) CD56+ CD3− cells were then lysed and assessed for expression of BMP-2, -4, -6 and -7 ligands (B) and BMPRIA, IB and BMPRII (C) by qRT-PCR. In (D), NK cells were purified by MACS (purity typically >95%) from 3 donors then lysed after 4 hours of culture in media without cytokines or in culture media supplemented with IL-2 alone, or with IL-2 and IL-12 with or without addition of poly I:C. RNA was then extracted and qRT-PCR performed. RNA isolated from skeletal muscle served as positive controls (data not shown). Data are shown as the mean ± 1SD of three separate donors. *p<0.01 vs. media only and †<0.01 vs. media only, IL-2 alone or IL-2 with IL-12.
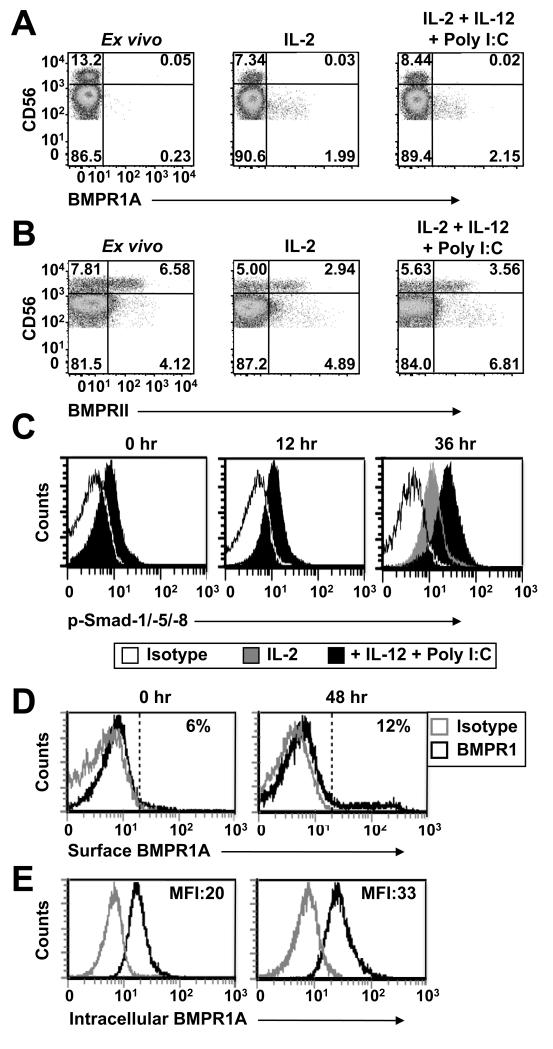
To better understand the relationship between NK cells BMP receptor mRNA expression and cell surface receptor expression we screened purified NK cells by flow cytometry for BMPRIA and BMPRII expression at the protein level ex vivo and after exposure to cytokines with or without addition of poly I:C. Immediately following purification from blood, NK cells BMPRIA expression ranged between donors from barely detectable (Fig. 2A) to low expression (Fig. 2D). Culture in IL-2 induced some up-regulation of BMPRIA on NK cells but this was unaffected by the further addition of IL-12 and poly I:C over the 20 hour culture period (Fig. 2A). These NK cells did however significantly up-regulate their expression of the activation marker CD69 when exposed to IL-12 and poly I:C (not shown). BMPRII expression differed and was relatively abundant on CD56hi NK cells with levels approaching 50% for some donors (Fig. 2B). As with NK cells BMPRIA expression, culture in IL-2, -12 and poly I:C had no significant impact on their expression of BMPRII (Fig. 2B).
Fig. 2. NK cells express BMPRIA and BMPRII on their surface.
MACS purified NK cells (purity ≥95%) were stained with monoclonal antibodies against CD3, CD56, BMPRIA, BMPRII or p-Smads-1/-5/-8 either immediately after purification or after 12 - 48 hours of culture in 96 well plates at 1 × 105 cells/well in media supplemented with IL-2 with or without addition of IL-12 and poly I:C. The dot plots show CD56int and CD56hi CD3− NK cells with the exclusion of a small (<5%) population of CD56− cells. NK cells surface expression of BMPRIA is shown in (A and D), intracellular BMPRIA expression in (E), BMPRII expression in (B) and intracellular p-Smads-1/-5/-8 expression in (C). Data are representative of 3 - 6 donors.
NK cells interactions with DCs or target cells can significantly influence their phenotype. To test if this was the case for NK cells expression of BMP receptors we co-cultured NK cells with activated autologous CD1c+ myeloid DCs or with K562 target cells and measured BMP receptor expression by flow cytometry. The results demonstrated that neither of these stimulatory conditions had any effect on NK cells expression of either BMPRIA or BMPRII (not shown). Thus, stimuli typically associated with the induction of NK cell activation did not impact NK cells BMP receptor expression.
To confirm mRNA and protein expression of BMP receptors and ligands facilitated autocrine BMP signaling we next measured NK cells intracellular p-Smad-1/-5/-8 levels by flow cytometry. Immediately after isolation a significant proportion of NK cells stained p-Smad-1/-5/-8 positive and these levels increased through culture in IL-2 alone and further increased through addition of IL-12 and poly I:C (Fig. 2C). While these results are in agreement with those showing increased expression of mRNAs encoding BMPRIA and Smad-1 by NK cells cultured under the same conditions (Supplemental Fig. 1), they were not fully reconcilable with our data showing more limited cell surface expression of BMPRIA and differential expression of BMPRII i.e. between CD56int and CD56hi NK cells (Fig. 2 A and B). To understand this more fully we explored the possibility that NK cells may have an alternative mechanism to facilitate BMP signaling. Others have shown in transfected cell lines that BMPRIA can be present intracellularly and to test if this was relevant to NK cells, we compared cell surface and intracellular BMPRIA staining immediately after isolation and following culture in IL-2, -12 and poly I:C. Consistent with our previous data (see Fig. 2A), BMPRIA was up-regulated on the surface of a minority (range 2 - 15%) of NK cells after stimulation (Fig. 2D). However, in complete contrast to this the intracellular staining revealed that the majority of NK cells stained BMPRIA positive ex vivo and that these levels increased upon in vitro stimulation with IL-2, -12 and poly I:C (Fig. 2E). The analysis also demonstrated that both the CD56int and CD56hi NK cell subsets each expressed similar levels of intracellular BMPRIA ex vivo and that each subset increased their expression of intracellular BMPRIA and p-Smads-1/-5/-8 upon stimulation with IL-2, -12 and poly I:C (Supplemental Fig. 2 A and B).
Autocrine BMP signaling confers optimal NK cell effector functions
To address our hypothesis that BMP signaling in NK cells may alter their effector functions we first utilised Compound C/dorsomorphin, a chemical known to inhibit BMP signaling by binding to BMPRIA, BMPRIB and ActRIA 44. In summary, addition of Compound C/dorsomorphin to IL-2 and IL-12 stimulated NK cells resulted in their reduced expression of NKG2D, and in a dose dependant manner, inhibition of IFN-γ release (Supplemental Fig. 3 A and B). Furthermore, addition of Compound C/dorsomorphin to IL-2, -12 and poly I:C stimulated NK cells resulted in the near complete inhibition of their capacity to kill K562 target cells (Supplemental Fig. 3C).
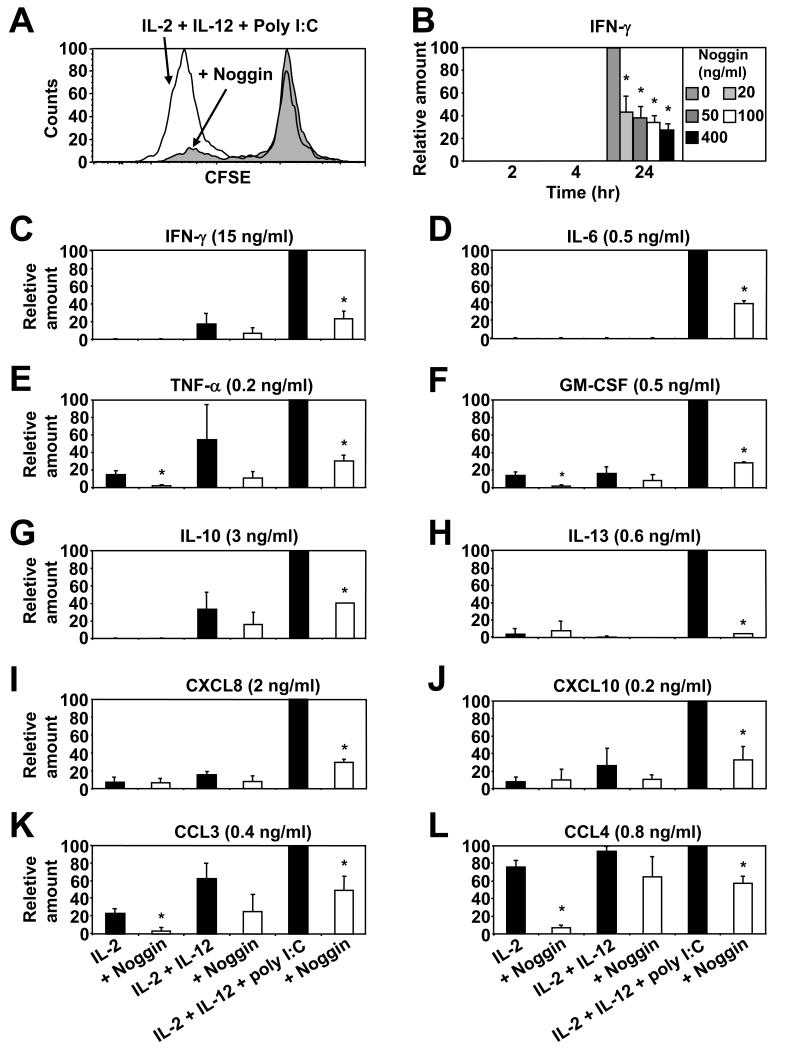
Given the potentially confounding effects of Compound C/dorsomorphin on other kinases, we reassessed our findings by utilising the well-documented natural BMP antagonist, noggin 45. We first assessed the effects of the addition of noggin on NK cells proliferation, as this is an important mechanism to amplify anti-tumour defences. To do this we CFSE labelled NK cells and cultured them for 5 days in IL-2, -12 and poly I:C with or without noggin. The results demonstrate that this stimulatory combination of cytokines, together with a TLR-3 agonist, was effective at inducing NK cell proliferation (especially in the CD56hi subpopulation; data not shown) but most importantly, inhibition of BMP signaling via the addition of noggin significantly inhibited proliferation globally (Fig. 3A). Furthermore, in agreement with our findings using dorsomorphin/compound C (Supplemental Fig. 3B), the addition of noggin (even at low concentrations) significantly inhibited IFN-γ release by IL-2, -12 and poly I:C stimulated NK cells (Fig. 3B). To extend our analysis, additional donors NK cells were cultured in IL-2 alone or together with IL-12 with or without the addition of poly I:C or noggin before the supernatants were interrogated for cytokine and chemokine content by LUMINEX. Firstly, the results demonstrate that the addition of poly I:C (together with IL-2 and IL-12) induced significant increases in NK cells release of the majority of cytokine’s and chemokine’s tested i.e. above that induced by IL-2 and IL-12 stimulation alone (Fig. 3 C - L). This method confirmed and extended our ELISA data by again demonstrating that inhibition of autocrine BMP signaling through the addition of noggin to IL-2, -12 and poly I:C activated NK cells significantly inhibited their IFN-γ production (Fig. 3C), but also revealed that the addition of noggin suppressed NK cells production of Th-1 associated IL-6, TNF-α and GM-CSF (Fig. 3 D - F), Th-2 associated IL-10 and IL-13 (Fig. 3 G and H) and the chemokine’s CXCL8, CXCL10, CCL3 and CCL4 (Fig. 3 I - L).
Fig. 3. The natural BMP antagonist noggin inhibits NK cells proliferation and cytokine and chemokine production.
In (A) MACS purified NK cells (purity ≥95%) were CFSE labelled then cultured at 2 × 105 cells/well in 96 well round bottomed plates in media supplemented with IL-2, -12 and poly I:C in the absence or presence of noggin (100 ng/ml) for 5 days. NK cells proliferation was assessed through dilution of CFSE intensity by flow cytometry. In (B) purified NK cells were cultured at 1 × 105 cells/well in 96 well round bottomed plates in media supplemented with IL-2, -12 and poly I:C with or without the indicated concentrations of noggin for 2 - 24 hours and IFN-γ levels in the NK cells supernatants were measured by ELISA. Similaraly, in (C - L) purified NK cells were cultured in IL-2 alone with or without addition of IL-12 or poly I:C or noggin (100 ng/ml) for 20 hours and the indicated cytokine’s and chemokine’s were measured by Luminex. One representative donor of three is shown in (A) while in (B - L) the data are the mean ± 1SD of three separate donors. *p<0.01 vs. IL-2, -12 and poly I:C activated NK cells without addition of noggin.
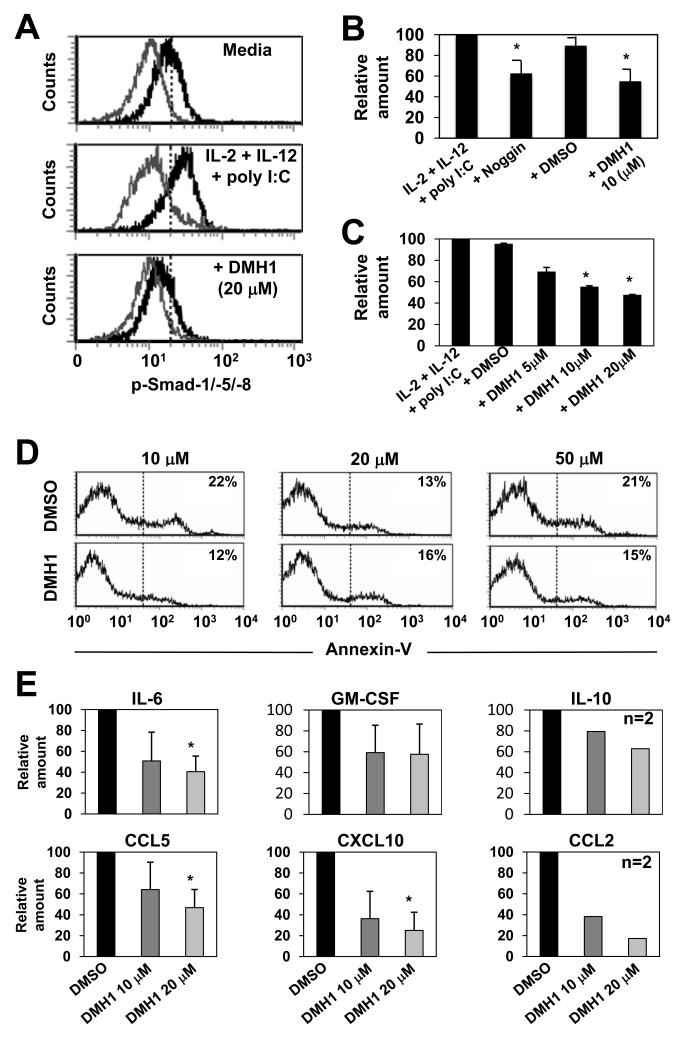
Noggin neutralises a number of BMPs (including BMP-2, -4, -5, -6 and -7) but its binding affinity varies e.g. very high affinity for BMP-2 but moderate affinity for BMP-7. Therefore, to further substantiate our findings we modified our experimental method by incorporating the use of DMH1, a second-generation Compound C/dorsomorphin homologue known to be a highly specific inhibitor of BMPRIA (and also ActRIA). We first tested if addition of DMH1 to IL-2, -12 and poly I:C stimulated NK cells resulted in altered p-Smad-1/-5/-8 expression. The results demonstrate that stimulation with IL-2, -12 and poly I:C increased p-Smad-1/-5/-8 levels in NK cells above that expressed by NK cells cultured in media alone, but most importantly, the addition of DMH1 effectively inhibited p-Smad-1/-5/-8 induction in each instance (Fig. 4A).
Fig. 4. The BMPRIA inhibitor DMH1 decreases NK cells cytokine and chemokine production.
MACS purified NK cells (purity ≥95%) were cultured in IL-2, -12 and poly I:C with or without addition of DMH1 (5 - 50 μM) or DMSO as vehicle control or noggin (100 ng/ml). In (A) p-Smads-1/-5/-8 expression were determined after 20 hours by intracellular staining and flow cytometry. In (B and C) NK cell supernatants were collected after 20 hours of culture and IFN-γ production determined by ELISA. In (D) annexin-V expression was determined by flow cytometry and in (E) the indicated cytokine and chemokine levels were determined using a cytometric bead array system. In (A and D) one representative donor of three is shown and in (B), (C) and (E) the data are the mean ± 1SD of two or three separate donors. *p<0.01 vs. no addition of noggin or DMH1.
Next, to test whether inhibition of NK cells BMP signaling via DMH1 supported our findings using noggin we compared the two reagents side by side. The results demonstrated that addition of DMH1 to IL-2, -12 and poly I:C stimulated NK cells inhibited their IFN-γ release, and importantly, this was comparable to the inhibition induced by noggin (Fig. 4B), was dose dependent (Fig. 4C), and was not superficially due to inhibitor toxicity (Fig. 4D). Intracellular IFN-γ staining also revealed that both CD56int and CD56hi NK subsets contained IFN-γ+ cells upon stimulation with IL-2, -12 and poly I:C and that the proportion of each diminished upon DMH1 treatment (Supplemental Fig. 4A). Also, similarly to noggins inhibitory effects on NK cell proliferation, blocking autocrine BMP signaling through the addition of DMH1 to either IL-2 or IL-15 stimulated NK cells also resulted in inhibition of proliferation (Supplemental Fig. 4 B and C). Finally, further analysis also confirmed that addition of DMH1 to IL-2, -12 and poly I:C stimulated NK cells resulted in a very similar pattern of cytokine (IL-6, -10 and GM-CSF) and chemokine (CCL5, CXCL10 and CCL2) inhibition as that induced by BMP neutralisation through addition of noggin (compare Fig. 3 C - L to Fig. 4E). Thus, inhibition of BMP signaling through addition of a naturally occurring antagonist or prevention of BMP signaling through chemically mediated receptor blockade each resulted in a decreased capacity of NK cells to produce inflammatory cytokines and chemokines. Given that each method of BMP signaling inhibition resulted in the same functional outcome, and taking into consideration that small molecule inhibitors have several advantages over their endogenous counterparts (including lower cost and more consistent activity), we concluded our studies using DMH1.
As mentioned previously, the importance of bi-directional communication between NK cells and DCs for Th-1 mediated anti-tumour immunity is well documented. Therefore, we wished to identify if inhibition of BMP signaling in NK cells resulted in their altered capacity to activate autologous DCs. To do this we cultured NK cells in the presence or absence of IL-2, -12 and poly I:C with or without addition of DMH1 for 12 hours before the NK cells were washed thoroughly and then co-cultured with autologous MoDCs. After 24 hours we then assessed the phenotype of the MoDCs and measured cytokine production. In agreement with the literature, activated NK cells had an enhanced capacity to induce CD80 and CD83 expression by MoDCs, but importantly, DMH1 treated NK cells were compromised in this regard (Fig. 5A). In line with these findings, DMH1 treated IL-2, -12 and poly I:C activated NK cells completely failed to induce IL-12p70 production by MoDC and DMH1 treatment inhibited global TNF-α production (Fig. 5B). Taken together, these results show that the auto-activatory effects of BMP signaling in NK cells are important not only for their own capacity to produce inflammatory cytokine’s and chemokine’s but also for their paracrine capacity to activate DCs.
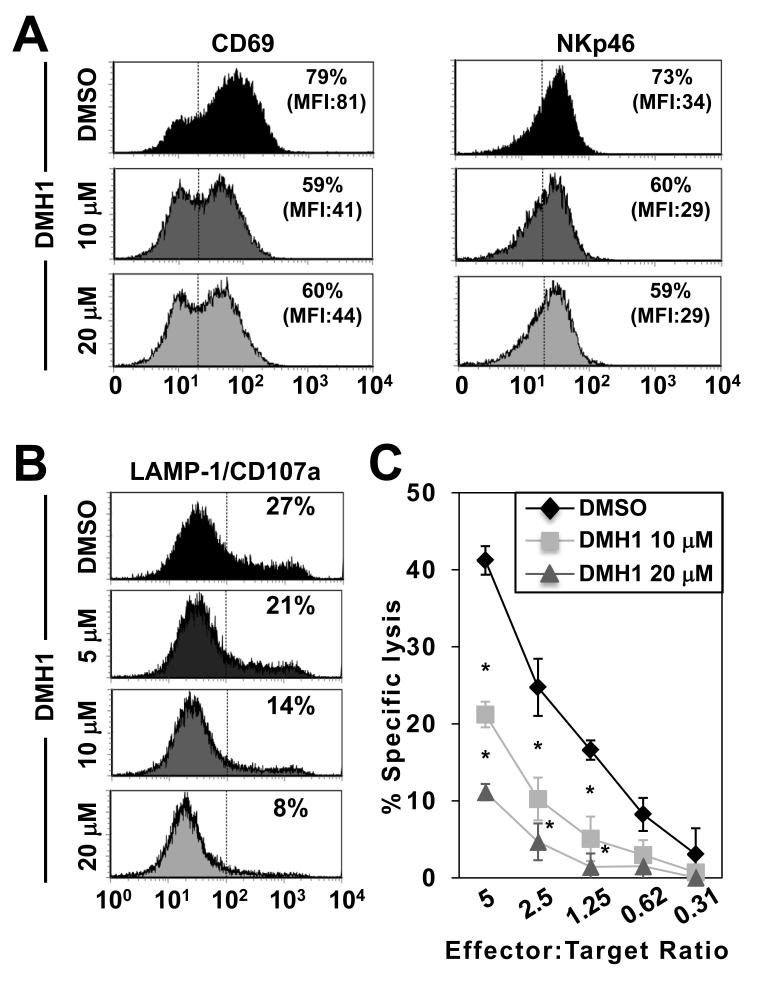
The engagement of activatory and inhibitory receptors allows NK cells to discriminate between healthy cells and those requiring execution. Therefore, we next examined the significance of autocrine BMP signaling in this character defining function. These studies first identified that autocrine BMP signaling in NK cells is required for their optimal expression of the activation markers CD69 and NKp46 (Fig. 6A). Moreover, NK cells co-cultured with K562 target cells in the presence of DMH1 completely failed to release IFN-γ (Supplemental Fig. 5A). Finally, NK cells activated with IL-2, -12 and poly I:C in the presence of DMH1 had reduced expression of the cytotoxic granule marker LAMP1/CD107a upon subsequent co-culture with K562 target cells (Fig. 6B), which most importantly, directly correlated with a dramatic reduction in killing capacity (Fig. 6C). However, the addition of DMH1 during the 4-hour cytotoxicity assay had no inhibitory effect on NK cells cytolytic capacity (Supplemental Fig. 5B). Taken together, our data define autocrine BMP signaling as an inherent pathway underpinning NK cells most defining property, killing.
Fig. 6. DMH1-treated NK cells are less phenotypically activated and have a reduced capacity to kill.
In (A) NK cells were stimulated with IL-2, -12 and poly I:C for 12 hours in the absence or presence of the indicated doses of DMH1 or DMSO as vehicle control and the expression of CD69 and NKp46 was determined by flow cytometry. In (B and C) NK cells were treated as in (A) then co-cultured for 4-hours with K562 target cells before (B) LAMP1/CD107a expression was determined by flow cytometry and (C) cytotoxicity determined by specific lysis. One representative donor of three is shown in (A and B) while in (C) the specific lysis data are the mean ± 1SD of three separate donors. *p<0.01 vs. DMSO.
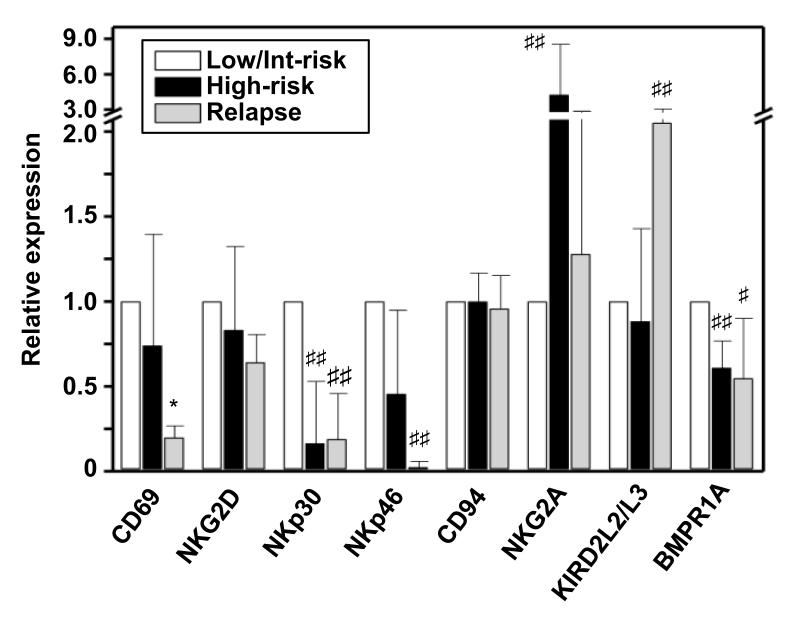
In our concluding analyses we wished to understand if NK cells from cancer patients displayed some changed phenotypic expression within the BMP signaling system and whether this correlated with other important markers of NK cell activation. To this end we collected bone marrow samples from ALL patients who were assessed as being at low/intermediate or high risk of relapse or those having already relapsed. The analysis demonstrated that NK cells from relapsed patients had significantly reduced expression of the activation marker CD69 as well as the natural cytotoxicity receptors NKp30 and NKp46 (Fig. 7). Conversely, high risk and relapsed patients co-expressed increased levels of the inhibitory receptors NKG2A and KIRD2L2/L3, respectively. Most importantly, the analysis demonstrated that bone marrow NK cells from high-risk patients had significantly reduced (p<0.05) expression of the high affinity BMP binding receptor BMPRIA and relapsed patients had reduced (p=0.06) expression (Fig.7). Thus, lower levels of BMPRIA expression correlates with an NK cell phenotype representing reduced activatory marker and increased inhibitory marker expression.
Fig. 7. Human acute lymphoblastic leukemia patients NK cells display reduced expression of the high affinity BMP binding receptor BMPRIA.
Bone marrow aspirates were taken from ALL patients diagnosed as low/intermediate or high risk of relapse or relapsed (n=7, 5 and 5 respectively). Bone marrow cells were stained with combinations of flourochrome labeled antibodies against CD3, CD45, CD56, CD69, NKG2D, NKp30 and 46, CD94, NKG2A, KIR2DL2/L3 and BMPRIA before the cells were analysed by flow cytometry. All p values are calculated against the low/intermediate group and *p<0.01, ##p<0.05 and #p<0.06.
Discussion
A major distinguishing feature separating the BMPs from the TGF-βs and activins is that the BMPs predominantly signal via a complex of p-Smads-1/-5/-8 while the TGF-βs and activins share the use of Smads-2 and -3 14. It was these differences in Smad usage that led to the synthesis of our hypothesis that BMP signaling might influence NK cells effector functions differently to the inhibitory signals provided by TGF-β1 10,46 or activin-A 11. Our first PCR based screen aimed to identify whether blood circulating human NK cells (and CD4+ and CD8+ T cells) express a genetic signature indicative of a capacity to respond to and/or produce specific BMP ligands. These results identified that NK cells express mRNAs for BMPRIA (the principal BMP ligand binding receptor) as well as mRNAs for BMPRII (the exclusive BMP type II receptor) 14. It is interesting that neither NK cells nor CD4+ or CD8+ T cells expressed mRNAs for BMPRIB. These data are therefore the first to demonstrate that NK cells have the genetic makeup to express the prototypic BMP type I/II receptor pair. Furthermore, the analysis revealed the novel findings that these same NK cells contained mRNAs for distinct BMP ligands i.e. BMP-2 and -6 but not BMP-4 or -7. In comparison with CD4+ or CD8+ T cells NK cells were exclusive in their expression of BMP-2 mRNAs, but interestingly, each lymphocyte population expressed BMP-6 mRNAs. Taken together, this information formed the foundation of our investigations which led to our demonstration that inhibition of autocrine BMP signaling in cytokine and TLR-3 stimulated NK cells confers optimal (i) IFN-γ and global cytokine and chemokine production; (ii) phenotypic activation and proliferation; (iii) autologous DC activation and (iv) cytotoxicity.
To date, the literature describing roles for TGF-β superfamily members in the mediation of NK cells effector functions has almost exclusively focused on TGF-β1. However, we recently demonstrated that activin-A displays some similarities in function to TGF-β1 in that it inhibits NK cells proliferation and cytokine and chemokine production, but unlike TGF-β1, does not directly affect their cytolytic capacity 11. This is particularly relevant considering we have found that activated human CD1c+ myeloid DCs and MoDCs produce large amounts of activin-A yet questionable levels of TGF-β1 47. These findings are intriguing and raise the possibility that it is DC derived activin-A that is the most relevant negative regulator of NK cells functions during bi-direction DC/NK crosstalk.
Most interestingly, our current findings open the possibility that individual TGF-β superfamily members may play very distinct (as yet unrealised) roles in NK cell biology. Contextually, our current studies complement and extend our recent report which shows that the subpopulation of early human intrathymic CD34+CD1a− progenitor cells that express BMPRIA contains a large population of NK cell lineage committed precursor cells 12. Indeed, in the presence of exogenous IL-15 autocrine BMP signaling promotes human intrathymic progenitor differentiation in vitro into an immature CD56lowCD161low population that remains predominantly BMPRIA+ and then a major mature CD56int/hiCD161hi population that has mostly lost BMPRIA expression 12. Likewise, our analysis of human lymph node resident NK cells demonstrated that BMPRIA expression diminished sequentially from stage 2 pre-NK cells to stage 4 mature NK cells.
Consistent with these observations, our new data show that freshly isolated blood circulating NK cells express mRNAs for BMPRIA but that these cells only express low levels of cell surface BMPRIA. This finding was surprising considering BMPRIA is thought to be the dominant ligand binding receptor and that these same donors CD56hi NK cells expressed significant levels of cell surface BMPRII (see Fig. 2B). These results prompted a more in depth investigation that led to the striking findings that the majority of freshly isolated (cell surface BMPRIA low) NK cells have intracellular stores of BMPRIA and that a significant proportion of these express p-Smads-1/-5/-8. Interestingly, our BMPRIA intracellular staining data is in line with Song et al who have shown similar results in transfected mouse C2C12 myoblast cell lines and human HEK 293 embryonic kidney cells 48. These intracellular stores of BMPRIA may be essential for NK cells further BMP responsiveness, a suggestion supported by our findings that addition of DMH1 profoundly inhibits NK cells effector functions. Moreover, it is noteworthy that the addition of potent activatory signals to NK cells such as IL-2 in combination with IL-12 and poly I:C or co-culture with K562 targets or activated DCs had no effect on NK cells surface expression of BMPRIA nor their net expression of BMPRII. Considering such stimuli are well known to induce or reduce the expression of a number of important receptors on NK cells, including IL-12p70 and TGF-β receptors (respectively) 11, it stands out that the only factor we have tested that results in altered (increased) BMPRIA expression was the addition of recombinant BMP-2 (data not shown). It is certainly possible that BMPs produced by NK cells or other immune or stromal cells could trigger receptor exocytosis thus making the receptor available to bind exogenous ligand. Alternatively, a mechanism may be in place whereby BMPRIA containing “compartments” could fuse with ligand and BMPRII containing endosomes (or other forms of vesicles) to facilitate signaling. In this regard Shi et al described that the FYVE-domain protein endofin, which largely localises in early endosomes, binds Smad-1 preferentially and enhances Smad-1 phosphorylation and nuclear localisation upon BMP stimulation 49. This result suggests that BMP signaling could well be initiated in an early endocytic compartment. Similarly, two other FYVE-domain proteins (SARA and Hgs) also localise in early endosomes and mediate the initiation of TGF-β/activin signaling favouring receptor-induced activation of Smads-2 and -3 50,51.
Our initial findings that Compound C/dorsomorphin profoundly inhibited NK cells capacity to kill the K562 human leukemic tumour cell line and inhibited their production of IFN-γ significantly raised our awareness of the potential importance of this pathway to NK cells. Indeed, it was this data that prompted our more exhaustive investigations of the effects of BMP signaling inhibition via complementary usage of its natural antagonist noggin and the BMPRIA and ActRIA inhibitor DMH1. Our data demonstrates that autocrine BMP signaling confers a number of character defining functions to NK cells thus supporting our original hypothesis i.e. that BMP signaling in NK cells may result in functionally distinct outcomes to those induced by activin-A or TGF-β. Indeed, in the absence of autocrine BMP signaling NK cells capacity to proliferate was severely compromised as was their capacity to: i) produce inflammatory cytokines and chemokines; ii) express phenotypic markers of activation that are known to play important co-activatory roles; iii) bi-directionally activate autologous DCs, and finally and most importantly; iv) kill.
The sum of this inhibition could be devastating and life threatening. For example, an NK cell encountering a BMP antagonist-producing tumour or oncogenic virus-infected cell in vivo may become incapacitated and thus functionally defunct. An outcome such as this could clearly have substantial “knock-on” effects potentially resulting in significantly compromised immunity. This raises the very important question as to the potential production of various BMPs by distinct immune cell populations and to the potential production of BMP antagonists by tumour cells. Indeed, in the context of tumourigenesis our findings are intriguing and clearly point to the value of a detailed analysis of human immune cell and tumour cell specific expression of BMP receptors, ligands and antagonists.
This is especially relevant considering our finding that NK cells resident in the bone marrow of high risk of relapse ALL patients have reduced expression of ligand binding BMPRIA. This raises the possibility that NK cells from patients with some forms of cancer may have a reduced capacity to bind BMP ligands thus leading to a reduced capacity to receive the activatory signal provided by p-Smad-1/-5/-8 signaling. Clearly we need to extend this analysis and assess the expression of BMP receptors (cell surface and intracellular) and levels of p-Smad-1/-5/-8 signaling in NK cell populations from patients with other types of cancer. Finally, given inhibition of BMP signaling can so potently inhibit NK cells effector functions, deliberate activation of this pathway is a logical next step to understand whether this approach may generate hyper-activated NK cells that are more potent in the killing of tumour cells for immunotherapy. Taken together, our findings have identified a major new piece in the complex puzzle that is the biology of NK cells.
Supplementary Material
Acknowledgements
We wish to thank Dr Suzanne Graham for helpful discussion and for proof reading the manuscript. N.R. was supported by an Industry Fellowship Grant from the Australian National Health and Medical Research Council (NHMRC), The Ludwig Institute for Cancer Research, a Wellcome Trust Travelling Fellowship and a Lord Kelvin Adam Smith Fellowship from the University of Glasgow. L.H. and A.E. are supported by pre-doctoral fellowships (AP2009-4324 and AP2010-0795, respectively) from the Ministerio de Educación, Cultura y Deporte. E.M. is an employee of CSL limited and an Honorary Senior Research Fellow of the Ludwig Institute for Cancer Research. J.C. is an NHMRC Practitioner Fellow. This work was supported by grants SAF2012-33180 (Ministerio de Economía y Competitividad), S2010/BMD-2420 (Comunidad de Madrid) and RD12/0019/0007 (Instituto de Salud Carlos III) to A.Va. and A.Vi.
Footnotes
Conflict of interest: None.
References
- 1.Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011 Jan 7;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986 Jun 15;136(12):4480–4486. [PubMed] [Google Scholar]
- 3.Fehniger TA, Cooper MA, Nuovo GJ, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003 Apr 15;101(8):3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 4.Vitale M, Della Chiesa M, Carlomagno S, et al. The small subset of CD56brightCD16- natural killer cells is selectively responsible for both cell proliferation and interferon-gamma production upon interaction with dendritic cells. Eur J Immunol. 2004 Jun;34(6):1715–1722. doi: 10.1002/eji.200425100. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez LA, Pope B, Lee C, Zayed E. Aggressive natural killer cell leukemia in an adult with establishment of an NK cell line. Blood. 1986 Apr;67(4):925–930. [PubMed] [Google Scholar]
- 6.Chan A, Filer A, Parsonage G, et al. Mediation of the proinflammatory cytokine response in rheumatoid arthritis and spondylarthritis by interactions between fibroblast-like synoviocytes and natural killer cells. Arthritis Rheum. 2008 Mar;58(3):707–717. doi: 10.1002/art.23264. [DOI] [PubMed] [Google Scholar]
- 7.Banchereau J, Pascual V, O’Garra A. From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat Immunol. 2012 Oct;13(10):925–931. doi: 10.1038/ni.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moretta A, Vitale M, Sivori S, et al. Human natural killer cell receptors for HLA-class I molecules. Evidence that the Kp43 (CD94) molecule functions as receptor for HLA-B alleles. J Exp Med. 1994 Aug 1;180(2):545–555. doi: 10.1084/jem.180.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agaugue S, Marcenaro E, Ferranti B, Moretta L, Moretta A. Human natural killer cells exposed to IL-2, IL-12, IL-18, or IL-4 differently modulate priming of naive T cells by monocyte-derived dendritic cells. Blood. 2008 Sep 1;112(5):1776–1783. doi: 10.1182/blood-2008-02-135871. [DOI] [PubMed] [Google Scholar]
- 10.Castriconi R, Cantoni C, Della Chiesa M, et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):4120–4125. doi: 10.1073/pnas.0730640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robson NC, Wei H, McAlpine T, Kirkpatrick N, Cebon J, Maraskovsky E. Activin-A attenuates several human natural killer cell functions. Blood. 2009 Apr 2;113(14):3218–3225. doi: 10.1182/blood-2008-07-166926. [DOI] [PubMed] [Google Scholar]
- 12.Hidalgo L, Martinez VG, Valencia J, et al. Expression of BMPRIA on human thymic NK cell precursors: role of BMP signaling in intrathymic NK cell development. Blood. 2012 Feb 23;119(8):1861–1871. doi: 10.1182/blood-2011-07-370650. [DOI] [PubMed] [Google Scholar]
- 13.Mueller TD, Nickel J. Promiscuity and specificity in BMP receptor activation. FEBS Lett. 2012 Jul 4;586(14):1846–1859. doi: 10.1016/j.febslet.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 14.Sieber C, Kopf J, Hiepen C, Knaus P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009 Oct-Dec;20(5-6):343–355. doi: 10.1016/j.cytogfr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Liu F, Hata A, Baker JC, et al. A human Mad protein acting as a BMP-regulated transcriptional activator. Nature. 1996 Jun 13;381(6583):620–623. doi: 10.1038/381620a0. [DOI] [PubMed] [Google Scholar]
- 16.Urist MR. Bone: formation by autoinduction. Science. 1965 Nov 12;150(3698):893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 17.Wozney JM, Rosen V, Celeste AJ, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988 Dec 16;242(4885):1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 18.Plikus MV, Mayer JA, de la Cruz D, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008 Jan 17;451(7176):340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu A, Niswander LA. Bone morphogenetic protein signalling and vertebrate nervous system development. Nat Rev Neurosci. 2005 Dec;6(12):945–954. doi: 10.1038/nrn1805. [DOI] [PubMed] [Google Scholar]
- 20.Francis PH, Richardson MK, Brickell PM, Tickle C. Bone morphogenetic proteins and a signalling pathway that controls patterning in the developing chick limb. Development. 1994 Jan;120(1):209–218. doi: 10.1242/dev.120.1.209. [DOI] [PubMed] [Google Scholar]
- 21.Vukicevic S, Latin V, Chen P, Batorsky R, Reddi AH, Sampath TK. Localization of osteogenic protein-1 (bone morphogenetic protein-7) during human embryonic development: high affinity binding to basement membranes. Biochem Biophys Res Commun. 1994 Jan 28;198(2):693–700. doi: 10.1006/bbrc.1994.1100. [DOI] [PubMed] [Google Scholar]
- 22.Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995 Nov 15;9(22):2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- 23.Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 2003 Sep;21(9):1025–1032. doi: 10.1038/nbt864. [DOI] [PubMed] [Google Scholar]
- 24.Ozkaynak E, Schnegelsberg PN, Oppermann H. Murine osteogenic protein (OP-1): high levels of mRNA in kidney. Biochem Biophys Res Commun. 1991 Aug 30;179(1):116–123. doi: 10.1016/0006-291x(91)91342-a. [DOI] [PubMed] [Google Scholar]
- 25.Kingsley DM, Bland AE, Grubber JM, et al. The mouse short ear skeletal morphogenesis locus is associated with defects in a bone morphogenetic member of the TGF beta superfamily. Cell. 1992 Oct 30;71(3):399–410. doi: 10.1016/0092-8674(92)90510-j. [DOI] [PubMed] [Google Scholar]
- 26.Gong Y, Krakow D, Marcelino J, et al. Heterozygous mutations in the gene encoding noggin affect human joint morphogenesis. Nat Genet. 1999 Mar;21(3):302–304. doi: 10.1038/6821. [DOI] [PubMed] [Google Scholar]
- 27.Lehmann K, Seemann P, Stricker S, et al. Mutations in bone morphogenetic protein receptor 1B cause brachydactyly type A2. Proc Natl Acad Sci U S A. 2003 Oct 14;100(21):12277–12282. doi: 10.1073/pnas.2133476100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shore EM, Xu M, Feldman GJ, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006 May;38(5):525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 29.Israel DI, Nove J, Kerns KM, et al. Heterodimeric bone morphogenetic proteins show enhanced activity in vitro and in vivo. Growth Factors. 1996;13(3-4):291–300. doi: 10.3109/08977199609003229. [DOI] [PubMed] [Google Scholar]
- 30.Kotzsch A, Nickel J, Seher A, et al. Structure analysis of bone morphogenetic protein-2 type I receptor complexes reveals a mechanism of receptor inactivation in juvenile polyposis syndrome. J Biol Chem. 2008 Feb 29;283(9):5876–5887. doi: 10.1074/jbc.M706029200. [DOI] [PubMed] [Google Scholar]
- 31.Harth S, Kotzsch A, Hu J, Sebald W, Mueller TD. A selection fit mechanism in BMP receptor IA as a possible source for BMP ligand-receptor promiscuity. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0013049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alarcon C, Zaromytidou AI, Xi Q, et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell. 2009 Nov 13;139(4):757–769. doi: 10.1016/j.cell.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onichtchouk D, Chen YG, Dosch R, et al. Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature. 1999 Sep 30;401(6752):480–485. doi: 10.1038/46794. [DOI] [PubMed] [Google Scholar]
- 34.Takase M, Imamura T, Sampath TK, et al. Induction of Smad6 mRNA by bone morphogenetic proteins. Biochem Biophys Res Commun. 1998 Mar 6;244(1):26–29. doi: 10.1006/bbrc.1998.8200. [DOI] [PubMed] [Google Scholar]
- 35.Hata A, Lagna G, Massague J, Hemmati-Brivanlou A. Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 1998 Jan 15;12(2):186–197. doi: 10.1101/gad.12.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casellas R, Brivanlou AH. Xenopus Smad7 inhibits both the activin and BMP pathways and acts as a neural inducer. Dev Biol. 1998 Jun 1;198(1):1–12. doi: 10.1006/dbio.1998.8893. [DOI] [PubMed] [Google Scholar]
- 37.Zhu H, Kavsak P, Abdollah S, Wrana JL, Thomsen GH. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature. 1999 Aug 12;400(6745):687–693. doi: 10.1038/23293. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Chang C, Gehling DJ, Hemmati-Brivanlou A, Derynck R. Regulation of Smad degradation and activity by Smurf2, an E3 ubiquitin ligase. Proc Natl Acad Sci U S A. 2001 Jan 30;98(3):974–979. doi: 10.1073/pnas.98.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katsuno Y, Hanyu A, Kanda H, et al. Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene. 2008 Oct 23;27(49):6322–6333. doi: 10.1038/onc.2008.232. [DOI] [PubMed] [Google Scholar]
- 40.Langenfeld E, Hong CC, Lanke G, Langenfeld J. Bone morphogenetic protein type I receptor antagonists decrease growth and induce cell death of lung cancer cell lines. PLoS One. 2013;8(4):e61256. doi: 10.1371/journal.pone.0061256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hardwick JC, Van Den Brink GR, Bleuming SA, et al. Bone morphogenetic protein 2 is expressed by, and acts upon, mature epithelial cells in the colon. Gastroenterology. 2004 Jan;126(1):111–121. doi: 10.1053/j.gastro.2003.10.067. [DOI] [PubMed] [Google Scholar]
- 42.Piccirillo SG, Reynolds BA, Zanetti N, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006 Dec 7;444(7120):761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 43.Martinez-Laperche C, Gomez-Garcia AM, Lassaletta A, et al. Detection of occult cerebrospinal fluid involvement during maintenance therapy identifies a group of children with acute lymphoblastic leukemia at high risk for relapse. Am J Hematol. 2013 May;88(5):359–364. doi: 10.1002/ajh.23407. [DOI] [PubMed] [Google Scholar]
- 44.Yu PB, Hong CC, Sachidanandan C, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008 Jan;4(1):33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996 Aug 23;86(4):599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 46.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol. 2005 Jun;6(6):600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 47.Robson NC, Phillips DJ, McAlpine T, et al. Activin-A: a novel dendritic cell-derived cytokine that potently attenuates CD40 ligand-specific cytokine and chemokine production. Blood. 2008 Mar 1;111(5):2733–2743. doi: 10.1182/blood-2007-03-080994. [DOI] [PubMed] [Google Scholar]
- 48.Song GA, Kim HJ, Woo KM, et al. Molecular consequences of the ACVR1(R206H) mutation of fibrodysplasia ossificans progressiva. J Biol Chem. 2010 Jul 16;285(29):22542–22553. doi: 10.1074/jbc.M109.094557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi W, Chang C, Nie S, Xie S, Wan M, Cao X. Endofin acts as a Smad anchor for receptor activation in BMP signaling. J Cell Sci. 2007 Apr 1;120(Pt 7):1216–1224. doi: 10.1242/jcs.03400. [DOI] [PubMed] [Google Scholar]
- 50.Itoh F, Divecha N, Brocks L, et al. The FYVE domain in Smad anchor for receptor activation (SARA) is sufficient for localization of SARA in early endosomes and regulates TGF-beta/Smad signalling. Genes Cells. 2002 Mar;7(3):321–331. doi: 10.1046/j.1365-2443.2002.00519.x. [DOI] [PubMed] [Google Scholar]
- 51.Miura S, Takeshita T, Asao H, et al. Hgs (Hrs), a FYVE domain protein, is involved in Smad signaling through cooperation with SARA. Mol Cell Biol. 2000 Dec;20(24):9346–9355. doi: 10.1128/mcb.20.24.9346-9355.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.