Abstract
The cornea is highly sensitive to oxidative stress, a process that can lead to lipid peroxidation. Ultraviolet light B (UVB) and nitrogen mustard (mechlorethamine) are corneal toxicants known to induce oxidative stress. Using a rabbit air-lifted corneal organ culture model, the oxidative stress responses to these toxicants in the corneal epithelium was characterized. Treatment of the cornea with UVB (0.5 J/cm2) or nitrogen mustard (100 nmol) resulted in the generation of 4-hydroxynonenal (4-HNE), a reactive lipid peroxidation end product. This was associated with increased expression of the antioxidant, heme oxygenase-1 (HO-1). In human corneal epithelial cells in culture, addition of 4-HNE or 9-nitrooleic acid, a reactive nitrolipid formed during nitrosative stress, caused a time-dependent induction of HO-1 mRNA and protein; maximal responses were evident after 10 hr with 30 μM 4-HNE or 6 hr with 10 μM 9-nitrooleic acid. 4-HNE and 9-nitrooleic acid were also found to activate Erk1/2, JNK and p38 MAP kinases, as well as phosphoinositide-3-kinase (PI3)/Akt. Inhibition of p38 blocked 4-HNE- and 9-nitrooleic acid-induced HO-1 expression. Inhibition of Erk1/2, and to a lesser extent, JNK and PI3K/Akt, suppressed only 4-HNE-induced HO-1, while inhibition of JNK and PI3K/Akt, but not Erk1/2, partly reduced 9-nitrooleic acid-induced HO-1. These data indicate that the actions of 4-HNE and 9-nitrooleic acid on corneal epithelial cells are distinct. The sensitivity of corneal epithelial cells to oxidative stress may be an important mechanism mediating tissue injury induced by UVB or nitrogen mustard.
Keywords: cornea, nitrogen mustard, UVB, 4-hydroxynonenal, 9-nitrooleic acid, lipid peroxidation, nitrosative stress
Introduction
As the outermost layer of the eye, the cornea is highly sensitive to injury induced by environmental insults. These result in alterations in the cornea’s structural integrity, which can impair vision (Lu et al., 2001; Cejka et al., 2010). The mechanisms by which environmental insults damage the cornea are not well understood. Our laboratories have been investigating the pathogenesis of corneal injury induced by ultraviolet B light (UVB) and vesicants, such as nitrogen mustard and sulfur mustard (Gordon et al., 2010; Black et al., 2011; Malaviya et al., 2012). These agents induce oxidative and nitrosative stress which can cause aberrant epithelial cell growth and differentiation, and cytotoxicity via necrosis and apoptosis (Heck et al., 2003; Laskin et al., 2010). In the cornea, this results in opacities, ulcerative diseases and keratitis (Cejkova et al., 2004; Kadar et al., 2013).
An important consequence of oxidative and nitrosative stress is the generation of excessive amounts of toxic reactive intermediates, including superoxide anion, hydrogen peroxide, hydroxyl radicals and nitric oxide (Hughes, 2008). Recent studies have suggested that modification of lipids by these reactive oxygen and nitrogen species is one of the major contributors to tissue damage (Roberts et al., 2010). For example, during oxidative stress, reactive oxygen species can initiate lipid peroxidation, a process that generates highly reactive electrophilic species such as α, β-unsaturated hydroxyalkenals (Niki, 2009). Additional reactive oxidants are also generated when superoxide anion chemically reacts with nitric oxide to produce intermediates such as peroxynitrite (Pacher et al., 2007). Subsequent reactions of nitric oxide-derived oxidants with double bonds of fatty acids, particularly the highly abundant oleic, linoleic, and arachidonic acids, lead to the generation of nitro-fatty acids, such as 9- and 10-nitrooleic acid (Devasagayam et al., 2004; Jain et al., 2008). Both α, β-unsaturated hydroxyalkenals and the nitrooleic acids are known to directly modify structural components in cells via Michael additions leading to toxicity (Esterbauer et al., 1991; Trostchansky and Rubbo, 2008). These lipid-derived intermediates have been identified as endogenous signaling molecules and their ability to cause inappropriate or altered cellular signal transduction can contribute to tissue injury (Niki, 2009).
The present studies were aimed at assessing the consequences of UVB- and vesicant-induced oxidative stress in the cornea. For these studies we used an air lifted rabbit cornea organ culture, a model system that has previously been used to characterize corneal wound healing (Gordon et al., 2010). We found that exposure of the cornea to UVB or nitrogen mustard resulted in the appearance of 4-hydroxynonenal (4-HNE), a relatively abundant α, β-unsaturated hydroxyalkenal. This was associated with increased expression of the antioxidant heme oxygenase-1 (HO-1), a stress protein important in protecting the cornea from injury induced by oxidative and nitrosative stress (Neil et al., 1995; Patil et al., 2008). Mechanisms by which 4-HNE and a related nitro-fatty acid derived mediator, 9-nitrooleic acid, induce HO-1 were analyzed using human corneal epithelial cells in culture. Our data demonstrate that reactive lipids formed during the pathogenesis of corneal injury are important in regulating the cytotoxic actions of UVB and nitrogen mustard. Results from these studies provide additional support for the key contribution of oxidative stress and lipid peroxidation to the actions of xenobiotics in the cornea.
Materials and Methods
Reagents
Alexa Fluor 488 conjugated goat anti-mouse IGg, ProLong® Gold antifade reagent, 4′-6-diamidino-2-phenylindole (DAPI), Dulbecco’s Modified Eagle’s Medium (DMEM), keratinocyte serum-free (KSF) medium, epidermal growth factor, bovine pituitary extract and fetal bovine serum (FBS) were from Invitrogen (Carlsbad, CA). Mouse monoclonal 4-HNE antibody was from R&D Systems (Minneapolis, MN), rabbit anti-HO-1 polyclonal antibody from Stressgen Biotechnology (Victoria, BC, Canada) and mouse monoclonal anti-HO-1 antibody from Abcam (Cambridge, MA). Rabbit polyclonal p38, phospho-p38, JNK, phospho-JNK, Erk1/2, phospho-Erk1/2, AKT and phospho-AKT antibodies were from Cell Signaling Technology (Beverly, MA). The Detergent Compatible (DC) protein assay kit was purchased from Bio-Rad Laboratories (Hercules, CA) and the Western Lightning enhanced chemiluminescence kit (ECL) from Perkin Elmer Life Sciences (Boston, MA). SYBR Green Master Mix and other PCR reagents were from Applied Biosystems (Foster City, CA). 4-HNE, PD 98059, SP600125 and Wortmannin were from Calbiochem (La Jolla, CA) and 9-nitrooleic acid from Cayman Chemical (Ann Arbor, MI). Nitrogen mustard (mechlorethamine), SB203580, protease inhibitor cocktail which contained 4-(2-aminoethyl) benzenesulfonyl fluoride, aprotinin, bestatin hydrochloride, N-(trans-epoxysuccinyl)-L-leucine 4-guanidinobutylamide, EDTA and leupeptin, and all other chemicals were from Sigma-Aldrich (St. Louis, MO).
Rabbit cornea organ culture and treatments
Eyes from young adult New Zealand white rabbits were purchased from Pel-Freez (Rogers, AR). Preparation of corneal organ cultures has been described previously (Gordon et al., 2010). Briefly, corneas with a 2 mm surrounding scleral rim were dissected from the rabbit eyes. The endothelial sides of the corneas were filled with 1.5% agar in DMEM, and after solidification, the cornea was inverted epithelial side up. DMEM supplemented with 1% non-essential amino acids, 0.01 mg/ml ciprofloxacin, and 0.1 mg/ml ascorbic acid was added up to the scleral rim. The corneas were incubated in a 5% CO2 incubator at 37 ºC and routinely wetted with DMEM every 6 hr. Nitrogen mustard (100 nmol in PBS) or PBS control was applied directly onto one set of cornea in volumes no greater than 20 μl. After 1 hr, the corneas were washed and refed with fresh medium. Other corneas were exposed to 0.5 J/cm2 UVB as previously described (Po, 2012). Control corneal organ cultures were placed under the light and covered with black vinyl to block UVB irradiation. At 3 hr and 6 hr post treatment, corneas exposed to nitrogen mustard or UVB were placed in optimal cutting temperature (OCT) medium and frozen until 10 μm sections were cut on a Microm HM505E cryostat. Cornea sections were then transferred to glass slides and stored at −80 ºC until analysis.
Immunofluorescence
Sections of corneas were fixed in cold methanol for 10 min, air dried, then rinsed with PBS and blocked with 5% goat serum at room temperature. After 60 min, sections were washed with PBS and incubated with a 1:100 dilution of HO-1 antibody or a 1:200 dilution of 4-HNE antibody in 1.5 % goat serum overnight at 4 ºC. Sections were then washed with PBS containing 0.2 % Tween-20 and incubated with a 1:1,000 dilution of goat anti-mouse Alexa-Fluor 488 secondary antibody for 1 hr at room temperature. The nuclei were counterstained with DAPI and the section treated with ProLong® Gold antifade reagent. Fluorescent images of corneas were captured with a ZEISS X-cite series 120Q fluorescence microscope (Thornwood, NY).
Cell cultures and treatments
The origin of the human corneal epithelial (HCE) cells has been described previously (Black et al., 2011). Cells were maintained in keratinocyte serum-free (KSF) medium supplemented with 5% fetal bovine serum, 0.1% gentamicin, 0.05 μg/ml epidermal growth factor and 0.05 mg/ml bovine pituitary extract. For RNA and protein analysis, monolayers of cells were grown to 90% confluency in six well plates and treated with either vehicle control, 4-HNE (30 μM) or 9-nitrooleic acid (10 μM). For mRNA analysis, cells were washed with PBS and total RNA was extracted using Tri-Reagent. For protein analysis, cells were washed with PBS and 300 μl of lysis buffer (10 mM Tris-base, pH 7.6, supplemented with 1% SDS and protease inhibitor cocktail) was added to the culture dish wells; after transfer to 1.5 ml Eppendorf microcentrifuge tubes, the cells were sonicated on ice. Lysates were centrifuged (300 x g, 10 min at 4° C) and the supernatants prepared for western blotting analysis. Total protein concentrations in supernatants were determined using the DC protein assay kit with bovine serum albumin as the standard. For kinase inhibition experiments, cells were pretreated for 3 hr with p38 MAP kinase inhibitor SB203580 (10 μM), JNK kinase inhibitor SP600125 (20 μM), Erk1/2 kinase inhibitor PD98059 (10 μM) or PI3/Akt kinase inhibitor Wortmannin (0.1 μM). 4-HNE, 9-nitrooleic acid or vehicle control was then added to the medium for a 6 hr incubation. Cells were then lysed and analyzed for protein expression by western blotting.
Analysis of mRNA expression
Total RNA was converted to cDNA using M-MLV reverse transcriptase as previously described (Black et al., 2011). For PCR analysis, a standard curve was generated from serial dilutions of cDNA mixtures of the samples. Real-time PCR was conducted on an ABI Prism 7900 Sequence Detection System using 96-well optical reaction plates. SYBR-Green was used as the fluorescent detection signal and the standard curve method was used for relative quantitative analysis. Primer sequences were generated using Primer Express software (Applied Biosystems) and the oligonucleotides were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). The house keeping gene, β-actin, was used to normalize the values. Forward and reverse (5′-3′) primer sequences were: HO-1, GCT CAA AAA GAT TGC CCA GA and GCG GTA GAG CTG CTT GAA CT; β-actin, AAA GAC CTG TAC GCC AAC AC and GTC ATA CTC CTG CTT GCT GAT.
Western blotting
Western blotting was performed as previously described (Black et al., 2008). Briefly, cell lysates were electrophoresed on 10% SDS-polyacrylamide gels, transferred to nitrocellulose membranes, and blocked in Tris buffer supplemented with 5% milk for 60 min at room temperature. The blots were then incubated overnight at 4°C with primary antibodies, washed with tTBS (Tris-buffered saline supplement with 0.1% Tween 20), and incubated for 60 min at room temperature with horseradish peroxidase-conjugated secondary antibodies. Proteins were visualized by ECL chemiluminescence.
Analysis of 4-HNE metabolism
HCE cells (2 × 106 cells/ml) were suspended in Eppendorf tubes in 1 ml serum-free KSF medium and incubated with 100 μM 4-HNE at 37 °C. 4-HNE and metabolites were extracted from the samples after 0–180 min by the addition of an equal volume of acetonitrile/acetic acid (96:4, v/v) and rapid mixing. After centrifugation (1000 x g, 10 min at 4° C), acetonitrile/acetic acid-containing supernatants were analyzed by HPLC as described by Hartley et al (1995) with some modifications, using a Jasco HPLC system (Jasco Corporation, Tokyo, Japan). 4-HNE and its metabolites were separated on a Phenomenex 5 μ C18 column (250 × 2.00 mm) using a mobile phase consisting of 70% 50 mM potassium phosphate buffer (pH 2.7) and 30% acetonitrile (v/v). Samples were analyzed isocratically at a flow rate of 0.25 ml/min, monitoring the HPLC effluent at 224 nm.
Statistical analysis
Data were evaluated using the two-way ANOVA. P < 0.05 was considered statistically significant. All experiments were repeated two times.
Results
Effects of UVB or nitrogen mustard on the formation of 4-HNE-adducts and HO-1 expression in cultured rabbit corneas
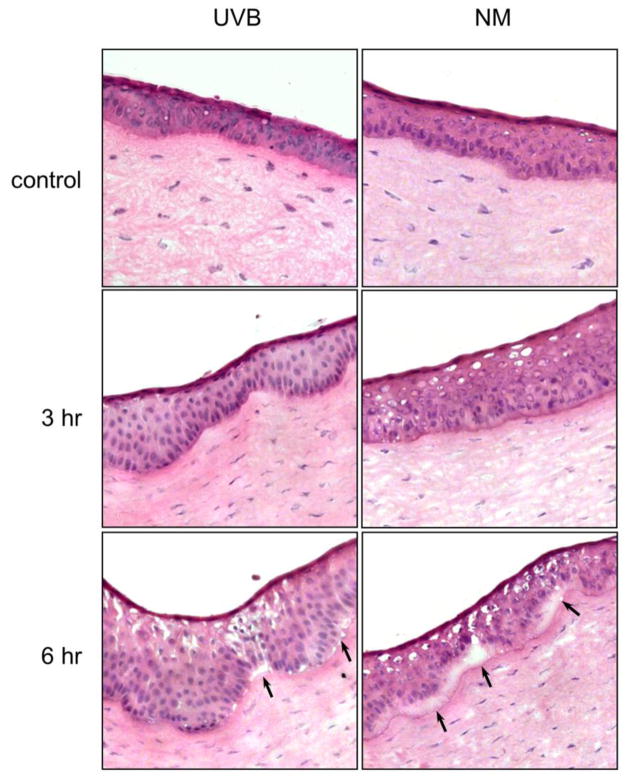
Consistent with previous studies (Maumenee and Scholz, 1948; Koliopoulos and Margaritis, 1979), we found that UVB or nitrogen mustard caused significant damage to the epithelial layer of the cornea. Figure 1 (upper panel) shows the 5–7 layer epithelium of unexposed rabbit cornea. Treatment with UVB or nitrogen mustard resulted in a thickening of the epithelial layer with a downward hyperplasia within 3 hr (Fig. 1, center panels). At 6 hr post exposure, areas of separation appeared between the epithelial and stromal layers of the corneas (Fig. 1, lower panels).
Figure 1. Morphology of rabbit corneas treated with UVB or nitrogen mustard.
Cornea organ cultures were exposed to control, UVB (0.5 J/cm2) or nitrogen mustard (NM, 100 nmol). Black arrows indicate areas of separation of epithelium from the stroma following UVB or NM treatment. After 3 hr and 6 hr, histological sections were prepared and stained with hematoxylin and eosin. Original magnification x 400
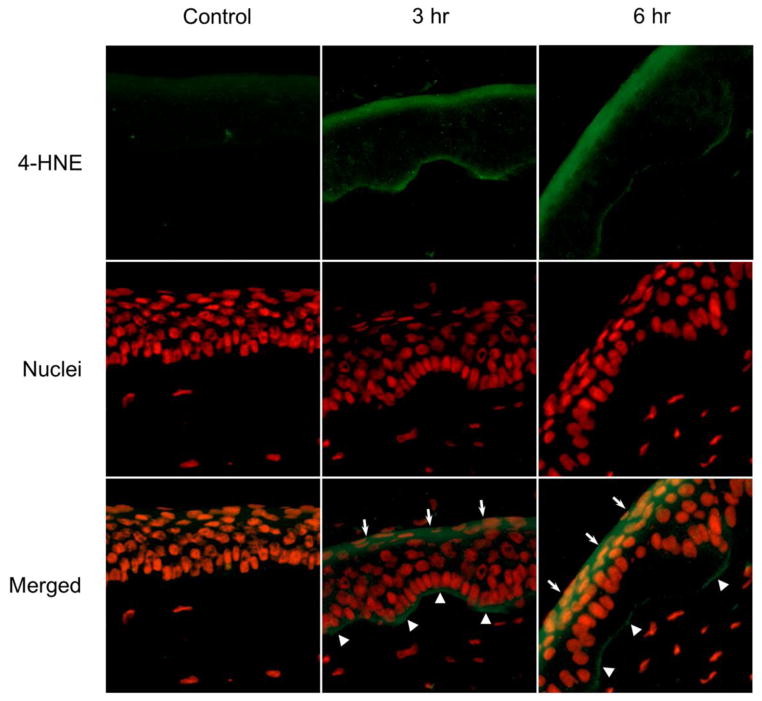
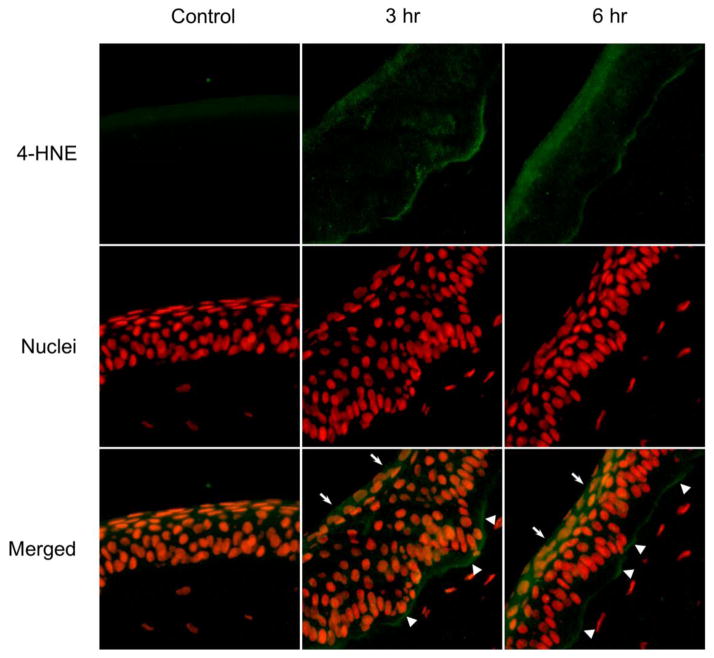
As a highly reactive end product of lipid peroxidation, 4-HNE forms stable protein adducts with histidine, lysine, and cysteine side chains which can be used as biomarkers for oxidative tissue damage (Gutteridge, 1995). Using an antibody that detects 4-HNE-histidine adducts, we found that corneas treated with UVB (Fig. 2) or nitrogen mustard (Fig. 3) for 3 hr or 6 hr readily generated 4-HNE in a time related manner. 4-HNE-adducts were detected at the apical surface of the corneal epithelium and at the basal epithelial surface at the basement membrane (Figs. 2 and 3, middle and right panels). Greater amounts of 4-HNE adducts were detected in intermediate areas of the epithelium in UVB or nitrogen mustard treated corneas with increasing periods of time. In contrast, minimal 4-HNE adducts were found in control corneas (Figs. 2 and 3, left panels).
Figure 2. Effects of UVB on 4-HNE formation in rabbit corneas.
Cornea organ cultures were exposed to control or UVB (0.5 J/cm2). After 3 hr and 6 hr, histological sections were prepared and the central portions of the corneas were analyzed for 4-HNE using mouse monoclonal 4-HNE primary antibody and Alexa-Fluor 488 labeled secondary antibody. Nuclei were visualized using DAPI staining. White arrows and arrowheads indicate areas of 4HNE adduct formation on the apical epithelial surface and basal epithelial surface, respectively. Original magnification x 400
Figure 3. Effects of nitrogen mustard on 4-HNE formation in rabbit corneas.
Cornea organ cultures were treated with control or 100 nmol nitrogen mustard. After 3 hr and 6 hr, histological sections were prepared and the central portions of the corneas were analyzed for 4-HNE using mouse monoclonal primary 4-HNE antibody and Alexa-Fluor 488-labeled secondary antibody. Nuclei were visualized using DAPI staining. White arrows and arrowheads indicate areas of 4-HNE adduct formation on the apical epithelial surface and basal epithelial surface, respectively. Original magnification x 400
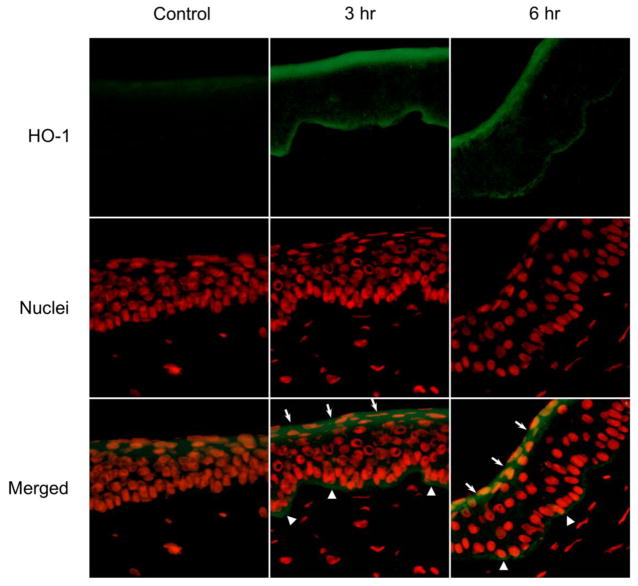
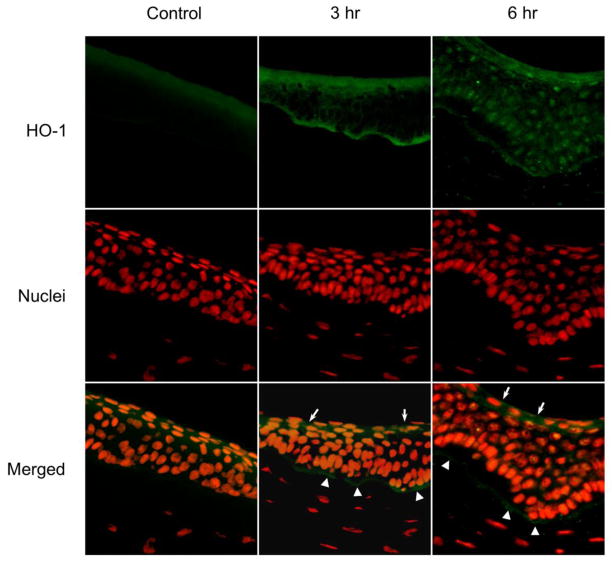
HO-1 is an inducible enzyme synthesized in tissues in response to oxidative stress (Gozzelino et al., 2010). Control corneas expressed low levels of HO-1 on their apical and basal epithelial surfaces (Figs. 4 and 5, left panels). A marked upregulation in HO-1 expression was observed 3 hr and 6 hr after UVB or nitrogen mustard treatment (Figs. 4 and 5, middle and right panels). HO-1 was expressed at the apical epithelial surface and at the basal surface above the basement membrane. HO-1 was also observed in the central cornea 6 hr post UVB or nitrogen mustard treatment. Of note was that upregulation of HO-1 was found to be associated with its appearance in the nuclei of the corneal epithelial cells.
Figure 4. Effects of UVB on HO-1 expression in rabbit corneas.
Cornea organ cultures were exposed to control or UVB (0.5 J/cm2). After 3 hr and 6 hr, histological sections were prepared and the central portions of corneas analyzed for HO-1 expression using mouse monoclonal primary HO-1 antibody and Alexa-Flour 488-labeled secondary antibody. Nuclei were visualized using DAPI staining. White arrows and arrowheads indicate areas of HO-1 formation on the apical epithelial surface and basal epithelial surface, respectively. Original magnification x 400
Figure 5. Effects of nitrogen mustard on HO-1 expression in rabbit corneas.
Cornea organ cultures were treated with control or 100 nmol nitrogen mustard. After 3 hr and 6 hr, histological sections were prepared and the central portions of corneas analyzed for HO-1 expression using mouse monoclonal HO-1 antibody and Alexa-Flour 488-labeled secondary antibody. Nuclei were visualized using DAPI staining. White arrows and arrowheads indicate areas of HO-1 formation on the apical epithelial surface and basal epithelial surface, respectively. Original magnification x 400
Effects of 4-HNE on cultured corneal epithelial cells
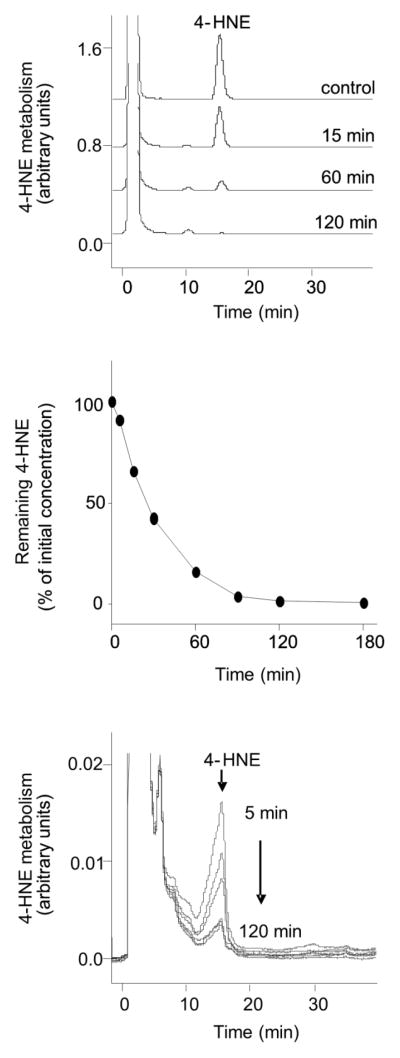
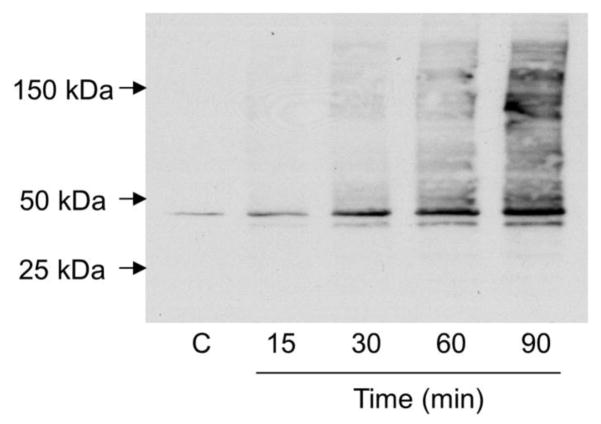
We next used cultured human corneal epithelial cells to analyze the effects of 4-HNE on HO-1 expression. 4-HNE was readily taken up by the corneal epithelial cells, as reflected by its rapid disappearance from the medium; its half-life in the medium was approximately 25 min (Fig. 6, upper and middle panels). 4-HNE was detected inside the cells with levels declining rapidly after 5 min (Fig. 6, bottom panel). Potential metabolites of 4-HNE were also observed in the cells, as measured by the appearance of a broad shoulder on the 4-HNE HPLC peak. As observed with 4-HNE, these metabolites degraded over time. Figure 7 shows that 4-HNE treatment also caused a time-dependent appearance of protein adducts in the corneal epithelial cells. A prominent band with a molecular weight of 45 kDa appeared in the cells 15 min after 4-HNE treatment (Fig. 7). With increasing time (30–90 min), a 43 kDa molecular weight band appeared, as well as a number of relatively lower abundance higher molecular weight bands, ranging in molecular weight from 50 kDa to greater than 200 kDa.
Figure 6. 4-HNE metabolism in human corneal epithelial cells.
Cell suspensions (2 × 106/ml) were incubated with 100 μM 4-HNE. At the indicated time points, reactions were stopped by the addition of an equal volume of acetonitrile/acetic acid (96:4, v/v). Top panel: HPLC analysis of 4-HNE metabolism. After stopping the reaction, cells were pelleted and clear supernatants analyzed by HPLC. Middle panel: Kinetics of 4-HNE metabolism. Bottom panel: Cells were extracted and analyzed by HPLC after treatment with 100 μM 4-HNE for 5, 15, 30, 60 and 120 min.
Figure 7. Formation of 4-HNE-protein adducts in human corneal epithelial cells.
Cells were treated with vehicle control (C) or 4-HNE (30 μM) for 0, 15, 30, 60 and 90 min. Cell lysates were then prepared, and protein analyzed by western blotting using mouse monoclonal antibody to 4-HNE. Each lane contained 10 μg of corneal epithelial protein.
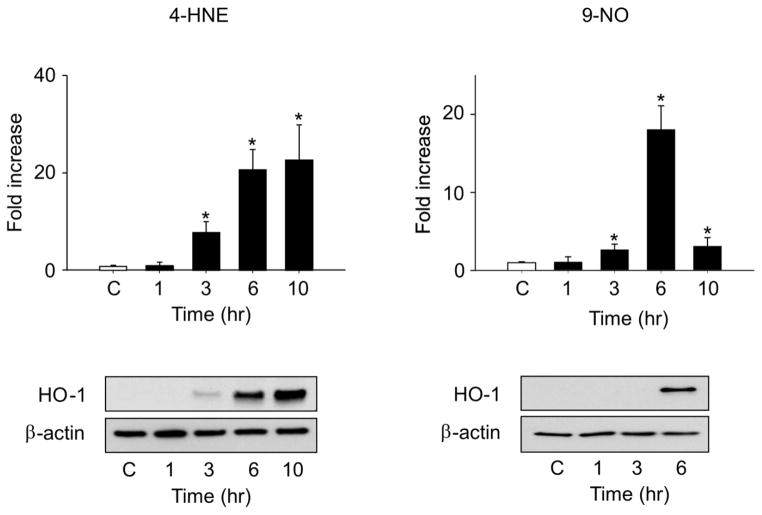
Treatment of corneal epithelial cells with 4-HNE resulted in a time-dependent induction of HO-1 mRNA and protein (Fig. 8, left panels). A several-fold increase was evident 6–10 hr after treatment with 30 μM 4-HNE. We next compared the actions of 4-HNE with the nitrosative stress lipid mediator, 9-nitrooleic acid, which is known to be generated following nitration of oleic acid (Jain et al., 2008). As observed with 4-HNE, 9-nitrooleic acid induced expression of mRNA and protein for HO-1 in human corneal epithelial cells (Fig. 8, right panels). However, the effects of 9-nitrooleic acid on HO-1 mRNA were transient; maximal levels were attained after 6 hr with 10 μM 9-nitrooleic acid.
Figure 8. Effects of electrophilic lipid peroxidation products on HO-1 expression in human corneal epithelial cells.
Cells were treated with vehicle control (C), 30 μM 4-HNE (left panels) or 10 μM 9-nitrooleic acid (9-NO, right panels). mRNA or protein was extracted from the cells at the indicated times and analyzed by real time PCR (upper panels) and western blotting (lower panels), respectively. β-actin was used as a protein loading control. Data are presented as the mean ± SE (n = 3). *Significantly different from control (P < 0.05).
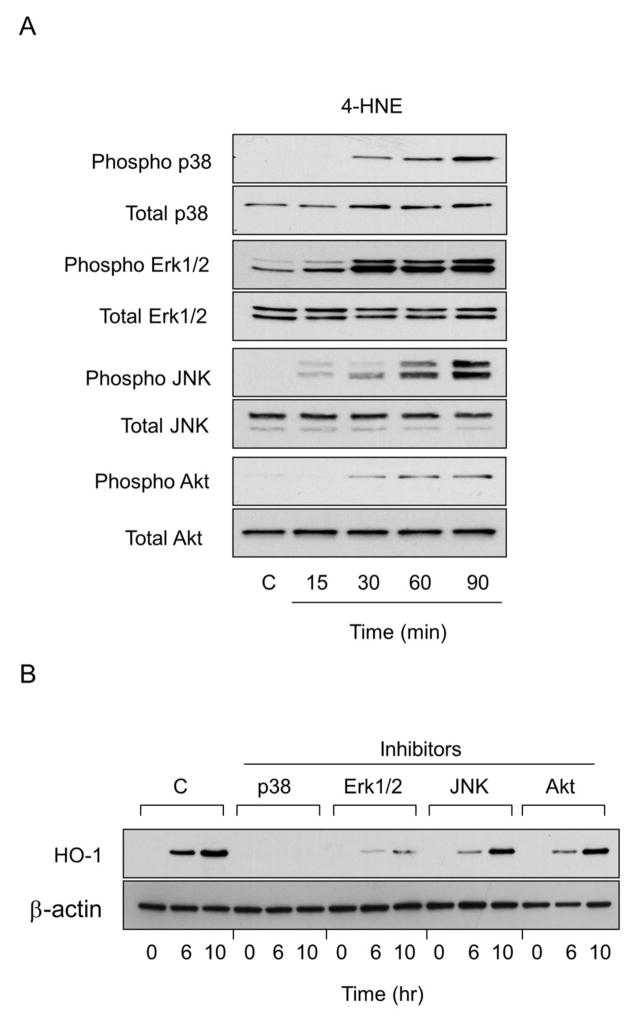
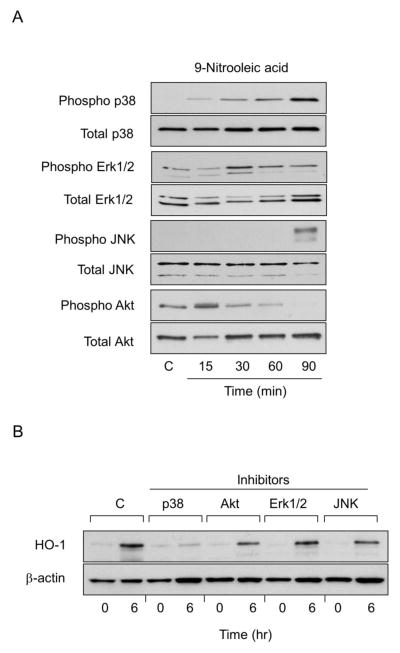
In further studies we analyzed the role of MAP kinase and PI3/Akt signaling in 4-HNE- and 9-nitrooleic acid-induced HO-1 expression. Both fatty acid derived lipids were found to activate corneal ERK1/2, p38 and JNK MAP kinases, as measured by increases in the phosphorylated forms of these enzymes (Figs. 9 and 10). The effects of 4-HNE were time-dependent, reaching a maximum after 60–90 min with each enzyme. The stimulatory effects of 9-nitrooleic acid on p38 kinase were generally similar to 4-HNE. In contrast, JNK was only activated after 90 min, while ERK1/2 expression increased after 30 min and declined thereafter. Both 4-HNE and 9-nitrooleic acid also activated PI3/Akt signaling; these increases were time-dependent and maximal after 90 min with 4-HNE and 15 min with 9-nitrooleic acid. To determine if 4-HNE and 9-nitrooleic acid-induced upregulation of HO-1 expression in corneal epithelial cells was dependent on MAP kinases and PI3/Akt, we used specific enzyme inhibitors. Treatment of the cells with the p38 MAP kinase inhibitor, SB203580, or the ERK-1/2 kinase inhibitor, PD98059, markedly suppressed 4-HNE-induced expression of HO-1. A JNK inhibitor, SP600125, and a PI3K/Akt inhibitor, wortmannin, only partially inhibited HO-1 expression. In contrast, the p38 kinase inhibitor only blocked 9-nitrooleic acid-induced expression of HO-1; partial inhibition of HO-1 expression was evident with the JNK and PI3K/Akt inhibitors. Minimal effects were observed on 9-nitrooleic acid-induced expression of HO-1 with the ERK1/2 inhibitor (Fig. 10).
Figure 9. Role of MAP kinases and PI3/Akt kinase signaling in 4-HNE-induced HO-1 expression in human corneal epithelial cells.
Panel A: Effects of 4-HNE on expression of MAP kinases and PI3K/Akt kinase. Cells were treated with control (C) or 30 μM 4HNE for 15, 30, 60 and 90 min. Cell lysates were prepared and analyzed for total and phosphorylated p38, JNK, Erk1/2, or PI3/Akt by western blotting. Panel B: Effects of MAP kinase and PI3K/Akt inhibitors on 4-HNE-induced HO-1 expression. Cells were pre-incubated with inhibitors to p38 (SB203580,10 μM), JNK (SP600125, 20 μM), Erk1/2 (PD98059, 10 μM) or PI3K (wortmannin, 0.1 μM) for 3 hr and then treated with 30 μM 4-HNE for additional 6 hr or 10 hr. Total cell lysates were prepared and analyzed for HO-1 protein expression by western blotting.
Figure 10. Role of MAP kinases and PI3/Akt kinase signaling in 9-nitrooleic acid-induced HO-1 expression in human corneal epithelial cells.
Panel A: Effects of 9-nitrooleic acid on expression of MAP kinases and PI3K/Akt kinases. Cells were treated with control (C) or 10 μM 9-nitrooleic acid for 15, 30, 60 and 90 min. Cell lysates were prepared and analyzed for total and phosphorylated p38, JNK, Erk1/2, or PI3/Akt using western blotting. Panel B: Effects of MAP kinase and PI3/Akt inhibitors on 9-nitrooleic acid-induced HO-1 expression. Human epithelial cells were pre-incubated with inhibitors to p38 (SB203580,10 μM), JNK (SP600125, 20 μM), Erk1/2 (PD98059, 10 μM) or PI3/Akt kinase (wortmannin, 0.1 μM) for 3 hr and then treated with 10 μM 9-nitrooleic acid for additional 6 hr. Total cell lysates were then prepared and analyzed for HO-1 protein expression by western blotting.
Discussion
Earlier studies have shown that ocular exposures to UVB or vesicants results in damage to the corneal epithelium, which involves both acute and chronic keratopathies (Bergmanson, 1990; Ghabili et al., 2010). Depending on dose, inflammation and edema are observed as well as increasing corneal thickness, and sloughing of epithelial cells. Detachment of epithelial cells is thought to be due to degradation of the basement membrane and basal cell hemidesmosome transmembraneous components, which are critical for the attachment of the epithelium to the basement membrane (Petrali et al., 1997; McNutt et al., 2012; Kadar et al., 2013). Our laboratories have been characterizing the effects of ocular toxicants including UVB and vesicants on rabbit cornea using an air-lifted organ culture model (Gordon et al., 2010). In this model, the corneal epithelium retains its typical differentiated phenotype with an intact basement membrane separating the epithelial and stromal cell layers (Foreman et al., 1996; Gordon et al., 2010). Using this organ culture system, the present studies demonstrate that both UVB and nitrogen mustard, a sulfur mustard analog, cause damage to the cornea. This is characterized by a thickening of the epithelium, largely due to epithelial hyperplasia, and localized separation of the epithelium from the basement membrane zone. These data are consistent with the effects of UVB or sulfur mustard on rabbit cornea in vivo (Petrali et al., 1997; Cejka et al., 2010) and demonstrate the utility of the cornea organ culture model for mechanistic studies on the action of xenobiotics.
Using UVB or nitrogen mustard which generates similar corneal damage in the rabbit organ cultures, we found evidence of 4-HNE production by corneal epithelium by immunofluorescence. This was confirmed by our identification of 4-HNE-modified proteins in the corneal epithelial cell cultures. These proteins were most abundant at the apical and basal surfaces of the corneal epithelia. The apical expression was not simply autofluorescence since the intensity of the signal was far greater than that of the unexposed control corneas. With time, 4-HNE modified proteins were also evident in other suprabasal layers of the corneal epithelium, although at reduced amounts relative to the apical and basal expression patterns. These data indicate that UVB- or nitrogen mustard-induced corneal toxicity is associated with oxidative stress and lipid peroxidation. This is consistent with earlier studies showing that these toxicants can generate superoxide anion and hydrogen peroxide (Korkmaz et al., 2006; Black et al., 2011). A similar induction of 4-HNE production has previously been described in mouse keratinocytes treated with UVB (Zhaorigetu et al., 2003). Our observation that the 4-HNE was localized in cells near the corneal surface is consistent with the fact that these cells are the first to encounter the toxicants, and likely receive the highest doses resulting in significant oxidative stress. The reason for increased expression in the basal epithelial cells following treatment with UVB or nitrogen mustard is not known, although it is known that both agents have a profound effect here. In the skin, basal keratinocytes are a major target for both nitrogen mustard and sulfur mustard, possibly due to their ability to stimulate the production of proteases that degrade the basement membrane (Papirmeister, 1991). In this regard, sulfur mustard has been reported to induce a variety of basement membrane degrading enzymes in the skin including elastase, tryptase, calpain, matrix metalloproteinase-2 and matrix metalloproteinase-9 (Shakarjian et al., 2010). Matrix metalloproteinases are also known to be induced in the cornea in different diseases and these or related proteases may also contribute to UVB- and nitrogen mustard-induced injury in basal corneal epithelial cells (Sakimoto and Sawa, 2012).
HO-1 is an important adaptive response protein induced in cells subject to oxidative stress. It functions to protect cells by inhibiting apoptosis and inflammation (Gozzelino et al., 2010). We found that areas of 4-HNE production in the cornea after UVB or nitrogen mustard treatment were associated with increased expression of HO-1. These data are consistent with earlier studies demonstrating that corneal injury in mice results in increased HO-1 expression and activity, which contributes to reduced inflammation and increased wound healing (Patil et al., 2008), and that UVB is an effective inducer of HO-1 in human corneal epithelial cells in culture (Black et al., 2011). Both nitrogen mustard and sulfur mustard have also been reported to upregulate HO-1 in vivo (Malaviya et al., 2010; Malaviya et al., 2012). The ability of UVB or nitrogen mustard to modulate corneal epithelial cell expression of antioxidants such as HO-1 is likely to be important in regulating inflammation and protecting the cornea against oxidative stress. Of interest were our findings that following UVB or nitrogen mustard treatment, HO-1 translocated into the nucleus of the corneal epithelial cells. Earlier studies demonstrated that nuclear HO-1 can serve in the regulation of gene transcription and may be involved in cell growth regulation and tumor progression (Elguero et al., 2012; Gandini et al., 2012). In the cornea, nuclear HO-1 may serve to regulate expression of genes important in controlling the resolution of inflammation and wound healing. Further studies are needed to determine the precise functions of nuclear HO-1 in regulating corneal responses to UVB or nitrogen mustard.
4-HNE is known to modulate HO-1 expression in many cell types (Zhang and Forman, 2009; Ishikado et al., 2010). To investigate its mechanism of action in the cornea, a human corneal epithelial cell line known to be sensitive to UVB was used (Black et al., 2011). In these cells, UVB regulates HO-1 expression via MAP kinase signaling. We found that human corneal epithelial cells readily take up 4-HNE, and that the 4-HNE was effective in inducing HO-1 mRNA and protein. These data indicate that one level of HO-1 regulation is mRNA expression. In addition to reactive oxygen species, many tissues generate reactive nitrogen species during oxidative stress. Earlier studies have shown that exposure of skin to UVB, or lung to nitrogen mustard, leads to oxidative, as well as nitrosative stress (Malaviya et al., 2012; Terra et al., 2012). In human corneal epithelial cells and in rabbit cornea, UVB has been shown to stimulate expression of nitric oxide synthases (Cejkova et al., 2005; Black et al., 2011). One consequence of nitrosative stress is nitration of unsaturated fatty acids. Generated in nitric oxide-dependent oxidative reactions, several of these lipid products are electrophilic fatty acid nitroalkenes and include nitrolinoleic acid and nitrooleic acid derivatives. Like 4-HNE, these nitro-fatty acids react via Michael additions and form adducts with many cellular components; their reaction with signaling proteins can regulate their function and control gene expression (Freeman et al., 2008). Of interest was our finding that 9-nitrooleic acid, a relatively abundant nitro-fatty acid, was comparable to 4-HNE in inducing HO-1 mRNA and protein expression in corneal epithelial cells, although the kinetics of the responses to the two reactive lipids were distinct. Thus, induction of HO-1 mRNA and protein by 4-HNE was more rapid and persistent when compared to 9-nitrooleic acid. Differences in the activities of these lipid mediators in inducing HO-1 in corneal epithelial cells may be due to their relative reactivity with signaling molecules important for controlling expression of stress response genes (Schwobel et al., 2010) (see further below), and/or differences in their uptake and metabolism. Importantly, our data suggest that nitrosative stress and subsequent generation of reactive nitrolipids plays a role in regulating an adaptive response in corneal epithelial cells. Further studies are needed to determine if nitro-fatty acids such as 9-nitrooleic acid can be generated in the cornea after treatment with stressors such as UVB and nitrogen mustard.
Reactive aldehydes including 4-HNE activate MAP kinase and PI3K/Akt signaling cascades, key processes regulating HO-1 expression (Schmitz et al., 2002; Salinas et al., 2003; Iles et al., 2005). Both 4-HNE and 9-nitrooleic acid were found to activate these pathways in corneal epithelial cells; 4-HNE was more effective than 9-nitrooleic acid, a finding consistent with its increased ability to induce HO-1. Studies with kinase inhibitors demonstrated that p38 MAP kinase was the most active in regulating 4-HNE- and 9-nitrooleic acid-induced HO-1 expression. Erk1/2, and to a lesser extent, JNK and PI3K/Akt, were also involved in 4-HNE-induced HO-1 expression, while JNK and PI3K/Akt, but not Erk1/2, were partly effective in regulating the activity of 9-nitrooleic acid. These data indicate 4-HNE and 9-nitrooleic acid modulate HO-1 expression by distinct mechanisms. It is well recognized that additional signaling pathways control HO-1 expression including Nrf2/Keap-1 and NF-κB (Nguyen et al., 2003; Wijayanti et al., 2004). In this regard, 4-HNE has been shown to activate Nrf2 and NF-κB (Ruef et al., 2001; Kansanen et al., 2011). It remains to be determined if 4-HNE and 9-nitrooleic acid also control the activity of Nrf2 and NF-κB in corneal epithelial cells, and consequent HO-1 expression. In earlier studies using human corneal epithelial cells, we found that UVB-induced expression of HO-1 mRNA was associated with activation of Erk1/2 JNK and p38 MAP kinases (Black et al., 2011). In contrast to 4-HNE and 9-nitrooleic acid, the effects of UVB were mediated by JNK and not p38 MAP kinase. These data indicate that mechanisms regulating HO-1 by 4-HNE and 9-nitrooleic acid are also distinct from UVB.
Of interest were our findings that 4-HNE was rapidly metabolized by the corneal epithelial cells. This is evidenced by 1) its rapid disappearance from the medium of treated cells, 2) its rapid intracellular metabolism, 3) the appearance of both intra- and extracellular metabolites, and 4) the formation of 4-HNE-protein adducts. Metabolism of 4-HNE and related reactive aldehydes to inactive products is an important mechanism for protecting cells from oxidative stress. Several pathways mediate 4-HNE metabolism including oxidation to 4-hydroxy-2-nonenoic acid by aldehyde dehydrogenase, reduction to non-2-ene-1,4-diol by alcohol dehydrogenase, and conjugation by glutathione-S-transferases (Canuto et al., 1994; Spycher et al., 1996; Tjalkens et al., 1998). The cornea contains both aldehyde dehydrogenases and alcohol dehydrogenases; of interest is the fact that aldehyde dehydrogenase is a highly abundant corneal crystallin, a structural protein important in maintaining clarity of the cornea (Cooper et al., 1993; Chen et al., 2013). The metabolism of 4-HNE by isoforms of this enzyme including ALDH3A1 in humans, and most other mammals, and ALDH1A1 in rabbits, has been well characterized (Chen et al., 2013). Metabolism of 4-HNE by glutathione-S-transferases in human and bovine corneas has also been reported (Srivastava et al., 1994; Singhal et al., 1995). At the present time, the precise identity of the 4-HNE-derived metabolites, as well as the enzymes mediating 4-HNE metabolism in the corneal epithelial cells used in our studies are not known.
As described above, 4-HNE forms protein adducts via Michael additions across its carbon-carbon double bond (Esterbauer et al., 1991). In cultured corneal epithelial cells, a number of proteins with molecular weights ranging from 43 to 300 kDa were modified by 4-NHE. That adducts can be formed in corneal cells is consistent with our findings of 4-HNE-protein adducts in the epithelium of rabbit cornea in organ culture following treatment with UVB or nitrogen mustard. The identity of the modified proteins in either intact cornea or the corneal epithelial cell cultures is not known. 4-HNE can directly or indirectly modify signaling proteins controlling expression of adaptive response genes and these may be important in regulating expression of HO-1 (Leonarduzzi et al., 2004; Siow et al., 2007; Rudolph and Freeman, 2009; Huang et al., 2012). Further studies are needed to identify proteins modified by 4-HNE in the cornea and to determine their role in mediating injury induced by UVB or nitrogen mustard.
In summary, our data demonstrate that in the rabbit cornea organ culture model, injury following exposure to UVB or nitrogen mustard results in oxidative stress, as exemplified by the generation of 4-HNE modified proteins and expression of HO-1. Using corneal epithelial cell cultures, MAP kinase and PI3K/Akt signaling were found to be important mechanisms by which 4-HNE modulates expression of HO-1. Similar results were observed in corneal cell cultures with 9-nitrooleic acid, a lipid-derived product resulting from nitrosative stress, although 4-HNE and 9-nitrooleic acid appear to act by distinct mechanisms. Earlier studies have shown that oxidative and nitrosative stress induced by UVB or mustards contribute to toxicity in target tissues. Both stress processes can arise by a variety of mechanisms in injured tissues including increases in enzymes that generate reactive oxygen and reactive nitrogen species, disruption of mitochondrial function, and changes in intracellular antioxidants such as sulfhydryl-containing amino acids, small molecular weight peptides such as glutathione, and various antioxidant enzymes (Niki, 2009). A more precise definition of the mechanisms by which UVB or nitrogen mustard induce oxidative and nitrosative stress in the cornea is needed as is the role of antioxidants in protecting against tissue injury.
Highlights.
UVB or nitrogen mustard causes rabbit corneal epithelial injury.
4-hydroxynonenal (4-HNE) was formed and heme oxygenase-1 (HO-1) was increased.
4-HNE induced HO-1 mRNA and protein expression in human corneal epithelial cells.
The induction of HO-1 by 4-HNE was through MAP kinase activation.
Acknowledgments
Supported by NIH grants U54AR055073, EY09056 and ES005022.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergmanson JP. Corneal damage in photokeratitis--why is it so painful? Optom Vis Sci. 1990;67:407–413. doi: 10.1097/00006324-199006000-00004. [DOI] [PubMed] [Google Scholar]
- Black AT, Gordon MK, Heck DE, Gallo MA, Laskin DL, Laskin JD. UVB light regulates expression of antioxidants and inflammatory mediators in human corneal epithelial cells. Biochemical pharmacology. 2011;81:873–880. doi: 10.1016/j.bcp.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black AT, Gray JP, Shakarjian MP, Laskin DL, Heck DE, Laskin JD. Distinct effects of ultraviolet B light on antioxidant expression in undifferentiated and differentiated mouse keratinocytes. Carcinogenesis. 2008;29:219–225. doi: 10.1093/carcin/bgm242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canuto RA, Ferro M, Muzio G, Bassi AM, Leonarduzzi G, Maggiora M, Adamo D, Poli G, Lindahl R. Role of aldehyde metabolizing enzymes in mediating effects of aldehyde products of lipid peroxidation in liver cells. Carcinogenesis. 1994;15:1359–1364. doi: 10.1093/carcin/15.7.1359. [DOI] [PubMed] [Google Scholar]
- Cejka C, Platenik J, Sirc J, Ardan T, Michalek J, Brunova B, Cejkova J. Changes of corneal optical properties after UVB irradiation investigated spectrophotometrically. Physiol Res. 2010;59:591–597. doi: 10.33549/physiolres.931867. [DOI] [PubMed] [Google Scholar]
- Cejkova J, Ardan T, Cejka C, Kovaceva J, Zidek Z. Irradiation of the rabbit cornea with UVB rays stimulates the expression of nitric oxide synthases-generated nitric oxide and the formation of cytotoxic nitrogen-related oxidants. Histol Histopathol. 2005;20:467–473. doi: 10.14670/HH-20.467. [DOI] [PubMed] [Google Scholar]
- Cejkova J, Stipek S, Crkovska J, Ardan T, Platenik J, Cejka C, Midelfart A. UV Rays, the prooxidant/antioxidant imbalance in the cornea and oxidative eye damage. Physiol Res. 2004;53:1–10. [PubMed] [Google Scholar]
- Chen Y, Thompson DC, Koppaka V, Jester JV, Vasiliou V. Ocular aldehyde dehydrogenases: Protection against ultraviolet damage and maintenance of transparency for vision. Prog Retin Eye Res. 2013;33:28–39. doi: 10.1016/j.preteyeres.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DL, Isola NR, Stevenson K, Baptist EW. Members of the ALDH gene family are lens and corneal crystallins. Adv Exp Med Biol. 1993;328:169–179. doi: 10.1007/978-1-4615-2904-0_19. [DOI] [PubMed] [Google Scholar]
- Devasagayam TP, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. Free radicals and antioxidants in human health: current status and future prospects. J Assoc Physicians India. 2004;52:794–804. [PubMed] [Google Scholar]
- Elguero B, Gueron G, Giudice J, Toscani MA, De Luca P, Zalazar F, Coluccio-Leskow F, Meiss R, Navone N, De Siervi A, Vazquez E. Unveiling the association of STAT3 and HO-1 in prostate cancer: role beyond heme degradation. Neoplasia. 2012;14:1043–1056. doi: 10.1593/neo.121358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Foreman DM, Pancholi S, Jarvis-Evans J, McLeod D, Boulton ME. A simple organ culture model for assessing the effects of growth factors on corneal re-epithelialization. Exp Eye Res. 1996;62:555–564. doi: 10.1006/exer.1996.0065. [DOI] [PubMed] [Google Scholar]
- Freeman BA, Baker PR, Schopfer FJ, Woodcock SR, Napolitano A, d’Ischia M. Nitro-fatty acid formation and signaling. J Biol Chem. 2008;283:15515–15519. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandini NA, Fermento ME, Salomon DG, Blasco J, Patel V, Gutkind JS, Molinolo AA, Facchinetti MM, Curino AC. Nuclear localization of heme oxygenase-1 is associated with tumor progression of head and neck squamous cell carcinomas. Exp Mol Pathol. 2012;93:237–245. doi: 10.1016/j.yexmp.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Ghabili K, Agutter PS, Ghanei M, Ansarin K, Shoja MM. Mustard gas toxicity: the acute and chronic pathological effects. J Appl Toxicol. 2010;30:627–643. doi: 10.1002/jat.1581. [DOI] [PubMed] [Google Scholar]
- Gordon MK, Desantis A, Deshmukh M, Lacey CJ, Hahn RA, Beloni J, Anumolu SS, Schlager JJ, Gallo MA, Gerecke DR, Heindel ND, Svoboda KK, Babin MC, Sinko PJ. Doxycycline hydrogels as a potential therapy for ocular vesicant injury. J Ocul Pharmacol Ther. 2010;26:407–419. doi: 10.1089/jop.2010.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819–1828. [PubMed] [Google Scholar]
- Hartley DP, Ruth JA, Petersen DR. The Hepatocellular Metabolism of 4-Hydroxynonenal by Alcohol-Dehydrogenase, Aldehyde Dehydrogenase, and Glutathione-S-Transferase. Arch Biochem Biophys. 1995;316:197–205. doi: 10.1006/abbi.1995.1028. [DOI] [PubMed] [Google Scholar]
- Heck DE, Vetrano AM, Mariano TM, Laskin JD. UVB light stimulates production of reactive oxygen species: unexpected role for catalase. J Biol Chem. 2003;278:22432–22436. doi: 10.1074/jbc.C300048200. [DOI] [PubMed] [Google Scholar]
- Huang Y, Li W, Kong AN. Anti-oxidative stress regulator NF-E2-related factor 2 mediates the adaptive induction of antioxidant and detoxifying enzymes by lipid peroxidation metabolite 4-hydroxynonenal. Cell Biosci. 2012;2:40. doi: 10.1186/2045-3701-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MN. Chemistry of nitric oxide and related species. Methods Enzymol. 2008;436:3–19. doi: 10.1016/S0076-6879(08)36001-7. [DOI] [PubMed] [Google Scholar]
- Iles KE, Dickinson DA, Wigley AF, Welty NE, Blank V, Forman HJ. HNE increases HO-1 through activation of the ERK pathway in pulmonary epithelial cells. Free Radic Biol Med. 2005;39:355–364. doi: 10.1016/j.freeradbiomed.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikado A, Nishio Y, Morino K, Ugi S, Kondo H, Makino T, Kashiwagi A, Maegawa H. Low concentration of 4-hydroxy hexenal increases heme oxygenase-1 expression through activation of Nrf2 and antioxidative activity in vascular endothelial cells. Biochem Biophys Res Commun. 2010;402:99–104. doi: 10.1016/j.bbrc.2010.09.124. [DOI] [PubMed] [Google Scholar]
- Jain K, Siddam A, Marathi A, Roy U, Falck JR, Balazy M. The mechanism of oleic acid nitration by *NO(2) Free Radic Biol Med. 2008;45:269–283. doi: 10.1016/j.freeradbiomed.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Kadar T, Cohen M, Cohen L, Fishbine E, Sahar R, Brandeis R, Dachir S, Amir A. Endothelial cell damage following sulfur mustard exposure in rabbits and its association with the delayed-onset ocular lesions. Cutan Ocul Toxicol. 2013;32:115–123. doi: 10.3109/15569527.2012.717571. [DOI] [PubMed] [Google Scholar]
- Kansanen E, Bonacci G, Schopfer FJ, Kuosmanen SM, Tong KI, Leinonen H, Woodcock SR, Yamamoto M, Carlberg C, Yla-Herttuala S, Freeman BA, Levonen AL. Electrophilic nitro-fatty acids activate NRF2 by a KEAP1 cysteine 151-independent mechanism. J Biol Chem. 2011;286:14019–14027. doi: 10.1074/jbc.M110.190710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliopoulos JX, Margaritis LH. Response of the cornea to far ultraviolet light: an ultrastructural study. Ann Ophthalmol. 1979;11:765–769. [PubMed] [Google Scholar]
- Korkmaz A, Yaren H, Topal T, Oter S. Molecular targets against mustard toxicity: implication of cell surface receptors, peroxynitrite production, and PARP activation. Arch Toxicol. 2006;80:662–670. doi: 10.1007/s00204-006-0089-x. [DOI] [PubMed] [Google Scholar]
- Laskin JD, Black AT, Jan YH, Sinko PJ, Heindel ND, Sunil V, Heck DE, Laskin DL. Oxidants and antioxidants in sulfur mustard-induced injury. Ann N Y Acad Sci. 2010;1203:92–100. doi: 10.1111/j.1749-6632.2010.05605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonarduzzi G, Robbesyn F, Poli G. Signaling kinases modulated by 4-hydroxynonenal. Free Radic Biol Med. 2004;37:1694–1702. doi: 10.1016/j.freeradbiomed.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med (Maywood) 2001;226:653–664. doi: 10.1177/153537020222600711. [DOI] [PubMed] [Google Scholar]
- Malaviya R, Sunil VR, Cervelli J, Anderson DR, Holmes WW, Conti ML, Gordon RE, Laskin JD, Laskin DL. Inflammatory effects of inhaled sulfur mustard in rat lung. Toxicol Appl Pharmacol. 2010;248:89–99. doi: 10.1016/j.taap.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya R, Venosa A, Hall L, Gow AJ, Sinko PJ, Laskin JD, Laskin DL. Attenuation of acute nitrogen mustard-induced lung injury, inflammation and fibrogenesis by a nitric oxide synthase inhibitor. Toxicol Appl Pharmacol. 2012;265:279–291. doi: 10.1016/j.taap.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maumenee AE, Scholz RO. The histopathology of the ocular lesions produced by the sulfur and nitrogen mustard. Bull Johns Hopkins Hosp. 1948;82:121–147. [PubMed] [Google Scholar]
- McNutt P, Hamilton T, Nelson M, Adkins A, Swartz A, Lawrence R, Milhorn D. Pathogenesis of acute and delayed corneal lesions after ocular exposure to sulfur mustard vapor. Cornea. 2012;31:280–290. doi: 10.1097/ICO.0B013E31823D02CD. [DOI] [PubMed] [Google Scholar]
- Neil TK, Stoltz RA, Jiang S, Laniado-Schwartzman M, Dunn MW, Levere RD, Kappas A, Abraham NG. Modulation of corneal heme oxygenase expression by oxidative stress agents. J Ocul Pharmacol Ther. 1995;11:455–468. doi: 10.1089/jop.1995.11.455. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med. 2009;47:469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- Papirmeister B. Medical defense against mustard gas : toxic mechanisms and pharmacological implications. CRC Press; Boca Raton: 1991. [Google Scholar]
- Patil K, Bellner L, Cullaro G, Gotlinger KH, Dunn MW, Schwartzman ML. Heme oxygenase-1 induction attenuates corneal inflammation and accelerates wound healing after epithelial injury. Invest Ophthalmol Vis Sci. 2008;49:3379–3386. doi: 10.1167/iovs.07-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrali JP, Miskena FJ, Hamilton TA, Finger AV, Janny SJ. Sulfur mustard toxicity of the rabbit eye: An ultrastructural study. J Toxicol-Cutan Ocul. 1997;16:227–237. [Google Scholar]
- Po I. A comparison of corneal wound healing after UVB and nitrogen mustard exposure, Toxicology. Rutgers University; 2012. pp. 1–95. [Google Scholar]
- Roberts RA, Smith RA, Safe S, Szabo C, Tjalkens RB, Robertson FM. Toxicological and pathophysiological roles of reactive oxygen and nitrogen species. Toxicology. 2010;276:85–94. doi: 10.1016/j.tox.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph TK, Freeman BA. Transduction of redox signaling by electrophile-protein reactions. Sci Signal. 2009;2:re7. doi: 10.1126/scisignal.290re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruef J, Moser M, Bode C, Kubler W, Runge MS. 4-hydroxynonenal induces apoptosis, NF-kappaB-activation and formation of 8-isoprostane in vascular smooth muscle cells. Basic Res Cardiol. 2001;96:143–150. doi: 10.1007/s003950170064. [DOI] [PubMed] [Google Scholar]
- Sakimoto T, Sawa M. Metalloproteinases in corneal diseases: degradation and processing. Cornea. 2012;31(Suppl 1):S50–56. doi: 10.1097/ICO.0b013e318269ccd0. [DOI] [PubMed] [Google Scholar]
- Salinas M, Diaz R, Abraham NG, Ruiz de Galarreta CM, Cuadrado A. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J Biol Chem. 2003;278:13898–13904. doi: 10.1074/jbc.M209164200. [DOI] [PubMed] [Google Scholar]
- Schmitz ML, Bacher S, Droge W. Molecular analysis of mitogen-activated protein kinase signaling pathways induced by reactive oxygen intermediates. Methods Enzymol. 2002;352:53–61. doi: 10.1016/s0076-6879(02)52006-1. [DOI] [PubMed] [Google Scholar]
- Schwobel JA, Wondrousch D, Koleva YK, Madden JC, Cronin MT, Schuurmann G. Prediction of michael-type acceptor reactivity toward glutathione. Chem Res Toxicol. 2010;23:1576–1585. doi: 10.1021/tx100172x. [DOI] [PubMed] [Google Scholar]
- Shakarjian MP, Heck DE, Gray JP, Sinko PJ, Gordon MK, Casillas RP, Heindel ND, Gerecke DR, Laskin DL, Laskin JD. Mechanisms mediating the vesicant actions of sulfur mustard after cutaneous exposure. Toxicol Sci. 2010;114:5–19. doi: 10.1093/toxsci/kfp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal SS, Awasthi S, Srivastava SK, Zimniak P, Ansari NH, Awasthi YC. Novel human ocular glutathione S-transferases with high activity toward 4-hydroxynonenal. Invest Ophthalmol Vis Sci. 1995;36:142–150. [PubMed] [Google Scholar]
- Siow RC, Ishii T, Mann GE. Modulation of antioxidant gene expression by 4-hydroxynonenal: atheroprotective role of the Nrf2/ARE transcription pathway. Redox Rep. 2007;12:11–15. doi: 10.1179/135100007X162167. [DOI] [PubMed] [Google Scholar]
- Spycher S, Tabataba-Vakili S, O’Donnell VB, Palomba L, Azzi A. 4-hydroxy-2,3-trans-nonenal induces transcription and expression of aldose reductase. Biochem Biophys Res Commun. 1996;226:512–516. doi: 10.1006/bbrc.1996.1386. [DOI] [PubMed] [Google Scholar]
- Srivastava SK, Singhal SS, Bajpai KK, Chaubey M, Ansari NH, Awasthi YC. A group of novel glutathione S-transferase isozymes showing high activity towards 4-hydroxy-2-nonenal are present in bovine ocular tissues. Exp Eye Res. 1994;59:151–159. doi: 10.1006/exer.1994.1093. [DOI] [PubMed] [Google Scholar]
- Terra VA, Souza-Neto FP, Pereira RC, Xavier Da Silva TN, Ramalho LN, Luiz RC, Cecchini R, Cecchini AL. Nitric oxide is responsible for oxidative skin injury and modulation of cell proliferation after 24 hours of UVB exposures. Free Radic Res. 2012;46:872–882. doi: 10.3109/10715762.2012.686036. [DOI] [PubMed] [Google Scholar]
- Tjalkens RB, Luckey SW, Kroll DJ, Petersen DR. Alpha,beta-unsaturated aldehydes increase glutathione S-transferase mRNA and protein: correlation with activation of the antioxidant response element. Arch Biochem Biophys. 1998;359:42–50. doi: 10.1006/abbi.1998.0895. [DOI] [PubMed] [Google Scholar]
- Trostchansky A, Rubbo H. Nitrated fatty acids: mechanisms of formation, chemical characterization, and biological properties. Free Radic Biol Med. 2008;44:1887–1896. doi: 10.1016/j.freeradbiomed.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Wijayanti N, Huber S, Samoylenko A, Kietzmann T, Immenschuh S. Role of NF-kappaB and p38 MAP kinase signaling pathways in the lipopolysaccharide-dependent activation of heme oxygenase-1 gene expression. Antioxid Redox Signal. 2004;6:802–810. doi: 10.1089/ars.2004.6.802. [DOI] [PubMed] [Google Scholar]
- Zhang H, Forman HJ. Signaling pathways involved in phase II gene induction by alpha, beta-unsaturated aldehydes. Toxicol Ind Health. 2009;25:269–278. doi: 10.1177/0748233709102209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhaorigetu S, Yanaka N, Sasaki M, Watanabe H, Kato N. Inhibitory effects of silk protein, sericin on UVB-induced acute damage and tumor promotion by reducing oxidative stress in the skin of hairless mouse. J Photochem Photobiol B. 2003;71:11–17. doi: 10.1016/s1011-1344(03)00092-7. [DOI] [PubMed] [Google Scholar]