Abstract
Many children diagnosed with attention deficit hyperactivity disorder are treated with methylphenidate (MPH), despite limited information on later vulnerability to drug abuse. A previous study in adolescent monkeys treated with MPH for 1 year did not reveal differences in acquisition to cocaine reinforcement compared to controls (Gill et al., 2012). The present study extended this characterization to include MPH self-administration. Adolescent male rhesus monkeys previously treated with a sustained-release formulation of MPH (beginning at ∼ 30 months old) and control monkeys (n=8/group) were used. All had prior experience of self-administering cocaine under a fixed-ratio (FR) 30 schedule of reinforcement. Responding was maintained by food (1.0-g banana-flavored pellets) and MPH (saline, 0.001 – 0.1 mg/kg per injection) was substituted for food for at least 5 consecutive sessions. MPH functioned as a reinforcer in all monkeys; there were no differences between groups in MPH self-administration. These findings extend earlier research with cocaine reinforcement showing that MPH treatment in adolescent monkeys does not increase future reinforcing effects of stimulant drugs.
Keywords: methylphenidate, cocaine, abuse liability, drug abuse, rhesus monkey
Introduction
The number of adolescents that meet DSM criteria for various mental disorders has increased over the past 20 years (cf. Merikangas et al., 2010). Consistent with these trends, recent United States estimates for the diagnosis of attention deficit hyperactivity disorder (ADHD) in adolescents is approximately 10% (NIH, 2000). The diagnosis is frequently accompanied by pharmacotherapies, despite the limited amount of data on the long-term consequences of treating adolescents with psychoactive drugs. Stimulants, such as methylphenidate (MPH) are the first choice of medication for the management of ADHD (Antshel et al., 2011; Swanson et al., 2011). In fact, MPH is the most widely prescribed stimulant for children, adolescents, and adults with ADHD, owing to its positive effects on academic performance, inattention, impulsivity, and hyperactivity (Solanto, 2002; Crawford et al., 2011; Swanson et al., 2011). With a 3% annual increase in the percentage of children diagnosed with ADHD from 1997 to 2006 (Pastor and Reuben, 2008), there has been increasing concern about the effects of MPH on the developing brain and long-term behavioral consequences.
Two recent studies in adolescent rhesus monkeys reported no significant effects on several growth measures following 12-18 months of chronic MPH treatment (Gill et al., 2012; Soto et al., 2012). In addition, neither study reported any significant effect on several measures of dopamine (DA) receptor function as measured with positron emission tomography. When cocaine reinforcement was studied, prior MPH treatment did not increase vulnerability (Gill et al., 2012). In addition to its therapeutic effects, MPH has abuse liability, especially in young adults (Boyd et al., 2006), and can function as a reinforcer under several conditions in animal models (e.g., Johanson and Schuster, 1975; Bergman et al., 1989; Lile et al., 2003; Alvers et al., 2012; see Kollins et al., 2001 for review). However, the effect of adolescent MPH treatment on subsequent MPH self-administration has not been examined, and was the goal of the present study. The hypothesis was that prior exposure to MPH would not differentially affect MPH self-administration when compared to control monkeys.
Methods
Subjects
Sixteen young adult male rhesus monkeys (approximately 6 yr old at the start of this study) with a history of MPH (Metadate CD) or vehicle treatment (n=8/group) and cocaine self-administration (average total cocaine intake 32.4 mg/kg; Gill et al., 2012) were used for the present study. During the previous study, MPH-treated monkeys received clinically relevant doses of 10-15 ng/ml (Swanson and Volkow, 2003), as determined from monthly blood samples, in pudding, while control monkeys received unadulterated pudding, daily-, for a total of 12 months, beginning at a mean age of 39 months (see Gill et al., 2012). Monkeys were fed enough Purina Monkey Chow daily to accommodate growth and maintain food-reinforced responding. Water was freely available in the home cage. All monkeys had previously been surgically implanted with a chronic, indwelling intravenous catheter and associated vascular access port (VAP). Animal housing and handling and all experimental procedures were performed in accordance with the 2003 National Research Council Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research and were approved by the Animal Care and Use Committee of Wake Forest University. Environmental enrichment was provided as outlined in the Animal Care and Use Committee of Wake Forest University Non-Human Primate Environmental Enrichment Plan.
Apparatus
Experimental sessions were conducted in ventilated, sound-attenuating chambers (1.5 × 0.74 × 0.76 m; Med Associates, St. Albans, VT). Prior to placement in the chamber, the monkey's back was cleaned with 95% ethanol and chlorhexidine, and the VAP was connected to an infusion pump (Cole-Parmer Instrument Co., Niles, IL) located outside the chamber via a 20-gauge Huber Point Needle (Access Technologies) and tubing. The pump was operated for approximately 3 s to fill the port and catheter with the concentration of solution available for that experimental session. On one side of the experimental chamber were two photo-optic switches (Model 117-1007; Stewart Ergonomics, Inc., Furlong, PA) with a horizontal row of three stimulus lights positioned 14 cm above each switch. A food receptacle was located between the switches and connected to a pellet dispenser (Med Associates) located on the top of the chamber for delivery of 1.0-g banana-flavored food pellets (Bio-Serv, Frenchtown, NJ). Monkeys responded on only one of the switches for food or drug and this was counterbalanced between monkeys within each group.
Procedure
All monkeys were trained to respond on either the right or left photo-optic switch under a fixed-ratio (FR) 30 schedule of food reinforcement. Illumination of a green stimulus light served as a discriminative stimulus for food or drug availability. The 30th response extinguished the green light and illuminated a white stimulus light above a centrally located food receptacle, followed by the delivery of a food pellet and a 10 s timeout. Sessions ended after 10 or 30 reinforcers (depending on the monkey) or after 60 min had elapsed, whichever occurred first. When saline or MPH (National Institute on Drug Abuse, Bethesda, MD, dissolved in sterile 0.9% saline up to a concentration of 100 mg/ml) was substituted for food, sessions ended after 30 injections or 60 min. First, saline was substituted for food until responding decreased by at least 80% of food-maintained response rates for 3 consecutive sessions. After a return to food-maintained responding, half log-unit doses of MPH (0.001 to 0.1 mg/kg per injection) were substituted for food for at least 5 consecutive sessions and until responding was deemed stable. MPH doses were tested in random order and each dose was separated by at least two days of food-maintained responding as described above.
Data Analysis
The primary dependent variables were response rates, reinforcement frequency and total MPH intake per session. For each animal, data were averaged across the last three days of availability of each dose. A two-way ANOVA (treatment group × MPH dose) was used and post-hoc Bonferroni analyses were also conducted. In all cases, differences were considered statistically significant at the 95% level of confidence (p<0.05). Data are presented as mean (± SEM) for MPH-treated and control monkeys (n=8/group).
Results
There were no differences in food-maintained responding between groups, nor were there differences in the number of sessions required for responding to extinguish (<80% food-maintained response rates when saline was substituted; Table 1). For both the MPH-treated and control monkeys, response rates varied significantly as a function of MPH dose (F5,84 = 3.75; p < 0.005) and was characterized as an inverted U-shaped function of dose in both groups (Figure 1A). Similarly, reinforcement frequency (Figure 1B) varied significantly as a function of MPH dose (F5,84 = 4.76; p < 0.001). MPH intake increased in a dose-dependent manner and was not different between groups (Figure 1C). For all analyses, there was no significant main effect of treatment group and no significant interaction.
Table 1.
Baseline performance of MPH-treated and Control Monkeys¶.
| MPH-Treated | Controls | |
|---|---|---|
| Food Response Rates§ | 0.93± 0.26 | 1.34± 0.31 |
| Saline Response Rates§ | 0.04± 0.03 | 0.02± 0.01 |
| Number of Sessions to Ext | 6.57± 0.53 | 8.38± 1.41 |
Each point is the mean (± SEM) of 8 monkeys.
Values are responses per second
Figure 1.
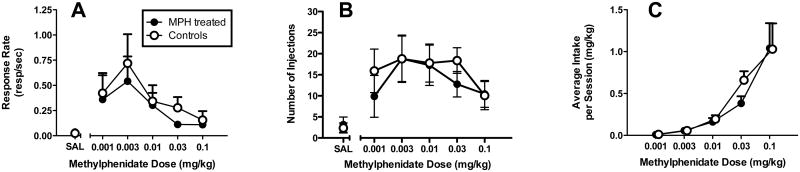
Methylphenidate self-administration in MPH-treated and control monkeys. Panel A: Response rates (responses per sec) as a function of MPH dose. Panel B: Number of injections as a function of MPH dose. Panel C: Total Methylphenidate intake (mg/kg per session) as a function of dose. Each point represents the mean ± S.E.M. (n=8).
Discussion
The present study examined the effects of chronic exposure to MPH, within the range administered to children, in adolescent monkeys, more than 1.5 years after terminating MPH treatment. MPH functioned as a reinforcer in all monkeys, with response rates characterized as an inverted U-shaped function of dose and intake increasing in a monotonic fashion. Importantly, there were no differences in response rates, number of injections or intake of MPH between the MPH-treated monkeys and controls. These results confirm earlier findings in these monkeys (Gill et al., 2012) that MPH treatment in adolescent monkeys does not enhance the reinforcing effects of stimulants.
This is the first animal study to examine the effects of adolescent MPH treatment on later MPH self-administration. It has been hypothesized that MPH has lower abuse liability in humans because of pharmacokinetics (Volkow et al., 1995), although it is not clear from animal studies that MPH and cocaine have different reinforcing effects. For example, Johanson and Schuster (1975) showed that monkeys with double-lumen catheters would choose equally between MPH and cocaine. Similarly, using rhesus monkeys, Lile et al. (2003) showed that the breakpoints for cocaine and MPH were not significantly different. The present findings are consistent with this finding and extend the research to include the examination of MPH history on subsequent self-administration. In an earlier study using these monkeys, it was reported that MPH treatment did not increase sensitivity to the reinforcing effects of cocaine (Gill et al., 2012). The present results showing no differences in MPH self-administration in these same monkeys are consistent with mediation of the reinforcing effects of the two stimulant drugs by similar mechanisms.
There are some limitations to the study. It is important to note that prior exposure of these monkeys to cocaine (Gill et al., 2012) may have decreased the likelihood of observing differences when MPH was made available. Future research examining the effects of adolescent MPH treatment on later MPH self-administration in animals without prior cocaine exposure is warranted. In addition, it remains possible that the early MPH history has effects on other behaviors, such as cognition. Clearly, much research is needed to understand the effects of chronic MPH treatment on the developing brain. Nonetheless, the present findings showing that adolescent MPH treatment does not enhance later stimulant reinforcement are encouraging in relation to the large number of youths being treated with MPH.
Acknowledgments
This research was supported by National Institute on Drug Abuse grants DA20648 and DA06634. The authors report no conflict of interest and would like to acknowledge the technical assistance of Tonya Calhoun and the helpful contributions to experimental design provided by Kathryn Gill, Allyson Bennett and Peter Pierre. We dedicate this to the memory of our colleague and friend William Woolverton.
Funding: This research was supported by National Institutes of Health grants R01 DA20648 and P50 DA006634
Footnotes
Conflicting Interests: None declared
References
- Alvers KM, Marusich JA, Gipson CD, Beckmann JS, Bardo MT. Environmental enrichment during development decreases intravenous self-administration of methylphenidate at low unit doses in rats. Behav Pharmacol. 2012;23:650–657. doi: 10.1097/FBP.0b013e3283584765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antshel KM, Hargrave TM, Simonescu M, Kaul P, Hendricks K, Faraone SV. Advances in understanding and treating ADHD. BMC Med. 2011;9:72. doi: 10.1186/1741-7015-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther. 1989;251:150–155. [PubMed] [Google Scholar]
- Boyd CJ, McCabe SE, Cranford JA, Young A. Adolescents' motivations to abuse prescription medications. Pediatrics. 2006;118:2472–2480. doi: 10.1542/peds.2006-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford CA, Baella SA, Farley CM, Herbert MS, Horn LR, Campbell RH, Zavala AR. Early methylphenidate exposure enhances cocaine self-administration but not cocaine induced conditioned place preference in young adult rats. Psychopharmacology. 2011;213:43–52. doi: 10.1007/s00213-010-2011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KE, Pierre PJ, Daunais J, Bennett AJ, Martelle S, Gage HD, Swanson JM, Nader MA, Porrino LJ. Chronic treatment with extended release methylphenidate does not alter dopamine systems or increase vulnerability for cocaine self-administration: a study in nonhuman primates. Neuropsychopharmacology. 2012;37:2555–2565. doi: 10.1038/npp.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Schuster CR. A choice procedure for drug reinforcers: cocaine and methylphenidate in the rhesus monkey. J Pharmacol Exp Ther. 1975;193:676–688. [PubMed] [Google Scholar]
- Kollins SH, MacDonald EK, Rush CR. Assessing the abuse potential of methylphenidate in nonhuman and human subjects: a review. Pharmacol Biochem Behav. 2001;68:611–627. doi: 10.1016/s0091-3057(01)00464-6. [DOI] [PubMed] [Google Scholar]
- Lile JA, Wang Z, Woolverton WL, France JE, Gregg TC, Davies HM, Nader MA. The reinforcing efficacy of psychostimulants in rhesus monkeys: the role of pharmacokinetics and pharmacodynamics. J Pharmacol Exp Ther. 2003;307:356–366. doi: 10.1124/jpet.103.049825. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication – adolescent supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. National Institutes of Health Consensus Development Conference Statement: diagnosis and treatment of attention-deficit/hyperactivity disorder (ADHD) J Am Acad Child Adolesc Psychiatry. 2000;39:182–193. doi: 10.1097/00004583-200002000-00018. [DOI] [PubMed] [Google Scholar]
- Pastor PN, Reuben CA. Diagnosed attention deficit hyperactivity disorder and learning disability: United States, 2004-2006. Vital Health Stat. 2008;10:1–14. [PubMed] [Google Scholar]
- Solanto MV. Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research. Behav Brain Res. 2002;130:65–71. doi: 10.1016/s0166-4328(01)00431-4. [DOI] [PubMed] [Google Scholar]
- Soto PL, Wilcox KM, Zhou Y, Ator NA, Riddle MA, Wong DF, Weed MR. Long-term exposure to oral methylphenidate or dl-amphetamine mixture in peri-adolescent rhesus monkeys: effects on physiology, behavior, and dopamine system development. Neuropsychopharmacology. 2012;37:2566–2579. doi: 10.1038/npp.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Serum and brain concentrations of methylphenidate: implications for use and abuse. Neurosci Biobehav Rev. 2003;27:615–621. doi: 10.1016/j.neubiorev.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Swanson J, Baler RD, Volkow ND. Understanding the effects of stimulant medications on cognition in individuals with attention-deficit hyperactivity disorder: a decade of progress. Neuropsychopharmacology. 2011;36:207–226. doi: 10.1038/npp.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley JS, Dewey S, Ashby C, Liebermann J, Hitzemann R, Wolf AP. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry. 1995;52:456–463. doi: 10.1001/archpsyc.1995.03950180042006. [DOI] [PubMed] [Google Scholar]


