Abstract
We propose mechanisms by which the transmembrane domain of vesicular stomatitis virus (VSV-TMD) promotes both initiation of fusion and formation of a fusion pore. Time courses of polyethyleneglycol (PEG)-mediated fusion of 25 nm small unilamellar vesicles composed of dioleoylphosphatidylcholine, dioleoylphosphatidylethanolamine (DOPE), bovine brain sphingomyelin, and cholesterol (35:30:15:20 molar ratio) were recorded at pH 7.4 at five different temperatures (from 17°C to 37°C) and compared with time courses obtained with the same vesicles containing the fusion-active TMD of the G protein of VSV. Multiple time courses were fitted globally to a one-intermediate ensemble kinetic model to estimate the rate constants for conversion of the aggregated state to an intermediate hemifused state (k1, stalk, or I1) that rapidly transits to an unstable intermediate (I2 state) that converts to a final fusion pore state with a combined rate k3. The probabilities of lipid mixing, contents mixing, and contents leakage in the three states were also obtained from this analysis. The activation thermodynamics for each step were consistent with previously published models of lipid rearrangements during intermediate and pore formation. The influences of VSV-TMD, hexadecane, and VSV-TMD + hexadecane on the kinetics, activation thermodynamics, and membrane structure support the hypothesis that these two agents do not catalyze fusion by a common mechanism, except possibly at the lowest temperatures examined. VSV-TMD primarily catalyzed initial intermediate formation, although it substantially increased the probability of contents mixing in the intermediate state. Our results support the hypothesis that the catalytic influence of VSV-TMD on the initial-intermediate- and pore-forming steps of PEG-mediated fusion derives from its ability to impose a positive intrinsic curvature and thereby stress small unilamellar vesicle outer leaflets as well as the periphery of intermediate microstructures.
Introduction
Biological membrane fusion is observed in many vital intra- and intercellular events, such as viral infection, neurotransmission, protein trafficking, and fertilization. Since membranes with mammalian membrane-like compositions do not generally fuse spontaneously, membrane fusion is mediated by protein machinery in living systems. The entry of enveloped viruses into cells requires the fusion of cellular and viral membranes triggered by conformational changes in viral glycoproteins. Based on their molecular architecture, viral fusion proteins fall into one of three classes. In class I fusion proteins (e.g., influenza hemagglutinin and paramyxovirus F protein) the post-fusion trimer displays a six-helix bundle with a fusion peptide at the N-terminus of the central helices and the transmembrane domains (TMDs) at the C-terminus of the antiparallel outer helices existing in the same common membrane postfusion (1,2). Class II fusion proteins are composed mainly of β sheets and their internal fusion peptides are embedded in the ectodomain (3). Class III is a comparatively newly identified group whose members include fusion proteins from rhabdoviruses, herpesviruses, vesicular stomatitis virus (VSV), and baculoviruses. Despite some diversity, all characterized viral fusion proteins convert from a fusion-competent state to a state that embeds the fusion peptide in the target membrane, whereas the TMD remains attached to the viral membrane. The free energy associated with a conformational change in the fusion protein is proposed to bring these two membranes into close proximity (4), a condition required for fusion (5).
Several reports have demonstrated the importance of the TMD region of viral fusion proteins in the fusion process. Replacement of the TMD of influenza hemagglutinin (6,7) and VSV G protein (8) by a glycerylphosphatidylinositol (GPI) anchor abolished fusion. Several studies showed that certain residues in TMD sequences of HIV (9–11) or VSV (12,13) are critical for fusion. All of these studies demonstrated the importance of viral fusion protein TMDs to fusion, but in general failed to address the detailed mechanisms by which these TMDs promote fusion. Although some reports pointed to a role of TMD in formation of hemifusion intermediates (6,7,14), others indicate a possible role later in the fusion process (12,13). Some have suggested that they might deform the lipids surrounding them, which would result in dimples and thus favor formation of a stalk (15,16), whereas others have suggested that they might occupy interstice space (a region of imperfect acyl-chain packing at the interface between lamellar and nonlamellar regions of fusing membranes (17) to stabilize fusion intermediates (13). Here, we present a detailed analysis of the kinetics of polyethyleneglycol (PEG)-triggered fusion of an ensemble of DOPC/DOPE/SM/CH (35:30:15:20) small unilamellar vesicles (SUVs) at pH 7.4 at five temperatures in the presence of VSV-TMD. The activation thermodynamics for fusion of these vesicles in the absence of VSV-TMD at pH 7.4 are reported in an accompanying paper (18) and are consistent with mechanistic models we proposed earlier for pH 5 (19) once the altered hydration of DOPE at pH 7.4 is taken into account.
VSV, a member of the Rhabdoviridae viruses, enters the cell via an endocytic pathway and subsequently fuses with a cellular membrane within the acidic environment of the endosome (20), where fusion is mediated by a single transmembrane viral glycoprotein (G) that is trimeric and forms the spikes that protrude from the viral surface (21). Earlier studies showed that mutation of two Gly to Ala (see sequence in Materials section) in the TMD of this G protein inhibited fusion in our model system (13) as it did in ex vivo studies (12). A crude analysis of the initial rates of lipid mixing (LM) and contents mixing (CM) tentatively suggested that VSV-TMD influenced mainly the final step of pore formation (CM) as opposed to the step of initial intermediate formation (LM) (13). However, both the initial and final steps of fusion involve both LM and CM (22,23), so such a simplified analysis cannot yield mechanistic detail. In this study, we measured LM, CM, and contents leakage (CL) at five different temperatures in the presence and absence of the VSV-TMD peptide, and fitted the data to our previously well-documented ensemble kinetic model of the fusion process (19,22). In this way, we determined the rate constants for the interconversion of states as well as the probability of observing CM, LM, or CL in each state, and revealed in detail the effects of VSV-TMD on each step of the fusion process. In addition, we report the activation thermodynamics for all steps and use this information to propose mechanisms by which the VSV-TMD peptide might catalyze each step. To aid in interpreting the effects of VSV-TMD on the activation thermodynamics, we also recorded the effects of VSV-TMD on the membrane bilayer properties, as well as the fusion kinetics in the presence of both VSV-TMD and 2 mol % hexadecane (a known interstice-occupying agent). Finally, we propose possible mechanisms by which VSV-TMD might catalyze fusion in our model system, and use our findings to test the previously proposed hypothesis that VSV-TMD might promote fusion by occupying interstice space (13).
Materials and Methods
Materials
The VSV-TMD peptide with sequence (K)KSSIASFFFIIGLIIGLFLVLR(R) used in this study was synthesized by Invitrogen. Chloroform stock solutions of 1,2-dioleoyl-3-phosphatidylcholine (DOPC), 1,2-dioleoyl-3-sn-phosphatidylethanolamine (DOPE), and bovine brain sphingomyelin (SM) were purchased from Avanti Polar Lipids (Birmingham, AL) and used without further purification. The concentrations of all of the stock phospholipids were determined by a phosphate assay (24). Cholesterol (CH) was purchased from Avanti and then purified as described previously (25). We obtained 2-(4,4-difluoro-5,7-diphenyl-4-bora-3a,4a-diaza-s-indacene-3-dodecanoyl)-1-hexadecanoyl-sn-glycero-3-phosphoethanolamine (BODIPY530-PE) and 2-(4,4-difluoro-5,7-diphenyl-4-bora-3a,4a-diaza-s-indacene-3-dodecanoyl)-1-hexadecanoyl-sn-glycero-3-phosphocholine (BODIPY500-PC) from Molecular Probes (Eugene, OR). Terbium chloride and N-[tris(hydroxymethyl)methyl]2-2-aminoethane sulphonic acid (TES) were purchased from Sigma Chemical (St. Louis, MO). PEG of molecular weight 7000–9000 (PEG 8000) was purchased from Fisher Scientific (Fairlane, NJ) and further purified as previously described (26). Dodecyl octaethylene glycol monoether (C12E8) was purchased from Calbiochem (La Jolla, CA). All other reagents were of the highest purity grade available.
Methods
Most methods used in this study have been reported previously and are also described in an accompanying article (18). VSV-TMD peptide was dissolved in trifluoroethanol/water (95:5 vol/vol) mixture. We incorporated VSV-TMD into SUVs by adding it at a 1:600 peptide/lipid ratio to a chloroform mixture of phospholipids prior to freeze-drying and sonication. This is the highest mol fraction that could be used to recover intact SUVs. Because so little peptide could be incorporated, we were unable to reliably determine the orientation of the peptide in the bilayer without a very expensive custom synthesis of fluorescently tagged peptides. Because the packing constraints of SUVs are considerable and force other TMD peptides we have studied to insert roughly asymmetrically, we assume this is the case for VSV-TMD as well.
Fusion model
We analyze our data in terms of a sequential, multistep model that was previously described in detail (19,22). In our experiments, SUVs are brought rapidly into close contact (the aggregation rate is 10-fold higher than the rate of any fusion event) by addition of PEG to form an aggregated state (referred to as the A state in our model). This avoids the complication of vesicle diffusion in our data analysis. The A state converts to a fusion intermediate (I) state at a rate k1. This involves a change in system topology, since contacting or cis bilayer leaflets merge during this step (hemifusion; see Fig. S3 in the Supporting Material). Finally, the I state converts slowly (rate k3) to a final fusion pore (FP) state, an event that also occasions a change in system topology. Experimentally, we track the process by monitoring LM between vesicle membranes, CM between vesicle compartments, and CL from the vesicle compartments, all of which can occur in the A, I, or FP state with probabilities αi (CM), βi (LM), and λi (CL), where i is 0 for the A state, 1 for the I state, and 3 for the FP state. Because of the normalization conditions used and experimental observations, a total of seven parameters (two ki, α, and β, and three λi) are required to describe three double-exponential curves, which guarantees that the model will not be underdetermined as long as all three data sets are analyzed globally. The probabilistic quantities α and β, as well as the leakage rates λi, are required because our kinetic model describes the passage of an ensemble of vesicles through thermodynamic states leading to fusion. Individual fusing vesicle pairs or aggregates are not identical structurally, and they do not travel through phase space in concert. In our ensemble measurements, we average over many events that occur between vesicles brought rapidly into close contact by addition of PEG. To obtain kinetics from single-event studies, one must record many traces of events that follow initiation of fusion between docked membranes (vesicles attached to other vesicles or to membranes) and then analyze the time probability distributions (dwell-time distributions) to distinguish distinct events and obtain their characteristic times (rates). Elsewhere (27), we describe in detail the relationship between the ensemble approach and a well-documented single-event study of fusion of docked vesicles (28), and show that pictures of fusion obtained by the two methods are consistent. Although both approaches have advantages and disadvantages, ensemble measurements have the advantage of requiring much shorter experiment times. This allowed us to examine the influence of many variables (e.g., pH (18) and fusion peptides (27)) on the kinetics and activation thermodynamics for each step of the fusion process by performing experiments at five different temperatures (17°C, 22°C, 27°C, 32°C, and 37°C). It also allowed us to collect precise data, in that all experiments were repeated twice for any given sample and then repeated for three different sample preparations at a given temperature. The kinetic parameter values are the averages of all these determinations. In an accompanying article (18), we document the kinetics and activation thermodynamics of PEG-mediated fusion of the DOPC/DOPE/SM/CH (35:30:15:20) SUVs used in our experiments at pH 7.4. In addition, we explore the role of pH in determining the mechanism of each step of the fusion process, as well as the influence of hexadecane on each step. Detailed descriptions of the ensemble kinetic model, the kinetic parameters for the control vesicles, and the equations used to obtain the activation thermodynamics are also given in that work.
Results
Effect of VSV-TMD on PEG-mediated fusion
Fig. 1 shows in red the time courses of LM, CM, and CL for SUVs containing VSV-TMD at a peptide/lipid ratio of 1:600 at 22°C and pH 7.4 as compared with data obtained with control vesicles (green) (18). Data obtained at each temperature were analyzed globally according to a single-intermediate model. Kinetic parameters obtained at 22°C and 37°C are summarized in Table 1 and parameters obtained at other temperatures are presented in Table S1. When compared with results obtained with control vesicles lacking VSV-TMD (18), the results show that VSV-TMD had no substantial effect on the extent of LM (FLM) or CM (FCM) or on the probability of LM in the intermediate state (β). It had the greatest effects on the rate of initial intermediate formation (k1), the rate of leakage from unfused vesicles (λ0), and the probability of CM in the intermediate state (α). VSV-TMD also increased k3 slightly at low (but not high) temperatures and had moderate effects on leakage rates λ1 and λ3. To understand the role of VSV-TMD in promoting the rate of I formation and the extent of pore formation, we examined the activation thermodynamics of I formation and the nature of pore formation from I.
Figure 1.
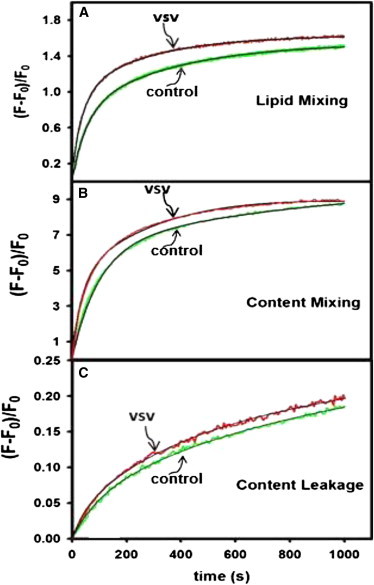
Effect of VSV-TMD peptides on the kinetics of (A) LM, (B) CM, and (C) CL during 5 wt % PEG-mediated SUV fusion at 22°C. Time courses are shown for peptide-free vesicles (green) as well as vesicles containing VSV-TMD (red) at a P/L ratio of 1:600. The black lines are the best fit of the data obtained using the one-intermediate model (18). To see this figure in color, go online.
Table 1.
Kinetic constants for PEG-mediated fusion of PC/PE/SM/CH (35:30:15:20) SUVs containing or not containing VSV-TMD, and in the presence or absence of 2% hexadecane at low and high temperatures
| k1 × 103 (s−1) | k3 × 103 (s−1) | α | β | λ0 × 104 | λ1 × 104 | λ3 × 104 | fCM | (1 − α) × fCM | fLM | |
|---|---|---|---|---|---|---|---|---|---|---|
| 22°C_con | 16.0 ± 0.5 | 1.69 ± 0.01 | 0.18 ± 0.010 | 0.66 ± 0.012 | 2.28 ± 0.07 | 2.09 ± 0.1 | 0.49 ± 0.17 | 0.25 ± 0.01 | 0.21 ± 0.004 | 0.43 ± 0.02 |
| 22°C_VSV | 26.3 ± 0.28 64% | 2.48 ± 0.03 47% | 0.44 ± 0.021 | 0.63 ± 0.015 | 7.87 ± 0.21 | 3.8 ± 0.1 | 0.72 ± 0.16 | 0.26 ± 0.01 | 0.15 ± 0.01 | 0.45 ± 0.01 |
| 22°C_hex | 17.0 ± 0.35 6.25% | 2.21 ± 0.03 31% | 0.57 ± 0.025 | 0.64 ± 0.022 | 7.73 ± 0.06 | 3.49 ± 0.05 | 0.32 ± 0.02 | 0.37 ± 0.01 | 0.16 ± 0.02 | 0.44 ± 0.02 |
| 22°C_hex + VSV | 26.0 ± 0.46 0% | 2.79 ± 0.04 12% | 0.60 ± 0.01 | 0.64 ± 0.01 | 18.3 ± 0.02 | 4.8 ± 0.1 | 0.5 ± 0.2 | 0.36 ± 0.02 | 0.14 ± 0.02 | 0.43 ± 0.02 |
| 37°C_con | 66.8 ± 1.5 | 3.03 ± 0.04 | 0.36 ± 0.021 | 0.72 ± 0.03 | 40.28 ± 0.35 | 7.24 ± 0.07 | 8.99 ± 0.28 | 0.33 ± 0.01 | 0.21 ± 0.01 | 0.46 ± 0.02 |
| 37°C_VSV | 117.9 ± 2.0 76% | 3.36 ± 0.03 11% | 0.56 ± 0.011 | 0.77 ± 0.04 | 51.9± 0.5 | 8.62 ± 0.07 | 11.50 ± 0.38 | 0.34 ± 0.02 | 0.15 ± 0.02 | 0.47 ± 0.03 |
| 37°C_hex | 89.0 ± 2.3 33% | 3.83 ± 0.15 26% | 0.68 ± 0.023 | 0.75 ± 0.03 | 87.87 ± 0.31 | 7.87 ± 0.36 | 4.96 ± 0.45 | 0.43 ± 0.03 | 0.14 ± 0.01 | 0.46 ± 0.02 |
| 37°C_hex + VSV | 95.9 ± 1.6 18.7% | 3.64 ± 0.11 0% | 0.66 ± 0.02 | 0.74 ± 0.03 | 83.9 ± 0.2 | 9.1 ± 0.2 | 8.6 ± 0.5 | 0.42 ± 0.01 | 0.14 ± 0.02 | 0.47 ± 0.03 |
The percentages beside the k1 and k3 values for VSV-TMD and hexadecane alone are changes relative to control, and the percentages below VSV-TMD + hexadecane (shown in bold italics) are relative to control + VSV-TMD.
Effect of VSV-TMD on the activation thermodynamics of PEG-mediated fusion
We similarly determined the temperature dependence of the activation free energy for formation of the I1 state and FP state in the presence of VSV-TMD at pH 7.4 and compared the results with the activation thermodynamics of control DOPC/DOPE/SM/CH (35:30:15:20 ) (18). Fig. 2, A and C, show the change in activation free energy associated with the presence of VSV-TMD in vesicle membranes () for formation of the I state and FP state, respectively. The changes in activation enthalpy () and activation entropy (T), as plotted in Fig. 2, B and D, for formation of the I state and FP state, respectively, were obtained as derivatives of as described elsewhere (18,19). The signs of and relative magnitudes of define whether the catalytic effect was entropic ( > 0) or enthalpic ( < 0). The insets in Fig. 2, B and D, show the changes in activation heat capacity () associated with the presence of VSV-TMD. The temperature dependence of reflects the nonlinear dependence of and on temperature. There are substantial similarities but also differences between the changes in activation thermodynamics due to VSV-TMD associated with the first and second steps. Among the similarities, and both switch from positive to negative with increasing temperature. The most noticeable difference is that VSV-TMD catalyzes step 1 entropically (i.e., ) over the entire temperature range, whereas it catalyzes step 3 enthalpically (i.e., ). This difference indicates that VSV-TMD catalyzes steps 1 and 3 by different mechanisms, as discussed below.
Figure 2.
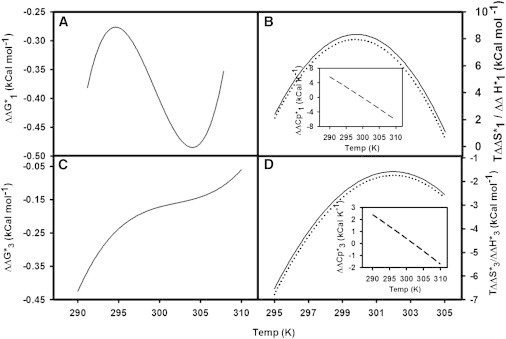
Temperature dependence of changes in activation free energy (), activation entropy (), and activation enthalpy () of control vesicles induced by the presence of VSV-TMD peptide. (A and C) Activation free energy () versus temperature for steps 1 and 3, respectively. (B) (solid line) and (dotted line) for intermediate formation. (D) (solid line) and (dotted line) for pore formation. Insets show the temperature dependence of changes in activation heat capacity () in the presence of the peptide for each step. Coefficients of the polynomials obtained from the raw plots of versus T for the vesicles containing TMD are given in Table S2. The plots in B and D were obtained from those in A and C by differentiation.
Combined catalytic influence of hexadecane and VSV-TMD
Fig. 3 illustrates how VSV-TMD and hexadecane influence fusion individually and in each other’s presence. One can see the very minimal catalytic influence of hexadecane on step 1 at low temperatures (18) in Fig. 3 A by comparing ΔG1∗ for control (●) versus hexadecane-containing vesicles (▼). VSV-TMD catalyzes step 1 over the entire temperature range (○). Of particular note is the fact that hexadecane interferes with the catalytic effect of VSV-TMD at the highest two temperatures examined (▵), where hexadecane itself has a catalytic effect. As shown in Fig. 3 C and elsewhere (18), both hexadecane and VSV-TMD catalyze step 3 over the entire temperature range, but hexadecane enhances the catalytic influence of VSV-TMD only at the two lowest temperatures examined (Fig. 3 B). At the highest temperature, hexadecane has a greater catalytic influence than VSV-TMD, and the effect of both together is indistinguishable from that of hexadecane alone (Fig. 3 B). Clearly, the effect of hexadecane on the catalytic influence of VSV-TMD varies substantially with temperature for both steps 1 and 3, with the mechanism of hexadecane’s influence differing from that of VSV-TMD at high temperatures, but perhaps being similar at low temperatures.
Figure 3.
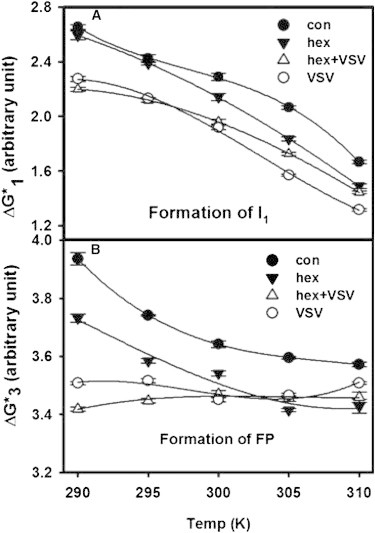
(A and B) Temperature dependence of the free-energy barrier for formation of (A) I1 state () and (B) pore formation () for control vesicles (●), vesicles in the presence of VSV-TMD (○), vesicles containing 2 mol % hexadecane (▼), and vesicles containing 2 mol % hexadecane in the presence of VSV-TMD (▵).
Effect of VSV-TMD and hexadecane on bilayer properties
We examined the influence of VSV-TMD on the bilayer properties of SUVs in the absence and presence of 2 mol % hexadecane. We explored the bilayer properties using three different fluorescent probes that report on different regions and properties of the membrane: C6NBDPC, DPH, and TMA-DPH. Because C6NBDPC partitions between its own micelles and membranes, its fluorescence lifetime components reveal the partition coefficient between these two phases, with the mol fraction of C6NBDPC in the membrane providing a partition coefficient that is sensitive to the free volume within membrane outer leaflets (29). The / ratio of TMA-DPH is not altered by the presence of either VSV-TMD or hexadecane at any temperature (Fig. S1), so exposure of its amine group to water remains unaltered by these two perturbants. Except at the lowest temperature examined (17°C), neither VSV-TMD nor hexadecane ordered the bilayer interior, and even at this temperature the ordering effects were barely significant (Fig. S2). The lifetime of C6NBDPC is sensitive to the polarity of its environment, which one can alter either by altering the extent of water penetration to the probe or by relocating the probe. Normally, C6NBDPC is located just below the interface region of the bilayer (30), but bilayer perturbation can cause it to penetrate deeper (29). The temperature dependencies of the mol fraction and fluorescence lifetime of C6NBDPC in control SUVS (●) and in membranes containing VSV-TMD (○) or hexadecane (▼), or both VSV-TMD and hexadecane (▵) are presented in Fig. 4, A and B, respectively. VSV-TMD occupied the bilayer free volume at all temperatures, but its space-filling ability was greatest at the three lowest temperatures and decreased with temperature (Fig. 4 A). It reduced surface packing at all temperatures (Fig. 4 C) and promoted water penetration into the headgroup region (Fig. 4 D). Consistent with these effects, the NBD moiety of C6NBDPC was in a less polar environment in the presence of VSV-TMD (i.e., either protected from water or penetrating deeper into the bilayer; Fig. 4 B). In summary, VSV-TMD seems to occupy the bilayer space in such a way that it spreads headgroups, which increases positive curvature stress. This could reflect a tilt of the VSV-TMD helix relative to the bilayer normal or simply a mismatch of protein structure with lipid packing. Since the native VSV-TMD appears to be a helix bent at the helix-breaking GXXXG sequence (13), and the peptide we used has sufficient nonpolar residues (20) to span the bilayer, the latter explanation is most likely.
Figure 4.
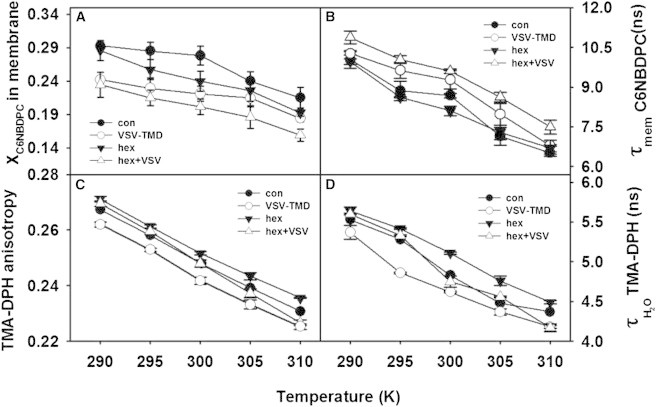
Effect of VSV-TMD, hexadecane, and hexadecane + TMD on the temperature dependence of (A) the mol fraction of C6NBDPC in membranes, (B) the lifetime of C6NBDPC in membranes, (C) TMA-DPH anisotropy, and (D) the fluorescence lifetime of TMA-DPH in membranes. Control SUVs (●) are compared with membranes containing hexadecane (▼), TMD peptide (○), and both hexadecane and TMD (Δ).
Hexadecane produced no change in the polarity of the NBD environment (Fig. 4 B), but did reduce the bilayer free volume at all but the lowest temperature, and its ability to do so increased and then decreased with increasing temperature (Fig. 4 A). It did not influence interfacial order and water penetration at low temperatures, but increased interfacial order (Fig. 4 C) and decreased water penetration into the interface with increasing temperature (Fig. 4 D). The temperature dependence of hexadecane’s structural influence (Fig. 4) suggests different hexadecane conformational ensembles at low and high temperatures, i.e., aligned with acyl chains and having little influence on membrane structure at low temperature, but having an increasingly broad conformational ensemble with increasing temperature. At the highest temperatures examined, this conformational ensemble appears to increase the acyl-chain cross section deep in the bilayer sufficiently to decrease water penetration of the interface (Fig. 4 D) and order the interface (Fig. 4 C), i.e., to produce an intrinsic negative curvature.
Of greatest interest was the combined effect of hexadecane and VSV-TMD. At the lowest temperature (290 K), hexadecane did not significantly reduce the bilayer free volume in the presence or absence of VSV-TMD (Fig. 4 A). With increasing temperature, the volume-reducing effects of hexadecane and VSV-TMD became increasingly additive. Given what we have observed regarding their individual influences, this is not surprising, since they should alter packing in different parts of the bilayer at these temperatures (with hexadecane occupying space deep in the bilayer and VSV-TMD doing so in the upper regions). This is especially evident in the temperature dependence of TMA-DPH anisotropy (Fig. 4 C) and lifetime (Fig. 4 D), where the surface-expanding influence of VSV-TMD and the surface-contracting effect of hexadecane completely nullify each other at high temperature. Whereas hexadecane had no effect on the polarity experienced by the NBD probe, hexadecane enhanced the probe-shielding effect of VSV-TMD (Fig. 4 B). This is consistent with VSV-TMD disrupting interfacial packing but also filling the bilayer space and promoting penetration of the NBD probe to deeper regions of the bilayer, where hexadecane would fill the hydrophobic space and reduce polarity.
To summarize, the individual and combined effects of VSV-TMD and hexadecane support the hypothesis that hexadecane aligns with acyl chains at low temperatures and has little influence on bilayer structure except to occupy space, whereas it appears to alter membrane structure with increasing temperatures and disproportionately occupies the interior hydrophobic space. VSV-TMD, on the other hand, has the same membrane-altering influence at all temperatures, namely, filling space but disrupting interfacial packing and promoting water penetration into the interface. These observations highlight the different space-filling abilities of a flexible agent such as hexadecane, which is free to locate in different regions of the bilayer, as opposed to the more rigid VSV-TMD, which adopts a bent but roughly helical (∼50%) conformation in a membrane (13).
Discussion
We have found that a sequential, two-intermediate model for fusion is able to account for the ensemble kinetics of the fusion process for a variety of membrane systems (19,22,31,32) over a range of temperatures (19). Our ensemble kinetic treatment is based on the expanded stalk structural model for fusion (33) as modified to treat the highly curved, space-encapsulating membranes of SUVs (17). The model is illustrated in Fig. S3. Based on earlier observations (19,27), formation of an initial fusion intermediate (I1 in Fig. S3) was proposed to occur through an unstable transition state (TS1) in which acyl chains are somewhat exposed to the dehydrated interbilayer space, thereby disrupting water-water and water-headgroup interactions in that space. Coarse-grained (34) and all-atom (35) MD simulations support this proposal. These observations also support the hypothesis that the final step of the fusion process (FP formation) involves passage through another unstable transition state (TS3), which is distinguished from the I2 semistable intermediate (Fig. S3) only by the probability of coordinated multilipid fluctuations in which lipids from both the cis and trans leaflets of the I2 intermediate cooperatively enter the highly unfavorable interstice region (17) of this semistable intermediate (19,27). In this work, we ask whether the effects of VSV-TMD on the kinetics and activation thermodynamics of the fusion process at pH 7.4, as well as on the measured bilayer structure, can be interpreted in terms of these mechanistic pictures of the individual steps of the fusion process.
Step 1: initial intermediate formation
The catalytic effect of VSV-TMD on step 1 was substantial, with k1 being increased by 64% at 22°C and by 76% at 37°C (Tables 1 and S1). Catalysis by VSV-TMD was entropic () over the entire temperature range (Fig. 2 B), implying that the presence of VSV-TMD either increased the number of energetically closely spaced microstructures in the transition state (TS1) leading to the stalk intermediate or stabilized a few microstructures in state A. The positive at low temperature is consistent with increased water ordering in TS1 due to increased acyl-chain migration into the interbilayer space. The fact that became increasingly negative with increasing temperature (Fig. 2 B, inset) is consistent with the expected reduction of water ordering by hydrocarbon with increasing temperature and with hexadecane decreasing the width of the energy distribution in TS1. According to our membrane structural measurements (Fig. 4, C and D), VSV-TMD does not appear to accommodate well to the packing of acyl chains and thus disorders and permits water entry into the interface, thereby increasing positive curvature stress in contacting outer leaflets of SUVs at all temperatures. This should encourage escape of acyl chains into the interbilayer space, an event that is also consistent with the observed and increasingly negative with increasing temperature.
We note that VSV-TMD’s catalytic influence on step 1 is entropic at all temperatures at pH 7.4 (Fig. 2 B), whereas that of hexadecane is entropic only at moderate temperatures and is enthalpic or inhibitory at high temperatures (18). This difference suggests that hexadecane and VSV-TMD have different mechanisms of action. Support for this proposal derives from the different mutual and individual effects of hexadecane and VSV-TMD on as plotted in Fig. 3 A as a function of temperature. Based on our membrane-structure measurements, VSV-TMD and hexadecane appear to alter bilayer structure in opposite ways at higher temperatures, with hexadecane reducing positive curvature stress and VSV-TMD increasing it (Fig. 4). At low temperature, where hexadecane has little influence on bilayer structure, it does not influence catalysis by VSV-TMD (Fig. 3 A). However, as temperature increases, the catalytic influence of hexadecane actually reduces the catalytic influence of VSV-TMD (Fig. 3 A). We propose that VSV-TMD catalyzes step 1 by increasing positive curvature stress in SUV outer leaflets in state A, whereas hexadecane decreases the outer-leaflet curvature stress of SUVs but catalyzes step 1 by promoting hydrocarbon-water contact in the TS1 intermediate (18). This hypothesis offers an explanation for the competing effects of VSV-TMD and hexadecane on step 1 (Fig. 3 A).
Step 3: conversion of intermediate to an FP state
The second semistable intermediate of the fusion process (I2 in Fig. S3), which is detected at pH 5, is not detected at pH 7.4 because the free energy of the I2 intermediate should increase at pH 7.4 due to reduced SUV outer-leaflet positive curvature stress at this pH (17,18). Thus, we propose that pores form at pH 7.4 by expansion of a stalk intermediate (I1, stalk radius = rS ≿ 0) via an unstable second intermediate (I2) with an expanded trans-membrane contact (ETMC) geometry to an ETMC geometry with a critical stalk radius (rS∗) at which transient pore expansion is more likely than pore reversal (see Fig. S3 for a detailed discussion of this proposal). The presence of VSV-TMD did not cause a reappearance of the second intermediate, so fusion at pH 7.4 is discussed in terms of a two-step process in the presence or absence of VSV-TMD (Tables 1 and S1).
We previously suggested that VSV-TMD might catalyze pore formation by occupying hydrophobic space and lowering the energy associated with the mismatch between lamellar and nonlamellar geometries (13) (interstice energy (17)). Hexadecane is known to lower this energy (36). At the lowest temperatures, hexadecane apparently aligns with acyl chains in hemifused cis leaflets, thereby contributing a positive intrinsic curvature that further destabilizes the ETMC circumference (18,27). However, with increasing temperatures, hexadecane apparently catalyzes pore formation by lowering interstice energy and promoting expansion of ETMC microstructures at both pH 5 (19) and 7.4. At pH 7.4, ΔΔH3∗ for hexadecane varies from negative at the lowest temperatures to positive at intermediate temperatures and negative at the highest temperatures (18). If the catalytic influence of VSV-TMD on step 3 at pH 7.4 reflects its ability to fill interstice space and increase rS toward rS∗, the activation thermodynamics and catalytic influence of hexadecane and VSV-TMD should be similar, especially at higher temperatures. However, these were similar only at lower temperatures. In this temperature range (290–300 K), ΔΔH3∗ for VSV-TMDs was more than twice as negative as that for hexadecane (compare Fig. 2 B with Fig. 4 F of our companion article (18)). In addition, VSV-TMD’s influence was clearly enthalpic at all temperatures, whereas that of hexadecane at pH 7.4 was less enthalpic and varied from enthalpic to entropic and then to enthalpic with increasing temperature (18). The simplest interpretation is that hexadecane and VSV-TMD catalyze pore formation by somewhat different mechanisms. The variation of hexadecane’s catalytic influence with temperature likely reflects the variation of its conformational ensemble with temperature, i.e., being more aligned with acyl chains at lower temperatures, but being disordered and occupying hydrophobic interior space within the membrane at high temperatures (18,19,27). This suggests a possible interpretation of the observation that the catalytic effects of VSV-TMD and hexadecane were not additive at lower temperatures (Fig. 3 B). Thus, it may be that hexadecane and VSV-TMD catalyze pore formation by similar mechanisms at low temperature (i.e., they align with acyl chains and increase curvature stress at the ETMC circumference), but the influence of VSV-TMD is much greater than that of hexadecane, so that when both are present, the influence of VSV-TMD dominates. At higher temperatures, the catalytic influences of VSV-TMD and hexadecane were again both enthalpic. We interpret the enthalpic influence of VSV-TMD at this temperature as still reflecting its ability to increase curvature stress in hemifused cis leaflets at the ETMC circumference. On the other hand, it is likely that the influence of hexadecane in this temperature range is to increase the ETMC circumference by increasing the stalk radius (18,27). We conclude that VSV-TMD and hexadecane catalyze fusion by different mechanisms, except at lower temperatures, where the ability of VSV-TMD to increase curvature stress at the ETMC circumference is much greater than that of hexadecane.
We tested these interpretations by considering the effects of hexadecane and VSV-TMD on the total extent of CM (fCM) and the probability of CM in the intermediate (α). Hexadecane increased the total extent of CM (fCM), whereas VSV-TMD did not (Tables 1 and S1). In addition, whereas both hexadecane and VSV-TMD increased the probability of CM in the intermediate (α), hexadecane was more effective in this regard (Tables 1 and S1). We note that the total amount or extent of CM that occurs in the FP state [] was the same for both perturbants (∼0.15; Table 1). According to our hypothesis for VSV-TMD action, it achieves catalysis not by reducing interstice energy but by increasing positive curvature stress at a fixed ETMC diameter (rS), i.e., by increasing the probability of CM earlier in the fusion process (i.e., it should increase α but not fCM, as observed). By contrast, hexadecane promotes fusion by increasing the radius of the ETMC intermediate, which increases the number of fluctuations that can lead to transient pores before final pore formation (i.e., increasing α and fCM). These observations support our hypothesis regarding the fundamentally different ways in which hexadecane and VSV-TMD catalyze fusion: VSV-TMD destabilizes the ETMC intermediate periphery at smaller stalk radii (i.e., it moves rS∗ to smaller rS), and hexadecane promotes expansion of the ETMC intermediate to a point where it has a much larger periphery and thus an increased number of fluctuations that can form pores (i.e., it expands rS toward rS∗).
Conclusions
The data and analysis presented here for PEG-mediated fusion of DOPC/DOPE/SM/CH SUVs at pH 7.4 are all consistent with the hypothesis that VSV-TMD catalyzes step 1 of the fusion process by occupying the upper bilayer space to facilitate acyl-chain excursions into the interbilayer space, thereby promoting the conversion of closely opposed monolayers to stalk intermediate microstructures, and catalyzes step 3 of the fusion process by occupying the upper bilayer space so as to increase curvature stress at the periphery of expanded intermediate microstructures. We previously proposed that this stress promotes correlated lipid fluctuations into interstice regions to produce transiently stable pores (19). In addition, we have shown that VSV-TMD promotes stable pore formation by a mechanism quite different from that proposed for hexadecane (18).
Finally, although hexadecane and VSV-TMD share similar activation thermodynamics for initial intermediate formation at low temperature, it appears that they do not share a common mechanism for catalyzing this step of fusion at any temperature, with VSV-TMD enthalpically destabilizing the contacting bilayers of the A state, and hexadecane entropically stabilizing the water-hydrocarbon environment of the TS1 state (18). This is in contrast to the previous suggestion that VSV-TMD might act by filling the interstice space, as does hexadecane at higher temperatures (13).
Acknowledgments
We thank Dr. Pradip Tarafdar for many useful discussions and especially acknowledge the efforts of two reviewers whose careful and substantial comments allowed us to greatly improve the presentation of our results and conclusions.
This work was supported by U.S. Public Health Service grant GM32707 to B.R.L.
Footnotes
Hirak Chakraborty and Tanusree Sengupta contributed equally to this work.
Hirak Chakraborty’s present address is CSIR-Centre for Cellular & Molecular Biology, Hyderabad 500007, India.
Tanusree Sengupta’s present address is Department of Biotechnology, Indian Institute of Technology Madras, Chennai 600036, India.
Supporting Material
References
- 1.Bullough P.A., Hughson F.M., Wiley D.C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- 2.Yin H.S., Paterson R.G., Jardetzky T.S. Structure of the uncleaved ectodomain of the paramyxovirus (hPIV3) fusion protein. Proc. Natl. Acad. Sci. USA. 2005;102:9288–9293. doi: 10.1073/pnas.0503989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison S.C. Viral membrane fusion. Nat. Struct. Mol. Biol. 2008;15:690–698. doi: 10.1038/nsmb.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White J.M., Delos S.E., Schornberg K. Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme. Crit. Rev. Biochem. Mol. Biol. 2008;43:189–219. doi: 10.1080/10409230802058320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgess S.W., McIntosh T.J., Lentz B.R. Modulation of poly(ethylene glycol)-induced fusion by membrane hydration: importance of interbilayer separation. Biochemistry. 1992;31:2653–2661. doi: 10.1021/bi00125a004. [DOI] [PubMed] [Google Scholar]
- 6.Kemble G.W., Danieli T., White J.M. Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell. 1994;76:383–391. doi: 10.1016/0092-8674(94)90344-1. [DOI] [PubMed] [Google Scholar]
- 7.Nüssler F., Clague M.J., Herrmann A. Meta-stability of the hemifusion intermediate induced by glycosylphosphatidylinositol-anchored influenza hemagglutinin. Biophys. J. 1997;73:2280–2291. doi: 10.1016/S0006-3495(97)78260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odell D., Wanas E., Ghosh H.P. Influence of membrane anchoring and cytoplasmic domains on the fusogenic activity of vesicular stomatitis virus glycoprotein G. J. Virol. 1997;71:7996–8000. doi: 10.1128/jvi.71.10.7996-8000.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyauchi K., Curran R., Matsuda Z. Mutations of conserved glycine residues within the membrane-spanning domain of human immunodeficiency virus type 1 gp41 can inhibit membrane fusion and incorporation of Env onto virions. Jpn. J. Infect. Dis. 2006;59:77–84. [PubMed] [Google Scholar]
- 10.Owens R.J., Burke C., Rose J.K. Mutations in the membrane-spanning domain of the human immunodeficiency virus envelope glycoprotein that affect fusion activity. J. Virol. 1994;68:570–574. doi: 10.1128/jvi.68.1.570-574.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shang L., Yue L., Hunter E. Role of the membrane-spanning domain of human immunodeficiency virus type 1 envelope glycoprotein in cell-cell fusion and virus infection. J. Virol. 2008;82:5417–5428. doi: 10.1128/JVI.02666-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cleverley D.Z., Lenard J. The transmembrane domain in viral fusion: essential role for a conserved glycine residue in vesicular stomatitis virus G protein. Proc. Natl. Acad. Sci. USA. 1998;95:3425–3430. doi: 10.1073/pnas.95.7.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dennison S.M., Greenfield N., Lentz B.R. VSV transmembrane domain (TMD) peptide promotes PEG-mediated fusion of liposomes in a conformationally sensitive fashion. Biochemistry. 2002;41:14925–14934. doi: 10.1021/bi0203233. [DOI] [PubMed] [Google Scholar]
- 14.Melikyan G.B., Markosyan R.M., Cohen F.S. A point mutation in the transmembrane domain of the hemagglutinin of influenza virus stabilizes a hemifusion intermediate that can transit to fusion. Mol. Biol. Cell. 2000;11:3765–3775. doi: 10.1091/mbc.11.11.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen F.S., Melikyan G.B. The energetics of membrane fusion from binding, through hemifusion, pore formation, and pore enlargement. J. Membr. Biol. 2004;199:1–14. doi: 10.1007/s00232-004-0669-8. [DOI] [PubMed] [Google Scholar]
- 16.Chernomordik L.V., Kozlov M.M. Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 2003;72:175–207. doi: 10.1146/annurev.biochem.72.121801.161504. [DOI] [PubMed] [Google Scholar]
- 17.Malinin V.S., Lentz B.R. Energetics of vesicle fusion intermediates: comparison of calculations with observed effects of osmotic and curvature stresses. Biophys. J. 2004;86:2951–2964. doi: 10.1016/S0006-3495(04)74346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakraborty H., Sengupta T., Lentz B.R. pH alters PEG-mediated fusion of phosphatidylethanolamine-containing vesicles. Biophys. J. 2014 doi: 10.1016/j.bpj.2014.07.048. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakraborty H., Tarafdar P.K., Lentz B.R. Activation thermodynamics of poly(ethylene glycol)-mediated model membrane fusion support mechanistic models of stalk and pore formation. Biophys. J. 2012;102:2751–2760. doi: 10.1016/j.bpj.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Blanc I., Luyet P.P., Gruenberg J. Endosome-to-cytosol transport of viral nucleocapsids. Nat. Cell Biol. 2005;7:653–664. doi: 10.1038/ncb1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roche S., Rey F.A., Bressanelli S. Structure of the prefusion form of the vesicular stomatitis virus glycoprotein G. Science. 2007;315:843–848. doi: 10.1126/science.1135710. [DOI] [PubMed] [Google Scholar]
- 22.Weinreb G., Lentz B.R. Analysis of membrane fusion as a two-state sequential process: evaluation of the stalk model. Biophys. J. 2007;92:4012–4029. doi: 10.1529/biophysj.106.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J., Lentz B.R. Evolution of lipidic structures during model membrane fusion and the relation of this process to cell membrane fusion. Biochemistry. 1997;36:6251–6259. doi: 10.1021/bi970404c. [DOI] [PubMed] [Google Scholar]
- 24.Chen P.S., Toribara T.Y., Warner H. Microdetermination of phosphate. Anal. Chem. 1956;28:1756–1758. [Google Scholar]
- 25.Schwenk E., Werthessen N.T. Studies on the biosynthesis of cholesterol. III. Purification of C14-cholesterol from perfusions of livers and other organs. Arch. Biochem. Biophys. 1952;40:334–341. doi: 10.1016/0003-9861(52)90119-7. [DOI] [PubMed] [Google Scholar]
- 26.Lentz B.R., McIntyre G.F., Massenburg D. Bilayer curvature and certain amphipaths promote poly(ethylene glycol)-induced fusion of dipalmitoylphosphatidylcholine unilamellar vesicles. Biochemistry. 1992;31:2643–2653. doi: 10.1021/bi00125a003. [DOI] [PubMed] [Google Scholar]
- 27.Chakraborty H., Tarafdar P.K., Lentz B.R. Wild-type and mutant hemagglutinin fusion peptides alter bilayer structure as well as kinetics and activation thermodynamics of stalk and pore formation differently: mechanistic implications. Biophys. J. 2013;105:2495–2506. doi: 10.1016/j.bpj.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyoung M., Srivastava A., Brunger A.T. In vitro system capable of differentiating fast Ca2+-triggered content mixing from lipid exchange for mechanistic studies of neurotransmitter release. Proc. Natl. Acad. Sci. USA. 2011;108:E304–E313. doi: 10.1073/pnas.1107900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J., Lentz B.R. Outer leaflet-packing defects promote poly(ethylene glycol)-mediated fusion of large unilamellar vesicles. Biochemistry. 1997;36:421–431. doi: 10.1021/bi9622332. [DOI] [PubMed] [Google Scholar]
- 30.Ho C., Slater S.J., Stubbs C.D. Hydration and order in lipid bilayers. Biochemistry. 1995;34:6188–6195. doi: 10.1021/bi00018a023. [DOI] [PubMed] [Google Scholar]
- 31.Tarafdar P.K., Chakraborty H., Lentz B.R. Phosphatidylserine inhibits and calcium promotes model membrane fusion. Biophys. J. 2012;103:1880–1889. doi: 10.1016/j.bpj.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haque M.E., Chakraborty H., Lentz B.R. Hemagglutinin fusion peptide mutants in model membranes: structural properties, membrane physical properties, and PEG-mediated fusion. Biophys. J. 2011;101:1095–1104. doi: 10.1016/j.bpj.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siegel D.P. The modified stalk mechanism of lamellar/inverted phase transitions and its implications for membrane fusion. Biophys. J. 1999;76:291–313. doi: 10.1016/S0006-3495(99)77197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smirnova Y.G., Marrink S.J., Knecht V. Solvent-exposed tails as prestalk transition states for membrane fusion at low hydration. J. Am. Chem. Soc. 2010;132:6710–6718. doi: 10.1021/ja910050x. [DOI] [PubMed] [Google Scholar]
- 35.Kasson P.M., Lindahl E., Pande V.S. Atomic-resolution simulations predict a transition state for vesicle fusion defined by contact of a few lipid tails. PLOS Comput. Biol. 2010;6:e1000829. doi: 10.1371/journal.pcbi.1000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozlov M.M., Leikin S., Rand R.P. Bending, hydration and interstitial energies quantitatively account for the hexagonal-lamellar-hexagonal reentrant phase transition in dioleoylphosphatidylethanolamine. Biophys. J. 1994;67:1603–1611. doi: 10.1016/S0006-3495(94)80633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


