Abstract
Reactions of ambient particles collected from four sites within the Los Angeles, CA air basin and Beijing, China with a mixture of N2O5, NO2, and NO3 radicals were studied in an environmental chamber at ambient pressure and temperature. Exposures in the chamber system resulted in the degradation of particle-bound PAHs and formation of molecular weight (mw) 247 nitropyrenes (NPYs) and nitrofluoranthenes (NFLs), mw 273 nitrotriphenylenes (NTPs), nitrobenz[a]anthracenes (NBaAs), and nitrochrysene (NCHR), and mw 297 nitrobenzo[a]pyrene (NBaP). The distinct isomer distributions resulting from exposure of filter-adsorbed deuterated fluoranthene to N2O5/NO3/NO2 and that collected from the chamber gas-phase suggest that formation of NFLs in ambient particles did not occur by NO3 radical-initiated reaction, but from reaction of N2O5, presumably subsequent to its surface adsorption. Accordingly, isomers known to result from gas-phase radical-initiated reactions of parent PAHs, such as 2-NFL and 2- and 4-NPY, were not enhanced from the exposure of ambient particulate matter to N2O5/NO3/NO2. The reactivity of ambient particles toward nitration by N2O5/NO3/NO2, defined by relative 1-NPY formation, varied significantly, with the relative amounts of freshly emitted particles versus aged particles (particles that had undergone atmospheric chemical processing) affecting the reactivity of particle-bound PAHs toward heterogeneous nitration. Analyses of unexposed ambient samples suggested that, in nighttime samples where NO3 radical-initiated chemistry had occurred, heterogeneous formation of 1-NPY on ambient particles may have contributed to the ambient 1-NPY concentrations at downwind receptor sites. These results, together with observations that 2-NFL is consistently the dominant particle-bound nitro-PAH measured in ambient atmospheres, suggest that for PAHs that exist in both the gas- and particle-phase, the heterogeneous formation of particle-bound nitro-PAHs is a minor formation route compared to gas-phase formation.
Keywords: nitro-PAHs, heterogeneous N2O5, NO3 radical, ambient particles, 2-nitrofluoranthene
Introduction
Polycyclic aromatic hydrocarbons (PAHs) and their nitrated derivatives (nitro-PAHs) are mutagenic in bacterial and mammalian assays and are classified as probable human carcinogens.1-4 Their presence in ambient atmospheres has been shown to contribute to the mutagenicity of ambient gas-phase and particulate matter (PM).5,6 Consequently, a wide-range of studies have investigated the sources and atmospheric chemical transformations of both PAHs (ref.7 and references therein) and nitro-PAHs.8 PAHs are ubiquitous air pollutants resulting from incomplete combustion processes, while sources of ambient nitro-PAHs include atmospheric reactions of their parent PAHs as well as primary emissions.7 PAHs found, at least partially, in the gas-phase (those containing 2-4 rings) can react via gas-phase radical-initiated reactions in the presence of NO2 to form nitro-PAHs, and both the OH and NO3 radical-initiated reactions of gas-phase fluoranthene (FL) have been shown to produce 2-nitrofluoranthene (2-NFL)9, generally the most abundant nitro-PAH in ambient atmospheres (refs.10,11 and references therein). PAHs have been measured in ambient PM worldwide12 and have been shown to undergo long-range transport from source regions (e.g., Asia to the western USA13-15) with the possibility of heterogeneous reactions occurring during transport. Accordingly, there has been renewed interest in the potential for heterogeneous formation of nitro-PAH.16-20 However, these previous studies did not use NFL and nitropyrene (NPY) product isomer-distributions to aid in assessing the mechanism and importance of heterogeneous nitro-PAH formation in ambient atmospheres.
Because FL is a nonalternant hydrocarbon, it has been proposed that the radical-initiated product, 2-NFL, can be utilized as a mechanistic probe for distinguishing between radical and electrophilic nitration.11,21,22 Specific isomer distributions of the molecular weight (mw) 247 NFLs and NPYs have previously been used to distinguish between the contributions of direct emissions vs gas-phase atmospheric reactions to ambient concentrations of nitro-PAHs (refs.10,11,23 and references therein). For example, the dominant NPY and NFL isomers observed in diesel exhaust are 1-NPY and 3-NFL, similar to the isomers formed from electrophilic nitration of PY and FL, respectively,24,25 while the isomers observed from the atmospheric gas-phase radical-initiated reactions of FL and PY include 2-NFL (from both gas-phase OH and NO3 radical reactions) and 2-NPY (only from OH radical reactions under ambient NO2 concentrations).9,11
Previous studies of heterogeneous nitration of PAHs with N2O5/NO3/NO2 have used PAHs adsorbed on Teflon (or Teflon impregnated) filters and disks, particles composed of diesel and wood soot, and coated azelaic acid particles.16,18,19,26,27 To the authors’ knowledge, only one other study has examined the formation of the nitro-PAHs from these reactions on ambient particulate matter.20 The present study investigates the heterogeneous formation of nitro-PAHs in ambient PM collected in Beijing, China, and at sites located within the Los Angeles air basin. Ambient particulate samples, along with filters coated with FL-d10 , PY-d10 and the less volatile deuterated triphenylene (TP-d12), chrysene (CHR-d12), benz[a]anthracene (BaA-d12), and benzo[a]pyrene (BaP-d12) were exposed to a mix of N2O5/NO3/NO2 with a focus on the formation of nitro-PAH. Isomer distributions of deuterated NFLs and NPYs formed in these chamber reactions provide insights into reaction pathways and their importance in ambient atmospheres.
Experimental Methods
Ambient Filter Sample Collection
Los Angeles Basin
Ambient filter samples were collected during a photochemical pollution episode in Los Angeles, Azusa, Riverside, and Banning, CA on August 22-23, 1997 as part of the SCOS97-NARSTO Intensive Sampling Periods campaign28 (see Figure S1 in Supporting Information (SI)). Samples were collected at an average flow rate of ~0.6 m3 min-1 over the time periods 0600-1200, 1200-1800, and 1800-0600 hr at Los Angeles and Azusa, and 0600-1800 and 1800-0600 hr at Riverside and Banning. The filter samples were archived in a sub-zero freezer and data on gas-phase PAH and semi-volatile nitro-PAH were also collected and reported.28 More information on sampling and analysis is presented in the SI. Data for NOx and O3 were obtained from the California Air Resources Board historical air quality archives.29
Riverside 2012
Ambient PM was collected using Hi-vol samplers in Riverside during four consecutive daytime (May 11-14, 0600-1800 hr) and nighttime (May 10-13, 1800-0600 hr) periods in 2012 as described in the SI. Co-pollutant data for this sampling period were obtained from the California Air Resources Board current Air Quality Data Query Tool.29
Beijing
Particles were collected at Peking University in Beijing, China from May 2009 to February 2010 and from April 20-22, 2011 using a three-stage High Volume Cascade Impactor (Series 230, Tisch Environmental, Cleves, OH) equipped with quartz filters (no. 1851-865, Tisch Environmental, Cleves, OH; 8 in × 10 in) during 24-hour collection intervals at a flow rate of ~1.0 m3 min-1.30 Samples were stored frozen until shipment to the United States and then stored at -20 °C until this study. A summary of all ambient samples collected and used in chamber studies is given in Table S1.
Environmental Chamber Exposures
Ambient Particulate Matter and PAH-dx Coated Filters
Experiments were performed at ~296 K and ~735 Torr of dry purified air in a ~7 m3 collapsible Teflon chamber equipped with a Teflon-coated fan. The ambient filters were divided into equal portions to provide an unexposed control for each exposed filter portion, thus allowing the initial particle-bound PAH and nitro-PAH concentrations to be compared with those following exposure to N2O5/NO3/NO2 in the chamber (Figure S2). NO3 radicals were generated in the dark from the thermal decomposition of N2O5 in the presence of NO2. Initial concentrations of N2O5 and NO2 were 0.42 - 0.48 ppmv and ~1 ppmv, respectively (1 ppmv = 2.4 ×1013 molecule cm-3 at 296 K and 735 Torr total pressure). The chamber was continually flushed at 100 L min-1 to avoid build-up of NO2, resulting in an average residence time of ~70 min for gaseous species in the chamber. N2O5 and NO2 were introduced hourly into the chamber at the above concentrations a total of 8 times during each experiment (Table S2 gives details of individual experiments, including experiments in which PAH-dx coated filters were co-exposed with the ambient filters).
For
| (1) |
| (2) |
| (I) |
at 298 K with k1 and k2 being rate constants for reactions 1 and 2, respectively, and concentrations in molecule cm-3.31 Based on Equation I and the decay of reactants as a result of wall loss and flush out, the NO3 exposure in this chamber system was estimated to be equivalent to ~45 pptv (1 × 109 molecule cm-3) for seven 12-hr nighttime periods, simulating a maximum ambient NO3 concentration exposure of ambient particles during a possible week-long transport event. Calculations for this approximation as well as a discussion of ambient NO3 formation are shown in the SI.
Deuterated PAH-Coated Filter Exposures
Mixtures of PY-d10, FL-d10, and TP-d12 in methanol (50 μg each) were deposited onto clean Teflon-impregnated glass fiber (TIGF) filters (Pallflex T60A20, 8 in × 10 in), which were then dried in a clean atmosphere for ~30 min. In addition, separate filters coated with solutions containing 50 μg of BaA-d12, CHR-d12, or BaP-d12 were exposed in two experiments. NO3 radicals were generated as described above, with initial concentrations of N2O5 and NO2: 0.21-0.48 ppmv and 0.5-1.0 ppmv, respectively. The number of additions of N2O5 and NO2 varied among individual experiments, with a maximum number of 8 additions during a single experiment (Table S2). After exposure, filters were removed from the chamber and stored at -20° C until analysis. Several filters, coated and dried as described above, were stored unreacted at -20 °C until analysis for determination of loss of deuterated PAHs during the deposition and drying processes. A polyurethane foam (PUF) plug was inserted into the chamber outflow vent for several experiments to monitor any gas-phase deuterated PAHs and nitro-PAHs in the chamber.
Analysis
Filter and PUF samples were extracted in dichloromethane and analyzed by gas-chromatography mass-spectrometry (GC-MS, Agilent Technologies, 5973N MSD) with electron impact (EI) ionization and positive (PCI) and negative chemical ionization (NCI). Full details are given in the SI.
Statistical Analysis
The paired t-test analysis was performed for ambient samples for which there existed replicate measurements [i.e., Beijing 1, Beijing 2, Beijing 3 (n=3), and R97MS (n=9)]. This test made it possible to distinguish between differences in PAH and nitro-PAHs caused by exposure to N2O5 and those resulting from variability within experiments and GC-MS measurements. Cluster analysis of 2-NFL/BeP ratios and reactivity values was performed using Ward’s minimum-variance clustering method. Since the two variables were measured on different scales and do not have equal variance, procedure ACECLUS in the SAS statistical program was first used to transform and standardize the data.
Chemicals
The chemicals used are listed in the SI.
Results
Deuterated Nitro-PAH Formation
Clean TIGF filters coated with deuterated PAHs and exposed to N2O5/NO3/NO2 were analyzed for the formation of deuterated nitro- and dinitro-PAH derivatives. These reactions confirmed earlier studies in which specific isomers were observed as filter-adsorbed nitro-products11,26,32,33 and gas-phase NO3 radical-initiated nitro-products (2-NFL from the gas-phase NO3 radical-initiated reaction of volatilized FL and, with a significantly lower yield, 4-NPY from PY9,11,33). It was also demonstrated that under the conditions used here for the ambient PM exposures, nearly complete reaction would be expected for fully available PAHs (see Figure S3) and reactions of filter-bound PY-d10 and FL-d10 with N2O5/NO2/NO3 formed deuterated nitro-PAH with a relative isomer distribution of 1-NPY-d9 >3-NFL-d9 ~8-NFL-d9 ~7-NFL-d9 >1-NFL-d9 (Figure 1, top and Figure S4).11,33 This isomer distribution may be contrasted with the dominance of 2-NFL-d9 in the gas-phase sample collected on the PUF plug, which is attributed to desorbed FL-d10 undergoing gas-phase reaction with NO3 (Figure 1, bottom). The very minor amount of 2-NFL-d9 observed on the filters (Figure 1, top) likely resulted from desorption of the parent FL-d10 from the filter surfaces, followed by gas-phase NO3 radical-initiated reaction to form 2-NFL-d9, with subsequent deposition onto the filter. This process is consistent with the presence of 2-NFL-d9 and FL-d10, as well as 4-NPY-d9 and PY-d10, on the PUF plugs inserted into the chamber outflow air. The 4-NPY-d9 on the PUF is attributed to gas-phase reaction of PY-d10 with NO3.9,11 Because the gas-phase reaction of NO3 radicals with PY has not been shown to form 1-NPY,9,11 the small amount of 1-NPY-d9 relative to 2-NFL-d9 on the outflow PUF plugs (Figure 1, bottom) may have been formed by heterogeneous reaction of PY-d10 with N2O5 on the surface of the PUF plug. The formation of 3-, 8-, 7- and 1-NFL-d9 on the filter suggests that the heterogeneous nitration of FL did not occur via reaction with the NO3 radical, which would be expected to lead to 2-NFL formation.
Figure 1.
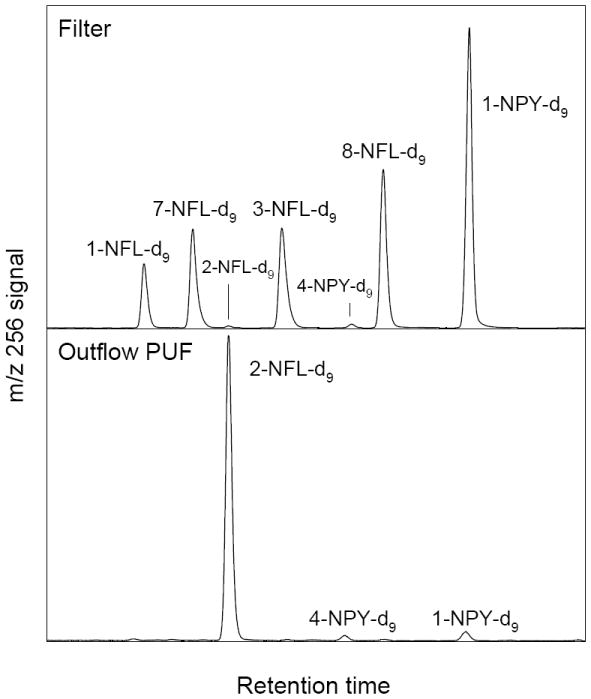
Comparison of the isomer distribution of mw 256 deuterated nitro-PAHs extracted from the filter (top) and from the PUF plug placed in the chamber outflow (bottom). The filter ion chromatogram (top) comes from deuterated PAHs exposed in MTC 2419 (see Table S2) and the PUF plug ion chromatogram is from a combination of PUF plug extracts from experiments MTC 2419-2422 (Table S2). Note that due to sensitivity differences, the filter-bound nitro-PAH-d9 were analyzed with EI ionization and those from the PUF plug were analyzed using NCI; thus, the two chromatograms are shown on different relative scales. A 60 m DB-17 capillary GC column was used to provide good separation of all isomers.
Other deuterated nitro-PAH products on the filters included 1- and 2-NTP-d11, 7- and 12-NBaA-d11, 6-NCHR-d11, and 6-NBaP-d11, formed from the heterogeneous reactions of TP-d12, BaA-d12, CHR-d12, and BaP-d12, respectively (Figures S4 and S5). These nitro-PAH isomer distributions agree with previous studies that examined reactions of surface-bound PAHs with N2O5.11,26 For a more complete discussion of the various exposure conditions and formation of dinitro-FLs and PYs see the SI and Figure S6.
Ambient PM Results
Ambient Particulate PAH Concentrations
The concentrations of selected particulate PAHs from ambient samples collected in Beijing, China, at four sites in southern California in August 1997 during the Southern California Ozone study (SCOS97), and in Riverside, CA (October 1997 and May 2012) are given in Table S3. Note that reporting only the particulate concentrations of FL and PY, as collected on filters, is an underestimation of their total atmospheric concentrations because FL and PY are predominantly present in the gas-phase.34 PAHs are primary emissions resulting from incomplete combustion processes and Beijing is clearly highly impacted by direct emissions.35 In the SCOS97 study, emissions were highest in heavily traffic-impacted Los Angeles and significantly decreased for the simultaneously sampled downwind sites of Riverside and Banning.
Ambient Particulate Nitro-PAH Concentrations
Initial ambient concentrations of particle-bound molecular weight (mw) 247 and 273 nitro-PAHs were measured at all sites and are reported in Table S4. While the 2-NFL/1-NPY ratios varied, 2-NFL was consistently the mw 247 nitro-PAH isomer observed in greatest abundance. Other isomers, such as 2-NPY, 4-NPY, 3-NFL and 8-NFL were identified and measured in most, but not all, samples. The mw 273 nitro-PAHs were present in lower concentrations relative to 2-NFL, and 7-NBaA was the most consistently measured mw 273 nitro-PAH in each sample (Table S4). Other isomers observed included 12-NBaA, 1- and 2-NTP, with 6-NCHR being less frequently detected. 6-NBaP was detected only in the particulate matter collected in Beijing, China, and in one Azusa morning sample.
Ambient PM Exposed to N2O5/NO3/NO2
After exposure to 8 additions of N2O5/NO2/NO3 over 8 hr in the environmental chamber, the ambient PM samples were examined for degradation of particle-bound PAHs and formation/losses of particle-bound nitro-PAHs. Exposures of most ambient PM samples were accompanied by the exposure of a FL-d10, PY-d10, and TP-d12 coated filter to verify consistency in nitrating agent concentrations, as shown by similar losses of deuterated PAHs and formation of deuterated nitro-PAHs (Figures S3 and S4, respectively).
PAH Degradation
The relative degradation of a given particle-bound PAH upon exposure to N2O5/NO3/NO2 is defined as: [(Δ[PAH]/[PAH]0) ×100] with Δ[PAH] the concentration difference between the unreacted and reacted filter samples and [PAH]0 the unreacted concentration of the specified PAH. Calculated degradations of these PAHs are given in Table S5. Degradation was observed for all reported particle-bound PAHs in Beijing PM, although the relative degradation of these PAHs differed among the three ambient Beijing samples. Results of paired t-test analyses of unreacted and reacted PAH concentrations (Table S5) show that statistical significance of PAH degradation was sample-dependent, with only pyrene (PY) concentrations changing significantly (p-value <0.05) for all three Beijing samples. Variable PAH degradation was also observed for PM collected during SCOS97 and in Riverside in 2012, with no loss detected for several PAHs in these samples. Therefore, considering the large losses of deuterated PAHs in these exposures (Figure S3), it is clear that some fraction of the PAHs present in ambient particles collected on filters was unavailable for reaction with N2O5/NO3/NO2.
Degradation of benzo[a]pyrene (BaP) was measured in all but one of the exposed PM samples, while no loss of benzo[e]pyrene (BeP) was observed for several samples (Table S5). BeP has previously been shown in laboratory studies to be a relatively unreactive PAH, while BaP is considered reactive.36,37 Our data are consistent with these findings as shown in Table S6, where the BaP/BeP ratios of the unexposed PM were higher than the corresponding ratio for the exposed PM for 78% of the samples. Therefore, when comparing relative extents of atmospheric processing in samples from various sites and times, we have chosen to ratio the PAHs and their atmospheric reaction products to the initial (i.e., unexposed) BeP concentration as a way of normalizing the ambient samples for the strength of direct emissions and meteorological differences such as inversion heights.
Nitro-PAH Formation and Loss
For the majority of exposed samples, 1-NPY was formed in the greatest amounts (Table S7). Other isomers formed consistently, but in lower amounts, included 3-NFL, 8-NFL, 6-NCHR, 7-NBaA, 1-NTP and 2-NTP (Figure 2 and Figure S7). Results of paired t-test analyses of unreacted and reacted nitro-PAH concentrations (Table S7) showed statistically significant (p<0.05) formation of 1-NPY, 7-NBaA, 6-NCHR, 2-NTP, and 6-NBaP in all three Beijing samples and, interestingly, the 6-NBaP formation in these samples exceeded that of 1-NPY. Thus the nitro-isomers formed in the exposed ambient PM were the same isomers observed from the exposed PAH-dx. In contrast, the amounts of 2-NFL and 4-NPY, nitro-PAHs formed from gas-phase reaction of FL and PY with NO3 radicals, respectively, were not enhanced by exposure and generally decreased somewhat (see Table S7). The changes for 2-NFL and 2-NPY in the Beijing samples and the R97 MS sample were shown to be statistically insignificant. In contrast to the deuterated PAH study, dinitro-PYs or dinitro-FLs were not formed in any of the exposed ambient PM samples.
Figure 2.
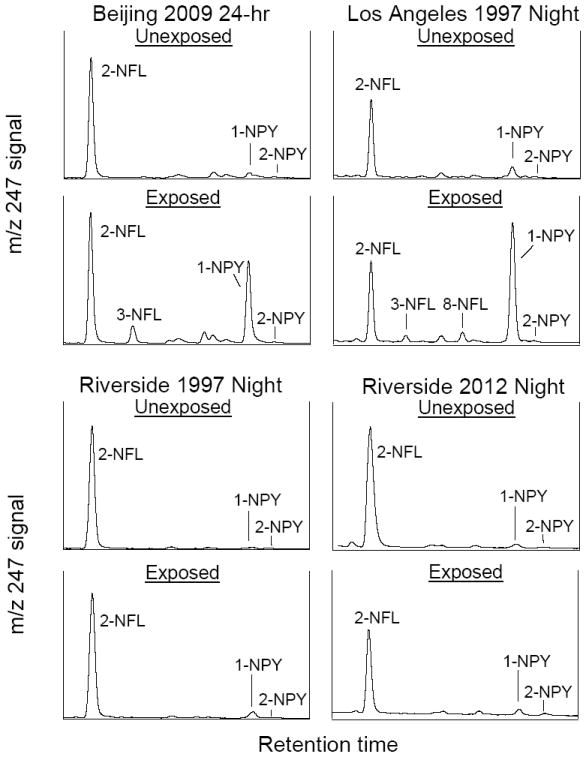
Effects of exposing ambient particulate matter collected in Beijing 2009 (top left), Los Angeles 1997 night (top right), Riverside 1997 night (bottom left), and Riverside 2012 night (bottom right) to N2O5/NO3/NO2 . GC-MS/NCI ion chromatograms for mw 247 NFLs and NPYs for the unexposed and exposed PM for each site are shown on the same scale relative to one another.
Reactivity Toward Nitro-PAH Formation and 2-NFL/BeP Ratios
Because 1-NPY was consistently formed when ambient PM was exposed to N2O5/NO2/NO3, 1-NPY was used as a representative nitro-PAH to describe the differing relative formation of nitro-PAHs among the various PM samples. The “reactivity” of ambient particles in this study, with respect to nitration by N2O5, is expressed as:
| (II) |
where Δ[1-NPY] is the change (exposed filter – corresponding unexposed filter) in 1-NPY and [PY]0 is the original amount of PY measured on each unexposed filter (a measure of the amount of PY potentially available for nitration in our chamber system). The calculated reactivity values varied among the PM samples, with relatively high calculated values for the averaged 24-hour samples collected in Beijing and much lower values for the daytime samples collected in the SCOS97 study and Riverside 2012 (Figure 3; Table S8). The reactivity values for the 12-hr nighttime SCOS97 samples are higher in the source regions of Los Angeles and Azusa relative to the Riverside and Banning receptor regions (Figure 3).
Figure 3.
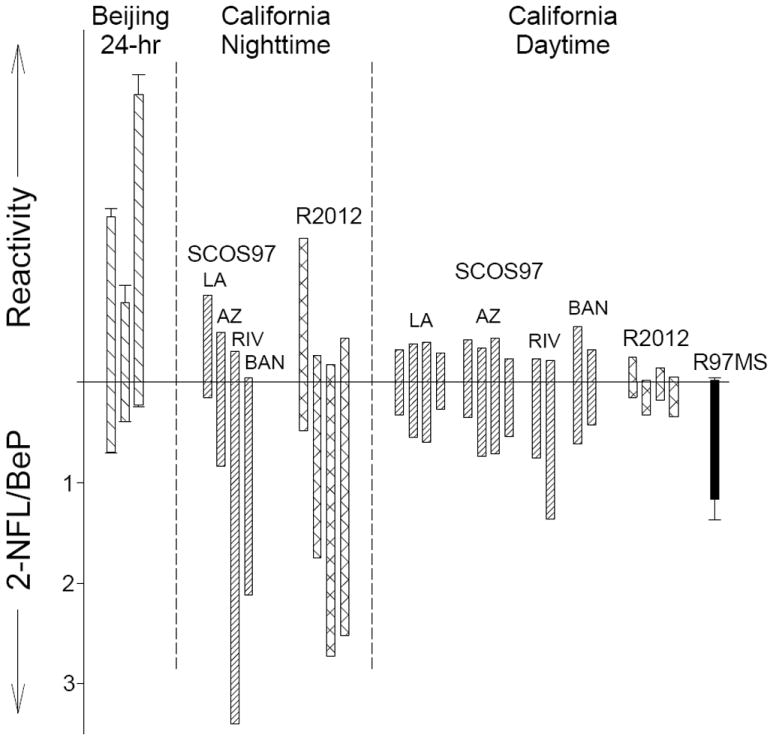
Comparison of calculated reactivity toward nitration in chamber exposures (Reactivity = (Δ[1-NPY]/[PY]0) × 100) and ambient 2-NFL/BeP ratios for samples from: Beijing, collected 2009- 2011 with 24-hr sampling periods (Beijing 24-hr;
 ), Los Angeles, Azusa, Riverside, and Banning during the SCOS97 intensive (SCOS97;
), Los Angeles, Azusa, Riverside, and Banning during the SCOS97 intensive (SCOS97;
 ), Riverside 2012 (R2012;
), Riverside 2012 (R2012;
 ), and Riverside 1997 using a Mega-Sampler (R97MS;
), and Riverside 1997 using a Mega-Sampler (R97MS;
 ). Error bars represent the standard deviation of the mean for replicate filter exposures. See Table S8 for data and Table S1 for ambient sampling details.
). Error bars represent the standard deviation of the mean for replicate filter exposures. See Table S8 for data and Table S1 for ambient sampling details.
Aged air masses contain primary and secondary particles which have experienced heterogeneous reactions with gas-phase oxidants at the surface of the particle and/or have accumulated the products of gas-phase reactions of volatile organic compounds (VOCs) with OH radicals, O3, and in some cases, NO3 radicals.38 Recent studies have shown that particle ageing may change the phase-state of ambient PM, affecting the diffusion of gas-phase nitrating species into particle bulk, as well as the accessibility of reactants to surface reactions.39-42 Thus primary particles containing PAHs and nitro-PAHs may be modified by particle aging, which may inhibit the availability of particle-bound PAHs for further nitration.
The presence of 2-NFL in ambient PM has been attributed to atmospheric gas-phase reactions of FL with both OH and NO3 radicals.9,11 In contrast, BeP is a direct emission and is expected to be relatively stable with respect to atmospheric chemical degradation.36,37 Thus, ambient 2-NFL/BeP ratios can be used to assess the relative contribution of atmospheric chemical aging or primary emissions to ambient PM, with higher 2-NFL/BeP ratios indicating greater atmospheric processing. Figure 3 shows a plot of the reactivity toward nitration for each PM sample based on the result of the chamber experiments (Equation II), together with the initial 2-NFL/BeP ratio of each unexposed PM sample. In general, PM samples with high reactivity, (such as those from Beijing) had low 2-NFL/BeP ratios, suggesting that air masses that had undergone little atmospheric processing had greater reactivity with respect to nitro-PAH formation via reaction of particle-bound PAHs with N2O5/NO2/NO3. For daytime samples, 2-NFL/BeP ratios were relatively low, with all of the 17 daytime California samples having reactivities <20% of the maximum (Figure 3, Table S8), consistent with ubiquitous chemical processing by OH radical reaction under sunlit conditions. In contrast, the Beijing 24-hr samples (where nighttime PM was sampled on top of daytime PM) and the California nighttime samples have widely varying 2-NFL/BeP ratios and reactivity values. These ratios may fluctuate due to differing nighttime contributions from primary emissions (greater BeP in Beijing, Los Angeles, and Azusa) and early-evening processing by NO3 chemistry (efficiently producing 2-NFL in Riverside and Banning). Cluster analysis of the reactivity and 2-NFL/BeP data show that 4 clusters account for 90.7% of the variation within samples. These clusters are shown in Figure 4, and can be described as Cluster 1: mainly daytime samples (15/16 points in cluster) from both source (Los Angeles and Azusa) and receptor (Riverside and Banning) regions indicating aging by daytime OH and O3 chemistry, and hence lower reactivity in the chamber system; Cluster 2: consisting of five nighttime and two daytime samples, all from the downwind receptor sites of Riverside and Banning with the highest 2-NFL/BeP ratios, suggesting particles sampled from an aged air mass; and Clusters 3 and 4: consisting of one Riverside 2012 nighttime sample (discussed below), a SCOS97 Los Angeles nighttime sample, and the 24-hour Beijing samples characterized by high reactivity and low 2-NFL/BeP ratios, indicating a greater relative contribution from direct emissions and greater availability of PAHs for nitration.
Figure 4.
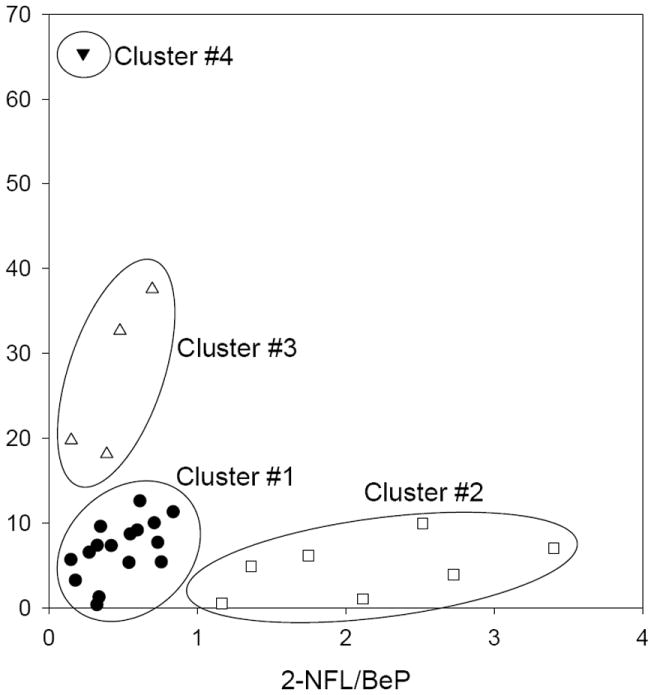
Cluster analysis of the relationship between 2-NFL/BeP ratios and calculated reactivity toward nitration for 28 PM samples. The first four clusters (shown here as different symbols) account for 90.7% of the variation.
The reactivities for the majority of the Riverside 2012 PM samples were similar to those of the Riverside samples collected in 1997. This suggests that archival storage of the SCOS97 filter samples did not significantly affect the nitration of ambient particle-bound PAHs. However, the reactivity of the first sample taken on May 10, 2012 from 1800-0600 in Riverside was high compared to those from the downwind receptor samples (Figure 3 and Table S8). The low 2-NFL/BeP ratio in this sample suggests fresh emissions during this sampling period, consistent with the relatively high NO and CO concentrations (markers of primary emissions) measured the day prior to commencing sampling (Figure S8). Thus, the differences in the nitration reactivity of ambient PM in our chamber system (Figures 2 and 3) can be explained by the degree of processing that a particle has undergone prior to collection and suggest that extrapolating from laboratory studies on pure PAH or adsorbents coated with PAH must be done very cautiously.
SCOS97- Ambient Case Study with Downwind NO3/N2O5 Chemistry
Archived PM filters collected during a southern California photochemical pollution episode that occurred on August 22-23, 1997 (Intensive #3 of the SCOS97 field campaign) were included in this study. Together with simultaneous measurements of volatile PAHs, semi-volatile nitro-PAHs and co-pollutants that have previously been reported,28,29 this forms a unique data set to examine the effects of downwind transport and atmospheric aging of ambient particles on the reactivity of their particle-bound PAH toward nitration. Additionally, because gas-phase NO3 radical chemistry was demonstrated to have occurred during this campaign,28 heterogeneous chemistry by NO3/N2O5 should also have occurred and its effects on 1-NPY concentrations under actual ambient conditions can be examined.
The sampling locations in Los Angeles (source site), Azusa (source and mid-basin receptor site), Riverside and Banning (downwind late-basin receptor sites) are shown on Figure S1 and the O3, NO2 and NO data for Aug. 22-23 at these sites are given in Figure S9. Higher and more sustained O3 levels were observed at the down-wind sites compared to Los Angeles. Unlike the Los Angeles site where O3 was rapidly titrated by fresh vehicular NO emissions after sunset, O3 and NO2 are clearly present after sunset at Riverside and Banning, allowing for NO3 radical formation.
The major PAH sources during this episode were vehicle emissions and, reflecting this, profiles of gas-phase naphthalene (measured immediately after collection28) and particle-associated benzo[ghi]perylene (B[ghi]P) and BeP (both measured from archived filters) look very similar to one another (Figure S10) and are consistent with the NO profiles (Figure S9) resulting from vehicle emissions. Thus, the highest PAH concentrations were in Los Angeles and Azusa and, because of lower emissions and dilution during downwind transport, lowest in Banning. Traffic was present throughout the day at the Los Angeles site and decreased PAH concentrations for the 1200-1800 hr samples largely reflect an increase in the inversion height.
Because certain nitro-PAH isomers are the products of both OH radical-initiated (dominantly daytime) and NO3 radical-initiated (early-evening and nighttime) gas-phase reactions of the parent PAH, their spatial and temporal trends reflect the radical chemistry that has occurred. However, particle lifetimes are sufficiently long that “carry-over” from night to day, or vice versa, may influence time-concentration profiles. The semi-volatile 3-nitrobiphenyl (3-NBPh) and particle-associated 2-NPY are only formed from gas-phase OH chemistry7 and their highest concentrations occurred in the daytime samples. When normalized as a ratio to BeP (Figure S11), the highest 3-NBPh/BeP ratios were the Azusa and Los Angeles 1200-1800 hr samples and a Riverside daytime (0600-1800 hr) sample. All eight of the daytime Los Angeles and Azusa samples (and 7 out of 9 of the Riverside and Banning daytime samples) fall into Cluster #1, namely, PM with low reactivity toward nitration and relatively low 2-NFL/BeP ratios. Therefore, daytime OH radical reactions, e.g., OH + VOCs followed by product adsorption onto PM, may decrease the availability of the PAH in PM toward nitration. The 2-NFL/BeP ratios of these samples were generally <1 (Figure 3), likely reflecting the lower yield of 2-NFL from the OH reaction vs the NO3 reaction of FL9.
The nighttime PAH concentrations at Los Angeles were similar to the daytime (0600-1200 hr) concentrations (Figure S10), but NO emissions from vehicle traffic removed any ground-level O3 present at sunset, and, therefore, NO3 could not form (Figure S9). Thus, neither OH nor NO3 radical reactions (nor reactions with O3) occurred and this lack of atmospheric reactions, combined with continued vehicle emissions during the Los Angeles nighttime sampling period, resulted in it having the lowest 2-NFL/BeP ratio and the highest reactivity (Figure 5) of any SCOS97 sample (note that this sample clusters with 2 of the Beijing samples).
Figure 5.
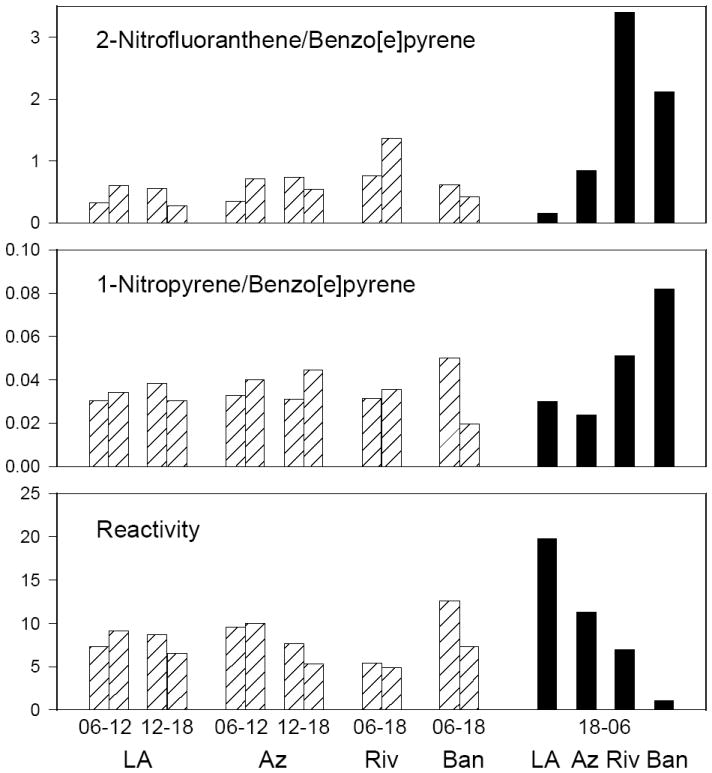
Ratios of 2-NFL/BeP (top), 1-NPY/BeP (middle), and reactivity of ambient samples toward nitration (bottom) from the SCOS97 field study. Daytime samples =
 ; nighttime samples =
; nighttime samples =
 . LA = Los Angeles; Az = Azusa; Riv = Riverside; Ban = Banning.
. LA = Los Angeles; Az = Azusa; Riv = Riverside; Ban = Banning.
The high 2-NFL/BeP ratios during SCOS97 Riverside and Banning nighttime samples (Figure 5, note these samples fall into Cluster #2 in Figure 4) are attributed to a significant contribution from NO3 radical chemistry to 2-NFL formation, consistent with the semi-volatile methylnitronaphthalene (MNN) and dimethylnitronaphthalene (DMNN) concentrations previously reported.28 Based on laboratory studies of relative OH vs NO3 formation profiles, certain nitro-PAH ratios have been identified as “markers” whose increase signifies a contribution from NO3 chemistry to ambient nitro-PAH formation.9,11,43-45 For example, 2-NFL is formed from both OH and NO3 radical species and, therefore, may be formed both during the daytime and evening, while 2-NPY is only formed from the OH radical reaction.7,9,11 Therefore the 2-NFL/2-NPY ratio will be high (typically >10) in atmospheres where significant NO3 formation of 2-NFL has occurred (see Riverside and Banning samples in Figure S12).10,11 Similarly, laboratory studies reporting different relative yields of MNNs and DMNNs from OH and NO3 chemistry have shown that increasing ratios of 2-methyl-4-nitronaphthalene/1-methyl-5-nitronaphthalene (2-M-4-NN/1-M-5-NN), and 2,7-dimethyl-4-nitronaphthalene/1,7-dimethyl-5-nitronaphthalene (2,7-DM-4-NN/1,7-DM-5-NN) are indicative of gas-phase NO3 chemistry.9,11,43-45 Figure S12 shows these three nitro-PAH ratios for the SCOS97 samples, along with the calculated reactivity observed toward nitration in the environmental chamber. All three marker ratios show significant increases in the Riverside and Banning samples demonstrating NO3 radical-initiated nitro-PAH formation. Because NO3 and NO2 are in thermal equilibrium with N2O5 (Equation I), atmospheres in which NO3 chemistry had occurred would also contain gas-phase N2O5. In our chamber exposures, heterogeneous reaction of PY-d10 produced 1-NPY-d9 and, hence, the SCOS97 study provides the opportunity to examine 1-NPY formation at downwind ambient sites.
1-NPY is generally the most abundant nitro-PAH in diesel emissions24,46 and is not formed from the OH or NO3 radical-initiated reactions of gas-phase PY.9,11 Consistent with 1-NPY resulting from direct emissions, the 1-NPY/BeP ratios were similar for all daytime samples and for the evening samples at Los Angeles and Azusa (Figure 5). However, 1-NPY/BeP ratios in the Riverside and Banning nighttime samples were higher relative to the corresponding samples from Los Angeles and Azusa. Increases in the 1-NPY/BeP ratio were observed for 27 of 28 PM samples upon exposure to N2O5 in our chamber system (Table S9) and, as noted, the presence of NO3 radical chemistry is demonstrated in both the Riverside and Banning nighttime samples from ambient 2-NFL/2-NPY ratios (Figure S12). We propose that (assuming negligible differences in the degradation rates of particulate phase 1-NPY and BeP) the elevated 1-NPY/BeP ratios in the Riverside and Azusa nighttime samples, although modest, may have been due to reactions of ambient particle-bound PY with N2O5 to form 1-NPY. However under these ambient conditions, gas-phase reaction of FL with NO3 producing 2-NFL clearly dominates over heterogeneous formation of 1-NPY (note the scale of the y-axes for 2-NFL/BeP vs 1-NPY/BeP in Figure 5) and this is consistent with the dominance of 2-NFL in ambient PM (ref.10,11 and references therein).
Mechanistic Implications
The kinetic dependence of the NO3 radical-initiated reaction of gas-phase naphthalene on NO2 was initially misinterpreted as a gas-phase reaction with N2O5.47 It has now been well established that this reaction involves addition of NO3 to form an NO3-naphthalene adduct which, in competition with back decomposition to reactants, adds NO2 and presumably loses HNO3 to form nitronaphthalenes.9,43,48 This experimental understanding is in agreement with the theoretical work of Ghigo et al.,49 who concluded that the gas-phase reaction of naphthalene is with the NO3 radical and not with N2O5 or NO2 alone. A similar mechanism for the gas-phase reaction of NO3 with FL would have the NO3 adding at the position of highest electron density (the 3-position), followed by addition of NO2 in the ortho position and loss of HNO3 to give 2-NFL. This is consistent with the formation of 2-NFL in high yield from the room temperature, solution-phase reaction of FL with N2O5 in the aprotic solvent, CCl4, while at subambient temperatures or in polar solvents, N2O5 is ionized [NO2+ NO3-] and 3-, 8-, 7- and 1-NFL are then the nitration products.32 A similar radical-initiated reaction producing 2-NFL has also been demonstrated for N2O4 in CCl4.21,22 Therefore, we assume that the heterogeneous reactions observed in ambient PM exposed to N2O5/NO3/NO2 which lead to 3-, 8-, 7- and 1-NFL are not radical-initiated, but rather nitration occurring after adsorption of N2O5. Similarly, since gas-phase reaction of PY with NO3 does not form 1-NPY (4-NPY is formed in very low yield9,11), the heterogeneous reaction of PY exposed to N2O5/NO3/NO2 to give 1-NPY is also unlikely to be NO3 radical-initiated.
Recently NO3 and N2O5 uptake coefficients on PAH surfaces including FL and PY were reported, and while the reactive uptake of NO3 was very fast, that of N2O5 was slow.17 A mechanism was suggested based on gas-phase chemistry of PAHs with NO3, but specific nitro-isomer products were not measured. As noted, the surface-bound deuterated PAH and the PM exposures to N2O5/NO3/NO2 reported here did not result in nitro-FL or nitro-PY radical-initiated products, suggesting that the heterogeneous nitration mechanism must be different from the gas-phase mechanism (see SI for further discussion).
In the present study, the [N2O5]/[NO3] ratios in the chamber experiments exceeded the corresponding ratios in ambient atmospheres due to the high NO2 concentrations employed. Furthermore, the concentrations of NO2 (~1 ppmv) and N2O5 (~0.5 ppmv) are factors of ≥100 higher than observed in ambient atmospheres.50-53 Thus, while the ambient PM was exposed to a NO3 concentration reasonably appropriate for a week-long transport event, the NO2 and N2O5 concentrations far exceed those expected in ambient for the same time period. Hence, our chamber exposures had high potential for heterogeneous N2O5 reactions, and it is also possible that the high concentrations of N2O5 and NO2 used could have blocked NO3 radicals from being accommodated on the surfaces and reacting. However, as noted above, the SCOS97 data are consistent with gas-phase NO3 radical formation of 2-ring nitro-PAHs and 2-NFL and very minor heterogeneous formation of 1-NPY.
Since NO3 is a secondary pollutant (formed from reaction of O3 with NO2) and our results suggest that the PAH in PM quickly become deactivated (or unavailable) toward nitration, heterogeneous formation of nitro-PAH by N2O5/NO3 is not likely to be important, in comparison with gas-phase nitro-PAH formation. Additional uptake and product studies, especially for FL, are needed to resolve the mechanism of heterogeneous nitro-PAH formation.
Supplementary Material
Acknowledgments
R.A., J.A. and K.Z. thank the U.S. Environmental Protection Agency Science to Achieve Results Program (Grant No. RD-83375201) and the University of California Agricultural Experiment Station for financial support. S.L.M.S. thanks the National Science Foundation (ATM-0841165) for funding this research, and the National Institute of Environmental Health Sciences (Grant No. P30ES00210) and the National Institute of Health (Grant P42 ES016465). Its contents are solely the responsibility of the authors and do not necessarily represent the official views and opinions of these agencies. Jill Schrlau is thanked for assistance during sample analysis.
Footnotes
Supporting Information Available
12 Figures, 9 tables, a detailed discussion of analytical techniques, and discussion of NO3 formation under chamber and ambient conditions. This information is available free of charge via the Internet at http://pubs.acs.org.
References Cited
- 1.International Agency for Research on Cancer (IARC) Evaluation of Carcinogenic Risks to Humans: Diesel and Gasoline Engine Exhausts and Some Nitroarenes. Vol. 46. World Health Organization; Lyon: 1989. Monographs in the series. [Google Scholar]
- 2.Durant JL, Busby WF, Jr, Lafleur AL, Penman BW, Crespi CL. Human cell mutagenicity of oxygenated, nitrated and unsubstituted polycyclic aromatic hydrocarbons associated with urban aerosols. Mutat Res. 1996;371:123–157. doi: 10.1016/s0165-1218(96)90103-2. [DOI] [PubMed] [Google Scholar]
- 3.IPCS. International Programme on Chemical Safety. World Health Organization; Geneva, Switzerland: 2003. Environmental Health Criteria 229: Selected Nitro- and Nitro-oxy-polycyclic Aromatic Hydrocarbons; pp. 47–68. [Google Scholar]
- 4.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Cogliano V. Carcinogenicity of polycyclic aromatic hydrocarbons. The Lancet Oncology. 2005;6:931–932. doi: 10.1016/s1470-2045(05)70458-7. [DOI] [PubMed] [Google Scholar]
- 5.Gupta P, Harger WP, Arey J. The contribution of nitro- and methylnitro-naphthalenes to the vapor-phase mutagenicity of ambient air samples. Atmos Environ. 1996;30:3157–3166. [Google Scholar]
- 6.Hannigan MP, Busby WF, Jr, Cass GR. Source contributions to the mutagenicity of urban particulate air pollution. J Air & Waste Manage Assn. 2005;55:399–410. doi: 10.1080/10473289.2005.10464633. [DOI] [PubMed] [Google Scholar]
- 7.Arey J. Atmospheric Reactions of PAHs Including the Formation of Nitroarenes. Chapter 9. In: Neilson AH, editor. The Handbook of Environmental Chemistry Vol.3 Part I, PAHs and Related Compounds. Springer-Berlag; Berlin: 1998. pp. 347–385. [Google Scholar]
- 8.Fan Z, Kamens RM, Zhang J, Hu J. Ozone-nitrogen dioxide-NPAH heterogeneous soot particle reactions and modeling NPAH in the atmosphere. Environ Sci Technol. 1996;30:2821–2827. [Google Scholar]
- 9.Atkinson R, Arey J, Zielinska B, Aschmann SM. Kinetics and nitro-products of the gas-phase OH and NO3 radical-initiated reactions of naphthalene-d8, fluoranthene-d10, and pyrene. Int J Chem Kin. 1990;22:999–1014. [Google Scholar]
- 10.Bamford HA, Baker JE. Nitro-polycyclic aromatic hydrocarbon concentrations and sources in urban and suburban atmospheres of the Mid-Atlantic region. Atmos Environ. 2003;37:2077–2091. [Google Scholar]
- 11.Zimmermann K, Atkinson R, Arey J, Kojima Y, Inazu K. Isomer distributions of molecular weight 247 and 273 nitro-PAHs in ambient samples, NIST diesel SRM, and from radical-initiated chamber reactions. Atmos Environ. 2012;55:431–439. [Google Scholar]
- 12.Baek SO, Field RA, Goldstone ME, Kirk PW, Lester JN, Perry R. A review of atmospheric polycyclic aromatic hydrocarbons: sources, fate, and behavior. Water, Air, & Soil Pollution. 1991;60:279–300. [Google Scholar]
- 13.Primbs T, Simonich S, Schmedding D, Wilson G, Jaffe D, Takami A, Kato S, Hatakeyama S, Kajii Y. Atmospheric outflow of anthropogenic semivolatile organic compounds from East Asia in Spring 2004. Environ Sci Technol. 2007;41:3551–3558. doi: 10.1021/es062256w. [DOI] [PubMed] [Google Scholar]
- 14.Genualdi S, Killin R, Woods J, Wilson G, Schmedding D, Massey Simonich S. Trans-Pacific and regional U.S. atmospheric transport of PAHs and pesticides in biomass burning emissions. Environ Sci Technol. 2009;43:1061–1066. doi: 10.1021/es802163c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Primbs T, Piekarz A, Wilson G, Schmedding D, Higginbotham C, Field J, Massey Simonich S. Influence of Asian and western United States urban areas and fires on the atmospheric transport of polycyclic aromatic hydrocarbons, polychlorinated biphenyls and fluorotelomer alcohols in the western United States. Environ Sci Technol. 2008;42:6385–6391. doi: 10.1021/es702160d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwamena N-OA, Abbatt JPD. Heterogeneous nitration reactions of polycyclic aromatic hydrocarbons and n-hexane soot by exposure to NO3/NO2/N2O5. Atmos Environ. 2008;42:8309–8314. [Google Scholar]
- 17.Gross S, Bertram AK. Reactive uptake of NO3, N2O5, NO2, HNO3, and O3 on three types of polycyclic aromatic hydrocarbon surfaces. J Phys Chem A. 2008;112:3104–3113. doi: 10.1021/jp7107544. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Yang B, Gan J, Liu C, Shu X, Shu J. Nitration of particle-associated PAHs and their derivatives (nitro-, oxy-, and hydroxy-PAHs) with NO3 radicals. Atmos Environ. 2011;45:2515–2521. [Google Scholar]
- 19.Liu C, Zhang P, Yang B, Wang Y, Shu J. Kinetic studies of heterogeneous reactions of polycyclic aromatic hydrocarbon aerosols with NO3 radicals. Environ Sci Technol. 2012;46:7575–7580. doi: 10.1021/es301403d. [DOI] [PubMed] [Google Scholar]
- 20.Ringuet J, Albinet A, Leoz-Garziandia E, Budzinski H, Villenave E. Reactivity of polycyclic aromatic compounds (PAHs, NPAHs, and OPAHs) adsorbed on natural aerosol particles exposed to atmospheric oxidants. Atmos Environ. 201261:15–22. [Google Scholar]
- 21.Squadrito GL, Church DF, Pryor WA. Anomalous nitration of fluoranthene with nitrogen dioxide in carbon tetrachloride. J Am Chem Soc. 1987;109:6535–6537. [Google Scholar]
- 22.Squadrito GL, Fronczek FR, Church DF, Pryor WA. A dichotomy in the nitration of fluoranthene with NO2/N2O4: mechanistic and toxicological implications. J Org Chem. 1990;55:2616–2621. [Google Scholar]
- 23.Reisen F, Arey J. Atmospheric reactions influence seasonal PAH and nitro-PAH concentrations in the Los Angeles Basin. Environ Sci Technol. 200539:64–73. [PubMed] [Google Scholar]
- 24.Bamford HA, Bezabeh DZ, Schantz MM, Wise SA, Baker JE. Determination and comparison of nitrated-polycyclic aromatic hydrocarbons measured in air and diesel particulate reference materials. Chemosphere. 2003;50:575–587. doi: 10.1016/s0045-6535(02)00667-7. [DOI] [PubMed] [Google Scholar]
- 25.Ruehle PH, Bosch LC, Duncan WP. Synthesis of Nitrated Polycyclic Aromatic Hydrocarbons. Chapter 4. In: White CM, editor. Nitrated Polycyclic Aromatic Hydrocarbons. Huethig; Heidelberg: 1985. pp. 169–235. [Google Scholar]
- 26.Pitts JN, Jr, Sweetman JA, Zielinska B, Atkinson R, Winer AM, Harger WP. Formation of nitroarenes from the reaction of polycyclic aromatic hydrocarbons with dinitrogen pentaoxide. Environ Sci Technol. 1985;19:1115–1121. doi: 10.1021/es00141a017. [DOI] [PubMed] [Google Scholar]
- 27.Kamens RM, Guo J, Guo Z, McDow SR. Polynuclear aromatic hydrocarbon degradation by heterogeneous reactions with N2O5 on atmospheric particles. Atmos Environ. 1990;24A:1161–1173. [Google Scholar]
- 28.Atkinson R, Arey J, Dodge MC, Harger WP, McElroy P, Phousongphouang PT. [April, 2013];Yields and Reactions of Intermediate Compounds Formed from the Initial Atmospheric Reactions of Selected VOCs. Contract No 96-306; Final Report to the California Air Resources Board; 2001. May be accessed from ( http://www.arb.ca.gov)
- 29.California Air Resources Board. [Sept. 3, 2012];Historical Air Quality Archives and Latest Ozone, Air Quality Data, Air Quality Data- Today. ( http://www.arb.ca.gov)
- 30.Wang W, Primbs T, Tao S, Massey Simonich SL. Atmospheric particulate matter pollution during the 2008 Beijing Olympics. Environ Sci Technol. 2009;43:5314–5320. doi: 10.1021/es9007504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atkinson R, Baulch DL, Cox RA, Crowley JN, Hampson RF, Hynes RG, Jenkin ME, Rossi MJ, Troe J. Evaluated kinetic and photochemical data for atmospheric chemistry: volume I- gas-phase reactions of Ox, HOx, NOx, and SOx species. Atmos Chem Phys. 2004;1 http://www.atmos-chem-phys.org/acp/4/1461 and http://www.iupac-kinetic.ch.cam.ac.uk/ [Google Scholar]
- 32.Zielinska B, Arey J, Atkinson R, Ramdahl T, Winer AM, Pitts JN., Jr Reaction of dinitrogen pentoxide with fluoranthene. J Am Chem Soc. 1986;108:4126–4132. [Google Scholar]
- 33.Sweetman JA, Zielinska B, Atkinson R, Ramdahl T, Winer AM, Pitts JN., Jr A possible formation pathway for the 2-nitrofluoranthene observed in ambient particulate matter. Atmos Environ. 1986;20:235–238. [Google Scholar]
- 34.Atkinson R, Arey J, Winer AM, Zielinska B, Dinoff TM, Harger WP, McElroy PA. [March 2013];A Survey of Ambient Concentrations of Selected Polycyclic Aromatic Hydrocarbons (PAH) at Various Locations in California. Contract No A5-185-32; Final Report to the California Air Resources Board; 1988. May be accessed from ( http://www.arb.ca.gov)
- 35.Wang W, Jariyasoit N, Schrlau J, Jia Y, Tao S, Yu T, Dashwood R, Zhang W, Wang X, Massey Simonich S. Concentration and photochemistry of PAHs, NPAHs, and OPAHs and toxicity of PM2.5 during the Beijing Olympic Games. Environ Sci Technol. 2011;45:6887–6895. doi: 10.1021/es201443z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butler JD, Crossley P. Reactivity of polycyclic aromatic hydrocarbons adsorbed on soot particles. Atmos Environ. 1981;15:91–94. [Google Scholar]
- 37.Nielsen T. Reactivity of polycyclic aromatic hydrocarbons towards nitrating species. Environ Sci Technol. 1984;18:157–163. doi: 10.1021/es00121a005. [DOI] [PubMed] [Google Scholar]
- 38.Rudich Y, Donahue NM, Mentel TF. Aging of organic aerosol: bridging the gap between laboratory and field studies. Annu Rev Phys Chem. 2007;58:321–352. doi: 10.1146/annurev.physchem.58.032806.104432. [DOI] [PubMed] [Google Scholar]
- 39.Virtanen A, Joutsensaari J, Koop T, Kannosto J, Yli-Pirila P, Leskinen J, Makela JM, Holopainen JK, Poschl U, Kulmala M, Worsnop DR, Laaksonen A. An amorphous solid state of biogenic secondary organic aerosol particles. Nature Letters. 2010;467:824–827. doi: 10.1038/nature09455. [DOI] [PubMed] [Google Scholar]
- 40.Shiraiwa M, Ammann M, Koop T, Poschl U. Gas uptake and chemical aging of semisolid organic aerosol particles. PNAS. 2011;108:11003–11008. doi: 10.1073/pnas.1103045108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou S, Lee AKY, McWhinney RD, Abbatt JPD. Burial effects of organic coatings on the heterogeneous reactivity of particle-borne benzo[a]pyrene (BaP) toward ozone. J Phys Chem A. 2012;116:7050–7056. doi: 10.1021/jp3030705. [DOI] [PubMed] [Google Scholar]
- 42.Zelenyuk A, Imre D, Beranek J, Abramson E, Wilson J, Shrivastava M. Synergy between secondary organic aerosols and long-range transport of polycyclic aromatic hydrocarbons. Environ Sci Technol. 2012;46:12459–12466. doi: 10.1021/es302743z. [DOI] [PubMed] [Google Scholar]
- 43.Atkinson R, Arey J. Mechanisms of the gas-phase reactions of aromatic hydrocarbons and PAHs with OH and NO3 radicals. Polycyclic Aromatic Compounds. 2007;27:15–40. [Google Scholar]
- 44.Wang L, Atkinson R, Arey J. Comparison of alkylnitronaphthalenes formed in NO3 and OH radical-initiated chamber reactions with those observed in ambient air. Environ Sci Technol. 2010;44:2981–2987. doi: 10.1021/es903369c. [DOI] [PubMed] [Google Scholar]
- 45.Zimmermann K, Atkinson R, Arey J. Effect of NO2 concentration on dimethylnitronaphthalene yields and isomer distribution patterns from the gas-phase OH radical-initiated reactions of selected dimethylnaphthalenes. Environ Sci Technol. 2012;46:7535–7542. doi: 10.1021/es3009826. [DOI] [PubMed] [Google Scholar]
- 46.Paputa-Peck MC, Marano RS, Schuetzle D, Riley TL, Hampton CV, Prater TJ, Skewes LM, Jensen TE, Ruehle PH. Determination of nitrated polynuclear aromatic hydrocarbons in particulate extracts by using capillary column gas chromatography with nitrogen selective detection. Anal Chem. 1983;55:1946–1954. [Google Scholar]
- 47.Pitts JN, Jr, Atkinson R, Sweetman JA, Zielinska B. The gas-phase reaction of naphthalene with N2O5 to form nitronaphthalenes. Atmos Environ. 1985;19:701–705. [Google Scholar]
- 48.Atkinson R, Tuazon EC, Arey J. Reactions of naphthalene in N2O5-NO3-NO2-air mixtures. Int J Chem Kinet. 1990;22:1071–1082. [Google Scholar]
- 49.Ghigo G, Causa M, Maranzana A, Tonachini G. Aromatic hydrocarbon nitration under tropospheric and combustion conditions: a theoretical mechanistic study. J Phys Chem A. 2006;110:13270–13282. doi: 10.1021/jp064459c. [DOI] [PubMed] [Google Scholar]
- 50.Marley NA, Gaffney JS, Ramos-Villegas R, Cardenas Gonzalez B. Comparison of measurements of peroxyacyl nitrates and primary carbonaceous aerosol concentrations in Mexico City determined in 1997 and 2003. Atmos Chem Phys. 2007;7:2277–2285. [Google Scholar]
- 51.Parrish DD, Singh HB, Molina L, Madronich S. Air quality progress in North American megacities: a review. Atmos Environ. 2011;45:7015–7025. [Google Scholar]
- 52.Heintz F, Platt U, Flentje H, Dubois R. Long-term observation of nitrate radicals at the Tor Station, Kap Arkona (Rugen) J Geophys Res. 1996;101:22891–22910. [Google Scholar]
- 53.Stutz J, Alicke B, Ackermann R, Geyer A, White A, Williams E. Vertical profiles of NO3, N2O5, O3, and NOx in the nocturnal boundary layer: 1. Observations during the Texas Air Quality Study 2000. J Geophys Res. 2004;109:D12306. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


