Abstract
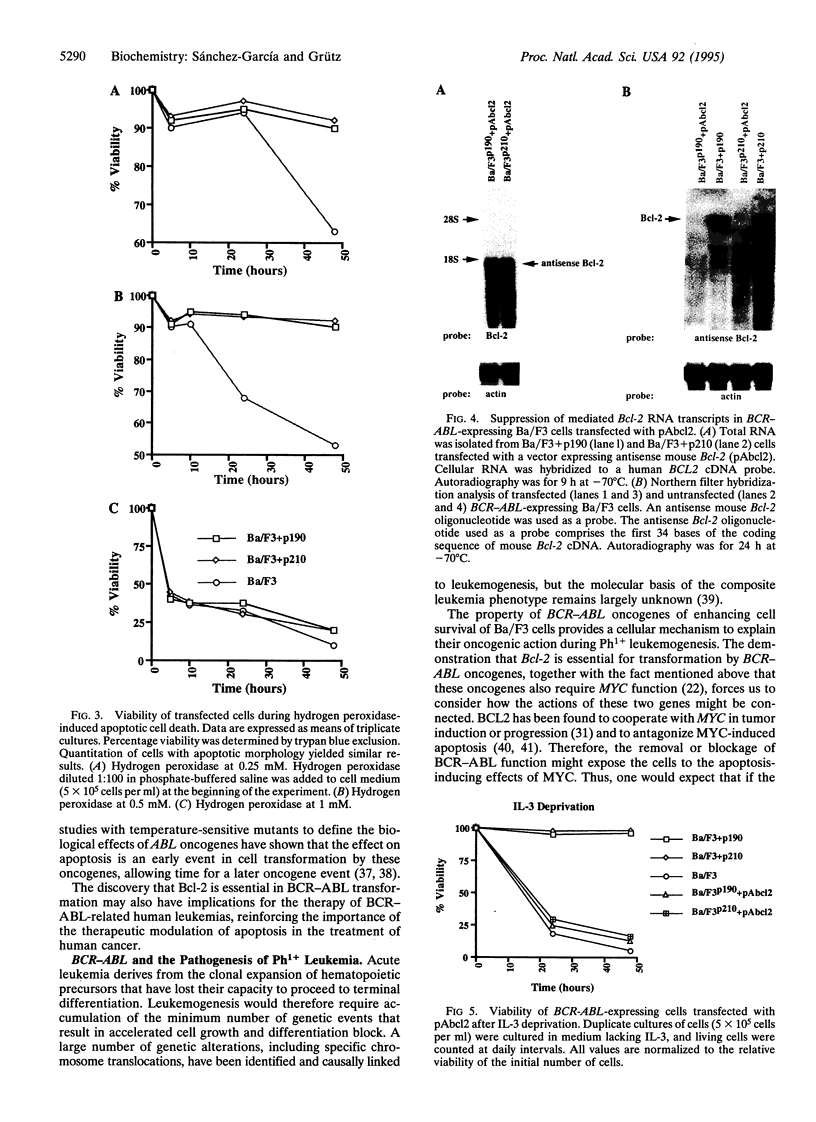
BCR-ABL is a chimeric oncogene generated by translocation of sequences from the c-abl protein-tyrosine kinase gene on chromosome 9 into the BCR gene on chromosome 22. Alternative chimeric proteins, p210BCR-ABL and p190BCR-ABL, are produced that are characteristic of chronic myelogenous leukemia and acute lymphoblastic leukemia, respectively. Their role in the etiology of human leukemia remains to be defined. Transformed murine hematopoietic cells can be used as a model of BCR-ABL function since these cells can be made growth factor independent and tumorigenic by the action of the BCR-ABL oncogene. We show that the BCR-ABL oncogenes prevent apoptotic death in these cells by inducing a Bcl-2 expression pathway. Furthermore, BCR-ABL-expressing cells revert to factor dependence and nontumorigenicity after Bcl-2 expression is suppressed. These results help to explain the ability of BCR-ABL oncogenes to synergize with c-myc in cell transformation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedi A., Zehnbauer B. A., Barber J. P., Sharkis S. J., Jones R. J. Inhibition of apoptosis by BCR-ABL in chronic myeloid leukemia. Blood. 1994 Apr 15;83(8):2038–2044. [PubMed] [Google Scholar]
- Bissonnette R. P., Echeverri F., Mahboubi A., Green D. R. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992 Oct 8;359(6395):552–554. doi: 10.1038/359552a0. [DOI] [PubMed] [Google Scholar]
- Carlesso N., Griffin J. D., Druker B. J. Use of a temperature-sensitive mutant to define the biological effects of the p210BCR-ABL tyrosine kinase on proliferation of a factor-dependent murine myeloid cell line. Oncogene. 1994 Jan;9(1):149–156. [PubMed] [Google Scholar]
- Choo Y., Sánchez-García I., Klug A. In vivo repression by a site-specific DNA-binding protein designed against an oncogenic sequence. Nature. 1994 Dec 15;372(6507):642–645. doi: 10.1038/372642a0. [DOI] [PubMed] [Google Scholar]
- Clark S. S., McLaughlin J., Crist W. M., Champlin R., Witte O. N. Unique forms of the abl tyrosine kinase distinguish Ph1-positive CML from Ph1-positive ALL. Science. 1987 Jan 2;235(4784):85–88. doi: 10.1126/science.3541203. [DOI] [PubMed] [Google Scholar]
- Cleveland J. L., Dean M., Rosenberg N., Wang J. Y., Rapp U. R. Tyrosine kinase oncogenes abrogate interleukin-3 dependence of murine myeloid cells through signaling pathways involving c-myc: conditional regulation of c-myc transcription by temperature-sensitive v-abl. Mol Cell Biol. 1989 Dec;9(12):5685–5695. doi: 10.1128/mcb.9.12.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley G. Q., Baltimore D. Transformation of an interleukin 3-dependent hematopoietic cell line by the chronic myelogenous leukemia-specific P210bcr/abl protein. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9312–9316. doi: 10.1073/pnas.85.23.9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley G. Q., McLaughlin J., Witte O. N., Baltimore D. The CML-specific P210 bcr/abl protein, unlike v-abl, does not transform NIH/3T3 fibroblasts. Science. 1987 Jul 31;237(4814):532–535. doi: 10.1126/science.2440107. [DOI] [PubMed] [Google Scholar]
- Dean M., Cleveland J. L., Rapp U. R., Ihle J. N. Role of myc in the abrogation of IL3 dependence of myeloid FDC-P1 cells. Oncogene Res. 1987 Aug;1(3):279–296. [PubMed] [Google Scholar]
- Delia D., Aiello A., Soligo D., Fontanella E., Melani C., Pezzella F., Pierotti M. A., Della Porta G. bcl-2 proto-oncogene expression in normal and neoplastic human myeloid cells. Blood. 1992 Mar 1;79(5):1291–1298. [PubMed] [Google Scholar]
- Dhut S., Chaplin T., Young B. D. Normal c-abl gene protein--a nuclear component. Oncogene. 1991 Aug;6(8):1459–1464. [PubMed] [Google Scholar]
- Elefanty A. G., Hariharan I. K., Cory S. bcr-abl, the hallmark of chronic myeloid leukaemia in man, induces multiple haemopoietic neoplasms in mice. EMBO J. 1990 Apr;9(4):1069–1078. doi: 10.1002/j.1460-2075.1990.tb08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Fairbairn L. J., Cowling G. J., Reipert B. M., Dexter T. M. Suppression of apoptosis allows differentiation and development of a multipotent hemopoietic cell line in the absence of added growth factors. Cell. 1993 Sep 10;74(5):823–832. doi: 10.1016/0092-8674(93)90462-y. [DOI] [PubMed] [Google Scholar]
- Fanidi A., Harrington E. A., Evan G. I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992 Oct 8;359(6395):554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- Gishizky M. L., Johnson-White J., Witte O. N. Efficient transplantation of BCR-ABL-induced chronic myelogenous leukemia-like syndrome in mice. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3755–3759. doi: 10.1073/pnas.90.8.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisterkamp N., Jenster G., ten Hoeve J., Zovich D., Pattengale P. K., Groffen J. Acute leukaemia in bcr/abl transgenic mice. Nature. 1990 Mar 15;344(6263):251–253. doi: 10.1038/344251a0. [DOI] [PubMed] [Google Scholar]
- Hermans A., Heisterkamp N., von Linden M., van Baal S., Meijer D., van der Plas D., Wiedemann L. M., Groffen J., Bootsma D., Grosveld G. Unique fusion of bcr and c-abl genes in Philadelphia chromosome positive acute lymphoblastic leukemia. Cell. 1987 Oct 9;51(1):33–40. doi: 10.1016/0092-8674(87)90007-9. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Kelliher M. A., McLaughlin J., Witte O. N., Rosenberg N. Induction of a chronic myelogenous leukemia-like syndrome in mice with v-abl and BCR/ABL. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6649–6653. doi: 10.1073/pnas.87.17.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H. P., Golde D. W. Chronic myelogenous leukemia--new concepts (first of two parts). N Engl J Med. 1981 May 14;304(20):1201–1209. doi: 10.1056/NEJM198105143042004. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Lugo T. G., Witte O. N. The BCR-ABL oncogene transforms Rat-1 cells and cooperates with v-myc. Mol Cell Biol. 1989 Mar;9(3):1263–1270. doi: 10.1128/mcb.9.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell T. J., Korsmeyer S. J. Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14; 18). Nature. 1991 Jan 17;349(6306):254–256. doi: 10.1038/349254a0. [DOI] [PubMed] [Google Scholar]
- McGahon A., Bissonnette R., Schmitt M., Cotter K. M., Green D. R., Cotter T. G. BCR-ABL maintains resistance of chronic myelogenous leukemia cells to apoptotic cell death. Blood. 1994 Mar 1;83(5):1179–1187. [PubMed] [Google Scholar]
- McLaughlin J., Chianese E., Witte O. N. Alternative forms of the BCR-ABL oncogene have quantitatively different potencies for stimulation of immature lymphoid cells. Mol Cell Biol. 1989 May;9(5):1866–1874. doi: 10.1128/mcb.9.5.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhirter J. R., Wang J. Y. Activation of tyrosinase kinase and microfilament-binding functions of c-abl by bcr sequences in bcr/abl fusion proteins. Mol Cell Biol. 1991 Mar;11(3):1553–1565. doi: 10.1128/mcb.11.3.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Mizushima S., Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990 Sep 11;18(17):5322–5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez G., London L., Hockenbery D., Alexander M., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990 May 1;144(9):3602–3610. [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Tarduchy G., Collins M., López-Rivas A. Regulation of apoptosis in interleukin-3-dependent hemopoietic cells by interleukin-3 and calcium ionophores. EMBO J. 1990 Sep;9(9):2997–3002. doi: 10.1002/j.1460-2075.1990.tb07492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyers C. L., Callahan W., Witte O. N. Dominant negative MYC blocks transformation by ABL oncogenes. Cell. 1992 Sep 18;70(6):901–910. doi: 10.1016/0092-8674(92)90241-4. [DOI] [PubMed] [Google Scholar]
- Shi Y., Glynn J. M., Guilbert L. J., Cotter T. G., Bissonnette R. P., Green D. R. Role for c-myc in activation-induced apoptotic cell death in T cell hybridomas. Science. 1992 Jul 10;257(5067):212–214. doi: 10.1126/science.1378649. [DOI] [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Spooncer E., Fairbairn L., Cowling G. J., Dexter T. M., Whetton A. D., Owen-Lynch P. J. Biological consequences of p160v-abl protein tyrosine kinase activity in a primitive, multipotent haemopoietic cell line. Leukemia. 1994 Apr;8(4):620–630. [PubMed] [Google Scholar]
- Sánchez-García I., Rabbitts T. H. LIM domain proteins in leukaemia and development. Semin Cancer Biol. 1993 Dec;4(6):349–358. [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Williams G. T., Smith C. A., Spooncer E., Dexter T. M., Taylor D. R. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature. 1990 Jan 4;343(6253):76–79. doi: 10.1038/343076a0. [DOI] [PubMed] [Google Scholar]