Abstract
Background. The mechanisms by which α-thalassemia and sickle cell traits confer protection from severe Plasmodium falciparum malaria are not yet fully elucidated. We hypothesized that hemoglobinopathic erythrocytes reduce the intraerythrocytic multiplication of P. falciparum, potentially delaying the development of life-threatening parasite densities until parasite clearing immunity is achieved.
Methods. We developed a novel in vitro assay to quantify the number of merozoites released from an individual schizont, termed the “intraerythrocytic multiplication factor” (IMF).
Results. P. falciparum (3D7 line) schizonts produce variable numbers of merozoites in all erythrocyte types tested, with median IMFs of 27, 27, 29, 23, and 23 in control, HbAS, HbSS, and α- and β-thalassemia trait erythrocytes, respectively. IMF correlated strongly (r2 = 0.97; P < .001) with mean corpuscular hemoglobin concentration, and varied significantly with mean corpuscular volume and hemoglobin content. Reduction of IMFs in thalassemia trait erythrocytes was confirmed using clinical parasite isolates with different IMFs. Mathematical modeling of the effect of IMF on malaria progression indicates that the lower IMF in thalassemia trait erythrocytes limits parasite density and anemia severity over the first 2 weeks of parasite replication.
Conclusions. P. falciparum IMF, a parasite heritable virulence trait, correlates with erythrocyte indices and is reduced in thalassemia trait erythrocytes. Parasite IMF should be examined in other low-indices erythrocytes.
Keywords: hemoglobinopathy, malaria, Plasmodium falciparum, sickle hemoglobin, thalassemia
The hemoglobinopathies comprise a group of heritable disorders that can be clinically benign or malignant. Haldane's “malaria hypothesis,” that β-thalassemia trait protects against severe Plasmodium falciparum malaria [1], has been extended to hemoglobin (Hb) S heterozygosity (HbAS), α-thalassemia trait, and other hemoglobinopathies that are prevalent in malaria endemic areas. Epidemiological data indicate that HbAS and α-thalassemia trait protect against life-threatening P. falciparum malaria syndromes (reviewed in [2]). Cellular and molecular mechanisms of this protection have not been fully elucidated (reviewed in [3, 4]) but likely include impaired cytoadherence of parasitized erythrocytes to microvascular endothelial cells and uninfected erythrocytes, two interactions associated with the development of severe disease [5–7]. Weakened host-pathogen interactions may minimize microvascular inflammation and associated pathologies (reviewed in [8]). Additional mechanisms of protection may limit parasite densities in individuals with sickle cell trait (HbAS) and disease (HbSS): restricted parasite development under low O2 tension [9], enhanced removal of parasitized erythrocytes in the spleen [10], impaired parasite egress from dehydrated erythrocytes [11], and inhibition of parasite replication by human erythrocyte miRNAs [12]. While only some of these specific mechanisms have been documented for α- or β-thalassemia traits, restriction of parasite growth in thalassemia trait erythrocytes has been observed (reviewed in [4]).
The association of severe P. falciparum malaria with high parasite densities [13] led us to hypothesize that hemoglobinopathic erythrocytes may protect against severe malaria by producing fewer merozoites per schizont than normal erythrocytes, thereby slowing parasite replication. While this mechanism has previously been considered, few studies have addressed the multiplication of P. falciparum within individual erythrocytes in vitro [14–17]. Previous approaches in these studies involved counting merozoites in 2-dimensional images of schizonts, which has the potential to underestimate merozoite number. We developed a novel in vitro method to accurately count the number of merozoites released from an individual schizont, which we term the “intraerythrocytic multiplication factor” (IMF). Here we investigate the effect of HbAS, HbSS, and α- and β-thalassemia trait erythrocytes on the IMF of a laboratory-adapted line and 2 clinical isolates of P. falciparum in vitro. We show that all 3 parasite strains produce a significantly lower number of merozoites per cycle in thalassemia trait erythrocytes than in control erythrocytes, and that the IMF correlates significantly with mean corpuscular Hb concentration (MCHC), mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH) in all cell types. To explore how this reduction in IMF, in the presence of only the innate immune system, might affect the development of severe malaria, we used a mathematical model of host-parasite interaction. The model was used to predict increases in parasite densities and anemia over 14 days of parasite multiplication in vivo, after which the adaptive immune response presumably comes into play.
METHODS
P. falciparum Strains and Human Erythrocytes
The laboratory-adapted P. falciparum 3D7 line was obtained from MR4 (Manassas, VA). Two clinical P. falciparum isolates from Mali (KN1068-4) and Cambodia (CP803) were obtained from patients with uncomplicated malaria and adapted to in vitro culture for 3 weeks prior to use. All parasite strains were cultured in human erythrocytes in Roswell Park Memorial Institute 1640 medium (Invitrogen) supplemented with 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Invitrogen), 4.5 mg/mL glucose (Sigma), 0.1 mM hypoxanthine (Invitrogen), 25 µg/mL gentamicin (Invitrogen), and 0.5% Albumax II (Invitrogen). Freshly drawn erythrocytes (refrigerated <72 hours) were used to initiate synchronized experimental cultures, which were maintained at low parasite densities and hematocrit. Control erythrocytes were obtained from healthy National Institutes of Health Blood Bank donors with normal hematological parameters. Hemoglobinopathic erythrocytes were obtained under protocols approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (see Supplementary Table 1 for hematological data). HbS subjects did not have microcytosis or thalassemia; α-globin gene deletions were verified in α-thalassemia trait subjects; β-thalassemia trait was identified by microcytosis with elevated HbA2 levels; none of the subjects had iron deficiency. Because minor glucose-6-phosphate dehydrogenase deficiency in Subject 1 (Supplementary Table 1) did not lead to erythrocyte hemolysis in vitro, the subject's erythrocytes were used in experiments.
Quantifying the IMF of P. falciparum Strains
Parasite egress experiments used highly synchronized cultures of P. falciparum obtained by the following procedures: enrichment of schizonts from asynchronous stock cultures grown in control erythrocytes using 65% Percoll [18, 19]; coincubation of schizonts for 2 hours with freshly drawn control or hemoglobinopathic erythrocytes at 37°C to initiate a new cycle of parasite replication; elimination of residual schizonts by lysis in sorbitol; culture of parasites from the early-ring to the late-schizont stage; relocation of cultures (0.1%–0.2% hematocrit) into the special environmental chambers for microscopy (HybriWell HBW20, Grace Bio-Labs, Bend, OR) for 0.5–1 hour at 37°C to accumulate “sites of parasite egress.” These chambers preserve egress sites, composed of scattered merozoites released from individual schizonts [11]. Here we used this finding to quantify for the first time the number of merozoites produced per schizont (ie, IMF). Merozoites can be accurately counted in images of egress sites formed in chambers filled with cells suspended in 0.5% Albumax, but not in 10% human serum–containing medium, as the latter prevents stable adherence of parasites to the glass and thus impairs egress site formation. Images of egress sites were recorded by laser scanning microscope LSM 510 (Carl Zeiss AG, Oberkochen, Germany) with a 63× oil objective, 1.4 numerical aperture, and 488-nm laser.
Egress sites with 2 or more food vacuoles, indicating the presence of a multiply infected erythrocyte, or sites with clustered merozoites were excluded from analysis. We did not notice differences in the number of multiply infected erythrocytes or clustered merozoites in control, thalassemia trait, and HbAS samples, but we did observe an accumulation of merozoite clusters in HbSS cultures, as reported earlier [11]. Cell suspensions were diluted to avoid site overlap, but if overlap occurred, sites were ignored. The stability of merozoite numbers at egress sites was verified by following 10 randomly selected schizonts over 1 hour after merozoite egress. We noticed, however, that merozoites began deteriorating 1 hour after egress and thus could be missed by microscopy that uses objectives of low magnification and resolution. To minimize this artifact, prompt acquisition of images was performed at high magnification and light gathering capacity to operate at the theoretical limit of resolution for light microscopy. Most experiments using hemoglobinopathic erythrocytes were performed in parallel with control erythrocytes; additional experiments with control erythrocytes were performed to assess the range of 3D7 IMF in multiple donors. Pooled IMF data for blood type/parasite strain were used in final analyses. Two investigators independently analyzed the images for IMF datasets.
Statistical Analysis
The Kolmogorov–Smirnov test was implemented using a C++ coding of the algorithm given in [20]. All other statistical tests on experimental data were performed using SigmaPlot 8.02 (IBM, Armonk, NY) or Mathematica 8.0.1.0 software (Wolfram Research, Champaign, IL).
Modeling of P. falciparum Replication
To compare the population growth of P. falciparum in control and thalassemia trait erythrocytes, we used mathematical analysis based on models for within-host parasite growth [21–23]. The mathematical formulation is described in Supplementary Methods. In brief, the model assumes that merozoites trigger a quickly acting, quickly decaying innate immune response in humans, which models the pyrogenic response typical for an acute malaria episode [24]. The trigger occurs when parasite densities reach a threshold Th, at which point activated factors kill the intracellular parasites at a rate χ, which varies in time and quickly reaches a maximum rate χMax. The killing factor χ then decays at a rate of 0.5 per hour, tending to zero until the next buildup of parasites. Parameters Th and χMax vary from simulation to simulation, within the ranges of their values that emulate the time series of parasite densities seen in neurosyphilis patients infected with P. falciparum for malariotherapy [25]. Aiming to assess direct effects of differing IMF values on the growth of parasite densities, we assumed that both merozoite invasion of erythrocytes and immune responses are unaffected by thalassemia traits. In real P. falciparum infections, anemia is due to the destruction of parasitized erythrocytes and dyserythropoiesis [26], so the model incorporates a variable factor δ to counteract erythropoiesis. For simplicity, we ignored the destruction of uninfected erythrocytes by host immune responses, although we expect this would amplify the rate of erythrocyte loss in real infections [27]. Based on results shown below, we selected 2 IMF values, 26 and 22 (typical for control and thalassemia trait erythrocytes, respectively), and simulated the outcomes of 10 000 infections for each of them. We assume that merozoites produced in both normal and thalassemia trait erythrocytes have the same viability, and that approximately 94% of released merozoites initiate a new cycle of replication. The latter assumption is supported by a study of volunteers inoculated with P. falciparum strain 3D7. In more than 70% of tested individuals, the calculated multiplication factor was above 24 [27]. Simulated infections began with a “primary release” from the liver of 104 merozoites into 5 × 106 µL of blood. A Latin hypercube procedure was used to sample the model parameter space evenly [28].
RESULTS
P. falciparum Schizonts Produce Variable Numbers of Merozoites in Control Erythrocytes
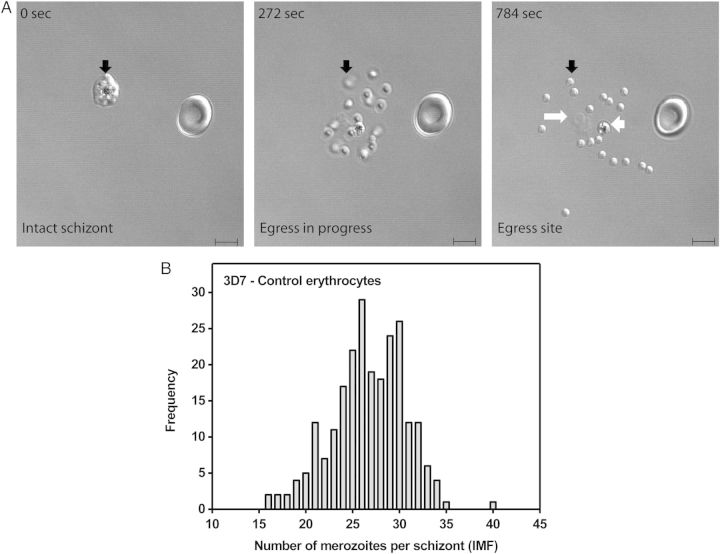
To calculate the IMF of P. falciparum parasites, we employed live cell microscopy of schizonts that have completed their life cycle by releasing merozoites in sealed environmental chambers. The glass surface of these chambers preserves a unique footprint of the egress site (Figure 1A), composed of released merozoites, a food vacuole, and membrane fragments [11, 29, 30]. Formed sites of egress were imaged and the total number of merozoites released from a schizont-infected erythrocyte (ie, IMF) was accurately counted (Figure 1). Only egress sites originating from a singly infected erythrocyte were analyzed to exclude potential effects of multiple infection of a single erythrocyte on the number of de novo produced merozoites in individual schizonts. The number of merozoites at an egress site thus represents a biological feature of the corresponding schizont/erythrocyte unit. To characterize this feature, we initially measured IMFs for a widely used, laboratory adapted P. falciparum line (3D7) in control erythrocytes under standard culture conditions. As reported for other parasite strains [15, 17], 3D7 schizonts produce variable numbers of merozoites in erythrocytes (Figure 1B, Table 1). Specifically, the non-Gaussian frequency distribution of 3D7 IMF contains all values between 16 and 35, ranges from 16 to 40, and has mean (± standard error of the mean) and median (interquartile range) IMF values of 26.68 (± 0.26) and 27 (24–30), respectively.
Figure 1.
P. falciparum 3D7 schizonts produce variable numbers of merozoites in control erythrocytes. A, Formation of a merozoite egress site by a 3D7 schizont infected control erythrocyte. Selected frames from a time-lapse recording of the egress process are shown. The 0-second frame shows an uninfected erythrocyte and an intact segmenting schizont (black arrow) containing multiple merozoites, which cannot be accurately counted. The 272-second frame shows merozoites in motion shortly after egress. Those that have not yet settled onto the glass surface are out of focus (black arrow). The 784-second frame shows a fully formed egress site littered with a single food vacuole (short white arrow) containing hemozoin, erythrocyte membrane fragments (long white arrow), and 18 merozoites (short black arrow), which have scattered and settled onto the glass surface. The “intraerythrocytic multiplication factor” (IMF) of this particular schizont is 18. DIC microscopy, scale bar = 5 µm. B, Non-Gaussian frequency distribution of 3D7 IMFs for 237 singly infected control erythrocytes. Data are derived from 17 independent experiments using erythrocytes from 13 healthy donors.
Table 1.
Comparison of Intraerythrocytic Multiplication Factor (IMF) Values for Different Plasmodium falciparum Strains in Control and Hemoglobinopathic Erythrocytes
| Parasite | Hb Type | IMF |
Donors, n | Egress Sites, n | P Value | |||
|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | Median | IQR | Range | |||||
| 3D7 | Control | 26.68 ± 0.26 | 27 | 24–30 | 16–40 | 13 | 237 | |
| 3D7 | AS | 27.08 ± 0.25 | 27 | 25–30 | 11–41 | 3 | 273 | .156 |
| 3D7 | SS | 27.95 ± 0.49 | 29 | 25–31 | 15–40 | 2 | 84 | .001 |
| 3D7 | α-thal traita | 23.19 ± 0.54 | 23 | 19–27 | 12–32 | 3 | 75 | <.001 |
| 3D7 | β-thal trait | 23.24 ± 0.57 | 23 | 20–26 | 15–34 | 3 | 59 | <.001 |
| KN1068–4 | Control | 24.56 ± 0.39 | 25 | 22–27.5 | 12–34 | 2 | 108 | |
| KN1068–4 | α-thal trait | 22.40 ± 0.37 | 22 | 20–24 | 14–34 | 1 | 101 | <.001 |
| CP803 | Control | 15.75 ± 0.19 | 15 | 14–17 | 7–28 | 5 | 322 | |
| CP803 | β-thal trait | 12.48 ± 0.16 | 12 | 11–14 | 5–19 | 1 | 235 | <.001 |
P values were calculated using the Kolmogorov–Smirnov test (comparisons made to control samples).
Abbreviations: 3D7, a laboratory adapted, clonal P. falciparum line; CP803, a short-term adapted, clonal P. falciparum isolate obtained from a Cambodian adult with malaria; Hb, hemoglobin; IQR, interquartile range; KN1068-4, a short-term adapted P. falciparum isolate obtained from a Malian child with malaria; thal, thalassemia.
a See Supplementary Table 1 for the molecular background of individual donors.
IMF is a P. falciparum Strain-Dependent Phenotype That is Affected by Hemoglobinopathic Erythrocytes
To test the hypothesis that erythrocyte phenotype affects IMF, we measured 3D7 IMF in α-thalassemia trait, β-thalassemia trait, HbAS, and HbSS erythrocytes, which differ from control erythrocytes in various biochemical and physical parameters [31, 32]. 3D7 IMF distributions in α-thalassemia trait, β-thalassemia trait, and HbSS (but not in HbAS) erythrocytes differ significantly from those in control erythrocytes (Table 1, Figure 2). On average, 3D7 schizonts produce 4 fewer merozoites in both α- and β-thalassemia trait erythrocytes than in control erythrocytes (median IMF, 23 for α- and β-thalassemia trait vs 27 for control; P < .001, IMF range 12–32 in α-thalassemia trait erythrocytes and 15–34 in β-thalassemia trait erythrocytes) (Table 1, Figure 2A). On average, 3D7 schizonts produce 2 more merozoites in HbSS than in control erythrocytes (median IMF, 29 vs 27; P = .001) (Table 1, Figure 2B). There is no statistically significant difference between 3D7 IMF in HbAS and control erythrocytes (Table 1, Figure 2B). These data indicate that schizonts from a single parasite line can produce 11–41 merozoites, depending on erythrocyte phenotype. The widths of IMF distributions for all erythrocyte types are >18, suggesting that erythrocyte properties contribute to the cell-to-cell variation in IMF values.
Figure 2.
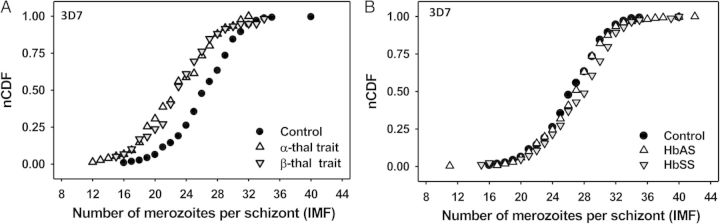
Hemoglobin type affects the intraerythrocytic multiplication factor (IMF) of the P. falciparum 3D7 line. Normalized cumulative distribution function (nCDF) plots of IMF values for 3D7 in normal (control) and hemoglobinopathic (α-thalassemia trait, β-thalassemia trait, HbAS, and HbSS) erythrocytes are shown. Lower and higher IMFs shift the curve to the left and right, respectively. Median IMFs of schizont populations correspond to nCDF values = 0.50. A, Distributions show that 3D7 schizonts produce fewer merozoites in both α- and β-thalassemia trait erythrocytes than in control erythrocytes. B, Distributions show that 3D7 schizonts produce more merozoites in both HbAS and HbSS erythrocytes than in control erythrocytes. Abbreviations: HbAS, hemoglobin A and S heterozygosity (individuals with sickle cell trait); HbSS, hemoglobin S homozygosity (individuals with sickle cell disease); thal, thalassemia.
To confirm that thalassemia trait erythrocytes reduce IMF, and to rule out idiosyncratic effects of extensive 3D7 parasite cultivation, we repeated the experiments using 2 clinical parasite isolates from patients with P. falciparum malaria (CP803 from Cambodia, KN1068-4 from Mali) and adapted to in vitro culture for only 3 weeks. While CP803 was identified by microsatellite analysis to be clonal [33], the clonality of KN1068-4 is not known. These 2 isolates have very different IMFs in control erythrocytes (median IMF, 25 for KN1068-4 vs 15 for CP803; P < .001) (Table 1, Figure 3). However, both parasite isolates produce fewer merozoites in thalassemia trait than in control erythrocytes (Table 1, Figure 3). KN1068-4 schizonts produce 3 fewer merozoites on average in α-thalassemia trait than in control erythrocytes (median IMF, 22 vs 25; P < .001) and CP803 schizonts produce 3 fewer merozoites on average in β-thalassemia trait than in control erythrocytes (median IMF, 12 vs 15; P < .001) (Table 1). Together, these results suggest that IMF is a parasite-heritable phenotype that can be modified by thalassemia trait erythrocytes.
Figure 3.
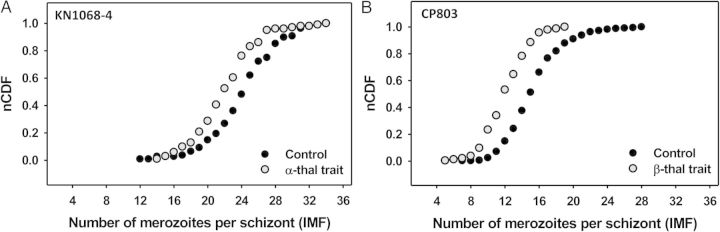
Hemoglobin type affects the intraerythrocytic multiplication factor (IMF) of clinical P. falciparum isolates. Normalized cumulative distribution function (nCDF) plots of IMF values for 2 clinical parasite isolates in normal (control) and hemoglobinopathic (α- and β-thalassemia trait) erythrocytes are shown. Lower and higher IMFs shift the curve to the left and right, respectively. Median IMFs of schizont populations correspond to nCDF values = 0.50. A, Distributions show that the parasite isolate KN1068-4 from Mali produces fewer merozoites per schizont in α-thalassemia trait erythrocytes. B, Distributions show that the parasite isolate CP803 from Cambodia produces fewer merozoites per schizont in β-thalassemia trait erythrocytes. Note that the mean IMF of CP803 is significantly lower than the mean IMFs of KN1068-4 and the P. falciparum 3D7 line. Abbreviation: thal, thalassemia.
P. falciparum IMF Strongly Correlates With MCHC
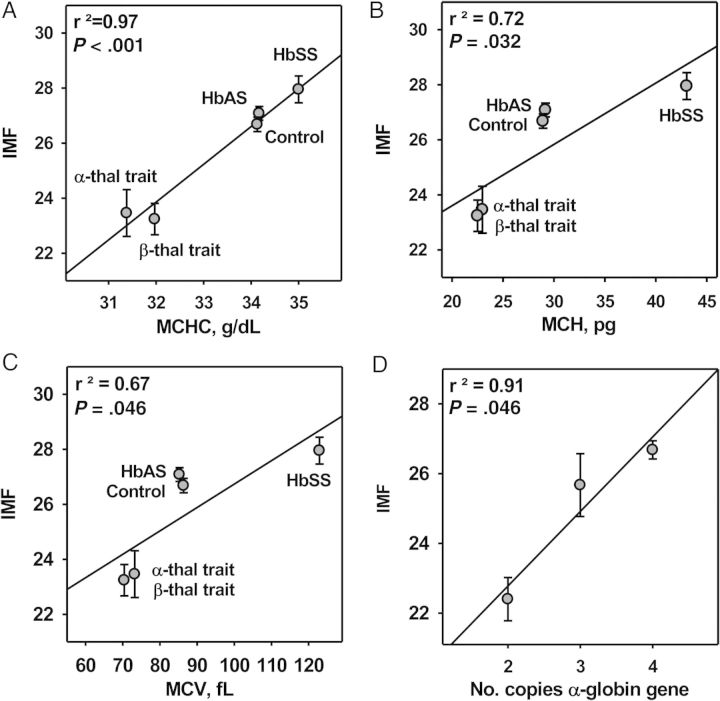
To identify erythrocyte properties that affect IMF, we performed linear regression analysis between the values of erythrocyte indices and the corresponding mean IMFs for the P. falciparum 3D7 line. IMF correlates positively with each of 3 erythrocyte properties: MCHC (r2 = 0.97; P < .001), MCH (r2 = 0.72; P = .032), and MCV (r2 = 0.67; P = .046) (Figure 4A–C). Thus, P. falciparum schizonts produce more merozoites in large, dense erythrocytes than in small, less dense erythrocytes. Indeed, HbSS erythrocytes have both the highest MCHC and IMF values (Figure 4A). Mean IMF values also correlated strongly (r2 = 0.91; P = .046) with the number of functional α-globin genes (ie, 2, 3, or 4) (Figure 4D). Together, these data indicate that the intraerythrocytic Hb concentration is a significant determinant of IMF.
Figure 4.
The intraerythrocytic multiplication factor (IMF) correlates strongly with erythrocyte indices. A, Linear regression analysis showing a strong positive correlation between mean IMFs for the P. falciparum 3D7 line and mean MCHCs of control and hemoglobinopathic erythrocytes. B, Linear regression analysis showing a positive correlation between mean IMFs for the P. falciparum 3D7 line and mean MCHs of control and hemoglobinopathic erythrocytes. C, Linear regression analysis showing a positive correlation between mean IMFs for the P. falciparum 3D7 line and mean MCVs of control and hemoglobinopathic erythrocytes. D, Linear regression analysis showing a strong correlation between mean IMFs for the P. falciparum 3D7 line and the number of functional α-globin genes in normal and α-thalassemia trait erythrocytes. Abbreviations: HbAS, hemoglobin A and S heterozygosity (individuals with sickle cell trait); HbSS, hemoglobin S homozygosity (individuals with sickle cell disease); MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; thal, thalassemia.
Mathematical Modeling Suggests That Lower IMF in Thalassemia Trait Than in Normal Erythrocytes Benefits the Human Host During the Early Phase of Parasite Replication In Vivo
We explored how relatively small differences in P. falciparum IMF between normal (IMF approximately 26) and thalassemia-trait (IMF approximately 22) erythrocytes may affect the replication of blood-stage parasites in vivo over the first 2 weeks of blood infection, holding constant merozoite infectivity to isolate the effect of IMF. Our model presumes that these parasites induce a rapidly acting, innate immune response in naive hosts during the first 2 weeks of blood infection, before an adaptive immune response develops. In this model, host protection is determined by 2 variables: Th, the threshold parasite density that must be exceeded to trigger the innate immune response, and χMax, the maximum rate of parasite killing by this mechanism.
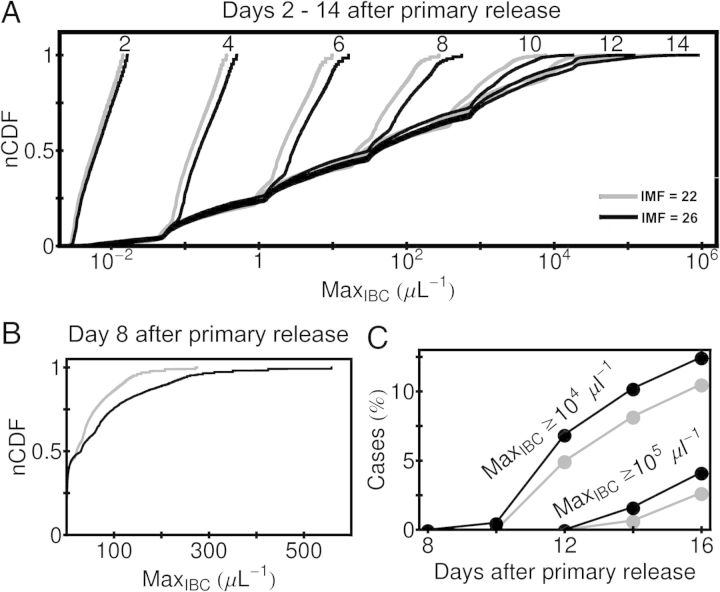
Using simulated infections, we estimated 2 clinically relevant outcomes: parasite densities and anemia. Figure 5A shows the normalized cumulative distribution frequencies of the maximum parasite densities achieved after 7 cycles of parasite replication (14 days). During the first 8 days, the median parasite densities achieved in “IMF = 22” cases is consistently lower than in “IMF = 26” cases; in some simulated infections (Figure 5B), the maximum parasite densities achieved in IMF = 22 cases is half that of IMF = 26 cases. Beyond 8 days of infection, the mean values of IMF = 22 and IMF = 26 distributions are similar (Figure 5A). Nonetheless, after 12 days, the fraction of simulations with parasite density >105/µL and >104/µL are consistently higher for IMF = 26 than for IMF = 22 cases (Figure 5C). These results were obtained by assuming that approximately 94% of released merozoites initiate the next erythrocyte cycle of replication. However, even with 50% of reinvasion success (see outlier in [27]), the main qualitative change in our model would be that the innate response is triggered 1 cycle later for larger values of Th.
Figure 5.
Predicted effect of intraerythrocytic multiplication factor (IMF) on parasite density in early simulated infections. A, Normalized cumulative distribution function (nCDF) plots of the maximum infected RBC (MaxIBC) observed for simulations up to 14 days after the beginning of asexual parasite replication (ie, after primary release from the liver). Simulated infections with IMF = 22 and IMF = 26 parasites are compared. Note that MaxIBC is plotted on a logarithmic scale. B, The cumulative distribution for MaxIBC at day 8 after primary release, with MaxIBC plotted on a linear scale. C, The fraction of simulations for which MaxIBC exceeds 104/µL and 105/µL at 8, 10, 12, 14, and 16 days after primary release. The same gray-scale code for IMF is used for all panels. Abbreviation: RBC, red blood cell.
The higher parasite densities achieved in IMF = 26 cases, however, may benefit individuals by enabling earlier malaria diagnosis than in IMF = 22 cases. In the parameter space of our model, over 50% of the IMF = 26 cases have a parasite density exceeding 10/µL of whole blood (the visual threshold for accurate microscopic diagnosis) within 6 days after initial erythrocyte infection, but none of the IMF = 22 cases did during that period (Supplementary Figure 1). This diagnostic benefit for IMF = 26 cases will disappear by day 10 when the proportions of detectable cases are nearly identical for the 2 values of IMF (Supplementary Figure 1). Thus, IMF = 22 cases will experience slower parasite density increases than IMF = 26 cases, but delayed malaria diagnosis of about 4 days.
Because high parasite densities are associated with severe malarial anemia [26], our modeling suggests that lower IMF in thalassemia trait carriers might benefit patients by reducing the incidence of this life-threatening manifestation. Specifically, the fraction of simulated infections in which uninfected erythrocyte density falls by either 1% (5 × 104/μL) or 5% (2.5 × 105/μL) from basal erythrocyte density is smaller in IMF = 22 than in IMF = 26 cases (Figure 6A). The area of immune-parameter space in which the uninfected erythrocyte density falls by 5% after 2 weeks of infection is more extensive in IMF = 26 than in IMF = 22 cases (Figure 6B).
Figure 6.
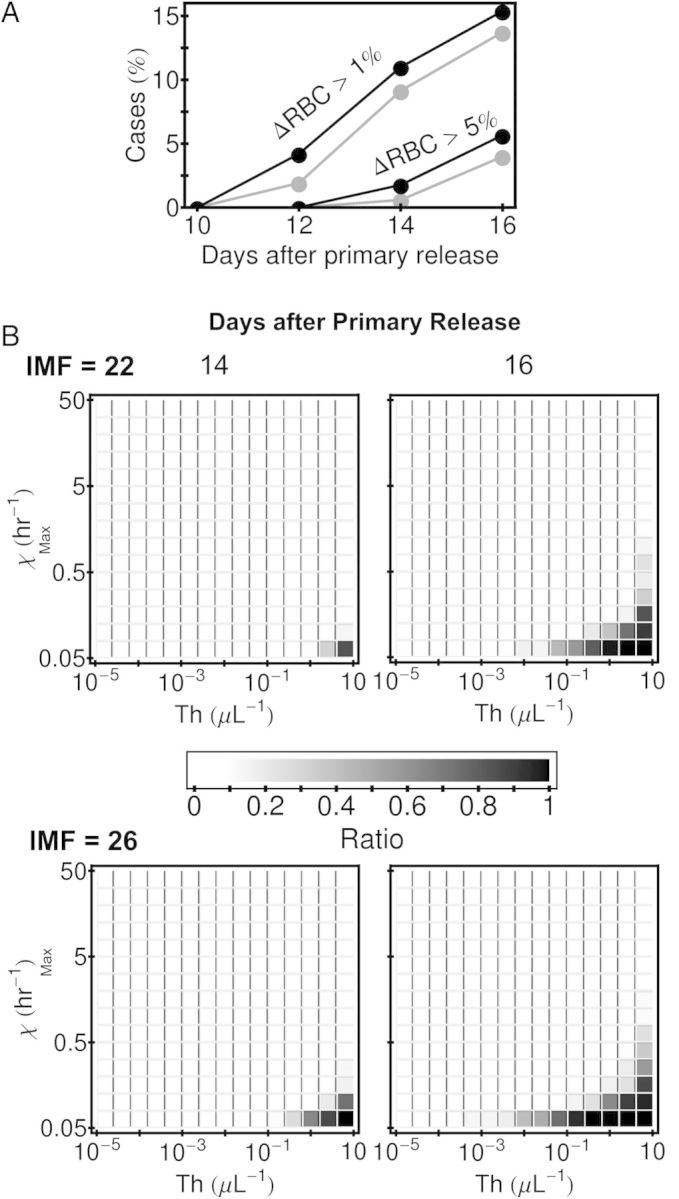
Predicted effect of intraerythrocytic multiplication factor (IMF) on the degree of anemia in early simulated infections. A, The fraction of simulations for which ΔRBC (the difference between basal RBC count, 5 × 106/µL, prior to infection and the RBC count during infection) exceeds 5 × 104/µL (1% of the basal RBC count) and 2.5 × 105/µL (5% of the basal RBC count) at 10, 12, 14, and 16 days after the beginning of asexual parasite replication (ie, after primary release from the liver). The gray-scale code for IMF is used here as in Figure 5A. B, The fraction of simulations for which ΔRBC exceeds 2.5 × 105/μL in different parts of the parameter space of the model immune response at 14 and 16 days after primary release. The 10 000 simulations done for each IMF are binned by their values of threshold density of merozoites that triggers the response, Th, and maximum killing rate of infected RBCs, χMax. The horizontal extent of the blocks shows the bin size Th, and the vertical extent shows the bin size χMax. Note that 2.5 × 104/µL is 5% of the basal RBC count. Abbreviation: RBC, red blood cell.
DISCUSSION
Here we have explored the intraerythrocytic multiplication characteristics of the human malaria parasite P. falciparum within hemoglobinopathic erythrocytes in vitro to test the hypothesis that hemoglobinopathies affect parasite multiplication in vivo. We used a novel assay that accurately quantifies the number of merozoites released from individual schizont-infected erythrocytes, the “intraerythrocytic multiplication factor” (IMF). The median IMF of the laboratory adapted P. falciparum 3D7 line assessed in control erythrocytes conforms well with the initial 3D7 parasite growth rates in the blood of volunteers [27]. We found that mean IMFs are higher in HbSS, equal in HbAS, and lower in α- and β-thalassemia trait erythrocytes, than in control erythrocytes. Similar IMF reductions in thalassemia trait erythrocytes were also seen for 2 clinical parasite isolates.
Because HbAS and HbSS erythrocytes are associated with equal or higher IMF values, this parameter is unlikely to account for the lower parasite densities found in HbAS and HbSS African children or the malaria protective effects of HbAS (reviewed in [2]). Other protective mechanisms, such as impaired cytoadherence of parasitized erythrocytes to microvascular endothelial cells [5–7], early removal of infected cells from the circulation [10], and defective parasite egress from dehydrated sickle cell erythrocytes [11] may negate the virulent effects of elevated IMF in HbAS and HbSS erythrocytes. However, lower IMFs in thalassemia trait erythrocytes suggest that α- and β-thalassemia traits may confer protection against severe malaria by limiting the rapid development of high parasite densities and associated severe anemia episodes, both of which are associated with fatal malaria. This logical conclusion is in accord with the results of our mathematical modeling, in which isogenic parasites produced either 26 or 22 merozoites per schizont in normal and thalassemia trait individuals, respectively, in the presence of an innate immune response. In addition to decreased IMF, a higher microerythrocyte count may protect against severe anemia in homozygous alpha(+)-thalassemia trait subjects [34].
Our results suggest new directions to explore parasite virulence. First, our findings that erythrocyte indices, especially MCHC, strongly influence IMF suggest that other hemoglobinopathic erythrocytes differing in these parameters may also significantly affect IMF—a readily testable hypothesis. For example, HbAC and HbCC erythrocytes have relatively high MCHC values, and HbE erythrocytes, which are also inherently β-thalassemic [35], have relatively low MCV and MCH but normal MCHC values [35, 36]. The combination of these characteristics, and the apparent selective invasion of HbE erythrocyte subsets by merozoites [37], may limit parasite replication in vivo. Second, our data predict that IMFs are reduced in the microcytic, hypochromic erythrocytes associated with iron-deficiency anemia. If true, this finding may help to explain how iron-deficiency anemia protects against P. falciparum malaria [38, 39], and how iron supplementation increases the morbidity and mortality of this disease (reviewed in [40]). Third, we found that IMF of various parasite strains can differ substantially (nearly 2-fold) in both control and hemoglobinopathic erythrocytes, suggesting that IMF is a parasite-heritable trait that can be mapped in phenotype-genotype association studies of parasite virulence. Finally, it is likely that hemoglobinopathies may also affect the IMF of P. vivax and other Plasmodium species that infect humans.
How the intraerythrocytic Hb concentration affects merozoite biomass requires further investigation. P. falciparum parasites digest more Hb than they utilize to harvest amino acids [41], extruding excess amounts in a likely effort to preserve erythrocyte osmotic stability [42]. Erythrocytes with low Hb concentration probably provide suboptimal conditions both for the synthesis of parasite proteins and maintenance of osmotic homeostasis. The lower IMF in thalassemia trait erythrocytes, as compared with normal cells, was observed for parasite isolates having both low and high IMFs, suggesting that the physical parameters of these erythrocytes are likely to affect IMF independently of the type of thalassemia trait. Factors other than Hb concentration may affect the merozoite biomass, as exemplified by the high IMFs we observed for the P. falciparum 3D7 line in HbSS erythrocytes. Paradoxically, deviations of HbSS cells from normal physiology [43] may be beneficial to merozoite production.
To explore whether putative lower IMFs in thalassemia trait carriers may help to protect patients from developing severe malaria, we used techniques of population modeling to follow 2 measures of disease progression—parasite density and erythrocyte loss. Our model assumes that normal and thalassemic individuals are infected with the same parasite, and that their erythrocytes produce 26 and 22 merozoites per schizont, respectively. In the absence of an immune response, “IMF = 26” cases are expected to have higher parasite densities than “IMF = 22” cases. We asked whether the innate immune response, being triggered earlier in IMF = 26 cases, blunts the rise in parasite density to that of IMF = 22 cases. The simulations predict that lower IMF values will benefit the majority of individuals early in infection, due to delayed onset of high parasite densities and slower progression to anemia, potentially preventing the development of severe malaria early in infection. As a consequence, the adaptive immune responses needed to rapidly clear patients' parasite densities and resolve their anemia will be developed. Additional refinement of the model, coupled with IMF data obtained ex vivo from hemoglobinopathic patients with malaria, could further improve our understanding of parasite virulence.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank A. Rabel, A.T. Melpolder, and patient volunteers for the collection of erythrocyte samples. This study utilized the high-performance computational capabilities of the Biowulf Linux cluster at the National Institutes of Health (NIH), Bethesda, MD (http://biowulf.nih.gov).
Financial support. This work was supported by the Intramural Research Programs of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Biomedical Imaging and Bioengineering (NIBIB), Center for Information Technology (CIT), and National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Haldane JB. The rate of mutation of human genes. Hereditas. 1949;35:267–73. [Google Scholar]
- 2.Taylor SM, Parobek CM, Fairhurst RM. Haemoglobinopathies and the clinical epidemiology of malaria: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:457–68. doi: 10.1016/S1473-3099(12)70055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunn HF. The triumph of good over evil: protection by the sickle gene against malaria. Blood. 2013;121:20–5. doi: 10.1182/blood-2012-08-449397. [DOI] [PubMed] [Google Scholar]
- 4.Taylor SM, Cerami C, Fairhurst RM. Hemoglobinopathies: slicing the Gordian knot of Plasmodium falciparum malaria pathogenesis. PLOS Pathog. 2013;9:e1003327. doi: 10.1371/journal.ppat.1003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cholera R, et al. Impaired cytoadherence of Plasmodium falciparum–infected erythrocytes containing sickle hemoglobin. Proc Natl Acad Sci USA. 2008;105:991–6. doi: 10.1073/pnas.0711401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fairhurst RM, et al. Abnormal display of PfEMP-1 on erythrocytes carrying haemoglobin C may protect against malaria. Nature. 2005;435:1117–21. doi: 10.1038/nature03631. [DOI] [PubMed] [Google Scholar]
- 7.Krause MA, et al. alpha-Thalassemia impairs the cytoadherence of Plasmodium falciparum–infected erythrocytes. PLOS One. 2012;7:e37214. doi: 10.1371/journal.pone.0037214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fairhurst RM, Bess CD, Krause MA. Abnormal PfEMP1/knob display on Plasmodium falciparum–infected erythrocytes containing hemoglobin variants: fresh insights into malaria pathogenesis and protection. Microbes Infect. 2012;14:851–62. doi: 10.1016/j.micinf.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasvol G, Weatherall DJ, Wilson RJ. Cellular mechanism for the protective effect of haemoglobin S against P. falciparum malaria. Nature. 1978;274:701–3. doi: 10.1038/274701a0. [DOI] [PubMed] [Google Scholar]
- 10.Ayi K, et al. Enhanced phagocytosis of ring-parasitized mutant erythrocytes: a common mechanism that may explain protection against falciparum malaria in sickle trait and beta-thalassemia trait. Blood. 2004;104:3364–71. doi: 10.1182/blood-2003-11-3820. [DOI] [PubMed] [Google Scholar]
- 11.Glushakova S, et al. New stages in the program of malaria parasite egress imaged in normal and sickle erythrocytes. Curr Biol. 2010;20:1117–21. doi: 10.1016/j.cub.2010.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaMonte G, et al. Translocation of sickle cell erythrocyte microRNAs into Plasmodium falciparum inhibits parasite translation and contributes to malaria resistance. Cell Host Microbe. 2012;12:187–99. doi: 10.1016/j.chom.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chotivanich K, et al. Parasite multiplication potential and the severity of Falciparum malaria. J Infect Dis. 2000;181:1206–9. doi: 10.1086/315353. [DOI] [PubMed] [Google Scholar]
- 14.Dorin-Semblat D, et al. Disruption of the PfPK7 gene impairs schizogony and sporogony in the human malaria parasite Plasmodium falciparum. Eukaryot Cell. 2008;7:279–85. doi: 10.1128/EC.00245-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reilly HB, et al. Quantitative dissection of clone-specific growth rates in cultured malaria parasites. Int J Parasitol. 2007;37:1599–607. doi: 10.1016/j.ijpara.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Read M, Hyde JE. Simple in vitro cultivation of the malaria parasite Plasmodium falciparum (erythrocytic stages) suitable for large-scale preparations. Methods Mol Biol. 1993;21:43–55. doi: 10.1385/0-89603-239-6:43. [DOI] [PubMed] [Google Scholar]
- 17.Thomson JG, Thomson D. The growth and sporulation of the benign and malignant tertaian malarial parasites in the culture tube and in the human host. Proc R Soc Lond. 1913;87:77–87. [Google Scholar]
- 18.Dluzewski AR, et al. A simple method for isolating viable mature parasites of Plasmodium falciparum from cultures. Trans R Soc Trop Med Hyg. 1984;78:622–4. doi: 10.1016/0035-9203(84)90221-9. [DOI] [PubMed] [Google Scholar]
- 19.Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–20. [PubMed] [Google Scholar]
- 20.Press W, et al. In Numerical recipes in C1992. Cambridge, UK: Cambridge University Press; [Google Scholar]
- 21.McQueen PG, McKenzie FE. Age-structured red blood cell susceptibility and the dynamics of malaria infections. Proc Natl Acad Sci USA. 2004;101:9161–6. doi: 10.1073/pnas.0308256101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McQueen PG, McKenzie FE. Host control of malaria infections: constraints on immune and erythropoeitic response kinetics. PLOS Comput Biol. 2008;4:e1000149. doi: 10.1371/journal.pcbi.1000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McQueen PG. Population dynamics of a pathogen: the conundrum of vivax malaria. Biophys Rev. 2010;2:111–20. doi: 10.1007/s12551-010-0034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karunaweera ND, et al. The paroxysm of Plasmodium vivax malaria. Trends Parasitol. 2003;19:188–93. doi: 10.1016/s1471-4922(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 25.Simpson JA, et al. Population dynamics of untreated Plasmodium falciparum malaria within the adult human host during the expansion phase of the infection. Parasitology. 2002;124(Pt 3):247–63. doi: 10.1017/s0031182001001202. [DOI] [PubMed] [Google Scholar]
- 26.Wickramasinghe SN, Abdalla SH. Blood and bone marrow changes in malaria. Baillieres Best Pract Res Clin Haematol. 2000;13:277–99. doi: 10.1053/beha.1999.0072. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence G, et al. Effect of vaccination with 3 recombinant asexual-stage malaria antigens on initial growth rates of Plasmodium falciparum in non-immune volunteers. Vaccine. 2000;18:1925–31. doi: 10.1016/s0264-410x(99)00444-2. [DOI] [PubMed] [Google Scholar]
- 28.McKay M, Beckman R, Conover W. A comparison of three methods for selecting values of input variables in the analysis of output from a computer code. Technometrics. 1979;21:239–45. [Google Scholar]
- 29.Glushakova S, et al. Membrane transformation during malaria parasite release from human red blood cells. Curr Biol. 2005;15:1645–50. doi: 10.1016/j.cub.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 30.Glushakova S, et al. Quantification of malaria parasite release from infected erythrocytes: inhibition by protein-free media. Malar J. 2007;6:61. doi: 10.1186/1475-2875-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orkin SH, Nathan DG, editors. Nathan and Oski's hematology of infancy and childhood. Philadelphia: Sanders/Elsevier; 2009. Disorders of hemoglobin; pp. 911–1109. [Google Scholar]
- 32.Steinberg MH. Pathophysiology of hemoglobin and its disorders. In: Steinberg MH, editor. Disorders in hemoglobin: genetics, pathophysiology, and clinical management. New York: Cambridge University Press; 2009. pp. 137–224. [Google Scholar]
- 33.Amaratunga C, et al. Artemisinin-resistant Plasmodium falciparum in Pursat province, western Cambodia: a parasite clearance rate study. Lancet Infect Dis. 2012;12:851–8. doi: 10.1016/S1473-3099(12)70181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fowkes FJ, et al. Increased microerythrocyte count in homozygous alpha(+)-thalassaemia contributes to protection against severe malarial anaemia. PLOS Med. 2008;5:e56. doi: 10.1371/journal.pmed.0050056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orkin SH, et al. Abnormal RNA processing due to the exon mutation of beta E-globin gene. Nature. 1982;300:768–9. doi: 10.1038/300768a0. [DOI] [PubMed] [Google Scholar]
- 36.Frischer H, Bowman J. Hemoglobin E, an oxidatively unstable mutation. J Lab Clin Med. 1975;85:531–9. [PubMed] [Google Scholar]
- 37.Chotivanich K, et al. Hemoglobin E: a balanced polymorphism protective against high parasitemias and thus severe P falciparum malaria. Blood. 2002;100:1172–6. [PubMed] [Google Scholar]
- 38.Gwamaka M, et al. Iron deficiency protects against severe Plasmodium falciparum malaria and death in young children. Clin Infect Dis. 2012;54:1137–44. doi: 10.1093/cid/cis010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kabyemela ER, et al. Decreased susceptibility to Plasmodium falciparum infection in pregnant women with iron deficiency. J Infect Dis. 2008;198:163–6. doi: 10.1086/589512. [DOI] [PubMed] [Google Scholar]
- 40.Spottiswoode N, et al. Implications of malaria on iron deficiency control strategies. Adv Nutr. 2012;3:570–8. doi: 10.3945/an.111.001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldberg DE. Hemoglobin degradation. Curr Top Microbiol Immunol. 2005;295:275–91. doi: 10.1007/3-540-29088-5_11. [DOI] [PubMed] [Google Scholar]
- 42.Mauritz JM, et al. The homeostasis of Plasmodium falciparum–infected red blood cells. PLOS Comput Biol. 2009;5:e1000339. doi: 10.1371/journal.pcbi.1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lew VL, Bookchin RM. Ion transport pathology in the mechanism of sickle cell dehydration. Physiol Rev. 2005;85:179–200. doi: 10.1152/physrev.00052.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.