Abstract
Aims
To estimate the 3 month prevalence of adverse drug events (ADEs), categories of ADEs and preventable ADEs, and the preventability of ADEs among adults in Sweden. Further, to identify drug classes and organ systems associated with ADEs and estimate their seriousness.
Methods
A random sample of 5025 adults in a Swedish county council in 2008 was drawn from the Total Population Register. All their medical records in 29 inpatient care departments in three hospitals, 110 specialized outpatient clinics and 51 primary care units were reviewed retrospectively in a stepwise manner, and complemented with register data on dispensed drugs. ADEs, including adverse drug reactions (ADRs), sub-therapeutic effects of drug therapy (STEs), drug dependence and abuse, drug intoxications from overdose, and morbidities due to drug-related untreated indication, were detected during a 3 month study period, and assessed for preventability.
Results
Among 4970 included individuals, the prevalence of ADEs was 12.0% (95% confidence interval (CI) 11.1, 12.9%), and preventable ADEs 5.6% (95% CI 5.0, 6.2%). ADRs (6.9%; 95% CI 6.2, 7.6%) and STEs (6.4%; 95% CI 5.8, 7.1%) were more prevalent than the other ADEs. Of the ADEs, 38.8% (95% CI 35.8–41.9%) was preventable, varying by ADE category and seriousness. ADEs were frequently associated with nervous system and cardiovascular drugs, but the associated drugs and affected organs varied by ADE category.
Conclusions
The considerable burden of ADEs and preventable ADEs from commonly used drugs across care settings warrants large-scale efforts to redesign safer, higher quality healthcare systems. The heterogeneous nature of the ADE categories should be considered in research and clinical practice for preventing, detecting and mitigating ADEs.
Keywords: adverse drug event, medical records, medication error, pharmacoepidemiology, prevalence
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Adverse drug events (ADEs) are common and often preventable among hospitalized patients, but evidence outside hospitals and in the general population is lacking.
Previous studies have focused on all ADEs combined or on adverse drug reactions (ADRs), a category of ADEs, potentially limiting the understanding of ADEs.
WHAT THIS STUDY ADDS
During 3 months, 12% of adults across care settings experienced ADEs, over one-third of which were potentially preventable, warranting further efforts in healthcare to tackle the problem, also in primary and other outpatient care.
Associated drugs, affected organs, preventability, and seriousness differ by ADE category, such as ADRs and sub-therapeutic effects, which should be considered in future research and in clinical practice.
Introduction
Improving patient safety and reducing preventable patient harm, including adverse drug events (ADEs), are emphasized by national, regional and global health authorities [1–4]. An ADE is commonly defined as ‘an injury resulting from medical intervention related to a drug’ [5], although definitions vary [6,7]. Approximately 5% of patients at or during hospitalization [6,8–10], and a median of 13% of ambulatory care patients [9] are reported to experience ADEs, and 11–90% of the ADEs are estimated preventable [8–14]. The few previous studies including outpatients without a hospitalization are commonly small, or limited to certain sub-populations or exclusively self-reports [15–26]. Even though ADEs are commonly described to include not only adverse drug reactions (ADRs), but also intoxications from overdoses, sub-therapeutic effects for example due to patient non-adherence, and events due to lack of therapy [6,8–12,25–32], these diverse event categories are rarely reported separately, possibly limiting the characterization of ADEs. Therefore, the burden of ADEs and categories of ADEs across care settings is largely unknown, in particular outside hospitals. The primary objective of this study was to estimate the 3 month prevalence of ADEs, categories of ADEs, and preventable ADEs, and the preventability of ADEs using medical records of a random sample of the adult general public in Sweden. Secondary objectives were to identify drug classes and organ systems associated with ADEs, to assess the seriousness of ADEs, and to estimate the prevalence of serious ADEs and preventable serious ADEs.
Methods
Setting and participants
A random sample of 5025 adult residents (≥18 years on 31 December 2007) in the county council of Östergötland, Sweden, was drawn from the Total Population Register of Statistics Sweden. The sample included all adults with a registered address in the county, including people living in nursing homes etc. We calculated the sample size based on a conservative 8% expected prevalence and for estimating a 50% proportion among individuals with ADEs, with a maximum width of ±5% for the 95% confidence interval (CI), requiring a minimum of 384 individuals with ADEs. Medical care in all care units of the study population was reviewed retrospectively for 3 months in 2008. To account for seasonal variation, the study population was randomly divided into four groups for each quarter of the year.
Outcome measures
The primary outcome measure was an ADE, defined as ‘an injury resulting from medical intervention related to a drug’ [5], which could be associated with prescribed, non-prescribed or complementary, but not illicit drugs. Some consider ADEs to consist of non-preventable ADRs, and medication errors that are by definition preventable [33], while others consider also part of ADRs preventable [34,35]. In our study, an ADR could be preventable and was defined according to the World Health Organization [36] as ‘a response to a drug which is noxious and unintended, and which occurs at doses normally used in man for the prophylaxis, diagnosis, or therapy of disease, or for the modification of physiological function’. We excluded drug dependence (DD) from ADRs, as DD could occur in higher doses than normally used. Apart from ADRs, medication errors such as omission of a dose [37] may result in other types of injury, which could be included in the broad definition for ADEs [5], but are not detailed in most studies on ADEs. Thus, we identified additional, mutually exclusive ADE categories from the literature [6,8–12,25–32,38–40]. To differentiate from ADRs, we defined drug intoxications from overdose (DIs) as ‘a noxious, intended or unintended drug reaction that occurs at higher doses than normally used in man for prophylaxis, diagnosis or treatment. The intention for administrating the drug(s) may or may not be therapeutic’. DD and drug abuse (DA) were defined according to the American Psychiatric Association as ‘a maladaptive pattern of substance use leading to clinically significant impairment or distress’, which had to be manifested according to specific criteria [41]. Sub-therapeutic effects of drug therapy (STEs) included absence of therapeutic response that could be linked causally either to dose that was too low, drug non-compliance, recent dose reduction/discontinuation or inadequate monitoring [40]. We also included STEs due to improper drug selection or when treatment had been rational (e.g. first line treatment not effective). Morbidity due to drug-related untreated indication (UTI) occurred when a person had a clinical condition that under normal circumstances would have required pharmacological therapy but none was received. Secondary outcome measures were preventable [42] and serious [34] ADEs.
Data sources and case assessment
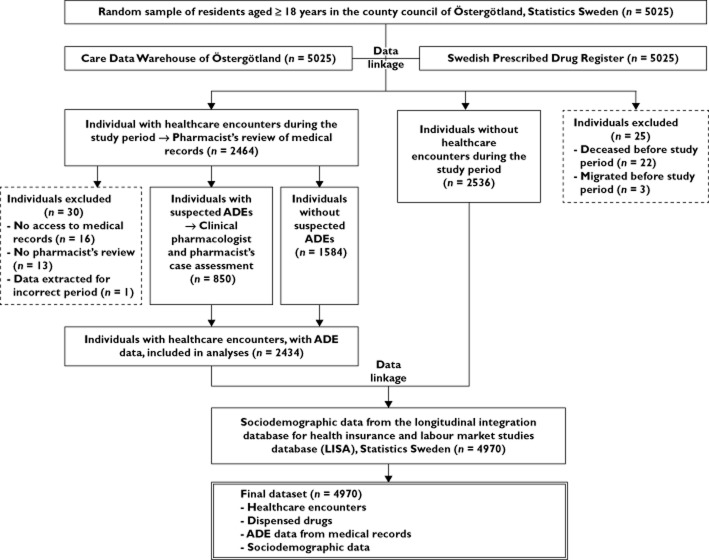
Data from multiple sources were linked using the personal identity number (Figure 1). Data on all individuals' dispensed drugs were retrieved from the Swedish Prescribed Drug Register (SPDR) [43], which covers all prescribed drugs dispensed in pharmacies (also, for example, low dose acetylic salicylic acid and small benzodiazepine packages), including prescription drugs for residential care. The SPDR excludes non-prescription and complementary drugs bought without a prescription, drugs administered in hospitals and emergency drugs administered in residential care. Data on healthcare encounters were retrieved from the regional patient register, Care Data Warehouse of Östergötland [44], including administrative data on all inpatient and outpatient care provided in the county in all medical specialities. In the study area, private outpatient care (including dental care) constituted 3% of all healthcare expenditure in 2008 [45], practically all of which is recorded (personal communication from Lars Svensson, Östergötland County Council). Thus, the coverage of the care data is considered full. Based on the administrative care data, electronic medical records in all care units were retrieved for individuals with one or more healthcare encounters (nurse or physician, visit or telephone contact, outpatient or inpatient, specialized or not, excluding dental and paramedical care) during the 3 month study period. Healthcare providers were contacted for paper copies when electronic records were missing, including private outpatient care providers (Östergötland had no private inpatient care providers in 2008).
Figure 1.
Study flow diagram. ADE, adverse drug event. Data sources were linked using the unique personal identity number
ADEs were detected in the medical records in a stepwise manner similar to previous studies [5,17], using manuals and standardized, pilot-tested data extraction tools. Pharmacists extracted information on suspected ADEs from the medical records for the 3 month study period, and 9 months before and 3 months after it. For prescribed drugs, data were extracted both from the SPDR and from the medical record, as medical records include drug use in inpatient care and emergency drug use in residential care, which are excluded from the SPDR. Information on non-prescribed and complementary drugs was extracted exclusively from the medical records. Used triggers included diagnoses (e.g. urticaria) [33], drugs (e.g. warfarin) [33] and drug–drug interactions [46]. A clinical pharmacologist and another pharmacist independently assessed the causality [47] between the suspected ADEs and drug therapies, detected possible additional ADEs, and assessed preventability [42], contribution to hospitalization [42] and seriousness [34]. Conflicting assessments were solved by consensus. Suspected ADEs with at least possible causality [47] were considered ADEs, and ADEs with at least possible preventability [42] preventable ADEs. An ADE was considered to contribute to a hospitalization if its significance for the admission was ‘dominant’, ‘partially contributing’, or ‘less important’ [42]. This assessment was also used for determining seriousness, among other seriousness criteria [34]. All assessors were trained in the process. Data from the medical records were combined with register data for all individuals (Figure 1).
Aggregated data
The individuals' sociodemographic characteristics were compared with the adult general population in Sweden by retrieving aggregated data on age, gender, marital status, area of residence, country of birth, education and income from Statistics Sweden.
Data analysis
In the main analyses, the 3 month prevalences and 95% confidence intervals were calculated for different categories of ADEs and preventable ADEs. Individuals with at least one ADE were used in the numerator in the prevalence calculations. All individuals in the study population were chosen as the denominator in the prevalence calculations, because ADEs could occur without dispensed drugs (non-prescription drugs or stockpile), and UTIs, STEs and prolonged ADEs do not require current drug use. The ADE prevalences were compared by age group using χ2 or Fisher's exact test, choosing cut-off ages based on changing patterns in morbidity and mortality: 18–44, 45–64 and ≥65 years [48]. The prevalences of serious ADEs, and preventable serious ADEs were also calculated. For the prevalence of hospitalizations contributed by ADEs, individuals with hospitalizations during the study period were used in the denominator. For preventability and seriousness, the number of preventable and/or serious ADEs was divided by the number of all ADEs. STATA software version 11.2 was used.
Drugs associated with each ADE category were classified according to the Anatomical Therapeutic Chemical (ATC) Classification [49], including main groups (first level) and pharmacological subgroups (third level) representing >1% of the ADE category, and chemical substances (fifth level) representing ≥20% of the given pharmacological subgroups. Psycholeptics (N05) and psychoanaleptics (N06) were also classified into the fourth level drug classes. For comparison, the most common drugs dispensed to all individuals were described, from 6 months before the study period until the last day before the study period.
According to the Medical Dictionary for Regulatory Activities (MedDRA) [50], organ systems (System Organ Classes [50]) and symptoms (Preferred Terms [50]) representing >1% of all or preventable ADEs were presented. For ADRs and STEs, chemical substances [49] associated with the Preferred Terms at least twice were reported.
As sensitivity analyses, the ADE prevalences were calculated varying the denominator: individuals with dispensed drugs during 6 months before the study period, and individuals with ≥1 healthcare encounters during the study period. In further sensitivity analyses, the ADE prevalence was calculated without UTIs and non-preventable STEs, to mimic previous ADE definitions. This was done using different denominators: all individuals, individuals with dispensed drugs and individuals with healthcare encounters.
Ethical considerations
An ethical approval was received from the Regional Ethical Review Board in Gothenburg (644-08). According to Swedish legislation, no informed consents from the participants were required, because participation could not change the participants' healthcare or health status and the results were expected to improve care for future patients.
Results
Study population
After excluding 55 individuals (Figure 1), the study population consisted of 4970 individuals, of which 2434 (49.0%) had healthcare encounters, in 29 departments of inpatient care in three hospitals, 110 specialized outpatient clinics, and 51 primary care units. The sociodemographic characteristics of the study population and the general population were similar (Table 1), although a larger proportion of the study population was born in Sweden.
Table 1.
Characteristics of the study population (n = 4970) compared with the adult general population in Sweden (n = 7 251 275)
| Variable | Study population n (%) | General population n (%) |
|---|---|---|
| Age* | ||
| Mean (SD) | 48.9 (19.0) | 48.9 (18.9) |
| 18–47 years | 2359 (47.5) | 3 605 647 (49.7) |
| 48–67 years | 1693 (34.1) | 2 322 001 (32.0) |
| ≥68 years | 918 (18.5) | 1 323 627 (18.3) |
| Missing | 0 (0.0) | 0 (0.0) |
| Gender | ||
| Male | 2427 (48.8) | 3 572 603 (49.3) |
| Missing | 0 (0.0) | 0 (0.0) |
| Marital status* | ||
| Single | 1914 (38.5) | 2 762 464 (38.1) |
| Married or registered partnership | 2203 (44.2) | 3 134 181 (43.2) |
| Separated | 526 (10.6) | 859 956 (11.9) |
| Widowed | 327 (6.6) | 494 674 (6.8) |
| Missing | 0 (0.0) | 0 (0.0) |
| Area of residence* | ||
| Cities and commuting municipalities | 3336 (67.1) | 4 820 495 (66.5) |
| Others | 1634 (32.9) | 2 430 780 (33.5) |
| Missing | 0 (0.0) | 0 (0.0) |
| Country of birth | ||
| Sweden | 4437 (89.3) | 6 135 688 (84.6) |
| OECD country, including Sweden | 4648 (93.5) | 6 677 580 (92.1) |
| Missing | 1 (0.0) | 1525 (0.0) |
| Highest level of education† | ||
| Mandatory school | 1264 (25.4) | 1 614 887 (22.3) |
| Secondary/high school | 2147 (43.2) | 3 250 209 (44.8) |
| High education | 1456 (29.3) | 2 198 568 (30.3) |
| Missing | 103 (2.1) | 187 611 (2.6) |
| Disposable monthly income‡ | ||
| Median | 1817 | 1905 |
| 0–1351 USD | 1268 (25.5) | 1 797 882 (24.8) |
| 1352–1903 USD | 1354 (27.2) | 1 800 638 (24.8) |
| 1904–2757 USD | 1237 (24.9) | 1 799 378 (24.8) |
| >2858 USD | 1111 (22.4) | 1 800 562 (24.8) |
| Missing | 0 (0.0) | 52 815 (0.7) |
OECD, Organization for Economic Co-operation and Development; NA, not applicable; SD, standard deviation; USD, United States dollar.
On 31 December 2007, apart from age for the study individuals in the beginning of the study period.
In 2008.
Average in 2008, weighted for number of children. Yearly average exchange rate in 2008 from Swedish krona to United States dollar 6.5808.
Prevalences of persons with ADEs
ADEs were detected in 596 of the 4970 individuals, resulting in a total prevalence of 12.0% (95% CI 11.1, 12.9%) in the total general population (Table 2). As described in Table 2, ADRs and STEs were more prevalent than the other ADE categories. The prevalences differed by age group for all ADEs, ADRs and STEs, being higher in older age groups. The prevalence of preventable ADEs was 5.6% (95% CI 5.0, 6.2%), also differing by age group. The 3 month prevalence of serious ADEs in the general population was 1.2% (95% CI 0.9, 1.6%), and preventable serious ADEs 0.7% (95% CI 0.5, 1.0%). ADEs contributed to admissions for 0.6% (95% CI 0.4, 0.8%) of the general population, and for 22.1% (95% CI 15.1, 29.1%) of 136 individuals with hospitalizations during the 3 month study period. When only preventable ADEs were analyzed, they contributed to admissions for 0.4% (95% CI 0.2, 0.6%) of the general population, and for 14.0% (95% CI 8.1, 19.8%) of individuals with hospitalizations.
Table 2.
Three month prevalence of persons with ADEs and preventable ADEs, by ADE category and age group
| Age 18–44 years (n = 2217) | Age 45–64 years (n = 1600) | Age ≥65 years (n = 1153) | All ages (n = 4970) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Prevalence % (95% CI) | n | Prevalence % (95% CI) | n | Prevalence % (95% CI) | P Value† | n | Prevalence % (95% CI) | |
| Any ADE* | 130 | 5.9 (4.9, 6.8) | 210 | 13.1 (11.5, 14.8) | 256 | 22.2 (19.8, 24.6) | <0.001 | 596 | 12.0 (11.1, 12.9) |
| ADRs | 76 | 3.4 (2.7, 4.2) | 107 | 6.7 (5.5, 7.9) | 159 | 13.8 (11.8, 15.8) | <0.001 | 342 | 6.9 (6.2, 7.6) |
| DIs | 3 | 0.1 (0.0, 0.3) | 0 | 0 (–) | 4 | 0.3 (0.0, 0.7) | 0.04 | 7 | 0.1 (0.0, 0.2) |
| DD or DA | 7 | 0.3 (0.1, 0.5) | 9 | 0.6 (0.2, 0.9) | 4 | 0.3 (0.0, 0.7) | 0.46 | 20 | 0.4 (0.2, 0.6) |
| STEs | 67 | 3.0 (2.3, 3.7) | 121 | 7.6 (6.3, 8.9) | 132 | 11.4 (9.6, 13.3) | <0.001 | 320 | 6.4 (5.8, 7.1) |
| UTIs | 14 | 0.6 (0.3, 1.0) | 17 | 1.1 (0.6, 1.6) | 16 | 1.4 (0.7, 2.1) | 0.08 | 47 | 0.9 (0.7, 1.2) |
| Any preventable ADE* | 58 | 2.6 (2.0, 3.3) | 88 | 5.6 (4.4, 6.6) | 132 | 11.4 (9.6, 13.3) | <0.001 | 278 | 5.6 (5.0, 6.2) |
| Preventable ADRs | 16 | 0.7 (0.4, 1.1) | 24 | 1.5 (0.9, 2.1) | 66 | 5.7 (4.4, 7.1) | <0.001 | 106 | 2.1 (1.7, 2.5) |
| Preventable DIs | 3 | 0.1 (0.0, 0.3) | 0 | 0 (–) | 4 | 0.3 (0.0, 0.7) | 0.04 | 7 | 0.1 (0.0, 0.2) |
| Preventable DD or DA | 6 | 0.3 (0.1, 0.5) | 9 | 0.6 (0.2, 0.9) | 3 | 0.3 (0.0, 0.6) | 0.27 | 18 | 0.4 (0.2, 0.5) |
| Preventable STEs | 31 | 1.4 (0.9, 1.9) | 57 | 3.6 (2.7, 4.5) | 64 | 5.6 (4.2, 6.9) | <0.001 | 152 | 3.1 (2.6, 3.5) |
| Preventable UTIs | 9 | 0.4 (0.1, 0.7) | 12 | 0.8 (0.3, 1.2) | 14 | 1.2 (0.6, 1.8) | 0.03 | 35 | 0.7 (0.5, 0.9) |
ADE, adverse drug event; ADR, adverse drug reaction; CI, confidence interval; DA, drug abuse; DD, drug dependence; DI, drug intoxication from overdose; STE, sub-therapeutic effect of drug therapy; UTI, morbidity due to drug-related untreated indication.
As one person could have multiple ADEs, the combined prevalence is lower than the sum of the prevalences of the ADE categories.
For testing the statistical significance between all three age groups using χ2 test, with the exception of using Fisher's exact test for DIs due to low number of cases.
Numbers of events
Of all 981 ADEs, 52.4% were ADRs, 38.8% STEs, 5.3% UTIs, 2.6% DD and DA cases and 0.8% DIs. Of the 596 individuals with ADEs, 30.4% had two to three, and 6.5% four or more ADEs. Among individuals with ADRs, 30.7% had two or more ADRs, while 15.3% of individuals with STEs had two or more STEs. Of individuals with ADEs, 78.5% experienced exclusively one ADE category.
Preventability and seriousness of events
The preventability of all ADEs was 38.8% (95% CI 35.8, 41.9%), varying by ADE category (Table 3). Serious ADEs represented 9.5% (95% CI 7.6, 11.3%) of all ADEs, of which DIs were the most and ADRs the least serious. Of all serious ADEs, 55.9% (95% CI 45.8, 66.0%) were preventable. By ADE category, the preventability of serious ADEs was similar to all ADEs, apart from the 54.8% (95% CI 36.3, 73.4%) preventability of serious ADRs. Of the ADEs contributing to hospitalizations, 62.8% (95% CI 48.3, 77.2%) were judged preventable.
Table 3.
Preventability and seriousness of events and the preventability of serious events, by ADE category
| Preventability | Seriousness | Preventability of serious ADEs | ||||
|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | |
| Any ADE (n = 981) | 379 | 38.8 (35.8, 41.9) | 93 | 9.5 (7.6, 11.3) | 52 | 55.9 (45.8, 66.0) |
| ADRs (n = 514) | 135 | 26.3 (22.4, 30.1) | 31 | 6.0 (4.0, 8.1) | 17 | 54.8 (36.3, 73.4) |
| DIs (n = 8) | 8 | 100.0 (67.6, 100.0) | 5 | 62.5 (29.0, 96.0) | 5 | 100.0 (56.6, 100.0) |
| DD or DA (n = 26) | 24 | 92.3 (75.9, 97.9) | 8 | 30.8 (13.0, 48.5) | 7 | 87.5 (52.9, 97.8) |
| STEs (n = 381) | 172 | 45.1 (40.1, 50.2) | 44 | 11.5 (8.3, 14.8) | 19 | 43.2 (27.9, 58.4) |
| UTIs (n = 52) | 40 | 76.9 (65.1, 88.8) | 5 | 9.6 (1.6, 17.6) | 4 | 80.0 (37.6, 96.4) |
ADE, adverse drug event; ADR, adverse drug reaction; CI, confidence interval; DA, drug abuse; DD, drug dependence; DI, drug intoxication from overdose; STE, sub-therapeutic effect of drug therapy; UTI, morbidity due to drug-related untreated indication.
Drugs associated with events
Drugs for the nervous system were associated with 39.3% of ADRs, 30.4% of STEs, all DD and DA cases and 62.5% of DIs (Table 4). Also cardiovascular drugs attributed to 29.6% of ADRs and 28.3% of STEs. Among nervous system drugs, psychoanaleptics were the most common (19.8%) among ADRs and analgesics (12.1%) among STEs (Table S1).
Table 4.
Drug classes associated with ADE categories*, ordered according to the most commonly dispensed drugs to all individuals
| Drug class† (ATC code) | Dispensed to all individuals‡ (n = 4970) n (%)§ | ADRs (n = 514) n (%)¶ | STEs (n = 381) n (%)¶ | DD or DA (n = 26) n (%)¶ | DIs (n = 8) n (%)¶ |
|---|---|---|---|---|---|
| Cardiovascular system (C) | 1242 (25.0) | 152 (29.6) | 108 (28.3) | – | 1 (12.5) |
| Nervous system (N) | 1136 (22.9) | 202 (39.3) | 116 (30.4) | 26 (100.0) | 5 (62.5) |
| Alimentary tract and metabolism (A) | 867 (17.4) | 37 (7.2) | 54 (14.2) | – | 2 (25.0) |
| Blood and blood forming organs (B) | 728 (14.6) | 38 (7.4) | 7 (1.8) | – | – |
| Anti-infectives for systemic use (J) | 697 (14.0) | 18 (3.5) | 24 (6.3) | – | – |
| Genitourinary system and sex hormones (G) | 651 (13.1) | 28 (5.4) | 6 (1.6) | – | – |
| Respiratory system (R) | 640 (12.9) | 24 (4.7) | 27 (7.1) | – | 2 (25.0) |
| Musculoskeletal system (M) | 548 (11.0) | 29 (5.6) | 37 (9.7) | 1 (3.9) | – |
| Systemic hormonal preparations** (H) | 349 (7.0) | 20 (3.9) | 14 (3.7) | – | – |
| Dermatologicals (D) | 325 (6.5) | – | 8 (2.1) | – | – |
| Sensory organs (S) | 276 (5.6) | – | 5 (1.3) | – | – |
| Antineoplastic and immunomodulating agents (L) | 73 (1.5) | 26 (5.1) | – | – | – |
| No dispensed drugs during the past 6 months | 1965 (39.5) | NA | NA | NA | NA |
ADE, adverse drug event; ADR, adverse drug reaction; ATC, Anatomical Therapeutic Chemical; DA, drug abuse; DD, drug dependence; DI, drug intoxication from overdose; NA, not applicable; STE, sub-therapeutic effect of drug therapy; –, ≤1% of the ADE category.
Excluding morbidities due to drug-related untreated indication.
Categorized according to the Anatomical Therapeutic Chemical (ATC) Classification System [49] main groups (1st level).
Dispensed drugs from the Swedish Prescribed Drug Register, for all study individuals from 6 months before the study period until the last day before the study period.
Representing >1% of the dispensed drugs. ¶Representing >1% of the ADE category.
Excluding sex hormones and insulins.
By and large, the main drug classes associated with all and preventable ADRs and STEs were similar. Drugs for the nervous system contributed to 43.7% of preventable ADRs (psychoanaleptics 17.8%, psycholeptics 15.6%, analgesics 14.1%), and drugs for the cardiovascular system to 37.8% (β-adrenoceptor blocking agents 15.6%, diuretics 14.1%, agents acting on the renin-angiotensin system 11.9%). Drugs for blood and blood forming organs composed 9.6% (antithrombotic agents 8.1%) of preventable ADRs, and drugs for the musculoskeletal system 8.2% (anti-inflammatory and anti-rheumatic products 7.4%). Of preventable STEs, cardiovascular drugs represented 31.4% (β-adrenoceptor blocking agents 16.9%, agents acting on the renin-angiotensin system 15.1%, diuretics 11.1%), nervous system drugs 21.5% (analgesics 8.1%, psychoanaleptics 8.1%, psycholeptics 4.1%), alimentary tract and metabolism drugs 20.4% (drugs used in diabetes 18.0%), and drugs for the musculoskeletal system 9.3% (anti-inflammatory and anti-rheumatic products 9.3%).
Organs affected by events
ADRs were most frequently gastrointestinal (21.6%) or general disorders (12.3%) (Table 5, Table S2), the most frequent ADR symptoms being fatigue, nausea, dizziness and increased weight. Analogously to all ADRs, preventable ADRs were the most frequently gastrointestinal (20.7%) or general disorders (13.3%).
Table 5.
Organs affected by ADRs and preventable ADRs, with ADR symptoms
| Organ system* and symptom† | ADRs (n = 514) n (%)‡ | Preventable ADRs (n = 135) n (%)‡ |
|---|---|---|
| Gastrointestinal disorders | 111 (21.6) | 28 (20.7) |
| Nausea | 32 (6.2) | 4 (3.0) |
| Dry mouth | 12 (2.3) | 6 (4.4) |
| Constipation | 12 (2.3) | 6 (4.4) |
| Diarrhoea | 11 (2.1) | – |
| Dyspepsia | 9 (1.8) | 3 (2.2) |
| Abdominal pain upper | 8 (1.6) | 2 (1.5) |
| General disorders and administration site conditions | 63 (12.3) | 18 (13.3) |
| Fatigue | 38 (7.4) | 9 (6.7) |
| Hyperhidrosis | 8 (1.6) | 2 (1.5) |
| Asthenia | – | 2 (1.5) |
| Withdrawal syndrome | – | 2 (1.5) |
| Cardiac disorders | 46 (8.9) | 12 (8.9) |
| Dizziness | 22 (4.3) | 4 (3.0) |
| Oedema peripheral | 9 (1.8) | 4 (3.0) |
| Palpitations | 8 (1.6) | – |
| Bradycardia | – | 2 (1.5) |
| Nervous system disorders | 45 (8.8) | 14 (10.4) |
| Tremor | 9 (1.8) | 3 (2.2) |
| Headache | 8 (1.6) | 3 (2.2) |
| Dizziness | 7 (1.4) | – |
| Depressed level of consciousness | – | 2 (1.5) |
| Vascular disorders | 45 (8.8) | 12 (8.9) |
| Hypotension | 10 (1.9) | 7 (5.2) |
| Psychiatric disorders | 40 (7.8) | 4 (3.0) |
| Sleep disorder | 9 (1.8) | – |
| Anxiety | 8 (1.6) | – |
| Investigations | 30 (5.8) | 8 (5.9) |
| Weight increased | 17 (3.3) | 2 (1.5) |
| International normalized ratio increase | 8 (1.6) | 5 (3.7) |
| Respiratory, thoracic and mediastinal disorders | 24 (4.7) | 6 (4.4) |
| Cough | 12 (2.3) | 4 (3.0) |
| Skin and subcutaneous tissue disorders | 23 (4.5) | 3 (2.2) |
| Rash | 7 (1.4) | – |
| Renal and urinary disorders | 16 (3.1) | 6 (4.4) |
| Renal failure | 6 (1.2) | 2 (1.5) |
| Urinary retention | – | 2 (1.5) |
| Reproductive system and breast disorders | 14 (2.7) | – |
| Musculoskeletal and connective tissue disorders | 13 (2.5) | 5 (3.7) |
| Myalgia | 6 (1.2) | 4 (3.0) |
| Metabolism and nutrition disorders | 13 (2.5) | 5 (3.7) |
| Hyperkalaemia | – | 2 (1.5) |
| Injury, poisoning and procedural complications | 10 (1.9) | 7 (5.2) |
| Fall | 10 (1.9) | 7 (5.2) |
| Endocrine disorders | 8 (1.6) | 4 (3.0) |
| Hypoglycaemia | – | 3 (2.2) |
| Blood and lymphatic system disorders | 6 (1.2) | 3 (2.2) |
| Anaemia | – | 3 (2.2) |
STEs were most frequently vascular (18.9%), dominated by hypertension (Table 6, Table S3). Preventable STEs were frequently vascular (23.8%), psychiatric (12.2%), or musculoskeletal (9.9%), as for all STEs, but endocrine disorders were more common among preventable (14.5%) than all STEs (8.7%).
Table 6.
Organs affected by STEs and preventable STEs, with STE symptoms
| Organ system*> and symptom† | STEs (n = 381) n (%)‡ | Preventable STEs (n = 172) n (%)‡ |
|---|---|---|
| Vascular disorders | 72 (18.9) | 41 (23.8) |
| Hypertension | 71 (18.6) | 41 (23.8) |
| Psychiatric disorders | 59 (15.5) | 21 (12.2) |
| Depression | 15 (3.9) | 7 (4.1) |
| Anxiety | 11 (2.9) | 4 (2.3) |
| Sleep disorder | 9 (2.4) | – |
| Depressed mood | 5 (1.3) | 2 (1.2) |
| Panic disorder | 5 (1.3) | – |
| Insomnia | 4 (1.0) | 2 (1.2) |
| Musculoskeletal and connective tissue disorders | 48 (12.6) | 17 (9.9) |
| Back pain | 14 (3.7) | 3 (1.7) |
| Arthralgia | 11 (2.9) | 4 (2.3) |
| Pain in extremity | 5 (1.3) | 3 (1.7) |
| Endocrine disorders | 33 (8.7) | 25 (14.5) |
| Hyperglycaemia | 31 (8.1) | 24 (14.0) |
| Cardiac disorders | 29 (7.6) | 8 (4.7) |
| Oedema peripheral | 11 (2.9) | 2 (1.2) |
| Cardiac failure | 6 (1.6) | 2 (1.2) |
| Angina pectoris | 4 (1.0) | – |
| Respiratory, thoracic and mediastinal disorders | 27 (7.1) | 9 (5.2) |
| Asthma | 10 (2.6) | 5 (2.9) |
| Sinusitis | 5 (1.3) | 2 (1.2) |
| Gastrointestinal disorders | 20 (5.3) | 5 (2.9) |
| Abdominal pain upper | 4 (1.0) | – |
| Constipation | – | 2 (1.2) |
| Skin and subcutaneous tissue disorders | 18 (4.7) | 11 (6.4) |
| Eczema | – | 3 (1.7) |
| Nervous system disorders | 14 (3.7) | 5 (2.9) |
| Migraine | 5 (1.3) | 2 (1.2) |
| Headache | – | 2 (1.2) |
| General disorders and administration site conditions | 12 (3.2) | 4 (2.3) |
| Pain | 11 (2.9) | 3 (1.7) |
| Renal and urinary disorders | 11 (2.9) | 7 (4.1) |
| Urinary tract infection | 7 (1.8) | 5 (2.9) |
| Ureteritis | – | 2 (1.2) |
| Metabolism and nutrition disorders | 10 (2.6) | 8 (4.7) |
| Hyperlipidaemia | 4 (1.0) | 4 (2.3) |
| Investigations | 8 (2.1) | 5 (2.9) |
| Reproductive system and breast disorders | 7 (1.8) | – |
| Eye disorders | 5 (1.3) | – |
| Blood and lymphatic system disorders | – | 2 (1.2) |
| Anaemia | – | 2 (1.2) |
STE, sub-therapeutic effect of drug therapy; –, ≤1% of STEs.
System Organ Classes according to the Medical Dictionary for Regulatory Activities (MedDRA) [50].
According to the Preferred Terms of the Medical Dictionary for Regulatory Activities (MedDRA) [50].
Representing >1% of all or preventable STEs.
UTIs were the most commonly psychiatric (17.3%) or vascular (13.5%), hypertension being the most common individual symptom (12.5%). All DD and DA cases were psychiatric (100%), while DIs were distributed in different organ classes.
Sensitivity analyses for total prevalence of persons with ADEs
The prevalences of ADEs and their categories were higher compared with the main analysis when alternative denominators were used (Table 7), with 18.3% (95% CI 16.9, 19.7%) total ADE prevalence for individuals with dispensed drugs, and 24.5% (95% CI 22.8, 26.2%) for individuals with healthcare encounters. The prevalence of all ADEs remained similar to the main analysis when UTIs were omitted from ADEs: 11.4% (95% CI 10.5, 12.3%) for all individuals, 17.6% (95% CI 16.2, 19.0%) for individuals with dispensed drugs, and 23.2% (95% CI 21.5, 24.9%) for individuals with healthcare encounters. When both UTIs and non-preventable STEs were omitted from ADEs, the prevalence was lower: 9.2% (95% CI 8.4, 10.0%) for all individuals, 14.3% (95% CI 13.1, 15.5%) for individuals with dispensed drugs, and 18.9% (95% CI 17.3, 20.4%) for individuals with healthcare encounters.
Table 7.
Sensitivity analyses, by varying the denominator, for the 3 month prevalence of persons with ADEs and preventable ADEs
| Main analysis | Sensitivity analyses | |||||
|---|---|---|---|---|---|---|
| Denominator all individuals (n = 4970) | Denominator individuals with dispensed drugs† (n = 3005) | Denominator individuals with healthcare encounters (n = 2434) | ||||
| n | Prevalence % (95% CI) | n | Prevalence % (95% CI) | n | Prevalence % (95% CI) | |
| Any ADE* | 596 | 12.0 (11.1, 12.9) | 550 | 18.3 (16.9, 19.7) | 596 | 24.5 (22.8, 26.2) |
| ADRs | 342 | 6.9 (6.2, 7.6) | 323 | 10.7 (9.6, 11.9) | 342 | 14.1 (12.7, 15.4) |
| DIs | 7 | 0.1 (0.0, 0.2) | 6 | 0.2 (0.0, 0.4) | 7 | 0.3 (0.1, 0.5) |
| DD or DA | 20 | 0.4 (0.2, 0.6) | 20 | 0.7 (0.4, 1.0) | 20 | 0.8 (0.5, 1.2) |
| STEs | 320 | 6.4 (5.8, 7.1) | 301 | 10.0 (8.9, 11.1) | 320 | 13.1 (11.8, 14.5) |
| UTIs | 47 | 0.9 (0.7, 1.2) | 36 | 1.2 (0.8, 1.6) | 47 | 1.9 (1.4, 2.5) |
| Any preventable ADE* | 278 | 5.6 (5.0, 6.2) | 256 | 8.5 (7.5, 9.5) | 278 | 11.4 (10.2, 12.7) |
| Preventable ADRs | 106 | 2.1 (1.7, 2.5) | 101 | 3.4 (2.7, 4.0) | 106 | 4.4 (3.5, 5.2) |
| Preventable DIs | 7 | 0.1 (0.0, 0.2) | 6 | 0.2 (0.0, 0.4) | 7 | 0.3 (0.1, 0.5) |
| Preventable DD or DA | 18 | 0.4 (0.2, 0.5) | 18 | 0.6 (0.3, 0.9) | 18 | 0.7 (0.4, 1.1) |
| Preventable STEs | 152 | 3.1 (2.6, 3.5) | 141 | 4.7 (3.9, 5.4) | 152 | 6.2 (5.3, 7.2) |
| Preventable UTIs | 35 | 0.7 (0.5, 0.9) | 28 | 0.9 (0.6, 1.3) | 35 | 1.4 (1.0, 1.9) |
ADE, adverse drug event; ADR, adverse drug reaction; CI, confidence interval; DA, drug abuse; DD, drug dependence; DI, drug intoxication from overdose; STE, sub-therapeutic effect of drug therapy; UTI, morbidity due to drug-related untreated indication.
As one person could have multiple ADEs, the combined prevalence is lower than the sum of the prevalences of the ADE categories.
Dispensed drugs from the Swedish Prescribed Drug Register, for all study individuals from 6 months before the study period until the last day before the study period.
Discussion
Our study demonstrates that the prevalence of ADEs is considerable in the entire healthcare, with more than one-third of ADEs potentially preventable. By large, commonly dispensed drugs were commonly associated with ADEs and preventable ADEs, but the associated drugs and affected organs differed by ADE category.
Strengths and weaknesses
This is the first study investigating ADEs in both inpatient and outpatient settings of a random population sample, making our results generalizable to the county and by and large the entire nation. However, the under-representation of persons born outside Sweden in our study population somewhat limits generalizability to the entire nation. We chose a retrospective study design, because recruiting a representative population prospectively for detecting their ADEs in healthcare units would have been practically infeasible and resulted in drop-outs, limiting generalizability. As the retrospective data collection enabled assessing symptoms of ADEs based only on the medical records, symptoms of ADEs that patients had not communicated to care providers or care providers had not recorded are underestimated in our study. To minimize the underestimation, our comprehensive case assessment with clinical experts' causality assessment was designed to detect ADEs that were not recognized, diagnosed, or reported as ADEs, but were otherwise detectable, for example, based on free text or diagnostic tests in the medical records. ADEs from prescribed drugs were probably detected to a greater extent than ADEs from other drugs, because non-prescribed and complementary drugs bought without a prescription are excluded from the SPDR and also less commonly recorded in medical records.
Our study benefited from a thorough ADE definition with categories, enabling the investigation of all adverse events related to drugs combined or divided into categories. However, differing definitions in other studies hindered direct comparisons. We applied established methods for case detection and assessing causality, preventability and seriousness, but the varying reliability and the lack of validation of the current methods [51–54] warrant cautious interpretation of the results.
Comparison to prior research
Our finding that 12% of the adult general population experienced ADEs during a 3 month period was, considering our methods, of similar magnitude with most previous studies [9,17,20–26,55], demonstrating that ADEs are a significant burden in the entire healthcare setting. Our lower prevalence than the recently reported 19% 1 month prevalence of self-reported ADEs among Swedish adults [25] may be explained by our retrospective study design [9], the incompleteness of medical records, and patients' unique ability to report events [21,56]. In particular, the higher prevalence of UTIs in self-reports [25] implies their insufficient detection from medical records exclusively. Our inclusive ADE definition and thorough case detection facilitated by access to all medical records, including before and after the study period, probably contributed in our reasonably high 25% ADE prevalence among individuals with healthcare encounters, compared with studies on outpatients [9,17,20–24,26,55]. The higher proportion of admissions contributed by ADEs in our study, 22%, compared with prior studies [6,9,10], may in addition be explained by the ambition in Sweden to hospitalize only the most severely ill, at high risk of ADEs. Despite our relatively high ADE prevalences compared with previous observational studies, expert panels have estimated ADEs even more common [57–59], indicating that our prevalences are not overestimations.
Drug classes and organ systems associated with ADEs among the general public varied between the ADE categories, but were by and large similar to previous descriptions for ambulatory care patients [9,12,15,17,25,26] and different from hospitalized patients [60–62]. As reported previously [9,17,25,26], the most commonly dispensed drugs, nervous system and cardiovascular drugs, contributed to ADEs the most frequently. Within the nervous system drugs, antidepressants dominated ADRs and analgesics STEs, as found before for self-reports [25], while analgesics, hypnotics and sedatives, and anxiolytics caused DD and DA. Although gastrointestinal ADRs have been described as common [12,15,25], we found them the most common among ADRs. However, if nervous system and psychiatric ADRs and fatigue were combined in our study, ADRs affecting the ‘central nervous system’ would become the most common, in line with others' findings [12,15]. ADRs or ADEs related to electrolyte, renal, hepatic, and haematologic functions are reported more common among hospitalized patients [60–62] than in our and others' general population samples [15,25], probably due to the differing nature of outpatient care, patients' age, and possibly overseeing such events in retrospective studies or studies using patient reports. Similarly to disease specific studies [63,64], but unlike in studies on all ADEs [12], we found hypertension and hyperglycaemia as STEs of antihypertensives and antidiabetics, and psychiatric and musculoskeletal STEs common in the general population. ADEs have previously been described to constitute of heterogeneous events [6,8–12,25–32], but our results illustrate that also reporting the associated drugs and affected organs by ADE category further contributes in understanding their nature.
Our 39% preventability of ADEs in the general population is comparable with previous estimates [8–12,14], as is the similarity of all and preventable ADEs [25,26]. As for all ADEs, nervous system and cardiovascular drugs were the most commonly associated with preventable ADEs, in line with the findings of others [12,25,26,65]. Complementary findings to previous research were our high frequencies of preventable STEs of antihypertensives (resulting in hypertension) and antidiabetics (resulting in hyperglycaemia), which in most studies have not been separated from all preventable ADEs from antidiabetics and antihypertensives [12]. These results reveal the usefulness of categorizing ADEs also for investigating preventability and developing preventive strategies.
Implications and future research
The heterogeneous nature of ADEs in our study reinforces the demand for improving and harmonizing definitions and classifications for ADEs and preventable ADEs, and methods for assessing them [7,51,52,66,67]. Apart from the traditionally emphasized ADRs, the other categories of ADEs combined caused harm more frequently, and differed in their nature from each other and from ADRs in terms of associated drugs, affected organs, preventability and seriousness. The ADE categories should therefore be considered in research and clinical practice for preventing, detecting and mitigating ADEs. Considering the reduction of ADEs more strongly as part of patient safety and quality of care would probably also benefit conceptualizing ADEs.
Although the results of this study reflect the Swedish healthcare system and the prevalence and pattern of ADEs vary depending on the patient population, settings, ADE definitions and methods [6], ADEs are most likely a significant health concern also in other countries and regions. Our results are the most generalizable to other countries with a similar disease burden dominated by non-communicable diseases [68], a similar pattern of drug use with a high annual prevalence of anti-infective, anti-inflammatory, cardiovascular and nervous system drug use [69], and a similarly structured publicly funded healthcare system [70]. Further, ADEs are unlikely to be exceptionally common in Sweden, considering the high quality of the Swedish healthcare system concerning patient safety indicators, compared with other high-income countries [71].
Despite the higher preventability of serious ADEs and ADEs in hospitals, also described by others [9,26], ADEs should be prevented, detected and mitigated in the entire healthcare system, because a large quantity of non-serious events combined may result in considerable direct resource consumption [72], and indirect costs [73]. Further, the high burden of ADEs and preventable ADEs from widely used drugs warrants large scale efforts to redesign safer, higher quality healthcare systems, as urged previously [74–76]. Significant improvements in quality and safety require the commitment of clinicians and care units, collaboration with patients, researchers and safety experts, and strong political will and leadership. As framed by Charles Vincent on improving patient safety: ‘Only very few systems have probably understood the nature and scale of capacity development that is actually needed; most have relied on enthusiasm, culture change and people doing quality improvement work in their non-existent spare time’ [74].
In conclusion, the considerable burden of ADEs and preventable ADEs from commonly used drugs in the adult general public warrants large-scale efforts to redesign safer, higher quality healthcare systems, across care settings. The heterogeneous nature of the ADE categories should be considered in research and clinical practice for preventing, detecting and mitigating ADEs.
Acknowledgments
The research was conducted as part of the project Drug-Related Morbidity in Sweden (DRUMS), and was funded through grants from the National Corporation of Swedish Pharmacies (Apoteket AB), the Region Västra Götaland, and Östergötland County Council. The funders had no role in the design and conduct of the stud, collection, management, analysis, and interpretation of the data and preparation, review, or approval of the manuscript. Persons other than the authors who substantially contributed to the work are: Anders Carlsten (PhD, Nordic School of Public Health NHV/Medical Products Agency), Ingela Jacobsson (BScRN, County Council of Östergötland), Ellinor Ottosson (MScPharm, Nordic School of Public Health NHV), Josefina Lindstén (MScPharm, Nordic School of Public Health NHV), Johnny Pettersson (MSc, Nordic School of Public Health NHV), Clas Rehnberg (PhD, Nordic School of Public Health NHV/Karolinska Institutet), Parshin Saadatirad (MScPharm, Nordic School of Public Health NHV), Staffan Svensson (PhD, Angered Family Medicine Unit), Karin Tunér (MScPharm, Nordic School of Public Health NHV/Region Halland), Annika Yeiter (MMed, Nordic School of Public Health NHV), and Tatiana Zverkova Sandström (BSocSc, Nordic School of Public Health NHV). These contributors were employed for contributing in the study design (AC, CR), data collection (IJ, EO, JL, JP, PS, SS, KT, AY, TZS) and data management (TZS).
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Table S1
Pharmacological sub-groups and drugs associated with ADE categories, ordered according to the most commonly dispensed drugs to all study individuals
Table S2
Drugs associated with symptoms of ADRs or preventable ADRs ≥2 times
Table S3
Drugs associated with symptoms of STEs and preventable STEs ≥2 times
References
- 1.Department of Health. Safety First: A Report for Patients, Clinicians and Healthcare Managers. London: Department of Health; 2006. [Google Scholar]
- 2.World Health Organization. WHO Patient Safety Research – Better Knowledge for Safer Care. Geneva: World Health Organization; 2009. [Google Scholar]
- 3.European Medicines Agency. Medication-Errors Workshop Report. London: European Medicines Agency; 2013. [Google Scholar]
- 4.Ministry of Health and Social Affairs. National Medication Strategy. Stockholm: Ministry of Health and Social Affairs; 2011. [in Swedish] [Google Scholar]
- 5.Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, Laffel G, Sweitzer BJ, Shea BF, Hallisey R. Incidence of adverse drug events and potential adverse drug events: implications for prevention. ADE Prevention Study Group. JAMA. 1995;274:29–34. [PubMed] [Google Scholar]
- 6.Leendertse AJ, Visser D, Egberts AC, van den Bemt PM. The relationship between study characteristics and the prevalence of medication-related hospitalizations: a literature review and novel analysis. Drug Saf. 2010;33:233–244. doi: 10.2165/11319030-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Aronson JK, Ferner RE. Clarification of terminology in drug safety. Drug Saf. 2005;28:851–870. doi: 10.2165/00002018-200528100-00003. [DOI] [PubMed] [Google Scholar]
- 8.Krähenbuhl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krähenbuhl S. Drug-related problems in hospitals: a review of the recent literature. Drug Saf. 2007;30:379–407. doi: 10.2165/00002018-200730050-00003. [DOI] [PubMed] [Google Scholar]
- 9.Tache SV, Sonnichsen A, Ashcroft DM. Prevalence of adverse drug events in ambulatory care: a systematic review. Ann Pharmacother. 2011;45:977–989. doi: 10.1345/aph.1P627. [DOI] [PubMed] [Google Scholar]
- 10.Winterstein AG, Sauer BC, Hepler CD, Poole C. Preventable drug-related hospital admissions. Ann Pharmacother. 2002;36:1238–1248. doi: 10.1345/aph.1A225. [DOI] [PubMed] [Google Scholar]
- 11.Kanjanarat P, Winterstein AG, Johns TE, Hatton RC, Gonzalez-Rothi R, Segal R. Nature of preventable adverse drug events in hospitals: a literature review. Am J Health Syst Pharm. 2003;60:1750–1759. doi: 10.1093/ajhp/60.17.1750. [DOI] [PubMed] [Google Scholar]
- 12.Thomsen LA, Winterstein AG, Sondergaard B, Haugbolle LS, Melander A. Systematic review of the incidence and characteristics of preventable adverse drug events in ambulatory care. Ann Pharmacother. 2007;41:1411–1426. doi: 10.1345/aph.1H658. [DOI] [PubMed] [Google Scholar]
- 13.Cano FG, Rozenfeld S. Adverse drug events in hospitals: a systematic review. Cad Saude Publica. 2009;25(Suppl. 3):S360–372. doi: 10.1590/s0102-311x2009001500003. [DOI] [PubMed] [Google Scholar]
- 14.Hakkarainen KM, Hedna K, Petzold M, Hägg S. Percentage of patients with preventable adverse drug reactions and preventability of adverse drug reactions – a meta-analysis. PLoS ONE. 2012;7:e33236. doi: 10.1371/journal.pone.0033236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isacson D, Johansson L, Bingefors K. Nationwide survey of subjectively reported adverse drug reactions in Sweden. Ann Pharmacother. 2008;42:347–353. doi: 10.1345/aph.1K488. [DOI] [PubMed] [Google Scholar]
- 16.Weingart SN, Gandhi TK, Seger AC, Seger DL, Borus J, Burdick E, Leape LL, Bates DW. Patient-reported medication symptoms in primary care. Arch Intern Med. 2005;165:234–240. doi: 10.1001/archinte.165.2.234. [DOI] [PubMed] [Google Scholar]
- 17.Gandhi TK, Weingart SN, Borus J, Seger AC, Peterson J, Burdick E, Seger DL, Shu K, Federico F, Leape LL, Bates DW. Adverse drug events in ambulatory care. N Engl J Med. 2003;348:1556–1564. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- 18.Chrischilles EA, Segar ET, Wallace RB. Self-reported adverse drug reactions and related resource use. A study of community-dwelling persons 65 years of age and older. Ann Intern Med. 1992;117:634–640. doi: 10.7326/0003-4819-117-8-634. [DOI] [PubMed] [Google Scholar]
- 19.Hutchinson TA, Flegel KM, Kramer MS, Leduc DG, Kong HH. Frequency, severity and risk factors for adverse drug reactions in adult out-patients: a prospective study. J Chronic Dis. 1986;39:533–542. doi: 10.1016/0021-9681(86)90198-0. [DOI] [PubMed] [Google Scholar]
- 20.Oladimeji O, Farris KB, Urmie JG, Doucette WR. Risk factors for self-reported adverse drug events among Medicare enrollees. Ann Pharmacother. 2008;42:53–61. doi: 10.1345/aph.1K073. [DOI] [PubMed] [Google Scholar]
- 21.Gandhi TK, Burstin HR, Cook EF, Puopolo AL, Haas JS, Brennan TA, Bates DW. Drug complications in outpatients. J Gen Intern Med. 2000;15:149–154. doi: 10.1046/j.1525-1497.2000.04199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green JL, Hawley JN, Rask KJ. Is the number of prescribing physicians an independent risk factor for adverse drug events in an elderly outpatient population? Am J Geriatr Pharmacother. 2007;5:31–39. doi: 10.1016/j.amjopharm.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Chrischilles E, Rubenstein L, Van Gilder R, Voelker M, Wright K, Wallace R. Risk factors for adverse drug events in older adults with mobility limitations in the community setting. J Am Geriatr Soc. 2007;55:29–34. doi: 10.1111/j.1532-5415.2006.01034.x. [DOI] [PubMed] [Google Scholar]
- 24.Shiyanbola OO, Farris KB. Concerns and beliefs about medicines and inappropriate medications: an Internet-based survey on risk factors for self-reported adverse drug events among older adults. Am J Geriatr Pharmacother. 2010;8:245–257. doi: 10.1016/j.amjopharm.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Hakkarainen KM, Andersson Sundell K, Petzold M, Hägg S. Prevalence and perceived preventability of self-reported adverse drug events – a population-based survey of 7099 adults. PLoS ONE. 2013;8:e73166. doi: 10.1371/journal.pone.0073166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurwitz JH, Field TS, Harrold LR, Rothschild J, Debellis K, Seger AC, Cadoret C, Fish LS, Garber L, Kelleher M, Bates DW. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289:1107–1116. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 27.Al-Olah YH, Al Thiab KM. Admissions through the emergency department due to drug-related problems. Ann Saudi Med. 2008;28:426–429. doi: 10.5144/0256-4947.2008.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zargarzadeh AH, Emami MH, Hosseini F. Drug-related hospital admissions in a generic pharmaceutical system. Clin Exp Pharmacol Physiol. 2007;34:494–498. doi: 10.1111/j.1440-1681.2007.04600.x. [DOI] [PubMed] [Google Scholar]
- 29.Raschetti R, Morgutti M, Menniti-Ippolito F, Belisari A, Rossignoli A, Longhini P, La Guidara C. Suspected adverse drug events requiring emergency department visits or hospital admissions. Eur J Clin Pharmacol. 1999;54:959–963. doi: 10.1007/s002280050582. [DOI] [PubMed] [Google Scholar]
- 30.Franceschi A, Tuccori M, Bocci G, Vannozzi F, Di Paolo A, Barbara C, Lastella M, Blandizzi C, Del Tacca M. Drug therapeutic failures in emergency department patients. A university hospital experience. Pharmacol Res. 2004;49:85–91. doi: 10.1016/j.phrs.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Roughead EE. The nature and extent of drug-related hospitalisations in Australia. J Qual Clin Pract. 1999;19:19–22. doi: 10.1046/j.1440-1762.1999.00288.x. [DOI] [PubMed] [Google Scholar]
- 32.Hallas J, Gram LF, Grodum E, Damsbo N, Brosen K, Haghfelt T, Harvald B, Beck-Nielsen J, Worm J, Jensen KB. Drug related admissions to medical wards: a population based survey. Br J Clin Pharmacol. 1992;33:61–68. doi: 10.1111/j.1365-2125.1992.tb04001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morimoto T, Gandhi TK, Seger AC, Hsieh TC, Bates DW. Adverse drug events and medication errors: detection and classification methods. Qual Saf Health Care. 2004;13:306–314. doi: 10.1136/qshc.2004.010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356:1255–1259. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 35.Goettler M, Schneeweiss S, Hasford J. Adverse drug reaction monitoring – Cost and benefit considerations. Part II: cost and preventability of adverse drug reactions leading to hospital admission. Pharmacoepidemiol Drug Saf. 1997;6(Suppl. 3):S79–90. doi: 10.1002/(sici)1099-1557(199710)6:3+<s79::aid-pds294>3.3.co;2-f. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. Technical Report Series. International Drug Monitoring: The Role of National Centres. Geneva: World Health Organization; 1972. [PubMed] [Google Scholar]
- 37.van Gijssel-Wiersma DG, van den Bemt PM, Walenbergh-van Veen MC. Influence of computerised medication charts on medication errors in a hospital. Drug Saf. 2005;28:1119–1129. doi: 10.2165/00002018-200528120-00006. [DOI] [PubMed] [Google Scholar]
- 38.Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2007;64:566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- 39.Jönsson AK, Spigset O, Tjäderborn M, Druid H, Hägg S. Fatal drug poisonings in a Swedish general population. BMC Clin Pharmacol. 2009;9:7. doi: 10.1186/1472-6904-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hallas J, Harvald B, Worm J, Beck-Nielsen J, Gram LF, Grodum E, Damsbo N, Schou J, Kromann-Andersen H, Frolund F. Drug related hospital admissions: results from an intervention program. Eur J Clin Pharmacol. 1993;45:199–203. doi: 10.1007/BF00315383. [DOI] [PubMed] [Google Scholar]
- 41.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4th edn. Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- 42.Hallas J, Harvald B, Gram LF, Grodum E, Brosen K, Haghfelt T, Damsbo N. Drug related hospital admissions: the role of definitions and intensity of data collection, and the possibility of prevention. J Intern Med. 1990;228:83–90. doi: 10.1111/j.1365-2796.1990.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 43.Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad Olausson P, Bergman U, Persson I, Sundstrom A, Westerholm B, Rosen M. The new Swedish Prescribed Drug Register – Opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16:726–735. doi: 10.1002/pds.1294. [DOI] [PubMed] [Google Scholar]
- 44.Wirehn AB, Karlsson HM, Carstensen JM. Estimating disease prevalence using a population-based administrative healthcare database. Scand J Public Health. 2007;35:424–431. doi: 10.1080/14034940701195230. [DOI] [PubMed] [Google Scholar]
- 45.Östergötland County Council. Annual Report 2008. Linköping: Östergötland County Council; 2009. [in Swedish] [Google Scholar]
- 46.Böttiger Y, Laine K, Andersson ML, Korhonen T, Molin B, Ovesjo ML, Tirkkonen T, Rane A, Gustafsson LL, Eiermann B. SFINX – A drug-drug interaction database designed for clinical decision support systems. Eur J Clin Pharmacol. 2009;65:627–633. doi: 10.1007/s00228-008-0612-5. [DOI] [PubMed] [Google Scholar]
- 47.Howard RL, Avery AJ, Howard PD, Partridge M. Investigation into the reasons for preventable drug related admissions to a medical admissions unit: observational study. Qual Saf Health Care. 2003;12:280–285. doi: 10.1136/qhc.12.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.National Board of Health and Welfare. Public Health Report. Västerås: National Board of Health and Welfare; 2009. [In Swedish] [Google Scholar]
- 49.World Health Organization. Guidelines for ATC Classification and DDD Assignment 2010. Oslo: WHO Collaborating Centre for Drug Statistics Methodology; 2009. [Google Scholar]
- 50.Medical Dictionary for Regulatory Activities. 2011. Introductory Guide MedDRA Version 14.0, document MSSO-DI-6003-14.0.0, Geneva: Medical Dictionary for Regulatory Activities.
- 51.Hakkarainen KM, Andersson Sundell K, Petzold M, Hägg S. Methods for assessing the preventability of adverse drug events: a systematic review. Drug Saf. 2012;35:105–126. doi: 10.2165/11596570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 52.Ferner RE, Aronson JK. Preventability of drug-related harms – Part I: a systematic review. Drug Saf. 2010;33:985–994. doi: 10.2165/11538270-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 53.Agbabiaka TB, Savovic J, Ernst E. Methods for causality assessment of adverse drug reactions: a systematic review. Drug Saf. 2008;31:21–37. doi: 10.2165/00002018-200831010-00003. [DOI] [PubMed] [Google Scholar]
- 54.Kunac DL, Reith DM, Kennedy J, Austin NC, Williams SM. Inter- and intra-rater reliability for classification of medication related events in paediatric inpatients. Qual Saf Health Care. 2006;15:196–201. doi: 10.1136/qshc.2005.014407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanlon JT, Schmader KE, Koronkowski MJ, Weinberger M, Landsman PB, Samsa GP, Lewis IK. Adverse drug events in high risk older outpatients. J Am Geriatr Soc. 1997;45:945–948. doi: 10.1111/j.1532-5415.1997.tb02964.x. [DOI] [PubMed] [Google Scholar]
- 56.Weissman JS, Schneider EC, Weingart SN, Epstein AM, David-Kasdan J, Feibelmann S, Annas CL, Ridley N, Kirle L, Gatsonis C. Comparing patient-reported hospital adverse events with medical record review: do patients know something that hospitals do not? Ann Intern Med. 2008;149:100–108. doi: 10.7326/0003-4819-149-2-200807150-00006. [DOI] [PubMed] [Google Scholar]
- 57.Gyllensten H, Hakkarainen KM, Jönsson AK, Andersson Sundell K, Hägg S, Rehnberg C, Carlsten A. Modelling drug-related morbidity in Sweden using an expert panel of pharmacists. Int J Clin Pharmacol. 2012;34:538–546. doi: 10.1007/s11096-012-9641-3. [DOI] [PubMed] [Google Scholar]
- 58.Johnson JA, Bootman JL. Drug-related morbidity and mortality. A cost-of-illness model. Arch Intern Med. 1995;155:1949–1956. [PubMed] [Google Scholar]
- 59.Hakkarainen KM, Alström D, Hägg S, Carlsten A, Gyllensten H. Modelling drug-related morbidity in Sweden using an expert panel of physicians. Eur J Clin Pharmacol. 2012;68:1309–1319. doi: 10.1007/s00228-012-1244-3. [DOI] [PubMed] [Google Scholar]
- 60.Wiffen P, Gill M, Edwards J, Moore A. Adverse drug reactions in hospital patients – A systematic review of the prospective and retrospective studies. Bandolier Extra. 2002:1–16. June. [Google Scholar]
- 61.Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, Farrar K, Park BK, Breckenridge AM. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329:15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR, Pirmohamed M. Adverse drug reactions in hospital in-patients: a prospective analysis of 3695 patient-episodes. PLoS ONE. 2009;4:e4439. doi: 10.1371/journal.pone.0004439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cuijpers P. The patient perspective in research on major depression. BMC Psychiatry. 2011;11:89. doi: 10.1186/1471-244X-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ho PM, Rumsfeld JS, Masoudi FA, McClure DL, Plomondon ME, Steiner JF, Magid DJ. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166:1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 65.Howard RL, Avery AJ, Slavenburg S, Royal S, Pipe G, Lucassen P, Pirmohamed M. Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol. 2007;63:136–147. doi: 10.1111/j.1365-2125.2006.02698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pintor-Marmol A, Baena MI, Fajardo PC, Sabater-Hernandez D, Saez-Benito L, Garcia-Cardenas MV, Fikri-Benbrahim N, Azpilicueta I, Faus MJ. Terms used in patient safety related to medication: a literature review. Pharmacoepidemiol Drug Saf. 2012;21:799–809. doi: 10.1002/pds.3296. [DOI] [PubMed] [Google Scholar]
- 67.Yu KH, Nation RL, Dooley MJ. Multiplicity of medication safety terms, definitions and functional meanings: when is enough enough? Qual Saf Health Care. 2005;14:358–363. doi: 10.1136/qshc.2005.014159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Danielsson M, Talback M. Public health: an overview: health in Sweden: the National Public Health Report 2012. Chapter 1. Scand J Public Health. 2012;40:6–22. doi: 10.1177/1403494812459457. [DOI] [PubMed] [Google Scholar]
- 69.Weitoft GR, Ericsson O, Fastbom J. Prescription drugs: health in Sweden: the National Public Health Report 2012. Chapter 18. Scand J Public Health. 2012;40:293–304. doi: 10.1177/1403494812459623. [DOI] [PubMed] [Google Scholar]
- 70.Anell A, Glenngård AH, Merkur S. Sweden: health system review. Health Syst Transit. 2012;14:1–159. [PubMed] [Google Scholar]
- 71.Organisation for Economic Co-operation and Development. 2009. Health care quality indicators project: patient safety indicators report 2009 (OECD Health Working Paper No. 47), Paris: Organisation for Economic Co-operation and Development.
- 72.Runciman WB, Edmonds MJ, Pradhan M. Setting priorities for patient safety. Qual Saf Health Care. 2002;11:224–229. doi: 10.1136/qhc.11.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gyllensten H, Rehnberg C, Jönsson AK, Petzold M, Carlsten A, Andersson Sundell K. Cost of illness of patient-reported adverse drug events: a population-based cross-sectional survey. BMJ Open. 2013;3:e002574. doi: 10.1136/bmjopen-2013-002574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vincent C. High performing healthcare systems. In: Vincent C, editor. Patient Safety. 2nd edn. West Sussex: Wiley-Blackwell; 2010. pp. 390–404. [Google Scholar]
- 75.Nolan TW. System changes to improve patient safety. BMJ. 2000;320:771–773. doi: 10.1136/bmj.320.7237.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leape LL. System analysis and redesign: the foundation of medical error prevention. In: Cohen MR, editor. Medication Errors. 2nd edn. Washington DC: American Pharmacists' Association; 2006. pp. 3–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Pharmacological sub-groups and drugs associated with ADE categories, ordered according to the most commonly dispensed drugs to all study individuals
Table S2
Drugs associated with symptoms of ADRs or preventable ADRs ≥2 times
Table S3
Drugs associated with symptoms of STEs and preventable STEs ≥2 times