Abstract
We examined the functional consequences of cellular transformation of rat IAR-2 epithelial cells, by a mutant N-ras oncogene, on the dynamics of active lamellae, structures that play an important role in cell motility, adhesion, and surface-receptor capping. Lamellar activity was assessed by measuring the rate of outer-edge pseudopodial activity and by analyzing the motility of Con A-coated beads placed on lamellar surfaces with optical tweezers. Although transformation dramatically affected the shape and size of active cellular lamellae, there was little detectable effect on either pseudopodial activity or bead movement. To investigate the potential relationship between functional lamellar activity and the microtubule cytoskeleton, lamellar activity was examined in nontransformed and transformed cells treated with the microtubule-disrupting drug nocodazole. In the absence of microtubules, transformed cells were less polarized and possessed decreased rates of pseudopodial and bead motility. On the basis of these observations, it is suggested that ras-induced transformation of epithelial cells consists of two cytoskeletal modifications: overall diminished actin cytoskeletal dynamics in lamellae and reorganization of the microtubule cytoskeleton that directs pseudopodial activity to smaller polarized lamellae.
Full text
PDF

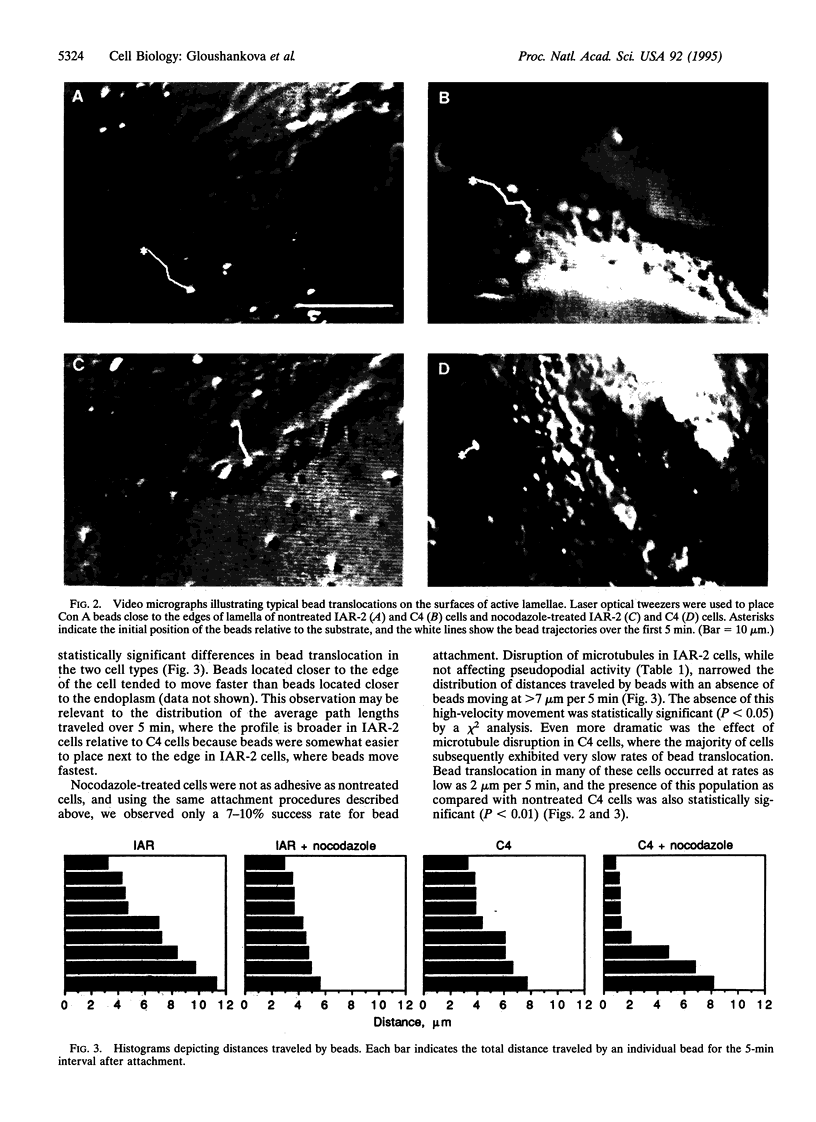

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandrova A. Y., Dugina V. B., Paterson H., Bershadsky A. D., Vasiliev J. M. Motility of intracellular particles in rat fibroblasts is greatly enhanced by phorbol ester and by over-expression of normal p21N-ras. Cell Motil Cytoskeleton. 1993;25(3):254–266. doi: 10.1002/cm.970250306. [DOI] [PubMed] [Google Scholar]
- Ashkin A., Dziedzic J. M., Yamane T. Optical trapping and manipulation of single cells using infrared laser beams. Nature. 1987 Dec 24;330(6150):769–771. doi: 10.1038/330769a0. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D., Feramisco J. R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986 Sep 5;233(4768):1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- Bershadsky A. D., Vaisberg E. A., Vasiliev J. M. Pseudopodial activity at the active edge of migrating fibroblast is decreased after drug-induced microtubule depolymerization. Cell Motil Cytoskeleton. 1991;19(3):152–158. doi: 10.1002/cm.970190303. [DOI] [PubMed] [Google Scholar]
- Condeelis J. Life at the leading edge: the formation of cell protrusions. Annu Rev Cell Biol. 1993;9:411–444. doi: 10.1146/annurev.cb.09.110193.002211. [DOI] [PubMed] [Google Scholar]
- Gloushankova N. A., Lyubimova A. V., Tint I. S., Feder H. H., Vasiliev J. M., Gelfand I. M. Role of the microtubular system in morphological organization of normal and oncogene-transfected epithelial cells. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8597–8601. doi: 10.1073/pnas.91.18.8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucik D. F., Kuo S. C., Elson E. L., Sheetz M. P. Preferential attachment of membrane glycoproteins to the cytoskeleton at the leading edge of lamella. J Cell Biol. 1991 Sep;114(5):1029–1036. doi: 10.1083/jcb.114.5.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi L., Ballinari D., Bongarzone I., Migliari M., Mondellini P., Traversari C., Modina S. Ultrastructural cytoskeleton alterations and modification of actin expression in the NIH/3T3 cell line after transformation with Ha-ras-activated oncogene. Cell Motil Cytoskeleton. 1990;15(4):220–229. doi: 10.1002/cm.970150405. [DOI] [PubMed] [Google Scholar]
- Montesano R., Saint Vincent L., Drevon C., Tomatis L. Production of epithelial and mesenchymal tumours with rat liver cells transformed in vitro. Int J Cancer. 1975 Oct 15;16(4):550–558. doi: 10.1002/ijc.2910160405. [DOI] [PubMed] [Google Scholar]
- Prendergast G. C., Gibbs J. B. Pathways of Ras function: connections to the actin cytoskeleton. Adv Cancer Res. 1993;62:19–64. doi: 10.1016/s0065-230x(08)60314-0. [DOI] [PubMed] [Google Scholar]
- Schmidt C. E., Horwitz A. F., Lauffenburger D. A., Sheetz M. P. Integrin-cytoskeletal interactions in migrating fibroblasts are dynamic, asymmetric, and regulated. J Cell Biol. 1993 Nov;123(4):977–991. doi: 10.1083/jcb.123.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliev J. M., Gelfand I. M., Domnina L. V., Zacharova O. S., Ljubimov A. V. Contact inhibition of phagocytosis in epithelial sheets: alterations of cell surface properties induced by cell-cell contacts. Proc Natl Acad Sci U S A. 1975 Feb;72(2):719–722. doi: 10.1073/pnas.72.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliev J. M. Polarization of pseudopodial activities: cytoskeletal mechanisms. J Cell Sci. 1991 Jan;98(Pt 1):1–4. doi: 10.1242/jcs.98.1.1. [DOI] [PubMed] [Google Scholar]
- Wirth P. J., Luo L. D., Fujimoto Y., Bisgaard H. C. Two-dimensional electrophoretic analysis of transformation-sensitive polypeptides during chemically, spontaneously, and oncogene-induced transformation of rat liver epithelial cells. Electrophoresis. 1992 May;13(5):305–320. doi: 10.1002/elps.1150130163. [DOI] [PubMed] [Google Scholar]




