Abstract
Context
The initial report of the Selenium and Vitamin E Cancer Prevention Trial (SELECT) found no reduction in risk of prostate cancer with either selenium or vitamin E supplements but a non-statistically significant increase in prostate cancer risk with vitamin E. Longer follow-up and more prostate cancer events provide further insight into the relationship of vitamin E and prostate cancer.
Objective
To determine the long-term effect of vitamin E and selenium on risk of prostate cancer in relatively healthy men.
Design, Setting and Participants
SELECT randomized 35,533 men from 427 study sites in the United States, Canada and Puerto Rico in a double-blind manner between August 22, 2001 and June 24, 2004. Eligible men were 50 years or older (African Americans) or 55 years or older (all others) with a PSA ≤4.0 ng/mL and a digital rectal examination not suspicious for prostate cancer. Included in the analysis are 34,887 men randomly assigned to one of four treatment groups: selenium (n=8752), vitamin E (n=8737), both agents (n=8702), or placebo (n=8696). Data reflect the final data collected by the study sites on their participants through July 5, 2011.
Interventions
Oral selenium (200 μg/day from L-selenomethionine) with matched vitamin E placebo, vitamin E (400 IU/d of all rac-α-tocopheryl acetate) with matched selenium placebo, both agents, or both matched placebos for a planned follow-up of a minimum of 7 and maximum of 12 years.
Main Outcome Measures
Prostate cancer incidence.
Results
This report includes 54,464 additional person-years of follow-up since the primary report. Hazard ratios (99% confidence intervals [CI]) and numbers of prostate cancers were 1.17(99% CI 1.004-1.36, p=.008, n=620) for vitamin E, 1.09 (99% CI 0.93-1.27, p=.18, n=575) for selenium, 1.05 (99%CI 0.89-1.22, p=.46, n=555) for selenium + vitamin E vs. 1.00 (n=529) for placebo.The absolute increase in risk compared with placebo for vitamin E, selenium and the combination were 1.6, 0.9 and 0.4 cases of prostate cancer per 1,000 person-years.
Conclusions
Dietary supplementation with Vitamin E significantly increases the risk of prostate cancer among healthy men.
Trial registration
clinicaltrials.gov identifier: NCT00006392
Lifetime risk of prostate cancer in the United Sates is currently estimated to be 16%.1 While most cases are found at an early, curable stage, treatment is costly and urinary, sexual, and bowel-related side effects are common.2 Even men who choose active surveillance as an initial management strategy face anxiety, uncertain prognosis, and a measurable risk of sepsis with follow-up biopsies3, and more than one-third of those who initially defer therapy are ultimately treated.4,5 With such a high prevalence, risk of morbidity from treatment, and treatment-related costs, primary prevention of prostate cancer is an attractive option.
With considerable preclinical and epidemiologic evidence that selenium and vitamin E may reduce prostate cancer risk, we conducted and reported the results of a prospective randomized trial examining the effect of these two agents for prostate cancer prevention.6 Coordinated by SWOG, a federally funded cancer research cooperative group, the Selenium and Vitamin E Cancer Prevention Trial (SELECT) began accrual on August 22, 2001 and randomized 35,533 men into four groups: (1) selenium with matching placebo, (2) vitamin E with matching placebo, (3) both agents, or (4) placebo.
Based on a preplanned interim analysis, the independent data and safety monitoring committee (DSMC) met on September 15, 2008 and recommended the early discontinuation of study supplements because of lack of efficacy for risk reduction and because futility analysis demonstrated no possibility of benefit to the planned degree with additional follow-up.6 With a median follow-up of 5.5 years, the number of prostate cancers detected, hazard ratios [HR] and 99% confidence intervals [CI] were n=473, HR=1.13, 99% CI 0.95-1.35 for vitamin E; n=432, HR=1.04,99% CI 0.87-1.24for selenium; n=437, HR=1.05, 99% CI 0.88-1.25for selenium + vitamin E; and n=416, HR=1.0 for placebo. While these results were not statistically significant, the DSMC expressed concern about the increased risk of prostate cancer observed in the vitamin E + placebo group which approached statistical significance (p=0.06) and a statistically non-significant increased risk of type 2 diabetes mellitus in the selenium + placebo group (p=0.16). Since that time participant follow-up has continued, allowing observation of additional events. On May 20, 2011, the DSMC reviewed trial data and recommended reporting the finding regarding increased risk of prostate cancer with vitamin E. This recommendation was based on final data collection from the study sites and coincided with the preplanned final analysis at 7 years after the last participant was randomized.
Methods
Detailed descriptions of the rationale, design, conduct, and initial results of SELECT have been previously published.6,7 The study enrolled healthy men at average risk of prostate cancer based on a baseline PSA of ≤ 4 ng/mL and normal digital rectal exam (DRE) commencing at age 50 for African Americans or age 55 for all others. Subjects were randomized into one of four groups: (1) selenium (200 μg/day from L-selenomethionine) with matching vitamin E placebo, (2) vitamin E (400 IU/day of all rac-α-tocopherol acetate) with matching selenium placebo, (3) both agents, or (4) placebo + placebo. Participants without prostate cancer were monitored every 6 months with an annual limited physical examination including blood pressure, weight, and smoking status; participants who developed prostate cancer while on study were monitored annually thereafter. Participants were recommended to undergo PSA and DRE testing and prostate biopsy based on the standard of care in their community and in accordance with the participant's preference. To facilitate adherence, a multivitamin containing no selenium or vitamin E was offered. All participants were required to provide written informed consent and the local institutional review board of each study site approved the study. At study visits, men were asked about new medical events in the previous 6 months. The primary endpoint of the study was prostate cancer incidence as determined by routine clinical management and confirmed by central pathology review. Blinded follow-up continued until October 23, 2008, at which time participants discontinued use of study supplements. Prostate cancer status was determined by self-report at each 6-month study visit. Medical records were obtained thereafter and clinical stage and diagnostic method abstracted. The pathology report and tissue were forwarded to the SELECT central pathology laboratory for confirmation of diagnosis and for assignment of Gleason score. Median baseline and follow up plasma vitamin E and selenium levels are included in our original report.
Follow-up continued in an unblinded fashion at study sites from October 2008 until July 2011.The final study site visits included follow-up for study endpoints, and a blood sample for participants diagnosed with prostate cancer. An independent DSMC met yearly commencing with study inception, reviewing data on safety, adherence, and prostate and other cancer diagnoses. On September 15, 2008 the DSMC recommended reporting initial results related to the lack of efficacy of the agents on prevention of prostate cancer. Since that time the DSMC has continued to meet yearly via teleconference.
Statistical Analysis
The primary endpoint of SELECT was prostate cancer incidence resulting from routine community care. Cancers not centrally confirmed (17% of the total) are included in the analysis. Five pre-specified comparisons of the four study groups were conducted: (1) selenium vs placebo, (2) vitamin E vs placebo, (3) selenium + vitamin E vs placebo, (4) selenium vs selenium + vitamin E, and (5) vitamin E vs selenium + vitamin E. Although a one-sided significance level of 0.005 was specified to test for the preventive effect for each supplement comparison and thus 99% confidence intervals are reported, we have reported two-sided p-values throughout because the comparison of prevention vs. increased risk of cancer is a two-sided question.6 A proportional hazards model was used to compare prostate cancer and other cancer incidence between placebo and each of the three arms with active agents. Those without the endpoint of interest were censored at their last contact date. An additional analysis was performed on all the data using a variable for selenium supplementation, a variable for vitamin E supplementation, and an interaction term. In all cases, the proportional hazards assumption was evaluated by assessing each study arm by time interaction. The cumulative incidence curves for prostate cancer were generated accounting for the competing risk of death.8 A chi-square test was used to test the difference in the relative risk of diabetes. Data were analyzed using SAS version 9.2 (SAS Institute Inc, Cary, North Carolina).
Results
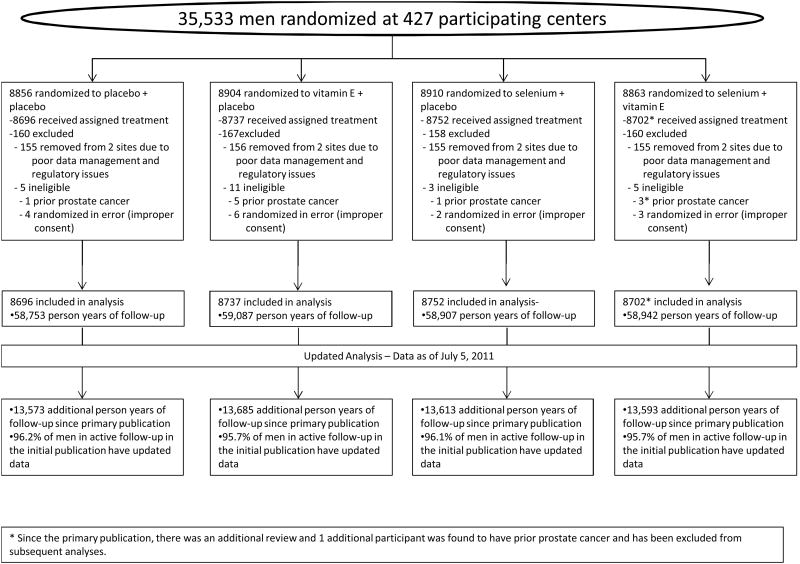
The current report includes data as of July 5, 2011. There are 54,464 additional person-years of follow-up since the primary report, an increase of 23%.A summary of baseline characteristics are displayed in Table 1 and an updated CONSORT diagram in Figure 1. The frequency of use of DRE and PSA is displayed in Table 2; no differences between arms are apparent in the intensity of PSA testing, absolute PSA levels, PSA change from study entry to year 1, nor rates of testing following study unblinding. A total of 521 additional prostate cancers have been diagnosed since the initial report: 113 in the placebo group, 147 in the Vitamin E group, 143 in the selenium group, and 118 in the combination group (Table 3). The rate of prostate cancer detection was greater in all treatment groups when compared with placebo but was statistically significant only in the vitamin E alone group (HR 1.17, 99% CI 1.004-1.36, p=0.008) (Table 3). After adjustment for the marginal effects of vitamin E and selenium, the interaction between vitamin E and selenium was statistically significant (p=0.021), indicating no increased risk of prostate cancer when vitamin E and selenium were taken together. The risk of Gleason 7 or greater disease was higher for all three interventions (vitamin E, HR = 1.16, 99% CI 0.86 – 1.58; selenium, HR = 1.21, 99% CI 0.90 – 1.63; combination, HR = 1.06, 99% CI 0.91 – 1.66) but did not reach statistical significance for any group (Table 3). The elevated risk estimate for vitamin E was consistent across both low and high grade disease. There was no difference in overall survival between any of the treatment groups (all p≥ 0.47, Table 5).
Table 1. Baseline participant characteristics.
| Placebo (n=8,696) |
Vitamin E alone (n=8,737) |
Selenium alone (n=8,752) |
Vitamin E + selenium (n=8,702) |
|||||
|---|---|---|---|---|---|---|---|---|
| AGE | ||||||||
| Median (interquartile range) | 63 | (58-67) | 62 | (58.0-67) | 63 | (58-68) | 62 | (58-67) |
| 50 - 54 years | 355 | 4% | 403 | 5% | 337 | 4% | 385 | 4% |
| 55 - 64 years | 5,078 | 58% | 5,142 | 59% | 5,075 | 58% | 5,051 | 58% |
| 65 - 74 years | 2,702 | 31% | 2,642 | 30% | 2,734 | 31% | 2,731 | 31% |
| 75+ years | 561 | 6% | 550 | 6% | 606 | 7% | 535 | 6% |
| RACE | ||||||||
| White | 6,862 | 79% | 6,893 | 79% | 6,944 | 79% | 6,872 | 79% |
| African American | 1,083 | 12% | 1,106 | 13% | 1,054 | 12% | 1,075 | 12% |
| Hispanic (Non-Afri. American) | 496 | 6% | 476 | 5% | 484 | 6% | 484 | 6% |
| Hispanic (Afri. American) | 76 | 1% | 103 | 1% | 86 | 1% | 96 | 1% |
| Aboriginal | 27 | 0% | 22 | 0% | 41 | 0% | 29 | 0% |
| Asian/Pacific Islander | 128 | 1% | 110 | 1% | 111 | 1% | 123 | 1% |
| Unknown | 24 | 0% | 27 | 0% | 32 | 0% | 23 | 0% |
| PSA (NG/ML) | ||||||||
| Median (interquartile range) | 1.1 | (0.6,1.9) | 1.1 | (0.6,1.9) | 1.1 | (0.6,1.9) | 1.1 | (0.6,1.8) |
| 0.1 - 1.0 | 4,133 | 48% | 4,234 | 48% | 4,247 | 49% | 4,235 | 49% |
| 1.1 - 2.0 | 2,735 | 31% | 2,648 | 30% | 2,652 | 30% | 2,657 | 31% |
| 2.1 - 3.0 | 1,153 | 13% | 1,222 | 14% | 1,199 | 14% | 1,147 | 14% |
| 3.1 - 4.0 | 668 | 8% | 627 | 7% | 649 | 7% | 656 | 7% |
| >4.0 | 5 | 0% | 3 | 0% | 2 | 0% | 1 | 0% |
| Missing | 2 | 0% | 3 | 0% | 3 | 0% | 6 | 0% |
Figure 1. CONSORT Diagram.
Table 2. Diagnostic Testing.
| Placebo (n=8,696) |
Vitamin E alone (n=8,737) |
Selenium alone (n=8,752) |
Vitamin E + selenium (n=8,702) |
|
|---|---|---|---|---|
| Prostate biopsy (men ever having biopsy) | ||||
| Prior to unblinding1 | 1,041 | 1,046 | 1,003 | 1,014 |
| Post unblinding | 256 | 268 | 267 | 254 |
| Number of DREs/participant | ||||
| Prior to unblinding | 3.20 | 3.20 | 3.20 | 3.21 |
| Post unblinding | 0.64 | 0.63 | 0.64 | 0.64 |
| Number of PSA tests/participant | ||||
| Prior to unblinding | 3.87 | 3.88 | 3.87 | 3.90 |
| Post unblinding | 0.84 | 0.85 | 0.86 | 0.86 |
| Geometric mean PSA (95% CI) | ||||
| Year 0 | 1.13 (0.24, 4.41) | 1.12 (0.23, 4.37) | 1.12 (0.23, 4.41) | 1.13 (0.23, 4.42) |
| Year 1 | 1.16 (0.22, 4.82) | 1.14(0.21, 4.75) | 1.14 (0.21, 4.89) | 1.15 (0.21, 4.91) |
| Year 2 | 1.18 (0.21, 5.08) | 1.15 (0.21, 4.95) | 1.17 (0.21, 5.12) | 1.16 (0.21, 5.08) |
| Year 3 | 1.19 (0.21, 5.25) | 1.17 (0.20, 5.26) | 1.20 (0.21, 5.31) | 1.19 (0.21, 5.25) |
| Year 4 | 1.23 (0.21,5.62) | 1.19 (0.20, 5.40) | 1.23 (0.21, 5.61) | 1.23 (0.21, 5.62) |
| Year 5 | 1.25 (0.21, 5.81) | 1.23 (0.21, 5.62) | 1.26 (0.21, 5.89) | 1.23 (0.21, 5.81) |
| Year 6 | 1.28 (0.21, 6.03) | 1.23 (0.20, 5.83) | 1.26 (0.20, 5.98) | 1.25 (0.21, 6.03) |
| Year 7 | 1.30 (0.21, 6.27) | 1.26 (0.21, 5.91) | 1.30 (0.21, 6.22) | 1.28 (0.21, 6.27) |
| Year 8 | 1.31 (0.20, 6.52) | 1.29 (0.20, 6.30) | 1.39 (0.23, 6.59) | 1.35 (0.20, 6.52) |
| PSA velocity Year 0 – Year 1 median (Q1, Q3) | 0 (-0.20, 0.30) | 0 (-0.20, 0.22) | 0 (-0.20, 0.28) | 0 (-0.20, 0.30) |
Trial was unblinded on October 23, 2008. Data in the primary paper are as of this date.
Table 3. Number and risk of prostate cancers.
| Placebo (n=8,696) |
Vitamin E alone (n=8,737) |
Selenium alone (n=8,752) |
Vitamin E + selenium (n=8,702) |
|
|---|---|---|---|---|
| Number of prostate cancers | ||||
| As of 10/2008 | 416 | 473 | 432 | 437 |
| As of 07/2011 | 529 | 620 | 575 | 555 |
| Hazard ratio, (99% CI), p-value | ||||
| As of 10/2008 | 1.13 (0.95 – 1.35), p=.06 | 1.05 (0.88 – 1.25), p=.52 | 1.04 (0.87 – 1.24), p=.62 | |
| As of 07/2011 | 1.17 (1.004 – 1.36), p=.008 | 1.09 (0.93 – 1.27), 0=.18 | 1.05 (0.89 – 1.22), p=.46 | |
| Absolute risk† | 9.3 | 10.9 | 10.1 | 9.7 |
| Gleason ≥ 7 (n) | 133 | 155 | 161 | 164 |
| Hazard ratio (99% CI), p-value | 1.16 (0.86, 1.58), p=.20 | 1.21 (0.90, 1.63), p=.11 | 1.23 (0.91, 1.66), p=.08 |
Prostate cancers per 1,000 person years
Table 5. Secondary Endpoints.
| Placebo (n=8,696) |
Vitamin E alone (n=8,737) |
Selenium alone (n=8,752) |
Vitamin E + selenium (n=8,702) |
|
|---|---|---|---|---|
| Colorectal Cancer | 75 | 85 | 74 | 93 |
| Hazard ratio (99% CI) | 1.09 (0.72, 1.64) | 0.96 (0.63, 1.46) | 1.21 (0.81, 1.81) | |
| p-value | p=0.60 | p=0.79 | p=0.22 | |
| Lung Cancer | 92 | 104 | 94 | 104 |
| Hazard ratio (99% CI) | 1.11 (0.76, 1.61) | 1.02 (0.70, 1.50) | 1.11 (0.76, 1.62) | |
| p-value | p=0.49 | p=0.89 | p=0.48 | |
| All other primary cancers (excludes prostate, includes colorectal and lung) | 579 | 570 | 557 | 594 |
| Hazard ratio (99% CI) | 0.97 (0.83, 1.14) | 0.96 (0.83, 1.13) | 1.02 (0.88, 1.19) | |
| p-value | p=0.65 | p=0.54 | p=0.74 | |
| All cancers (including prostate) | 1108 | 1190 | 1132 | 1149 |
| Hazard ratio (99% CI) | 1.07 (0.96, 1.19) | 1.02 (0.92, 1.14) | 1.02 (0.92, 1.14) | |
| p-value | p=0.13 | p=0.59 | p=0.60 | |
| Deaths (all cause) | 564 | 571 | 551 | 542 |
| Hazard ratio (99% CI) | 1.01 (0.86, 1.17) | 0.98 (0.84, 1.14) | 0.96 (0.82, 1.12) | |
| p-value | p=.91 | p=.67 | p=.47 | |
| October 23, 2008† | ||||
| Diabetes* | 669 | 700 | 724 | 660 |
| Relative Risk (99% CI) | 1.04 (0.90 – 1.18) | 1.07 (0.94 – 1.22) | 0.97 (0.86 – 1.11) | |
| p-value | p = 0.47 | p = 0.16 | p = 0.61 | |
| July 5, 2011 | ||||
| Diabetes* | 869 | 918 | 913 | 875 |
| Relative Risk (99% CI) | 1.05 (0.93 – 1.17) | 1.04 (0.93 – 1.17) | 0.99 (0.89 – 1.12) | |
| p-value | p = 0.29 | p = 0.34 | p = 0.91 | |
| Cardiovascular events, grade 4 or higher** | 969 | 909 | 939 | 943 |
| Hazard Ratio (99% CI) | 0.93 (0.83,1.05) | 0.97 (0.86,1.09) | 0.97 (0.86,1.09) | |
| p-value | p = 0.11 | p = 0.45 | p = 0.51 |
Date of data freeze for initial publication
Prevalent cases at baseline and men who never submitted a form with a diabetes assessment are excluded from the analysis.
Time to first reported cardiovascular event, cardiovascular procedure (e.g., CABG), or hemorrhagic stroke, all men. Cardiovascular endpoints were not centrally adjudicated.
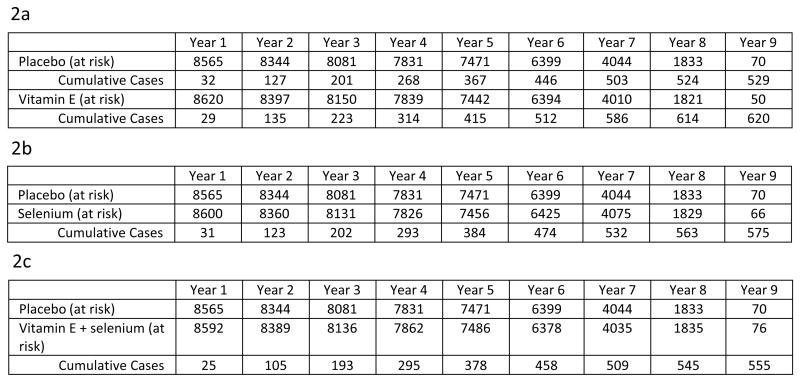
The cumulative incidence curves of prostate cancer by supplement arm compared to placebo are presented in Figures 2a-c. The difference in rates of prostate cancer between vitamin E and placebo became apparent during participants' third year on the trial, at which point the HR was 1.10, and increased slightly each year thereafter. The proportional hazards assumption was reasonable for each study arm (all p≥ 0.17). The unadjusted absolute increase in risk compared with placebo for vitamin E, selenium, and the combination were 1.6, 0.9, and 0.4 cases of prostate cancer per 1,000 person-years.
Figure 2.
a: Cumulative Incidence of Prostate Cancer, Vitamin E vs. Placebo
b: Cumulative Incidence of Prostate Cancer, Selenium vs. Placebo
c: Cumulative Incidence of Prostate Cancer, Combination vs. Placebo
Virtually all men with prostate cancer were without metastases at diagnosis (Table 4). Gleason 6 was the most common grade with the most common form of more aggressive disease being Gleason score 7. Stage and grade distributions were similar among groups.
Table 4. Clinical and Pathological Characteristics of Incident Prostate Cancers.
| Placebo (n=8,696) |
Vitamin E alone (n=8,737) |
Selenium alone (n=8,752) |
Vitamin E + selenium (n=8,702) |
|||||
|---|---|---|---|---|---|---|---|---|
| TOTAL PROSTATE CANCERS DIAGNOSED | 529 | 620 | 575 | 555 | ||||
| Number as of unblinding (primary paper) | 416 | 79% | 473 | 76% | 432 | 75% | 437 | 79% |
| Number diagnosed prior to unblinding but data received afterwards | 26 | 5% | 33 | 5% | 22 | 4% | 17 | 3% |
| Number diagnosed after unblinding | 87 | 16% | 114 | 18% | 121 | 21% | 101 | 18% |
| CONFIRMATION STATUS | ||||||||
| Confirmed by central pathology* | 444 | 84% | 506 | 82% | 474 | 82% | 475 | 86% |
| T-STAGE | ||||||||
| TX | 5 | 1% | 9 | 1% | 7 | 1% | 8 | 1% |
| T1a-c | 375 | 72% | 460 | 75% | 425 | 76% | 391 | 72% |
| T2a-b | 143 | 27% | 138 | 23% | 127 | 23% | 144 | 26% |
| T3a-b | 1 | 0% | 3 | 0% | 3 | 1% | 2 | 0% |
| Not staged | 5 | 10 | 13 | 10 | ||||
| N-STAGE | ||||||||
| NX | 378 | 72% | 441 | 73% | 397 | 70% | 393 | 72% |
| N0 | 145 | 28% | 167 | 27% | 166 | 29% | 154 | 28% |
| N1 | 0 | 0% | 0 | 0% | 2 | 0% | 1 | 0% |
| Not staged | 6 | 12 | 10 | 7 | ||||
| M-STAGE | ||||||||
| MX | 364 | 70% | 435 | 72% | 399 | 71% | 391 | 72% |
| M0 | 159 | 30% | 170 | 28% | 159 | 28% | 153 | 28% |
| M1a-c | 0 | 0% | 3 | 0% | 7 | 1% | 3 | 1% |
| Not staged | 6 | 12 | 10 | 8 | ||||
| GLEASON SCORE | ||||||||
| 4 - 6 | 286 | 69% | 310 | 67% | 281 | 64% | 281 | 63% |
| 7 | 102 | 24% | 118 | 25% | 135 | 31% | 124 | 28% |
| 8 - 10 | 31 | 7% | 37 | 8% | 26 | 6% | 40 | 9% |
| Not graded | 110 | 155 | 134 | 110 | ||||
There were no disagreements. The cases not confirmed by central pathology review were either because no materials or inadequate materials were sent for review.
In the initial SELECT report a non-statistically significant increased risk of type 2 diabetes mellitus (as defined by self-report or new use of glitazone medications) was observed in the selenium supplementation group (HR=1.07). In the updated results the hazard ratio has moved closer to 1(HR=1.04) and is not statistically significant (p=0.34) (Table 5). Table 5 also displays updated data on the pre-specified secondary endpoints of lung, colorectal, and total other cancers, deaths, and grade 4 cardiovascular events. There are no statistically significant differences in the hazard ratios between groups, suggesting neither benefit nor harm for dietary supplementation with selenium or vitamin E for these endpoints.
Comment
Prostate cancer prevention remains an important public health goal because of its incidence, high likelihood of curative-intent treatment even when indolent disease is present,9 and treatment related costs and morbidity. Although two large randomized trials have demonstrated that 5α-reductase inhibitors reduce risk by 20 – 25%,10,11 their use is controversial because of concerns related to an observed increased risk of high grade disease.12 SELECT was designed to assess the effect of selenium and vitamin E alone and in combination as supplements to a normal diet on their ability to prevent prostate cancer in men at average risk. Other randomized studies have shown no benefit to dietary supplementation with selenium, lycopene, or soy in reducing the risk of risk of invasive cancer in men with high- grade prostatic intraepithelial neoplasia (HGPIN) on biopsy.13,14
In this report we detail an observation of serious public health concern that has emerged with continued follow-up of SELECT participants. With primary endpoint ascertainment based on contemporary community practice across the US, Canada, and Puerto Rico using PSA and DRE as indications for biopsy, the risk of prostate cancer at 7 years of median follow-up was increased by 17% in men randomized to supplementation with vitamin E alone a difference which started to appear about two years post randomization. While there is debate over how to best handle accumulating results after the publication of the primary findings and the appropriate threshold for statistical significance, the increased rate of prostate cancer in the vitamin E arm was seen as early as 2006 and continued until the present analysis (HR ranged from 1.12 to 1.17) suggesting that the current results are not an outlier observation due to multiple looks at the data. Extended follow-up with additional events has resulted in narrowed confidence intervals. A biological explanation for the observed increased risk of prostate cancer in the vitamin E arm is not apparent from these data; it does not appear to be due to an increased biopsy rate prompted by changes in DRE, PSA, or unblinding. There was not a statistically significant increased risk of prostate cancer in the vitamin E and selenium combination group (HR=1.05; p=0.46), suggesting that selenium has a protective effect by dampening the increased risk associated with vitamin E alone, a hypothesis reinforced by the p-value (0.021) of the interaction term in the marginal analysis. Tests of this hypothesis and other potential explanations for the results will be addressed by analysis of the effects of baseline plasma vitamin E levels and their interaction with baseline plasma and toenail selenium levels from samples collected from participants at study entry. Despite the lack of a mechanistic explanation, the findings show that vitamin E supplementation in the general population of healthy men significantly increases the risk of being diagnosed with prostate cancer.
The current findings of SELECT are at odds with other large randomized intervention trials that examined the effects of vitamin E supplementation on prostate cancer risk. The Alpha-Tocopherol, Beta Carotene (ATBC) trial reported a 35% risk reduction for prostate cancer in men taking 50mg/day of Vitamin E for a median of 6.1 years,15 although there are important differences with SELECT: 1) the participants of ATBC were all long term smokers (36 years on average), compared to 43% never smokers and 8% current smokers in SELECT; 2) prostate cancer was a secondary endpoint in ATBC; and 3) men in ATBC were not screened, so that prostate cancer was diagnosed at more advanced stages than in SELECT. In the Physicians Health Study II (PHS II) conducted contemporaneously with SELECT, intervention with 400IU vitamin E every other day for a median of 8 years had no effect on the incidence of prostate cancer (HR = 0.97; 95% CI 0.85-1.09; P=.58), although like SELECT there was no effect on total cancer incidence (HR, 1.04; 95% CI 0.95-1.13; P=.41) or overall mortality (HR= 1.08. 95% CI 0.98-1.19).16 Furthermore, both ATBC and PHS II were designed and analyzed as factorial trials, so the reported effect of vitamin E is estimated across the secondary factor (beta carotene or vitamin C, respectively). In contrast, SELECT was designed as a four-arm trial because of concerns about the potential interaction of vitamin E and selenium, and a statistically significant interaction between these agents was indeed observed.
Given that more than 50% of individuals 60 or older are taking supplements containing vitamin E and that 23% of them are taking ≥400 IU per day17 despite a recommended daily dietary allowance of only 22.4 IU for adult men,18 the implications of our observations are substantial. Consistent with the original SELECT report, longer follow-up did not demonstrate a benefit to selenium or vitamin E supplementation on risk of colorectal or lung cancer or cardiovascular events. While modest benefits for vitamin E supplementation have been observed in a limited number of randomized clinical trials for Alzheimer's disease19 and (as one part of a cocktail of oral antioxidants) for age-related macular degeneration,20 no benefits were demonstrated for prevention of cardiac events or mortality,21,22,23 colorectal adenomas,24 respiratory infections in elderly individuals,25 pre-eclampsia in women with type 1 diabetes,26 or prevention or progression of cataracts or macular degeneration.27,28 Furthermore, the increased incidence of prostate cancer seen in SELECT, the previously reported increased incidence of lung cancer with high dose beta-carotene in both ATBC15and the Beta-Carotene and Retinol Efficacy Trial (CARET),29 and the increased risk of colon polyps seen in a trial administering high dose folate,30 suggest that caution should be used when recommending or studying high doses of micronutrients. As opposed to synthetic pharmaceuticals, these naturally occurring dietary constituents are part of normal physiology, and a “U” shaped dose response curve may exist where either deficiency or supraphysiologic doses are harmful.
The experiences of SELECT, ATBC, and CARET emphasize the importance of large scale, population-based, randomized trials in accurately assessing the benefits and harms of micronutrients as dietary supplements. Because a statistically significant interaction was observed between vitamin E and selenium, we believe that caution should be used when designing factorial prevention trials in the future. Although factorial designs are appealing because of their statistical efficiency, interactions can make it difficult to evaluate the underlying effects of each treatment component.31 Furthermore, the fact that the increased risk of prostate cancer in the vitamin E group of SELECT was only apparent after extended follow-up (allowing for additional events) suggests that health effects from these agents may continue even after the intervention is stopped, emphasizing the need for long-term follow-up even in trials closed before the planned intervention period is completed. Consenting SELECT participants have the opportunity to transition to a Centralized Follow-Up study where annual updates to general health and cancer status are obtained either via a mailed questionnaire or data entered by the participant on the SELECT participant website, which will allow additional follow up to further address these issues.
Conclusion
Extended follow-up of SELECT participants shows that healthy men with average risk of prostate cancer subjected to contemporary community standards of screening and biopsy who took a common dose and formulation of vitamin E (400 IU per day) have a significantly increased risk of prostate cancer. The observed 17% increase in prostate cancer incidence demonstrates the potential for seemingly innocuous yet biologically active substances such as vitamins to cause harm. The lack of benefit from dietary supplementation with vitamin E or other agents with respect to preventing common health conditions and cancers or improving overall survival, and their potential harm, underscore the need for consumers to be skeptical of health claims for unregulated over-the-counter products in the absence of strong evidence of benefit from clinical trials.
Acknowledgments
Dr. Gaziano reported receiving grant support (to his institution) from Wyeth (now Pfizer) in the form of vitamin and placebo pills and packaging. Dr. Chin reported receiving consultancy fees from Janssen, Amgen, Novartis and Firmagon; receiving payment for lectures from Firmagon; and payment for development of educational presentations from Astra Zeneca, Novartis and Firmagon. Dr. Meyskens reported being a co-founder of Cancer Prevention Pharmaceuticals. Dr. Baker reported Board Membership for Merck (no compensation). Dr. Karp reported receiving grants (to his institution) from Pfizer.
Funding/Support: This work was supported in part by Public Health Service Cooperative Agreement grant CA37429 awarded by the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and in part by the National Center for Complementary and Alternative Medicine (National Institutes of Health). Study agents and packaging were provided by Perrigo Company (Allegan, Michigan), Sabinsa Corporation (Piscataway, New Jersey), Tishcon Corporation (Westbury, New York), and DSM Nutritional Products Inc. (Parsipanny, New Jersey).
Role of the Sponsor: This work was supported by the National Institutes of Health (primarily the National Cancer Institute (NCI) and the National Center for Complimentary and Alternative Medicine. Representatives from the NIH (NCI) participated in design, oversight, and reporting of this study.
Footnotes
Financial Disclosures: The following had no disclosures to report: Dr. Crowley, Ms. Darke, Dr. Ford, Dr. G. Goodman, Ms. P. Goodman, Dr. Klein, Dr. Klotz, Dr. Lieber, Lippman, Dr. Lucia, Dr. Minasian, Dr. Parnes, Dr. Parsons, Dr. Tangen, Dr. Thompson, Dr. Walther
Data Access and Responsibility: Catherine Tangen had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 3.Zaytoun OM, Vargo EH, Rajan R, Berglund R, Gordon S, Jones JS. Emergence of fluoroquinolone-resistant Escherichia coli as cause of postprostate biopsy infection: implications for prophylaxis and treatment. Urology. 2011;77(5):1035–41. doi: 10.1016/j.urology.2010.12.067. [DOI] [PubMed] [Google Scholar]
- 4.Latini DM, Hart SL, Knight SJ, et al. The relationship between anxiety and time to treatment for patients with prostate cancer on surveillance. J Urol. 2007;178(3 Pt 1):826–31. doi: 10.1016/j.juro.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 5.Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29(16):2185–90. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 6.Lippman SM, Klein EA, Goodman PJ, et al. Effect of Selenium and Vitamin E on Risk of Prostate Cancer and Other Cancers. The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lippman SM, Goodman PJ, Klein EA, et al. Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT) J Natl Cancer Inst. 2005;97(2):94–102. doi: 10.1093/jnci/dji009. [DOI] [PubMed] [Google Scholar]
- 8.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statist Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 9.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28(7):1117–23. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andriole GL, Bostwick DG, Brawley OW, et al. REDUCE Study Group: Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362(13):1192–202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 11.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349(3):215–24. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 12.Theoret MR, Ning YM, Zhang JJ, Justice R, Keegan P, Pazdur R. The Risks and Benefits of 5α-Reductase Inhibitors for Prostate-Cancer Prevention. N Engl J Med. 2011;365(2):97–9. doi: 10.1056/NEJMp1106783. [DOI] [PubMed] [Google Scholar]
- 13.Fleshner NE, Kapusta L, Donnelly B, et al. Progression from high-grade prostatic intraepithelial neoplasia to cancer: a randomized trial of combination vitamin-e, soy, and selenium. J Clin Oncol. 2011;29(17):2386–90. doi: 10.1200/JCO.2010.32.0994. [DOI] [PubMed] [Google Scholar]
- 14.Marshall JR, Tangen CM, Sakr WA, et al. Phase III Trial of Selenium to Prevent Prostate Cancer in Men with High-Grade Prostatic Intraepithelial Neoplasia: SWOG S9917. Cancer Prev Res. doi: 10.1158/1940-6207.CAPR-10-0343. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group: The Effect of Vitamin E and Beta Carotene on the Incidence of Lung Cancer and Other Cancers in Male Smokers. N Engl J Med. 1994;330(15):1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 16.Gaziano JM, Glynn RJ, Christen WG, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2009;301(1):52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford ES, Ajani UA, Mokdad AH. Brief communication: The prevalence of high intake of vitamin E from the use of supplements among U.S. adults. Ann Intern Med. 2005;143(2):116–20. doi: 10.7326/0003-4819-143-2-200507190-00010. [DOI] [PubMed] [Google Scholar]
- 18.Office of Dietary Supplements, National Institutes of Health. [accessed August 26, 2011];Dietary Supplement Fact Sheet: Vitamin E. http://ods.od.nih.gov/factsheets/VitaminE-QuickFacts (reviewed June 24, 2011)
- 19.Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. The Alzheimer's Disease Cooperative Study. N Engl J Med. 1997;336(17):1216–22. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 20.The Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss. Arch Ophthalmol. 2001;119:1417–36. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yusuf S, Dagenais G, Pogue J, Bosch J, Slight P. Vitamin E supplementation and cardiovascular events in high risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–60. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 22.Sesso HD, Buring JE, Christen WG, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2008;300(18):2123–33. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294(1):56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg ER, Baron JA, Tosteson TD, et al. A clinical trial of antioxidant vitamins to prevent colorectal adenoma. Polyp Prevention Study Group. N Engl J Med. 1994;331(3):141–7. doi: 10.1056/NEJM199407213310301. [DOI] [PubMed] [Google Scholar]
- 25.Graat JM, Schouten EG, Kok FJ. Effect of daily vitamin E and multivitamin-mineral supplementation on acute respiratory tract infections in elderly persons: a randomized controlled trial. JAMA. 2002;288(6):715–21. doi: 10.1001/jama.288.6.715. [DOI] [PubMed] [Google Scholar]
- 26.McCance DR, Holmes VA, Maresh MJ, et al. Diabetes and Pre-eclampsia Intervention Trial (DAPIT) Study Group.Vitamins C and E for prevention of pre-eclampsia in women with type 1 diabetes (DAPIT): a randomised placebo-controlled trial. Lancet. 2010;376(9737):259–66. doi: 10.1016/S0140-6736(10)60630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNeil JJ, Robman L, Tikellis G, Sinclair MI, McCarty CA, Taylor HR. Vitamin E supplementation and cataract: randomized controlled trial. Ophthalmology. 2004;111(1):75–84. doi: 10.1016/j.ophtha.2003.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Taylor HR, Tikellis G, Robman LD, McCarty CA, McNeil JJ. Vitamin E supplementation and macular degeneration: randomised controlled trial. BMJ. 2002;325(7354):11. doi: 10.1136/bmj.325.7354.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omenn GS, Goodman GE, Thornquist MD, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996;88(21):1550–9. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 30.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297(21):2351–9. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 31.Green S, Liu PY, O'Sullivan J. Factorial design considerations. J Clin Oncol. 2002;20(16):3424–30. doi: 10.1200/JCO.2002.03.003. [DOI] [PubMed] [Google Scholar]