Abstract
A rapidly emerging concept is that the vascular adventitia acts as a biological processing center for the retrieval, integration, storage, and release of key regulators of vessel wall function. It is the most complex compartment of the vessel wall and comprises a variety of cells including fibroblasts, immunomodulatory cells, resident progenitor cells, vasa vasorum endothelial cells, and adrenergic nerves. In response to vascular stress or injury, resident adventitial cells are often the first to be activated and reprogrammed to then influence tone and structure of the vessel wall. Experimental data indicate that the adventitial fibroblast, the most abundant cellular constituent of adventitia, is a critical regulator of vascular wall function. In response to vascular stresses such as overdistension, hypoxia, or infection, the adventitial fibroblast is activated and undergoes phenotypic changes that include proliferation, differentiation, and production of extracellular matrix proteins and adhesion molecules, release of reactive oxygen species, chemokines, cytokines, growth factors, and metalloproteinases that, collectively, affect medial smooth muscle cell tone and growth directly and that stimulate recruitment and retention of circulating inflammatory and progenitor cells to the vessel wall. Resident dendritic cells also participate in “sensing” vascular stress and actively communicate with fibroblasts and progenitor cells to simulate repair processes that involve expansion of the vasa vasorum, which acts as a conduit for further delivery of inflammatory/progenitor cells. This review presents the current evidence demonstrating that the adventitia acts as a key regulator of pulmonary vascular wall function and structure from the “outside in.”
Introduction
The arterial wall is a heterogeneous, three-layered structure comprising an intima, a media, and an adventitia. Each layer exhibits specific histological, biochemical, and functional characteristics and, as such, each contributes in unique ways to maintaining vascular homeostasis and to regulating the vascular response to stress or injury. Endothelial cells and smooth muscle cells (SMCs), the principal cellular constituents of the intima and media, respectively, have received much attention from vascular biologists, while the adventitia in general and the principal cell contained therein, the fibroblast, have been largely overlooked. However, an increasing volume of experimental data indicates that the adventitial compartment of blood vessels, in both the pulmonary and systemic circulations, like the connective tissue stroma in tissues throughout the body, is a critical regulator of vessel wall function in health and disease. A rapidly emerging concept is that the vascular adventitia acts as a biological processing center for the retrieval, integration, storage, and release of key regulators of vessel wall function. Indeed, the adventitial compartment is now suggested by many to be the principal “injury-sensing tissue” of the vessel wall and the adventitial fibroblast to be a “sentinel cell.” In response to hormonal, inflammatory, and environmental stresses such as hypoxia/ischemia, or vascular distention, resident adventitial cells (fibroblasts, dendritic cells, progenitor cells) are the first vascular wall cells to exhibit evidence of “activation.” Such adventitial activation is denoted by increases in cell proliferation, the expression of contractile and extracellular matrix (ECM) proteins, as well as in the secretion of chemokines, cytokines, and growth and angiogenic factors capable of directly affecting resident vascular wall cell growth and initiating inflammation in a manner that influences overall vascular tone and wall structure. Thus, the adventitia is considered by many as capable of regulating vascular function and structure from the “outside in.”
The purpose of this review is to provide evidence that, in response to injury, resident adventitial stromal cells (fibroblasts in particular) are activated and ultimately exhibit phenotypic characteristics that contribute significantly to pulmonary vascular remodeling. Data will be reviewed supporting the concept that fibroblasts (in some cases, only specific subpopulations of fibroblasts) within the adventitial compartment are able to (i) proliferate with greater propensity than SMCs in response to injury or stress, (ii) differentiate into SM-like cells (i.e., myofibroblasts), which can accumulate in the adventitia and/or migrate to the medial and intimal layers of the vessel wall, (iii) increase and alter their profile of ECM production and deposition, (iv) synthesize and release growth factors and reactive oxygen species (ROS) that have potent paracrine effects on neighboring SMCs and endothelial cells, (v) initiate and perpetuate chronic vascular inflammation through the production of chemokines and cytokines, leading to the recruitment and retention of circulating leukocytes and progenitor cells to the vessel wall, and (iv) synthesize and release angiogenic chemokines and molecules, which support neovascular growth of the vasa vasorum and thus perpetuate the inflammatory response. The emerging role of dendritic cells as well as resident and circulating mesenchymal (fibroblast/myofibroblast) progenitor cells in vascular remodeling will also be discussed.
Adventitia: Cellular Composition
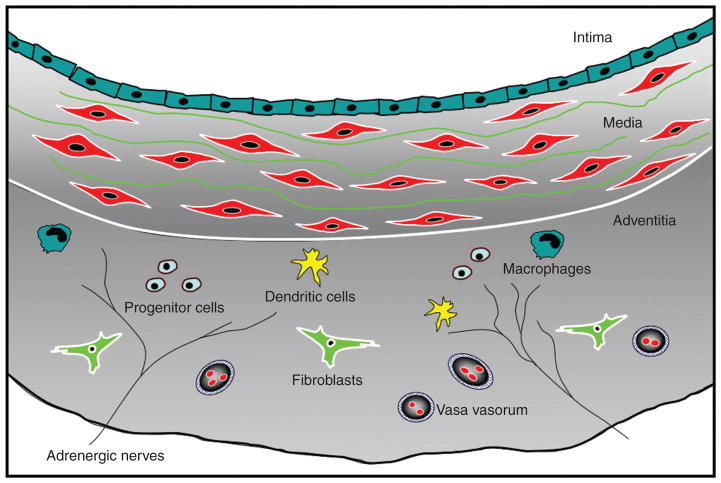
The connective tissue stroma is an important structural component of all tissues in vertebrate animals. Stromal cells (in the pulmonary circulation, we refer to cells principally located in the adventitia) are responsible for defining the specialized architecture of organs and tissues via secretion of ECM and characteristic cytokines and chemokines. The adventitial stroma, consisting of an ECM scaffold containing fibroblasts, progenitor cells, blood and lymphatic vessels, nerves, and immune cells, is the most complex and heterogeneous compartment of the vessel wall (Fig. 1). Collectively, the cells within the stroma are capable of sensing and directing responses to a wide array of stimuli through reciprocal communication with other stromal cells as well as with neighboring cells, such as SMCs and epithelium, to orchestrate tissue responses.
Figure 1.
Complex cellular composition of the vascular adventitia. Unlike the normal intima and media, which are composed of endothelial and smooth muscle cells, respectively, the normal adventitia comprises a wide variety of cell types, including fibroblasts, resident progenitor cells, immunomodulatory cells (dendritic cells, macrophages, T lymphocytes), vasa vasorum endothelial cells, and adrenergic nerves.
Fibroblast
Definition
The most abundant cell type in the connective tissue stroma is the fibroblast. Fibroblasts are ubiquitous cells that provide mechanical strength to tissues by producing ECM, which forms a supporting framework. They were first described in the late 19th century based on their location and microscopic appearance (220). Fibroblasts are often defined morphologically as spindle-shaped, elongated cells, commonly exhibiting extensive cytoplasmic extensions that readily adhere to and migrate over tissue culture substrates. This morphologic definition of fibroblasts is often expanded by describing functional characteristics of cells that (i) synthesize and secrete a complex array of structural (e.g., collagens and fibronectin) and nonstructural ECM molecules (e.g., matricellular family of molecules such as thrombospondins and osteopontin), (ii) actively organize and remodel ECM through the production of proteinases, and (iii) converse with nearby cells (e.g., endothelial cells, SMCs, epithelial cells) through paracrine and autocrine mechanisms. Unfortunately, beyond these morphologic and functional descriptions, a reliable and specific marker for the fibroblast has yet to be found. Therefore, to identify fibroblasts, the lack of markers for other cell lineages (nonlymphoid, nonendothelium, and nonepithelium) as well as morphologic, functional, and biochemical characteristics are often used. A number of markers for fibroblasts, including vimentin, desmin, FSP-1, discoidin-domain receptor 2, and prolyl-4-hydroxylase, among others, have been and/or are currently utilized to identify the fibroblast (Table 1). However, all currently utilized markers are potentially problematic, as they are also expressed in other cell types and are not present in all fibroblasts.
Table 1.
| Marker | Function | Other cell types in which it is expressed |
|---|---|---|
| Vimentin | Intermediate-filament protein | Endothelial cells, myoepithelial cells, and neurons |
| Fibroblast activation protein | Serine protease | Activated melanocytes |
| α-SM-actin | Cytoskeletal protein | Vascular smooth muscle cells, pericytes, and myoepithelial cells |
| Prolyl-4-hydroxylase | Collagen biosynthesis | Endothelial cells, cancer cells, and epithelial cells |
| 2α(1)-Procollagen | Collagen-1 biosynthesis | Osteoblasts and chondroblasts |
| α1β 1-integrin | Collagen receptor | Monocytes and endothelial cells |
| FSP1 (S100A4, mts-1) | Intermediate-filament-associated protein | Invasive carcinoma cells |
| Desmin (found in skin fibs) | Intermediate-filament protein | Muscle cells and vascular smooth muscle cells |
| Discoidin-domain receptor 2 (found in cardiac fibs) | Collagen receptor | Endothelial cells |
Origins
Stromal fibroblasts are believed to arise from at least three distinct cellular origins: primary mesenchyme, local epithelial-mesenchymal transition (EMT), and bone-marrow-derived precursors (156, 196). At present, it would appear that the principal origin of fibroblasts, in most tissue stroma, is primary mesenchymal cells. Upon appropriate stimulation, it is thought that these fibroblasts proliferate to generate new fibroblasts. However, additional sources of fibroblasts that are found in both normal and pathologic conditions have been described and are relevant to stromal function. Studies initiated by Friedenstein et al., and more recently by the Drake laboratory have established that certain organs, including the lung, contain fibroblast/myofibroblast populations that are of hematopoietic stem cell origin (52, 139). This fact is important because it relates to heterogeneity of fibroblast phenotypes and functions discussed below. Another source of fibroblasts, in both normal and pathologic conditions in a variety of tissues, is EMT. During development, local EMT has been shown to be a central mechanism for diversifying cells in the formation of complex tissues (93, 95). Fibroblasts can be derived by this process in adult tissue following epithelial stress such as inflammation or tissue injury [for review, see (1, 94)]. In addition, there is accumulating evidence that endothelial cells can undergo an EMT and that this could be a relevant source of fibroblasts in lung and lung vascular disease (3, 67, 157, 240).
Phenotypic and functional heterogeneity
The numerous sources of fibroblasts probably contribute to the now well-accepted notion of fibroblast heterogeneity. It is well documented that fibroblasts exhibit site-specific gene expression (25, 196). Extensive analysis of expression profiles from primary human fibroblasts from distinct anatomical sites has shown that gene expression patterns are as divergent as those observed among distinct lineages of white blood cells (25). In addition, heterogeneity of fibroblast populations within specific tissues, including the lung, is well documented (45, 83, 90, 150, 152, 195, 196). Significant diversity in the fibroblast populations comprising the pulmonary artery adventitia has also been reported (34). One hypothesis receiving increasing attention is that only certain fibroblast subsets within a tissue respond to injury or stress with heightened proliferative or dysregulated fibrogenic responses (62, 83, 90, 150, 153). Differences among subsets of normal fibroblasts have been identified on the basis of surface markers, cytoskeletal composition, lipid content, and cytokine profile (62, 150, 152). The most extensively characterized marker, which has been utilized to differentiate specific lung fibroblast subpopulations, is the glycosyl phosphatidyl inositol (GPI)-anchored protein Thy-1 (9). Thy-1 is localized to lipid rafts and signals to Src family kinases to regulate cell adhesion and cytoskeletal organization (9, 10). Thy-1(−) and Thy-1(+) mouse lung fibroblasts differ morphologically and have different secretory profiles (153). Thy-1(−) cells, at least within the lung, appear to be a more consistent fibrogenic subtype (61, 62, 165, 242). They exhibit greater proliferative responses to PDGF-AA and connective tissue growth factor (9, 61). Additionally, they secrete twice as much latent TGF-β as Thy-1(+) cells, show increased shedding of syndecan-2, and can express five times more α-SM-actin, a characteristic of the myofibroblast phenotype than Thy-1(+) fibroblasts (242). Recent data show that Thy-1(−/−) mice exhibit more severe lung fibrosis (increased collagen accumulation) and increased Smad 2/3 phosphorylation (indicating higher levels of active TGF-β) in response to intratracheal bleomycin than do Thy-1(+) control mice (62).
That selective expansion of specific fibroblast subsets can occur in response to injury is supported by studies demonstrating that fibroblasts from within a fibrogenic milieu differ from those in normal tissues. Fibroblasts isolated from lungs with active fibrotic disease have increased proliferative capacity, are capable of anchorage-independent growth, and are morphologically distinct (152, 163, 210). Interestingly, the myofibroblasts in fibroblastic foci in lung tissues from individuals with idiopathic pulmonary fibrosis (IPF)/usual interstitial pneumonia are Thy-1(−) despite the fact that the majority of fibroblasts from normal lungs are Thy-1(+) (62). Fibroblasts derived from the pulmonary hypertensive arteries exhibit exaggerated growth properties that may be the result of selective expansion of a distinct population of fibroblasts and/or emergence of fibroblast populations with abnormalities in the pathways normally utilized to control growth (33–35, 211, 232). Collectively, these findings suggest the existence within the lung and lung vasculature of fibroblast subsets, with an increased propensity to contribute to fibrotic responses. Studies are needed to characterize the various populations of fibroblasts present in the adventitia of different vessels and to specifically evaluate their role in vascular remodeling.
At present, and perhaps unfortunately, the vast majority of work contained in the literature related to the pulmonary (or systemic) vasculature describes responses of “the adventitial fibroblast” to stress or injury. Experiments in cell culture most often describe the responses of “aggregate populations” of adventitial fibroblasts. The remainder of this review will focus on work that describes generalized responses of the “adventitial fibroblast” keeping in mind the caveat that not all adventitial fibroblasts may be exhibiting equally the responses described.
Dendritic cells and macrophages
The adventitia is now recognized to function critically in immune recognition and the regulation of vascular inflammation (114). It has been shown that dendritic cells (DCs) reside in the adventitia (often at the media-adventitial border) of medium and large sized vessels of many mammalian species including humans. DCs act to recognize injury and/or pathogen-derived molecular patterns and to initiate adaptive immune responses in an otherwise immunopriviledged niche (64, 114). These cells are the most powerful antigen-presenting cells known and are critically involved in priming immune responses (197). Vascular DCs derive from two sources, the endogeneous population of wall-residing and circulating DCs, a mobile force of potent immunoregulators. DCs, in many vessels (aorta, subclavian, carotid, mesenteric, iliac, and temporal arteries), colocalize with the vasa vasorum network and lymphatics and express pattern recognition receptors of the Toll-like receptor (TLR) family, which exhibit highly specific injury- and pathogen-sensing functions. Their importance in initiating/sustaining inflammation is emphasized by studies showing that depletion of DCs abrogates vasculitis (113). Interestingly, the pattern of TLRs is vessel specific, which contributes to vessel-specific risk for inflammatory vasculopathies (160). DCs are also observed in the pulmonary arteries of man and animals (21, 148). Increases in their numbers are observed in pulmonary artery in the setting of pulmonary hypertension, raising the possibility for a role in perpetuating inflammation just as in the systemic circulation (21, 148).
Vasa vasorum
Vasa vasorum (VV) consists of small arteries that enter the vessel wall either from the abluminal surface (VV externa) or from the luminal surfaces (VV interna) and then arborize to the outer media. Venous vasa vasorum drain a network of capillaries/venules laid down around the outer media to veins in close proximity to the arteries. The function of the vasa vasorum is both to deliver nutrients and oxygen to the arterial and venous walls and to remove “waste products,” either produced by cells in the wall or introduced by diffusional transport through the endothelium of the artery or vein. In a classic paper, Wolinsky and Glagov reported that the extent, distribution, and vascularization of the vessel by vasa vasorum depend on the vessel wall thickness and that in blood vessels larger than 0.5 mm in diameter, nourishment of the media is supplemented by vasa vasorum (231).
Recently, there has been great interest in vascular diseases where expansion of the vasa vasorum takes place, leading to the hypothesis that the vasa vasorum contributes to vascular remodeling (103, 167). It has been shown that hypertension not only induces medial and adventitia thickening but also significantly increases adventitial vasa vasorum density (11, 60, 167). Additional studies have emphasized the involvement of vasa vasorum in the inflammation and progression associated with atherosclerosis (103). There are also data indicating that the inflammatory reactions observed in vasculitis appear to begin in the adventitia where inflammatory cells are located close to the vasa vasorum (137). Experimental evidence demonstrates that in the setting of Takayasu arteritis, the vessel wall is infiltrated by inflammatory cells derived from its own nutritive (i.e., vasa vasorum) vascular system, which destroy the medial and intimal structures (103). Furthermore, although controversial, experiments also suggest an active role of vasa vasorum expansion in contributing to vascular remodeling and chronic inflammation in atherosclerosis (167). Moulton et al. showed that blocking neovascularization with angiostatin reduces progression of advanced atherosclerosis (129). Increasingly, the role of vasa vasorum is being identified in the vascular remodeling associated with at least the hypoxic forms of pulmonary hypertension (discussed below).
Resident progenitor cells
There is increasing experimental evidence demonstrating that both the developing and the adult arterial and venous vessel walls can serve as niches for various stem and progenitor cells (81, 83, 146, 222, 240). A vasculogenic zone has been described as being present at the adventitial-medial border and has been variously described as containing progenitor cells such as endothelial progenitor cells, smooth muscle progenitors, hematopoietic stem cells, mesenchymal stem cells, and the so-called mesangial cells coexpressing both endothelial and myogenic markers (222, 240). At present, the exact nature of the progenitor cells residing in the vascular walls in different tissues is undetermined. Furthermore, the exact differentiation potential of these cells is also largely undetermined. It is speculated that these progenitor cells are probably involved in both physiologically beneficial and detrimental processes during homeostatic and pathogenic conditions. Their potential role in vascular remodeling in pulmonary and systemic blood vessels is discussed below.
Adrenergic nerves
The pulmonary vascular bed is innervated by the adrenergic nervous system, and norepinephrine has been shown to be one of the major signaling molecules released by the nerves residing in the adventitia and outer media of blood vessels (91, 92, 218, 222). In the human lung, Meyrick and Reid demonstrated that innervation of the pulmonary arterial wall is confined largely to the adventitia, although occasional fibers are reported in the outer layer of the media (122). It has been demonstrated that in at least some patients with pulmonary hypertension, increases in the numbers of adrenergic axons are observed in the adventitial layer of even intra-acinar muscular arteries (122). Although little recent work has been reported regarding this observation, emerging evidence suggests that angiogenic responses are often associated with new nerve growth.
Adventitial Fibroblasts: Primary Responders and Initiators of Vascular Remodeling
Early activation in response to vascular stress
Systemic circulation
One of the most consistent findings in experimental models of systemic vascular injury and hypertension is early and often dramatic adventitial remodeling (69, 173, 199). Evidence of early adventitial fibroblast proliferation, along with monocyte/macrophage accumulation, has been reported in hypertension, atherosclerosis, and vascular injury (73, 104, 147, 173, 177, 179, 190, 230, 238). In a model of chronic nitric oxide (NO) inhibition (resulting in systemic hypertension), Arribas et al. showed that adventitial thickness and cell number increased long before changes in the media or intima (5). Increases in adventitial fibroblast proliferation, which precede and exceed endothelial and SMC proliferation, are a common denominator in these studies. In fact, when quantitative analysis of cell numbers and density is applied to animal models of hypertensive remodeling, the most consistent, yet unexpected, findings that emerge are increases in adventitial cell density and decreases in SMC density (5, 96, 120). Studies evaluating the mechanical behavior of the adventitia support the idea that under conditions of elevated blood pressure, the adventitia becomes the predominant wall component due to its pronounced stiffening behavior (176, 189). Thus, the adventitial fibroblast has been suggested to be the most appropriate cell for “sensing” hypertensive states (120, 176). These observations of unique adventitial mechanical properties and early increases in fibroblast proliferation have stimulated the hypothesis that the adventitia plays an essential role in the regulatory systems that control vascular remodeling and vascular tone at least under conditions of high wall stress.
Pulmonary circulation
Similar findings have emerged from experimental studies in the pulmonary circulation. In hypoxia-induced pulmonary hypertension, there is early and often dramatic evidence of adventitial remodeling (12, 121, 201, 202). Less pronounced but still significant adventitial changes are noted in high-flow and monocrotaline models of pulmonary hypertension as well as in idiopathic forms of human pulmonary hypertension (26, 104, 123, 154). In hypoxic models of pulmonary hypertension, adventitial fibroblasts have been noted to undergo the earliest and most significant increases in proliferation of all vascular wall cell types (12, 121, 142). Thus, like the systemic arterial adventitial fibroblast, the pulmonary arterial fibroblast seems poised to undergo early changes in proliferation in response to a variety of injurious stimuli.
Molecular signaling controlling fibroblast proliferation
Proproliferative signals
Because of the early dramatic increases in proliferation of the pulmonary adventitial fibroblast in hypoxic models of pulmonary hypertension and observations suggesting a distinct environmental “stimulus-sensing” capability of the fibroblast, many investigators have sought to understand the molecular basis of this process. It has been established that even heterogeneous populations of pulmonary artery adventitial fibroblasts proliferate in response to hypoxic conditions, a response not observed in pulmonary artery SMCs when cultured and tested under identical experimental conditions (31, 33, 34, 225–227). Some have even suggested that this is a unique (compared with systemic) response of pulmonary artery fibroblasts (227). Activation of Gαi and Gq family members, perhaps in a ligand-independent fashion, and subsequent stimulation of protein kinase C and mitogen-activated protein kinase (MAPK) family members are important regulators of hypoxia-induced adventitial fibroblast proliferation (31–33, 201). Activation of phosphatidylinositol-3-kinase, and synergistic interaction with Akt, mammalian target of rapamycin (mTOR), and p70 ribosomal protein S6 kinase, has also recently been demonstrated to be necessary for the proliferative responses of pulmonary artery adventitial fibroblasts in response to hypoxia (58, 100). Therefore, a complex network of signaling pathways initiated by G-protein-mediated signaling is responsible for the stimulation and proliferation of pulmonary artery adventitial fibroblasts in response to hypoxia, a response that is distinct among vascular wall cells. It is currently unclear as to why hypoxia fails to initiate the same set of proliferative signals in pulmonary arterial SMCs or for that matter even in certain subsets of adventitial fibroblasts.
Downstream of the aforementioned signaling pathways lay transcription factors, which are involved in the proliferative response. Hypoxia is known to activate a diverse array of transcription factors and thus has a profound impact on the cellular transcriptome (29). However, it is likely that the degree and nature of the global transcriptional response to hypoxia in vivo are both cell-type and cell-state specific. Although the transcription factor hypoxia-inducible factor-1α (HIF-1α) plays a major role in controlling the ubiquitous transcriptional response to hypoxia, it is clear that a number of other transcriptional activators and repressors are also activated either directly or indirectly under hypoxic conditions. While activation of both H1F-1α and HIF-2α is observed in human pulmonary artery adventitial fibroblasts in response to hypoxia, activation of HIF-1α appears to be the major regulator of replication of fibroblasts under hypoxic conditions and that of HIF-2α appears to be important in the enhanced migration (44, 100). Hypoxia also induces a significant increase in the expression and activity of early growth response-1 (Egr-1) transcription factor in adventitial fibroblasts (57). Attenuation of Egr-1 protein with antisense oligonucleotides reduces the hypoxia-induced proliferation of pulmonary arterial fibroblasts (7). Egr-1 contributes to the proliferative phenotype induced by hypoxia, at least in part, by regulating expression of cyclin D and epidermal growth factor receptor (7). Hypoxia-induced upregulation of Egr-1 has been demonstrated to be an important contributor to the pathogenesis of pulmonary vascular remodeling (234). Thus, both HIF-1α and Egr-1 are important transcription factors participating in hypoxia-induced adventitial fibroblasts proliferation. They are also likely involved in regulating other phenotypic changes induced by hypoxia (described below).
Hypoxia or other stressors may also affect local increases in adventitial fibroblast proliferation by inducing the secretion of various autocrine/paracrine factors (27). For example, increased release of ATP from pulmonary artery adventitial fibroblasts has been demonstrated under hypoxic conditions (57). Extracellular ATP can stimulate adventitial fibroblast proliferation by itself and can also act synergistically with other growth factors released by fibroblasts, endothelial cells, or macrophages, including IGF-1 and PDGF (57). The proliferative effects of ATP appear to be mediated largely through G-protein-coupled P2Y receptors and downstream signaling intermediaries strikingly similar to those included by hypoxia. Angiotensin II (Ang-II) is another autocrine/paracrine factor released in response to hypoxia, under the control of HIF-1α, which has also recently been demonstrated to act in a positive feedback loop and to facilitate pulmonary artery adventitial fibroblast proliferation under hypoxic conditions (100, 127). Specific increases in angiotensin-converting enzyme expression are observed in the adventitia of hypoxic pulmonary hypertensive animals (128). This system could operate to control adventitial fibroblast proliferation in response to a variety of stresses and probably deserves the same attention that it has received with regard to fibroblasts in the systemic circulation (144, 168).
Antiproliferative signals
Excessive proliferation of fibroblasts to brief or unsustained stimuli such as hypoxia or stretch could lead to unwanted changes in vascular structure. Therefore, it is likely that many stimuli could also activate replication repressor signals in fibroblasts to limit or control replication. The existence of growth-limiting signaling pathways has been described in many cell types in response to other growth-promoting stimuli (209, 239). Protein tyrosine phosphatases are regulators of growth factor signaling in vascular remodeling. They exist in adventitial fibroblasts and undergo upregulation, concomitant with promitogenic signaling pathways, in response to vascular injury presumably to mitigate proliferative responses (124). Protein kinase Cζ (PKCζ) and MAPK phosphatase-1 (MKP-1) have also been identified as proliferative suppressors in several cell types. Short et al. tested the hypothesis that hypoxia would also activate PKCζ and MKP-1 to repress proliferative signals in normal adventitial fibroblasts and thereby limit acute hypoxia-induced proliferation of fibroblasts. Using multiple molecular and pharmacological strategies, they showed that hypoxia-induced proliferation of pulmonary artery adventitial fibroblasts is indeed negatively regulated by the atypical PKCζ isozyme (184, 187). They also demonstrated that PKCζ attenuates the phosphorylation of ERK1/2 through the regulation of MKP-1 in fibroblasts exposed for more than 24 to 48 hours to hypoxia supporting the idea that PKCζ and MKP-1 can act as signal terminators of ERK1/2 activation and thus limit hypoxia-induced proliferation of pulmonary artery adventitial fibroblasts derived from normal animals.
As noted above, fibroblasts derived from fibrogenic foci in the lung exhibit a different phenotype than the majority of fibroblasts from the normal lung. Similarly, a significant modulation in the phenotype of pulmonary artery adventitial fibroblasts derived from chronically (≥2 weeks) hypoxic animals has been observed, including far greater growth responses to a number of growth-promoting stimuli such as hypoxia compared with fibroblasts from normoxic animals (33, 34, 225, 227). Changes in the signaling pathways used to elicit proliferation are observed in these cells compared with controls (33, 184, 185, 225). Interestingly, Das et al. have found that the functional role of the atypical PKCζ isozyme in the proliferative responses is significantly altered in fibroblasts from chronically hypoxic animals. In these cells, PKCζ acts as a proproliferative kinase for adventitial fibroblasts, as opposed to its antiproliferative actions in fibroblasts derived from normoxic control animals described above (32). These observations raise the possibility that chronic hypoxia leads to the emergence of fibroblasts in the adventitia that have lost their ability to limit stimulus-induced proliferation. Whether chronic hypoxia, or any stimulus for that matter, modulates intracellular signaling patterns to change fibroblast phenotype or causes expansion of unique fibroblast subsets, or both, remains unclear.
Contribution of ROS to fibroblast activation
In addition to early evidence of proliferation, other markers of “activation” are observed in fibroblasts in response to injury, as well as to a variety of pathophysiologic stimuli. One early marker of activation, observed in response to a number of stressors, is an increase in the production of ROS through vascular NADPH oxidases (6, 28, 69, 143, 144, 179). Furthermore, inflammatory cells also produce ROS via the phagocytic NADPH oxidase; thus, recruitment of inflammatory cells is another source of adventitial ROS. This early and often dramatic change in both extra- and intracellular concentrations of ROS can have profound acute and chronic effects on vascular function (108, 212). The ROS, superoxide (O2−), for example, can act directly on selected targets or can rapidly dismutate via superoxide dismutase to hydrogen peroxide, a well-recognized signaling molecule that is capable of diffusing into cells (e.g., fibroblasts or SMCs) and initiating signaling pathways resulting in contraction, growth, or migration (4, 69, 212). Superoxide produced in the adventitial compartment can react with and inactivate NO, thus lowering its bioavailability and contributing to increases in vascular tone, even in the presence of normal NO production by the vascular endothelium (166). Although most of the in vivo evidence demonstrating that adventitial fibroblasts produce ROS via an NADPH oxidase has been examined in fibroblasts isolated from systemic arteries. Li et al. have recently reported that isolated pulmonary artery adventitial fibroblasts generate NADPH-oxidase-derived ROS in response to hypoxia, and this source of ROS is responsible for hypoxia-induced fibroblast proliferation (106).
In addition to direct effects of the ROS on the adventitial fibroblast, the release of ROS by the adventitial fibroblast can also have paracrine effects on neighboring SMCs to increase their contraction (212). Superoxide can directly increase ERK activity, ultimately leading to increases in intracellular calcium and pulmonary artery SMC contraction (59). In vivo evidence that activated adventitial fibroblasts play an important paracrine role in regulating vascular remodeling was recently provided by experiments in the systemic circulation showing that targeted perivascular delivery of a novel NADPH oxidase inhibitor against GP91phox effectively attenuated angioplasty-induced neointimal formation of the rat carotid artery and Ang-II-induced medial thickening (109). Liu et al. showed that hypoxia-induced pulmonary hypertension and vascular remodeling were completely abolished in NADPH oxidase (GP91phox) knockout mice, although these investigators did not specifically address the vascular compartment responsible for the NADPH oxidase activity (109). The role of adventitial ROS in the regulation of vascular tone and pulmonary vascular dysfunction has been further supported by evidence that overexpression of the extracellular form of superoxide dismutase (EC-SOD or SOD3), highly expressed in the adventitia, including adventitial remodeling and medial wall proliferation, attenuates chronic hypoxic pulmonary hypertension in mice (136). The specific role of the adventitia and the adventitial fibroblast in the oxidant-antioxidant imbalance in pulmonary hypertension will be important both to understand the pathogenesis of vascular remodeling and potentially to develop novel therapeutic approaches.
In addition, adventitial fibroblasts, in response to ROS and other microenvironmental stimuli, are capable of releasing a number of mediators, which could affect vascular tone. These include ET-1, PDGF, EGF, FGF-2, PGH-2, HSP-90, and cyclophilins (72, 85). Activated resident fibroblasts (as well as recruited monocytes and macrophages) also release cytokines and growth factors capable of directly affecting SMC proliferation and ECM production (200, 224). Factors released include ET-1, Ang-II, TGF-β, IGF-I, FGF-2, and PDGF-BB, all of which have significant effects on growth and matrix production of fibroblasts and on the underlying SMCs. Specifically, pulmonary artery adventitial fibroblasts, in response to hypoxia, produce paracrine factors through HIF-dependent mechanisms, which have potent stimulatory effects on SMC proliferation (170). Thus, the activated adventitial fibroblast appears to play a significant role in influencing the tone and structure of the vascular wall following a variety of injuries or stresses both directly, through the secretion of vasoactive and growth-promoting molecules, and indirectly by producing chemokines, which promote accumulation of leukocytes and progenitor cells. Indeed, fibroblasts are the directors of numerous responses involved in vascular remodeling (Fig. 2).
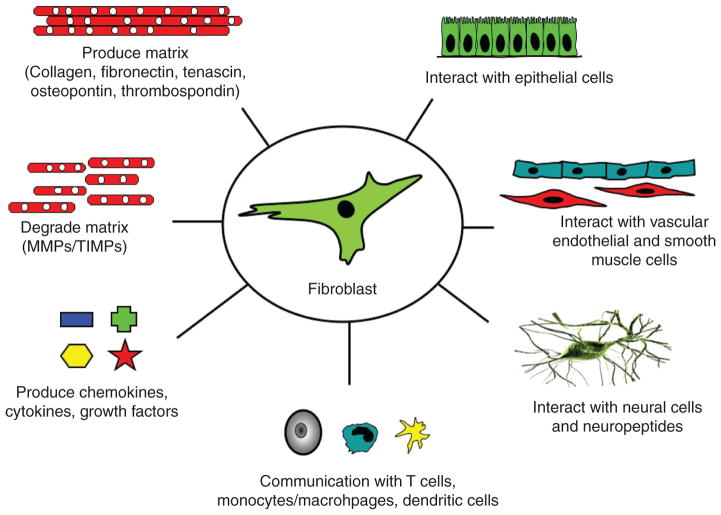
Figure 2.
Fibroblasts play a central role in the control of vascular function. Fibroblasts produce and organize elements of extracellular matrix (ECM) and also degrade structural elements of the ECM; they secrete a complex mixture of growth factors, cytokines, chemokines; they communicate with neural cells with cells of hematopoietic origin (dendritic cells, macrophages, T lymphocytes), with SMCs and endothelial and epithelial cells; importantly, this communication is reciprocal. Abbreviations: MMP, matrix metalloproteinase; TIMP, tissue inhibitor of matrix metalloproteinases. Adapted from reference 196.
Emerging concepts regarding control of fibroblast phenotype
As noted, there is increasing evidence that adventitial fibroblasts in the pulmonary hypertensive vessel wall acquire a hyperproliferative, inflammatory, and invasive phenotype. Questions arise as to mechanisms regulating this phenotype. Intriguingly, this phenotype resembles in certain ways the phenotypic characteristics of rheumatoid arthritis synovial fibroblasts (RASFs) and cancer-associated myofibroblasts. It has been demonstrated that SFs, perhaps more than other types of fibroblasts, acquire phenotypic characteristics commonly associated with transformed cells (97, 102). RASFs show “spontaneous” or “constitutive” activities associated with aggressive behavior and they differ from SFs of patients with osteoarthritis or normal SFs. For example, RASFs upregulate proto-oncogenes, matrix-specific degrading enzymes [matrix metalloproteinases (MMPs)], adhesion molecules, and cytokines (47, 97).
DNA methylation
Recent studies have provided potential insight into the mechanisms involved in this intrinsically activated cellular phenotype. 5-Methylcytosine DNA levels are reduced both in RASF tissues and in cultured RASFs (97, 134). Specifically, the promoter of an L1 element was partially demethylated, confirming a global genomic hypomethylation in RASFs. It was proposed that the hyperaggressive and proinflammatory phenotype of RASFs is the result of a progressive loss of methylation marks and that tissue-specific transcription factors, which are not normally expressed, are upregulated and are responsible for the activation of many genes involved in the pathogenesis of rheumatoid arthritis. This concept was confirmed in experiments where 5-azaC (a DNA-hypomethylator) treatment of normal SFs lead to a phenotype identical to RASFs. Over 186 genes were upregulated in 5-azaC-treated cells by greater than twofold by hypomethylation including growth factors and growth factor receptors, ECM proteins, adhesion molecules, and matrix-degrading enzymes. Furthermore, hypomethylation of certain receptors, specifically the death receptor, could explain the relative resistance to apoptosis, which has been reported in RASFs in certain patients (97, 206). In addition, there is also work suggesting that global genomic hypomethylation can be accompanied or followed by specific promoter hypermethylation (43).
There is growing evidence for epigenetic alterations in other fibrotic diseases (65, 99). A recent study demonstrated epigenetic silencing of Thy-1 by DNA hypermethylation specifically within fibroblast foci in patients with IPF, suggesting that this may be an important mechanism for pathogenetic fibroblast alterations since the absence of Thy-1 correlates with a profibrotic phenotype. Importantly, treatment with DNA methyltransferase restored Thy-1 expression in Thy-1(−) fibroblasts. Together, these data indicate that epigenetical control of Thy-1 may contribute to the fibrotic phenotype in lung fibroblast and that targeting the methylation status of DNA may be a treatment option. Clearly, investigations into the methylation status of fibroblast in the setting of pulmonary hypertension need to be performed.
Micro RNA activity
In addition to DNA methylation, other mechanisms of epigenetic control, including histone modification and micro RNA activity, may be involved in the control of fibroblast phenotype. Specific micro RNAs are expressed in RASFs. Micro RNAs are single-stranded RNA molecules of 21 to 23 nucleotides in length that can be complementary to multiple mRNAs and induce silencing of multiple transcripts. They have been found to regulate reprogramming of gene expression in several types of cancer and in other organs such as the heart (65, 138, 216). Given their role in physiologic and pathological abnormalities in the immune system including cancer and autoimmune diseases, it is likely that they play a role in fibrotic abnormalities in other organs, including the lung. It is known that there are specific micro RNA genes that are expressed in the lung, potentially implicating them as candidate regulatory factors in development or disease. For instance, when the miR-223 gene was deleted in vivo, mice developed a progressive pulmonary inflammation consisting of neutrophils that have increased oxidative responses to challenge (86). The role of micro RNAs in regulating fibroblast phenotype in the adventitia has not been investigated and may be a fertile area of future investigations.
Telomeres and telomerases
Another point of regulation of the fibroblast phenotype may come at the level of the telomere. Telomeres are guanine-rich repeat sequences at the ends of chromosomes and are regulated by telomerase, a ribonucleoprotein complex with an RNA component (TERC and hTR) that serves as a template for the addition of repeat sequences and a reverse transcriptase catalytic subunit (TERT). Telomerase is upregulated in many malignancies, and it has been shown that cells transfected with the telomerase gene can proliferate indefinitely. With regard to lung fibrotic diseases, about 15% of patients with familial IPF exhibit telomerase mutations, as do some patients with sporadic IPF (2, 213). Induction of telomerase activity has been noted in rat lung fibroblasts in bleomycin-induced fibrosis (135). Furthermore, telomerase (TERT)-deficient mice are protected from bleomycin-induced fibrosis; transplantation of wild-type bone marrow into TERT-null mice restores sensitivity to bleomycin, and transplantation of TERT-null bone marrow into wild-type mice is protective (110). These studies indicate that telomerase expression is required for profibrotic fibroblast phenotype and that bone-marrow-derived cells have a critical role in bleomycin fibrosis. It will be important to determine the activity of telomerases in the fibroblast derived from the vessels of animals and patients with severe pulmonary hypertension.
In summary, the adventitial fibroblast is activated early in response to vascular stress and plays an important role in remodeling through proliferation, migration, and production of ROS, cytokines, and growth factors that can modulate the contractile, proliferative, and matrix-producing capabilities of neighboring SMCs as well as other cells in the vessel wall and in the adjacent lung parenchyma (Fig. 2). Many of the signaling pathways and transcription factors involved have been identified and may be potential targets for therapy. Unfortunately, but most importantly, the mechanisms contributing to the fibroblasts ability to act as a “sentinel” cell remain unknown. Investigations aimed at identifying the mechanisms utilized by the fibroblast to sense changes in the environment are needed, as this may open further opportunities for the treatment of vascular remodeling.
Differentiation of Adventitial Fibroblasts into Myofibroblasts: Contribution to Vascular Pathology
Myofibroblast markers
Activation of fibroblasts by a variety of stimuli can result in their differentiation into a myofibroblast phenotype, a process shown to be critical to a variety of fibrotic diseases including those of the lung (37, 53, 149). It is now recognized that a variety of other cells including epithelial, endothelial, and resident and circulating progenitor cells can also differentiate into myofibroblasts (Fig. 3). No matter their origin, myofibroblasts express α-SM-actin, the most frequently used marker for myofibroblast identification, which allows monitoring of this cell type during experimental and clinical conditions (37, 38). Three major ultrastructural features discriminate myofibroblasts from quiescent fibroblasts in tissues: (i) bundles of contractile microfilaments, (ii) extensive cell-to-matrix attachment sites, and (iii) intracellular adherence and gap junctions (46, 75). However, this definition has its limits when myofibroblasts need to be discriminated from other contractile cell types, such as SMCs, and, in this instance, requires additional molecular markers. The most frequently employed myofibroblast marker, α-SM-actin, unfortunately fails to distinguish between myofibroblasts and SMCs in situations that exhibit mixed populations of activated cells. This becomes particularly important when one considers the potential role of the myofibroblast in contributing to vascular pathology. Contractile SMCs specifically express SM-myosin heavy chain, h-caldesmon, and desmin; however, SMCs lose these markers when acquiring a synthetic phenotype in vivo and after being placed in culture. Until recently, smoothelin was suggested to be a late differentiation marker for SMCs that is not expressed in myofibroblasts. However, studies have demonstrated smoothelin as well as other late SMC markers in TGF-β̄ 1-treated lung fibroblasts (24). The 41g-isoform of the stress fiber protein paladin has also been proposed as a novel marker for myofibroblast differentiation, but Western blotting analysis indicates expression of this isoform also in SMCs (169). Hence, at present, no single cytoskeletal protein allows reliable discrimination between a myofibroblast and a de-differentiated SMC (203).
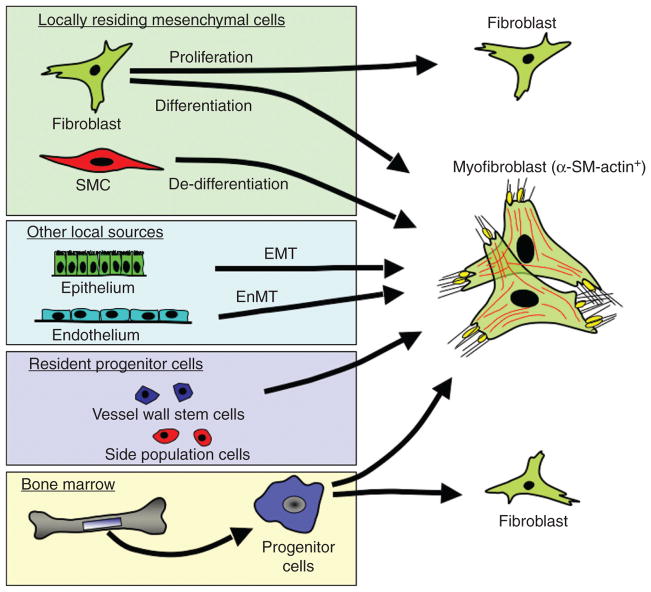
Figure 3.
Multiple origins for myofibroblasts in the vasculature: α-SM-actin-expressing myofibroblasts are believed to originate via: (i) differentiation of tissue resident fibroblasts, (ii) de-differentiation of resident smooth muscle cell (SMC), (iii) epithelial-to-mesenchymal trans-differentiation (EMT), (iv) endothelial-to-mesenchymal transition (EnMT), (v) differentiation of resident or bone marrow-derived circulating progenitor cells. Adapted from Hinz B, et al. The myofibroblast: one function, multiple origins. Am J Pathol 170(6): 1807–1816, 2007.
Myofibroblast functions
Early and dramatic increases in the appearance of α-SM-actin expressing myofibroblasts in the adventitia are observed in hypoxia-induced pulmonary hypertension as well as in numerous other vasculopathies (194, 198, 229). Myofibroblasts are implicated as key participants in tissue remodeling because of their ability to perform multiple physiologic functions in response to change in the local environment (53). Myofibroblasts are the principal producers of collagen and other ECM proteins including fibronectin (particularly the splice variant ED-A fibronectin), tenascin-C, osteopontin, and elastin in the fibrotic tissues including the remodeled pulmonary artery adventitia (53, 180, 201, 202). In addition, activated myofibroblasts produce a variety of growth factors, cytokines, and ROS that can have autocrine as well as paracrine effects on medial SMCs. Myofibroblasts exhibit significant contractile capabilities with slow-onset and sustained contraction in response to a variety of agonists. In addition, their responses to vasodilatory stimuli differ from those of SMCs and thus myofibroblast accumulation could contribute to the abnormalities of vasorelaxation observed in the setting of chronic pulmonary hypertension. Thus, the myofibroblast accumulation can directly contribute to exert changes in the tone and structure of the vessel wall under pathophysiologic conditions (75, 183).
Based on the work in systemic models of hypertension and vascular injury, the myofibroblast appears capable of migrating from the adventitia to the media, or even the intima, and thus contributing to the thickening of these vascular wall compartments, observed in response to injury (105, 173, 179–182, 190, 223). Studies using in vivo gene transfer techniques to directly label adventitial fibroblasts prior to balloon injury showed that the fibroblasts transitioned to a myofibroblast and within 3 days labeled cells were observed in the media and after 7 days in the intima. Subsequent studies showed that overexpression of SMAD7 or inhibition of PDGF in adventitial fibroblasts abrogated their migration to the media and the intima (115, 116). Accumulation of myofibroblasts in the intima of patients with pulmonary hypertension has been well documented and consistently observed (191, 236). However, the origin of the myofibroblast in the intima in this disease is unclear and is likely complex with multiple cell origins (see the “Adventitia: A Depot for Vascular Progenitor Cells” section).
Regulation of myofibroblast phenotype
The differentiation of fibroblasts into myofibroblasts is regulated by a complex microenvironment consisting of growth factors, cytokines, adhesion molecules, and ECM molecules. TGF-β is a well-known cytokine capable of inducing transition of a fibroblast into a myofibroblast phenotype by stimulating α-SM-actin expression and collagen production (55, 117). Thrombin, ET-1, Ang-II, IL-6, and Fizz-1 have also been reported to induce differentiation of normal lung fibroblasts into a myofibroblast phenotype (15, 54, 111, 183, 205). All of these factors are upregulated by hypoxia and have been observed in the pulmonary artery adventitia of chronically hypoxic animals (201). Furthermore, it has recently been demonstrated that hypoxia alone can stimulate a fibroblast-myofibroblast transition along with the induction of proliferation of pulmonary artery adventitial fibroblasts (186). However, these two distinct cellular responses to hypoxia were found to be regulated by different intracellular signaling modules. Hypoxia-induced proliferative responses in fibroblasts utilize a Gαi-initiated, ERK1/2-dependent signaling pathway. In contrast, hypoxia-induced α-SM-actin expression, though also dependent on Gαi activation, utilizes JNK rather than ERK1/2 signaling to achieve the response. It remains unclear as to whether myofibroblasts can further differentiate into SMCs, although this intriguing possibility has been raised and would have significant implications for vascular patho-physiology (24).
Adventitia: Role in Vascular Inflammation
Vascular inflammation has traditionally been considered an “inside-out” response centered on leukocyte/monocyte recruitment to the intima of blood vessels. In this hypothesis, injured vascular cells on the intimal surface of blood vessels express surface adhesion molecules and inflammatory mediators that participate in monocyte homing to the endothelium and eventual transmigration into the media and/or the intima. However, growing experimental evidence supports a new paradigm of an “outside-in” hypothesis in which vascular inflammation is initiated in the adventitia and progresses inward toward the intima. For a long period of time, most immunologists regarded fibroblast activation as relatively insignificant in regulating immune responses and concentrated primarily on immune interactions between lymphocytes, macrophages, and dendritic cells. However, it is now becoming clear that many danger signals are not antigen specific and current models have begun to focus on an extended immune system in which stromal cells, including fibroblasts, play a key role in innate immune responses. It has become evident that small peptides released during activation of the coagulation or complement cascades as well as during remodeling of the ECM have profound effects on fibroblast immune responses (20, 48, 114, 193).
Upon activation, fibroblasts are not only stimulated to proliferate and differentiate into myofibroblasts and to produce ECM proteins but also rapidly upregulate production of cytokines, chemokines, and adhesion molecules. They have been shown to regulate the behavior of hematopoietic cells that infiltrate damaged tissues through a number of mechanisms and most specifically through the CD40/CD40-ligand interactions (193). It is also now recognized that fibroblasts express pattern recognition receptors, such as the TLRs, and receptor for advanced glycosylation endpoints. These receptors allow the fibroblast to act as a part of the innate immune system through the induction of proinflammatory cytokines and MMPs (125).
In support of the “outside-in” hypothesis regarding adventitial regulation of inflammation is the fact that in the systemic circulation, soon after vascular injury but prior to established disease, the adventitia becomes highly populated with neutrophils, macrophages, and apoptotic cells (13, 141, 177). It has also been demonstrated that following a wide variety of vascular injuries, there is an almost immediate influx of leukocytes into the adventitial compartment (68, 114). Similarly, studies in the pulmonary circulation have shown that both chronic hypoxic exposure and monocrotaline treatment lead to the early appearance and persistence of inflammatory/progenitor cells in the adventitia of both large and small pulmonary arteries followed by prominent remodeling (39, 50, 172, 200, 215).
Studies in the systemic circulation have demonstrated upregulation of chemokines, chemokine receptors, and adhesion molecules in the adventitia, which likely regulates both the recruitment and retention of circulating inflammatory cells in the vessel wall (82, 141). Fibroblasts, in response to stress or injury (including hypoxia), are known to be capable of producing a wide array of chemokines that facilitate recruitment of leukocytes from the vasculature response, which is probably regulated by NAPH oxidase (22, 28). A recent study in the hypoxic pulmonary circulation demonstrated a complex, time-dependent, and pulmonary-artery-specific upregulation of several cytokines/chemokines, their receptors, and adhesion molecules, which appear to be primarily produced/expressed by resident adventitial fibroblasts and monocytes and are likely involved in the initiation and perpetuation of the inflammatory response (21). Chemotactic factors documented to increase in the adventitia during the development of hypoxia-induced pulmonary hypertension include SDF-1, MCP-1, VEGF, ET-1, TGF-β, and osteopontin (21, 35, 200). Importantly, there are likely numerous cytokines and chemokines that fibroblasts release in a stimulus-specific manner and that are capable of regulating influx of specific leukocyte subtypes. The newly recruited leukocytes produce ROS and cytokines, which, in turn, activate adventitial fibroblasts and/or the underlying SMCs, thus augmenting the inflammatory response.
Since perivascular inflammation appears chronic in many forms of pulmonary hypertension, questions arise as to what perpetuates the inflammation. In systemic circulation, there is convincing evidence that fibroblasts taken from diseased tissue display a fundamentally different phenotype compared with fibroblasts taken from normal tissues at the same anatomical site (18, 145). For example, SFs derived from patients with rheumatoid arthritis display unique properties and secrete a distinct pattern of cytokines compared with SFs from noninflamed joints (48, 145). Interestingly, this distinct phenotype is stable in the absence of any external stimulation over many passages in culture, implying a stable “imprinted” alteration in cell function. In the inflamed lung, fibroblasts have been shown to regulate the Th1-Th2 balance (77). The molecular basis for this persistently activated fibroblast phenotype at sites of chronic inflammation remains unclear, although recent findings suggest that NF-κB signaling pathway plays a critical role in perpetuating chronic inflammatory responses (20). Studies in RelB-knockout mice support a critical role for the fibroblast in regulating the switch from acute to chronic inflammation (112). In these mice, endotoxin triggers the sustained and persistent production of a wide variety of inflammatory chemokines by tissue fibroblasts, leading to a rapid (within hours) accumulation of a large number of inflammatory cells. This hyperactive fibroblast phenotype is suppressed by transfection of wild-type RelB, which appears to selectively stabilize the NF-κB inhibitor I-κB in fibroblasts, proving that the severe inflammation is directly caused in large part by a fibroblast’s RelB deficiency (233). Additionally, these findings provide evidence that a failure in the normal “switch-off signal” in fibroblasts directly contributes to the persistence of an immune response in chronic inflammation. In such cases, a switch from innate to acquired immunity is subverted, leading to persistent accumulation of leukocytes within the inflamed tissue, a finding consistent with the observations in several forms of chronic pulmonary hypertension.
The transition to a chronic inflammatory phenotype also requires changes in the adhesion molecule and chemokine receptor expression of fibroblasts and recruited hematopoietic cells, respectively. Fibroblasts express and upregulate adhesion molecules, including ICAM-1 and VCAM-1, that cause cell adhesion of leukocytes in response to a variety of stimuli. Furthermore, secretion of cytokines, including TGF-β, by the activated fibroblast causes activation and upregulation of receptors such as CXCR4 in newly recruited hematopoietic cells (19). Activated fibroblasts also secrete SDF-1, the cognate ligand for CXCR4. Thus, an environment is created in chronically inflamed tissues whereby the stroma (adventitia) appears to act as a “foster home” for leukocytes, leading to their inappropriate retention and survival (Fig. 4) (20).
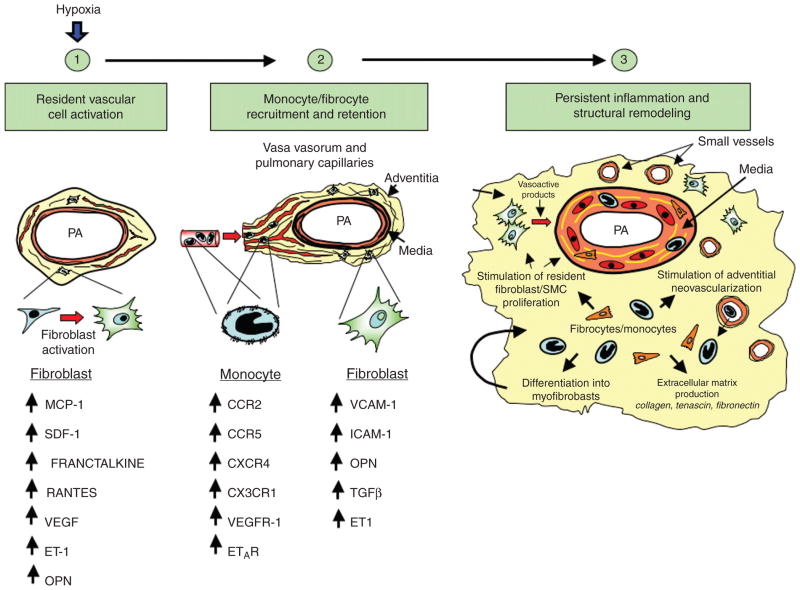
Figure 4.
Essential role of the adventitial fibroblast in initiating and perpetuating vascular inflammation and, consequently, vascular remodeling. In response to hormonal, infectious, or environmental (hypoxia, hemodynamic stress, etc.) stimuli, the fibroblast is activated and secretes chemokines, cytokines, and matricellular proteins involved in the recruitment of monocytes, lymphocytes, and progenitor cells. With time, fibroblasts upregulate adhesion molecule expression, which promotes retention of leukocytes and progenitor cells within the adventitia. Some of the newly recruited cells can differentiate into fibroblasts and myofibroblasts, which perpetuate the cycle, thus leading to persistent inflammation and structural vascular remodeling. Abbreviation: PA, pulmonary artery.
Adventitia: A Depot for Vascular Progenitor Cells
Resident adventitial progenitor cells
It was previously thought that the resident adventitial fibroblast was the sole contributor to the increases in fibroblast/myofibroblast mass observed in vascular remodeling. However, recent studies suggest that a population of mesenchymal progenitor cells (MPCs) exists in the adventitia and may give rise to fibroblasts, myofibroblasts, and even SMCs in response to vascular injury. MPCs are described as cells that are capable of differentiating into at least one of the mesenchymal lineages: adipocyte, osteoblast, or myofibroblast (158). Recent studies have shown that MPCs are stored in nearly all tissues and organs of the body, including bone marrow, adipose tissue, skin, umbilical cord blood, brain, spleen, liver, kidney, lung, muscle, thymus, and pancreas, as well as in large blood vessels such as the aorta and vena cava (30, 78). Hoshino et al. demonstrated that the pulmonary artery contains fibroblasts capable of multipotent differentiation including myogenic differentiation (characterized by increased expression of α-SM-actin and calponin) (78). Interestingly, lung interstitial fibroblasts were not found to have these capabilities (78). In addition, Zengin et al. have described another population of progenitor cells residing at the medial-adventitial border in what they describe as a “vasculogenic zone” (241). These cells have been termed adult mesodermal/multipotent vascular wall resident stem cells. They are CD34+/Tie-2+/KDR(Flk-1)+/CD45+ and VE-cadherin negative. In vitro studies demonstrate that they have the capability of assuming an endothelial phenotype under certain conditions and potentially participate in the regeneration of vascular endothelial cells. It is suggested they might participate in tumor vascularization and arteriogenesis responses. Interestingly, some of these cells also have the capability of expressing hematopoietic markers and assuming a monocyte/macrophage phenotype. They thus seem to be readily available to participate in immune responses within the vessel wall. In addition, these cells are capable of giving rise to cells exhibiting a fibroblast/myofibroblast-like phenotype (241). Potentially a different population of progenitor cells was observed by Hu et al., who showed that the adventitia contains progenitor cells capable of differentiating into SMCs or endothelial cells (80, 211). These cells could migrate to the media and the neointima, contributing to lesion progression. These cells express stem cell markers, including Sca-1, cKit, CD34, and Flk-1. Explanted cultures of adventitial tissues, using stem cell medium, resulted in the outgrowth of heterogeneous cell populations, some of which were shown to differentiate into SMCs in response to PDGF-BB stimulation. When these same Sca-1+ cells carrying the Lac-Z gene were transferred to the adventitial side of vein grafts in Apo-E-deficient mice, Lac-Z cells were found in atherosclerotic lesions of the intima in these vessels. One possibility to explain the presence of resident progenitor cells in the adventitia is that these cells are remnants of earlier developmental stages and that mesenchymal precursors that remain located in the adventitia are capable of differentiating into different types of vascular cells under the appropriate circumstances. It is also possible that different progenitor cells reside in different vessels, each with individualized roles in response to vascular injury. In the future it will be important to examine the exact molecular nature of the various progenitor cells that likely reside in the vessel wall.
Adventitial accumulation of circulating progenitor cells
The adventitia may also serve as a repository for circulating progenitor cells following vascular injury. Circulating progenitor cells have been implicated in the pathophysiology of a number of systemic and pulmonary vascular diseases (63, 74, 107, 174, 200). In sex-mismatched bone marrow transplant patients, Caplice et al. demonstrated that some SMCs in the atherosclerotic plaque originate from cells administered at bone marrow transplantation (23). Interestingly, these cells appear to cluster not only in the intima but also in and around adventitial microvessels. Others have demonstrated that circulating or bone-marrow-derived progenitor cells participate in the neovascularization of the adventitia and the intima and may be essential for atherosclerotic disease progression (79, 129). We have recently demonstrated the appearance of cells expressing stem cell antigen cKit in the pulmonary artery adventitia of chronically hypoxic animals (35). Many of these cells were observed in and around the rapidly expanding adventitial vasa vasorum blood vessels. Expansion of the adventitial vasa vasorum appears important in the progression of many vascular diseases, as it provides a conduit for delivery of inflammatory and progenitor cells to the vascular wall (129, 200).
Inflammation is an important component of systemic and pulmonary vascular diseases (81, 200, 214). Inflammatory cells, specifically monocytes, dendritic cells, and some T lymphocytes but not B lymphocytes or neutrophils, are observed in the adventitia in the development of hypoxic pulmonary hypertension (21, 50). Contained within this inflammatory cell influx is a subset of monocytic cells, which have been termed fibrocytes. They are characterized by the dual expression of both leukocytic markers (CD45, CD34, CD11b, CD14) and mesenchymal markers (α1-procollagen) (161, 204). Fibrocytes are rapidly recruited at sites of tissue injury and have been shown to differentiate into collagen-producing fibroblasts and/or myofibroblasts (140, 151, 161, 175, 204, 235). We have observed a rapid and dramatic influx of fibrocytes into the adventitia of animals (rats and calves) exposed to chronic hypoxia (49). When these cells were depleted in the circulation, hypoxia-induced pulmonary vascular remodeling was inhibited (49). Thus, fibrocyte recruitment appears necessary for the exuberant remodeling seen in some models of hypoxic pulmonary hypertension. In further support of the idea that recruited cells contribute to the vascular remodeling process in the setting of hypoxic pulmonary hypertension are recent observations regarding the appearance of cells expressing progenitor markers (cKit) and hematopoietic and monocytic markers (CD45, CD11b) in the media of distal pulmonary arteries of neonatal calves with severe hypoxic pulmonary hypertension (51). It was shown that a population of morphologically distinct cells (rhomboid in shape, therefore termed “R” cells) that transiently expressed CD11b and constitutively expressed the mesenchymal marker type I procollagen could be consistently grown in culture from the remodeled but not from control vessels (the latter yielded only a highly uniform and differentiated population of SMCs). These cells expressed high mRNA levels of the progenitor markers cKit, CD34, and CD73, as well as of the inflammatory mediators IL-6, MCP1, and motility-associated antigen S100A4. “R” cells exhibited highly augmented proliferative (including autocrine growth), migratory, invasive, and promitogenic capabilities. The autocrine as well as paracrine growth properties were due, at least in part, to the production of PDGF-A/B, SDF-1, and S100A4 (51). In this regard, Young et al. (237) recently showed that blocking SDF-1 and/or CXCR4 signaling reduced the appearance of cKit+ cells in the hypoxic vessel wall and nearly completely abrogated hypoxia-induced pulmonary hypertension and remodeling.
Collectively, these observations support the idea that adventitia serves as a source of mesenchymal cells critically involved in vascular repair, not only through differentiation of resident fibroblasts but also through differentiation of resident adventitial progenitors as well as through the recruitment and differentiation of circulating MPCs.
Adventitia: Cell-Matrix Interactions
The composition of the adventitial ECM is principally regulated by fibroblasts. In response to stress or injury, the fibroblast dramatically changes its production of ECM molecules, the accumulation of which can have a profound effect on vascular structure and function. The fibrillar collagens are a major component of the adventitial matrix, and the most abundant type I and III collagens are the principal matrix proteins produced by adventitial fibroblasts (159). ECM-cell interactions affect the physical coupling of cells via the regulation of cell-surface adhesion molecules and may also regulate, via a positive feedback loop, the deposition of ECM (17). Thus, under normal conditions, there must be a homeostatic relationship between resident fibroblasts and this collagen ECM to maintain fibroblasts in a quiescent, undifferentiated state (188). Activation of the fibroblast leads to alterations in the production and relative composition of ECM components, which, in turn, ultimately contributes to further changes in cell growth, behavior, and differentiation.
Recent findings have revealed that adventitial ECM composition is altered during the progression of diseases such as atherosclerosis and restenosis (164). Excessive and progressive deposition of ECM proteins in the adventitia is common in remodeled vessels, including those in pulmonary arterial hypertension (41, 87, 198, 202). Marked increases in the production and accumulation of collagens and elastin are observed in the adventitia during the development of pulmonary hypertension (41). Although not well studied, the accumulation of collagen likely affects stiffness of the vessel wall, which can have profound effects on flow dynamics in the vessel and ultimately on right ventricular function (40, 171). In addition, marked increases in the accumulation of cellular fibronectin (ED-A fibronectin), tenascin-C (TN-C), and osteopontin in the adventitial compartment of models of hypoxia-induced pulmonary hypertension are observed (21, 41, 202). Fibronectin, in particular ED-A fibronectin, appears to play a critical role in facilitating proliferation of fibroblasts as well as their differentiation into myofibroblasts (37, 131). TN-C expression has been shown to be upregulated in pulmonary hypertensive vessels (88). TN-C, like fibronectin, is associated with fibroblast and SMC proliferation and has also been shown to contribute to differentiation of a fibroblast to a myofibroblast (89, 207). In addition, fibronectin and TN-C deposition coincides with the expression and activity of MMPs, a family of zinc enzymes responsible for the degradation of the ECM components including basement membrane collagen, interstitial collagen, fibronectin, and various proteoglycans (56, 84). Excessive or inappropriate expression of MMPs may contribute to the pathogenesis of tissue destructive processes in a wide variety of diseases including pulmonary hypertension (16). MMPs are produced by a number of adventitial cells including fibroblasts and macrophages. Indeed, upregulated expression of these MMPs may be necessary for the fibroblast to move through the adventitial matrix into the media and even into the intima (182). MMP activity is upregulated in adventitial fibroblasts from animals with hypoxia-induced pulmonary hypertension. The potent proteolytic activities of MMPs are regulated by specific tissue inhibitors of MMPs (TIMPs), the activity of which have been reported to be decreased in various vasculopathies, thus creating an environment in the adventitia conducive to cell migration (219). Inhibition of MMP activity attenuates monocrotaline- and hypoxia-induced pulmonary hypertension (71, 219). It is thus clear that adventitial fibroblasts dramatically change their production of ECM proteins in response to vascular stress. These changes facilitate fibroblast proliferation, migration, and differentiation. Each of these changes, in turn, affects vascular function and structure. Thus cell-matrix interactions may provide novel therapeutic targets in the prevention of remodeling that characterizes pulmonary hypertension.
Adventitia: Role of Vasa Vasorum in Vascular Disease
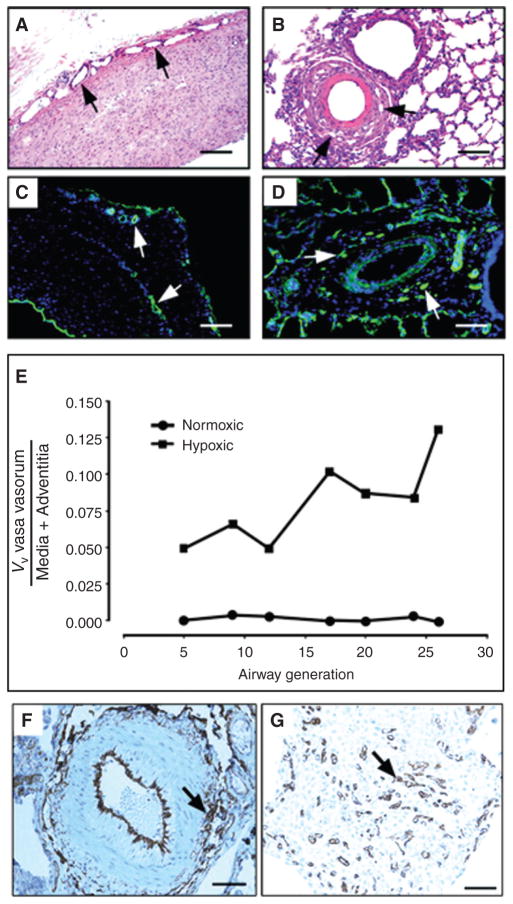
Since chronic inflammation and fibrosis are known to be associated with angiogenic responses, the possibility exists that the vascular remodeling associated with at least some forms of pulmonary hypertension would involve angiogenic responses in or around the vessel wall. In the systemic circulation, the adventitial vasa vasorum undergoes marked neovascularization in a number of vasculopathies, including atherosclerosis, type II diabetes, metabolic syndrome, restenosis, and vasculitis (8, 66, 70, 72, 129, 167). This neovascularization process is thought to play a direct role in the remodeling (167). In the setting of pulmonary hypertension, expansion of the vasa vasorum network in the adventitia and the media has also been described (35, 36, 126). In fact, expansion of the pulmonary artery vasa vasorum is commonly observed in the setting of pulmonary artery obstruction (126). For instance, Kimura et al. in patients with chronic thromboembolic obstruction of the pulmonary arteries, reported that the volume of pulmonary adventitia vasa vasorum increases and the core of the nonresolving clots is recannulized by neovascular endothelialized structures that originate from the vasa vasorum (98). Increased density of capillaries in the adventitial and periadventitial regions of pulmonary arteries in patients with severe IPF and pulmonary hypertension has also been described (133). In an animal model (i.e., the hypoxic neonatal calf) of severe hypoxia-induced pulmonary hypertension, marked expansion of the vasa vasorum network in the adventitia and within the outer aspects of the media of vessels all along the longitudinal axis of the pulmonary circulation has been described (Fig. 5) (35).
Figure 5.
(A–E), Angiogenic expansion of the vasa vasorum in the pulmonary artery adventitia of calves with severe hypoxia-induced pulmonary hypertension. Histopathology of large (A, C) and small (B, D) pulmonary arteries. Both histological, H&E (A, B) and immunofluorescent, PECAM-1/CD31 (C, D) stainings demonstrate marked expansion of the vasa vasorum capillary network in adventitial, perivascular regions. Quantitative morphometric analyses demonstrated that the volume density (VV) of vasa vasorum is significantly greater in neonatal calves with severe hypoxia-induced pulmonary hypertension compared with normoxic controls (E). (F, G) Angiogenic responses in the adventitia of a human patient with pulmonary fibrosis and associated pulmonary hypertension. Medium sized pulmonary artery stained with CD31, demonstrating evidence for capillary network expansion in the perivascular area (medial/adventitial region, arrow) (F). CD31 immunohistochemical evidence of capillary proliferation (arrow) (G). Bars, 500 μm in A and C, 100 μm in B, D, F, and 25 μm in G.
Role of fibroblasts in controlling vasa network expansion/function
The mechanisms controlling expansion of the vasa vasorum network in the pulmonary and systemic circulations are not well understood. However, it is increasingly appreciated that activation of fibroblasts must play a critical role since stromal fibroblasts have been clearly implicated in the angiogenesis that accompanies tumor progression both in cancers of epithelial origin and in chronic inflammatory diseases such as rheumatoid arthritis (14, 42, 95, 130, 162). As noted, fibroblasts are capable of producing many factors involved in angiogenic responses including VEGF, PDGF, ET-1, and TGF-β. Thus, Davie et al. carried out experiments to determine whether fibroblasts were involved in the expansion of the vasa vasorum seen in pulmonary hypertensive vessels. Using both coculture and conditioned media approaches, they found that adventitial fibroblasts, especially those from pulmonary hypertensive animals, were capable of stimulating vasa vasorum endothelial cell (VVEC) proliferation (36). Furthermore, conditioned media from adventitial fibroblasts was capable of augmenting both the self-assembly and integrity of cord-like networks formed by VVEC in matrigel. Exposure to hypoxia (3% O2) augmented all these responses. Several angiogenic factors are observed in the hypoxic adventitia including VEGF, fibronectin, thrombin, and S100A4 (21). Interestingly, all these molecules have been found to be upregulated in the pulmonary arteries of patients with various forms of pulmonary arterial hypertension (215, 221). In addition, studies in the systemic circulation have also suggested that ET-1, a factor well known to be upregulated in pulmonary hypertension, plays a critical role in coronary vasa vasorum neovascularization in the setting of experimental hypercholesterolemia through local upregulation of VEGF (192, 228). These observations are in accordance with a number of previous studies in which hypoxic conditions have been shown to induce angiogenic phenotypes in a number of stromal cell types (76, 155, 178). Moreover, these observations are consistent with the well-established paradigm that hypoxia is a common feature of many pathological conditions associated with neovascular growth. Importantly, previous studies in our laboratory have established that adventitial fibroblasts exhibit the earliest and most dramatic activation responses among all cells in the vessel wall (199). These data are consistent with the emerging concept that the endothelium of de novo forming microvessels receives and integrates proangiogenic signals from a number of nonendothelial cells, including fibroblasts (66, 101, 119, 132, 217).
Fibroblasts, cultured on ECM proteins, have been shown to secrete cytokines and proangiogenic growth factors that regulate the formation of capillary-like networks by human umbilical vein endothelial cells and systemically derived microvascular endothelial cells (66, 118, 132, 208). Other studies have shown that stromal cells, including fibroblast-like cells, not only provide initial stimuli for the angiogenic cascade but also provide a stabilizing force to newly formed vessels (66, 101, 118, 119, 132, 208, 217). Tissue fibroblasts have also been described to exhibit proangiogenic capabilities at sites of wound healing and inflammation. These cells respond to chemotactic cytokines released in the tissue environment and are frequently the first cell type to migrate to the wound site where they orchestrate reparative neovascularization (119). Thus, activated adventitial fibroblasts may regulate angiogenic responses of the resident endothelial cells in the adventitia and stimulate a process of neovascular growth be it normal or disordered.
Summary
It is becoming increasingly clear that the adventitia should no longer be considered a nearly inert support structure for the vessel wall. Rather, accumulating evidence now supports the idea that the adventitia is a biological processing center for a wide variety of pathologic stimuli. Resident adventitial fibroblasts because of their distinct biochemical properties and interactions, with a unique matrix protein environment, are poised to be activated in response to a variety of potentially injurious stimuli. The activated fibroblast, in a process that appears to occur largely through NAPH-oxidase-dependent signaling, is capable of a wide array of responses including proliferation, differentiation, migration, and secretion of paracrine factors, which have profound influences on vascular wall structure and function. Participating in the responses to stress in the adventitia are immunomodulatory and progenitor cells. In addition, expansion of the vasa vasorum, in a process regulated by local fibroblasts, provides a conduit for continued delivery of leukocytes and progenitor cells. Thus, the adventitia, under many conditions, should be considered the conductor of a symphony of events that occur in the vessel wall to impart functional and structural changes. A better understanding of adventitial cell activation could ultimately lead to targeted drug delivery aimed at reducing adventitial fibroblast activation.
Acknowledgments
The authors thank Marcia McGowan and Stephen Hofmeister for their work on the manuscript.
References
- 1.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, Vulto I, Xie M, Qi X, Tuder RM, Phillips JA, III, Lansdorp PM, Loyd JE, Armanios MY. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA. 2008;105:13051–13056. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: | Potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1–L8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 4.Ardanaz N, Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: Regulation and signaling leading to dysfunction. Exp Biol Med (Maywood) 2006;231:237–251. doi: 10.1177/153537020623100302. [DOI] [PubMed] [Google Scholar]
- 5.Arribas SM, Hillier C, Gonzalez C, McGrory S, Dominiczak AF, McGrath JC. Cellular aspects of vascular remodeling in hypertension revealed by confocal microscopy. Hypertension. 1997;30:1455–1464. doi: 10.1161/01.hyp.30.6.1455. [DOI] [PubMed] [Google Scholar]
- 6.Azumi H, Inoue N, Takeshita S, Rikitake Y, Kawashima S, Hayashi Y, Itoh H, Yokoyama M. Expression of NADH/NADPH oxidase p22phox in human coronary arteries. Circulation. 1999;100:1494–1498. doi: 10.1161/01.cir.100.14.1494. [DOI] [PubMed] [Google Scholar]
- 7.Banks MF, Gerasimovskaya EV, Tucker DA, Frid MG, Carpenter TC, Stenmark KR. Egr-1 antisense oligonucleotides inhibit hypoxia-induced proliferation of pulmonary artery adventitial fibroblasts. J Appl Physiol. 2005;98:732–738. doi: 10.1152/japplphysiol.00821.2004. [DOI] [PubMed] [Google Scholar]
- 8.Barger AC, Beeuwkes R, III, Lainey LL, Silverman KJ. Hypothesis: Vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med. 1984;310:175–177. doi: 10.1056/NEJM198401193100307. [DOI] [PubMed] [Google Scholar]
- 9.Barker TH, Hagood JS. Getting a grip on Thy-1 signaling. Biochim Biophys Acta. 2009;1793:921–923. doi: 10.1016/j.bbamcr.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker TH, Pallero MA, MacEwen MW, Tilden SG, Woods A, Murphy-Ullrich JE, Hagood JS. Thrombospondin-1-induced focal adhesion disassembly in fibroblasts requires Thy-1 surface expression, lipid raft integrity, and Src activation. J Biol Chem. 2004;279:23510–23516. doi: 10.1074/jbc.M402169200. [DOI] [PubMed] [Google Scholar]
- 11.Bayer IM, Caniggia I, Adamson SL, Langille BL. Experimental angiogenesis of arterial vasa vasorum. Cell Tissue Res. 2002;307:303–313. doi: 10.1007/s00441-002-0512-4. [DOI] [PubMed] [Google Scholar]
- 12.Belknap JK, Orton EC, Ensley B, Tucker A, Stenmark KR. Hypoxia increases bromodeoxyuridine labeling indices in bovine neonatal pulmonary arteries. Am J Respir Cell Mol Biol. 1997;16:366–371. doi: 10.1165/ajrcmb.16.4.9115746. [DOI] [PubMed] [Google Scholar]
- 13.Best PJ, Hasdai D, Sangiorgi G, Schwartz RS, Holmes DR, Jr, Simari RD, Lerman A. Apoptosis. Basic concepts and implications in coronary artery disease. Arterioscler Thromb Vasc Biol. 1999;19:14–22. doi: 10.1161/01.atv.19.1.14. [DOI] [PubMed] [Google Scholar]
- 14.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogatkevich GS, Tourkina E, Abrams CS, Harley RA, Silver RM, Ludwicka-Bradley A. Contractile activity and smooth muscle alpha-actin organization in thrombin-induced human lung myofibroblasts. Am J Physiol Lung Cell Mol Physiol. 2003;285:L334–L343. doi: 10.1152/ajplung.00417.2002. [DOI] [PubMed] [Google Scholar]
- 16.Brauer PR. MMPs—role in cardiovascular development and disease. Front Biosci. 2006;11:447–478. doi: 10.2741/1810. [DOI] [PubMed] [Google Scholar]
- 17.Brooke BS, Karnik SK, Li DY. Extracellular matrix in vascular morphogenesis and disease: Structure versus signal. Trends Cell Biol. 2003;13:51–56. doi: 10.1016/s0962-8924(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 18.Brouty-Boye D, Pottin-Clemenceau C, Doucet C, Jasmin C, Azzarone B. Chemokines and CD40 expression in human fibroblasts. Eur J Immunol. 2000;30:914–919. doi: 10.1002/1521-4141(200003)30:3<914::AID-IMMU914>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 19.Buckley CD, Amft N, Bradfield PF, Pilling D, Ross E, Arenzana-Seisdedos F, Amara A, Curnow SJ, Lord JM, Scheel-Toellner D, Salmon M. Persistent induction of the chemokine receptor CXCR4 by TGF-beta 1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. J Immunol. 2000;165:3423–3429. doi: 10.4049/jimmunol.165.6.3423. [DOI] [PubMed] [Google Scholar]
- 20.Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, Salmon M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001;22:199–204. doi: 10.1016/s1471-4906(01)01863-4. [DOI] [PubMed] [Google Scholar]
- 21.Burke DL, Frid MG, Kunrath CL, Karoor V, Anwar A, Wagner BD, Strassheim D, Stenmark KR. Sustained hypoxia promotes the development of a pulmonary artery-specific chronic inflammatory microenvironment. Am J Physiol Lung Cell Mol Physiol. 2009;297:L238–250. doi: 10.1152/ajplung.90591.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capers Qt, Alexander RW, Lou P, De Leon H, Wilcox JN, Ishizaka N, Howard AB, Taylor WR. Monocyte chemoattractant protein-1 expression in aortic tissues of hypertensive rats. Hypertension. 1997;30:1397–1402. doi: 10.1161/01.hyp.30.6.1397. [DOI] [PubMed] [Google Scholar]
- 23.Caplice NM, Bunch TJ, Stalboerger PG, Wang S, Simper D, Miller DV, Russell SJ, Litzow MR, Edwards WD. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc Natl Acad Sci USA. 2003;100:4754–4759. doi: 10.1073/pnas.0730743100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chambers RC, Leoni P, Kaminski N, Laurent GJ, Heller RA. Global expression profiling of fibroblast responses to transforming growth factor-beta1 reveals the induction of inhibitor of differentiation-1 and provides evidence of smooth muscle cell phenotypic switching. Am J Pathol. 2003;162:533–546. doi: 10.1016/s0002-9440(10)63847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chazova I, Loyd JE, Zhdanov VS, Newman JH, Belenkov Y, Meyrick B. Pulmonary artery adventitial changes and venous involvement in primary pulmonary hypertension. Am J Pathol. 1995;146:389–397. [PMC free article] [PubMed] [Google Scholar]
- 27.Conrad PW, Conforti L, Kobayashi S, Beitner-Johnson D, Rust RT, Yuan Y, Kim HW, Kim RH, Seta K, Millhorn DE. The molecular basis of O2-sensing and hypoxia tolerance in pheochromocytoma cells. Comp Biochem Physiol B Biochem Mol Biol. 2001;128:187–204. doi: 10.1016/s1096-4959(00)00326-2. [DOI] [PubMed] [Google Scholar]
- 28.Csanyi G, Taylor WR, Pagano PJ. NOX and inflammation in the vascular adventitia. Free Radic Biol Med. 2009;47:1254–1266. doi: 10.1016/j.freeradbiomed.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cummins EP, Taylor CT. Hypoxia-responsive transcription factors. Pflugers Arch. 2005;450:363–371. doi: 10.1007/s00424-005-1413-7. [DOI] [PubMed] [Google Scholar]
- 30.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 31.Das M, Bouchey DM, Moore MJ, Hopkins DC, Nemenoff RA, Stenmark KR. Hypoxia-induced proliferative response of vascular adventitial fibroblasts is dependent on g protein-mediated activation of mitogen-activated protein kinases. J Biol Chem. 2001;276:15631–15640. doi: 10.1074/jbc.M010690200. [DOI] [PubMed] [Google Scholar]
- 32.Das M, Burns N, Wilson SJ, Zawada WM, Stenmark KR. Hypoxia exposure induces the emergence of fibroblasts lacking replication repressor signals of PKCzeta in the pulmonary artery adventitia. Cardiovasc Res. 2008;78:440–448. doi: 10.1093/cvr/cvn014. [DOI] [PubMed] [Google Scholar]
- 33.Das M, Dempsey EC, Bouchey D, Reyland ME, Stenmark KR. Chronic hypoxia induces exaggerated growth responses in pulmonary artery adventitial fibroblasts: potential contribution of specific protein kinase c isozymes. Am J Respir Cell Mol Biol. 2000;22:15–25. doi: 10.1165/ajrcmb.22.1.3536. [DOI] [PubMed] [Google Scholar]
- 34.Das M, Dempsey EC, Reeves JT, Stenmark KR. Selective expansion of fibroblast subpopulations from pulmonary artery adventitia in response to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2002;282:L976–L986. doi: 10.1152/ajplung.00382.2001. [DOI] [PubMed] [Google Scholar]
- 35.Davie NJ, Crossno JT, Jr, Frid MG, Hofmeister SE, Reeves JT, Hyde DM, Carpenter TC, Brunetti JA, McNiece IK, Stenmark KR. Hypoxia-induced pulmonary artery adventitial remodeling and neovascularization: contribution of progenitor cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L668–L678. doi: 10.1152/ajplung.00108.2003. [DOI] [PubMed] [Google Scholar]
- 36.Davie NJ, Gerasimovskaya EV, Hofmeister SE, Richman AP, Jones PL, Reeves JT, Stenmark KR. Pulmonary artery adventitial fibroblasts cooperate with vasa vasorum endothelial cells to regulate vasa vasorum neovascularization: A process mediated by hypoxia and endothelin-1. Am J Pathol. 2006;168:1793–1807. doi: 10.2353/ajpath.2006.050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 38.Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: A key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:509–517. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- 39.Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J. 2003;22:358–363. doi: 10.1183/09031936.03.00038903. [DOI] [PubMed] [Google Scholar]
- 40.Durmowicz AG, Orton EC, Stenmark KR. Progressive loss of vasodilator responsive component of pulmonary hypertension in neonatal calves exposed to 4,570 m. Am J Physiol. 1993;265:H2175–H2183. doi: 10.1152/ajpheart.1993.265.6.H2175. [DOI] [PubMed] [Google Scholar]
- 41.Durmowicz AG, Parks WC, Hyde DM, Mecham RP, Stenmark KR. Persistence, re-expression, and induction of pulmonary arterial fibronectin, tropoelastin, and type I procollagen mRNA expression in neonatal hypoxic pulmonary hypertension. Am J Pathol. 1994;145:1411–1420. [PMC free article] [PubMed] [Google Scholar]
- 42.Egeblad M, Littlepage LE, Werb Z. The fibroblastic coconspirator in cancer progression. Cold Spring Harb Symp Quant Biol. 2005;70:383–388. doi: 10.1101/sqb.2005.70.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 44.Eul B, Rose F, Krick S, Savai R, Goyal P, Klepetko W, Grimminger F, Weissmann N, Seeger W, Hanze J. Impact of HIF-1alpha and HIF-2alpha on proliferation and migration of human pulmonary artery fibroblasts in hypoxia. FASEB J. 2006;20:163–165. doi: 10.1096/fj.05-4104fje. [DOI] [PubMed] [Google Scholar]
- 45.Eyden B. Fibroblast phenotype plasticity: Relevance for understanding heterogeneity in “fibroblastic” tumors. Ultrastruct Pathol. 2004;28:307–319. doi: 10.1080/019131290882204. [DOI] [PubMed] [Google Scholar]
- 46.Eyden B. The myofibroblast: A study of normal, reactive and neoplastic tissues, with an emphasis on ultrastructure, Part 1: Normal and reactive cells. J Submicrosc Cytol Pathol. 2005;37:109–204. [PubMed] [Google Scholar]
- 47.Firestein GS, Alvaro-Gracia JM, Maki R. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990;144:3347–3353. [PubMed] [Google Scholar]
- 48.Flavell SJ, Hou TZ, Lax S, Filer AD, Salmon M, Buckley CD. Fibroblasts as novel therapeutic targets in chronic inflammation. Br J Pharmacol. 2008;153 (suppl 1):S241–S246. doi: 10.1038/sj.bjp.0707487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frid M, Brunetti J, Burke D, Carpenter T, Davie N, Reeves J, Roedersheimer M, van Rooijen N, Stenmark K. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of monocyte/macrophage lineage. Am J Pathol. 2006;168:659–669. doi: 10.2353/ajpath.2006.050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol. 2006;168:659–669. doi: 10.2353/ajpath.2006.050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frid MG, Li M, Gnanasekharan M, Burke DL, Fragoso M, Strassheim D, Sylman JL, Stenmark KR. Sustained hypoxia leads to the emergence of cells with enhanced growth, migratory, and pro-mitogenic potentials within the distal pulmonary artery wall. Am J Physiol Lung Cell Mol Physiol. 2009;297(6):L1059–L1072. doi: 10.1152/ajplung.90611.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luria EA, Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92. [PubMed] [Google Scholar]
- 53.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 54.Gallucci RM, Lee EG, Tomasek JJ. IL-6 modulates alpha-smooth muscle actin expression in dermal fibroblasts from IL-6-deficient mice. J Invest Dermatol. 2006;126:561–568. doi: 10.1038/sj.jid.5700109. [DOI] [PubMed] [Google Scholar]
- 55.Gao PJ, Li Y, Sun AJ, Liu JJ, Ji KD, Zhang YZ, Sun WL, Marche P, Zhu DL. Differentiation of vascular myofibroblasts induced by transforming growth factor-beta1 requires the involvement of protein kinase Calpha. J Mol Cell Cardiol. 2003;35:1105–1112. doi: 10.1016/s0022-2828(03)00207-4. [DOI] [PubMed] [Google Scholar]
- 56.Gebb SA, Jones PL. Hypoxia and lung branching morphogenesis. Adv Exp Med Biol. 2003;543:117–125. doi: 10.1007/978-1-4419-8997-0_8. [DOI] [PubMed] [Google Scholar]
- 57.Gerasimovskaya EV, Ahmad S, White CW, Jones PL, Carpenter TC, Stenmark KR. Extracellular ATP is an autocrine/paracrine regulator of hypoxia-induced adventitial fibroblast growth. Signaling through extracellular signal-regulated kinase-1/2 and the Egr-1 transcription factor. J Biol Chem. 2002;277:44638–44650. doi: 10.1074/jbc.M203012200. [DOI] [PubMed] [Google Scholar]
- 58.Gerasimovskaya EV, Tucker DA, Stenmark KR. Activation of phosphatidylinositol 3-kinase, Akt, and mammalian target of rapamycin is necessary for hypoxia-induced pulmonary artery adventitial fibroblast proliferation. J Appl Physiol. 2005;98:722–731. doi: 10.1152/japplphysiol.00715.2004. [DOI] [PubMed] [Google Scholar]
- 59.Gonzalez MC, Arribas SM, Molero F, Fernandez-Alfonso MS. Effect of removal of adventitia on vascular smooth muscle contraction and relaxation. Am J Physiol Heart Circ Physiol. 2001;280:H2876–H2881. doi: 10.1152/ajpheart.2001.280.6.H2876. [DOI] [PubMed] [Google Scholar]
- 60.Gossl M, Rosol M, Malyar NM, Fitzpatrick LA, Beighley PE, Zamir M, Ritman EL. Functional anatomy and hemodynamic characteristics of vasa vasorum in the walls of porcine coronary arteries. Anat Rec. 2003;272:526–537. doi: 10.1002/ar.a.10060. [DOI] [PubMed] [Google Scholar]
- 61.Hagood JS, Lasky JA, Nesbitt JE, Segarini P. Differential expression, surface binding, and response to connective tissue growth factor in lung fibroblast subpopulations. Chest. 2001;120:64S–66S. doi: 10.1378/chest.120.1_suppl.s64. [DOI] [PubMed] [Google Scholar]
- 62.Hagood JS, Prabhakaran P, Kumbla P, Salazar L, MacEwen MW, Barker TH, Ortiz LA, Schoeb T, Siegal GP, Alexander CB, Pardo A, Selman M. Loss of fibroblast Thy-1 expression correlates with lung fibrogenesis. Am J Pathol. 2005;167:365–379. doi: 10.1016/S0002-9440(10)62982-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han CI, Campbell GR, Campbell JH. Circulating bone marrow cells can contribute to neointimal formation. J Vasc Res. 2001;38:113–119. doi: 10.1159/000051038. [DOI] [PubMed] [Google Scholar]
- 64.Han JW, Shimada K, Ma-Krupa W, Johnson TL, Nerem RM, Goronzy JJ, Weyand CM. Vessel wall-embedded dendritic cells induce T-cell autoreactivity and initiate vascular inflammation. Circ Res. 2008;102:546–553. doi: 10.1161/CIRCRESAHA.107.161653. [DOI] [PubMed] [Google Scholar]
- 65.Hardie WD, Glasser SW, Hagood JS. Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol. 2009;175:3–16. doi: 10.2353/ajpath.2009.081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hartlapp I, Abe R, Saeed RW, Peng T, Voelter W, Bucala R, Metz CN. Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J. 2001;15:2215–2224. doi: 10.1096/fj.01-0049com. [DOI] [PubMed] [Google Scholar]
- 67.Hashimoto N, Phan SH, Imaizumi K, Matsuo M, Nakashima H, Kawabe T, Shimokata K, Hasegawa Y. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;43:161–172. doi: 10.1165/rcmb.2009-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54:S10–S19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 69.Haurani MJ, Pagano PJ. Adventitial fibroblast reactive oxygen species as autocrine and paracrine mediators of remodeling: Bellwether for vascular disease? Cardiovasc Res. 2007;75:679–689. doi: 10.1016/j.cardiores.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 70.Hayden MR, Tyagi SC. Vasa vasorum in plaque angiogenesis, metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: A malignant transformation. Cardiovasc Diabetol. 2004;3:1. doi: 10.1186/1475-2840-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herget J, Novotna J, Bibova J, Povysilova V, Vankova M, Hampl V. Metalloproteinase inhibition by Batimastat attenuates pulmonary hypertension in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol. 2003;285:L199–L208. doi: 10.1152/ajplung.00167.2002. [DOI] [PubMed] [Google Scholar]
- 72.Herrmann J, Best PJ, Ritman EL, Holmes DR, Lerman LO, Lerman A. Chronic endothelin receptor antagonism prevents coronary vasa vasorum neovascularization in experimental hypercholesterolemia. J Am Coll Cardiol. 2002;39:1555–1561. doi: 10.1016/s0735-1097(02)01798-9. [DOI] [PubMed] [Google Scholar]
- 73.Herrmann J, Samee S, Chade A, Porcel MR, Lerman LO, Lerman A. Differential effect of experimental hypertension and hypercholesterolemia on adventitial remodeling. Arterioscler Thromb Vasc Biol. 2005;25:447–453. doi: 10.1161/01.ATV.0000152606.34120.97. [DOI] [PubMed] [Google Scholar]
- 74.Hillebrands J, Van Den Hurk BM, Klatter FA, Popa ER, Nieuwenhuis P, Rozing J. Recipient origin of neointimal vascular smooth muscle cells in cardiac allografts with transplant arteriosclerosis. J Heart Lung Transplant. 2000;19:1183–1192. doi: 10.1016/s1053-2498(00)00209-6. [DOI] [PubMed] [Google Scholar]
- 75.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 76.Hlatky L, Tsionou C, Hahnfeldt P, Coleman CN. Mammary fibroblasts may influence breast tumor angiogenesis via hypoxia-induced vascular endothelial growth factor up-regulation and protein expression. Cancer Res. 1994;54:6083–6086. [PubMed] [Google Scholar]
- 77.Hogaboam CM, Bone-Larson CL, Lipinski S, Lukacs NW, Chensue SW, Strieter RM, Kunkel SL. Differential monocyte chemoattractant protein-1 and chemokine receptor 2 expression by murine lung fibroblasts derived from Th1- and Th2-type pulmonary granuloma models. J Immunol. 1999;163:2193–2201. [PubMed] [Google Scholar]
- 78.Hoshino A, Chiba H, Nagai K, Ishii G, Ochiai A. Human vascular adventitial fibroblasts contain mesenchymal stem/progenitor cells. Biochem Biophys Res Commun. 2008;368:305–310. doi: 10.1016/j.bbrc.2008.01.090. [DOI] [PubMed] [Google Scholar]
- 79.Hu Y, Davison F, Zhang Z, Xu Q. Endothelial replacement and angiogenesis in arteriosclerotic lesions of allografts are contributed by circulating progenitor cells. Circulation. 2003;108:3122–3127. doi: 10.1161/01.CIR.0000105722.96112.67. [DOI] [PubMed] [Google Scholar]
- 80.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 82.Jabs A, Okamoto E, Vinten-Johansen J, Bauriedel G, Wilcox JN. Sequential patterns of chemokine- and chemokine receptor-synthesis following vessel wall injury in porcine coronary arteries. Atherosclerosis. 2007;192:75–84. doi: 10.1016/j.atherosclerosis.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 83.Jelaska A, Strehlow D, Korn JH. Fibroblast heterogeneity in physiological conditions and fibrotic disease. Springer Semin Immunopathol. 1999;21:385–395. [PubMed] [Google Scholar]
- 84.Jian B, Jones PL, Li Q, Mohler ER, III, Schoen FJ, Levy RJ. Matrix metalloproteinase-2 is associated with tenascin-C in calcific aortic stenosis. Am J Pathol. 2001;159:321–327. doi: 10.1016/S0002-9440(10)61698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jin ZG, Melaragno MG, Liao DF, Yan C, Haendeler J, Suh YA, Lambeth JD, Berk BC. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res. 2000;87:789–796. doi: 10.1161/01.res.87.9.789. [DOI] [PubMed] [Google Scholar]
- 86.Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD, Camargo FD. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature. 2008;451:1125–1129. doi: 10.1038/nature06607. [DOI] [PubMed] [Google Scholar]
- 87.Jones FS, Jones PL. The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev Dyn. 2000;218:235–259. doi: 10.1002/(SICI)1097-0177(200006)218:2<235::AID-DVDY2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 88.Jones FS, Meech R, Edelman DB, Oakey RJ, Jones PL. Prx1 controls vascular smooth muscle cell proliferation and tenascin-C expression and is upregulated with Prx2 in pulmonary vascular disease. Circ Res. 2001;89:131–138. doi: 10.1161/hh1401.093582. [DOI] [PubMed] [Google Scholar]
- 89.Jones PL, Rabinovitch M. Tenascin-C is induced with progressive pulmonary vascular disease in rats and is functionally related to increased smooth muscle cell proliferation. Circ Res. 1996;79:1131–1142. doi: 10.1161/01.res.79.6.1131. [DOI] [PubMed] [Google Scholar]
- 90.Jordana M, Schulman J, McSharry C, Irving LB, Newhouse MT, Jordana G, Gauldie J. Heterogeneous proliferative characteristics of human adult lung fibroblast lines and clonally derived fibroblasts from control and fibrotic tissue. Am Rev Respir Dis. 1988;137:579–584. doi: 10.1164/ajrccm/137.3.579. [DOI] [PubMed] [Google Scholar]
- 91.Kadowitz PJ, Hyman AL. Effect of sympathetic nerve stimulation on pulmonary vascular resistance in the dog. Circ Res. 1973;32:221–227. doi: 10.1161/01.res.32.2.221. [DOI] [PubMed] [Google Scholar]
- 92.Kadowitz PJ, Knight DS, Hibbs RG, Ellison JP, Joiner PD, Brody MJ, Hyman AL. Influence of 5- and 6-hydroxydopamine on adrenergic transmission and nerve terminal morphology in the canine pulmonary vascular bed. Circ Res. 1976;39:191–199. doi: 10.1161/01.res.39.2.191. [DOI] [PubMed] [Google Scholar]
- 93.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 96.Kantachuvesiri S, Fleming S, Peters J, Peters B, Brooker G, Lammie AG, McGrath I, Kotelevtsev Y, Mullins JJ. Controlled hypertension, a transgenic toggle switch reveals differential mechanisms underlying vascular disease. J Biol Chem. 2001;276:36727–36733. doi: 10.1074/jbc.M103296200. [DOI] [PubMed] [Google Scholar]
- 97.Karouzakis E, Gay RE, Michel BA, Gay S, Neidhart M. DNA hypomethylation in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2009;60:3613–3622. doi: 10.1002/art.25018. [DOI] [PubMed] [Google Scholar]
- 98.Kimura H, Okada O, Tanabe N, Tanaka Y, Terai M, Takiguchi Y, Masuda M, Nakajima N, Hiroshima K, Inadera H, Matsushima K, Kuriyama T. Plasma monocyte chemoattractant protein-1 and pulmonary vascular resistance in chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2001;164:319–324. doi: 10.1164/ajrccm.164.2.2006154. [DOI] [PubMed] [Google Scholar]
- 99.Kottmann RM, Hogan CM, Phipps RP, Sime PJ. Determinants of initiation and progression of idiopathic pulmonary fibrosis. Respirology. 2009;14:917–933. doi: 10.1111/j.1440-1843.2009.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krick S, Hanze J, Eul B, Savai R, Seay U, Grimminger F, Lohmeyer J, Klepetko W, Seeger W, Rose F. Hypoxia-driven proliferation of human pulmonary artery fibroblasts: Cross-talk between HIF-1alpha and an autocrine angiotensin system. FASEB J. 2005;19:857–859. doi: 10.1096/fj.04-2890fje. [DOI] [PubMed] [Google Scholar]
- 101.Kroon ME, Koolwijk P, Van Der Vecht B, van Hinsbergh VW. Hypoxia in combination with FGF-2 induces tube formation by human microvascular endothelial cells in a fibrin matrix: Involvement of at least two signal transduction pathways. J Cell Sci. 2001;114:825–833. doi: 10.1242/jcs.114.4.825. [DOI] [PubMed] [Google Scholar]
- 102.Lafyatis R, Remmers EF, Roberts AB, Yocum DE, Sporn MB, Wilder RL. Anchorage-independent growth of synoviocytes from arthritic and normal joints. Stimulation by exogenous platelet-derived growth factor and inhibition by transforming growth factor-beta and retinoids. J Clin Invest. 1989;83:1267–1276. doi: 10.1172/JCI114011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Langheinrich AC, Kampschulte M, Buch T, Bohle RM. Vasa vasorum and atherosclerosis—quid novi? Thromb Haemost. 2007;97:873–879. [PubMed] [Google Scholar]
- 104.Langleben D, Szarek JL, Coflesky JT, Jones RC, Reid LM, Evans JN. Altered artery mechanics and structure in monocrotaline pulmonary hypertension. J Appl Physiol. 1988;65:2326–2331. doi: 10.1152/jappl.1988.65.5.2326. [DOI] [PubMed] [Google Scholar]
- 105.Li G, Chen SJ, Oparil S, Chen YF, Thompson JA. Direct in vivo evidence demonstrating neointimal migration of adventitial fibroblasts after balloon injury of rat carotid arteries. Circulation. 2000;101:1362–1365. doi: 10.1161/01.cir.101.12.1362. [DOI] [PubMed] [Google Scholar]
- 106.Li S, Tabar SS, Malec V, Eul BG, Klepetko W, Weissmann N, Grimminger F, Seeger W, Rose F, Hanze J. NOX4 regulates ROS levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxid Redox Signal. 2008;10:1687–1698. doi: 10.1089/ars.2008.2035. [DOI] [PubMed] [Google Scholar]
- 107.Liu C, Nath KA, Katusic ZS, Caplice NM. Smooth muscle progenitor cells in vascular disease. Trends Cardiovasc Med. 2004;14:288–293. doi: 10.1016/j.tcm.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 108.Liu J, Ormsby A, Oja-Tebbe N, Pagano PJ. Gene transfer of NAD(P)H oxidase inhibitor to the vascular adventitia attenuates medial smooth muscle hypertrophy. Circ Res. 2004;95:587–594. doi: 10.1161/01.RES.0000142317.88591.e6. [DOI] [PubMed] [Google Scholar]
- 109.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: Role of superoxide and NADPH oxidase (gp91phox) Am J Physiol Lung Cell Mol Physiol. 2006;290:L2–L10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- 110.Liu T, Chung MJ, Ullenbruch M, Yu H, Jin H, Hu B, Choi YY, Ishikawa F, Phan SH. Telomerase activity is required for bleomycin-induced pulmonary fibrosis in mice. J Clin Invest. 2007;117:3800–3809. doi: 10.1172/JCI32369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu T, Dhanasekaran SM, Jin H, Hu B, Tomlins SA, Chinnaiyan AM, Phan SH. FIZZ1 stimulation of myofibroblast differentiation. Am J Pathol. 2004;164:1315–1326. doi: 10.1016/S0002-9440(10)63218-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lo D, Feng L, Li L, Carson MJ, Crowley M, Pauza M, Nguyen A, Reilly CR. Integrating innate and adaptive immunity in the whole animal. Immunol Rev. 1999;169:225–239. doi: 10.1111/j.1600-065x.1999.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 113.Ma-Krupa W, Jeon MS, Spoerl S, Tedder TF, Goronzy JJ, Weyand CM. Activation of arterial wall dendritic cells and breakdown of self-tolerance in giant cell arteritis. J Exp Med. 2004;199:173–183. doi: 10.1084/jem.20030850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc Res. 2007;75:640–648. doi: 10.1016/j.cardiores.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mallawaarachchi CM, Weissberg PL, Siow RC. Smad7 gene transfer attenuates adventitial cell migration and vascular remodeling after balloon injury. Arterioscler Thromb Vasc Biol. 2005;25:1383–1387. doi: 10.1161/01.ATV.0000168415.33812.51. [DOI] [PubMed] [Google Scholar]
- 116.Mallawaarachchi CM, Weissberg PL, Siow RC. Antagonism of platelet-derived growth factor by perivascular gene transfer attenuates adventitial cell migration after vascular injury: New tricks for old dogs? FASEB J. 2006;20:1686–1688. doi: 10.1096/fj.05-5435fje. [DOI] [PubMed] [Google Scholar]
- 117.Malmstrom J, Lindberg H, Lindberg C, Bratt C, Wieslander E, Delander EL, Sarnstrand B, Burns JS, Mose-Larsen P, Fey S, Marko-Varga G. Transforming growth factor-beta 1 specifically induce proteins involved in the myofibroblast contractile apparatus. Mol Cell Proteomics. 2004;3:466–477. doi: 10.1074/mcp.M300108-MCP200. [DOI] [PubMed] [Google Scholar]
- 118.Martin TA, Harding KG, Jiang WG. Regulation of angiogenesis and endothelial cell motility by matrix-bound fibroblasts. Angiogenesis. 1999;3:69–76. doi: 10.1023/a:1009004212357. [DOI] [PubMed] [Google Scholar]
- 119.Martin TA, Harding K, Jiang WG. Matrix-bound fibroblasts regulate angiogenesis by modulation of VE-cadherin. Eur J Clin Invest. 2001;31:931–938. doi: 10.1046/j.1365-2362.2001.00914.x. [DOI] [PubMed] [Google Scholar]
- 120.McGrath JC, Deighan C, Briones AM, Shafaroudi MM, McBride M, Adler J, Arribas SM, Vila E, Daly CJ. New aspects of vascular remodelling: The involvement of all vascular cell types. Exp Physiol. 2005;90:469–475. doi: 10.1113/expphysiol.2005.030130. [DOI] [PubMed] [Google Scholar]
- 121.Meyrick B, Reid L. Hypoxia and incorporation of 3H-thymidine by cells of the rat pulmonary arteries and alveolar wall. Am J Pathol. 1979;96:51–70. [PMC free article] [PubMed] [Google Scholar]
- 122.Meyrick B, Reid L. Ultrastructural findings in lung biopsy material from children with congenital heart defects. Am J Pathol. 1980;101:527–542. [PMC free article] [PubMed] [Google Scholar]
- 123.Meyrick BO, Reid LM. Crotalaria-induced pulmonary hypertension. Uptake of 3H-thymidine by the cells of the pulmonary circulation and alveolar walls. Am J Pathol. 1982;106:84–94. [PMC free article] [PubMed] [Google Scholar]
- 124.Micke P, Hackbusch D, Mercan S, Stawowy P, Tsuprykov O, Unger T, Ostman A, Kappert K. Regulation of tyrosine phosphatases in the adventitia during vascular remodelling. Biochem Biophys Res Commun. 2009;382:678–684. doi: 10.1016/j.bbrc.2009.03.078. [DOI] [PubMed] [Google Scholar]
- 125.Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, Drexler S, Sofat N, Kashiwagi M, Orend G, Brennan F, Foxwell B. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15:774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 126.Mitzner W, Wagner EM. Vascular remodeling in the circulations of the lung. J Appl Physiol. 2004;97:1999–2004. doi: 10.1152/japplphysiol.00473.2004. [DOI] [PubMed] [Google Scholar]
- 127.Morrell NW, Atochina EN, Morris KG, Danilov SM, Stenmark KR. Angiotensin converting enzyme expression is increased in small pulmonary arteries of rats with hypoxia-induced pulmonary hypertension. J Clin Invest. 1995;96:1823–1833. doi: 10.1172/JCI118228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morrell NW, Morris KG, Stenmark KR. Role of angiotensin-converting enzyme and angiotensin II in development of hypoxic pulmonary hypertension. Am J Physiol. 1995;269:H1186–H1194. doi: 10.1152/ajpheart.1995.269.4.H1186. [DOI] [PubMed] [Google Scholar]
- 129.Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, Lo KM, Gillies S, Javaherian K, Folkman J. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci USA. 2003;100:4736–4741. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 131.Muro AF, Moretti FA, Moore BB, Yan M, Atrasz RG, Wilke CA, Flaherty KR, Martinez FJ, Tsui JL, Sheppard D, Baralle FE, Toews GB, White ES. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:638–645. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nakatsu MN, Sainson RC, Aoto JN, Taylor KL, Aitkenhead M, Perezdel-Pulgar S, Carpenter PM, Hughes CC. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: The role of fibroblasts and angiopoietin-1. Microvasc Res. 2003;66:102–112. doi: 10.1016/s0026-2862(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 133.Nathan S, Noble P, Tuder R. Idiopathic pulmonary fibrosis and pulmonary hypertension: Connecting the dots. Am J Respir Crit Care Med. 2007;175:875–880. doi: 10.1164/rccm.200608-1153CC. [DOI] [PubMed] [Google Scholar]
- 134.Neidhart M, Rethage J, Kuchen S, Kunzler P, Crowl RM, Billingham ME, Gay RE, Gay S. Retrotransposable L1 elements expressed in rheumatoid arthritis synovial tissue: Association with genomic DNA hypomethylation and influence on gene expression. Arthritis Rheum. 2000;43:2634–2647. doi: 10.1002/1529-0131(200012)43:12<2634::AID-ANR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 135.Nozaki Y, Liu T, Hatano K, Gharaee-Kermani M, Phan SH. Induction of telomerase activity in fibroblasts from bleomycin-injured lungs. Am J Respir Cell Mol Biol. 2000;23:460–465. doi: 10.1165/ajrcmb.23.4.3958. [DOI] [PubMed] [Google Scholar]
- 136.Nozik-Grayck E, Suliman HB, Majka S, Albietz J, Van Rheen Z, Roush K, Stenmark KR. Lung EC-SOD overexpression attenuates hypoxic induction of Egr-1 and chronic hypoxic pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2008;295:L422–L430. doi: 10.1152/ajplung.90293.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Numano F. Vasa vasoritis, vasculitis and atherosclerosis. Int J Cardiol. 2000;75(suppl 1):S1–S8. doi: 10.1016/s0167-5273(00)00196-0. discussion S17–S19. [DOI] [PubMed] [Google Scholar]
- 138.O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 10:111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- 139.Ogawa M, LaRue AC, Drake CJ. Hematopoietic origin of fibroblasts/myofibroblasts: Its pathophysiologic implications. Blood. 2006;108:2893–2896. doi: 10.1182/blood-2006-04-016600. [DOI] [PubMed] [Google Scholar]
- 140.Okada H, Kalluri R. Cellular and molecular pathways that lead to progression and regression of renal fibrogenesis. Curr Mol Med. 2005;5:467–474. doi: 10.2174/1566524054553478. [DOI] [PubMed] [Google Scholar]
- 141.Okamoto E, Couse T, De Leon H, Vinten-Johansen J, Goodman RB, Scott NA, Wilcox JN. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation. 2001;104:2228–2235. doi: 10.1161/hc4301.097195. [DOI] [PubMed] [Google Scholar]
- 142.Orton EC, LaRue SM, Ensley B, Stenmark K. Bromodeoxyuridine labeling and DNA content of pulmonary arterial medial cells from hypoxia-exposed and nonexposed healthy calves. Am J Vet Res. 1992;53:1925–1930. [PubMed] [Google Scholar]
- 143.Pagano PJ, Chanock SJ, Siwik DA, Colucci WS, Clark JK. Angiotensin II induces p67phox mRNA expression and NADPH oxidase superoxide generation in rabbit aortic adventitial fibroblasts. Hypertension. 1998;32:331–337. doi: 10.1161/01.hyp.32.2.331. [DOI] [PubMed] [Google Scholar]
- 144.Pagano PJ, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: Enhancement by angiotensin II. Proc Natl Acad Sci USA. 1997;94:14483–14488. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pap T, Muller-Ladner U, Gay RE, Gay S. Fibroblast biology. Role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arthritis Res. 2000;2:361–367. doi: 10.1186/ar113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL, Majesky MW. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci USA. 2008;105:9349–9354. doi: 10.1073/pnas.0711382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Patel S, Shi Y, Niculescu R, Chung EH, Martin JL, Zalewski A. Characteristics of coronary smooth muscle cells and adventitial fibroblasts. Circulation. 2000;101:524–532. doi: 10.1161/01.cir.101.5.524. [DOI] [PubMed] [Google Scholar]
- 148.Perros F, Dorfmuller P, Souza R, Durand-Gasselin I, Mussot S, Mazmanian M, Herve P, Emilie D, Simonneau G, Humbert M. Dendritic cell recruitment in lesions of human and experimental pulmonary hypertension. Eur Respir J. 2007;29:462–468. doi: 10.1183/09031936.00094706. [DOI] [PubMed] [Google Scholar]
- 149.Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002;122:286S–289S. doi: 10.1378/chest.122.6_suppl.286s. [DOI] [PubMed] [Google Scholar]
- 150.Phan SH. Fibroblast phenotypes in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29:S87–S92. [PubMed] [Google Scholar]
- 151.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Phipps RE. Pulmonary Fibroblast Heterogeneity. Boca Raton, FL: CRC Press; 1992. [Google Scholar]
- 153.Phipps RP, Penney DP, Keng P, Quill H, Paxhia A, Derdak S, Felch ME. Characterization of two major populations of lung fibroblasts: Distinguishing morphology and discordant display of Thy 1 and class II MHC. Am J Respir Cell Mol Biol. 1989;1:65–74. doi: 10.1165/ajrcmb/1.1.65. [DOI] [PubMed] [Google Scholar]
- 154.Pinto RF, de Higuchi ML, Aiello VD. Decreased numbers of T-lymphocytes and predominance of recently recruited macrophages in the walls of peripheral pulmonary arteries from 26 patients with pulmonary hypertension secondary to congenital cardiac shunts. Cardiovasc Pathol. 2004;13:268–275. doi: 10.1016/j.carpath.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 155.Popovici RM, Irwin JC, Giaccia AJ, Giudice LC. Hypoxia and cAMP stimulate vascular endothelial growth factor (VEGF) in human endometrial stromal cells: potential relevance to menstruation and endometrial regeneration. J Clin Endocrinol Metab. 1999;84:2245–2248. doi: 10.1210/jcem.84.6.5886. [DOI] [PubMed] [Google Scholar]
- 156.Postlethwaite AE, Shigemitsu H, Kanangat S. Cellular origins of fibroblasts: Possible implications for organ fibrosis in systemic sclerosis. Curr Opin Rheumatol. 2004;16:733–738. doi: 10.1097/01.bor.0000139310.77347.9c. [DOI] [PubMed] [Google Scholar]
- 157.Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer. 2008;99:1375–1379. doi: 10.1038/sj.bjc.6604662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science (New York) 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 159.Prockop DJ, Kivirikko KI. Collagens: Molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 160.Pryshchep O, Ma-Krupa W, Younge BR, Goronzy JJ, Weyand CM. Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation. 2008;118:1276–1284. doi: 10.1161/CIRCULATIONAHA.108.789172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: Collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 162.Radisky E, Radisky D. Stromal induction of breast cancer: Inflammation and invasion. Rev Endocr Metab Disord. 2007;8:279–287. doi: 10.1007/s11154-007-9037-1. [DOI] [PubMed] [Google Scholar]
- 163.Raghu G, Chen YY, Rusch V, Rabinovitch PS. Differential proliferation of fibroblasts cultured from normal and fibrotic human lungs. Am Rev Respir Dis. 1988;138:703–708. doi: 10.1164/ajrccm/138.3.703. [DOI] [PubMed] [Google Scholar]
- 164.Raines EW. The extracellular matrix can regulate vascular cell migration, proliferation, and survival: Relationships to vascular disease. Int J Exp Pathol. 2000;81:173–182. doi: 10.1046/j.1365-2613.2000.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rege TA, Pallero MA, Gomez C, Grenett HE, Murphy-Ullrich JE, Hagood JS. Thy-1, via its GPI anchor, modulates Src family kinase and focal adhesion kinase phosphorylation and subcellular localization, and fibroblast migration, in response to thrombospondin-1/hep I. Exp Cell Res. 2006;312:3752–3767. doi: 10.1016/j.yexcr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 166.Rey FE, Pagano PJ. The reactive adventitia: Fibroblast oxidase in vascular function. Arterioscler Thromb Vasc Biol. 2002;22:1962–1971. doi: 10.1161/01.atv.0000043452.30772.18. [DOI] [PubMed] [Google Scholar]
- 167.Ritman EL, Lerman A. The dynamic vasa vasorum. Cardiovasc Res. 2007;75:649–658. doi: 10.1016/j.cardiores.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rondelet B, Kerbaul F, Van Beneden R, Hubloue I, Huez S, Fesler P, Remmelink M, Brimioulle S, Salmon I, Naeije R. Prevention of pulmonary vascular remodelling and of decreased BMPR-2 expression by losartan therapy in shunt-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2005;289(6):H2319–H2324. doi: 10.1152/ajpheart.00518.2005. [DOI] [PubMed] [Google Scholar]
- 169.Ronty MJ, Leivonen SK, Hinz B, Rachlin A, Otey CA, Kahari VM, Carpen OM. Isoform-specific regulation of the actin-organizing protein palladin during TGF-beta1-induced myofibroblast differentiation. J Invest Dermatol. 2006;126:2387–2396. doi: 10.1038/sj.jid.5700427. [DOI] [PubMed] [Google Scholar]
- 170.Rose F, Grimminger F, Appel J, Heller M, Pies V, Weissmann N, Fink L, Schmidt S, Krick S, Camenisch G, Gassmann M, Seeger W, Hanze J. Hypoxic pulmonary artery fibroblasts trigger proliferation of vascular smooth muscle cells: Role of hypoxia-inducible transcription factors. FASEB J. 2002;16:1660–1661. doi: 10.1096/fj.02-0420fje. [DOI] [PubMed] [Google Scholar]
- 171.Rosenbloom J, Abrams WR, Mecham R. Extracellular matrix 4: The elastic fiber. FASEB J. 1993;7:1208–1218. [PubMed] [Google Scholar]
- 172.Sahara M, Sata M, Morita T, Nakamura K, Hirata Y, Nagai R. Diverse contribution of bone marrow-derived cells to vascular remodeling associated with pulmonary arterial hypertension and arterial neointimal formation. Circulation. 2007;115:509–517. doi: 10.1161/CIRCULATIONAHA.106.655837. [DOI] [PubMed] [Google Scholar]
- 173.Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: From innocent bystander to active participant. Circ Res. 2001;89:1111–1121. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 174.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 175.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 176.Schulze-Bauer CA, Regitnig P, Holzapfel GA. Mechanics of the human femoral adventitia including the high-pressure response. Am J Physiol Heart Circ Physiol. 2002;282:H2427–H2440. doi: 10.1152/ajpheart.00397.2001. [DOI] [PubMed] [Google Scholar]
- 177.Scott NA, Cipolla GD, Ross CE, Dunn B, Martin FH, Simonet L, Wilcox JN. Identification of a potential role for the adventitia in vascular lesion formation after balloon overstretch injury of porcine coronary arteries. Circulation. 1996;93:2178–2187. doi: 10.1161/01.cir.93.12.2178. [DOI] [PubMed] [Google Scholar]
- 178.Sharkey AM, Day K, McPherson A, Malik S, Licence D, Smith SK, Charnock-Jones DS. Vascular endothelial growth factor expression in human endometrium is regulated by hypoxia. J Clin Endocrinol Metab. 2000;85:402–409. doi: 10.1210/jcem.85.1.6229. [DOI] [PubMed] [Google Scholar]
- 179.Shi Y, Niculescu R, Wang D, Patel S, Davenpeck KL, Zalewski A. Increased NAD(P)H oxidase and reactive oxygen species in coronary arteries after balloon injury. Arterioscler Thromb Vasc Biol. 2001;21:739–745. doi: 10.1161/01.atv.21.5.739. [DOI] [PubMed] [Google Scholar]
- 180.Shi Y, O’Brien JE, Fard A, Mannion JD, Wang D, Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation. 1996;94:1655–1664. doi: 10.1161/01.cir.94.7.1655. [DOI] [PubMed] [Google Scholar]
- 181.Shi Y, O’Brien JE, Jr, Fard A, Zalewski A. Transforming growth factor-beta 1 expression and myofibroblast formation during arterial repair. Arterioscler Thromb Vasc Biol. 1996;16:1298–1305. doi: 10.1161/01.atv.16.10.1298. [DOI] [PubMed] [Google Scholar]
- 182.Shi Y, Patel S, Niculescu R, Chung W, Desrochers P, Zalewski A. Role of matrix metalloproteinases and their tissue inhibitors in the regulation of coronary cell migration. Arterioscler Thromb Vasc Biol. 1999;19:1150–1155. doi: 10.1161/01.atv.19.5.1150. [DOI] [PubMed] [Google Scholar]
- 183.Shi-Wen X, Chen Y, Denton CP, Eastwood M, Renzoni EA, Bou-Gharios G, Pearson JD, Dashwood M, du Bois RM, Black CM, Leask A, Abraham DJ. Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell. 2004;15:2707–2719. doi: 10.1091/mbc.E03-12-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Short M, Fox S, Lam F, Stenmark K, Das M. Protein Phosphorylation and Cell Signaling. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2005. Hypoxia-induced proliferative responses of vascular adventitital fibroblasts: Role of PKC-zeta and MAP kinase phosphatases-1. [Google Scholar]
- 185.Short M, Fox S, Stenmark K, Das M. Hypoxia-induced alterations in PKC-zeta signaling result in augmented fibroblast proliferation. Aspen Lung Conference; Aspen, CO. 2004; [DOI] [PubMed] [Google Scholar]
- 186.Short M, Nemenoff RA, Zawada WM, Stenmark KR, Das M. Hypoxia induces differentiation of pulmonary artery adventitial fibroblasts into myofibroblasts. Am J Physiol Cell Physiol. 2004;286:C416–C425. doi: 10.1152/ajpcell.00169.2003. [DOI] [PubMed] [Google Scholar]
- 187.Short MD, Fox SM, Lam CF, Stenmark KR, Das M. Protein kinase Czeta attenuates hypoxia-induced proliferation of fibroblasts by regulating MAP kinase phosphatase-1 expression. Mol Biol Cell. 2006;17:1995–2008. doi: 10.1091/mbc.E05-09-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shuttleworth CA. Type VIII collagen. Int J Biochem Cell Biol. 1997;29:1145–1148. doi: 10.1016/s1357-2725(97)00033-2. [DOI] [PubMed] [Google Scholar]
- 189.Silver FH, Horvath I, Foran DJ. Viscoelasticity of the vessel wall: The role of collagen and elastic fibers. Crit Rev Biomed Eng. 2001;29:279–301. doi: 10.1615/critrevbiomedeng.v29.i3.10. [DOI] [PubMed] [Google Scholar]
- 190.Siow RC, Mallawaarachchi CM, Weissberg PL. Migration of adventitial myofibroblasts following vascular balloon injury: Insights from in vivo gene transfer to rat carotid arteries. Cardiovasc Res. 2003;59:212–221. doi: 10.1016/s0008-6363(03)00292-x. [DOI] [PubMed] [Google Scholar]
- 191.Smith P, Heath D, Yacoub M, Madden B, Caslin A, Gosney J. The ultrastructure of plexogenic pulmonary arteriopathy. J Pathol. 1990;160:111–121. doi: 10.1002/path.1711600204. [DOI] [PubMed] [Google Scholar]
- 192.Smith PJ, Teichert-Kuliszewska K, Monge JC, Stewart DJ. Regulation of endothelin-B receptor mRNA expression in human endothelial cells by cytokines and growth factors. J Cardiovasc Pharmacol. 1998;31 (suppl 1):S158–S160. doi: 10.1097/00005344-199800001-00045. [DOI] [PubMed] [Google Scholar]
- 193.Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151:317–322. [PMC free article] [PubMed] [Google Scholar]
- 194.Sobin SS, Tremer HM, Hardy JD, Chiodi HP. Changes in arteriole in acute and chronic hypoxic pulmonary hypertension and recovery in rat. J Appl Physiol. 1983;55:1445–1455. doi: 10.1152/jappl.1983.55.5.1445. [DOI] [PubMed] [Google Scholar]
- 195.Sorrell JM, Caplan AI. Fibroblast heterogeneity: More than skin deep. J Cell Sci. 2004;117:667–675. doi: 10.1242/jcs.01005. [DOI] [PubMed] [Google Scholar]
- 196.Sorrell JM, Caplan AI. Fibroblasts—a diverse population at the center of it all. Int Rev Cell Mol Biol. 2009;276:161–214. doi: 10.1016/S1937-6448(09)76004-6. [DOI] [PubMed] [Google Scholar]
- 197.Steinman RM, Inaba K. Myeloid dendritic cells. J Leukoc Biol. 1999;66:205–208. doi: 10.1002/jlb.66.2.205. [DOI] [PubMed] [Google Scholar]
- 198.Stenmark K, Durmowicz A, Dempsey EC. Modulation of Vascular Wall Cell Phenotype in Pulmonary Hypertension. London: Portland Press; 1995. [Google Scholar]
- 199.Stenmark KR, Davie N, Frid M, Gerasimovskaya E, Das M. Role of the adventitia in pulmonary vascular remodeling. Physiology (Bethesda) 2006;21:134–145. doi: 10.1152/physiol.00053.2005. [DOI] [PubMed] [Google Scholar]
- 200.Stenmark KR, Davie NJ, Reeves JT, Frid MG. Hypoxia, leukocytes, and the pulmonary circulation. J Appl Physiol. 2005;98:715–721. doi: 10.1152/japplphysiol.00840.2004. [DOI] [PubMed] [Google Scholar]
- 201.Stenmark KR, Gerasimovskaya E, Nemenoff RA, Das M. Hypoxic activation of adventitial fibroblasts: Role in vascular remodeling. Chest. 2002;122:326S–334S. doi: 10.1378/chest.122.6_suppl.326s. [DOI] [PubMed] [Google Scholar]
- 202.Stenmark KR, Mecham RP. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu Rev Physiol. 1997;59:89–144. doi: 10.1146/annurev.physiol.59.1.89. [DOI] [PubMed] [Google Scholar]
- 203.Stevens T, Phan S, Frid MG, Alvarez D, Herzog E, Stenmark KR. Lung vascular cell heterogeneity: Endothelium, smooth muscle, and fibroblasts. Proc Am Thorac Soc. 2008;5:783–791. doi: 10.1513/pats.200803-027HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Strieter RM, Keeley EC, Burdick MD, Mehrad B. The role of circulating mesenchymal progenitor cells, fibrocytes, in promoting pulmonary fibrosis. Trans Am Clin Climatol Assoc. 2009;120:49–59. [PMC free article] [PubMed] [Google Scholar]
- 205.Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc Natl Acad Sci USA. 2005;102:437–442. doi: 10.1073/pnas.0408704102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Takami N, Osawa K, Miura Y, Komai K, Taniguchi M, Shiraishi M, Sato K, Iguchi T, Shiozawa K, Hashiramoto A, Shiozawa S. Hypermethylated promoter region of DR3, the death receptor 3 gene, in rheumatoid arthritis synovial cells. Arthritis Rheum. 2006;54:779–787. doi: 10.1002/art.21637. [DOI] [PubMed] [Google Scholar]
- 207.Tamaoki M, Imanaka-Yoshida K, Yokoyama K, Nishioka T, Inada H, Hiroe M, Sakakura T, Yoshida T. Tenascin-C regulates recruitment of myofibroblasts during tissue repair after myocardial injury. Am J Pathol. 2005;167:71–80. doi: 10.1016/S0002-9440(10)62954-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tille JC, Pepper MS. Mesenchymal cells potentiate vascular endothelial growth factor-induced angiogenesis in vitro. Exp Cell Res. 2002;280:179–191. doi: 10.1006/excr.2002.5635. [DOI] [PubMed] [Google Scholar]
- 209.Tonks NK. Redox redux: Revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–670. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 210.Torry DJ, Richards CD, Podor TJ, Gauldie J. Anchorage-independent colony growth of pulmonary fibroblasts derived from fibrotic human lung tissue. J Clin Invest. 1994;93:1525–1532. doi: 10.1172/JCI117131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Torsney E, Hu Y, Xu Q. Adventitial progenitor cells contribute to arteriosclerosis. Trends Cardiovasc Med. 2005;15:64–68. doi: 10.1016/j.tcm.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 212.Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: Implications in hypertension. Histochem Cell Biol. 2004;122:339–352. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- 213.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, Rosenblatt RL, Shay JW, Garcia CK. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci USA. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tuder RM. Pathology of pulmonary arterial hypertension. Semin Respir Crit Care Med. 2009;30:376–385. doi: 10.1055/s-0029-1233307. [DOI] [PubMed] [Google Scholar]
- 215.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med. 2007;28:23–42. vii. doi: 10.1016/j.ccm.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Velazquez OC, Snyder R, Liu ZJ, Fairman RM, Herlyn M. Fibroblast-dependent differentiation of human microvascular endothelial cells into capillary-like 3-dimensional networks. FASEB J. 2002;16:1316–1318. doi: 10.1096/fj.01-1011fje. [DOI] [PubMed] [Google Scholar]
- 218.Verity MA, Bevan JA. Fine structural study of the terminal effector plexus, neuromuscular and intermuscular relationships in the pulmonary artery. J Anat. 1968;103:49–63. [PMC free article] [PubMed] [Google Scholar]
- 219.Vieillard-Baron A, Frisdal E, Raffestin B, Baker AH, Eddahibi S, Adnot S, D’Ortho MP. Inhibition of matrix metalloproteinases by lung TIMP-1 gene transfer limits monocrotaline-induced pulmonary vascular remodeling in rats. Hum Gene Ther. 2003;14:861–869. doi: 10.1089/104303403765701150. [DOI] [PubMed] [Google Scholar]
- 220.Virchow R. Die cellularpathologie in ltrer begruendung auf physiologische und pathologische gewebelehre. Berlin: 1858. [Google Scholar]
- 221.Voelkel NF, Cool C. Pathology of pulmonary hypertension. Cardiol Clin. 2004;22:343–351. v. doi: 10.1016/j.ccl.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 222.Von Euler US, Lishajko F. Catechol amines in the vascular wall. Acta Physiol Scand. 1958;42:333–341. doi: 10.1111/j.1748-1716.1958.tb01567.x. [DOI] [PubMed] [Google Scholar]
- 223.Wallner K, Sharifi BG, Shah PK, Noguchi S, DeLeon H, Wilcox JN. Adventitial remodeling after angioplasty is associated with expression of tenascin mRNA by adventitial myofibroblasts. J Am Coll Cardiol. 2001;37:655–661. doi: 10.1016/s0735-1097(00)01117-7. [DOI] [PubMed] [Google Scholar]
- 224.Wang HD, Xu S, Johns DG, Du Y, Quinn MT, Cayatte AJ, Cohen RA. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ Res. 2001;88:947–953. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- 225.Welsh DJ, Peacock AJ, MacLean M, Harnett M. Chronic hypoxia induces constitutive p38 mitogen-activated protein kinase activity that correlates with enhanced cellular proliferation in fibroblasts from rat pulmonary but not systemic arteries. Am J Respir Crit Care Med. 2001;164:282–289. doi: 10.1164/ajrccm.164.2.2008054. [DOI] [PubMed] [Google Scholar]
- 226.Welsh DJ, Scott P, Plevin R, Wadsworth R, Peacock AJ. Hypoxia enhances cellular proliferation and inositol 1, 4, 5-triphosphate generation in fibroblasts from bovine pulmonary artery but not from mesenteric artery. Am J Respir Crit Care Med. 1998;158:1757–1762. doi: 10.1164/ajrccm.158.6.9706054. [DOI] [PubMed] [Google Scholar]
- 227.Welsh DJ, Scott PH, Peacock AJ. p38 MAP kinase isoform activity and cell cycle regulators in the proliferative response of pulmonary and systemic artery fibroblasts to acute hypoxia. Pulm Pharmacol Ther. 2006;19 (2):128–138. doi: 10.1016/j.pupt.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 228.Wilasrusmee C, Ondocin P, Bruch D, Shah G, Kittur S, Wilasrusmee S, Kittur DS. Amelioration of cyclosporin A effect on microvasculature by endothelin inhibitor. Surgery. 2003;134:384–389. doi: 10.1067/msy.2003.233. [DOI] [PubMed] [Google Scholar]
- 229.Wilcox JN, Okamoto EI, Nakahara KI, Vinten-Johansen J. Perivascular responses after angioplasty which may contribute to postangioplasty restenosis: A role for circulating myofibroblast precursors? Ann N Y Acad Sci. 2001;947:68–90. doi: 10.1111/j.1749-6632.2001.tb03931.x. discussion 90–62. [DOI] [PubMed] [Google Scholar]
- 230.Wilcox JN, Waksman R, King SB, Scott NA. The role of the adventitia in the arterial response to angioplasty: The effect of intravascular radiation. Int J Radiat Oncol Biol Phys. 1996;36:789–796. doi: 10.1016/s0360-3016(96)00299-4. [DOI] [PubMed] [Google Scholar]
- 231.Wolinsky H, Glagov S. Nature of species differences in the medial distribution of aortic vasa vasorum in mammals. Circ Res. 1967;20:409–421. doi: 10.1161/01.res.20.4.409. [DOI] [PubMed] [Google Scholar]
- 232.Xia H, Diebold D, Nho R, Perlman D, Kleidon J, Kahm J, Avdulov S, Peterson M, Nerva J, Bitterman P, Henke C. Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Exp Med. 2008;205:1659–1672. doi: 10.1084/jem.20080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Xia Y, Pauza ME, Feng L, Lo D. RelB regulation of chemokine expression modulates local inflammation. Am J Pathol. 1997;151:375–387. [PMC free article] [PubMed] [Google Scholar]
- 234.Yan SF, Lu J, Xu L, Zou YS, Tongers J, Kisiel W, Mackman N, Pinsky DJ, Stern DM. Pulmonary expression of early growth response-1: Biphasic time course and effect of oxygen concentration. J Appl Physiol. 2000;88:2303–2309. doi: 10.1152/jappl.2000.88.6.2303. [DOI] [PubMed] [Google Scholar]
- 235.Yang L, Scott PG, Giuffre J, Shankowsky HA, Ghahary A, Tredget EE. Peripheral blood fibrocytes from burn patients: Identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab Invest. 2002;82:1183–1192. doi: 10.1097/01.lab.0000027841.50269.61. [DOI] [PubMed] [Google Scholar]
- 236.Yi ES, Kim H, Ahn H, Strother J, Morris T, Masliah E, Hansen LA, Park K, Friedman PJ. Distribution of obstructive intimal lesions and their cellular phenotypes in chronic pulmonary hypertension. A morphometric and immunohistochemical study. Am J Respir Crit Care Med. 2000;162:1577–1586. doi: 10.1164/ajrccm.162.4.9912131. [DOI] [PubMed] [Google Scholar]
- 237.Young KC, Torres E, Hatzistergos KE, Hehre D, Suguihara C, Hare JM. Circ Res. 2009;104:1293–1301. doi: 10.1161/CIRCRESAHA.109.197533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zalewski A, Shi Y. Vascular myofibroblasts. Lessons from coronary repair and remodeling. Arterioscler Thromb Vasc Biol. 1997;17:417–422. doi: 10.1161/01.atv.17.3.417. [DOI] [PubMed] [Google Scholar]
- 239.Zebedee Z, Hara E. Id proteins in cell cycle control and cellular senescence. Oncogene. 2001;20:8317–8325. doi: 10.1038/sj.onc.1205092. [DOI] [PubMed] [Google Scholar]
- 240.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 241.Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, Weil J, Reichenspurner H, Kilic N, Ergun S. Vascular wall resident progenitor cells: A source for postnatal vasculogenesis. Development (Cambridge) 2006;133:1543–1551. doi: 10.1242/dev.02315. [DOI] [PubMed] [Google Scholar]
- 242.Zhou Y, Hagood JS, Murphy-Ullrich JE. Thy-1 expression regulates the ability of rat lung fibroblasts to activate transforming growth factor-beta in response to fibrogenic stimuli. Am J Pathol. 2004;165:659–669. doi: 10.1016/s0002-9440(10)63330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]