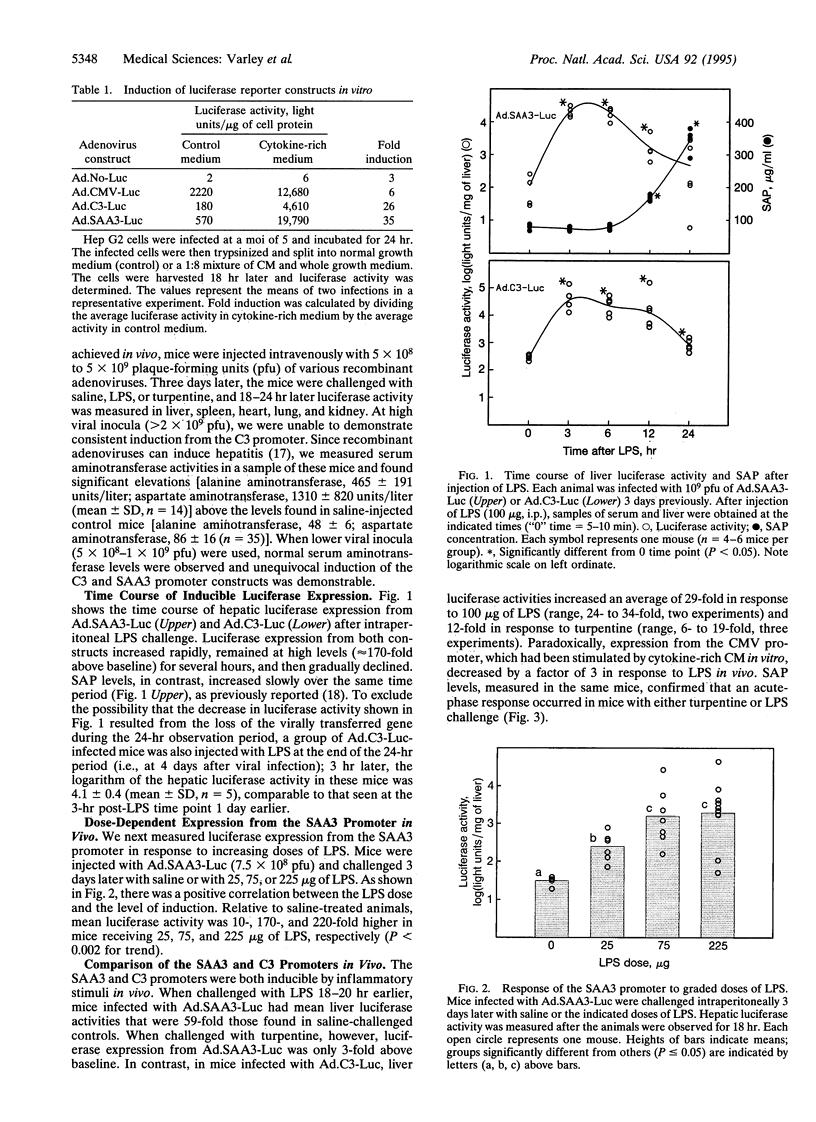
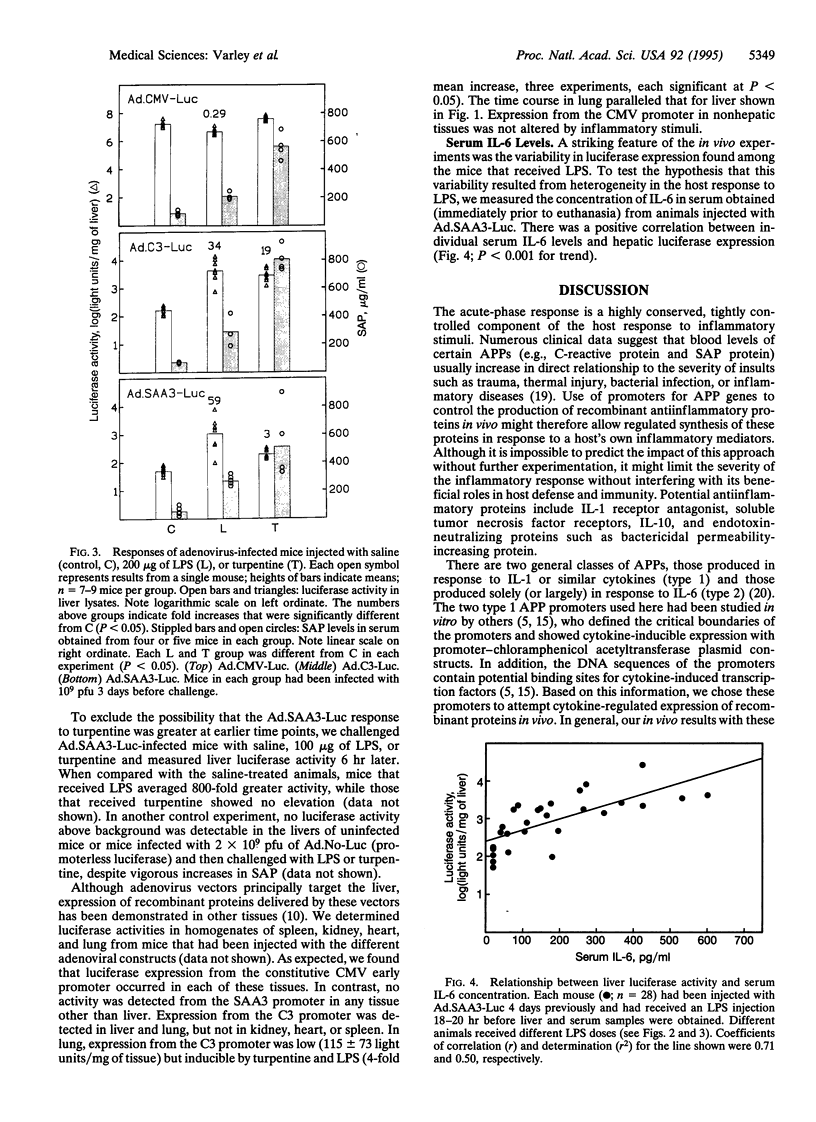
Abstract
We report that promoters for two murine acute-phase protein (APP) genes, complement factor 3 (C3) and serum amyloid A3 (SAA3), can increase recombinant protein expression in response to inflammatory stimuli in vivo. To deliver APP promoter-luciferase reporter gene constructs to the liver, where most endogenous APP synthesis occurs, we introduced them into a nonreplicating adenovirus vector and injected the purified viruses intravenously into mice. When compared with the low levels of basal luciferase expression observed prior to inflammatory challenge, markedly increased expression from the C3 promoter was detected in liver in response to both lipopolysaccharide (LPS) and turpentine, and lower-level inducible expression was also found in lung. In contrast, expression from the SAA3 promoter was found only in liver and was much more responsive to LPS than to turpentine. After LPS challenge, hepatic luciferase expression increased rapidly and in proportion to the LPS dose. Use of cytokine-inducible promoters in gene transfer vectors may make it possible to produce antiinflammatory proteins in vivo in direct relationship to the intensity and duration of an individual's inflammatory response. By providing endogenously controlled production of recombinant antiinflammatory proteins, this approach might limit the severity of the inflammatory response without interfering with the beneficial components of host defense and immunity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcorn J. L., Gao E., Chen Q., Smith M. E., Gerard R. D., Mendelson C. R. Genomic elements involved in transcriptional regulation of the rabbit surfactant protein-A gene. Mol Endocrinol. 1993 Aug;7(8):1072–1085. doi: 10.1210/mend.7.8.8232306. [DOI] [PubMed] [Google Scholar]
- Baumann H., Gauldie J. The acute phase response. Immunol Today. 1994 Feb;15(2):74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Brasier A. R., Ron D. Luciferase reporter gene assay in mammalian cells. Methods Enzymol. 1992;216:386–397. doi: 10.1016/0076-6879(92)16036-j. [DOI] [PubMed] [Google Scholar]
- Brissette L., Young I., Narindrasorasak S., Kisilevsky R., Deeley R. Differential induction of the serum amyloid A gene family in response to an inflammatory agent and to amyloid-enhancing factor. J Biol Chem. 1989 Nov 15;264(32):19327–19332. [PubMed] [Google Scholar]
- Conary J. T., Parker R. E., Christman B. W., Faulks R. D., King G. A., Meyrick B. O., Brigham K. L. Protection of rabbit lungs from endotoxin injury by in vivo hyperexpression of the prostaglandin G/H synthase gene. J Clin Invest. 1994 Apr;93(4):1834–1840. doi: 10.1172/JCI117169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas P., Ledoux D., Nys M., Vrindts Y., De Groote D., Franchimont P., Lamy M. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992 Apr;215(4):356–362. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenwald J. E., Fong Y. M., Fahey T. J., 3rd, Calvano S. E., Chizzonite R., Kilian P. L., Lowry S. F., Moldawer L. L. Interleukin 1 receptor blockade attenuates the host inflammatory response. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4966–4970. doi: 10.1073/pnas.87.13.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D. A relational database of transcription factors. Nucleic Acids Res. 1990 Apr 11;18(7):1749–1756. doi: 10.1093/nar/18.7.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Green M., Wold W. S. Human adenoviruses: growth, purification, and transfection assay. Methods Enzymol. 1979;58:425–435. doi: 10.1016/s0076-6879(79)58157-9. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., DePaoli A. M., Burant C. F., Refetoff S. Expression of a thyroid hormone-responsive recombinant gene introduced into adult mice livers by replication-defective adenovirus can be regulated by endogenous thyroid hormone receptor. J Biol Chem. 1994 Sep 30;269(39):23872–23875. [PubMed] [Google Scholar]
- Herz J., Gerard R. D. Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2812–2816. doi: 10.1073/pnas.90.7.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. H., Rienhoff H. Y., Jr, Liao W. S. Regulation of mouse serum amyloid A gene expression in transfected hepatoma cells. Mol Cell Biol. 1990 Jul;10(7):3619–3625. doi: 10.1128/mcb.10.7.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Kawamura N., Singer L., Wetsel R. A., Colten H. R. Cis- and trans-acting elements required for constitutive and cytokine-regulated expression of the mouse complement C3 gene. Biochem J. 1992 May 1;283(Pt 3):705–712. doi: 10.1042/bj2830705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N., Rosenthal E. R., Yoshimura K., Crystal R. G. Transfer of a constitutive viral promoter-cystic fibrosis transmembrane conductance regulator cDNA to human epithelial cells conveys resistance to down-regulation of cAMP-regulated Cl- secretion in the presence of inflammatory stimuli. Nucleic Acids Res. 1994 Oct 25;22(21):4470–4476. doi: 10.1093/nar/22.21.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolls J., Peppel K., Silva M., Beutler B. Prolonged and effective blockade of tumor necrosis factor activity through adenovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):215–219. doi: 10.1073/pnas.91.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. C., Hayes S., Young A. P. Transactivation of the 'promoterless' luciferase-encoding vectors pXP1 and pXP2 by C/EBP alpha. Gene. 1994 Jan 28;138(1-2):257–258. doi: 10.1016/0378-1119(94)90819-2. [DOI] [PubMed] [Google Scholar]
- Lowell C. A., Potter D. A., Stearman R. S., Morrow J. F. Structure of the murine serum amyloid A gene family. Gene conversion. J Biol Chem. 1986 Jun 25;261(18):8442–8452. [PubMed] [Google Scholar]
- Lowell C. A., Stearman R. S., Morrow J. F. Transcriptional regulation of serum amyloid A gene expression. J Biol Chem. 1986 Jun 25;261(18):8453–8461. [PubMed] [Google Scholar]
- Malone R. W., Hickman M. A., Lehmann-Bruinsma K., Sih T. R., Walzem R., Carlson D. M., Powell J. S. Dexamethasone enhancement of gene expression after direct hepatic DNA injection. J Biol Chem. 1994 Nov 25;269(47):29903–29907. [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- McPhaul M. J., Deslypere J. P., Allman D. R., Gerard R. D. The adenovirus-mediated delivery of a reporter gene permits the assessment of androgen receptor function in genital skin fibroblast cultures. Stimulation of Gs and inhibition of G(o). J Biol Chem. 1993 Dec 15;268(35):26063–26066. [PubMed] [Google Scholar]
- Meek R. L., Benditt E. P. Amyloid A gene family expression in different mouse tissues. J Exp Med. 1986 Dec 1;164(6):2006–2017. doi: 10.1084/jem.164.6.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg H. S., Rogy M. A., Lazarus D. D., Van Zee K. J., Keeler B. P., Chizzonite R. A., Lowry S. F., Moldawer L. L. Cachexia and the acute-phase protein response in inflammation are regulated by interleukin-6. Eur J Immunol. 1993 Aug;23(8):1889–1894. doi: 10.1002/eji.1830230824. [DOI] [PubMed] [Google Scholar]
- Steel D. M., Whitehead A. S. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol Today. 1994 Feb;15(2):81–88. doi: 10.1016/0167-5699(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Suffredini A. F. Current prospects for the treatment of clinical sepsis. Crit Care Med. 1994 Jul;22(7):S12–S18. [PubMed] [Google Scholar]
- Thompson J. F., Hayes L. S., Lloyd D. B. Modulation of firefly luciferase stability and impact on studies of gene regulation. Gene. 1991 Jul 22;103(2):171–177. doi: 10.1016/0378-1119(91)90270-l. [DOI] [PubMed] [Google Scholar]
- Yang Y., Nunes F. A., Berencsi K., Furth E. E., Gönczöl E., Wilson J. M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]


