Abstract
Demonstration of safe and stable reversal of blindness after a single unilateral subretinal injection of a recombinant adeno-associated virus (AAV) carrying the RPE65 gene (AAV2-hRPE65v2) prompted us to determine whether it was possible to obtain additional benefit through a second administration of the AAV vector to the contralateral eye. Readministration of vector to the second eye was carried out in three adults with Leber congenital amaurosis due to mutations in the RPE65 gene 1.7 to 3.3 years after they had received their initial subretinal injection of AAV2-hRPE65v2. Results (through 6 months) including evaluations of immune response, retinal and visual function testing, and functional magnetic resonance imaging indicate that readministration is both safe and efficacious after previous exposure to AAV2-hRPE65v2.
INTRODUCTION
Leber congenital amaurosis (LCA) is a group of hereditary retinal dystrophies characterized by profound impairment in retinal and visual function in infancy and early childhood followed by progressive deterioration and loss of retinal cells in the first few decades of life (1-3). LCA is usually inherited as an autosomal recessive trait, and mutations in 15 different genes have been reported so far (4, 5). One of the more common forms of LCA, LCA2, is due to mutations in the RPE65 gene (6, 7). This gene encodes an all-trans-retinyl ester isomerase, an enzyme critical to the function of the retinoid cycle (8, 9). Without RPE65, very little 11-cis-retinal, the vitamin A derivative that is the chromophore of rod and cone photoreceptor opsins, is made (8, 9). Without 11-cis-retinal, opsins cannot capture light and relay this into electrical responses to initiate vision (8, 10). Successful proof-of-principle studies in LCA2 murine and canine animal models using a replication-defective adeno-associated viral vector (rAAV) (11–14) demonstrated that the biochemical blockade of the visual cycle due to RPE65 deficiency could be overcome through gene augmentation. Safety and dosing studies in large animals then provided the pre-clinical safety and efficacy data that formed the impetus to test this approach in human clinical trials (15–17).
We reported safe and stable amelioration in retinal and visual function in all 12 patients treated in a phase 1/2 study at The Children’s Hospital of Philadelphia (CHOP) (16, 18-20). These individuals had been injected subretinally in the eye with worse vision in a dose-escalation study with doses ranging from 1.5 × 1010 to 1.5 × 1011 vector genomes (vg) of the AAV2 vector carrying the RPE65 gene (AAV2.hRPE65v2) (16, 18). Each one of the subjects showed improvement in multiple measures of retinal and visual function in the injected eye. Most of the subjects showed improvement in full-field light sensitivity and pupillary light reflex (PLR). About half of the subjects showed significant improvement in visual acuity, and all showed a trend toward improvement in visual fields. Five of the 12 patients (including all pediatric subjects age 8 to 11 years) developed the ability to navigate a standardized obstacle course (16, 18). The improvements were observed as early as 1 month after treatment and persisted through the latest time point (now 4 years for the initial subjects) (16, 18, 20). Functional magnetic resonance imaging (fMRI) studies carried out in subjects after they had received the injection also showed that the visual cortex became responsive to retinal input after this unilateral gene therapy, even after prolonged visual deprivation (20). Both the retina and the visual cortex became far more sensitive to dim light and lower-contrast stimuli.
The success of the unilateral injections begged the question of whether additional visual function could be further gained in the contralateral eye of these patients. Because the immune consequences of subretinal readministration of rAAV2 were unknown, we carried out contralateral eye readministration studies in two different large-animal models. Readministration resulted in efficacy in both eyes in the affected dogs and appeared safe in both affected dogs and unaffected nonhuman primates (21). However, there is little precedent for the ability to safely readminister rAAV in humans and obtain a therapeutic effect. There was also a concern that immune responses after readministration would diminish the benefits that the subjects had obtained in their previously injected eye. We therefore proceeded cautiously to test safety and efficacy of administration to the contralateral eye in three adult subjects who had already undergone unilateral subretinal injection in our phase 1/2 dose-escalation study (16, 18).
Through comparison of pre- and postsurgical testing, we demonstrate that delivery of AAV2-hRPE65v2 to the contralateral eye is safe even if years have passed since the initial treatment. Further, before and after comparisons of psychophysical data and fMRI results provide additional evidence for the effectiveness of gene therapy readministration in LCA2 patients and also reveal the magnitude and pattern of improvement. Results in two patients receiving different doses in each eye suggest a possible dose-response effect of the gene therapy vector.
RESULTS
Follow-on enrollment and study design
The readministration study was carried out as a “follow-on” (FO) study to the original phase 1/2 protocol (NCT01208389). The original protocol entailed injection into each subject’s more impaired eye (16, 18). The Institutional Review Board (IRB) had given approval for the contralateral eye administration as long as the first three subjects were adults. The first three adults enrolled in the FO study were CH12, CH11, and NP01, all of whom have missense mutations in RPE65 (Table 1), and these individuals self-selected on the basis of availability. The disease was advanced in each one of these subjects, the degree of which correlated with their age due to the degenerative nature of LCA2. These individuals had received their initial injection 1.7 to 3.4 years earlier and were enrolled sequentially (with an 8-week interval between each enrollment). After providing informed consent, the subjects underwent “FO baseline” immunological and retinal/visual testing before the readministration. The schedule of tests in the FO study was similar to but not identical to the schedule in the initial study (table S1). Some tests that had been used in the initial study were dropped (for example, electroretinograms). Other analyses had been added during the course of the initial study, and these were maintained in the FO study including the full-field light sensitivity threshold (FST) test. Subjects also consented separately to participate in an fMRI study.
Table 1.
Subject enrollment characteristics and injection details. Subjects are listed in the order that they were enrolled in the FO study. Eye #1, retina that was initially injected; Eye #2, retina that received the FO injection. All subjects were followed through FOd180. Visual acuity is expressed in LogMAR (log of the minimum angle of resolution). Higher values indicate poorer vision (see Supplementary Methods). Hand motion vision was assigned a conservative LogMAR of 2.6.
| Patient ID | Age at readministration |
Sex | Follow-up after initial injection (years) |
AAV2-hRPE65v2 dose (vg)/volume (μl) |
Visual acuity (pre/post) |
RPE65 mutation | ||
|---|---|---|---|---|---|---|---|---|
| Eye #1 | Eye #2 | Eye #1 | Eye #2 | |||||
| CH12 | 46 | F | 2.1 | 1.5 × 1011/300 (high/high) |
1.5 × 1011/300 (high/high) |
2.6/2.16 | 2.6/2.0 | K303X/W431C |
| CH11 | 27 | F | 2.3 | 4.8 × 1010/150 (medium/low) |
1.5 × 1011/300 (high/high) |
0.76/0.77 | 0.64/0.58 | V473D/V473D |
| NP01 | 29 | F | 3.7 | 1.5 × 1010/150 (low/low) |
1.5 × 1011/300 (high/high) |
1.5/1.6 | 1.83/1.6 | E102K/E102K |
As with the initial injection, the area targeted in the readministration was selected on the basis of the results of clinical evaluations and retinal imaging studies indicating that the tissue in that region had sufficient numbers of viable retinal cells. Although the subjects had received different doses and volumes of AAV2-hRPE65v2 in their initial administration, they all received 1.5 × 1011 vg in 300 μl for the readministration study in their previously uninjected (second) eye (Fig. 1A and Table 1). This was the same dose/volume that 46-year-old patient CH12 had received initially. The other two subjects (NP01 and CH11, 29 and 27 years, respectively) had previously received lower doses (1.5 × 1010 and 4.8 × 1010 vg, respectively) in a volume of 150 μl (Table 1). Post-injection safety, retinal/visual function, and fMRI imaging studies were carried out serially at prescribed FO time points through the latest evaluation time point, FO day 180 (FOd180) (table S1).
Fig. 1.
(A) Images of fundus photos compare the baseline (“Pre”) and d60 (“Post”) appearance and the predicted pre- and post-readministration visual field. There is extensive disease at baseline, with retinal pigment epithelial disturbance and geographical atrophy in the macula in patient CH12. Arrowheads indicate the lower border of the subretinal injection site, which was supratemporal and included the superior aspect of the macula in all three subjects. The lower border of the bleb was closer to the superior vascular arcade in CH12, whereas the lower borders for patients CH11 and NP01 were closer to the fovea. On the far right are the pre- and post-readministration visual fields. The predicted visual field changes based on the injection sites (and assuming a healthy retina) were similar for the three subjects (yellow shaded areas). Gray shaded areas denote scotomas (spots in the visual field in which vision is absent or decreased) that were altered in location at each different FO exam (only baseline scotomas are shown). (B) Full-field sensitivity threshold testing shows an increase in retinal light sensitivity (y axis shows sensitivity thresholds) in the left eyes of NP01 and CH11 by d30 persisting through the latest time point (d180), but no change in sensitivity of the previously injected eye for the three patients. There was no change in FST test results for either eye of patient CH12. (C) Improved PLR in the second eye to receive an injection of AAV2-hRPE65v2. Average pre-readministration PLR amplitudes of constriction are compared with those of post-readministration amplitudes (FOd30 to FOd180). PLR amplitudes were measured after illumination with light at 10 lux (CH12) or 0.4 lux (CH11 and NP01). *P = 0.08; **P = 0.009; ***P = 0.01.
Safety of subretinal readministration
There were no surgical complications resulting from vector readministration. Vector was delivered to the superotemporal retina, including the macular region superior to the fovea, in all three individuals (Table 1, Fig. 1, and Supplementary Methods). Although the regions of the retina that were targeted in the initially injected eye and the FO eye were similar, they were not entirely symmetrical except for patient CH12. The central retina of CH12 was scarred, and thus, the superior portions of the macula and retina were targeted. CH11’s second eye injection was slightly superior to the fovea, whereas the first injection encompassed the fovea; NP01’s second eye injection occupied the superior portion of the macula, whereas her first injection was superotemporal to the macula (16, 18). AAV readministration was well tolerated, and there was no inflammation in either eye of the subjects observed by clinical exam at any of the post-readministration time points (Fig. 1).
There were no serious adverse events related to vector readministration in any of the subjects. Adverse events included surface irritation of the eye between FOd30 and FOd60 (CH12), a sprained ankle in week 4 (CH11), and a headache on FOd2 (NP01). All were deemed minor.
Similar to previous results (18), blood and tear samples were positive at low levels for vector DNA sequences at early post-injection time points (table S2). Some of the polymerase chain reaction (PCR) results were nonquantitative. All samples were negative after FOd3. There was no clear relationship between leakage of vector into the blood and immune responses (Tables 2 and 3). There were no significant detectable T cell responses to either vector or transgene product (Table 2). Two subjects in this study had a transient positive enzyme-linked immunospot (ELISpot) result at a single time point (CH11, week 6, for AAV2 and RPE65; NP01, week 5, for RPE65). In both instances, the finding was isolated and was not confirmed in any other peripheral blood mononuclear cell (PBMC) samples collected subsequently from these subjects. Additionally, higher than normal background [>50 spotforming units (SFUs) per 106 PBMCs plated in the assay] may have influenced the readout of the ELISpot, making the relevance of these findings unclear. Neutralizing antibody (NAb) responses to AAV2 and RPE65 protein remained at or close to baseline in the postoperative period in each subject (Table 2). The minor variations were most likely due to the variability of the assay used to measure NAb. By comparison, NAb after the systemic administration of an AAV2 vector in humans increased by several logs (4). In summary, readministration of AAV2-hRPE65v2 to the contralateral eye appeared safe based on both clinical examination and immunological response.
Table 2.
Analysis of anti-AAV2 and anti-RPE65 Nab and responses over time after initial injection (bold) and after readministration. The exact time points evaluated differed for the initial and the FO study (table S1). There were no detectable anti-RPE65 Nabs detected after the initial injection (18). However, these data are not included in Table 2 because the assay was modified for the FO study measurements. Results are indicated as reciprocal dilutions of serum samples (see Supplementary Methods). Anti-AAV2 titers after the first injection were previously reported (18) and are shown here for comparison with the FO titers. The titers remained low throughout the course of the study, with a minor increase at week 8 for CH12 (italicized) followed by a return to baseline. High FO baseline NAbs directed against RPE65 protein were detectable in subjects CH12 and CH11. The positivity may have been due to cross-reaction with another RPE65-like protein or that the subject may produce a dysfunctional but immunologically detectable protein. The positive responses detected early on decreased slightly over time. NA, sample not available.
| Subject ID | Antibody assay |
Baseline/FO baseline |
FOd7 | d28/FOd28 | FOd60 | d90 | d180/ FOd180 |
d365 |
|---|---|---|---|---|---|---|---|---|
| CH12 | AAV2 | Neat–1:3.16/1:1 | 1:1 | Neat–1:3.16/1:1 | 1:3.16–1:10 | Neat–1:3.16 | 1:1 | Neat–1:3.16 |
| RPE65 | 1000 | 1000 | 1000 | 1000 | 100 | |||
| CH11 | AAV2 | 1:3.16–1:10/1:3.16–1:10 | 1:1 | 1:3.16–1:10/1:3.16–1:10 | 1:3.16–1:10 | 1:3.16–1:10 | 1:1 | 1:3.16–1:10 |
| RPE65 | 1000 | 1000 | 100 | 100 | 100 | |||
| NP01 | AAV2 | <1:3.16/1:3.16–1:10 | 1:1 | <1:3.16/1:1 | 1:1 | <1:3.16 | 1:1–1:3.16 | 1:3.16–1:10 |
| RPE65 | 100 | <100 | <100 | <100 | NA |
Table 3.
Analysis of T cell responses performed by IFN-γ ELISpot after initial injection (bold) and after readministration. The time points for study are described in table S1. Most of the samples tested for T cell responses to the AAV capsid or the RPE65 transgene product were negative throughout the initial (18) and FO studies. A few samples tested positive in the assay (for example, CH12, FO week 6); however, these samples were negative the following week, suggesting either that the positive readings were false positives or that there was weak or transient T cell activation. Thus, there were no cell-mediated T cell responses detectable in peripheral blood, a result in agreement with the lack of local inflammation. Pos, positive (>50 SFUs per million cells plated) and at least threefold the medium-only control; Neg, negative (<50 SFUs per million cells plated) or less than threefold the medium-only control; Bkg, high background/not interpretable (medium control >100 SFUs per million cells plated).
| Subject | Antigen | d0/FOd0 | FO week 1 |
Week 2/FO week 2 |
FO week 3 |
Week 4/FO week 4 |
FO week 5 |
FO week 6 |
FO week 7 |
FO week 8 |
d90/FOd90 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CH12 | AAV | Neg/Neg | Neg | Neg/Neg | Neg | Neg/Neg | Neg | Neg | Neg | Neg | Neg*/Neg |
| RPE65 | Neg/Neg | Neg | Neg/Neg | Neg | Neg/Neg | Neg | Neg | Neg | Neg | Neg*/Neg | |
| CH11 | AAV | Neg/Bkg | Bkg | Neg/Bkg | Bkg | Neg†/Bkg | Bkg | Pos† | Neg | Neg | Neg/Neg |
| RPE65 | Neg/Bkg | Bkg | Neg/Bkg | Bkg | Neg†/Bkg | Bkg | Pos† | Neg | Neg | Neg/Neg | |
| NP01 | AAV | Neg/Neg | Neg | Neg/Bkg | Neg | Neg/Bkg | Neg | Neg | Bkg | Neg | Neg/Bkg |
| RPE65 | Neg/Neg | Neg | Neg/Bkg | Neg | Neg/Bkg | Pos† | Neg | Bkg | Neg | Neg/Bkg |
Poor viability of cells.
Positive result likely due to high background reactivity.
Readministration and retinal/visual function
Each subject reported improvements in vision in the second (FO) eye extending over the entire period of observation beginning as early as FOd14. Testing revealed a trend toward improvement in visual acuity of the second eye in all three subjects, with the highest level of improvement in CH12. This patient also showed a trend toward improvement in the initially injected eye (Table 1). There was no change in the visual acuity of the previously injected eye of patients CH11 and NP01. There was a trend in improvement of the visual field correlating with the area of retina injected (Fig. 1A), although there was a high degree of intrasubject and intervisit variability in these subjects with low vision and nystagmus (involuntary, oscillating movements of the eyes). For CH12, the pre- and postvisual fields were limited to a very small central island. For CH11, the outer border of the FOd90 post-readministration visual fields was expanded compared to the FO baseline and FOd30 visual fields. For NP01, the visual fields showed expansion at FOd45 and FOd90 compared to baseline (Fig. 1A). There was also a trend regarding a decrease in the amplitude of nystagmus in the initially injected eye of all three subjects and in the newly injected eye of CH11 and NP01 (table S5). Two of the subjects (CH12 and NP01) showed reduced frequency of nystagmus, whereas CH11 showed increased frequency of nystagmus in both eyes after readministration (table S5).
The most significant improvements pertained to light sensitivity. Full-field light sensitivity, a subjective test of light perception, revealed sustained improvement in both white and chromatic (blue) light sensitivity in two of the three subjects (CH11 and NP01; Fig. 1B). One of these subjects (NP01) also showed increased sensitivity to red stimuli. The initially injected eyes retained their baseline white and blue light sensitivity with the exception of CH11, in whose initially injected eye there was diminished blue (but not white) light sensitivity after injection. The significance of this isolated finding is unknown. Similarly, there were fluctuations in sensitivity in the initially injected eyes of CH11 and NP01 between baseline and FOd30, but levels eventually returned to baseline.
Increases in light sensitivity for the newly injected eyes were also detected with pupillometry. The PLR test provides objective data relating to retinal function and the integrity of a major component of the retinal/central nervous system circuitry. We previously demonstrated that after unilateral injection of AAV2-hRPE65v2, the injected eye showed an improved PLR, whereas the noninjected eye remained defective (16, 18, 19). Here, we show that there is an increased amplitude of constriction after readministration in each of the three FO eyes (Fig. 1C). There were minimal changes in the amplitude of constriction of the initially injected eye after readministration at this same level of illuminance. Using pupillometry, we also show that in all three subjects after readministration, the second eye gains responses (fig. S1). Further, in at least two of the subjects, CH12 and CH11, the initially injected eye retains its PLRs at the previous threshold sensitivity. The net result was that with threshold or subthreshold illumination, the PLR waveform changed from one suggesting a relative afferent pupillary defect (rAPD; where the initially injected eye had a robust response, whereas the uninjected eye did not) to one that was more symmetrical for the left and right eyes (fig. S1). Although amelioration of the rAPD was apparent as early as FOd14, it can take months for patterns to stabilize and for symmetry to develop between the left and the right eyes. Additional follow-up testing will be necessary in these and other subjects to determine the long-term effects of the intervention on the pupillary responses of both eyes.
The ability of the subjects to accurately navigate a standardized course was also evaluated (16, 18). At and before the FO baseline, none of the subjects had been able to successfully negotiate an obstacle course using either eye. After readministration, both NP01 and CH11 avoided collisions with objects using their left, FO-injected eyes even in dim (10 lux) light for CH11 (P = 0.002 and 0.015, respectively; movies S1 to S4) and down to 5 lux for NP01 (P = 0.005). Improvements in navigation were noted within 1 month after injection and persisted throughout the course of the study. There were no improvements in navigation using the initially injected eye.
Readministration and cortical responses
fMRI analyses were performed with the general linear model and the contrast of active blocks (checkerboard stimuli) minus the rest blocks (black screen) (fig. S2) using the BrainVoyager QX software (22). To account for variability in the disease stage among subjects, we analyzed fMRI individually for each participant (20) (and not grouped as in most fMRI analyses). A single-subject analysis approach was especially suitable based on the fact that the three subjects differed by age and disease progression and thus differed in the area of the retina in which there was evidence of sufficient (albeit unhealthy) retinal cells. This approach also makes the correlation of fMRI results and clinical outcomes possible for each individual. All analyses were carried out to obtain significant results at high statistical thresholds that were corrected for false detection of any activation due to multiple-comparison type I errors (23); the thresholds were lowered if no activation was detected. At a lower statistical threshold, there was frontal activation responsible for eye movement (frontal eye fields), anterior cingulate (decision-making for button press), and premotor and sensory motor cortex (for button press).
fMRI results for newly treated eyes
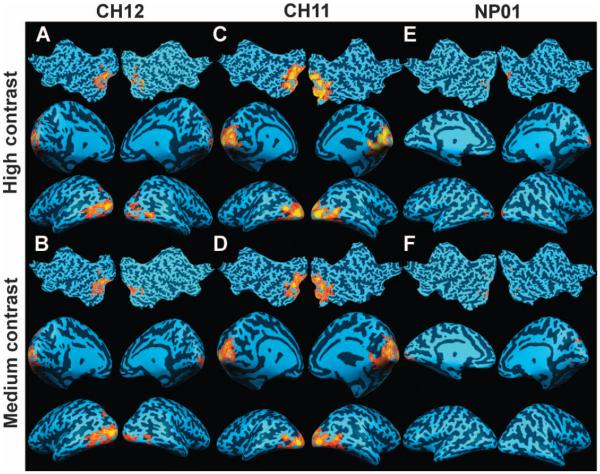
fMRI after gene therapy readministration showed significant cortical activation in and around the visual cortex for all three LCA2 subjects for full-field contrast-reversing (8 Hz) checkerboard stimuli at high and medium contrasts (Figs. 2 to 4). Presentation of the same stimuli at baseline, before readministration, did not result in significant cortical activation for either the high- or the medium-contrast stimulus. The results for each subject are as follows.
Fig. 2.
Subject CH12 fMRI results at baseline, FOd30, and FOd90. (A and B) Subject CH12 showed no cortical activation at baseline for high- and medium-contrast stimuli. (C and D) At FOd30, significant bilateral cortical activations were observed in response to the high-contrast stimulus (C), whereas no response was recorded for the medium-contrast stimulus (D). (E and F) At FOd90, CH12’s cortical responses to the same stimuli markedly increased especially for the high-contrast stimulus. Smaller clusters of activations are observed in response to medium-contrast stimulus at FOd90 (F).
Fig. 4.
Subject NP01 fMRI results at baseline, FOd45, and FOd90. (A and B) Subject NP01 showed no visual activation at baseline. (C and D) At FOd45, although significant cortical responses for the high-contrast stimulus were recorded (C), no response was observed for the medium-contrast stimulus (D). (E and F) At FOd90, NP01 showed significant activation for high-contrast (E) and medium-contrast (F) stimuli. Areas of activation at FOd90 were distributed in closer proximity to the primary visual cortex compared to FOd45 fMRI results [compare (E) and (C)].
CH12’s untreated eye before readministration was unresponsive to the high- and medium-contrast stimuli (Fig. 2, A and B) even at liberal statistical threshold levels. Significant bilateral cortical responses to the high-contrast stimulus were observed: false discovery rate (fdr) was <5% with a corrected P value (Pc) of <0.002 and continuously connected area (cca) of ≥100 mm2; no response to medium contrast was recorded at FOd30 (Fig. 2, C and D, respectively). Even though her FO baseline and posttreatment visual fields were limited to a very small central area (Fig. 1), CH12’s cortical responses to the high-contrast stimulus markedly increased at FOd90 (Fig. 2, E and F), especially for the high-contrast stimulus (fdr < 5%, Pc < 0.005, cca ≥ 1000 mm2). The medium-contrast stimulus showed unilateral but significant (fdr < 5%, Pc < 0.0002, cca ≥ 25 mm2) cortical activation.
CH11 showed no cortical activation, regardless of visual stimulus presented to her untreated (left) eye at FO baseline (Fig. 3, A and B). However, widespread bilateral activation was observed for the fMRI obtained on FOd30 in response to the high- and medium-contrast stimuli (fdr < 5%, Pc < 0.003, cca > 1000 mm2) (Fig. 3, C and D), and the areas of activation increased by FOd90 (Fig. 3, E and F). At FOd90 (Fig. 3E), there was greater bilateral cortical activation for the high-contrast stimulus (fdr < 5%, Pc < 0.003, cca ≥ 1000 mm2). Marked activation was also present in response to the medium-contrast stimulus (fdr < 5%, Pc < 0.003, cca ≥ 1000 mm2) (Fig. 3F). As depicted in Fig. 3, CH11’s FO visual activations were symmetrically distributed in both hemispheres as well as in the upper and lower banks of the calcarine fissure, comparable to a pattern predicted from her visual field distribution and the location of the subretinal injection (Fig. 1), given that the cells in the injected region were viable.
Fig. 3.
Subject CH11 fMRI results at baseline, FOd30, and FOd90. (A and B) Subject CH11 showed no baseline cortical activation to the high- or medium-contrast checkerboard stimuli. (C and D) Highly significant and widespread bilateral activation at FOd30 in response to both high- and medium-contrast stimuli, respectively. (E and F) A more marked increase in cortical activation was present at FOd90 for high-contrast (E) and medium-contrast (F) stimuli.
Similar to CH11 and CH12, NP01 did not present with any activation in response to the high- or medium-contrast stimuli for her untreated eye at FO baseline (Fig. 4, A and B). At FOd45, there was a response to the high-contrast stimulus (Fig. 4C; fdr < 5%, Pc < 0.001, cca ≥ 50 mm2), but not to the medium-contrast stimulus (Fig. 4D). The clusters of activation were bilaterally distributed and mainly located in the lateral and basal areas of the visual cortex, generally reflective of a pattern predicted by the FO visual fields (Fig. 1). At FOd90, NP01 showed increased bilateral activation in response to both the high-contrast (fdr < 5%, Pc < 0.0003, cca ≥ 100 mm2) and the medium-contrast (fdr < 5%, Pc < 0.001, cca ≥ 25 mm2) stimuli as depicted in Fig. 4, E and F, respectively.
Qualitative fMRI temporal changes for the FO studies of all three subjects are summarized in table S3. Results show that cortical responses increased in all subjects from baseline to FOd30 and continued to FOd90. Quantification of the fMRI results (areas of activation, mm2) for each hemisphere and total visual cortex for the FO studies are presented in table S4. Results show that the areas of visual cortex activation after visual stimulation increased in all three subjects through FOd90 (P < 0.0001, table S4). Steady increases in total cortical activation areas through FOd90 for all three subjects agreed with the increased light sensitivity measured with PLR testing and, for two of the subjects, with FST testing, in the same time frame (fig. S1 and Fig. 1C). This may reflect increasing expression of the RPE65 transgene over this time period. The largest relative gains were observed in CH12 and NP01, the oldest of the three subjects. All subjects presented with greater bilateral activation at FOd90. This is not surprising because the subretinal injections spanned the midline of the posterior pole of the eye and thus should affect both hemispheres. There was good correlation between the fMRI findings and the results of retinal and visual function testing. In particular, the incremental increase in total cortical activation areas through FOd90 correlated with average postsurgical pupil constriction amplitudes (P < 0.049).
In summary, results from fMRI showed an increase in cortical activation after readministration of gene therapy, and the pattern of visual cortex activation roughly correlated with the location of injection and visual field distribution. Temporal increases in cortical activation also generally correlated in time and magnitude with those that were measured using psychophysical testing.
fMRI results for previously treated eye
In addition to the newly treated eye, fMRI was also performed on the eye that had been initially injected at least 1.7 years earlier (see Table 1). This experiment was carried out to evaluate the functionality of the contralateral eye and to evaluate any potential toxicity associated with readministration of gene therapy. fMRI for the contralateral eye was carried out at FO baseline and FOd90.
As shown in Fig. 5, fMRI results at FO baseline for CH12 showed bilateral activation, distributed more extensively in the lateral aspects of the visual cortex, in response to high-contrast stimuli (fdr < 5%, Pc < 0.01, cca > 25 mm2) and at an uncorrected statistical level (P < 0.01, cca > 25 mm2) for medium-contrast stimuli. CH11 showed bilateral activation for high-contrast stimuli (fdr < 5%, Pc < 0.01, cca > 100 mm2) and no activation for medium-contrast stimuli. The fMRI results for NP01 were observed at an uncorrected fdr statistical level for high-contrast stimuli (P < 0.01, cca > 25 mm2), with no activation detected for medium-contrast stimuli.
Fig. 5.
fMRI results for initially injected eyes in response to high- and medium-contrast stimuli at FO baseline, before injection of the contralateral eyes. (A and B) CH12’s fMRI results for the high- and medium-contrast stimuli showed bilateral activation. (C and D) CH11 showed activation to the high-contrast stimuli (C) but did not respond to medium-contrast stimuli (D). (E and F) Similar to CH11, NP01 responded to the high-contrast but not to the medium-contrast stimulus. The lower cortical activation for NP01 may be due to the fact that subject received the lowest dose of AAV2-hRPE65v2 for her initial subretinal injection and that subject is a chronic smoker (smoking is known to abate cortical blood flow and thus the fMRI signal).
The fMRI results for the initially injected eyes at FOd90 are presented in Fig. 6. All three subjects demonstrated bilateral activation in response to the high- and medium-contrast stimuli in and around the visual cortex. The fMRI results for CH12 demonstrated bilateral activation in response to high-contrast (fdr < 5%, Pc < 0.003, cca > 100 mm2) and medium-contrast (fdr < 5%, Pc < 0.004, cca > 100 mm2) stimuli. CH11 also showed widespread activation for high-contrast (fdr < 5%, Pc < 0.004, cca > 100 mm2) and medium-contrast (fdr < 5%, Pc < 0.003, cca > 100 mm2) stimuli. NP01 showed activation at significant but fdr uncorrected statistical levels for high-contrast (P < 0.008, cca > 25 mm2) and medium-contrast (P < 0.008, cca > 25 mm2) stimuli. NP01 presented with lower cortical activation compared to CH12 and CH11.
Fig. 6.
fMRI results for initially injected eyes 90 days after readministration of the contralateral eyes. (A and B) CH12’s fMRI results to high- and medium-contrast stimuli demonstrated significant bilateral cortical activation. (C and D) CH11 also showed widespread activation for high- and medium-contrast stimuli. (E and F) Although NP01 also showed activation in response to the high- and medium-contrast stimuli, they were at an uncorrected statistical threshold. Lower activation in NP01 may be due to a lower dose of AAV2-hRPE65v2 for the initial subretinal injection and the fact that this subject is a chronic smoker.
Overall, the subjects demonstrated more extensive cortical activation for their initially treated eye after readministration of gene therapy to the second eye. Thus, the first injected eye retains and even shows ameliorated visual cortex activity after readministration. These results demonstrate that not only did each of the subjects retain retinal and visual function after injection of the first eye, but may have possibly gained retinal and visual function in both eyes.
DISCUSSION
Here, three adults who had each previously received a single, unilateral, subretinal injection of AAV2-hRPE65v2 underwent a repeat subretinal administration in their contralateral (previously uninjected) eye. After injection, each of these “second” eyes became far more sensitive to dim light as shown by full-field sensitivity testing, pupillometry, and fMRI even though they had been severely impaired for more than 2.5 decades (and more than 4.5 decades in one individual). Two of these individuals also developed greatly improved navigational abilities using the newly injected eye. The results may reflect an age effect whereby the individuals who were younger (and thus whose retinas had not undergone as much degeneration) showed larger gains than the older individual. The gains were stable through at least the FOd180 time point, and the treatment appeared safe in all subjects. Efficacy was due to AAV-mediated delivery of wild-type RPE65 into the retinal pigment epithelium (RPE) and subsequent restoration of the retinoid cycle.
The improvements in retinal and cortical responses after subretinal delivery of AAV2-hRPE65v2 are not instantaneous because the transgene delivered by the single-stranded AAV2 vector must become doublestranded to be competent for transcription. Similar to earlier results in large animals and also to results after injection of the first eye in humans, there is a gradual ramp-up period that plateaus between 1.5 and 3 months after subretinal delivery (14, 16, 18, 24). Similar temporal gains in subjective and objective measures of retinal and visual function and in the activation of the visual cortex are found over this same time frame after readministration in humans. Here, we have also evaluated the spatial pattern of activation of the visual cortex after readministration and have found that the activation patterns mirror the improvements identified through subjective and objective clinical testing of retinal and visual function. Until now, no one has measured the temporal-spatial patterns of improvement in retinal and visual function after gene therapy using fMRI.
Given the gains in retinal and visual function that these and nine other individuals have enjoyed since injection of their first eye, one may have predicted the same level and time course of improvement after injection of the second eye. However, there are several variables that might have interfered with successful additional transduction events. These individuals were exposed, during the first injection, to antigens on the AAV capsid as well as RPE65 protein encoded by the AAV cargo. The concern with readministration was that previous exposure could “vaccinate” the individual and result in an inflammatory response upon repeat exposure. Although harmful immune responses were not observed in affected dogs and unaffected nonhuman primates in preclinical readministration studies (21), efficacy after readministration of AAV in humans has only been described in one study (25). This was a study where AAV was used to produce an immune response to vaccinate against HIV (25). A modest number of the HIV patients indeed developed (the desired) immune responses after injection and readministration. Our study in LCA2 subjects describes efficacy after readministration of gene therapy in a genetic disease—a response that was not accompanied by a significant (and potentially damaging) immune response. In addition, test results showed that the gains in retinal and visual function that had resulted from the initial injection were maintained after the second eye was injected.
Most of the results of the preclinical studies were predictive of results of the human readministration studies. However, there was one result that we had not observed in earlier studies. This was that one of the subjects showed improved light sensitivity responses to red stimuli in the second eye after readministration. Red stimuli selectively stimulate cone photoreceptors. This result suggests that the chromophore generated with the help of RPE65 can activate cone photoreceptors. Previously, we had only observed improvements in rod photoreceptor responses (which were also stimulated by blue light) (16, 18). In general, there is a strong bias toward improvement in the short-wavelength (blue) spectrum, demonstrating greatest improvement in rod photoreceptors. This is analogous to the “Purkinje phenomenon,” which occurs during dark adaptation wherein the peak sensitivity of the retina shifts from the red (cone) to the blue (rod) photoreceptor population (26).
There were also some unexpected findings. The first relates to fMRI results in the initially injected eye. A concern before the study was that immune responses to readministration would dampen the gains in retinal and visual function of the initially injected eye. Surprisingly, the function of the initially injected eye was improved after readministration. Psychophysical testing did not reveal any change in function of the first injected eye. The improvement in cortical function may reflect plasticity of the neuronal connections in the brain. An increase in cortical activation at FOd90 for the initially injected eye may also be due to reduced nystagmus in the newly injected eye (improved eye movement synchrony) and a better ability to fixate the gaze during fMRI. Further study is necessary to unravel the role of nystagmus in the improvement of visual function. However, in support of this hypothesis, all three subjects showed a reduced amplitude of nystagmus in the eye receiving readministration after injection.
A second unexpected finding pertained to dose effects. In our studies of effects of unilateral “first eye” injection in subjects with LCA2, a dose response was not identified. Here, an interocular comparison of different doses was carried out because two of the subjects had previously received a lower dose than was administered in the readministration (Table 1). The responses in the second eye were significantly greater than those in the first injected eye in the two subjects who had previously received lower doses. This strongly argues for a dose/volume response. Such a response is difficult to elicit between subjects with different mutations, stages of disease, amblyopia (a condition wherein visual input is not recognized by the brain due to interference with retinal-cortical communication during development), and other complicating variables. Increases in both the dose and the volume likely contributed to the extent of improvement in the readministered eye of these two individuals. A third set of unexpected findings were variations in the timeline for improvement. As in our previous studies using psychophysical measures, fMRI showed improvements in cortical activation by the first month after injection, and there was a general ramp-up of improvement through FOd90. In CH11, the area of cortical activation was more extensive at d30 compared to the level of activation in the other two subjects. Another timeline anomaly was that there appeared to be improvement in light sensitivity in the previously injected eye at the FOd30 time point as shown through FST testing in two of the subjects (Fig. 1). This finding, which may have been a nonspecific effect of corticosteroids taken during the perioperative period, was transient, however, and the levels of light sensitivity returned to their FO baseline levels thereafter.
Finally, an unexpected finding that is more difficult to explain is the dichotomy between fMRI and the psychophysical results for CH12. With CH12, the fMRI responses were larger than would have been predicted on the basis of her improvements in visual acuity and light sensitivity (as judged by the PLR test). CH12 reported (through button press) seeing the stimuli during the period when responses were detected, and so, these responses were not an artifact. We can only speculate at this point why the fMRI responses in this subject appear to be more sensitive than the other outcome measures, at least with this individual. Cortical activation in this individual may reflect additional aspects of vision (such as motion detection or depth perception) that would not be recognized in the other test results, or these responses may reflect a heightened level of attention. Alternatively, in this individual who had received the same dose in each eye and had also received those doses in a symmetrical fashion, there may have been a binocular summation, where the previously treated eye becomes a better driver of the visual cortex when it is better correlated with signals from the other eye.
In summary, this study provides the first demonstration of improved retinal and visual function after gene therapy readministration in a genetic disease and also the first demonstration of efficacy after readministration to the contralateral eye. Two of the three subjects can now navigate in dim light, arguably a clinically meaningful result. The fMRI data also provide the first evaluation of cortical responses to visual stimuli before and after gene therapy and is the first demonstration of the temporal-spatial changes in retinal and cortical activation in humans, as reflected by response of the visual cortex. The strong safety profile in this readministration study is likely to be due, at least in part, to the immune-privileged nature of the target tissue, the low dose of vector used, and the use of a vector preparation from which empty capsid had been removed, resulting in a lower antigen load. Although longer periods of follow-up and evaluation in these and additional subjects will be required to determine with certainty that readministration is safe in humans, the current data confirm the results of preclinical laboratory studies (21), which demonstrated that subretinal administration to the contralateral eye in animals previously exposed to intraocular AAV2-hRPE65v2 is both safe and efficacious. The current data provide evidence for the safety of vector readministration in humans of up to 1.5 × 1011 vg but cannot be extrapolated to higher doses or to vector with higher antigen load.
MATERIALS AND METHODS
Surgery and retinal/visual function testing
All recombinant DNA and human studies were carried out in compliance with local and federal guidelines. The transgene cassette in the AAV2-hRPE65v2 vector carries a chicken β-actin promoter driving expression of the human RPE65 complementary DNA with an optimized Kozak sequence (14). The vector was manufactured by The Center for Cellular and Molecular Therapeutics at CHOP with current good manufacturing practices (16, 18). Surgery was performed as previously described (16, 18) with a standard three-port pars plana vitrectomy with removal of the posterior cortical vitreous (Supplementary Methods).
As per request by the IRB, the first three subjects were adults and the selection/order of these subjects was based on their availability. Subjects were evaluated before and at designated time points after surgery as was described (16, 18, 19). Efficacy for each subject was monitored with objective and subjective measures of vision (16, 18, 19). Statistical significance of mobility test results was evaluated with Fisher’s exact test. P values of <0.05 were considered significant.
NAb assay and anti-AAV2 antibody ELISA
Anti-AAV NAb titer, anti-RPE65 antibody titer, and interferon-γ (IFN-γ) ELISpot assay results were determined as previously described (Supplementary Methods) (16, 18).
Acknowledgments
We thank O. Zelenaia, B. Hauck, F. Bennett, K. Maguire, J. Tress, K. Brint, L. Pollack-Johnson, A. Faella, J. Rundio, P. Lam, R. Golembski, and X. Zhu for expert technical assistance; L. Raffini and A. Fossough for expert clinical support; and E. Traboulsi for clinical input.
Funding: Center for Cellular and Molecular Therapeutics at CHOP; Foundation Fighting Blindness–sponsored CHOP-PENN Pediatric Center for Retinal Degenerations; Clinical Translational Science Award NIH/National Center for Research Resources UL1-RR-024134, 1R21EY020662, and 1R01EY019014-01A2; Transdisciplinary Award Program in Translational Medicine and Therapeutics (TAPITMAT) from University of Pennsylvania; Research to Prevent Blindness; Hope for Vision; Howard Hughes Medical Institute; Paul and Evanina Mackall Foundation Trust at Scheie Eye Institute; anonymous donors; Italian Telethon Foundation; and F. M. Kirby Foundation.
Competing interests: J.B. and A.M.M. are co-inventors on a pending patent (2797-11-US) for a method to treat or slow the development of blindness, but both waived any financial interest in this technology in 2002. K.A.H. and J.F.W. are co-inventors on a patent regarding methods of making AAV vectors for clinical studies (U.S. patent 61/299,184, 2010). J.B. and J.F.W. serve on the scientific advisory board for Avalanche Technologies. J.B. served on a scientific advisory board for Sanofi-Aventis in 2010 to 2011. J.B. has consulted for GlaxoSmithKline. J.F.W. has consulted for Tacere Therapeutics, Genzyme, Novartis, and Genetix Inc. The other authors declare that they have no competing interests.
Footnotes
SUPPLEMENTARY MATERIAL
www.sciencetranslationalmedicine.org/cgi/content/full/4/120/120ra15/DC1
Materials and Methods
Results
Discussion
Table S1. Summary of the visual tests done at baseline before injection of the first eye, baseline before injection of the second eye [follow-on (FO), baseline], as well as after treatment of the first and then the second eye.
Table S2. Biodistribution data for subjects CH12 (A), CH11 (B), and NP01 (C) comparing results after injection in the first eye with those after readministration to the contralateral eye.
Table S3. Qualitative temporal changes in fMRI activation.
Table S4. Quantification of fMRI.
Table S5. Nystagmus parameters over time.
Fig. S1. Pupillary light reflex (PLR) testing shows an improved left eye response after readministration in all three subjects.
Fig. S2. fMRI stimuli and design.
Movie S1. NP01, follow-on baseline.
Movie S2. NP01, post-readministration.
Movie S3. CH11, follow-on baseline. Movie S4. CH11, post-readministration.
References
REFERENCES AND NOTES
- 1.Aleman TS, Jacobson SG, Chico JD, Scott ML, Cheung AY, Windsor EA, Furushima M, Redmond TM, Bennett J, Palczewski K, Cideciyan AV. Impairment of the transient pupillary light reflex in Rpe65−/− mice and humans with Leber congenital amaurosis. Invest. Ophthalmol. Vis. Sci. 2004;45:1259–1271. doi: 10.1167/iovs.03-1230. [DOI] [PubMed] [Google Scholar]
- 2.Lorenz B, Gyürüs P, Preising M, Bremser D, Gu S, Andrassi M, Gerth C, Gal A. Early-onset severe rod–cone dystrophy in young children with RPE65 mutations. Invest. Ophthalmol. Vis. Sci. 2000;41:2735–2742. [PubMed] [Google Scholar]
- 3.Simonelli F, Ziviello C, Testa F, Rossi S, Fazzi E, Bianchi PE, Fossarello M, Signorini S, Bertone C, Galantuomo S, Brancati F, Valente EM, Ciccodicola A, Rinaldi E, Auricchio A, Banfi S. Clinical and molecular genetics of Leber’s congenital amaurosis: A multicenter study of Italian patients. Invest. Ophthalmol. Vis. Sci. 2007;48:4284–4290. doi: 10.1167/iovs.07-0068. [DOI] [PubMed] [Google Scholar]
- 4.den Hollander AI, Black A, Bennett J, Cremers FP. Lighting a candle in the dark: Advances in genetics and gene therapy of recessive retinal dystrophies. J. Clin. Invest. 2010;120:3042–3053. doi: 10.1172/JCI42258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: Genes, proteins and disease mechanisms. Prog. Retin. Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, Dryja TP. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or Leber congenital amaurosis. Proc. Natl. Acad. Sci. U.S.A. 1998;95:3088–3093. doi: 10.1073/pnas.95.6.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson DA, Gyürüs P, Fleischer LL, Bingham EL, McHenry CL, Apfelstedt-Sylla E, Zrenner E, Lorenz B, Richards JE, Jacobson SG, Sieving PA, Gal A. Genetics and phenotypes of RPE65 mutations in inherited retinal degeneration. Invest. Ophthalmol. Vis. Sci. 2000;41:4293–4299. [PubMed] [Google Scholar]
- 8.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat. Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 9.Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc. Natl. Acad. Sci. U.S.A. 2005;102:13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumaramanickavel G, Denton MJ, Gal A. Mutations in RPE65 cause autosomal recessive childhood–onset severe retinal dystrophy. Nat. Genet. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- 11.Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J, Dejneka NS, Pearce-Kelling SE, Maguire AM, Palczewski K, Hauswirth WW, Jacobson SG. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol. Ther. 2005;12:1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, Jacobson SG, Hauswirth WW, Bennett J. Gene therapy restores vision in a canine model of childhood blindness. Nat. Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 13.Dejneka NS, Surace EM, Aleman TS, Cideciyan AV, Lyubarsky A, Savchenko A, Redmond TM, Tang W, Wei Z, Rex TS, Glover E, Maguire AM, Pugh EN, Jr., Jacobson SG, Bennett J. In utero gene therapy rescues vision in a murine model of congenital blindness. Mol. Ther. 2004;9:182–188. doi: 10.1016/j.ymthe.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Bennicelli J, Wright JF, Komaromy A, Jacobs JB, Hauck B, Zelenaia O, Mingozzi F, Hui D, Chung D, Rex TS, Wei Z, Qu G, Zhou S, Zeiss C, Arruda VR, Acland GM, Dell’Osso LF, High KA, Maguire AM, Bennett J. Reversal of blindness in animal models of Leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol. Ther. 2008;16:458–465. doi: 10.1038/sj.mt.6300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 16.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr., Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell’Osso L, Hertle R, Ma JX, Redmond TM, Zhu X, Hauck B, Zelenaia O, Shindler KS, Maguire MG, Wright JF, Volpe NJ, McDonnell JW, Auricchio A, High KA, Bennett J. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL, Flotte TR, Byrne BJ, Jacobson SG. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase I trial. Hum. Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, Mingozzi F, Bennicelli JL, Ying GS, Rossi S, Fulton A, Marshall KA, Banfi S, Chung DC, Morgan JI, Hauck B, Zelenaia O, Zhu X, Raffini L, Coppieters F, De Baere E, Shindler KS, Volpe NJ, Surace EM, Acerra C, Lyubarsky A, Redmond TM, Stone E, Sun J, McDonnell JW, Leroy BP, Simonelli F, Bennett J. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: A phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, Rossi S, Marshall K, Banfi S, Surace EM, Sun J, Redmond TM, Zhu X, Shindler KS, Ying GS, Ziviello C, Acerra C, Wright JF, McDonnell JW, High KA, Bennett J, Auricchio A. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol. Ther. 2010;18:643–650. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashtari M, Cyckowski LL, Monroe JF, Marshall KA, Chung DC, Auricchio A, Simonelli F, Leroy BP, Maguire AM, Shindler KS, Bennett J. The human visual cortex responds to gene therapy–mediated recovery of retinal function. J. Clin. Invest. 2011;121:2160–2168. doi: 10.1172/JCI57377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amado D, Mingozzi F, Hui D, Bennicelli JL, Wei Z, Chen Y, Bote E, Grant RL, Golden JA, Narfstrom K, Syed NA, Orlin SE, High KA, Maguire AM, Bennett J. Safety and efficacy of subretinal readministration of a viral vector in large animals to treat congenital blindness. Sci. Transl. Med. 2010;2:21ra16. doi: 10.1126/scitranslmed.3000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum. Brain Mapp. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nichols T, Hayasaka S. Controlling the familywise error rate in functional neuroimaging: A comparative review. Stat. Methods Med. Res. 2003;12:419–446. doi: 10.1191/0962280203sm341ra. [DOI] [PubMed] [Google Scholar]
- 24.Bennett J, Maguire AM, Cideciyan AV, Schnell M, Glover E, Anand V, Aleman TS, Chirmule N, Gupta AR, Huang Y, Gao GP, Nyberg WC, Tazelaar J, Hughes J, Wilson JM, Jacobson SG. Stable transgene expression in rod photoreceptors after recombinant adeno-associated virus-mediated gene transfer to monkey retina. Proc. Natl. Acad. Sci. U.S.A. 1999;96:9920–9925. doi: 10.1073/pnas.96.17.9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vardas E, Kaleebu P, Bekker LG, Hoosen A, Chomba E, Johnson PR, Anklesaria P, Birungi J, Barin B, Boaz M, Cox J, Lehrman J, Stevens G, Gilmour J, Tarragona T, Hayes P, Lowenbein S, Kizito E, Fast P, Heald AE, Schmidt C. A phase 2 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 vaccine based on adeno-associated virus. AIDS Res. Hum. Retroviruses. 2010;26:933–942. doi: 10.1089/aid.2009.0242. [DOI] [PubMed] [Google Scholar]
- 26.Davson H. Physiology of the Eye. Pergamon Press; Elmsford, NY: 1990. pp. 395–396. [Google Scholar]