Abstract
Murine resting (G0) T lymphocytes contained no detectable mRNA of 3-phosphoglycerate dehydrogenase (PHGDH) catalyzing the first step in the phosphorylated pathway of L-serine biosynthesis. Immobilized anti-CD3 activation of G0 T cells resulted in the expression of PHGDH mRNA in G1 with a maximal expression in S phase. G0 T cells activated with either immobilized anti-CD3 plus cyclosporine A or phorbol dibutyrate, which are known not to induce interleukin 2 (IL-2) production and thus fail to drive the activated G0 T cells to enter S phase, did not express the PHGDH mRNA. The addition of recombinant IL-2 (rIL-2) during these activation conditions, however, restored the cell cycle progression along with the expression of PHGDH mRNA. As compared to the [3H]TdR incorporation of G0 T cells following immobilized anti-CD3 activation in the complete RPMI 1640 medium containing L-serine (30 mg/L) and glycine (10 mg/L), it declined to the level of 68% or 52% when the medium was deprived of L-serine alone or deprived of both L-serine and glycine. The presence of antisense PHGDH oligonucleotide, which appeared to suppress the level of PHGDH protein, significantly reduced the [3H]TdR incorporation of activated G0 T cells. These results demonstrate that the expression of PHGDH gene, dictated by IL-2R signaling, is crucial for DNA synthesis during S phase of activated T cells.
Keywords: L-serine biosynthesis, PHGDH gene, T cell activation, IL-2R signaling, S phase, cell cycle
Introduction
L-serine serves as a building block for protein synthesis and can be modified in different metabolic pathways to generate several essential compounds including glycine, cysteine, D-serine, phosphatidylserine, sphingomyelins, and cerebrosides. It is also required for the synthesis of nucleotide precursors such as purines and thymidine, which is linked to cellular replication. Although L-serine is available from dietary sources, it can be endogenously synthesized from glycolytic intermediate by the phosphorylated pathway in mammals. The phosphorylated pathway starts at 3-phosphoglycerate and sequentially proceeds three stages of enzymatic reactions to synthesize L-serine [1]. The 3-phosphoglycerate dehydrogenase (PHGDH) catalyses the transition of 3-phosphoglycerate into 3-phosphohydroxy pyruvate, which is the first and rate-limiting step in the phosphorylated pathway, using NAD+/NADH as a cofactor. Phosphoserine aminotransferase (PSAT) catalyzes the conversion of 3-phosphohydroxy pyruvate to 3-phosphoserine which is subsequently dephosphorylated by phosphoserine phosphatase (PSP) to form L-serine.
It has been shown that the enzyme activity of PHGDH is elevated eight- to 50-fold in rat hepatoma [2], 10-fold in human colon carcinoma and 32-fold in rat sarcoma [3] as compared to individual normal control values. It has been also shown that PHGDH expression at the transcription level is upregulated in human colon carcinoma, and in most leukemias and lymphomas of human and murine origin [4]. This elevated activity of PHGDH, which leads to the enhanced capacity of L-serine biosynthesis, has been interpreted as an acquired growth advantage of tumor cells because of the metabolic importance of L-serine in nucleotide biosynthesis. The importance of L-serine in cellular replication and thus highest capacity of the phosphorylated pathway in S phase of the cell cycle are supported by our previous results, which demonstrate that the level of mRNA specific for both PHGDH and PSAT, abruptly down-regulated in accordance with growth arrest of U937 cells on 12-O-tetradecanoylphorbol 13-acete (TPA)-induced monocytic differentiation, can be recovered when PMA-treated cells restore cell growth by a retrodifferentiation process, and that the level of PHGDH- and PSAT-specific mRNA fluctuates during the cell cycle with a maximum in S phase of human Jurkat T cells [4, 5]. Although these previous results have suggested that the expression of PHGDH gene, which contributes to the phosphorylated pathway, might be a prerequisite for traversing S phase, there has been no direct evidence showing the requirement of PHGDH gene expression for S phase during the cell cycle progression.
In the present study, the expression of PHGDH was investigated during immobilized anti-CD3 activation of murine splenic resting (G0) T lymphocytes, with focusing on contribution of interleukin-2 receptor (IL-2R) signaling, which is known to induce the signal necessary for G1/S transition [6-8], to the induction of PHGDH gene expression. Using antisense PHGDH oligonucleotide, the requirement of PHGDH for S phase of activated G0 T cells was also investigated. We show that the PHGDH mRNA is not detectable in G0 T cells but is induced in G1 phase by IL-2R signaling and reaches a maximal level in S phase of activated T cells, along with a significant reduction in [3H]TdR incorporation into DNA of activated T cells in the presence of antisense PHGDH oligonucleotide that can specifically block the translation of PHGDH transcript. These results first demonstrate that the expression of PHGDH gene depends on IL-2R signaling and is required for S phase during the proliferation of activated T cells.
Materials and Methods
Animals
C57BL/6 male mice, 4 to 6 mo old, were purchased from the Jackson Laboratory (Bar Harbor, ME) and maintained at the Gerontology Research Center Animal Facility (Baltimore, MD). The Gerontology Research Center is fully accredited by the American Association of Laboratory Animal Care.
Chemicals, Antibodies, reagents, and medium
Phorbol dibutyrate (PBu2) was purchased from Calbiochem (La Jolla, Ca, USA). Radioactive materials including [α-32P]dCTP (≈3,000 Ci/mmol), and random primer labeling kit were purchased from Amersham (Arlington Heights, IL, USA). [3H]TdR (2 Ci/mmol) and Nylon membrane (GeneScreen Plus™) for Northern analysis was obtained from New England Nuclear (Boston, MA, USA). Mouse rIL-2 was purchased from Genzyme (Cambridge, MA, USA). Rabbit anti-mouse IgG were obtained from Jackson ImmunoResearch (West Grove, PA, USA), and monoclonal anti-CD3 of mouse was purchased from PharMingen (San Diego, Ca, USA). ECL kit was purchased from Amersham (Arlington, Heights, IL, USA). Immobilon-P transfer membrane for Western analysis was obtained from Millipore (Bedford, MA, USA). All reagents for electrophoresis were supplied by Sigma (St. Louis, MO, USA). The PHGDH antisense (AS-1, 5′-GCCATTGCTAGAGTC AG-3′; AS-2, 5′-CCAGGCACATGATCA T-3′;) and AS-3, 5′-GGACAGCTGATGACAT-3′; AS-4, 5′-GTGACATTGAGGCC-3′), and sense (S-1, 5′-CTG ACT CTA GCA ATG GC-3′; S-2, 5′-ATG ATC ATG TGC CTG G-3′; S-3, 5′-ATG TCA TCA GCT GTC C-3′; S-4, 5′-GGCCTCAATGTCAC-3′) phosphorothioate oligonucleotides were purchased from Oligos Etc. Inc. (Wilsonville, OR, USA). The culture medium used for activation of T lymphocytes was RPMI 1640 containing 10% FBS (HyClone, Logan Utah, USA), 20 mM HEPES, 5 × 10−5 M β-mercaptoethanol, and 100 μg/ml gentamycin. The culture media derived of either L-serine or L-serine and glycine were purchased from JBI (Daegu, Korea), and supplemented with 10% dialyzed FBS (JRH Biosciences, KS, USA). The culture medium for investigating the effect of antisense PHGDH oligonucleotide on activation of G0 T cells was the RPMI 1640 medium deprived of L-serine, and contained 10% dialyzed FBS.
Cell culture, activation of T cells, and [3H]TdR incorporation
The preparation and activation of murine resting T cells were performed as previously described [9, 10]. The incorporation of [3H]TdR into DNA by G0T cells (105) activated by immobilized anti-CD3 was determined as described elsewhere [11]. For activation in the presence of synthetic oligonucleotides, G0 T cells at a density of 5 × 105 cells/ml were pretreated with indicated amounts of antisense or nonsense PHGDH oligonucleotides at 37°C in the culture medium. After 4 h, cells were cultured for 40 h in 6-well plates (BD Biosciences, Bedford, MA, USA) or 96-well plates previously coated with anti-CD3.
Flow cytometry analysis
Approximately 1 × 106 T cells were fixed with 67% cold ethanol for 1 h. The cells were washed with PBS, and resuspended with 12.5 μg RNase in 250 μl of 1.12 % sodium citrate buffer (pH 8.45). Incubation was continued at 37 °C for 30 min before staining of the cellular DNA with 250 μl of propidium iodide, at 50 μg /ml, for 20 min. The stained cells were analyzed on a FACScan flow cytometer for relative DNA content, based on increased red fluorescence.
Northern blot analysis
Total RNA was extracted and isolated by solubilization in guanidine thiocyanate as described elsewhere [10]. Equivalent amounts of total RNA were electrophoresed on 1% formaldehyde-agarose gels and transferred to GeneScreen Plus membranes. The nylon membrane was hybridized overnight at 62°C with cDNA probe radiolabeled with [32P]dCTP by random primer labeling method [12], and washed at 65°C.
Western blot analysis
Cellular lysates were prepared by suspending 5 × 106 activated T cells in 100 μl of lysis buffer (137 mM NaCl, 15 mM EGTA, 1 mM sodium orthovanadate, 15 mM MgCl2, 0.1% Triton X-100, 25 mM MOPS, 2.5 μg/ml proteinase inhibitor E-64, and pH 7.2). The cells were disrupted by sonication and extracted at 4°C for 30 min. An equivalent amount of quantified protein lysate (20 μg) was subjected to electrophoresis on 11% SDS-polyacrylamide gel. The proteins were electro-transferred to immobilon-P membranes (Millipore Corporation, Bedford, MA, USA). Detection of protein was carried out with the ECL Western blotting kit (Amersham, Arlington, Heights, IL, USA) according to the manufacturer's instructions.
Statistical analysis
Unless otherwise indicated, each result in this paper is representative of at least three separate experiments. Values represent the mean ± standard deviation (SD) of these experiments. Statistical significance was calculated with Student's t-test. P values less than 0.05 were considered significant.
Results
Expression of PHGDH gene in anti-CD3-activated T cells
When murine G0 T cells were activated by immobilized anti-CD3, the cells appeared to enter S phase in 20 h and approximately 34% of the T cells were accumulated in S phase 25 h after activation, at which time the cells in G2/M phase began to be detected, as determined by flow cytometry (Fig. 1A). After activation for 30 h, the cell population was more heterogeneous, with approximately 59% of the cells in S phase, 30% in G1 phase, and 10% in G2/M phase. However, after activation for 40 h, only 3% of the cells in G2/M, 42% in G1, and 54% in S phase, suggesting that some population of cells completed the first round of the cell cycle and reentered into G1. The [3H]TdR-incorporation data also showed that the G0 T cells started S phase in 20 h following activation (Fig. 1B).
Fig. 1.
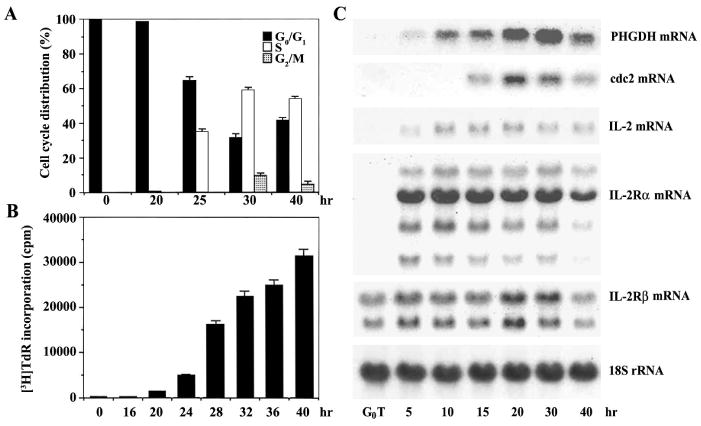
Kinetic analysis of the cell cycle progression (A), [3H]TdR- incorporation (B), and PHGDH mRNA (C) of during activation of G0 T cells by immobilized anti-CD3. Murine resting T cells were activated by immobilized anti-CD3. The cells were harvested at the indicated times for cell cycle analysis, which was performed on an equal number of the cells (104) by flow cytometry after staining of DNA with propidium iodide. For [3H]TdR-incorporation assay, murine G0 T cells (105/well) were activated by immobilized anti-CD3 in a 96-well plate, and pulsed for 1 h with 1 μCi of [3H]TdR at times indicated. Each value is expressed as mean ± SD (n = 3). For Northern analysis, total RNA (10 μg) from each time point were electrophoresed, transferred, and probed with 32P-labeled PHGDH, cdc2, IL-2, IL-2Rα, IL-2Rβ, and 18S rRNA cDNA.
During the polyclonal activation of murine G0 T cells by immobilized anti-CD3, the kinetic expression of 3-phosphoglycerate dehydrogenase (PHGDH) gene along with several important genes known to be essential for the completion of G1 and G1/S transition was assessed by Northern blot analysis (Fig. 1C). In unstimulated resting T cells, mRNA specific for IL-2Rβ was detectable, whereas there was no IL-2- or IL-2Rα-specific mRNA. After activation, mRNA specific for IL-2 and IL2Rα was first detected in 5 h. Since it is generally accepted that activated T cells express IL-2 and high affinity IL-2R at the early stage of G1, these results indicate that G0 T cells complete the G0/G1 transition and enter G1 by 5 h after activation. The cdc2-specific mRNA, of which expression was previously known to be dictated by IL-2R signaling during activation of murine G0 T cells [10, 11], was first detected in 15 h and reached maximum level 20 h after activation, and then slightly declined until 40 h. Under these conditions, the expression of PHGDH mRNA was first detectable in 5 h as a faint band and reached a maximum level in 30 h after activation, at which time the majority of cells were in S phase. These results indicate that the expression of PHGDH mRNA, which is undetectable in G0 T cells, is induced at early G1 and reaches the highest level in S phase during the activation of G0 T cells by immobilized anti-CD3.
Lack of PHGDH expression in T cells activated in the absence of IL-2 synthesis
Since the kinetic expression of PHGDH in murine G0 T cells following immobilized anti-CD3-activation indicated that the accumulation of its mRNA might be under the influence of IL-2R signaling that is required for the G1/S transition of activated T cells, this was further investigated by examining the expression of these genes in T cells activated by either immobilized anti-CD3 plus cyclosporine A (CsA) or phorbol dibutyrate (PBu2), both of which fail to induce the synthesis of IL-2 and thus render the T cells accumulated in G1 phase [9, 10].
G0 T cells were activated by immobilized anti-CD3 for 20 h and 40 h with or without CsA (100 nM) in the presence or absence of exogenously added rIL-2. Flow cytometric analysis of the cell cycle progression of G0 T cells activated by each condition is shown in Figure 2A. In the presence or absence of CsA, essentially all of the cells activated by immobilized anti-CD3 for 20 h were remained in G0/G1. However, 40 h after activation, only those T cells activated by anti-CD3 or anti-CD3 plus CsA in the presence of 1 nM rIL-2 continued to progress through the cell cycle. As shown in Figure 2B, G0 T cells following activation by anti-CD3 plus CsA failed to accumulate specific mRNA for PHGDH and cdc2. However, addition of 1 nM rIL–2 under the same conditions resulted in the expression of PHGDH- and cdc2-specific mRNA, and the pattern of gene expression was restored to that observed with anti-CD3 activation. In addition, G0 T cells activated by PBu2 failed to enter S phase unless 1 nM rIL-2 were exogenously added (Fig. 3A). Under the same conditions, G0 T cells activated by PBu2 did not induce the expression of PHGDH- and cdc2-specific mRNA during 40 h period after activation. When 1 nM rIL-2 was exogenously added during the PBu2 activation, the expression of PHGDH and cdc2 was induced (Fig. 3B). These results demonstrate that IL-2 signaling through IL-2R is a prerequisite for the expression of PHGDH gene in G1 phase of the activated T cells, and suggest that the expression of PHGDH gene is among the essential events required for the IL-2-mediated completion of the G1/S transition and proliferation of activated T cells.
Fig. 2.
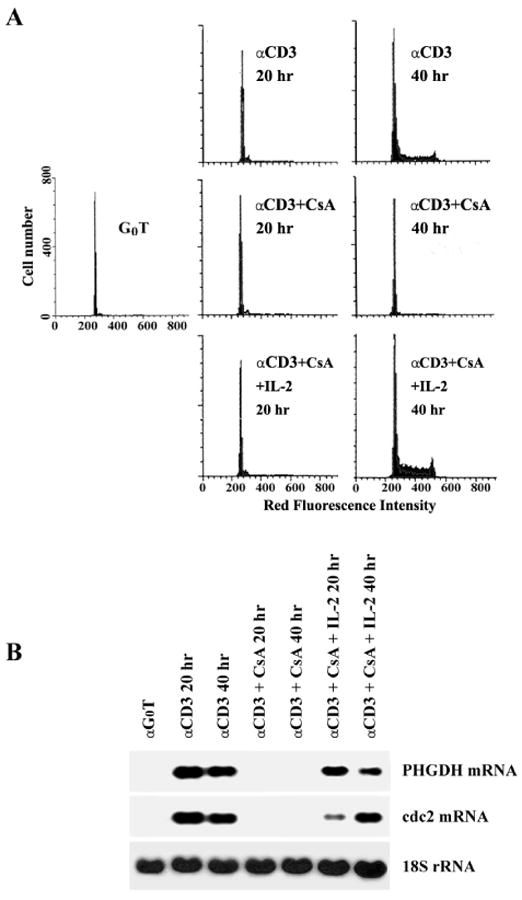
Effect of CsA on the cell cycle progression (A) and the expression of PHGDH mRNA (B) during immobilized anti-CD3 activation of G0 T cells. Murine G0 T cells were activated by immobilized anti-CD3 without or with CsA in the presence or absence of exogenous rIL-2. After staining of DNA with propidium iodide, an equal number of cells (104) were subjected to cell cycle analysis by flow cytometry. Ten micrograms of total RNA from individual equivalent cultures with were electrophoresed, transferred, and probed with 32P-labeled PHGDH, cdc2, and 18S rRNA cDNA.
Fig. 3.
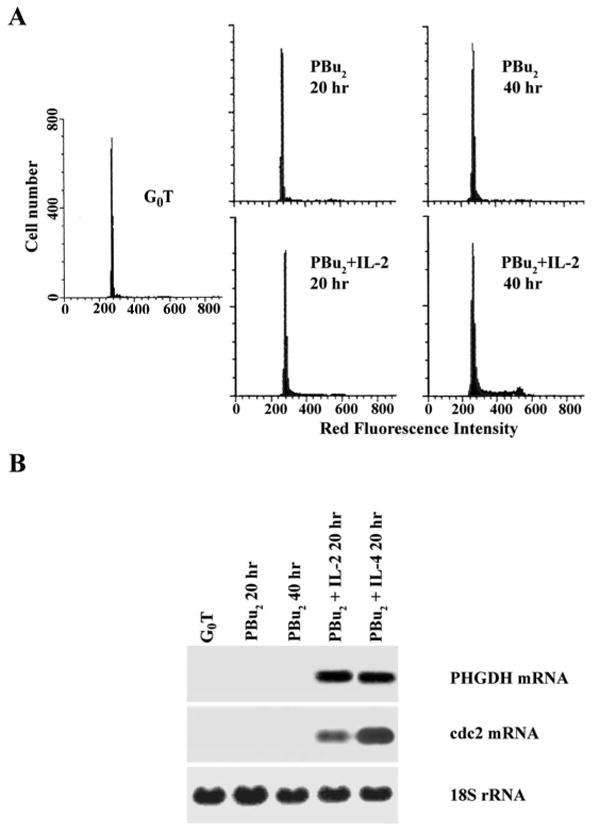
Lack of PHGDH mRNA expression in PBu2-activated G0 T cells. Murine G0 T cells were activated by PBu2 in the presence or absence of exogenous rIL-2. After staining of DNA with propidium iodide, an equal number of cells (104) were subjected to cell cycle analysis by flow cytometry. Ten micrograms of total RNA from individual equivalent cultures were electrophoresed, transferred, and probed with 32P-labeled PHGDH, cdc2, and 18S rRNA cDNA.
Inhibition of DNA synthesis of activated T cells by antisense PHGDH oligonucleotide
As an attempt to investigate the requirement of PHGDH expression for S phase in the activated T cells, the [3H]TdR incorporation of G0 T cells following immobilized anti-CD3 activation in the RPMI 1640 medium containing L-serine (30 mg/L) and glycine (10 mg/L) was compared with those observed in the RPMI 1640 medium deprived of either L-serine or L-serine and glycine. As shown in Figure 4, the [3H]TdR incorporation of activated G0 T cells in the medium without L-serine declined to the level of 68%, and it declined to the level of 52% when the culture medium was deprived of both L-serine and glycine. These results demonstrate that L-serine derived from either the phosphorylated pathway or exogenous source can commonly contribute to S phase during immobilized anti-CD3 activation of G0 T cells. In addition, the results representing more significant reduction in [3H]TdR incorporation of activated G0 T cells in the culture medium deprived of L-serine and glycine, as compared to that in the culture medium deprived of L-serine, indicate that exogenously added glycine, which serine hydroxymethyl transferase can convert to L-serine, also supports S phase in the activated T cells.
Fig. 4.
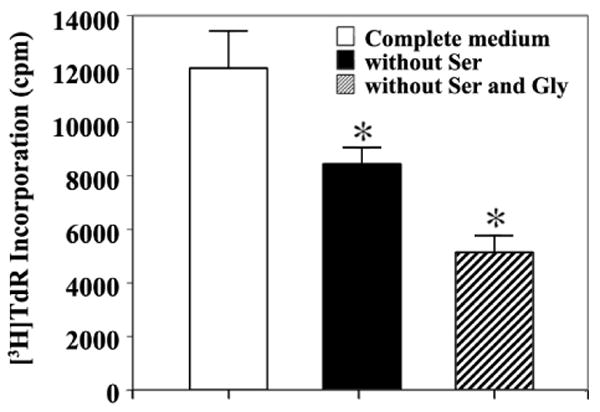
Effect of deprivation of either L-serine or L-serine and glycine on the [3H]TdR incorporation into DNA of G0 T cells following immobilized anti-CD3 activation. Murine G0 T cells were activated with immobilized anti-CD3 for 40 h in the complete RPMI 1640 medium (control) or the RPMI 1640 medium derived of either L-serine or L-serine and glycine. These culture media were purchased from JBI (Daegu, Korea), and supplemented with 10% dialyzed FBS (JRH Biosciences, KS, USA). For [3H]TdR incorporation assay, the last 2 h was pulsed with 1 μCi of [3H]TdR before the cells were harvested. Each value is expressed as mean ± SD (n = 3). *P < 0.05 as compared with the control.
To obtain more direct evidence for the requirement of PHGDH expression for S phase in the activated T cells, we decided to block the translation of PHGDH mRNA with synthetic phosphorothioate oligonucleotide and to examine the effect of depletion of PHGDH on the proliferation of G0 T cells following immobilized anti-CD3 activation. Four different synthetic phosphorothioate oligonucleotides complementary to different regions of the PHGDH coding sequence were prepared along with individual sense oligonucleotides as the control. The effectiveness of these oligonucleotides were tested by analyzing [3H]TdR incorporation of G0 T cells activated by immobilized anti-CD3 in the presence of each oligonucleotide at a concentration of 10 μM. As shown in Figure 5A–C, the antisense oligonucleotide (AS-2) directed against the region in exon 3 was the most effective in suppressing [3H]TdR incorporation of activated G0 T cells, indicating that AS-2 oligonucleotide was effective in inhibiting the synthesis of PHGDH protein. In order to examine further whether AS-2 oligonucleotide was able to deplete PHGDH protein, murine G0 T cells were treated with antisense AS-2 or sense S-2 oligonucleotide at the concentrations of 10, 25, or 50 μM for 4 h before activation by immobilized anti-CD3 for 40 h, and the incorporation of [3H]TdR was analyzed. As shown in Figure 6A, the incorporation of [3H]TdR into DNA of activated T cells treated with the antisense oligonucleotide AS-2 declined remarkably in a dose-dependent manner, whereas treatment of the cells with the sense oligonucleotide S-2 exerted a slight inhibitory effect only at a concentration of 50 μM. Under the same conditions, Western blot analysis revealed that there was a significant reduction in the amount of PHGDH protein detected in those G0 T cells that had been exposed to the antisense oligonucleotide AS-2 at concentrations of 25 or 50 μM (Fig. 6B). These results demonstrate that the expression of PHGDH, which is involved in the phosphorylated pathway of L-serine synthesis, is essential for S phase in the activated T cells.
Fig. 5.
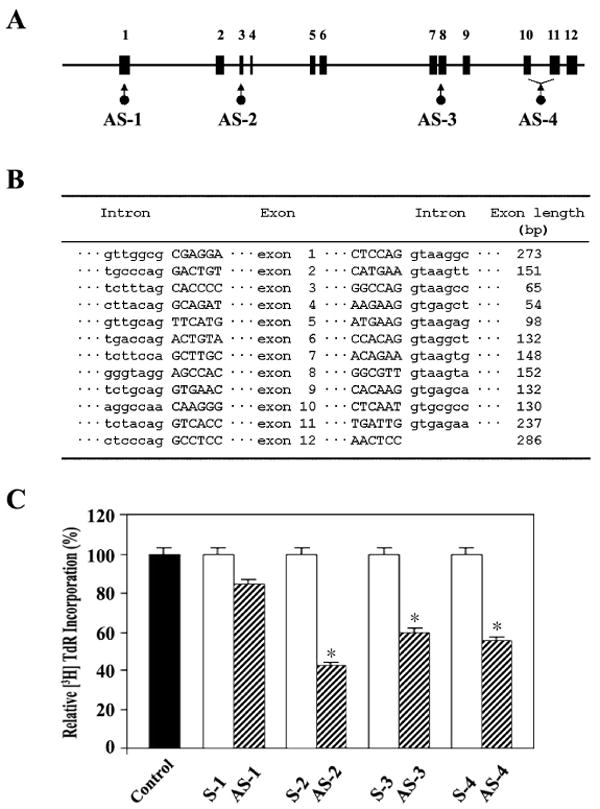
Exon-intron boundary (A) and genomic organization (B) of murine PHGDH gene, and Western blot analysis of the lysate of G0 T cells activated for 40 h by immobilized anti-CD3 with either no treatment or after treatment with PHGDH antisense or sense oligonucleotides. Murine G0 T cells were pretreated with individual antisense or sense phosphorothioate oligonucleotides at 25 μM for 4 hr before activation by immobilized anti-CD3 for 40 h. For [3H]TdR incorporation assay, the last 2 h was pulsed with 1 μCi of [3H]TdR before the cells were harvested. Each value is expressed as mean ± SD (n = 3). *P < 0.05 as compared with the control.
Fig. 6.
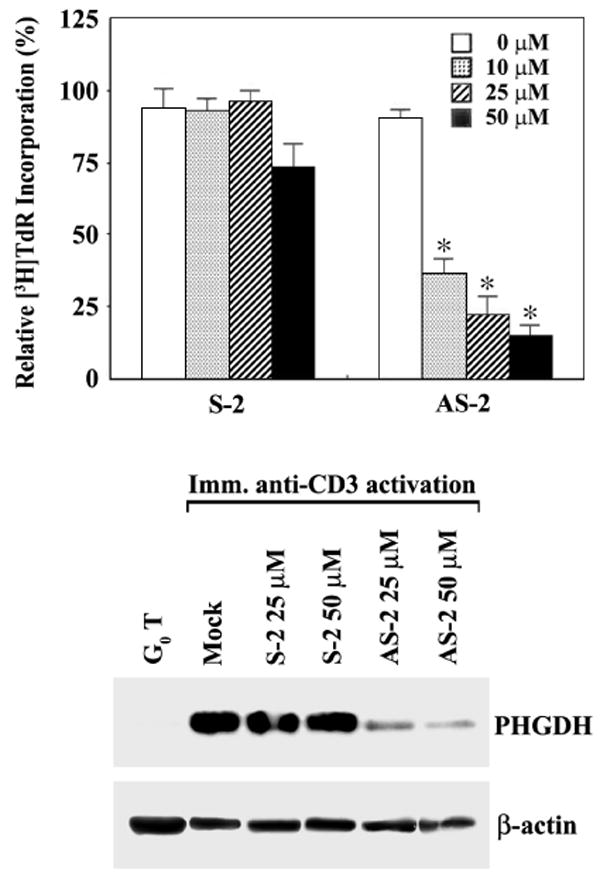
Effect of PHGDH antisense oligonucleotide on the [3H]TdR incorporation into DNA (A) and synthesis of PHGDH protein (B) in G0 T cells following immobilized anti-CD3 activation. Murine G0 T cells were treated with PHGDH antisense oligonucleotides at various concentrations for 4 h and then activated by immobilized anti-CD3 for 40 h. For [3H]TdR incorporation assay, the last 2 h was pulsed with 1 μCi of [3H]TdR before the cells were harvested. Each value is expressed as mean ± SD (n = 3). *P < 0.05 as compared with the control. Equivalent amounts of cell lysates were electrophoresed on 4-12% SDS gradient polyacrylamide gels and electrotransferred to Immobilon-P membrane. Western analysis was performed as described in Materials and Methods using ECL Western blotting detection system.
Discussion
After completing development in thymus, mature T lymphocytes migrate to the peripheral lymphoid organs and tissues, and remain in a resting (G0) state. The interaction of antigen or mitogenic lectin with specific receptors on G0 T cells initiates a cascade of biochemical events to induce the expression of a wide variety of genes essential for activation and proliferation, and subsequent immune functions [13–15]. The IL-2 and IL-2R are thought to be among the more critical genes for the proliferation of activated T cells, in that binding of IL-2 to IL-2R expressed on the surface of activated T cells induces the signal necessary for the G1/S transition [6-8]. In this report, we have employed the model of polyclonal activation of murine G0 T cells to determine the requirement of the expression of PHGDH gene for S phase of the cell cycle, because this cell model provides a population of cells that, following polyclonal activation, synchronously enter G1 phase and progress through the first round of the cell cycle. In response to immobilized anti-CD3, murine splenic G0 T cells appeared to progress out of G0 and traversed G1 over a time period of approximately 20 h before transition to S phase occurred, as determined by Flow cytometry as well as [3H]TdR incorporation assay. Although there was no detectable PHGDH mRNA in murine G0 T cells, it began to be detected 5 h after activation by immobilized anti-CD3, which corresponds to a point of G1 phase as justified by the detection of IL-2 and IL-2R transcripts, and reached high levels as the cells entered and traversed S phase. Under these conditions, the expression of cdc2 mRNA was first detected in 15 h, in consistent with a previous data showing the kinetics of the cdc2 gene expression in murine T cells following immobilized anti-CD3 activation [10].
The increase in the level of PHGDH mRNA in the activated T cells was abrogated in the presence of CsA, which functions, as an immunosuppressive agent, early during the activation of T cells [16–18]. The CsA, when associated with cyclophilin A, a 17-kDa intracellular protein, binds with and inhibits the phosphatase activity of calcineurin in T cells. This causes to prevent the transcription of IL-2 gene, and thus the activated G0 T cells remains in G1 phase due to the failure to enter S phase. Exogenous addition of IL-2 effectively reversed the CsA-induced suppression of T-cell proliferation, and addition of IL-2 during the activation of G0 T cells in the presence of CsA also reestablished the increase in the level of PHGDH. In addition, murine G0 T cells activated by PBu2, which has been shown not to induce the synthesis of IL-2 [9, 10], were unable to complete the G1/S transition of the cell cycle, and the expression of PHGDH was not induced. The addition of exogenous IL-2 during the activation of these cells by PBu2 restored the proliferative capacity of the cells and resulted in the similar pattern of increase in the PHGDH level as observed when G0 T cells were activated by immobilized anti-CD3. These results are all consistent with the interpretation that IL-2 signaling through IL-2R is necessary to induce the expression of PHGDH gene in activated T cells, which occurs in G1 phase and reaches a maximum in S phase in order to support L-serine biosynthesis required for the formation of nucleotide precursors of DNA.
Although current data representing the expression of PHGDH gene during polyclonal activation of G0 T cells indicated that the PHGDH expression could be a prerequisite for entering and traversing S phase, we used two experimental approaches to show more direct evidence for the requirement of PHGDH for S phase of the activated T cells; one is to measure the effect of exogenously added L-serine on the proliferation of G0 T cells following immobilized anti-CD3 activation, and the other one is to take advantage of the antisense phosphorothioate oligonucleotide to prevent the translation of PHGDH transcript. The supplement of L-serine during immobilized anti-CD3 activation enhanced the [3H]TdR-incorporation of activated T cells, confirming that L-serine synthesized via the phosphorylated pathway is required for S phase of the activated T cells. Since several literature have shown that the translation start site or translated region are good targets for antisense oligonucleotides [19, 20], four different oligonucleotides which are targeted to the AUG start site (AS-1), or sites in the coding region, such as the exon 3 (AS-2), the exon 8 (AS-3), and the region spanning over exon 10 and exon 11 (AS-4), respectively, were tested to compare their effectiveness in preventing [3H]TdR incorporation of activated T cells. The incorporation of [3H]TdR into DNA of activated T cells pretreated with antisense oligonucleotide AS-2 showed the most significant reduction. This reduction in the level of [3H]TdR-incorporation was induced in a dose-dependent fashion, when G0 T cells were pretreated with AS-2 at various concentrations of 10, 25, or 50 μM. At the same time, Western blot analysis verified that a significant reduction of the synthesis of PHGDH protein in the activated T cells treated with antisense oligonucleotide AS-2, indicating the correlation between the significant reduction in the level of PHGDH protein and failure of traversing S phase. These results demonstrated that the expression of PHGDH is necessary to traverse through S phase in the activated T cells. These results also indicate that PHGDH-dependent synthesis of L-serine via the phosphorylated pathway is a major route for production of endogenous L-serine in the activated T cells.
In summary, murine G0 T cells activated by means that prevented the production of endogenous IL-2 failed to induce the expression of PHGDH gene and arrested in G1 phase. The addition of a physiological level of rIL-2 (1 nM) was sufficient to recover the expression of PHGDH gene and to reverse the G1-arrest associated with the lack of endogenous IL-2. These results, together with the observation that antisense PHGDH oligonucleotide could significantly reduce the expression level of PHGDH protein in the activated T cells and prevented traversing S phase, suggest that the production of IL-2, which is induced within first 5 h of T cell activation, results in the expression of PHGDH in G1 phase and promotes the phosphorylated pathway for the synthesis of L-serine, which is metabolized to synthesize nucleotide precursors required for DNA replication in S phase.
Acknowledgments
This work was supported by a grant (02-PJ1-PG3-20908-0036) from the Ministry of Health and Welfare, Korea, and a grant (KRF-2003-005-C00007) from the Korean Research Foundation.
References
- 1.Snell K. The duality of pathways for serine biosynthesis is a fallacy. Trends Biochem Sci. 1986;11:241–243. [Google Scholar]
- 2.Snell K, Weber G. Enzymic imbalance in serine metabolism in rat hepatomas. Biochemical J. 1986;233:617–620. doi: 10.1042/bj2330617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snell K, Natsumeda Y, Eble JN, Glover JL, Weber G. Enzymic imbalance in serine metabolism in human colon carcinoma and rat sarcoma. Br J Cancer. 1988;57:87–90. doi: 10.1038/bjc.1988.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho HM, Jun DY, Bae MA, Ahn JD, Kim YH. Nucleotide sequence and differential expression of the human 3-phosphoglycerate dehydrogenase gene. Gene. 2000;245:193–201. doi: 10.1016/s0378-1119(00)00009-3. [DOI] [PubMed] [Google Scholar]
- 5.Baek JY, Jun DY, Taub D, Kim YH. Characterization of human phosphoserine aminotransferase involved in the phosphorylated pathway of L-serine biosynthesis. Biochem J. 2003;373:191–200. doi: 10.1042/BJ20030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stern JB, Smith KA. Interleukin 2 induction of T cell G1 progression and c-myb expression. Science. 1986;233:203–206. doi: 10.1126/science.3523754. [DOI] [PubMed] [Google Scholar]
- 7.Minami Y, Kono T, Miyazaki T, Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–268. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- 8.Nelson BH, Willerford DM. Biology of the interleukin-2 receptor. Adv Immunol. 1998;70:1–81. doi: 10.1016/s0065-2776(08)60386-7. [DOI] [PubMed] [Google Scholar]
- 9.Proust JJ, Shaper NL, Buchholz MJ, Nordin AA. T cell activation in the absence of interleukin 2 (IL-2) results in the induction of high affinity IL-2 receptors unable to transmit a proliferation signal. Eur J Immunol. 1991;21:335–341. doi: 10.1002/eji.1830210214. [DOI] [PubMed] [Google Scholar]
- 10.Kim YH, Proust JJ, Buchholz MJ, Chrest FJ, Nordin AA. Expression of the murine homologue of the cell cycle control protein p34cdc2 in T lymphocytes. J Immunol. 1992;149:17–23. [PubMed] [Google Scholar]
- 11.Kim YH, Buchholz MJ, Nordin AA. Murine T-lymphocyte proliferation induced by interleukin 2 correlates with a transient increase in p56lck kinase activity and the tyrosine phosphorylation of a 97-kDa protein. Proc Natl Acad Sci USA. 1993;90:3187–3191. doi: 10.1073/pnas.90.8.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feinberg AP, Volgelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1984;137:266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- 13.Ullman KS, Northrop JP, Verweij CL, Crabtree GR. Transmission of signal from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]
- 14.Firpo EJ, Koff A, Solomon MJ, Roberts JM. Inactivation of a cdk2 inhibitor during interleukin 2-induced proliferation of human T lymphocytes. Mol Cell Biol. 1994;14:4889–4901. doi: 10.1128/mcb.14.7.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hess K, Yang Y, Golech S, Sharov A, Becker KG, Weng NP. Kinetic assessment of general gene expression changes during human naive CD4+ T cell activation. Int Immunol. 2004;16:1711–1721. doi: 10.1093/intimm/dxh172. [DOI] [PubMed] [Google Scholar]
- 16.Bierer BE, Hollander G, Fruman D, Burakoff SJ. Cyclosporin A and FK506: molecular mechanism of immunosuppression and probes for transplantation biology. Curr Biol. 1993;5:763–773. doi: 10.1016/0952-7915(93)90135-f. [DOI] [PubMed] [Google Scholar]
- 17.Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 18.Emmel EA, Verwij CL, Durand DB, Higgin KM, Lacy E, Crabtree GR. Cyclosporine A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989;246:1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- 19.Sohail M, Southern EM. Selecting optimal antisense reagents. Adv Drug Deliv Rev. 2000;44:23–34. doi: 10.1016/s0169-409x(00)00081-8. [DOI] [PubMed] [Google Scholar]
- 20.Kurreck J. Antisense technologies. Improvement through novel chemical modifications. Eur J Biochem. 2003;270:1628–1644. doi: 10.1046/j.1432-1033.2003.03555.x. [DOI] [PubMed] [Google Scholar]


