Abstract
Objective
To evaluate serum interferon-α (IFNα) activity in the context of autoantibody profiles in patients with juvenile dermatomyositis (JDM).
Methods
Sera from 36 JDM patients were analyzed. Autoantibody profiles were determined by probing microarrays, fabricated with ~80 distinct autoantigens, with serum and a Cy3-conjugated secondary antibody. Arrays were scanned and analyzed to determine antigen reactivity. Serum IFNα activity was measured using a functional reporter cell assay. Sera were assayed alone or in combination with cellular material released from necrotic U937 cells to stimulate peripheral blood mononuclear cells from healthy donors in vitro, and IFNα production in culture was measured by a dissociation-enhanced lanthanide fluorescent immunoassay (DELFIA).
Results
Reactivity against at least 1 of 41 autoantigens on the microarray, including Ro 52, Ro 60, La, Sm, and RNP, was observed in 75% of the serum samples from patients with JDM. IFNα activity was detected in 7 samples by reporter cell assay. The reporter cell assay showed a significant association of reactivity against Ro, La, Sm, and proliferating cell nuclear antigen with serum IFNα activity (P = 0.005). Significance Analysis of Microarrays (SAM) identified increased reactivity against Sm, RNP, Ro 52, U1-C, and Mi-2 in these sera. Sixteen samples induced IFNα production as measured by DELFIA, and there was a significant association of reactivity against Ro, La, Sm, and RNP with the induction of IFNα by serum and necrotic cell material (P = 0.034). SAM identified increased reactivity against Ro 60 in these sera.
Conclusion
These data support the hypothesis that nucleic acid–associated autoantibodies, including the Ro/La and Sm/RNP complexes, may stimulate the production of active IFNα in children with JDM.
Juvenile dermatomyositis (JDM), the most common inflammatory myopathy of childhood, is a systemic vasculopathy with a mean age at onset of 6.7 years and a female predominance of 2.3:1 (1). Diagnosis is based on the Bohan and Peter criteria (2, 3).
The pathophysiology of JDM is likely autoimmune, and both genetic and environmental factors have been implicated. The majority of patients with JDM are positive for antinuclear antibodies (ANAs), although the ANA specificity remains unknown for most patients (4).
Type I interferons (IFNs) appear to play a role in JDM. Transcript profiling of muscle tissue from untreated patients with JDM demonstrated increased expression of several type I IFN–inducible genes (5). Transcript profiling has also shown up-regulation of type I IFN–inducible genes in peripheral blood mononuclear cells (PBMCs) from patients with JDM (6). Comparison of transcript profiles of PBMCs from healthy children, patients with JDM, and patients with pediatric systemic lupus erythematosus (SLE) demonstrated up-regulation of type I IFN–regulated transcripts in 50% of the patients with active JDM, and this type I IFN signature overlapped with, but was distinct from, the type I IFN signature identified in patients with pediatric SLE (6). In addition, IFNα activity in sera from untreated patients with JDM was increased compared to that in sera from age-matched controls (7). Furthermore, sera from adult myositis patients with Jo-1 or Ro 52/Ro 60 autoantibodies induced IFNα production in healthy donor PBMCs (8). Those studies suggest that both type I IFN proteins and IFN-inducible genes are up-regulated in myositis and may be critical in disease pathogenesis.
Autoantigen microarrays allow the comprehensive evaluation of autoantibodies directed against dozens of autoantigens using microliter quantities of serum (9). Antibody binding to autoantigens on microarrays has been shown to be 4–8 times more sensitive than enzyme-linked immunosorbent assays (ELISAs), with specific antibody binding demonstrated over a 1,000-fold range (10). The purpose of this pilot study was to characterize the autoantibodies present in the sera of patients with JDM using autoantigen microarrays and to determine whether serologic IFNα activity correlates with the presence of specific autoantibodies.
PATIENTS AND METHODS
Patients
Thirty-six patients (28 females and 8 males with a median age of 10.5 years) with definite/probable JDM (based on the Bohan and Peter criteria) and 10 controls (with a median age of 11 years) were included in the study. Patient demographics and clinical characteristics at the time of sample collection are shown in Supplementary Table 1 (available on the Arthritis & Rheumatism web site at http://onlinelibrary.wiley.com/doi/10.1002/art.38038/abstract). Skin and muscle disease activity were evaluated using the validated Disease Activity Score (DAS) (11). The Institutional Review Board at the Ann and Robert H. Lurie Children's Hospital of Chicago Research Center approved the study (IRB #2002-11762), and age-appropriate informed consent was obtained. Sera from 10 of the patients with JDM (28%) were positive for myositis-specific autoantibodies (MSAs) or myositis-associated autoantibodies (MAAs), including anti-Ro, anti–Mi-2, anti–U1 RNP, anti–U2 RNP, anti–Scl-70, anti–PM-Scl, and anti–signal recognition particle (12).
Autoantigen Microarrays
Autoantigen microarrays were generated by spotting distinct autoantigens in replicates using a VersArray ChipWriter Pro microarrayer (Bio-Rad) with a customized printhead and Silicon Microarray Spotting Pins (Parallel Synthesis Technologies) as previously described (10). A complete list of these autoantigens is provided in Supplementary Table 2 (available on the Arthritis & Rheumatism web site at http://onlinelibrary.wiley.com/doi/10.1002/art.38038/abstract). Microarrays were probed with 1:150 dilutions of serum and a Cy3-conjugated goat anti-human IgG/IgM secondary antibody (Jackson ImmunoResearch) using protocols established in our laboratory (13). Arrays were scanned using a GenePix 4000 scanner (Molecular Devices). Digital images were collected and stored in a database for analysis.
Reporter cell assay
Reporter cells (WISH epithelial cell line) were cultured for 6 hours with sera from patients with JDM, lysed, and RNA was isolated. Real-time quantitative polymerase chain reaction was used to measure the relative expression of 3 IFNα-induced genes (IFN-induced protein with tetratricopeptide repeats 1, myxovirus resistance protein 1, and RNA-dependent protein kinase) in the reporter cells (14). Relative expression data from these transcripts were normalized using the mean and SD of healthy donor sera (n = 10) that were run in the same assay, and data are presented as an IFNα activity score. The serum-induced reporter cell activity can be blocked with anti-IFNα monoclonal antibodies, and no significant functional inhibitors have been detected (14).
IFNα inducers and culture of PBMCs
PBMCs from healthy donors were cultured in 96-well plates with 4 different concentrations of patient or healthy control sera, ranging from 0.001% to 1%, alone or in combination with 10% necrotic material from U937 cells. Each serum sample was used to stimulate PBMCs from 2 healthy donors. Ultraviolet light–inactivated herpes simplex virus type 1 and serum from an SLE patient were used as positive controls. After 20 hours, IFNα levels in the cell culture supernatants were measured by a dissociation-enhanced lanthanide fluoroimmunoassay (15). The cutoff level, based on the level induced by the healthy control sera, was set at 21 units/ml.
Statistical analysis
For the autoantigen microarrays, quantitative analysis of the fluorescence intensity of individual features was performed using GenePix Pro software version 6.1 (Molecular Devices). The fluorescence intensity of each feature was determined based on the median pixel intensity within the circumscribed antigen feature minus the background. The median fluorescence intensity minus background (MFI-B) was calculated for each antigen. For each individual sample from patients with JDM, reactivity against an antigen was considered to be significant if the MFI-B for the antigen was more than 3 SD above the mean MFI-B for that antigen in the 10 control samples.
Significance Analysis of Microarrays (SAM) was used to determine differences between patients with JDM and controls and to perform subgroup analyses among the patients with JDM. SAM is an interactive algorithm developed at Stanford University that assigns a standardized score to each protein recognition event, and antigens with scores higher than an adjustable threshold are considered “potentially significant.” SAM can be used to correlate array data with clinical, demographic, and treatment data. SAM is described fully at http://www-stat.stanford.edu/~tibs/SAM (16).
Comparisons of the proportions of patients who were positive for particular autoantibodies and had a positive type I IFN assay were performed using Fisher's exact test, with P values calculated by the sum of small p's method. Odds ratios were calculated using standard methodology.
RESULTS
Evaluation of the MFI-B for each patient with JDM compared to the mean MFI-B of controls revealed that 27 patients with JDM (75%) had reactivity against at least 1 of 41 antigens on the microarray (Table 1). The number of patients with reactivity against each of these antigens ranged from 1 (3%) to 6 (17%). The majority of sera demonstrated reactivity against one or two antigens while some sera had a broader spectrum of reactivity (Table 1).
Table 1.
Autoantibody reactivity in sera from patients with JDM, as determined by autoantigen microarray*
| Antigen | No. (%) positive patients |
|---|---|
| Ro 52 | 6 (17) |
| Histone H2B | 5 (14) |
| Mi-2 | 5 (14) |
| U1-A | 5 (14) |
| CENP-B | 4 (11) |
| La | 4 (11) |
| Type II collagen | 3 (8) |
| Histone H3 | 3 (8) |
| Ro 60 | 3 (8) |
| Sm | 3 (8) |
| CENP-A | 2 (6) |
| Type VIII collagen | 2 (6) |
| EBNA-1(58–72) peptide | 2 (6) |
| EBNA-1(398–412) peptide | 2 (6) |
| Hsp25 | 2 (6) |
| Hsp70 | 2 (6) |
| LKM-1 | 2 (6) |
| PCNA | 2 (6) |
| U1-70K | 2 (6) |
| U1-C | 2 (6) |
| Actin | 1 (3) |
| BPI | 1 (3) |
| Cardiolipin | 1 (3) |
| EBNA-1(35–58) peptide | 1 (3) |
| Fibrinogen type IV | 1 (3) |
| Fibrinogen type IS | 1 (3) |
| GBM-u | 1 (3) |
| Grp78 | 1 (3) |
| Heparan sulfate | 1 (3) |
| Histones, whole | 1 (3) |
| Hsp90 | 1 (3) |
| Hsp47 | 1 (3) |
| Ku | 1 (3) |
| PDH | 1 (3) |
| PL-12 | 1 (3) |
| PR3 | 1 (3) |
| Ribosomal P | 1 (3) |
| Sm/RNP | 1 (3) |
| ssDNA | 1 (3) |
| TG | 1 (3) |
| TPO | 1 (3) |
JDM = juvenile dermatomyositis; EBNA-1 = Epstein-Barr nuclear antigen 1; LKM-1 = liver-kidney microsomal antibody 1; PCNA = proliferating cell nuclear antigen; BPI = bactericidal/permeability increasing protein; GBM-u = glomerular basement membrane, undissociated; PDH = pyruvate dehydrogenase; PR3 = proteinase 3; ssDNA = single-stranded DNA; TG = thyroglobulin; TPO = thyroid peroxidase.
Two MSAs, anti–Mi-2 and anti–PL-12, and several MAAs, including anti-La, anti–Ro 52, anti–Ro 60, anti–U1 RNPs, and anti-Ku antibodies, were identified in this JDM cohort (Table 1). Several of the other reactivities observed were against autoantigens associated with SLE, including histones, Sm, and proliferating cell nuclear antigen (PCNA) (Table 1). There was a correlation between ELISA results and microarray data in the sera from patients with JDM for Ro 52, Ro 60, and La, with Spearman's r values ranging from 0.44 to 0.63 (data not shown).
SAM did not reveal any significant difference in autoantibody reactivity between sera from patients with JDM as a whole and control sera. Similarly, the results of SAM subgroup analyses of ANA-positive versus ANA-negative patients, patients with high (≥3) versus those with low (≤2) skin or muscle DAS, and patients with versus those without a family history of SLE were not significant (data not shown).
Given that PBMCs from a subset of patients with JDM have an IFN signature, we used a reporter cell assay to further characterize sera from this JDM cohort for the ability to induce IFNα activity (14). Of the JDM patient sera assayed, most did not induce up-regulation of the IFNα-regulated genes, 2 induced high IFNα activity (+++), and 5 induced low activity (+), as shown in Figure 1A and Table 2. Typical values for normal pediatric and adult sera are between 0 and 2.
Figure 1.
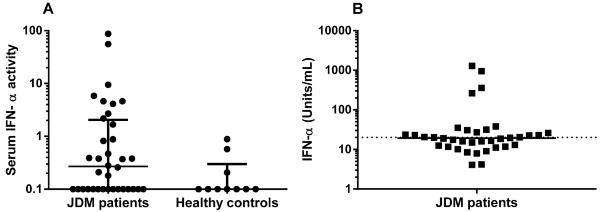
Interferon-α (IFNα) activity and production in sera from patients with juvenile dermatomyositis (JDM). A, Serum IFNα activity in patients with JDM and healthy controls, as determined by reporter cell assay. Each data point represents an individual subject; horizontal lines and error bars show the median and interquartile range. B, In vitro IFNα production in normal peripheral blood mononuclear cells (PBMCs) stimulated with necrotic cell material combined with sera from patients with JDM. Each data point represents the mean IFNα production in PBMCs from 2 healthy blood donors in response to serum from an individual patient. The horizontal line represents the median IFNα production for the JDM cohort. Samples with IFNα production above 21 units/ml (broken line) were considered positive based on assays run with healthy donor serum.
Table 2.
Autoantibody reactivity in, and induction of IFNα by, serum samples from patients with JDM*
| Sample | IFNα activitya | IFNα productionb | Ro 52c | Ro 60c | Lac | Other autoreactivity on microarray |
|---|---|---|---|---|---|---|
| 5353 | − | − | − | − | − | BPI, TG |
| 5417 | +++ | +++ | − | − | − | Sm,d U1-A,d U1-C,d EBNA-1 (58–72) peptided |
| 5757 | − | − | − | − | − | None |
| 6106 | − | + | − | − | − | PDH |
| 6120 | − | + | − | + | − | Cardiolipin, types II and VIII collagen, histone H2B,d histone |
| 6224 | + | + | + | − | + | CENP-B,d EBNA-1 (398–412) peptide,d Hsp70, Mi-2d |
| 6242 | − | + | − | − | − | Type VIII collagen, whole histonesd |
| 6255 | − | + | − | − | − | Grp78 |
| 6384 | − | − | − | − | − | None |
| 6661 | − | + | − | − | − | GBM-u, TPO |
| 6668 | − | − | − | − | − | None |
| 6735 | − | − | − | − | − | Mi-2d |
| 6868 | − | − | − | − | − | None |
| 7028 | − | − | − | − | − | Type II collagen |
| 7084 | + | + | − | − | − | Mi-2d |
| 7126 | − | − | − | − | − | None |
| 7310 | − | − | − | − | − | None |
| 7434 | − | + | − | − | + | U1-Ad |
| 7466 | − | + | − | − | − | None |
| 7660 | − | − | − | − | − | Mi-2d |
| 7786 | + | − | − | − | − | CENP-B,d EBNA-1(58–72) peptide,d histone H2B,d histone |
| 7834 | + | − | − | − | − | LKM-1, PCNAd |
| 7856 | − | + | − | − | − | Type II collagen, histone H2B,d histone H3,d PR3, Smd |
| 7857 | + | − | + | − | − | CENP-Bd |
| 7858 | − | + | − | − | − | Fibrinogen type IV, histone H2Bd |
| 7991 | − | − | − | − | − | CENP-A,d histone H2B,d Hsp25, Hsp47, Ku,d PL-12d |
| 7992 | − | − | − | − | − | None |
| 7993 | − | − | − | − | − | None |
| 7995 | − | − | − | − | − | Actin |
| 7997 | − | − | − | − | − | LKM-1, U1-Ad |
| 7999 | +++ | +++ | + | + | − | EBNA-1(35–58) peptide,d Ro 52,d Ro 60,d Sm/RNP,d U1-A,d |
| 8053 | − | − | + | − | − | None |
| 8112 | − | +++ | + | + | + | CENP-Bd |
| 8113 | − | − | − | − | + | EBNA-1(398–412) peptide,d fibrinogen type IS, U1-70K,d U1- |
| 8130 | − | + | − | − | − | CENP-A,d Mi-2d |
| 8147 | − | +++ | + | − | − | None |
See Table 1 for definitions.
Determined by reporter cell assay. − = no interferon-α (IFNα) activity; + = low IFNα activity; +++ = high IFNα activity.
Determined by dissociation-enhanced lanthanide fluoroimmunoassay. − = no IFNα production; + = low IFNα production; +++ = high IFNα production.
− = not reactive; + = reactive.
Nucleic acid-associated antigen.
There was an association between the induction of serum IFNα activity in the reporter cell assay and positivity for anti-Ro, anti-La, anti-Sm, or anti-PCNA specificities. Serum IFNα activity was induced by sera from 6 of the 13 patients who were positive for 1 of these autoantibodies versus only 1 of the 23 patients who were negative for these antibodies (P = 0.005). Similarly, there was an association between serum IFNα activity and autoantibody positivity in patients with reactivity against any DNA-associated antigen, with serum from 6 of the 16 patients who were positive for 1 of these antibodies inducing serum IFNα activity, as compared to serum from only 1 of the 21 patients who were negative for these antibodies (P = 0.039). The 2 serum samples with high IFNα activity also had strong reactivity against components of the Sm/RNP complex, and all of the samples with IFNα activity in the reporter cell assay demonstrated reactivity against at least 1 nucleic acid–associated autoantigen (Table 2). SAM identified increased reactivity against several of these antigens, including Ro 52, Sm/RNP, U1-C, and Mi-2, in sera positive for the receptor cell assay (data not shown).
Previous studies have demonstrated that SLE sera combined with apoptotic U937 cells induce healthy donor PBMCs to produce IFNα in vitro (15). We evaluated sera from patients in this JDM cohort for the ability to induce IFNα production using this assay. Sixteen serum samples induced IFNα production and thus had the capacity to generate interferogenic immune complexes, as seen in Figure 1B. There was an association between IFNα production and reactivity against Ro, La, Sm, and RNP antigens (P = 0.034). In addition, several of the 16 positive samples demonstrated reactivity against other nucleic acid–associated antigens, including Ro 52, La, CENP-B, Mi-2, and components of the Sm/RNP complex (Table 2). SAM identified increased reactivity against Ro 60 in sera positive for this assay (data not shown).
DISCUSSION
This is the first study to evaluate autoantibody reactivity against a large number of antigens in JDM using antigen microarrays. Using the stringent cutoff of MFI-B greater than 3 SD above the mean MFI-B in the control group to determine significant reactivity, we demonstrated an unexpectedly broad spectrum of autoantibodies in this JDM cohort. These included MSAs and MAAs, autoantibodies classically associated with SLE and other autoimmune diseases, as well as other autoantibodies not previously described in JDM. The frequency of MSAs in our cohort was similar to that found in previous studies, while our cohort had an increased frequency of certain MAAs, including anti-Ro, anti-La, and anti–U1 RNP (17). This likely reflects the increased sensitivity of the antigen microarrays compared to standard immunoassays such as ELISA (10).
Despite the presence of autoantibody reactivity in the majority of sera from patients with JDM, it is not surprising that SAM did not identify reactivity that distinguished the JDM cohort as a whole from the control subjects or that distinguished between ANA-positive and ANA-negative subgroups of JDM patients, due to the small percentage of patients with reactivity against any given antigen (3–17%) (Table 1). Interestingly, many of the autoantibodies identified were directed against nuclear antigens, and these reactivities could be the source of the positive ANAs without known specificity. However, these reactivities do not account for all 72% of the ANA positivity in this cohort, suggesting that additional nuclear antigens not present on our microarray are likely present in some patients with JDM. This is a known limitation of antigen microarray technology, which is a biased assay that only detects reactivity to the predetermined antigens printed on the microarray. In particular, the microarrays manufactured for this study did not include the p155/140 antigen that was recently identified in >20% of patients with JDM (18).
This study demonstrated that sera from some patients with JDM had the ability to induce IFNα activity or production in two in vitro assays (Figure 1). In fact, there was a correlation between the presence of antibodies against RNA-containing autoantigens and both serum IFNα activity and the capacity to generate interferogenic immune complexes. In particular, SAM identified Ro 52 and Ro 60 reactivity as being significantly associated with the ability of the sera to induce IFNα production in vitro, and Ro 52, Sm/RNP, and U1-C reactivity was associated with IFNα activity in the reporter cell assay. Consequently, antibodies to RNA-binding proteins seem to be critical elements in the process that leads to increased IFNα production in a subset of patients with JDM. However, there are some limitations of this study. For example, given that the family history of SLE is increased 5 times above background in children with JDM and is associated with increased IFNα activity (19), it is possible that unrecognized family linkages might be the source of the low levels of La, Ro 52, and Ro 60. Still, SAM did not identify an increased reactivity to any antigens when comparing JDM patients with a family history of SLE to those without a family history of SLE (data not shown).
In conclusion, a broad spectrum of autoantibodies is produced in patients with JDM, and sera with antibodies directed against RNA-containing autoantigens induce IFNα activity or production in vitro. We hypothesize that RNA-containing autoantigens, including Ro, La, Sm, and RNP, play a critical role in the activation of the type I IFN system in JDM and are an essential component of the autoimmune disease process. Further studies are needed to elucidate the mechanisms by which this activation contributes to the pathogenesis of JDM.
Supplementary Material
Acknowledgments
Grants and other financial supporters: Dr. Balboni's work was supported by the NIH (grant K08-AI-080945), The Stanford University Child Health Research Institute (Child Health Research Program Pilot Grant for Early Career Investigators) and the Arthritis Foundation (Postdoctoral Fellowship). Dr. Niewold's work was supported by the NIH (grants R01-AR-060861, K08-AI-083790, P30-DK-42086, NIAID Clinical Research Loan Repayment AI-071651, a CTSA Core Subsidy Grant and CTSA Pilot Grants UL1-RR-024999), the Lupus Research Institute (Novel Research Grant), and the Alliance for Lupus Research (Target Identification in Lupus Grant). Drs. Eloranta and Rönnblom's work was supported by the Swedish Research Council, the Swedish Society of Medicine, the Swedish Rheumatism Foundation, the Söderberg's Foundation, the King Gustaf V 80-year Foundation and COMBINE. Dr. Utz's work was supported by the Donald E. and Delia B. Baxter Foundation (Career Development Award), the NIH (grants 5 U19-AI-082719, 5 U19-AI-050864, 5 U19-AI-056363, 1 U19-AI-090019 and 4 U19-AI-090019, and the NHLBI Proteomics contract HHSN288201000034C), the Canadian Institutes of Health Research (grant 2 OR-92141), the Alliance for Lupus Research (grant 21858), the Ben May Trust, the Floren Family Trust, the European Union Seventh Framework Programme (FP7/2007-2013 grant 261382). Dr. Pachman's work was supported by the Cure JM Foundation and the NIH (grants R01-AR-48289 and R01-NR-012692.
REFERENCES
- 1.Feldman BM, Rider LG, Reed AM, Pachman LM. Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood. Lancet. 2008;371:2201–12. doi: 10.1016/S0140-6736(08)60955-1. [DOI] [PubMed] [Google Scholar]
- 2.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 3.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292:403–7. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 4.Pachman LM, Friedman JM, Maryjowski-Sweeney ML, Jonnason O, Radvany RM, Sharp GC, et al. Immunogenetic studies of juvenile dermatomyositis. III. Study of antibody to organ-specific and nuclear antigens. Arthritis Rheum. 1985;28:151–7. doi: 10.1002/art.1780280208. [DOI] [PubMed] [Google Scholar]
- 5.Tezak Z, Hoffman EP, Lutz JL, Fedczyna TO, Stephan D, Bremer EG, et al. Gene expression profiling in DQA1*0501+ children with untreated dermatomyositis: a novel model of pathogenesis. J Immunol. 2002;168:4154–63. doi: 10.4049/jimmunol.168.8.4154. [DOI] [PubMed] [Google Scholar]
- 6.Pascual V, Patel P, McVicker V, Abbott K, Gurhsahaney A, Pachman LM. Peripheral blood mononuclear cell gene expression profiles in children with juvenile dermatomyositis/polymyositis share type-1 interferon signatures with systemic lupus erythematosus but are distinct. Arthritis Rheum. 2006;54:S695–6. [Google Scholar]
- 7.Niewold TB, Kariuki SN, Morgan GA, Shrestha S, Pachman LM. Elevated serum interferon-alpha activity in juvenile dermatomyositis: associations with disease activity at diagnosis and after thirty-six months of therapy. Arthritis Rheum. 2009;60:1815–24. doi: 10.1002/art.24555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eloranta ML, Barbasso Helmers S, Ulfgren AK, Ronnblom L, Alm GV, Lundberg IE. A possible mechanism for endogenous activation of the type I interferon system in myositis patients with anti-Jo-1 or anti-Ro 52/anti-Ro 60 autoantibodies. Arthritis Rheum. 2007;56:3112–24. doi: 10.1002/art.22860. [DOI] [PubMed] [Google Scholar]
- 9.Balboni I, Chan SM, Kattah M, Tenenbaum JD, Butte AJ, Utz PJ. Multiplexed protein array platforms for analysis of autoimmune diseases. Annu Rev Immunol. 2006;24:391–418. doi: 10.1146/annurev.immunol.24.021605.090709. [DOI] [PubMed] [Google Scholar]
- 10.Robinson WH, DiGennaro C, Hueber W, Haab BB, Kamachi M, Dean EJ, et al. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat Med. 2002;8:295–301. doi: 10.1038/nm0302-295. [DOI] [PubMed] [Google Scholar]
- 11.Bode RK, Klein-Gitelman MS, Miller ML, Lechman TS, Pachman LM. Disease activity score for children with juvenile dermatomyositis: reliability and validity evidence. Arthritis Rheum. 2003;49:7–15. doi: 10.1002/art.10924. [DOI] [PubMed] [Google Scholar]
- 12.Targoff IN. Autoantibodies and their significance in myositis. Curr Rheumatol Rep. 2008;10:333–40. doi: 10.1007/s11926-008-0053-2. [DOI] [PubMed] [Google Scholar]
- 13.Balboni I, Limb C, Tenenbaum JD, Utz PJ. Evaluation of microarray surfaces and arraying parameters for autoantibody profiling. Proteomics. 2008;8:3443–9. doi: 10.1002/pmic.200800146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bave U, Alm GV, Ronnblom L. The combination of apoptotic U937 cells and lupus IgG is a potent IFN-alpha inducer. J Immunol. 2000;165:3519–26. doi: 10.4049/jimmunol.165.6.3519. [DOI] [PubMed] [Google Scholar]
- 16.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–21. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghirardello A, Zampieri S, Iaccarino L, Tarricone E, Bendo R, Gambari PF, et al. Anti-Mi-2 antibodies. Autoimmunity. 2005;38:79–83. doi: 10.1080/08916930400022681. [DOI] [PubMed] [Google Scholar]
- 18.Targoff IN, Mamyrova G, Trieu EP, Perurena O, Koneru B, O'Hanlon TP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54:3682–9. doi: 10.1002/art.22164. [DOI] [PubMed] [Google Scholar]
- 19.Niewold TB, Wu SC, Smith M, Morgan GA, Pachman LM. Familial aggregation of autoimmune disease in juvenile dermatomyositis. Pediatrics. 2011;127:e1239–46. doi: 10.1542/peds.2010-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


