Abstract
OBJECTIVES
To investigate the efficacy of a novel brain plasticity–based computerized cognitive training program in older adults and to evaluate the effect on untrained measures of memory and attention and participant-reported outcomes.
DESIGN
Multisite randomized controlled double-blind trial with two treatment groups.
SETTING
Communities in northern and southern California and Minnesota.
PARTICIPANTS
Community-dwelling adults aged 65 and older (N = 487) without a diagnosis of clinically significant cognitive impairment.
INTERVENTION
Participants were randomized to receive a broadly-available brain plasticity–based computerized cognitive training program (intervention) or a novelty- and intensity-matched general cognitive stimulation program modeling treatment as usual (active control). Duration of training was 1 hour per day, 5 days per week, for 8 weeks, for a total of 40 hours.
MEASUREMENTS
The primary outcome was a composite score calculated from six subtests of the Repeatable Battery for the Assessment of Neuropsychological Status that use the auditory modality (RBANS Auditory Memory/Attention). Secondary measures were derived from performance on the experimental program, standardized neuropsychological assessments of memory and attention, and participant-reported outcomes.
RESULTS
RBANS Auditory Memory/Attention improvement was significantly greater (P = .02) in the experimental group (3.9 points, 95% confidence interval (CI) = 2.7–5.1) than in the control group (1.8 points, 95% CI = 0.6–3.0). Multiple secondary measures of memory and attention showed significantly greater improvements in the experimental group (word list total score, word list delayed recall, digits backwards, letter–number sequencing; P < .05), as did the participant-reported outcome measure (P = .001). No advantage for the experimental group was seen in narrative memory.
CONCLUSION
The experimental program improved generalized measures of memory and attention more than an active control program.
Keywords: clinical trial, cognitive decline, computerized cognitive training, participant-reported outcomes, brain plasticity
Cognitive decline is associated with risk for functional decline, nursing home placement, and mortality.1–3 In older individuals, concerns about forgetfulness are widespread and are associated with depression and anxiety.4–6 Interventions that reliably improve cognitive function thus have the opportunity to substantially improve the health and quality of life of older individuals.
Two general approaches for maintaining or improving cognitive function in older adults have emerged. The first approach is focused on direct instruction of putatively useful strategies.7–12 Although improvement on cognitive tests is generally seen after direct strategy instruction, performance gains typically do not generalize beyond tasks corresponding directly to the strategies taught,13–15 and it is not clear that older adults continue to use learned strategies over time.15 As a result, strategy training programs have not been widely adopted.
A second approach is derived from studies in animals16 and humans17–20 that suggest that nonspecific cognitive stimulation reduces the risk of cognitive decline. This has led to the practice of encouraging older adults to engage in everyday cognitively stimulating activities,14,21,22 but the retrospective and observational designs of the human studies have led to difficulty interpreting the direction of causation between cognitive function and cognitively stimulating activities.22
Regardless of the design principles, large-scale randomized controlled trials of training programs that are broadly available for patient use are lacking, limiting the ability of physicians to make evidence-based recommendations to older adults experiencing cognitive decline.
In recent years, recognition of the importance of sensory system function to cognitive function has prompted the development of a novel approach for treating age-related cognitive decline. It has been proposed that age-related reductions in the quality of neural information flowing through peripheral and central sensory systems to cognitive systems contribute to age-related cognitive decline.23,24 Animal and human studies have demonstrated that the performance of sensory systems in the cerebral cortex can be substantially improved through intensive learning and practice and that plastic brain changes across networks of relevant cortical areas in the central nervous system mediate these improvements.25,26 Consequently, a cognitive training program designed to improve central sensory system function could potentially improve cognitive function in older adults.27
Results are reported from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) study, a large randomized controlled two-arm clinical trial using a broadly available cognitive training program (Brain Fitness Program, Posit Science, San Francisco, CA). The program is designed to improve the function of the auditory system through intensive brain plasticity–based learning and has shown promise in smaller-scale studies.27,28 The current study builds upon the earlier studies by broadening the outcome measures to include a positive control for task learning, more memory and attention measures, and participant-reported outcomes (PROs), as well as being powered to detect across group differences.
The primary objective was to evaluate the efficacy of this experimental treatment (ET) training program by comparing the magnitude of improvements on untrained measures of memory and attention between the ET training program and an active control (AC) training program that engaged learning processes but was not designed to improve auditory system function.
METHODS
Design
This was a multisite (Los Angeles, CA; Rochester, MN; San Francisco, CA) randomized controlled double-blind trial.
Participants
Inclusion criteria were aged 65 and older, Mini-Mental State Examination (MMSE29) score of 26 or greater, English fluency, and ability to make time commitment. Exclusion criteria were major neurological or psychiatric illness history, including any history of stroke, transient is-chemic attack, or traumatic brain injury; acetylcholinesterase inhibitor use; current substance abuse; significant communicative impairments; and concurrent enrollment in other studies. Recruitment took place through advertisements, flyers, direct mail, and presentations.
Procedures
Institutional review board approval and written participant consent describing the ET and AC programs were obtained. No reimbursement was offered, but computer equipment was provided to all participants at no cost during the training period. Interventions were self-administered at participants’ homes; assessments occurred in clinical offices. Participants completed 40 sessions (1 h/d, 5 d/wk, for 8 weeks).
Participants not adherent to the training regimen (completing < 10 sessions in the first month or skipping > 10 consecutive sessions thereafter), those who during the study no longer met the inclusion and exclusion criteria, or those voluntarily withdrawing consent were discontinued from the study. In all cases, their pretraining data were retained for the intention-to-treat (ITT) analysis.
Participants were given sequential study identification numbers and randomly assigned to an age-stratified treatment group (20% aged 65–69, 40% aged 70–79, 40% aged 80). A random sequence of ET and AC assignments within each age stratum was generated before study commencement. Sites requested randomization allocation through e-mail, and a single staff member fulfilled requests through concealed randomization allocation sequence administered. Randomization was blocked according to site and age.
During the initial visit, an unblinded trainer installed the computer and provided individualized instruction and pretraining as needed in the use of the equipment and training program for both groups. The trainers used standardized scripts to describe the rationales and benefits of both programs to maintain participant blinding. ET and AC training tasks were self-administered. Trainers contacted participants weekly to identify and resolve technical problems and record adverse events.
Participants and clinicians administering and scoring outcome measures were blinded. Effectiveness of blinding was evaluated by administering a posttraining questionnaire to compare ET and AC group self-reports of perceived change in cognitive function and comparing proportions of ET and AC participants who voluntarily withdrew consent.
Training Programs
Brain Plasticity–Based ET
The ET consisted of six computerized exercises designed to improve the speed and accuracy of auditory information processing. Exercises continuously adjusted difficulty to user performance to maintain an approximately 85% correct rate. Correct trials were rewarded with points and animations. Exercises contained stimulus sets spanning the acoustic organization of speech. The exercises included time order judgment of pairs of frequency-modulated sweeps, discrimination of confusable syllables, recognition of sequences of confusable syllables, matching pairs of confusable syllables, reconstruction of sequences of verbal instructions, and identification of details in a verbally presented story. During the initial stages of training in all exercises, all auditory stimuli were processed to exaggerate the rapid temporal transitions within the sounds by increasing their amplitude and stretching them in time. The goal of the processing was to increase the effectiveness by which these stimuli engage and drive plastic changes in brain systems that, in older adults, exhibit relatively poor temporal response properties.27 This exaggeration was gradually removed over the course of the training period such that, by the end of training, all auditory stimuli had temporal characteristics representative of real-world rapid speech. In each training session, a participant worked with four of the six exercises for 15 minutes per exercise. Adherence was monitored using electronic data upload after each training session.
Educational Training AC
AC training was required to have face validity; be consistent with common physician recommendations for cognitive stimulation; and match ET for training time, audiovisual presentation, and computer use. Thus the program employed a learning-based training approach in which participants used computers to view digital video disc (DVD)-based educational programs on history, art, and literature. Participants answered written quizzes after each training session that required the specific factual content knowledge presented by the DVD in that session. These quizzes served to ensure attention and learning during the training session and allowed quantitative measurement of compliance.
The trial design did not incorporate a no-contact control (NCC) condition based on comparisons of NCC and AC group auditory memory outcomes in previous work that showed equivalent cognitive improvements in NCC and AC groups.27,28
Pretraining Characterization Measures
Demographics (age, education, sex, ethnicity, first language), cognitive status (MMSE, estimated intelligence quotient (Wechsler Test of Adult Reading)30), depression (15-item Geriatric Depression Scale score31), and sensory functions (audiometric function, tinnitus, hearing aid, eyeglass use) were measured.
Outcome Measures
The primary outcome measure was derived from Repeatable Battery for the Assessment of Neuropsychological Status (RBANS32), a standardized neuropsychological assessment battery that is sensitive to mild cognitive deficits. Because the ET focused on improving auditory processing, the primary outcome measure (RBANS Auditory Memory/Attention) was derived from the six RBANS subtests that use orally presented speech as stimuli (list learning, story memory, digit span forward, delayed free list recall, delayed list recognition, delayed free story recall). Raw scores were converted to scaled scores based on look-up tables mapping normative RBANS population data to optimal Gaussian distributions. Delayed list recall and recognition were summed before scaling to allow inclusion of the skewed recognition data. The five equally weighted scaled scores were then summed and mapped to a composite index score (average = 100, standard deviation ± 15).
Because the RBANS may exhibit ceiling effects in highly functioning older adults,28 additional assessments of auditory memory and attention were used to provide further information about the robustness of generalization. Standardized published measures that met the following criteria were chosen: use of orally presented speech as stimuli, sensitivity to age-related cognitive decline, lack of test–retest effects through use of multiple forms or stimuli that are not remembered across assessment visits, and relevance to memory and attention. The measures used were Rey Auditory Verbal Learning Test (RAVLT33) total score (sum of trials 1–5) and word list delayed recall, Rivermead Behavioral Memory Test (RBMT34) immediate and delayed recall, and Wechsler Memory Scale (WMS-III35) letter-number sequencing (LNS) and digit span backwards tests. An overall composite score (Overall Memory) combining RAVLT total score and word list delayed recall, RBMT immediate and delayed recall, and LNS and digits backwards was derived as described for RBANS Auditory Memory/Attention; because of the lack of published co-normed data, standardization was based on the pretraining score distribution.
Secondary outcomes also included a directly trained measure of exercise performance derived from the ET processing speed exercise, as well as a pre–post PRO measure that assesses perceptions of cognitive abilities (Cognitive Self-Report Questionnaire, CSRQ-2536). The CSRQ-25 consists of 25 statements about cognition and mood in everyday life over the past 2 weeks, answered using a 5-point Likert scale. The CSRQ-25 was developed as a PRO, because existing PROs do not include questions relevant to cognitive training, are not appropriate for healthy older adult population, or were designed to measure cognitive impairment rather than be sensitive to improvement. The CSRQ-25 was validated using factor analysis on 207 healthy older adults before this study. Concurrent and divergent validity were established by examining correlations (P < .05) with subscales of the Life Satisfaction Scale (LSS),37 Cognitive Failures Questionnaire (CFQ),38 and Geriatric Depression Scale.31 Reliability measures include Cronbach alpha 0.91, Spearman-Brown split-half reliability 0.94, and 2-month test–retest reliability 0.85.
A posttraining questionnaire was used to assess the maintenance of participant blinding. The measure consisted of 64 statements addressing eight different cognitive performance abilities (e.g., recall) asking whether participants believed that they improved, remained the same, or worsened specifically because of being in the study. The sum across all questions was used as a measure of participants’ belief in the efficacy of their training arm.
Clinical assessors were trained using a standardized protocol, and their performance was monitored and corrected as necessary throughout the study. A second blinded assessor double-scored assessments.
Measures were collected at pre- and posttraining visits. Counterbalanced parallel forms of the RBANS, RAVLT, and RBMT were used to reduce the potential of test–retest effects.
Analysis
A predefined analysis plan specified sample size, the ITT population, and the statistical approach.
Sample size was calculated using data from smaller studies27,28 to detect an effect size of 0.25 at 80% power for the primary outcome measure. The ITT sample included all participants completing the initial training visit, including those discontinued for training nonadherence, dropped for the previously described inclusion or exclusion reasons, voluntarily withdrawn, lost to follow-up, or dead.
Individual linear mixed effects models were fit for the primary and each of the secondary outcomes measures. Missing data were accounted for using iterative full-information maximum likelihood estimation of relevant model parameters. This approach allows the use of the entire ITT group while optimally estimating treatment effects in the presence of missing data in a statistically unbiased manner. It is generally considered superior to simple imputation (e.g., last observation carry forward),39–41 and in the case of a complete data set is similar to a repeated-measures analysis of variance. For comparison of ET to AC in the ITT group, each model included treatment group and time as fixed factors and site as a random factor. An interaction term (training group × time) estimated the effect of cognitive training on outcome measure change.
To confirm that the statistical approach or missing data did not misrepresent the results, each outcome measure was also analyzed with an analysis of covariance (ANCOVA) of the pre- to posttraining difference score in the fully evaluable sample, using pretraining score as a covariate and training group as a factor. P-values from the training group × time interaction term (from the linear mixed model) or the training group factor (ANCOVA) were evaluated for significance. In all cases, the significance and effect sizes from the linear mixed model and analysis of covariance approach were similar (i.e., all outcome measures showing significance or nonsignificance in the linear mixed model analysis showed the same effect in the ANCOVA analysis). Data from the linear mixed models are reported in the Results section.
A single primary outcome measure (RBANS Memory/Attention) was predefined to conserve an overall alpha level of 0.05. No corrections for multiple comparisons were made on the secondary measures.
Program usage, assessed in terms of the number of hours spent in training, was compared across treatment groups using independent-samples t-tests. One-sample t-tests were used to test whether self-reports on the post-training questionnaire were significantly different from zero.
Adverse events (AEs) were collected at points of contact between study staff and participants and when volunteered by participants. A blinded medical monitor categorized events according to body system, severity, and training relatedness. The ET and AC groups were considered equivalent if the frequency of events were within 15 percentage points.
An independent data management contractor conducted analyses using SAS version 9.0 (SAS Institute Inc., Cary, NC) and SPSS version 15 (SPSS Inc., Chicago, IL).
RESULTS
Participants
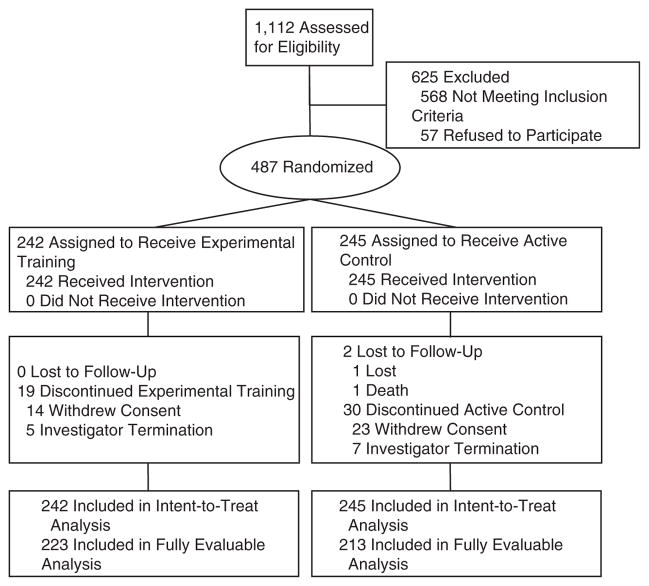
Of 1,112 individuals contacted, 487 (43.8%) were eligible, 568 (51.1%) were ineligible, and 57 (5.1%) refused participation (Figure 1). Recruitment was conducted from January 2006 to July 2007; the final participant completed posttraining assessment in November 2007.
Figure 1.
Flow of participants in Improvement in Memory with Plasticity-based Adaptive Cognitive Training Study.
The ITT sample consisted of 487 participants (ET = 242; AC = 245). Pretraining demographic, sensory, and overall cognitive function characteristics appear in Table 1. Pretraining performance on outcome measures is listed in Table 2. Except for sex (ET = 42.1% male, AC = 53.1% male, P = .02), there were no significant differences between the ET and AC samples.
Table 1.
Pretraining Data: Demographic, Cognitive, and Social Characteristics Including Baseline Outcomes Measures
| Measure | Experimental Treatment n = 242 | Active Control n = 245 |
|---|---|---|
| Demographic | ||
| Age, mean ± SD | 75.6 ± 6.6 | 75.0 ± 6.3 |
| Education, years mean ± SD | 15.7 ± 2.6 | 15.6 ± 2.6 |
| Male, n (%) | 102 (42.1) | 130 (53.1) |
| Caucasian, n (%) | 227 (93.8) | 234 (95.5) |
| First language English, n (%) | 238 (98.3) | 240 (98.0) |
| Cognitive | ||
| Mini-Mental State Examination score, mean ± SD (range 0–30) | 29.1 ± 1.1 | 29.2 ± 1.0 |
| Estimated intelligence quotient score, mean ± SD | 113.7 ± 8.0 | 113.6 ± 8.2 |
| 15-item Geriatric Depression Scale score, mean ± SD (range 0–15) | 1.3 ± 1.6 | 1.3 ± 1.7 |
| Sensory | ||
| Hearing function, 500 Hz mean ± SD | 27.0 ± 10.5 | 26.0 ± 10.4 |
| Tinnitus, n (%) | 46 (19.0) | 52 (21.2) |
| Hearing aid, n (%) | 39 (16.1) | 42 (17.1) |
| Glasses, n (%) | 229 (94.6) | 230 (93.9) |
t-tests were used for continuous variables and chi-square tests for categorical variables.
There were no significant differences between groups with the exception of sex, which was significantly different (P = .02).
SD = standard deviation.
Table 2.
Effect of Training on Primary and Secondary Outcome Measures in the Intention-to-Treat Group
| Measure | Experimental Training N = 242
|
Active Control N = 245
|
Change Difference (95% CI) | F Value (df) | P-Value† | Effect Size‡ | ||
|---|---|---|---|---|---|---|---|---|
| Baseline Mean ± SD (Range) | Change Mean (95% CI) | Baseline Mean ± SD (Range) | Change Mean (95% CI) | |||||
| Primary | ||||||||
|
| ||||||||
| Repeatable Battery for the Assessment of Neuropsychological Status Auditory Memory/Attention, index score | 96.0 ± 13.0 (53.0–122.0) | +3.9 (2.7 to 5.1) | 96.6 ± 12.7 (66.0–135.0) | +1.8 (0.6–3.0) | +2.1 (0.4–3.9) | 5.781 (1, 442.3) | .02 | 0.23 |
| Secondary | ||||||||
|
| ||||||||
| Exercise performance | ||||||||
|
| ||||||||
| Processing speed, ms* | 116 ± 84 (29–493) | − 68 (− 78 to − 58) | 117 ± 84 (25–500) | − 8 (− 16–0) | − 60 (− 72 to − 47) | 84.264 (1, 440.5) | < .001 | 0.87 |
|
| ||||||||
| Neuropsychological | ||||||||
|
| ||||||||
| Overall memory, index score | 99.6 ± 14.0 (53.0–138.0) | +4.2 (2.8–5.6) | 100.2 ± 15.9 (60.0–145.0) | +1.0 (− 0.4–2.4) | +3.2 (1.2–5.1) | 9.798 (1, 442.2) | .002 | 0.30 |
|
| ||||||||
| Rey Auditory Verbal Learning Test, raw score | ||||||||
|
| ||||||||
| Total‡ | 40.4 ± 9.6 (15.0–65.0) | +1.2 (0.2–2.2) | 41.2 ± 10.4 (15.0–70.0) | − 1.0 (− 2.0–0.0) | +2.2 (0.7–3.7) | 8.568 (1, 442.8) | .004 | 0.28 |
|
| ||||||||
| Word list delayed recall | 6.6 ± 3.5 (0.0–15.0) | +0.6 (0.2–1.0) | 6.9 ± 6.7 (0.0–15.0) | 0.0 (− 0.4–0.4) | +0.6 (0.0–1.2) | 4.415 (1, 448.2) | .04 | 0.20 |
|
| ||||||||
| Rivermead Behavioral Memory Test, raw score | ||||||||
|
| ||||||||
| Immediate recall | 7.9 ± 3.1 (1.0–17.0) | +0.6 (0.1–1.1) | 7.9 ± 3.3 (1.0–17.0) | +0.5 (0.0–1.0) | +0.1 (− 0.5–0.7) | 0.080 (1, 462.6) | .78 | 0.03 |
|
| ||||||||
| Delayed recall | 6.4 ± 3.1 (0.0–16.5) | +0.7 (0.2–1.2) | 6.6 ± 3.3 (0.0–17.5) | +0.6 (0.2–1.0) | +0.1 (− 0.5–0.7) | 0.263 (1, 458.5) | .61 | 0.05 |
|
| ||||||||
| Wechsler Memory Scale, raw score | ||||||||
|
| ||||||||
| Digit span backwards | 7.3 ± 2.2 (2.0–14.0) | +0.6 (0.4–0.8) | 7.2 ± 2.3 (2.0–14.0) | +0.1 (− 0.1–0.3) | +0.5 (0.2–0.8) | 7.528 (1, 450.7) | .006 | 0.26 |
|
| ||||||||
| Letter-number sequencing | 9.6 ± 2.4 (2.0–16.0) | +0.6 (0.3–0.9) | 9.6 ± 2.7 (0.0–17.0) | +0.2 (− 0.1–0.5) | +0.4 (0.0–0.8) | 5.871 (1, 448.1) | .02 | 0.23 |
|
| ||||||||
| Participant-reported outcome | ||||||||
|
| ||||||||
| Cognitive Self-Report Questionnaire-25 total, raw score | 2.226 ± 0.408 (1.300–3.300) | − 0.069 (− 0.108 to − 0.030) | 2.213 ± 0.460 (0.80–3.200) | +0.025 (− 0.014–0.064) | − 0.094 (− 0.153 to − 0.035) | 11.865 (1, 445.1) | .001 | 0.33 |
Lower scores represent better performance.
P-value from training group (experimental treatment (ET) vs active control (AC))-by-time interaction.
Effect size defined as Cohen d of the training group (ET vs AC)-by-time interaction.
SD = standard deviation; CI = confidence interval.
Noncompletion rates (training nonadherence, investigator drop for inclusion or exclusion reasons, voluntarily withdrawn, lost to follow-up, or dead) were not statistically different between the ET and AC groups (drop or withdrawal: ET = 19, 7.9%; AC = 32, 13.1%; P = .06, chi-square test), although there was a trend toward greater noncompletion in the AC group. There were no drops or withdrawals due to participant inability to learn to self-administer the ET or AC training programs. At baseline, the ET and AC noncompletion group was not significantly different in terms of demographic or sensory characteristics from the group that completed posttraining testing but scored significantly lower on cognitive function measures, including the MMSE (29.2 vs 28.6, P = .001).
Training Effects on Outcome Measures (ITT Sample)
The ET and AC difference in sex was corrected for by including a fixed factor for sex in all statistical analyses. The fixed sex factor did not significantly affect results, nor was sex found to interact with training effects. All other pre-training characteristics were equivalent across groups and were thus not included in the statistical analyses. Change scores, significance, and effect sizes (Cohen d) for ET and AC comparisons are reported in Table 2. The results presented below and in Table 2 pertain to training group (ET vs AC)-by-time interactions. For significant effects, the interaction indicates that training benefits were greater in the ET group than in the AC group.
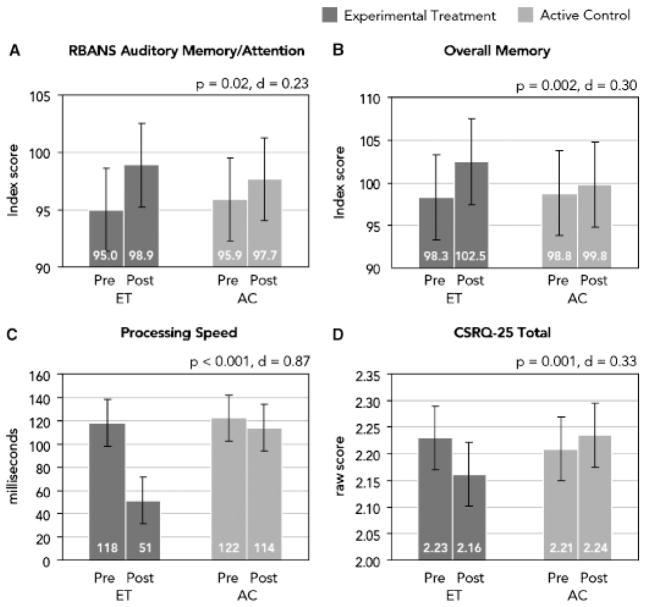
There was a significant effect (P = .02) on the primary outcome measure (RBANS Auditory Memory/Attention), indicating greater improvement in the ET group. Performance in the ET and AC groups improved by 3.9 and 1.8 index score units, respectively, yielding an effect size of 0.23.
Significant effects favored the ET group on the directly trained performance measure (P < .001; ET = − 68 and AC = − 8 ms, d = 0.87). On the untrained measures of memory and attention, there were significant effects favoring the ET group for overall memory (P = .002; +4.2 and +1.0 index score units, d = 0.30), digit span backwards (P = .006; +0.6 and +0.1 digits, d = 0.26), LNS (P = .02; +0.6 and +0.2 items, d = 0.23), RAVLT total (P = .004; +1.2 and − 1.0 raw score units, d = 0.28), and RAVLT word list delayed recall (P = .04; +0.6 and 0.0 words, d = 0.20). No significant differences were observed for RBMT immediate (P = .78, d = 0.03) and delayed (P = .61, d = 0.05) recall. Significant effects favored the ET group for the PRO measure (P = .001; − 0.069 and +0.025 raw score units, d = 0.33).
Figure 2 shows pre and post scores according to group for RBANS Auditory Memory/Attention, processing speed, overall memory, and the CSRQ-25 total.
Figure 2.
Pretraining and posttraining estimated means with 95% confidence intervals in the intention-to-treat group. For each outcome measure, the P-value and Cohen d effect size estimate is from the training group (experimental treatment (ET) vs active control (AC)) × time interaction, corrected for the significantly different sex distribution between groups. In (A) Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) and (B) overall memory, higher scores are better; in (C) processing speed and (D) Cognitive Self-Report Questionnaire-25 Total, lower scores are better.
To further characterize the results, reliable change score analysis was performed. A pre–posttraining change score criterion of +0.2 standard deviations of the pretraining scores was used to identify participants in the fully evaluable group showing reliable changes.15 Consistent with the results from the primary analyses of the outcomes measures as continuous variables, the analyses demonstrated that a larger percentage of participants in the ET group showed changes on each outcome measure than in the AC group (Table 3).
Table 3.
Percent of Participants Showing Reliable Improvement in the Fully Evaluable Group (0.2 Standard Deviations of the Mean Criterion for Reliable Improvement)
| Measures | Experimental Training n = 223 | Active Control n = 213 |
|---|---|---|
| % | ||
| Primary | ||
| Repeatable Battery for the Assessment of Neuropsychological Status Auditory Memory/Attention, index score | 56 | 43 |
| Secondary | ||
| Exercise performance | ||
| Processing speed, ms | 79 | 32 |
| Neuropsychological | ||
| Overall memory, index score | 57 | 46 |
| Rey Auditory Verbal Learning Test, raw score | ||
| Total score | 48 | 36 |
| Word list delayed recall | 51 | 39 |
| Rivermead Behavioral Memory Test, raw score | ||
| Immediate recall | 51 | 45 |
| Delayed recall | 50 | 46 |
| Wechsler Memory Scale III, raw score | ||
| Digits backwards | 49 | 42 |
| Letter-number sequencing | 48 | 43 |
| Participant-reported outcome | ||
| Cognitive Self-Report Questionnaire-25, total, raw score | 48 | 40 |
The effectiveness of the participant blind was evaluated in two ways. First, the posttraining questionnaire was analyzed as an indicator of participant belief in the effectiveness of training. Second, the rate of participant voluntary withdrawal of consent over the course of training was used as an indicator that participants did not believe the training was benefiting them. In the first analysis, both groups self-rated themselves as having improved cognitive function (both one-sample t-tests significantly different than 0, P < .001). For the second analysis, rates of participant voluntary withdrawal of consent were not statistically different between the ET (n = 14, 5.9%) and AC (n = 23, 9.7%) groups (P = .12, chi-square test).
Adverse Events
Of the 81 training-related AEs (77% mild, 22% moderate, 1% severe), 34 related to physical symptoms (musculoskeletal pain, fatigue, headache; ET = 19 incidents, AC = 15), 28 related to psychological symptoms (e.g., anxiety, boredom, depressed mood; ET = 15, AC = 13), and 19 related to frustration (ET = 16, AC = 3). Only in the frustration category was there greater than a 15 percentage point difference between the ET and AC groups.
DISCUSSION
The IMPACT study is the first large-scale randomized controlled clinical trial of a broadly available cognitive training program with older adults to show generalization of performance gains to untrained standardized measures of memory and attention.
Significant improvement favoring the ET group on a performance measure directly related to the trained tasks was expected and was consistent with the generally large effect sizes seen on directly trained tasks in other training programs.42,43 Unique to this study, performance improvements generalized to untrained standardized measures of memory and attention, implying that robust gains occurred across systems serving auditory-based cognition. Moreover, self-reported improvements by participants suggest that the changes may be behaviorally significant.
Characteristics of the IMPACT study design and results suggest that it is most likely that the specific approach taken in the brain plasticity–based training program account for the significantly greater improvements in performance observed for the ET group. First, the AC program mimicked recommendations for mental stimulation often made by healthcare providers, offered the real possibility of performance improvements on the outcome measures. This intentional incorporation of a learning-based AC program instead of an inactive placebo control set a high criterion for measuring success. Second, self-reports of posttraining benefit and participant voluntary withdrawal of consent rates were similar for the ET and AC groups, suggesting that the blinding procedures used were effective and, thus, that placebo effects were equivalent in both groups. In addition, although it is always possible in cognitive training studies that observed performance gains in the experimental group represent improved test-taking skills resulting from test-taking practice, this is an unlikely explanation in the IMPACT study. AC included paper-based written quizzes after each training session, whereas ET did not; thus, AC provided at least equal opportunities as the ET for developing the paper-based test-taking skills used during the neuropsychological assessment. Finally, it is unlikely that novelty of computer use, contact with staff, time spent being cognitively active, or nonspecific cognitive stimulation can explain the improvements in cognitive function, because these factors were all matched between the ET and AC groups.
The largest study of cognitive training in older adults (the Advanced Cognitive Training for Independent and Vital Elderly Study44) included a speed-of-processing training exercise sharing certain design principles with ET exercises in IMPACT, notably intensive practice, focus on perceptual speed and accuracy, use of adaptive algorithms, and emphasis on attention and reward. Training with this exercise showed a large effect size on the directly trained outcome measures43 and less risk of serious decline in health-related quality of life at follow-up visits.45,46 Additional studies have shown generalization of improvement to directly observed functional measures.47–50 Collectively, these results suggest that training programs incorporating intensive practice, focus on perceptual speed and accuracy, use of adaptive algorithms, and emphasis on attention and reward may represent a promising class of cognitive training approaches that will exhibit generalization and thus may be effective at countering age-related cognitive decline.
The magnitude of the effect sizes suggests that the results are clinically significant. Draft guidelines from the American Psychological Association15 have defined a 0.20 effect size as the threshold for clinical significance. In addition to the directly trained performance measure, seven of the nine generalized outcomes measures show a statistically significant effect size of 0.20 or larger (range 0.20–0.33) in the ITT population.
Study demographics (primarily Caucasian and well educated) limit the interpretation of data from IMPACT. In addition, no measures of executive function or overall functional status were included. It would clarify the clinical utility of the training to expand the assessment battery in future studies. Given the promise of this training approach, it would also be of interest to expand the training program to target executive functioning and functional status directly. Future analyses should also determine whether performance gains are maintained over time, which would provide evidence that the training produces plastic brain changes. Additionally, further research to explore the underlying mechanism of action contributing to improvements ascribed to the ET group through physiological mechanism such as functional magnetic resonance imaging may be warranted. Finally, the possibility cannot be excluded that the AC training program could show superior effects on other measures not employed in this study.
These results demonstrate that a cognitive training program designed to improve the speed and accuracy of central auditory system function while strongly engaging neuromodulatory systems can drive benefits that generalize to untrained measures of memory and attention and that the improvement is significantly larger than that seen with a program of general cognitive stimulation. Future research should evaluate the sensitivity of the program to preclinical cognitive decline and the effects of the experimental approach on the trajectory of cognitive decline in normal aging and for clinical disorders in which cognitive enhancement could yield improvements in patient outcomes.
Acknowledgments
The principal investigators wish to thank research staff members for their contributions in support of this study: Kimberly Baily, MS, Donna Felmlee, MS, Sherrie Hanna, MA, LLP, Tascha Helland, and Andrea Hillson-Jensen at the Mayo Clinic and Jennifer Dave, Caesar Gonzalez, Patricia Hanisee, William Mullane, Yohance Pickett, Sumera Raoof, MD, and Marissa R. Smith, PsyD, at the University of Southern California. We also wish to thank independent biostatistician Kevin DeLucci, PhD, of the University of California at San Francisco and Erika Jones, Natalie La-Boube, Andrea Roudebush, Jennifer Scroggins, and Sheila Stuteville of Quintiles Transnational Corp., who were responsible for the independent data analysis management and analyses reported in this study. Finally, we acknowledge the contributions of the administrative staff at Posit Science: Omar Ahsanuddin, Chuck Armstrong, Sharona Atkins, Soussan Behbahani, Natasha Belfor, PhD, Ilya Bezdezhskiy, Albert Boniske, Jennifer Borrow, Anne Bruce, PhD, Bonnie Connor, PhD, Jill Damon, PhD, Nicholas Joyce, Sarah Kim, MA, Molly Kluse, Ma’ayan Lieberman, Wasiem Mansour, Marissa Matthews, Todd McManus, PhD, Karen McWhirter, Dean Mengaz, Jason Minow, PhD, John Motzinger, Connor O’Sullivan, Cori Pansarasa, PhD, Wanda Rieman, MA, Brian Song, Laila Spina, PsyD, Cate Stasio, Daniel Tinker, Teresa Urquhart, Rudy Walter, Amy Walthall, PsyD, Lauren Wholey, Rick Wood, Jessica Young; and Kannan Raghavan of Research Pharmaceutical Services.
Footnotes
Portions of this research were presented as a poster presentation at the 60th Annual Scientific Meeting of the Gerontological Society of America, November 16–20, 2007, San Francisco, CA; 36th Annual Scientific Meeting of the International Neuropsychological Society, February 6–9, 2008, Waikoloa, HI; Annual Meeting of the American Academy of Neurology, April 12–19, 2008, Chicago, IL; 2008 American Geriatrics Society Annual Scientific Meeting (Encore), April 30–May 5, 2008, Washington, DC; and 6th International Conference of the International Society for Gerotechnology, June 4–6, 2008, Pisa, Italy.
Sponsor’s Role: Posit Science Corporation was the sponsor of this trial. The sponsor is the developer of the experimental training program (Brain Fitness) used in this study. Posit Science Corporation holds the patent for and a proprietary interest in this software. Henry W. Mahncke, PhD, was a contributor to the design and conduct of this study. Dr. Mahncke is an employee of and holds stock options in Posit Science Corporation, the developer of the experimental training program. Ultimate responsibility for the design and conduct of the trial resided with the co-principal investigators, Drs. Glenn Smith and Elizabeth Zelinski. Neither Dr. Smith nor Dr. Zelinski are employees of or hold equity in posit Science Corporation. In addition, the sponsor contracted with Quintiles Transnational Corp, and independent biostatistician Kevin DeLucci, PhD, of the University of California, San Francisco, to provide the independent data management and analyses reported in this study.
Conflict of Interest: The IMPACT Study was funded by Posit Science Corporation through research grants to Mayo Clinic Foundation and the University of Southern California. Patricia Housen received compensation from Posit Science for consulting services. Kristine Yaffe received compensation from Posit Science for consulting services and honoraria for speaking engagements. Ronald Ruff received compensation from Posit Science for consulting services. Henry W. Mahncke is an employee of and holds stock options in Posit Science Corporation, the developer of the experimental training program.
Author Contributions: Glenn E. Smith helped develop the study concept and design; assisted with the acquisition, statistical analysis and interpretation of data; with the drafting of the manuscript; with obtaining funding; and with study supervision. He also contributed to the critical revision of the manuscript for important intellectual content. Patricia Housen assisted with the statistical analysis and interpretation of data and with drafting of the manuscript; provided administrative, technical, or material support; and contributed to the critical revision of the manuscript for important intellectual content. Kristine Yaffe assisted with the statistical analysis and interpretation of the data and contributed to the critical revision of the manuscript for important intellectual content. Ronald Ruff helped develop the study concept and design, assisted with the statistical analysis and interpretation of the data, and contributed to the critical revision of the manuscript for important intellectual content. Robert F. Kennison assisted with the statistical analysis and interpretation of the data and contributed to the critical revision of the manuscript for important intellectual content. Henry W. Mahncke assisted with the statistical analysis and interpretation of the data and with study supervision; provided administrative, technical or material support; and contributed to the critical revision of the manuscript for important intellectual content. Elizabeth M. Zelinski helped develop the study concept and design; assisted with the acquisition, statistical analysis, and interpretation of data; drafting of the manuscript; obtaining funding; and study supervision. She also contributed to the critical revision of the manuscript for important intellectual content.
References
- 1.Sands LP, Yaffe K, Lui LY, et al. The effects of acute illness on ADL decline over 1 year in frail older adults with and without cognitive impairment. J Gerontol B Biol Sci Med Sci. 2002;57:M449–M454. doi: 10.1093/gerona/57.7.m449. [DOI] [PubMed] [Google Scholar]
- 2.Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287:2090–2097. doi: 10.1001/jama.287.16.2090. [DOI] [PubMed] [Google Scholar]
- 3.Yaffe K, Petersen RC, Lindquist K, et al. Subtype of mild cognitive impairment and progression to dementia and death. Demen Geriatr Cogn Disord. 2006;22:312–319. doi: 10.1159/000095427. [DOI] [PubMed] [Google Scholar]
- 4.Reese CM, Cherry KE, Norris LE. Practical memory concerns of older adults. J Clin Geropsychol. 1999;5:231–244. [Google Scholar]
- 5.Zelinski EM, Gilewski MJ. A 10-item Rasch modeled memory self-efficacy scale. Aging Ment Health. 2004;8:293–306. doi: 10.1080/13607860410001709665. [DOI] [PubMed] [Google Scholar]
- 6.Mol M, Carpay M, Ramakers I, et al. The effect of perceived forgetfulness on quality of life in older adults; a qualitative review. Int J Geriatr Psychiatry. 2007;22:393–400. doi: 10.1002/gps.1686. [DOI] [PubMed] [Google Scholar]
- 7.Naveh-Benjamin M, Brav TK, Levy O. The associative memory deficit of older adults: The role of strategy utilization. Psychol Aging. 2007;22:202–208. doi: 10.1037/0882-7974.22.1.202. [DOI] [PubMed] [Google Scholar]
- 8.Derwinger A, Stigsdotter Neely A, Bäckman L. Design your own memory strategies! Self-generated strategy training versus mnemonic training in old age: An 8-month follow-up. Neuropsychol Rehab. 2005;15:37–54. doi: 10.1080/09602010343000336. [DOI] [PubMed] [Google Scholar]
- 9.McDougall GJ., Jr Cognitive interventions among older adults. Annu Rev Nurs Res. 1999;17:219–240. [PMC free article] [PubMed] [Google Scholar]
- 10.Rebok GW, Balcerak LJ. Memory self-efficacy and performance differences in young and old adults—The effect of mnemonic training. Dev Psychol. 1989;25:714–721. [Google Scholar]
- 11.Willis SL, Nesselroade CS. Long-term effects of fluid ability training in old-old age. Dev Psychol. 1990;26:905–910. [Google Scholar]
- 12.O’Hara R, Brooks JO, Friedman L, et al. Long-term effects of mnemonic training in community-dwelling older adults. J of Psychiatr Res. 2007;41:585–590. doi: 10.1016/j.jpsychires.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Verhaeghen P, Marcoen A, Goossens L. Improving memory performance in the aged through mnemonic training: A meta-analytic study. Psychol Aging. 1992;7:242–251. doi: 10.1037//0882-7974.7.2.242. [DOI] [PubMed] [Google Scholar]
- 14.Fillit HM, Butler RN, O’Connell AW, et al. Achieving and maintaining cognitive vitality with aging. Mayo Clin Proc. 2002;77:681–696. doi: 10.4065/77.7.681. [DOI] [PubMed] [Google Scholar]
- 15.Rebok GW, Carlson MC, Langbaum JBS. Training and maintaining memory abilities in healthy older adults: Traditional and novel approaches. J Gerontol B Biol Sci Med Sci. 2007;62:53–61. doi: 10.1093/geronb/62.special_issue_1.53. [DOI] [PubMed] [Google Scholar]
- 16.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 17.Scarmeas N, Levy G, Tang MX, et al. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001;57:2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59:1910–1914. doi: 10.1212/01.wnl.0000036905.59156.a1. [DOI] [PubMed] [Google Scholar]
- 19.Wilson RS, Scherr PA, Schneider JA, et al. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69:1911–1920. doi: 10.1212/01.wnl.0000271087.67782.cb. [DOI] [PubMed] [Google Scholar]
- 20.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 21.Small GW. What we need to know about age related memory loss. BMJ. 2002;324:1502–1505. doi: 10.1136/bmj.324.7352.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hultsch DF, Hertzog C, Small BJ, et al. Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychol Aging. 1999;14:245–263. doi: 10.1037//0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- 23.Schneider BA, Pichora-Fuller MK. Implications of perceptual deterioration for cognitive aging research. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. pp. 155–219. [Google Scholar]
- 24.Wingfield A, Stine-Morrow EAL. Language and speech. In: Craik FIM, Salt-house TA, editors. Handbook of Aging and Cogntion. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. pp. 359–416. [Google Scholar]
- 25.Gilbert CD, Sigman M, Crist RE. The neural basis of perceptual learning. Neuron. 2001;31:681–697. doi: 10.1016/s0896-6273(01)00424-x. [DOI] [PubMed] [Google Scholar]
- 26.Buonomano DV, Merzenich MM. Cortical plasticity: From synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 27.Mahncke HW, Bronstone A, Merzenich MM. Brain plasticity and functional losses in the aged: Scientific bases for a novel intervention. Prog Brain Res. 2006;157:81–109. doi: 10.1016/S0079-6123(06)57006-2. [DOI] [PubMed] [Google Scholar]
- 28.Mahncke HW, Connor BB, Appelman J, et al. Memory enhancement in healthy older adults using a brain plasticity-based training program: A randomized, controlled study. Proc Natl Acad Sci USA. 2006;103:12523–12528. doi: 10.1073/pnas.0605194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.Wechsler D. Wechsler Test of Adult Reading Manual. San Antonio, TX: Harcourt Assessment; 2001. [Google Scholar]
- 31.Sheikh J, Yesavage J. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. In: Brink TL, editor. Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press; 1986. pp. 165–173. [Google Scholar]
- 32.Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status. San Antonio, TX: Psychological Corporation; 1998. [Google Scholar]
- 33.Schmidt M. Rey Auditory and Verbal Learning Test: A Handbook. Los Angeles, CA: Western Psychological Services; 1996. [Google Scholar]
- 34.Wilson B, Cockburn J, Baddeley A, et al. The Rivermead Behavioral Memory Test—II Supplement Two. San Antonio, TX: Harcourt Assessment; 2003. [Google Scholar]
- 35.Wechsler D. Wechsler Memory Scale-III. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 36.Spina LMR, Ruff RM, Mahncke HW. Cognitive Self-Report Questionnaire (CSRQ) Manual. San Francisco, CA: Posit Science Corporation; 2006. [Google Scholar]
- 37.Salamon MJ. Manual for the Life Satisfaction Scale (LSS): Formerly the Life Satisfaction in the Elderly Scale (LSES) Hewlett, NY: Adult Development Center; 2003. [Google Scholar]
- 38.Broadbent DE, Cooper PF, FitzGerald P, et al. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982;21(Pt 1):1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 39.Beunckens C, Molenberghs G, Kenward MG. Direct likelihood analysis versus simple forms of imputation for missing data in randomized clinical trials. Clin Trials. 2005;2:379–386. doi: 10.1191/1740774505cn119oa. [DOI] [PubMed] [Google Scholar]
- 40.Mallinckrodt CH, Clark WS, Carroll RJ, et al. Assessing Response Profiles from Incomplete Longitudinal Clinical Trial Data Under Regulatory Considerations. J Biopharm Stat. 2003;13:179–190. doi: 10.1081/BIP-120019265. [DOI] [PubMed] [Google Scholar]
- 41.Mallinckrodt CH, Watkin JG, Molenberghs G. Choice of the primary analysis in longitudinal clinical trials. Pharm Stat. 2004;3:161–169. [Google Scholar]
- 42.Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ball K, Edwards JD, Ross LA. The impact of speed of processing training on cognitive and everyday functions. J Gerontol B Psychol Sci Soc Sci. 2007;62(Spec No 1):19–31. doi: 10.1093/geronb/62.special_issue_1.19. [DOI] [PubMed] [Google Scholar]
- 44.Jobe JB, Smith DM, Ball K, et al. ACTIVE: A cognitive intervention trial to promote independence in older adults. Controll Clin Trials. 2001;22:453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolinsky FD, Unverzagt FW, Smith DM, et al. The ACTIVE cognitive training trial and health-related quality of life: Protection that lasts for 5 years. J Gerontol A Biol Sci Med Sci. 2006;61:1324–1329. doi: 10.1093/gerona/61.12.1324. [DOI] [PubMed] [Google Scholar]
- 46.Wolinsky FD, Unverzagt FW, Smith DM, et al. The effects of the ACTIVE cognitive training trial on clinically relevant declines in health-related quality of life. J Gerontol B Psychol Sci Soc Sci. 2006;61:S281–S287. doi: 10.1093/geronb/61.5.s281. [DOI] [PubMed] [Google Scholar]
- 47.Edwards JD, Wadley VG, Myers RS. Transfer of a speed of processing intervention to near and far cognitive functions. Gerontology. 2002;48:329–340. doi: 10.1159/000065259. [DOI] [PubMed] [Google Scholar]
- 48.Edwards JD, Wadley VG, Vance DE. The impact of speed of processing training on cognitive and everyday performance. Aging Ment Health. 2005;9:262–271. doi: 10.1080/13607860412331336788. [DOI] [PubMed] [Google Scholar]
- 49.Roenker DL, Cissell GM, Ball KK, et al. Speed-of-processing and driving simulator training result in improved driving performance. Hum Factors. 2003;45:218–233. doi: 10.1518/hfes.45.2.218.27241. [DOI] [PubMed] [Google Scholar]
- 50.Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]