Abstract
Atherosclerosis develops preferentially at branches and curvatures of the arterial tree, where blood flow pattern is disturbed rather than being laminar, and wall shear stress has an irregular distribution without defined directions. The endothelium in the atherosusceptible regions, in comparison to that in atheroresistant regions, shows activation of pro-proliferative and pro-inflammatory gene expressions, reduced production of nitric oxide (NO), increased leukocyte adhesion and permeability, as well as other atheroprone phenotypes. Differences in gene expressions and cell phenotypes have been detected in endothelia residing in native atherosusceptible and atheroresistant regions of the arteries, or in arteries from animal models with artificial creation of disturbed flow. Similar results have also been shown in in vitro systems that apply controlled shear stresses with or without clear directions to cultured endothelial cells (ECs) in fluid-dynamically designed flow-loading devices. The available evidence indicates that the coordination of multiple signaling networks, rather than individual separate pathways, link the mechanical signals to specific genetic circuitries in orchestrating the mechanoresponsive networks to evoke comprehensive genetic and functional responses.
Keywords: Shear stress, Mechanotransduction, Endothelial phenotype, Atherogenesis
1. Introduction
The interactions of blood flow with complex vessel geometry generate hemodynamic characteristics, including the heterogeneous spatial and temporal mechanical forces acting on the vessel wall. Vascular endothelial cells (ECs) covering the inner surface of blood vessels are constantly exposed to shear stress due to the frictional force created by the blood flow. ECs respond to the changes of local shear stress to modulate intracellular signaling, which leads to alterations of gene expression, cell morphology, and structural remodeling1, 2.
The correlation of atheroma location and ‘site-specific’ endothelial dysfunctional phenotype with the non-laminar characteristics of blood flow and shear stress at curved regions, branch points, and bifurcations indicates that such disturbed flow patterns are atheroprone. At these atheroprone regions, the flow departs from pulsatile, unidirectional shear stress to create flow-separation zones that include flow reversal, oscillating shear stress and occasional turbulence3. In contrast, the shear stress in the straight atheroresistant part of the arteries is pulsatile and has well-defined directions. The shear stresses resulting from different flow patterns initiate differential steps of signaling events in the endothelium, including mechanosensing, intracellular transmission of stress, conversion of mechanical force to biochemical signals, and feedback mechanisms3. As a result, the endothelium develops different adaptive phenotypes to differentially react with other coexistent risk factors, such as high cholesterol, hypertension, obesity, diabetes, and smoking, to contribute to the site-specific susceptibility for the initiation and progression of atherosclerosis. The aim of this review is to provide a brief summary of the current knowledge with a focus on the shear stress-initiated signaling and its regulation of endothelial function and dysfunction, as well as the communication between ECs and other vascular cells. The pathologic implications in atherosclerosis and future perspectives are also discussed. The effects of fluid shear stress on endothelial gene expression and the functional consequences have been previously reviewed in depth (e.g., by Chiu and Chien4 and Davies et al2).
2. Endothelial phenotypes in atherosusceptible regions in vivo
2.1 Endothelial phenotypes in native atherosusceptible regions
The earliest atherosclerotic lesions characteristically develop with focal patterns (i.e., preferentially at branches and curves of the arterial tree) where the local blood flow is disturbed4. The endothelium lining these atherosuscepetible regions have 1) increased permeability to plasma macromolecules, 2) increased turnover (proliferation and apoptosis), and 3) increased adhesiveness for monocytes that attach and migrate into the arterial wall, with subsequent alterations in EC morphology and structure4. Changes in expression or activation of signaling and functional molecules have been observed in the endothelium of atherosclerotic plaques or atherosusceptible regions (e.g., inner curvatures of aortic arch or carotid bifurcations) as compared with non-lesion regions or the straight segments (e.g., the descending thoracic aorta). Examples of molecules involved include the vascular factors related to homeostasis: endothelial nitric oxide synthase (eNOS)5, NF-E2-related factor 2 (Nrf2)6, Kruppel-like factor 2 (KLF2)7, pregnane X receptor (PXR)8, AMP-activated protein kinases (AMPKs)9, microRNA(miR)-10a10, angiopoietin-211, as well as other factors related to stress-responses: platelet-derived growth factors (PDGFs) and their receptors12, early growth response protein 1 (Egr-1)13, nuclear factor-κB (NF-κB)14-16, toll-like receptors (TLRs)17, p21-activated kinases (PAK)18, SHC (Src homology 2 domain containing) transforming protein 1 (Shc)19, c-Jun N-terminal kinase (JNK)20, x-box binding protein 1 (XBP-1)21, histone deacetylase 3 (HDAC3)22, bone morphogenetic protein-2/-4 (BMP2/4)23, 24, Smad1/525, monocyte chemoattractant protein-1 (MCP-1)26, intercellular adhesion molecule 1 (ICAM-1)27-29, 30, vascular cell adhesion protein 1 (VCAM-1)28-30, and endothelial leukocyte adhesion molecule 1 (E-selectin)27.
2.2 Endothelial phenotypes in experimental models of disturbed flow in vivo
Several animal models have been created to study the effects of disturbed flow with oscillatory and low shear stress on endothelial phenotypic responses. The strategies commonly employed to create disturbed flow are the following:
The introduction of a local stenosis by constricting a segment of a large vessel, such as the carotid artery or abdominal aorta of mice, rats or rabbits31-33. While ECs are well aligned and elongated in the direction of flow at the stenosis throat, where the flow is laminar with a relatively high shear stress, the cells immediately downstream to the stenosis throat are rounded and their proliferation is significantly increased25 in this post-stenotic region with flow separation and low-velocity recirculation31, 32. The vascular endothelial (VE)-cadherin in endothelial adherens junctions34 and the endothelial-protective KLF27 are highly expressed in ECs in regions with laminar flow, but not in the downstream regions with disturbed flow. In addition, bone morphogenetic protein receptor (BMPR)-specific Smad1/5 are found to be highly phosphorylated in ECs at poststenotic sites to cause cell cycle progression and cell proliferation25.
The partial ligation of a carotid artery of rats or mice. In this model, the three branches (external carotid, internal carotid, and occipital) of one carotid artery are ligated and the superior thyroid artery is left intact, resulting in low and oscillating shear stress in the common carotid artery of the operated side35. The partial ligation down-regulates KLF2 and eNOS while up-regulating ICAM-1, VCAM-1, and BMP4, and it impairs endothelium-dependent vasorelaxation and induces atherosclerosis in ApoE-/- mice fed a high-fat diet 35.
The creation of an arteriovenous fistula (AVF). This is often created between the carotid artery and the jugular vein or between the femoral artery and the femoral vein of rats or larger animals such as rabbit or swine, with juxta-anastomotic AVF. The AVF causes low and oscillating wall shear stress in zones where flow stagnation occurs on the outer wall of the artery and on the inner wall of the juxta-anastomotic site36, 37. In the venous segment of the rat AVF model, MCP-1 mRNA and protein increase in both the ECs and smooth muscle cells (SMCs), accompanied by increased activities of the transcription factors NF-κB and AP-138. In the swine AVF model, significant luminal stenosis and intima-media thickening are present as early as 28 days and 42 days post-surgery, respectively, in the juxta-anastomotic regions of the femoral artery and vein37.
All the in vivo results indicate that flow patterns play significant roles in vascular homeostasis. The mechanotransduction mechanisms involved have been analyzed by using in vitro flow systems; where the mechanical stimuli applied can be controlled and the molecular and functional responses can be studied in detail.
3. Shear stress-induced signal transduction, gene expression, and phenotypic changes in ECs
3.1 Mechanosensing and signaling in ECs
In vitro investigations have shown that application of shear stress to ECs can activate multiple mechanosensors located at the cell membrane (the biomolecules that are the initial responders to the changes in mechanical environment to trigger mechanotransduction). These include integrins39, 40, tyrosine kinase receptors (particularly vascular endothelial growth factor receptor-2, VEGFR-2)41, G proteins and G protein-coupled receptors42, ion channels43, and intercellular junction proteins44. Other possible mechanosensors are local membrane structures such as caveolae, gap junctions, membrane lipids, and glycocalyx45. The mechanosensing, transmitted via adaptor molecules, triggers a cascade of signaling pathways and modulates the expression of functional genes (e.g., genes concerned with proliferation or growth arrest, inflammation or anti-inflammation, and many others). For example, integrins (αvβ3, α2β1, α5β1, and α6β1), which mediate the effects of shear stress on cytoskeletal proteins (e.g., actin filaments), typically trigger both outside-in and inside-out signals to transmit and modulate the tensions among focal adhesion sites, membrane receptors, and the extracellular matrix1, 39, 40. Integrin activation results in phosphorylation of focal adhesion kinase (FAK), Paxillin and p130CAS (Crk-Associated Substrate), and leads to the activation of mitogen-activated protein kinases (MAPKs) via Ras GTPase46. The activation of VEGFR-2 by shear stress results in its association with casitas B-lineage lymphoma (Cbl), VE-cadherin, β-cadherin associated protein (catenin), and phosphatidylinositol-3-kinase (PI3K) to phosphorylate the downstream Akt (protein kinase B, PKB) 41. Shear stress regulates EC alignment and remodeling through the activation of Rho family GTPases (Cdc42, Rho, and Rac) that enhance the formation of stress fibers and focal adhesions, and regulates cytoskeletal reorganization42, 47. In addition, shear stress preferentially increases membrane fluidity at the upstream side of the ECs; this may induce the polarization of the cell and facilitate the lateral mobility of membrane proteins and enhance their interactions45, 48. Increasing evidence suggests that those mechanosensing and signaling mechanisms in ECs are not mutually exclusive, but are most likely interconnected. Recently, transmembrane proteins Piezo1 and Piezo2 have been identified as essential components of mechanically activated ion channels49, 50 in many cell types and may also serve as novel mechanotransduction molecules in ECs. Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) have also been identified recently as nuclear relays of mechanical signals exerted by extracellular matrix (ECM) rigidity and cell shape51. Therefore, YAP/TAZ are suggested to serve as novel sensors and mediators of mechanical cues51. Investigations on the involvement and functions of Piezo and YAP/TAZ in shear stress-induced signaling in ECs may be an attractive direction for mechano-biochemical transduction research.
3.2 Shear stress-induced gene expression and functional consequences in ECs
eNOS is the essential molecule for the synthesis and release of the potent vasodilator, anti-oxidant and anti-inflammatory mediator: nitric oxide. Impaired expression of eNOS is a crucial element for fluid shear stress regulation of site-specific endothelial functional phenotype. In both cultured ECs and intact vessels, atheroprotective laminar shear stress (LSS, mean stress ≥12 dyn/cm2 without oscillation) upregulates the protein expression level and/or activity of eNOS52, 53. The mechanism responsible for shear stress-regulation of eNOS remains unclear despite some suggestions of regulatory pathways based on in vitro investigation. The well-characterized residues for flow-regulated eNOS phosphorylation are active sites Ser 1177, Ser 633, Ser 635, and inhibitory sites Tyr 65754-57. LSS stimulates Akt phosphorylation, which in turn phosphorylates eNOS at Ser 1177, leading to eNOS activation and NO production54. LSS also increases the expression of proline-rich tyrosine kinase 2 (PYK2) and its association with eNOS, which phosphorylates eNOS at the repressive Tyr 657 and hence decreases eNOS activity56. The PYK2-dependent inhibition of NO production may serve to balance the eNOS activity and to limit the detrimental over-production of NO, i.e., the generation of cytotoxic peroxynitrite. Another key molecule for vascular endothelial homeostasis is the Krüppel-like factor (KLF) family zinc finger-containing transcription factor. KLF2 is abundantly expressed in ECs and positively regulates eNOS expression. Atheroprotective LSS or pulsatile shear stress (PSS, mean stress ≥12 dyn/cm2 with oscillation), but not atheroprone oscillatory shear stress (OSS, mean stress = 0∼0.5 dyn/cm2 with oscillation), causes a sustained increase of the mRNA level of KLF2 via the mediation of the signaling cascade comprising of extracellular-signal-regulated kinase (ERK) 5, myocyte enhancer factor-2 (MEF2), AMPK, and/or miR-92a to result in increased eNOS expression58, 59. Other mechanisms contributing to shear stress-mediated eNOS expression and activation include protein kinase A (PKA)55, NF-E2-related factor 2 (Nrf2)60, SIRT157, and other HDACs (e.g., HDAC5)61. The interactions among these signaling pathways remain to be elucidated.
Increased expression of biomarkers such as MCP-1, ICAM-1, VCAM-1, and E-selectin are a hallmark of the endothelial inflammatory phenotype in atherosusceptible regions. The initial application of LSS to static ECs causes a brief Ras GTPase and MAPK activation, which leads to the transient MCP-1 gene expression; prolonged LSS, however, decreases Ras activity and MCP-1 expression1. Such repression of MCP-1 by sustained LSS may be due to the eNOS expression and NO production that inhibit protein kinase C (PKC)-ε and ERK 1/2 62. Furthermore, prolonged PSS induces the AMPK / poly [ADP ribose] polymerase 1(PARP-1) / B-cell lymphoma-6 (Bcl-6) pathway to inhibit expressions of VCAM-1, MCP-1, and MCP-363. The Tie family of receptor tyrosine kinases (RTK) has been shown to contribute to the pro-inflammatory endothelial phenotype, as indicated by the findings that depletion of Tie1 augments the LSS-induced simulation of eNOS and -inhibitions of kappa B (I-κB) activity and ICAM-1 expression 64. In contrast, OSS induces MCP-1 and VCAM-1 activities; these are mediated by the miR-21/peroxisome proliferator-activated receptor alpha (PPARα) /activator protein 1 (AP-1) cascade65. OSS induces a sustained miR-21 expression to inhibit PPARα translation, leading to the activation of AP-1 and its association with the promoter regions of MCP-1 and VCAM-1 to increase their expressions65. Flow-sensitive miR-10a has been demonstrated to provoke endothelial inflammation via NF-κB-mediated activation of MCP-1, VCAM-1, E-selectin, and Interleukins IL-6, and IL-810. BMP-4 has been shown to exert proinflammatory effects on endothelium. OSS induces endothelial BMP-4 expression, which in turn activates ICAM-1 expression and monocyte adhesion through the production of reactive oxygen species (ROS)66. HDAC-3/5/7 have been found to mediate the inductive effect of OSS on VCAM-1 expression67. A recent investigation shows that the application of OSS to ECs activates sterol regulatory element binding protein 2 (SREBP2) to induce NLRP3 inflammation, and hence the increased innate immunity to cause topographical distribution of atherosclerotic lesions68.
Evidence from in vivo animal models, with the introduction of disturbed flow, suggests that increased EC proliferation is an early event of site-specific atherogenesis. In vitro studies verify that prolonged LSS or PSS reduces the number of ECs with bromodeoxyuridine (BrdU) incorporation and prevents ECs from entering S phase, with the majority of cells arrested in the G0 or G1 phase69, 70. In contrast, cells exposed to OSS, or at the disturbed flow reattachment area, have accelerated turnover rate with enhanced G0/G1-S transition25, 26. Among the important mediators involved in flow-regulated cell cycle progression are Smad1/5. Application of OSS to ECs causes the sustained phosphorylation of Smad1/5 through integrin/BMP receptor association and FAK/ERK cascade, resulting in activation of the mammalian target of rapamycin (mTOR)/p70S6 kinase, and leading to the up-regulation of cyclin A, down-regulation of p21 and p27, and consequential cell cycle progression25, 71. LSS stabilizes the tumor suppressor protein p53 and increases its phosphorylation by JNK, thus leading to the inhibition of EC growth69. LSS also increases the expression of the growth arrest proteins GADD45 (growth arrest and DNA damage inducible protein 45) and p21, and decreases the phosphorylation of the retinoblastoma (Rb), thus leading to cell cycle arrest69. MicroRNAs have been identified recently as important players in the regulation of endothelial proliferation under shear stress. We have shown with our collaborators that miR-19a72 and miR-23b70 mediate the LSS/PSS-induced cell cycle arrest via a decrease in E2F1 and hypophosphorylation of Rb, or directly targeting cyclin D1. All these findings demonstrate that shear flows with and without a clear direction differentially activate multiple signaling events to modulate EC growth (Fig. 1); these findings have helped to elucidate the molecular mechanisms underlying the focal nature of vascular diseases.
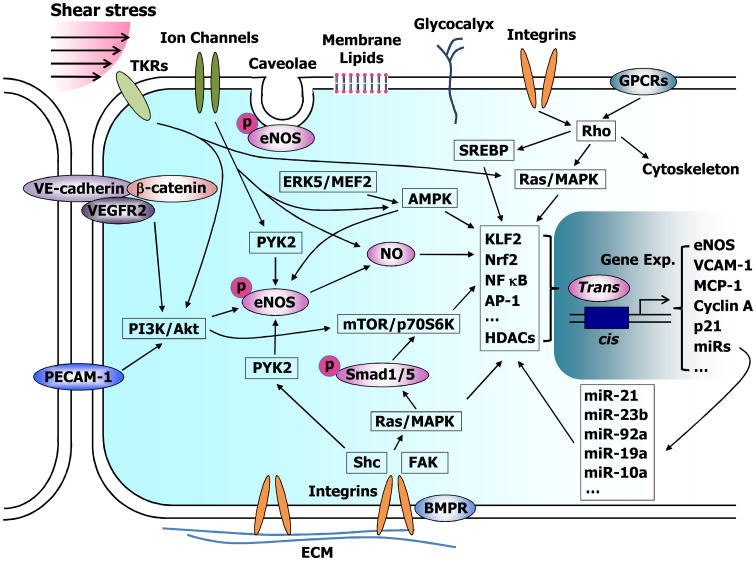
Figure 1.
Schematic diagram showing endothelial mechanotransduction and signaling induced by shear stress. Shear stress stimulates endothelial cells (ECs) through the activation of mechanosensors, including integrins, tyrosine kinase receptors (TKRs), G proteins and G protein-coupled receptors (GPCRs), ion channels, intercellular junction proteins (e.g., VE-cadherin and PECAM-1), caveolae, membrane lipids, and glycocalyx. These mechanosensors act through adaptor molecules (e.g., Shc) to trigger the activation of signaling molecules such as Ras, Rho, phosphatidylinositol-3-kinase (PI3K), and mitogen-activated protein kinases (MAPKs), which then activate eNOS, Smad1/5, and the transcription factors and cofactors (e.g., KLF2, NF kB, and AP-1) to regulate the expression of a number of functional genes such as eNOS, VCAM-1, and MCP-1, as well as microRNAs (miRs). This diagram illustrates that multiple signaling pathways coordinate to form mechanoresponsive networks to modulate EC phenotype and function.
Shear stresses with different flow patterns induce differential gene expression and functional consequences via complex mechanisms, which also include redox regulation73, endoplasmic reticulum-stress and protein unfolding74, and lipid metabolism75. Systems biology approaches such as high-throughput transcriptomics, proteomics, and miR-omics have been employed to investigate the shear stress-induced gene expressions and the consequential phenotypes in ECs in vitro or in vivo70, 76-79. In an in vitro flow model, miR profiling in ECs showed that 21 miRs were differentially expressed (8 up- and 13 down-regulated) in response to 24 hours of PSS as compared to static condition70. In an in vivo disturbed flow model, 62 and 523 genes were found to change significantly in the endothelium of mouse carotid arteries by 12 hours and 48 hours after partial ligation, respectively76. This study led to the discovery of novel mechanosentitive genes, including LMO4 (LIM domain only 4), KLK10 (Kallikrein-related peptidase 10) and DHH (desert hedgehog) 76. In native atherosusceptible and atheroresistant regions in porcine aorta, cDNA microarray was employed to study gene expressions in the endothelium, and ≈2,000 putatively differentially expressed genes were identified. Analyses of these differentially expressed gene indicated that atheroprone flow primes ECs toward inflammatory responses pending on other risk factors 80.However, proteomics and other comprehensive system-wide analyses such as metabolomics in atherosusceptible endothelium are restricted by the limited size of the specimen within the hemodynamic regions of interest and potential inclusion of multiple cell types. It would be of interest to overcome these technical hurdles to gain comprehensive insights for vascular disease progression.
4. Shear stress-modulation of communication between ECs and smooth muscle cells
In vivo investigations with animal models have shown an accelerated neointimal hyperplasia of the vessels after disturbance or cessation of blood flow in comparison with shame-operated controls with normal blood flow35, 81. In vitro experiments corroborate and greatly extend the in vivo findings by showing that application of atheroprotective shear stress to ECs co-cultured with vascular smooth muscle cells (SMCs) causes phenotypic switch of SMCs from de-differentiation to differentiation, induces contractile marker expression, and inhibits SMC migration and proliferation82-84. In contrast, application of atheroprone shear stress to ECs decreases SMC contractile marker genes (e.g., smooth muscle α-actin and myocardin), and induces SMC proinflammatory phenotype (e.g., expressions of VCAM-1, IL-8, and MCP-1)85. Several secretory molecules that mediate the flow-regulated effects via ECs on SMC gene expression and phenotype have been documented, including NO83, 86, prostacyclin (PGI2)87, PDGF-BB and TGF-β188, and miRs81, 89 (Fig. 2). MiR-143/-145 secreted by LSS-stimulated ECs have been shown to target gene expression and control phenotypes in co-cultured SMCs89. MiR-143/-145-containing extracellular vesicles derived from KLF2-expressing ECs can reduce atherosclerotic lesion formation in the aorta of ApoE-/- mice89. More recently, we also reported the findings of miRs in EC-SMC communication by showing that the endothelial miR-126/Argonaut 2 (Ago2) complex targets gene expression in the co-cultured SMCs to increase SMC turnover and that such effects are reduced by the application of atheroprotective LSS to ECs81. Systemic depletion of miR-126 in mice inhibited neointimal lesion formation of carotid arteries induced by cessation of blood flow81. These results suggest that the manipulation or interference of EC-secreted miRs provides a promising strategy to combat atherosclerosis.
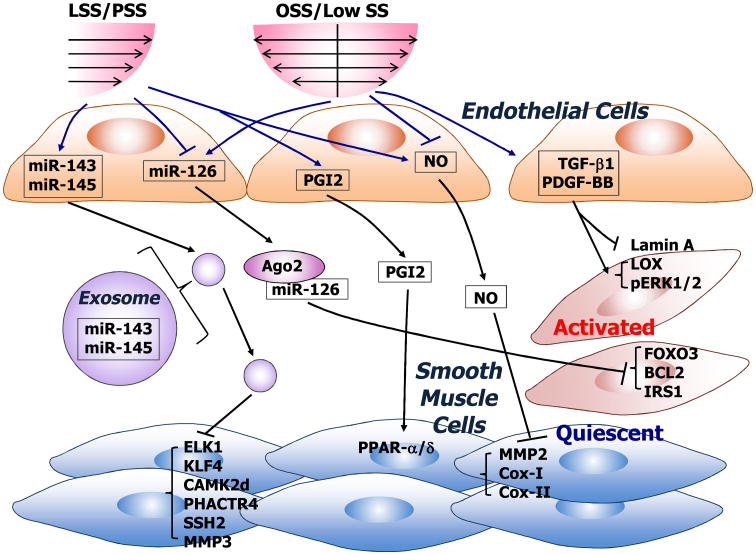
Figure 2.
Schematic diagram showing the modulation of gene expression and phenotype of smooth muscle cells (SMCs) by shear stress via ECs. Shear stress with sufficient magnitude and clear directions, i.e., laminar shear stress (LSS) and pulsatile shear stress (PSS), differ from that with a low magnitude and no clear direction, i.e., low shear stress and oscillatory shear stress (OSS) in their regulation of the release of secretory molecules such as NO, prostacyclin (PGI2), PDGF-BB and TGF-b1, and miRs. The uptake of NO, PGI2, and exosome-embedded miR-143/-145 by SMCs leads to functional targeting in SMCs with the promotion of a quiescent phenotype. In contrast, the PDGF-BB, TGF-b1, and Argonaute 2 (Ago2)-carried miR-126 released by ECs subjected to low shear stress or OSS induce an activated phenotype of SMCs.
Concluding remarks and perspectives
Hemodynamic forces can modulate the gene expression and phenotype of vascular ECs in vitro and in vivo. LSS and PSS that have well-defined directions are of utmost importance in maintaining vascular homeostasis. They activate signal transduction pathways and gene expression in ECs to suppress aberrant EC proliferation, inflammation, and atherosclerosis. In contrast, oscillating shear stress with a very low mean value and a reciprocating flow promotes atheroprone phenotype of ECs, with increases in EC proliferation, inflammation, leukocyte adhesion, lipoprotein uptake, and SMC migration and proliferation, thus contributing to atherogenesis. The diversity of endothelial functions is reflected in the variety of mechanisms of shear stress-induced signaling, and suggests that multiple mechanisms are coordinated in response to a given type of stimulus. A major challenge in this field is the integration of a large body of data on EC responses to shear stress with different flow patterns at the molecular level and the global scale to identify the shear stress-specific endothelial gene expression and phenotype. System-targeted high-throughput sequencing with increasingly sophisticated statistical and bioinformatics analyses are of particular importance in determining the intercellular and intracellular flow-responsive networks. Comparisons of genomic, epigenomic, translational, and post-translational profiles in the atherosusceptible and atheroresistant regions of arteries in vivo will enable the identification of genes and regulatory networks that may have direct pathophysiological relevance to endothelial function in health and disease. Current analyses have suggested that endothelial phenotypes are highly heterogeneous over different regions of the arterial trees. Analyses on homogenates of endothelium may mask and neutralize the changes in cells of interests due to the presence of cells with different behaviors in neighboring locations. Therefore, spatial and temporal studies on genomic and/or epigenomic profiling at single cell level would be a fruitful future direction. In addition, other biochemical risk factors (e.g., cholesterol, glucose, as well as blood cells) may also play important roles in regulating EC function, in conjunction with hemodynamic regulators. The interplays between mechanical and biochemical factors in atherogenesis remain to be elucidated. Therapeutic application of the currently identified target biomarkers for atherogenesis should not focus only on signal molecules, but rather to consider also the possible involvement of multiple targets in a variety of fundamental cellular processes. An attractive therapeutical option is the combined targeting of multiple molecules in the same or opposite pathways to achieve synergistic effects.
Significance.
Atherosclerosis is associated with the patterns of blood fluid shear stress and the ‘site-specific’ endothelial phenotypes. The aim of this review is to provide a brief summary of the current knowledge with a focus on the shear stress-initiated signaling and its regulation of endothelial function, as well as the communication between ECs and other vascular cells. The pathologic implications in atherosclerosis and future perspectives are also discussed. This review highlights some of the recent progress that has been made in our laboratory and others in the field of mechano-signal transduction in endothelial biology and their potential impacts as well as challenges.
Acknowledgments
None
Sources of Funding: This study is, in part, supported by NIH fundings HL106579, HL108735, and HL121365 (to S.C.)
Footnotes
Disclosure: None
References
- 1.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292(3):H1209–1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 2.Davies PF, Civelek M, Fang Y, Fleming I. The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res. 2013;99(2):315–327. doi: 10.1093/cvr/cvt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6(1):16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91(1):327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Won D, Zhu SN, Chen M, Teichert AM, Fish JE, Matouk CC, Bonert M, Ojha M, Marsden PA, Cybulsky MI. Relative reduction of endothelial nitric-oxide synthase expression and transcription in atherosclerosis-prone regions of the mouse aorta and in an in vitro model of disturbed flow. Am J Pathol. 2007;171(5):1691–1704. doi: 10.2353/ajpath.2007.060860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai G, Vaughn S, Zhang Y, Wang ET, Garcia-Cardena G, Gimbrone MA., Jr Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circ Res. 2007;101(7):723–733. doi: 10.1161/CIRCRESAHA.107.152942. [DOI] [PubMed] [Google Scholar]
- 7.Wang N, Miao H, Li YS, Zhang P, Haga JH, Hu Y, Young A, Yuan S, Nguyen P, Wu CC, Chien S. Shear stress regulation of Kruppel-like factor 2 expression is flow pattern-specific. Biochem Biophys Res Commun. 2006;341(4):1244–1251. doi: 10.1016/j.bbrc.2006.01.089. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Fang X, Zhou J, Chen Z, Zhao B, Xiao L, Liu A, Li YS, Shyy JY, Guan Y, Chien S, Wang N. Shear stress activation of nuclear receptor PXR in endothelial detoxification. Proc Natl Acad Sci U S A. 2013;110(32):13174–13179. doi: 10.1073/pnas.1312065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo D, Chien S, Shyy JY. Regulation of endothelial cell cycle by laminar versus oscillatory flow: distinct modes of interactions of AMP-activated protein kinase and Akt pathways. Circ Res. 2007;100(4):564–571. doi: 10.1161/01.RES.0000259561.23876.c5. [DOI] [PubMed] [Google Scholar]
- 10.Fang Y, Shi C, Manduchi E, Civelek M, Davies PF. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci U S A. 2010;107(30):13450–13455. doi: 10.1073/pnas.1002120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tressel SL, Huang RP, Tomsen N, Jo H. Laminar shear inhibits tubule formation and migration of endothelial cells by an angiopoietin-2 dependent mechanism. Arterioscler Thromb Vasc Biol. 2007;27(10):2150–2156. doi: 10.1161/ATVBAHA.107.150920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilcox JN, Smith KM, Williams LT, Schwartz SM, Gordon D. Platelet-derived growth factor mRNA detection in human atherosclerotic plaques by in situ hybridization. J Clin Invest. 1988;82(3):1134–1143. doi: 10.1172/JCI113671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCaffrey TA, Fu C, Du B, Eksinar S, Kent KC, Bush H, Jr, Kreiger K, Rosengart T, Cybulsky MI, Silverman ES, Collins T. High-level expression of Egr-1 and Egr-1-inducible genes in mouse and human atherosclerosis. J Clin Invest. 2000;105(5):653–662. doi: 10.1172/JCI8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brand K, Page S, Rogler G, Bartsch A, Brandl R, Knuechel R, Page M, Kaltschmidt C, Baeuerle PA, Neumeier D. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97(7):1715–1722. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci U S A. 2000;97(16):9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson SH, Caplice NM, Simari RD, Holmes DR, Jr, Carlson PJ, Lerman A. Activated nuclear factor-kappaB is present in the coronary vasculature in experimental hypercholesterolemia. Atherosclerosis. 2000;148(1):23–30. doi: 10.1016/s0021-9150(99)00211-7. [DOI] [PubMed] [Google Scholar]
- 17.Mullick AE, Soldau K, Kiosses WB, Bell TA, 3rd, Tobias PS, Curtiss LK. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J Exp Med. 2008;205(2):373–383. doi: 10.1084/jem.20071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orr AW, Stockton R, Simmers MB, Sanders JM, Sarembock IJ, Blackman BR, Schwartz MA. Matrix-specific p21-activated kinase activation regulates vascular permeability in atherogenesis. J Cell Biol. 2007;176(5):719–727. doi: 10.1083/jcb.200609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Sweet DT, Irani-Tehrani M, Maeda N, Tzima E. Shc coordinates signals from intercellular junctions and integrins to regulate flow-induced inflammation. J Cell Biol. 2008;182(1):185–196. doi: 10.1083/jcb.200709176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hahn C, Orr AW, Sanders JM, Jhaveri KA, Schwartz MA. The subendothelial extracellular matrix modulates JNK activation by flow. Circ Res. 2009;104(8):995–1003. doi: 10.1161/CIRCRESAHA.108.186486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng L, Zampetaki A, Margariti A, Pepe AE, Alam S, Martin D, Xiao Q, Wang W, Jin ZG, Cockerill G, Mori K, Li YS, Hu Y, Chien S, Xu Q. Sustained activation of XBP1 splicing leads to endothelial apoptosis and atherosclerosis development in response to disturbed flow. Proc Natl Acad Sci U S A. 2009;106(20):8326–8331. doi: 10.1073/pnas.0903197106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zampetaki A, Zeng L, Margariti A, Xiao Q, Li H, Zhang Z, Pepe AE, Wang G, Habi O, deFalco E, Cockerill G, Mason JC, Hu Y, Xu Q. Histone deacetylase 3 is critical in endothelial survival and atherosclerosis development in response to disturbed flow. Circulation. 2010;121(1):132–142. doi: 10.1161/CIRCULATIONAHA.109.890491. [DOI] [PubMed] [Google Scholar]
- 23.Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91(4):1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, Daemen MJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21(12):1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 25.Zhou J, Lee PL, Tsai CS, Lee CI, Yang TL, Chuang HS, Lin WW, Lin TE, Lim SH, Wei SY, Chen YL, Chien S, Chiu JJ. Force-specific activation of Smad1/5 regulates vascular endothelial cell cycle progression in response to disturbed flow. Proc Natl Acad Sci U S A. 2012;109(20):7770–7775. doi: 10.1073/pnas.1205476109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chien S. Molecular and mechanical bases of focal lipid accumulation in arterial wall. Prog Biophys Mol Biol. 2003;83(2):131–151. doi: 10.1016/s0079-6107(03)00053-1. [DOI] [PubMed] [Google Scholar]
- 27.Endres M, Laufs U, Merz H, Kaps M. Focal expression of intercellular adhesion molecule-1 in the human carotid bifurcation. Stroke. 1997;28(1):77–82. doi: 10.1161/01.str.28.1.77. [DOI] [PubMed] [Google Scholar]
- 28.Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, Cybulsky MI. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res. 1999;85(2):199–207. doi: 10.1161/01.res.85.2.199. [DOI] [PubMed] [Google Scholar]
- 29.Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18(5):842–851. doi: 10.1161/01.atv.18.5.842. [DOI] [PubMed] [Google Scholar]
- 30.Suo J, Ferrara DE, Sorescu D, Guldberg RE, Taylor WR, Giddens DP. Hemodynamic shear stresses in mouse aortas: implications for atherogenesis. Arterioscler Thromb Vasc Biol. 2007;27(2):346–351. doi: 10.1161/01.ATV.0000253492.45717.46. [DOI] [PubMed] [Google Scholar]
- 31.Hutchison KJ. Endothelial cell morphology around graded stenoses of the dog common carotid artery. Blood Vessels. 1991;28(5):396–406. doi: 10.1159/000158886. [DOI] [PubMed] [Google Scholar]
- 32.Levesque MJ, Liepsch D, Moravec S, Nerem RM. Correlation of endothelial cell shape and wall shear stress in a stenosed dog aorta. Arteriosclerosis. 1986;6(2):220–229. doi: 10.1161/01.atv.6.2.220. [DOI] [PubMed] [Google Scholar]
- 33.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113(23):2744–2753. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 34.Miao H, Hu YL, Shiu YT, Yuan S, Zhao Y, Kaunas R, Wang Y, Jin G, Usami S, Chien S. Effects of flow patterns on the localization and expression of VE-cadherin at vascular endothelial cell junctions: in vivo and in vitro investigations. J Vasc Res. 2005;42(1):77–89. doi: 10.1159/000083094. [DOI] [PubMed] [Google Scholar]
- 35.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297(4):H1535–1543. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ene-Iordache B, Remuzzi A. Disturbed flow in radial-cephalic arteriovenous fistulae for haemodialysis: low and oscillating shear stress locates the sites of stenosis. Nephrol Dial Transplant. 2012;27(1):358–368. doi: 10.1093/ndt/gfr342. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Krishnamoorthy M, Banerjee R, Zhang J, Rudich S, Holland C, Arend L, Roy-Chaudhury P. Venous stenosis in a pig arteriovenous fistula model--anatomy, mechanisms and cellular phenotypes. Nephrol Dial Transplant. 2008;23(2):525–533. doi: 10.1093/ndt/gfm547. [DOI] [PubMed] [Google Scholar]
- 38.Juncos JP, Grande JP, Kang L, Ackerman AW, Croatt AJ, Katusic ZS, Nath KA. MCP-1 contributes to arteriovenous fistula failure. J Am Soc Nephrol. 2011;22(1):43–48. doi: 10.1681/ASN.2010040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jalali S, del Pozo MA, Chen K, Miao H, Li Y, Schwartz MA, Shyy JY, Chien S. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc Natl Acad Sci U S A. 2001;98(3):1042–1046. doi: 10.1073/pnas.031562998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz MA. Integrin signaling revisited. Trends Cell Biol. 2001;11(12):466–470. doi: 10.1016/s0962-8924(01)02152-3. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Miao H, Li S, Chen KD, Li YS, Yuan S, Shyy JY, Chien S. Interplay between integrins and FLK-1 in shear stress-induced signaling. Am J Physiol Cell Physiol. 2002;283(5):C1540–1547. doi: 10.1152/ajpcell.00222.2002. [DOI] [PubMed] [Google Scholar]
- 42.Kuchan MJ, Jo H, Frangos JA. Role of G proteins in shear stress-mediated nitric oxide production by endothelial cells. Am J Physiol. 1994;267(3 Pt 1):C753–758. doi: 10.1152/ajpcell.1994.267.3.C753. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto K, Sokabe T, Matsumoto T, et al. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med. 2006;12(1):133–137. doi: 10.1038/nm1338. [DOI] [PubMed] [Google Scholar]
- 44.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437(7057):426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 45.Pan S. Molecular mechanisms responsible for the atheroprotective effects of laminar shear stress. Antioxid Redox Signal. 2009;11(7):1669–1682. doi: 10.1089/ars.2009.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 2002;91(9):769–775. doi: 10.1161/01.res.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- 47.Tzima E. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circ Res. 2006;98(2):176–185. doi: 10.1161/01.RES.0000200162.94463.d7. [DOI] [PubMed] [Google Scholar]
- 48.Butler PJ, Norwich G, Weinbaum S, Chien S. Shear stress induces a time- and position-dependent increase in endothelial cell membrane fluidity. Am J Physiol Cell Physiol. 2001;280(4):C962–969. doi: 10.1152/ajpcell.2001.280.4.C962. [DOI] [PubMed] [Google Scholar]
- 49.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330(6000):55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, Montal M, Patapoutian A. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483(7388):176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 52.Boo YC, Kim HJ, Song H, Fulton D, Sessa W, Jo H. Coordinated regulation of endothelial nitric oxide synthase activity by phosphorylation and subcellular localization. Free Radic Biol Med. 2006;41(1):144–153. doi: 10.1016/j.freeradbiomed.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 53.Jin ZG, Ueba H, Tanimoto T, Lungu AO, Frame MD, Berk BC. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ Res. 2003;93(4):354–363. doi: 10.1161/01.RES.0000089257.94002.96. [DOI] [PubMed] [Google Scholar]
- 54.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399(6736):601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 55.Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Lum H, Jo H. Shear stress stimulates phosphorylation of eNOS at Ser(635) by a protein kinase A-dependent mechanism. Am J Physiol Heart Circ Physiol. 2002;283(5):H1819–1828. doi: 10.1152/ajpheart.00214.2002. [DOI] [PubMed] [Google Scholar]
- 56.Fisslthaler B, Loot AE, Mohamed A, Busse R, Fleming I. Inhibition of endothelial nitric oxide synthase activity by proline-rich tyrosine kinase 2 in response to fluid shear stress and insulin. Circ Res. 2008;102(12):1520–1528. doi: 10.1161/CIRCRESAHA.108.172072. [DOI] [PubMed] [Google Scholar]
- 57.Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JY. Shear stress, SIRT1, and vascular homeostasis. Proc Natl Acad Sci U S A. 2010;107(22):10268–10273. doi: 10.1073/pnas.1003833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young A, Wu W, Sun W, Benjamin Larman H, Wang N, Li YS, Shyy JY, Chien S, Garcia-Cardena G. Flow activation of AMP-activated protein kinase in vascular endothelium leads to Kruppel-like factor 2 expression. Arterioscler Thromb Vasc Biol. 2009;29(11):1902–1908. doi: 10.1161/ATVBAHA.109.193540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu W, Xiao H, Laguna-Fernandez A, Villarreal G, Jr, Wang KC, Geary GG, Zhang Y, Wang WC, Huang HD, Zhou J, Li YS, Chien S, Garcia-Cardena G, Shyy JY. Flow-Dependent Regulation of Kruppel-Like Factor 2 Is Mediated by MicroRNA-92a. Circulation. 2011;124(5):633–641. doi: 10.1161/CIRCULATIONAHA.110.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takabe W, Warabi E, Noguchi N. Anti-atherogenic effect of laminar shear stress via Nrf2 activation. Antioxid Redox Signal. 2011;15(5):1415–1426. doi: 10.1089/ars.2010.3433. [DOI] [PubMed] [Google Scholar]
- 61.Wang W, Ha CH, Jhun BS, Wong C, Jain MK, Jin ZG. Fluid shear stress stimulates phosphorylation-dependent nuclear export of HDAC5 and mediates expression of KLF2 and eNOS. Blood. 2010;115(14):2971–2979. doi: 10.1182/blood-2009-05-224824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ni CW, Wang DL, Lien SC, Cheng JJ, Chao YJ, Hsieh HJ. Activation of PKC-epsilon and ERK1/2 participates in shear-induced endothelial MCP-1 expression that is repressed by nitric oxide. J Cell Physiol. 2003;195(3):428–434. doi: 10.1002/jcp.10259. [DOI] [PubMed] [Google Scholar]
- 63.Gongol B, Marin T, Peng IC, Woo B, Martin M, King S, Sun W, Johnson DA, Chien S, Shyy JY. AMPKalpha2 exerts its anti-inflammatory effects through PARP-1 and Bcl-6. Proc Natl Acad Sci U S A. 2013;110(8):3161–3166. doi: 10.1073/pnas.1222051110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woo KV, Qu X, Babaev VR, Linton MF, Guzman RJ, Fazio S, Baldwin HS. Tie1 attenuation reduces murine atherosclerosis in a dose-dependent and shear stress-specific manner. J Clin Invest. 2011;121(4):1624–1635. doi: 10.1172/JCI42040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou J, Wang KC, Wu W, Subramaniam S, Shyy JY, Chiu JJ, Li JY, Chien S. MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci U S A. 2011;108(25):10355–10360. doi: 10.1073/pnas.1107052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95(8):773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 67.Lee DY, Lee CI, Lin TE, Lim SH, Zhou J, Tseng YC, Chien S, Chiu JJ. Role of histone deacetylases in transcription factor regulation and cell cycle modulation in endothelial cells in response to disturbed flow. Proc Natl Acad Sci U S A. 2012;109(6):1967–1972. doi: 10.1073/pnas.1121214109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiao H, Lu M, Lin TY, et al. Sterol regulatory element binding protein 2 activation of NLRP3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility. Circulation. 2013;128(6):632–642. doi: 10.1161/CIRCULATIONAHA.113.002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin K, Hsu PP, Chen BP, Yuan S, Usami S, Shyy JY, Li YS, Chien S. Molecular mechanism of endothelial growth arrest by laminar shear stress. Proc Natl Acad Sci U S A. 2000;97(17):9385–9389. doi: 10.1073/pnas.170282597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang KC, Garmire LX, Young A, Nguyen P, Trinh A, Subramaniam S, Wang N, Shyy JY, Li YS, Chien S. Role of microRNA-23b in flow-regulation of Rb phosphorylation and endothelial cell growth. Proc Natl Acad Sci U S A. 2010;107(7):3234–3239. doi: 10.1073/pnas.0914825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou J, Lee PL, Lee CI, Wei SY, Lim SH, Lin TE, Chien S, Chiu JJ. BMP receptor-integrin interaction mediates responses of vascular endothelial Smad1/5 and proliferation to disturbed flow. J Thromb Haemost. 2013;11(4):741–755. doi: 10.1111/jth.12159. [DOI] [PubMed] [Google Scholar]
- 72.Qin X, Wang X, Wang Y, Tang Z, Cui Q, Xi J, Li YS, Chien S, Wang N. MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells. Proc Natl Acad Sci U S A. 2010;107(7):3240–3244. doi: 10.1073/pnas.0914882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, Jo H. Oscillatory shear stress stimulates endothelial production of O2- from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem. 2003;278(47):47291–47298. doi: 10.1074/jbc.M305150200. [DOI] [PubMed] [Google Scholar]
- 74.Feaver RE, Hastings NE, Pryor A, Blackman BR. GRP78 upregulation by atheroprone shear stress via p38-, alpha2beta1-dependent mechanism in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28(8):1534–1541. doi: 10.1161/ATVBAHA.108.167999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y, Chen BP, Lu M, Zhu Y, Stemerman MB, Chien S, Shyy JY. Shear stress activation of SREBP1 in endothelial cells is mediated by integrins. Arterioscler Thromb Vasc Biol. 2002;22(1):76–81. doi: 10.1161/hq0102.101822. [DOI] [PubMed] [Google Scholar]
- 76.Ni CW, Qiu H, Rezvan A, Kwon K, Nam D, Son DJ, Visvader JE, Jo H. Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood. 2010;116(15):e66–73. doi: 10.1182/blood-2010-04-278192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Son DJ, Kumar S, Takabe W, Woo Kim C, Ni CW, Alberts-Grill N, Jang IH, Kim S, Kim W, Won Kang S, Baker AH, Woong Seo J, Ferrara KW, Jo H. The atypical mechanosensitive microRNA-712 derived from pre-ribosomal RNA induces endothelial inflammation and atherosclerosis. Nat Commun. 2013;4:3000. doi: 10.1038/ncomms4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Civelek M, Manduchi E, Riley RJ, Stoeckert CJ, Jr, Davies PF. Chronic endoplasmic reticulum stress activates unfolded protein response in arterial endothelium in regions of susceptibility to atherosclerosis. Circ Res. 2009;105(5):453–461. doi: 10.1161/CIRCRESAHA.109.203711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Freed JK, Greene AS. Proteomic analysis of shear stress-mediated protection from TNF-alpha in endothelial cells. Microcirculation. 2010;17(4):259–270. doi: 10.1111/j.1549-8719.2010.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, Pritchard WF, Powell S, Chang GY, Stoeckert CJ, Jr, Davies PF. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci U S A. 2004;101(8):2482–2487. doi: 10.1073/pnas.0305938101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou J, Li YS, Nguyen P, Wang KC, Weiss A, Kuo YC, Chiu JJ, Shyy JY, Chien S. Regulation of vascular smooth muscle cell turnover by endothelial cell-secreted microRNA-126: role of shear stress. Circ Res. 2013;113(1):40–51. doi: 10.1161/CIRCRESAHA.113.280883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang HQ, Huang LX, Qu MJ, Yan ZQ, Liu B, Shen BR, Jiang ZL. Shear stress protects against endothelial regulation of vascular smooth muscle cell migration in a coculture system. Endothelium. 2006;13(3):171–180. doi: 10.1080/10623320600760282. [DOI] [PubMed] [Google Scholar]
- 83.Sakamoto N, Ohashi T, Sato M. Effect of fluid shear stress on migration of vascular smooth muscle cells in cocultured model. Ann Biomed Eng. 2006;34(3):408–415. doi: 10.1007/s10439-005-9043-y. [DOI] [PubMed] [Google Scholar]
- 84.Sakamoto N, Kiuchi T, Sato M. Development of an endothelial-smooth muscle cell coculture model using phenotype-controlled smooth muscle cells. Ann Biomed Eng. 2011;39(11):2750–2758. doi: 10.1007/s10439-011-0372-8. [DOI] [PubMed] [Google Scholar]
- 85.Hastings NE, Simmers MB, McDonald OG, Wamhoff BR, Blackman BR. Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-inflammatory priming. Am J Physiol Cell Physiol. 2007;293(6):C1824–1833. doi: 10.1152/ajpcell.00385.2007. [DOI] [PubMed] [Google Scholar]
- 86.Berceli SA, Davies MG, Kenagy RD, Clowes AW. Flow-induced neointimal regression in baboon polytetrafluoroethylene grafts is associated with decreased cell proliferation and increased apoptosis. J Vasc Surg. 2002;36(6):1248–1255. doi: 10.1067/mva.2002.128295. [DOI] [PubMed] [Google Scholar]
- 87.Tsai MC, Chen L, Zhou J, Tang Z, Hsu TF, Wang Y, Shih YT, Peng HH, Wang N, Guan Y, Chien S, Chiu JJ. Shear stress induces synthetic-to-contractile phenotypic modulation in smooth muscle cells via peroxisome proliferator-activated receptor alpha/delta activations by prostacyclin released by sheared endothelial cells. Circ Res. 2009;105(5):471–480. doi: 10.1161/CIRCRESAHA.109.193656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qi YX, Jiang J, Jiang XH, Wang XD, Ji SY, Han Y, Long DK, Shen BR, Yan ZQ, Chien S, Jiang ZL. PDGF-BB and TGF-{beta}1 on cross-talk between endothelial and smooth muscle cells in vascular remodeling induced by low shear stress. Proc Natl Acad Sci U S A. 2011;108(5):1908–1913. doi: 10.1073/pnas.1019219108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14(3):249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]