Abstract
Obesity is a pandemic and a serious global health concern. Obesity is a risk factor for multiple conditions and contributes to multi-morbidities, resulting in increased health costs and millions of deaths each year. Obesity has been associated with changes in brain structure, cognitive deficits, and dementia and Alzheimer's disease. Adipokines, defined as hormones, cytokines and peptides secreted by adipose tissue, may have more widespread influence and functionality in the brain than previously thought. In this review, six adipokines, and their actions in the obese and non-obese condition will be discussed. Included are: plasminogen activator inhibitor-1 (PAI-1), interleukin-6 (IL-6), tumor necrosis factors alpha (TNF-α), angiotensinogen (AGT), adiponectin and leptin. Their functionality in the periphery, their ability to cross the blood brain barrier (BBB) and their influence on dementia processes within the brain will be discussed.
Keywords: Obesity, adipokines, brain, leptin, Alzheimer, dementia
1. Introduction
Each year, obesity or obesity-related conditions lead to the death of 2.8 million adults around the world (WHO, 2012). This epidemic in Western societies, and burgeoning epidemic in non-Western societies, increase the risk for multiple adverse health conditions and contributes to multiple morbidities (Olde Dubbelink et al., 2008). Obesity has an obscure etiology but it is generally ascribed to an imbalance of energy intake versus energy output and a complex interplay between genes and environment, (Doherty, 2011) leading to the highest prevalence of overweight and obesity ever observed in the world's history (Bray and Popkin, 1998). Adipose tissue, mainly white adipose tissue (WAT), functions as the largest endocrine organ by secreting hundreds of hormones, peptides and cytokines which are collectively referred to as adipokines. These adipokines affect processes in the periphery and the central nervous system (CNS).
Obesity has been associated with alterations in brain structure and function, cognitive deficits and even dementia and Alzheimer's disease (AD) (Businaro et al., 2012; Delgado et al., 2011; Gustafson, 2008, 2010; Gustafson et al., 2004; Haltia et al., 2007). In 2003, the first association was reported between AD and being more overweight, (defined within the overweight category of body mass index (BMI) between 25-29.99 kg/m2) in women (Gustafson et al., 2003). Thus, the year 2013, marks 10 years of published epidemiologic reports relating higher mid-life and late-life BMI to dementia (Fitzpatrick et al., 2009; Gustafson et al., 2009; Gustafson et al., 2003; Hayden et al., 2006; Kivipelto et al., 2005; Whitmer et al., 2007; Whitmer et al., 2008). Levels of mid-life and late-life BMI associated with AD are in overweight and obese ranges based on traditional cutpoints used for cardiovascular disease and overall mortality. While higher levels of adult BMI may increase risk for chronic neurodegenerative diseases of aging, some studies show that the direction of the BMI-AD relationship changes direction, and BMI declines in association with AD, later in life (Besser, 2013, in press; Gustafson et al., 2012; Gustafson et al., 2009). Higher BMI in middle adult life could be reflecting higher vascular risk and declining BMI reflecting more neurodegenerative events in latest life. This theory is illustrated by observations of both higher absolute level of baseline BMI and more body weight decline among those with Mild Cognitive Impairment (MCI) being associated with clinical dementia progression (CDR-Sum of Boxes) (Besser, 2013, in press).
Accompanying the literature on dementia and AD, are observed alterations in brain structure and function including decreased total and gray matter volumes, increased white matter lesions and reduced white matter integrity (Brooks et al., 2012; Gustafson et al., 2004; Pannacciulli et al., 2006; Raji et al., 2010; Stanek et al., 2011). More specifically, abnormalities in brain regions such as the amygdala, hippocampus and frontal cortex (Cazettes et al., 2011; Widya et al., 2011); decreased cortical thickness (Haltia et al., 2007; Hassenstab et al., 2012); axonal degradation (Mueller et al., 2011); and decreased functional connectivity in the brain (Nummenmaa et al., 2012; Stoeckel et al., 2009) have been observed.
It is known that adipokines, secretory products of adipose tissue such as leptin, interact directly with specific nuclei in certain areas of the brain such as the hippocampus. This results inregulation of not only feeding behavior, but also neurodegeneration, synaptic plasticity, neurogenesis and memory consolidation (Doherty, 2011). Reviewed herein are the adipokines, plasminogen activator inhibitor-1 (PAI-1), interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), angiotensinogen (AGT), adiponectin and leptin. These adipokines are not exclusively secreted by adipose tissue, nor is adipose tissue the only or primary source of these compounds. However, the adipokines selected for review have been associated with obesity-related morbidities that have also been implicated in dementia and AD, such as chronic low levels of inflammation, hypertension, and direct impaired in regulation of energy metabolism (Gustafson, 2006; Harrison, 2013; Iadecola and Davisson, 2008; Jequier, 2002; Rocha and Folco, 2011; Xu et al., 2011). More specifically, AGT is an important mediator of hypertension; TNF-α, IL-6 and PAI-1 are involved in chronic inflammation and fibrinolysis; and leptin and adiponectin regulate several processes including energy metabolism (Diez and Iglesias, 2003; Gardes et al., 1982; Guerre-Millo, 2004; Jequier, 2002; Ouchi et al., 2003a; Rocha and Folco, 2011; Villarreal-Molina and Antuna-Puente, 2012). Before circulating peripheral adipokines are able to interact with the brain, they must cross the blood brain barrier (BBB) to enter the central nervous system (CNS). This large neurovascular interface controls the transport of a variety of blood-borne factors such as amino acids, peptides, polypeptides and proteins, as well as many other molecules, such as adipokines, into the CNS (Banks, 2006; Kastin et al., 1999a; Pan and Kastin, 2007).
To better understand the epidemiologic associations between obesity and dementia and AD, we focus this review on how adipose tissue may influence the brain via adipokine action, the biology of each adipokine in the periphery, as well as its ability to cross the BBB and influence brain processes. First will be described how adipokine levels are altered in the obese versus non-obese condition. Second will be described possible pathways and processes by which these adipokines might affect the BBB and brain in obesity. Because very little literature is available regarding detailed molecular pathways in humans, these last descriptions are mainly based on findings from in vitro (cell culture) studies and in vivo experiments using animal models.
2. Leptin
Leptin is a protein hormone that has drawn the most attention in obesity research since its discovery in 1994 (Zhang et al., 1994). Leptin was discovered as a hormone involved in long-term regulation of energy intake and expenditure, body weight, and neuroendocrine functions in mammals (Jequier, 2002). Furthermore, it has significantly broadened our understanding of the mechanisms underlying the development of obesity and its complications. In the non-obese condition, energy intake increases leptin secretion, and in the brain leptin induces a negative feedback on energy intake via stimulating the expression of anorexigenic neuropeptides. In children, plasma levels of leptin are positively correlated with body weight, thus a higher body weight is associated with a higher leptin level (Fleisch et al., 2007; Salbe et al., 2002). Obese adolescents show higher plasma leptin concentrations in comparison with non-obese adolescents (Foschini et al., 2008; Salbe et al., 2002). Similar positive correlations are observed between body weight or BMI in adults and elderly (Ahima, 2006; Considine et al., 1996; Gustafson, 2012; Zeki Al Hazzouri et al., 2012). Despite these positive correlations, levels of adipokines such as leptin are highly variable in adults (Gustafson, 2012). Moreover, leptin production is influenced by sex and BMI in humans (Wiesner et al., 1999).
Peripheral leptin is able to enter cerebrospinal fluid (CSF) and the central nervous system (CNS crossing the BBB and choroid plexus,), and subsequently in the CNS leptin interacts with specific areas of the brain such as the hypothalamus and hippocampus (Peiser et al., 2000; Zlokovic et al., 2000). However, besides leptin transport into the CNS and CSF, several studies indicated that leptin can also be produced in human and rodent brains, for example in the hypothalamus, cortex and cerebellum (Brown et al., 2007; Brown et al., 2008; Morash et al., 1999; Wiesner et al., 1999; Wilkinson et al., 2007). Leptin transport across the BBB occurs via a mechanism involving leptin receptor a (LepRa), and a second, not yet characterized, transport mechanism (Schulz et al., 2010). This transport system for leptin has been demonstrated to be diurnal, both in vivo in mice and in vitro in cell culture studies (Banks et al., 1996; Maresh et al., 2001; Pan and Kastin, 2001).
Within the brain, leptin regulates energy intake and expenditure via suppression and induction of the expression of selected neuropeptides (Ahima, 2006; Jequier, 2002). Within the hypothalamus leptin binds to leptin receptors located on two populations of hypothalamic neurons. One population of neurons produces orexigenic neuropeptides (neuropeptide Y (NPY) and the agouti-related peptide (AGRP)). The second population of neurons produces anorexigenic neuropeptides (α-melanocyte-stimulating hormone (α-MSH) & pro-opiomelanocortin (POMC)) (Jequier, 2002). Leptin inhibits the expression of orexigenic neuropeptides and stimulates the expression of anorexigenic neuropeptides, which results in inhibition of energy intake (Jequier, 2002). An important functional leptin receptor in the brain is leptin receptor b (LepRb), which is highly expressed in the specific brain regions as the neocortex, hypothalamus, medulla and cerebellum (Burguera et al., 2000b). LepRb is the full-length isoform of the leptin receptor, and in vivo and in vitro experiments have revealed that it is the only receptor that contains intracellular motifs required for activation of the janus kinase 2 and signal transducer and activator of transcription 3 (JAK-2/STAT-3) pathway (Baumann et al., 1996; Bjorbaek and Kahn, 2004; Bjorbaek et al., 1997; Fruhbeck, 2006; Myers, 2004; Tartaglia et al., 1995; White et al., 1997).
Besides a role in energy intake, the presence of leptin receptors in specific regions of the brain illustrates its potential for being involved in multiple mechanisms related to brain function and structure in many rodent models (Banks, 2006; Banks et al., 2000; Grill et al., 2002; Guan et al., 1997; Huang et al., 1996; Shioda et al., 1998). Thus, the multiple effects of leptin in experimental models on various aspects of memory, neurogenesis, neuroprotection and brain structure are not surprising (See Figure 2/Table 1) (Ahima et al., 1999; Farr et al., 2006). Mainly in experimental rodent models, these effects of leptin on brain structure are determined by its influence on neurogenesis, axon growth, synaptogenesis and dendritic morphology, which occur during both pre-and postnatal life, and are important for the establishment of hypothalamic, hippocampal and cortical pathways (Bouret, 2010; Paz-Filho et al., 2010). The effects of leptin on axonal growth are not restricted to the hippocampus, but are also evident in the cortex (Valerio et al., 2006). Leptin effects on memory are facilitated by the conversion of short term potentiation into long term potentiation (LTP). Leptin enhances N-methyl-D-aspartate (NMDA) receptor function and subsequently enhances LTP formation at hippocampal CA1 synapses and rapidly remodels dendrites, which partially explains its effect on improvement of hippocampal memory formation (Farr et al., 2006; Harvey, 2007; Oomura et al., 2006; Paz-Filho et al., 2010). In addition, several in vitro experiments and in vivo rodent studies suggest that leptin has neuroprotective actions by inhibiting apoptotic cell death, attenuating cell death, improving cell survival, protecting against glutametergic cytotoxicity, protecting against oxidative stress and promoting the proliferation of hippocampal progenitor cells (Morrison, 2009; Zhang et al., 2007). Thus, within the brain, leptin regulates energy intake and affects memory processes, neurogenesis, neuroprotection and brain structure.
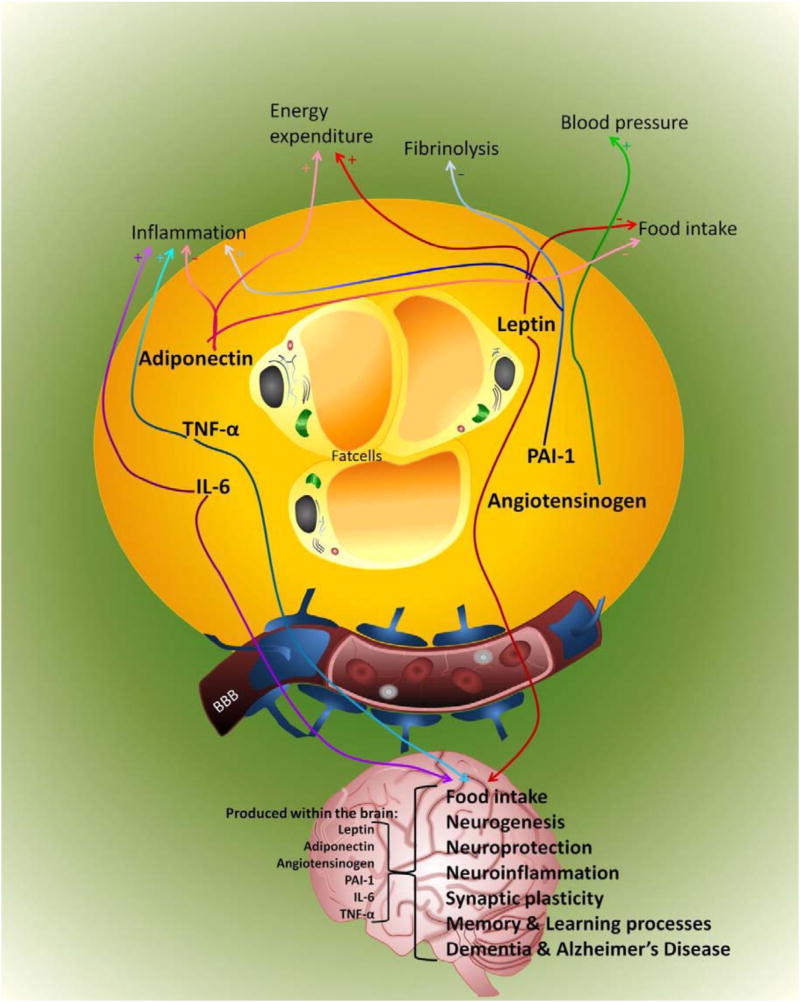
Figure 2. Effects of reviewed adipokines in the periphery and brain in obesity.
Fat cells produce and secrete adipokines like, leptin (red), PAI-1 (blue), angiotensinogen (green), adiponectin (pink), TNF-α (turquoise) and IL-6 (purple). In the periphery, TNF-α and IL-6 stimulate inflammation; these adipokines trigger the liver to produce acute phase proteins. Adiponectin attenuates the inflammatory response by inhibiting the production of TNF-α and IL-6. Furthermore, adiponectin modulating inflammatory responses, energy expenditure (CNS and periphery), food intake (CNS) and a number of metabolic processes, including glucose regulation and fatty acid catabolism. Angiotensinogen increases blood pressure. PAI-1 inhibits fibrinolysis and also stimulates the inflammatory response. Leptin increases energy expenditure and decreases food intake. Only three adipokines discussed in this review are able to cross the BBB and affect, positively or negatively depending on concentration and environment, brain processes such as food intake, synaptic plasticity, learning and memory, and development of dementia. Furthermore, angiotensinogen, PAI-1, IL-6, TNF-α are also produced within the brain by neurons, astrocytes and microglia, and several theories exist that leptin and adiponectin might also be produced in the brain. Several studies reported that, within the brain, adiponectin can be involved in regulating food intake and neuroprotection, while angiotensinogen is involved in learning and memory processes and PAI-1 regulates among other neuroinflammation. Il-6 and TNF-α produced by astrocytes and microglia are involved in neurogenesis, neuroinflammation, synaptic plasticity and learning and memory processes.
In obesity, the amount and function of adipokines are excessive, except for adiponectin and leptin. Adiponectin levels are decreased and it is hypothesized that leptin functionality could be decreased via leptin resistance. Therefore, obesity increases inflammation, increases blood pressure, and decreases fibrinolysis, energy expenditure and food intake in the periphery. In the brain, obesity results in impaired food intake, neurogenesis, synaptic plasticity and memory and learning processes possibly mediated through leptin, TNF-α and IL-6. Abbreviations: PAI-1: plasminogen activator inhibitor-1; IL-6: interleukin-6; TNF-α: tumor necrosis factor-alpha; CNS: central nervous system; BBB: blood brain barrier; +: increases/stimulates; -: decreases/inhibits
Table 1. Overview of reviewed adipokines: level in obesity, ability to cross the BBB, and roles in the periphery, non-obese and obese brain.
↑; Plasma levels of adipokine are upregulated in obesity. ↓; Plasma levels of adipokine are downregulated in obesity. Y; Adipokine is able to cross the BBB. N; Adipokine is not able to cross the BBB.
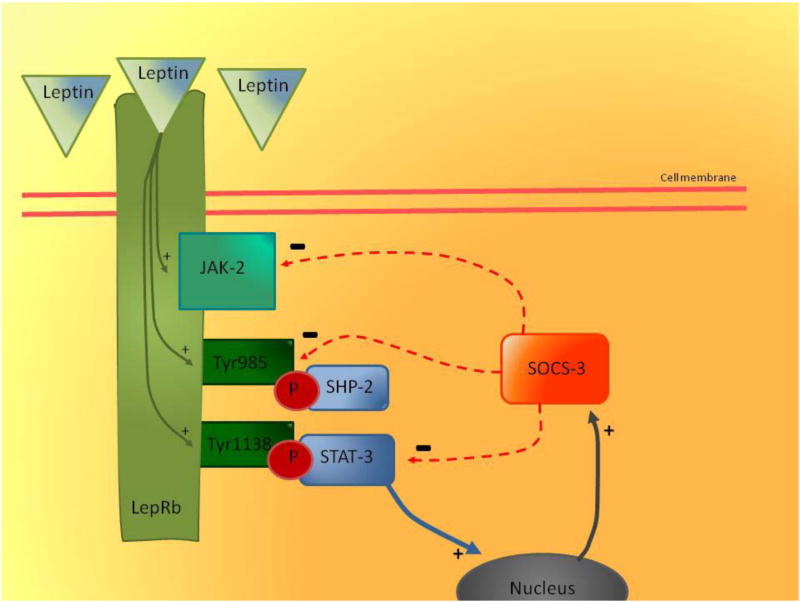
In contrast, rodent studies have shown that leptin often does not reach the CNS due to impaired transport across the BBB (Banks et al., 1999; Banks and Lebel, 2002; Burguera et al., 2000a; Kastin et al., 1999b). Leptin-resistance at the BBB decreases the transport of peripheral leptin into the brain. However, whether this leads to a decrease in total brain leptin levels also depends on leptin production within the brain. Impaired transport could contribute, in part, to the development of leptin-resistance observed in obesity (Caro et al., 1996; Friedman and Halaas, 1998). Additionally, animal research shows that leptin-resistance also develops in the brain and is associated with an impaired leptin receptor such as LepRb, tyrosine residues, and downstream neuronal circuitry via the JAK-2/STAT-3 pathway (Banks et al., 1999; El-Haschimi et al., 2000; Hileman et al., 2002; Wilsey et al., 2003; Yamashita et al., 1997). Tyrosine residues, Tyr985 and Tyr1138 are directly autophosphorylated after activation of LepRb in the acurate nucleus of the hypothalamus. Phosphorylated Tyr1138 mediates the activation of the transcription factor STAT3 (Munzberg and Myers, 2005). Among other targets, STAT-3 induces the transcription of suppressor of cytokine signaling 3 (SOCS-3) during LepRb signaling (See Figure 1) (Munzberg and Myers, 2005). Furthermore, Tyr985 plays a dual role in LepRb signaling, first binding tyrosine-protein phosphatase non-receptor type 11 (SHP-2) and second also providing an important site of interaction for SOCS-3 (Munzberg and Myers, 2005). SOCS-3 binding to the LepRb-JAK-2 complex attenuates LepRb-mediated signalling, and thereby LepRb-mediated signalling via the JAK-2/STAT-3 pathway (See Figure 1) (Bjorbaek et al., 1998; Munzberg and Myers, 2005). At high levels of circulating leptin as observed in obesity, LepRbs are highly activated and subsequently tyrosine residues are intensely phosphorylated. In case of LepRb this will lead to increased ‘leptin signalling’ via the JAK-2/STAT-3 pathway, while phosphorylation of the tyrosine residues will lead to increased expression of SOCS-3 which inhibits LepRb-mediated signalling (See Figure 1) (Munzberg and Myers, 2005). This inhibition could attenuate most of the expected increase in LepRb signalling (Munzberg and Myers, 2005). It is hypothesized that this inhibitory mechanism of SOCS-3 could be an underlying mechanism of leptin resistance in obesity (Munzberg and Myers, 2005). Moreover, rodent studies reveal that in the hypothalamus of rodents with diet-induced obesity, SOCS-3 expression is upregulated, while the leptin response via the JAK-2/STAT-3 signaling is attenuated (El-Haschimi et al., 2000; Munzberg and Myers, 2005).
Figure 1. Leptin resistance in obesity via SOCS-3 and JAK-2/STAT-3 pathway acurate nucleus of the hypothalamus.
Leptin binding to LepRb activates the LepRb-associated JAK-2 tyrosine kinase (green arrows), leading to the autophosphorylation of tyrosine residues on JAK-2 and the phosphorylation of Tyr985 and Tyr1138 on the intracellular tail of LepRb (green arrows). Tyr985 and Tyr1138 are directly autophosphorylated after activation of LepRb, and phosphorylated Tyr1138 mediates the activation of the transcription factor STAT-3, and phosphorylated Tyr985 binds to SHP-2. Among other targets, STAT-3 induces the transcription of SOCS-3 during LepRb signalling in the nucleus (blue arrow). SOCS-3 is produced and released from the nucleus (grey arrow). The binding of phosphorylated Tyr985 to SHP-2 provides an important site of inhibition for SOCS-3 (red arrow). Furthermore, SOCS-3 inhibits STAT-3 and via binding to the LepRb-JAK-2 complex it attenuates LepRb-mediated signalling, and thereby LepRb-mediated signalling via the JAK-2/STAT-3 pathway. At high levels of circulating leptin as observed in obesity, LepRbs are highly activated and subsequently tyrosine residues are intensely phosphorylated. In case of LepRb this will lead to increased ‘leptin signalling’ via the JAK-2/STAT-3 pathway, while phosphorylation of the tyrosine residues will lead to increased expression of SOCS-3 which inhibits LepRb-mediated signalling. This inhibition could attenuate most of the expected increase in LepRb signalling, and explain leptin resistance in obesity. (Munzberg and Myers, 2005)
Abbreviations: LepRb: Leptin receptor Rb; JAK-2: janus kinase 2; Tyr985/Tyr1138: tyrosine residues 985 and 1138; STAT-3: signal transducer and activator of transcription 3; P: phosphorylation; SHP-2: tyrosine-protein phosphatase non-receptor type 11; SOCS-3: suppressor of cytokine signaling 3; +: stimulation; -: inhibition
If assumed that obesity induces lower brain leptin levels or an attenuated leptin response, it could evoke a disrupted negative feedback loop in energy intake, impairments in memory processes, neurogenesis, neuroprotection and brain structure (See Figure 2/Table 1). However, leptin resistance within the CNS combined with impaired leptin transport at the BBB is not a waterproof explanation for all cases of human obesity. Leptin resistance is found in some animal models of obesity (Banks et al., 1999; El-Haschimi et al., 2000; Hileman et al., 2002; Yamashita et al., 1997), while for sporadic human obesity, there is often no evidence that leptin transport or response is impaired or deficient enough to be the primary cause of obesity (Arch et al., 1998; van Rossum et al., 2003).
3. Adiponectin
Adiponectin is a protein hormone, most well-described for modulating inflammatory responses, energy expenditure (CNS and periphery), food intake (CNS) and a number of metabolic processes, including glucose regulation and fatty acid catabolism in the periphery (See Figure 2) (Diez and Iglesias, 2003; Holland et al., 2013; Ouchi et al., 2003a; Qi et al., 2004; Villarreal-Molina and Antuna-Puente, 2012). In the periphery, adiponectin is released from adipose tissue into the blood circulation as full-length trimers, hexamers, high molecular weight (>500kDA, HMW) multimers and a globular fraction called globular adiponectin (Beltowski, 2003; Ouchi et al., 2003b). The two functional receptors of adiponectin, adipoR1 and adipoR2, contain seven transmembrane domains that serve as receptors for the multiple forms of globular and full length adiponectin. Activation of adiponectin receptors stimulates acetyl coenzyme-A carboxylase (ACC) phosphorylation and increases 5′ adenosine monophosphate-activated protein kinase (AMPK) in skeletal muscle and liver in animal models (Villarreal-Molina and Antuna-Puente, 2012). In humans, plasma adiponectin levels are, in contrast to most adipokines, inversely correlated to an increase in WAT, as well as to surrogate measures such as percentage body fat, waist-to-hip ratio and BMI (Cnop et al., 2003; Weyer et al., 2001). These correlations are less clear in children, adolescents and elderly (Ukkola and Santaniemi, 2002). In children, low adiponectin has been suggested to predict insulin resistance (Makni et al., 2012).
Adiponectin inhibits production of proinflammatory signals like TNF-α and IL-6. Adiponectin and TNF-α inhibit each other's production in adipose tissue, and adiponectin counteracts the proinflammatory effects of TNF-α in the vascular endothelium (Maeda et al., 2002; Matsuda et al., 2002; Ouchi et al., 1999; Ouchi et al., 2003a; Yokota et al., 2000). However, in contrast to IL-6, there is no evidence that plasma adiponectin levels are correlated with TNF-α levels in human plasma (Ouchi et al., 2003a). At the BBB, adiponectin affects proinflammatory signals by suppressing IL-6 release from endothelial cells in brain microvessels in mice(Spranger et al., 2006). Thus, interactions of adiponectin with the BBB seem to suppress proinflammatory signals, which is evidenced by decreased IL-6 production in rodent studies (Pan and Kastin, 2007). By inhibiting the production of IL-6 and TNF-α, adiponectin may indirectly affect inflammatory signalling across the BBB and within the brain. Thus, fewer proinflammatory cytokines might be able to cross the BBB due to high levels of circulating adiponectin. Peripheral plasma adiponectin levels are very low In human obesity (Cnop et al., 2003; Weyer et al., 2001). Thus, according to the afore mentioned hypothesis, more proinflammatory cytokines can cross the BBB, because of low circulating levels of adiponectin which result in an attenuation of the inhibitory effect on proinflammatory cytokine expression (Maeda et al., 2002; Matsuda et al., 2002; Ouchi et al., 1999; Ouchi et al., 2003a; Spranger et al., 2006; Yokota et al., 2000).
Based on rodent studies, it has been proposed that adiponectin has a neuroprotective function and may induce weight loss centrally by increasing energy expenditure (Qi et al., 2004; Qiu et al., 2011). Both adipoR1 and adipoR2 receptors are functional and widely found throughout the CNS in brain microvessels, hippocampus, hypothalamus and brainstem in humans and rodent models (Fry et al., 2006; Kos et al., 2007; Kubota et al., 2007; Qiu et al., 2011; Wilkinson et al., 2007). However, in humans the influx of adiponectin into the CNS is not sufficient to represent an active transport mechanism for adiponectin across the BBB. The observed 1000-fold lower cerebrospinal fluid (CSF)/serum adiponectin concentration implies the presence of an effective barrier (Kos et al., 2007). Thus, it is still a matter of debate from whence the adiponectin in the brain originates. Is it secreted within the brain after a trigger of peripheral adipokines such as leptin, or can the small functional forms of peripheral adiponectin also cross the BBB? Remarkably, the clinical study mentioned above did not determine which isoforms of adiponectin enter the CSF (Kos et al., 2007). Other studies have shown that the trimeric and low molecular weight adiponectin forms are detectable in the CSF of humans and rodents (Kubota et al., 2007; Kusminski et al., 2007; Qi et al., 2004). Lack of HMW adiponectin in CSF may imply that only smaller forms of adiponectin are able to cross the BBB (Kubota et al., 2007; Kusminski et al., 2007; Schulz et al., 2010).
Neuroprotective effects of adiponectin are emphasized by a study showing that adiponectin-knockout mice exhibit larger brain infarctions and more neurological deficits after ischemia reperfusion compared to wild-type mice, while adenovirus-mediated supplementation of adiponectin significantly reduces cerebral infarction size in both wild-type and adiponectin-deficient mice (Nishimura et al., 2008). Furthermore, preloading of adiponectin centrally via lateral ventricle injection in mice attenuates subsequent neuronal damage from kainic acid-induced seizure activity in hippocampal neurons (Jeon et al., 2009). These results emphasize that adiponectin has a neuroprotective function (See Figure 2/Table 1).
Low plasma levels of adiponectin commonly observed in human obesity may also disrupt blood glucose regulation and fatty acid catabolism, and attenuate inhibition of inflammatory signals in the periphery(Villarreal-Molina and Antuna-Puente, 2012). Rodent studies reveal that, in the brain, deficient levels of adiponectin disrupt the normal regulation of energy expenditure, decrease neuroprotective effects, and increase the risk of cerebral infarctions (Nishimura et al., 2008; Qi et al., 2004; Qiu et al., 2011). However, it remains a matter of debate if peripheral adiponectin is able to cross the BBB to affect brain processes.
4. Angiotensinogen
Almost every tissue in the human body, including adipose tissue and the brain contain a fully functional renin-angiotensin system (RAS) (Grobe et al., 2010; Weiland and Verspohl, 2008; Yvan-Charvet and Quignard-Boulange, 2011). Initially, the role of peripheral RAS in blood pressure regulation was described, but RAS is now recognized to regulate a variety of tissue-specific functions (Crowley and Coffman, 2012; Gonzalez-Villalobos et al., 2013). In the periphery, angiotensinogen (AGT), is the only known precursor for the production of the vasoconstriction-active peptide angiotensin II (ANG II). AGT is converted into angiotensin I by renin which is secreted by the kidneys, and subsequently converted into angiotensin II (ANG II) by angiotensin converting enzyme (ACE). Via binding to angiotensin receptor 2, ANG II increases blood pressure by increasing peripheral vascular resistance, increasing sympathetic nervous system activity, and controlling sodium homeostasis. Increased levels of ANG II are therefore associated with a higher risk for hypertension (Yiannikouris et al., 2012b). A positive correlation between blood pressure and circulating levels of AGT is consistently reported (Gardes et al., 1982; Kim et al., 1995; Ohkubo et al., 1990; Walker et al., 1979; Watt et al., 1992). The primary source of AGT is the liver, but WAT is considered as the major extrahepatic source of AGT which explains higher circulating plasma levels of AGT found in obese adults (Guerre-Millo, 2004; Van Harmelen et al., 2000a; van Harmelen et al., 2000b).
Studies in animal models provide evidence that production of AGT by WAT increases circulating AGT levels in obesity, thereby inducing hypertension (Yiannikouris et al., 2012a; Yiannikouris et al., 2012b). In wild-type mice, overexpression of AGT mRNA in WAT results in elevated plasma AGT and hypertension (Massiera et al., 2001). When overexpression of AGT is induced in AGT-knockout mice, which are hypotensive and lean, re-expression of AGT mRNA in WAT is sufficient to restore WAT mass and normal blood pressure (Massiera et al., 2001). However, some human studies report that plasma concentrations of AGT are similar in obese and lean humans (Goossens et al., 2007). These unaltered plasma AGT levels could alternatively be explained by β-adrenergic stimulation, which is shown to cause release of AGT from adipose tissue and increase plasma ANG II levels (Goossens et al., 2007). It is proposed that peripheral RAS is under control of the sympathetic nervous system (Hsueh et al., 1985). In the previous described study, the control of the sympathetic nervous system was mimicked by (3-adrenergic stimulation (Goossens et al., 2007). Plasma AGT levels are unaltered in obese individuals, but the effect of increased production of AGT by the WAT on peripheral RAS stays the same through an increase in plasma ANG II in obese individuals, which is in line with the results of the previous described animal studies.
Neither renin nor angiotensin peptides are able to cross the BBB (See Figure 2) (McKinley et al., 2003; Pardridge, 1983; Reid, 1979; Severs et al., 1978). Nevertheless, the brain contains an intrinsic brain RAS (Bader and Ganten, 2002; McKinley et al., 2003). The circumventricular organs are excluded from the brain RAS, because these organs lack a BBB and are directly influenced by the peripheral RAS (McKinley et al., 2003). All RAS components are produced within the brain, for example AGT is produced in most regions of the brain by glial cells, mainly astrocytes (Stornetta et al., 1988). Within the human brain AGT can also be converted into ANG I via renin and into ANG II via ACE. ANG II influences arterial pressure at a number of brain sites (Allen et al., 1988; Andreatta et al., 1988; Averill et al., 1987; Jensen et al., 1992; McKinley et al., 2003; Severs and Daniels-Severs, 1973; Simpson, 1981; Thornton and Nicolaidis, 1993). Moreover, ANG II can be converted into angiotensin III (ANG III) and angiotensin IV (ANG IV) by aminopeptidase A and N. When ANG IV interacts with angiotensin receptor 4 it influences learning and memory mechanisms within the brain (McKinley et al., 2003). AGT, the only precursor of angiotensin peptides, has a protective role in maintaining the integrity of the BBB (Goossens et al., 2007; Pan and Kastin, 2007; Yanai et al., 2000). A study in AGT knockout mice revealed that deletion of the AGT gene is involved in impairment of BBB function (Kakinuma et al., 1998). Impairment of the BBB results in movement of peptides and proteins into and out of the brain via leaky tight junctions between endothelial cells comprising the BBB (Janzer and Raff, 1987). In response to BBB impairment, reactive astrocytes migrate to the injured area, where they proliferate, produce extracellular matrix and reconstitute the BBB (Laywell et al., 1992; Ludwin, 1985). These astrocytes express AGT, suggesting that AGT also contributes to BBB reconstitution from the CNS (Kakinuma et al., 1998; Milsted et al., 1990; Stornetta et al., 1988).
In the periphery, increased AGT production by adipose tissue leads to higher plasma ANG II levels inducing hypertension in obese individuals. Subsequently, the vascular risk factor, hypertension, damages the vasculature (Novak et al., 2003). Higher levels of BMI are also associated with disrupted BBB integrity (Gustafson et al., 2007). Thus, increased AGT production in the periphery could result in increased ANG II levels, leading to hypertension (Yiannikouris et al., 2012b), which may impair BBB permeability (Youssef et al., 2012). On the other hand, peripheral AGT may protect BBB integrity, and AGT produced by astrocytes may contribute to BBB reconstruction (Goossens et al., 2007; Pan and Kastin, 2007; Yanai et al., 2000). Thus, AGT may be an important mediator in directly regulating BBB integrity in obesity, or indirectly increasing BBB permeability via elevated ANG II levels.
Overall, whether the effects of higher AGT levels in the periphery and at the BBB are advantageous among obese individuals should be a focus of future research. In the brain's RAS, the only precursor of angiotensin peptides, AGT, induces learning and memory processes via ANG IV.
5. Plasminogen activator inhibitor-1
Plasminogen activator inhibitor-1 (PAI-1) is a member of the serpin gene family. PAI-1 influences vascular health via inhibition of fibrinolysis, a process that prevents blood clots that clog arteries (Guerre-Millo, 2004). PAI-1 causes inhibition of fibrinolysis via inhibition of tissue type plasminogen activator (tPA) and urokinase plasminogen activator (uPA) (Guerre-Millo, 2004; Loskutoff et al., 1989). However, the biological role of PAI-1 extends beyond the regulation of inflammation and fibrinolysis, since PAI-1 has been shown to influence cell migration and angiogenesis by competing with integrin binding on the extracellular matrix (Guerre-Millo, 2004). In humans, the adipokine, PAI-1, is elevated in plasma of obese children, adolescents and adults (Giordano et al., 2011; Greenberg and Obin, 2006; Mantovani et al., 2011; Singh et al., 2012). Adipocytes are a main contributor to the elevated levels of PAI-1 seen in obesity. Furthermore, excess adipose tissue and especially central obesity in adults has been associated with decreased fibrinolysis, possibly via an elevated production of PAI-1 (Skurk and Hauner, 2004).
PAI-1 transport mechanisms across the BBB are still unknown. PAI-1 produced within the brain by microglia and astrocytes may regulate apoptosis, survival of neurites and migration of microglia(Ahn et al., 1999; Jeon et al., 2012; Soeda et al., 2008; Soeda et al., 2001). In vitro experiments examining the role of PAI-1 show that sufficient PAI-1 in the extracellular environments of neurons prevents apoptotic changes (Soeda et al., 2008; Soeda et al., 2001). Thus, PAI-1 inhibits apoptosis in neurons. PAI-1 in extracellular environments may be produced by neighbouring astrocytes (Soeda et al., 2001). Moreover, in vitro studies show that PAI-1 contributes to the survival of neurites, axons and/or dendrites. PAI-1 prevents the disintegration of formed neuronal networks by maintaining or promoting neuroprotective signalling through the MAPK/ERK pathway (Soeda et al., 2008). Furthermore, PAI-1 promotes the migration of microglial cells in culture via the low-density lipoprotein receptor-related protein (LRP) 1/Janus kinase (JAK)/signal transducer and activator of transcription (STAT)1 axis (Jeon et al., 2012). Several other cell culture studies indicate that glia-derived PAI-1 may regulate microglial migration in an autocrine or paracrine manner (Ahn et al., 1999; Jeon et al., 2012). Microglia, the resident macrophages of the CNS, constitute the brain's innate immune system and play a pivotal role in neuroinflammation and host defense against microbial agents (Block et al., 2007; Garden and Moller, 2006; Graeber and Streit, 2010; Jeon et al., 2012; Mallat et al., 2005; Woo et al., 2008). Thus, PAI-1 is an important adipokine involved in the regulation of microglial migration, thereby affecting neuroinflammation and the immune system in the brain.
Considering a function for PAI-1 in a non-obese hypothalamus, it is interesting to mention that transgenic mice overexpressing urokinase-type plasminogen activator (uPA) in several brain regions, including the paraventricular nucleus of the hypothalamus, also exhibit reduced energy intake, and body weight and size (Masos and Miskin, 1997; Miskin and Masos, 1997). PAI-1 is considered as the primary inhibitor of uPA (Masos and Miskin, 1997). Thus, overexpression of PAI-1 within the hypothalamus could attenuate uPA expression and thereby increase energy intake, and body weight and size, which are all three important parameters in obesity.
In the periphery, PAI-1 inhibits fibrinolysis and regulates cell migration and angiogenesis (See Figure 1/Table 1). Even today, PAI-1 transport mechanisms across the BBB are unknown, so only PAI-1 produced by microglia has been shown to regulate apoptosis, energy intake, survival of neurites, microglia migration, neuroinflammation and the brain's immune system (Ahn et al., 1999; Jeon et al., 2012; Masos and Miskin, 1997; Miskin and Masos, 1997; Soeda et al., 2008; Soeda et al., 2001). Although, it should be mentioned that these results are primarily obtained from in vitro experiments, thus it is still questionable if these processes are affected in human obesity. In the plasma of obese individuals, high levels of PAI-1 are found, which could lead to decreased fibrinolysis. If the effects of excessive PAI-1 levels within the brain in obesity are neuroprotective or even damaging is still under debate. Additionally, peripheral PAI-1 among other proteins produced by adipose tissue is not capable of affecting brain processes, since no transport mechanism for PAI-1 across the BBB has been discovered. However, PAI-1 produced by microglia may be modified by obesity. Therefore, more research is needed on the effects of obesity on PAI-1 levels in the brain.
6. Interleukin-6
The inflammatory cytokine, IL-6, is produced by adipocytes, macrophages and T-cells, and is involved in the acute phase reaction in inflammation (See Figure 2). In the liver, IL-6 stimulates the production of acute phase proteins such as C-reactive protein and fibrinogen. Obesity is marked by a peripheral chronic low inflammation state partly mediated via production of inflammatory adipokines such as IL-6 and TNF-α (Das, 2001). This chronic low inflammation state is already observed in obese children, manifested especially by elevated concentrations of IL-6 (Stelzer et al., 2012; Tam et al., 2010; Visser, 2001), which persist during adolescence and into adulthood (Stelzer et al., 2012). Furthermore, another inflammatory state is found in obese individuals, as examination of their brains via magnetic resonance imaging (MRI) showed increased gliosis in the mediobasal hypothalamus (Thaler et al., 2012).
Radioactively labelled IL-6 has been shown to cross the BBB by a saturable transport mechanism, entering both CSF and brain parenchyma. Approximately 50% of IL-6 in the CSF and 16% in brain parenchyma represent intact peripheral cytokine in male mice (Banks et al., 1994). However, excessive degradation of IL-6 is observed in the brain, thus the relative contribution of peripheral IL-6 on actions in the CNS is not clear. Nevertheless, relatively small amounts of intact IL-6 may be sufficient to produce biological effects (Banks et al., 1994). Rodent studies show that within the brain IL-6 is produced by glial cells, astrocytes and endothelial cells of the brain's microvessels (Fabry et al., 1993; Frei et al., 1989; Lieberman et al., 1989). A association exits between higher peripheral plasma IL-6 levels and lower hippocampal grey matter volume in middle-aged adults (Marsland et al., 2008; Yaffe et al., 2007). These findings possibly indicate that peripheral IL-6 mediates cognitive decline (Marsland et al., 2008). In addition, animal models show that peripheral inflammation increases the production of IL-6 in the brain, activating glial cells in the hippocampus, which in turn, inhibit neurogenesis and decrease synaptic plasticity, thereby disrupting learning and memory processes (Balschun et al., 2004; Gibertini et al., 1995; Monje et al., 2003; Poluektova et al., 2005).
Normally, IL-6 is expressed at relatively low levels within the brain (Gadient and Otten, 1997). When overexpression of IL-6 is stimulated in granule cells in a mouse hippocampus, the modulatory role of IL-6 on synaptic plasticity is revealed (Wei et al., 2012). IL-6 overexpression stimulates the formation of granule cell excitatory synapses, without affecting inhibitory synapses (Wei et al., 2012). Moreover, these cells are affected in granule cell adhesion and migration, suggesting that IL-6 is involved in the regulation of cell adhesion molecules that critically modulate excitatory synaptic formation (Wei et al., 2011). In mouse hippocampus, IL-6 elevation caused alterations in excitatory and inhibitory synaptic formations and disrupted the balance of excitatory/inhibitory synaptic transmissions, causing abnormal changes in the shape, length and distribution of dendritic spines (Wei et al., 2012). Another rodent study demonstrated that high IL-6 levels are neurotoxic and associated with maternal infection and neurodevelopmental damage (Samuelsson et al., 2006). It was even demonstrated that excessive prenatal exposure to IL-6 is noxious for CNS function, as it may play a role in the origin of neurodevelopmental and neurodegenerative diseases (Samuelsson et al., 2006).
Exercise in rodents increases the central anti-inflammatory response in the hypothalamus. IL-6 induces an anti-inflammatory environment by inducing the production of interleukin-10 (IL-10) (Ropelle et al., 2010). This phenomenon is crucial for restoring hypothalamic insulin and leptin signaling, and reorganizing the set point of energy (Ropelle et al., 2010). Moreover, another rodent study revealed that in the hypothalamus saturated fatty acids trigger the intracellular signaling network that induces an inflammatory response which includes IL-6, and determines resistance to anorexigenic signals (Milanski et al., 2009). Thus, in obesity IL-6 production is triggered by lack of exercise and monounsaturated fat-rich diets, which can lead to resistance to anorexigenic signals in the hypothalamus (Milanski et al., 2009; Ropelle et al., 2010). It remains to be seen what the influence of other dietary components and exercise may be.
The hippocampus is particularly vulnerable to the adverse effects of IL-6. IL-6 originating from the periphery, as well as glial and endothelial cells in the brain's microvessels, affects brain functions like synaptic plasticity and neurogenesis. In the hypothalamus, IL-6 is able to modify leptin signaling and other anorexic signals. Although it should be mentioned that peripheral IL-6 is quickly degraded in the brain. An early onset of IL-6 elevation and its persistence in aging obese individuals have been proposed to negatively affect brain functioning by inhibiting neurogenesis, decreasing synaptic plasticity and subsequently disrupting learning and memory processes (See Figure 2/Table 1) particularly in the hippocampus, which increases the risk of cognitive deficits in obese individuals (Yaffe et al., 2004).
7. TNF-α
TNF-α regulates the acute phase reaction of inflammation (See Figure 2), and is therefore an important mediator of the chronic inflammation found in obesity in the periphery and hypothalamus (Das, 2001; Thaler et al., 2012). In obese adults, elevated plasma levels of TNF-α have been observed when compared to normal weight adults (Mousa, 2005). In the periphery, TNF-α is produced by adipocytes and macrophages, and binds to two receptors, TNF-R1 and TNF-R2. TNF-R1 is expressed in most peripheral tissues and is activated via membrane bound soluble trimeric forms of TNF-α. Meanwhile, TNF-R2 is only expressed on cells of the immune system and is activated by the membrane bound homotrimer, TNF-α. In obese children, no increase in plasma TNF-α levels was observed as in obese adults, but obese children evidenced higher levels of TNF-R2, when compared to non-obese children (Schipper et al., 2012).
Rodent studies reveal that TNF-α is transported across the BBB (Di Simone et al., 2006), and can also be produced by astrocytes, microglia and some neurons within the brain (Chung and Benveniste, 1990; Lieberman et al., 1989; Morganti-Kossman et al., 1997). TNF-α receptors are expressed on neurons and glial cells throughout the CNS and can trigger processes like apoptosis (Montgomery and Bowers, 2012; Pickering et al., 2005). Within the brain TNF-α regulates synaptic transmission, synaptic plasticity and neurogenesis. As a result, it has a broad range of actions which can be either neuroprotective or neurotoxic.
TNF-α in vitro may protect neurons against metabolic, excitotoxic or oxidative insults by promoting maintenance of intracellular calcium homeostasis and suppression of reactive oxygen species (Barger et al., 1995; Cheng et al., 1994). TNF-α has direct effects on glutamate transmission by increasing the expression of AMPA receptors on synapses (Beattie et al., 2002). Furthermore, TNF-α originating from glial cells causes an increase in surface expression of neuronal AMPA receptors (Beattie et al., 2002). Thus, TNF-α is able to increase glutamate transmission by increasing the expression of AMPA receptors.
Examples of neurotoxic effects of TNF-α are its involvement in damage to myelin and oligodendrocytes (Selmaj and Raine, 1988). It also plays a facilitating role in glutamate excitotoxicity both directly as described above, and indirectly by inhibiting glial glutamate transporters on astrocytes. Decreased expression of glutamate transporters was caused by TNF-α inducing the classical I kappa B (IκB) degradation pathway to trigger NF-κB nuclear translocation and DNA binding to repress EAAT2 expression (Sitcheran et al., 2005). In this situation, the presence of elevated TNF-α concentrations led to an elevated extracellular glutamate concentration, thereby increasing the risk of glutamate excitotoxicity (Pickering et al., 2005). It was demonstrated that the combination of glutamate and TNF-α provoked an amplified neurotoxic effect on the AMPA receptor (Hermann et al., 2001). Furthermore, TNF-α mediates synaptic plasticity by inhibiting long-term potentiation (LTP) during the early phase of LTP by activation of TNF-R1 depending on p38 activation (Pickering et al., 2005). TNF-α also inhibits LTP in the CA1 and dentate gyrus regions of the rat hippocampus at pathophysiological levels (Butler et al., 2004; Cunningham et al., 1996; Tancredi et al., 1992). In humans and mice, differences in cognitive performance have been associated with TNF-α gene polymorphisms and TNF-α knockout mice display cognitive dysfunctions (Baune et al., 2008a; Baune et al., 2008b; Beste et al., 2010). Furthermore, TNF-α levels are elevated in several potential neuropathological states, and are associated with learning and memory deficits. In rodent models, it has been revealed that TNF-alpha, in a dose-dependent manner, modulates leptin signaling and action in the hypothalamus (Romanatto et al., 2007). Thus, in the hypothalamus, TNF-α produces a potent anorexic effect.
TNF-α may also have an anti-neurogenic effect during adult neurogenesis. In culture, microglia secreting TNF-α were found to be detrimental to hippocampal progenitor cells (HPC) by abruptly halting cell division which led to progenitor cell death (Cacci et al., 2005). Nevertheless, others have shown that striatal and hippocampal neurogenesis is compromised when an antibody to TNF-α is transiently infused into the lateral ventricle of a rat stroke model, indicating that TNF-α encourages the survival of neural progenitor cells (Hermann et al., 2001; Marchetti et al., 2004). These neuroprotective effects of TNF-α could be mediated through the TNF-R2 (Hermann et al., 2001; Marchetti et al., 2004). These findings demonstrate that TNF-α has positive and negative effects during adult neurogenesis as described above. Positive effects occur via encouraging survival of neural progenitor cells in a rat stroke model and negative effects occur via abruptly halting hippocampal progenitor cell division. These different effects could be triggered and dependent upon induction context and receptor sub-type engagement (Montgomery and Bowers, 2012).
The actions of TNF- α on neurons may be either neuroprotective or neurotoxic via its receptors and fluctuations in TNF- α levels. In obese individuals, excessive production of TNF-α by WAT could give onset to decreased adult neurogenesis by halting cell division of hippocampal progenitor cells, impaired LTP facilitation with subsequent memory and learning deficits, increased glutamate excitotoxicity and damaged myelin and oligodendrocytes (See Figure 2/Table 1).
8. Conclusion
After a decade of research on overweight and obesity in AD, a research focus on the endocrine aspects of adipose tissue and the brain has been birthed and escalated. Of the adipokines reviewed herein, it is known that peripheral leptin, TNF-α and IL-6 are able to cross the BBB and affect brain function (See Figure 2/Table 1). However, the other adipokines discussed including adiponectin, AGT and PAI-1, are either not able to cross the BBB, or possible transport mechanisms have not been identified. In obesity, excessive adipokine production by WAT mediates characteristic peripheral pathological processes like imbalanced energy metabolism, inflammation and hypertension. Several effects of adipokines on the brain increase vulnerability to the development of pathological processes in the brain. Adipose tissue secretes a multitude of compounds other than those discussed here, including for example, visfatin, resistin, apelin and chemerin (Adeghate, 2008; Guo et al., 2009; Lehr et al., 2012; Leivo-Korpela et al., 2011; MacDougald and Burant, 2007), for which even less is known about interactions with the brain. While epidemiologic studies have already shown a higher risk of dementia and brain-associated events in association with overweight and obesity during mid-life and to some extent late-life, the endocrine aspects of adipose tissue in relation to these outcomes are virtually unexplored, and could be useful for refining the adipose tissue exposure. Given secular increases in the prevalence of overweight and obesity due to a rise in the proportion obese (≥ 30 kg/m2) in particular(Ogden and Carroll, 2010), it remains to be seen whether the continued increasing occurrence of obesity continues to lead to impaired processes within the brain such as impaired neurogenesis and LTP. This would lead to more severe cognitive impairments and decline and perhaps even higher risk of dementia (Gustafson, 2008; Gustafson et al., 2007). Alternatively, improvements in health and care, education, and other social factors may dilute future observations of the influence of obesity on the brain. Further research must focus on the crosstalk between WAT-secreted adipokines and the brain to elucidate when and via which mechanisms brain processes become affected by these important endocrine mediators. In addition, whether the mechanisms associating these hormones in the obese condition with brain health, are protective or detrimental, remains to be further elucidated.
Highlights.
Adipokines from periphery and brain interact with obesity processes.
Obesity is associated with changes in brain structure, function and even dementia.
Adipokines have a widespread influence and function on brain health.
Adipokines may form a potential mechanism, which links obesity with brain health.
Acknowledgments
This study was supported by the EU 7th framework LipiDiDiet project (FP7/2007-2013) under grant agreement no211696; in part by grants NIH/NIAID 1R01MH076537, 1R01MH079880, and U01 318345; Swedish Research Council Diarienummer: 523-2005-8460, and the State University of New York Research Foundation.
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
Contributors: Deborah R. Gustafson was invited to submit this review. Subsequently, all contributors, Ilse AC Arnoldussen, Amanda J Kiliaan, and Deborah R Gustafson, participated equally in the drafting of this review manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ilse AC Arnoldussen, Email: I.Arnoldussen@anat.umcn.nl.
Amanda J Kiliaan, Email: A.Kiliaan@anat.umcn.nl.
Deborah R Gustafson, Email: deborah.gustafson@neuro.gu.se, deborah.gustafson@downstate.edu.
References
- Adeghate E. Visfatin: structure, function and relation to diabetes mellitus and other dysfunctions. Curr Med Chem. 2008;15:1851–1862. doi: 10.2174/092986708785133004. [DOI] [PubMed] [Google Scholar]
- Ahima RS. Adipose tissue as an endocrine organ. Obesity (Silver Spring) 2006;14(Suppl 5):242S–249S. doi: 10.1038/oby.2006.317. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Bjorbaek C, Osei S, Flier JS. Regulation of neuronal and glial proteins by leptin: implications for brain development. Endocrinology. 1999;140:2755–2762. doi: 10.1210/endo.140.6.6774. [DOI] [PubMed] [Google Scholar]
- Ahn MY, Zhang ZG, Tsang W, Chopp M. Endogenous plasminogen activator expression after embolic focal cerebral ischemia in mice. Brain Res. 1999;837:169–176. doi: 10.1016/s0006-8993(99)01645-5. [DOI] [PubMed] [Google Scholar]
- Allen AM, Dampney RA, Mendelsohn FA. Angiotensin receptor binding and pressor effects in cat subretrofacial nucleus. Am J Physiol. 1988;255:H1011–1017. doi: 10.1152/ajpheart.1988.255.5.H1011. [DOI] [PubMed] [Google Scholar]
- Andreatta SH, Averill DB, Santos RA, Ferrario CM. The ventrolateral medulla. A new site of action of the renin-angiotensin system. Hypertension. 1988;11:I163–166. doi: 10.1161/01.hyp.11.2_pt_2.i163. [DOI] [PubMed] [Google Scholar]
- Arch JR, Stock MJ, Trayhurn P. Leptin resistance in obese humans: does it exist and what does it mean? Int J Obes Relat Metab Disord. 1998;22:1159–1163. doi: 10.1038/sj.ijo.0800779. [DOI] [PubMed] [Google Scholar]
- Averill DB, Diz DI, Barnes KL, Ferrario CM. Pressor responses of angiotensin II microinjected into the dorsomedial medulla of the dog. Brain Res. 1987;414:294–300. doi: 10.1016/0006-8993(87)90009-6. [DOI] [PubMed] [Google Scholar]
- Bader M, Ganten D. It's renin in the brain: transgenic animals elucidate the brain renin angiotensin system. Circ Res. 2002;90:8–10. [PubMed] [Google Scholar]
- Balschun D, Wetzel W, Del Rey A, Pitossi F, Schneider H, Zuschratter W, Besedovsky HO. Interleukin-6: a cytokine to forget. FASEBJ. 2004;18:1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- Banks WA. Denial versus dualism: the blood-brain barrier as an interface of the gut-brain axis. Endocrinology. 2006;147:2609–2610. doi: 10.1210/en.2006-0335. [DOI] [PubMed] [Google Scholar]
- Banks WA, Clever CM, Farrell CL. Partial saturation and regional variation in the blood-to-brain transport of leptin in normal weight mice. Am J Physiol Endocrinol Metab. 2000;278:E1158–1165. doi: 10.1152/ajpendo.2000.278.6.E1158. [DOI] [PubMed] [Google Scholar]
- Banks WA, DiPalma CR, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity. Peptides. 1999;20:1341–1345. doi: 10.1016/s0196-9781(99)00139-4. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci Lett. 1994;179:53–56. doi: 10.1016/0304-3940(94)90933-4. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- Banks WA, Lebel CR. Strategies for the delivery of leptin to the CNS. Journal of drug targeting. 2002;10:297–308. doi: 10.1080/10611860290031895. [DOI] [PubMed] [Google Scholar]
- Barger SW, Horster D, Furukawa K, Goodman Y, Krieglstein J, Mattson MP. Tumor necrosis factors alpha and beta protect neurons against amyloid beta-peptide toxicity: evidence for involvement of a kappa B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc Natl Acad Sci U S A. 1995;92:9328–9332. doi: 10.1073/pnas.92.20.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, Lai CF, Tartaglia LA. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci U S A. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baune BT, Ponath G, Rothermundt M, Riess O, Funke H, Berger K. Association between genetic variants of IL-1beta, IL-6 and TNF-alpha cytokines and cognitive performance in the elderly general population of the MEMO-study. Psychoneuroendocrinology. 2008a;33:68–76. doi: 10.1016/j.psyneuen.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Baune BT, Wiede F, Braun A, Golledge J, Arolt V, Koerner H. Cognitive dysfunction in mice deficient for TNF- and its receptors. American journal of medical genetics. Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2008b;147B:1056–1064. doi: 10.1002/ajmg.b.30712. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Beltowski J. Adiponectin and resistin∼new hormones of white adipose tissue. Medical science monitor: international medical journal of experimental and clinical research. 2003;9:RA55–61. [PubMed] [Google Scholar]
- Besser LM, Gill DP, Monsell SE, Brenowitz W, Moranus D, Kukull WA, Gustafson DR. Body Mass Index, weight change, and clinical progression in Mild Cognitive Impairment and Alzheimer's Disease. Alz Dis Assoc Dis. 2013 doi: 10.1097/WAD.0000000000000005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste C, Baune BT, Falkenstein M, Konrad C. Variations in the TNF-alpha gene (TNF-alpha-308G-->A) affect attention and action selection mechanisms in a dissociated fashion. J Neurophysiol. 2010;104:2523–2531. doi: 10.1152/jn.00561.2010. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Molecular cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Kahn BB. Leptin signaling in the central nervous system and the periphery. Recent Prog Horm Res. 2004;59:305–331. doi: 10.1210/rp.59.1.305. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272:32686–32695. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nature reviews. Neuroscience. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Bouret SG. Neurodevelopmental actions of leptin. Brain Res. 2010;1350:2–9. doi: 10.1016/j.brainres.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray GA, Popkin BM. Dietary fat intake does affect obesity! Am J Clin Nutr. 1998;68:1157–1173. doi: 10.1093/ajcn/68.6.1157. [DOI] [PubMed] [Google Scholar]
- Brooks SJ, Benedict C, Burgos J, Kempton MJ, Kullberg J, Nordenskjold R, Kilander L, Nylander R, Larsson EM, Johansson L, Ahlstrom H, Lind L, Schioth HB. Late-life obesity is associated with smaller global and regional gray matter volumes: a voxel-based morphometric study. Int J Obes (Lond) 2012 doi: 10.1038/ijo.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R, Imran SA, Belsham DD, Ur E, Wilkinson M. Adipokine gene expression in a novel hypothalamic neuronal cell line: resistin-dependent regulation of fasting-induced adipose factor and SOCS-3. Neuroendocrinology. 2007;85:232–241. doi: 10.1159/000104248. [DOI] [PubMed] [Google Scholar]
- Brown R, Thompson HJ, Imran SA, Ur E, Wilkinson M. Traumatic brain injury induces adipokine gene expression in rat brain. Neurosci Lett. 2008;432:73–78. doi: 10.1016/j.neulet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burguera B, Couce ME, Curran GL, Jensen MD, Lloyd RV, Cleary MP, Poduslo JF. Obesity is associated with a decreased leptin transport across the blood-brain barrier in rats. Diabetes. 2000a;49:1219–1223. doi: 10.2337/diabetes.49.7.1219. [DOI] [PubMed] [Google Scholar]
- Burguera B, Couce ME, Long J, Lamsam J, Laakso K, Jensen MD, Parisi JE, Lloyd RV. The long form of the leptin receptor (OB-Rb) is widely expressed in the human brain. Neuroendocrinology. 2000b;71:187–195. doi: 10.1159/000054536. [DOI] [PubMed] [Google Scholar]
- Businaro R, Ippoliti F, Ricci S, Canitano N, Fuso A. Alzheimer's disease promotion by obesity: induced mechanisms-molecular links and perspectives. Curr Gerontol Geriatr Res. 2012;2012:986823. doi: 10.1155/2012/986823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MP, O'Connor JJ, Moynagh PN. Dissection of tumor-necrosis factor-alpha inhibition of long-term potentiation (LTP) reveals a p38 mitogen-activated protein kinase-dependent mechanism which maps to early-but not late-phase LTP. Neuroscience. 2004;124:319–326. doi: 10.1016/j.neuroscience.2003.11.040. [DOI] [PubMed] [Google Scholar]
- Cacci E, Claasen JH, Kokaia Z. Microglia-derived tumor necrosis factor-alpha exaggerates death of newborn hippocampal progenitor cells in vitro. J Neurosci Res. 2005;80:789–797. doi: 10.1002/jnr.20531. [DOI] [PubMed] [Google Scholar]
- Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–161. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- Cazettes F, Cohen JI, Yau PL, Talbot H, Convit A. Obesity-mediated inflammation may damage the brain circuit that regulates food intake. Brain Res. 2011;1373:101–109. doi: 10.1016/j.brainres.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B, Christakos S, Mattson MP. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron. 1994;12:139–153. doi: 10.1016/0896-6273(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Chung IY, Benveniste EN. Tumor necrosis factor-alpha production by astrocytes. Induction by lipopolysaccharide, IFN-gamma, and IL-1 beta. J Immunol. 1990;144:2999–3007. [PubMed] [Google Scholar]
- Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Crowley SD, Coffman TM. Recent advances involving the renin-angiotensin system. Exp Cell Res. 2012;318:1049–1056. doi: 10.1016/j.yexcr.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AJ, Murray CA, O'Neill LA, Lynch MA, O'Connor JJ. Interleukin-1 beta (IL-1 beta) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci Lett. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17:953–966. doi: 10.1016/s0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- Delgado TC, Violante IR, Nieto-Charques L, Cerdan S. Neuroglial metabolic compartmentation underlying leptin deficiency in the obese ob/ob mice as detected by magnetic resonance imaging and spectroscopy methods. J Cereb Blood Flow Metab. 2011;31:2257–2266. doi: 10.1038/jcbfm.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Simone N, Di Nicuolo F, Sanguinetti M, Castellani R, D'Asta M, Caforio L, Caruso A. Resistin regulates human choriocarcinoma cell invasive behaviour and endothelial cell angiogenic processes. J Endocrinol. 2006;189:691–699. doi: 10.1677/joe.1.06610. [DOI] [PubMed] [Google Scholar]
- Diez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 2003;148:293–300. doi: 10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- Doherty GH. Obesity and the ageing brain: could leptin play a role in neurodegeneration? Curr Gerontol Geriatr Res. 2011;2011:708154. doi: 10.1155/2011/708154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105:1827–1832. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabry Z, Fitzsimmons KM, Herlein JA, Moninger TO, Dobbs MB, Hart MN. Production of the cytokines interleukin 1 and 6 by murine brain microvessel endothelium and smooth muscle pericytes. J Neuroimmunol. 1993;47:23–34. doi: 10.1016/0165-5728(93)90281-3. [DOI] [PubMed] [Google Scholar]
- Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2006;27:1420–1425. doi: 10.1016/j.peptides.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O'Meara ES, Longstreth WT, Jr, Luchsinger JA. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch AF, Agarwal N, Roberts MD, Han JC, Theim KR, Vexler A, Troendle J, Yanovski SZ, Yanovski JA. Influence of serum leptin on weight and body fat growth in children at high risk for adult obesity. J Clin Endocrinol Metab. 2007;92:948–954. doi: 10.1210/jc.2006-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foschini D, Santos RV, Prado WL, de Piano A, Lofrano MC, Martins AC, Carnier J, Caranti DA, Sanches Pde L, Tock L, Mello MT, Tufik S, Damaso AR. Platelet and leptin in obese adolescents. J Pediatr (Rio J) 2008;84:516–521. doi: 10.2223/JPED.1845. [DOI] [PubMed] [Google Scholar]
- Frei K, Malipiero UV, Leist TP, Zinkernagel RM, Schwab ME, Fontana A. On the cellular source and function of interleukin 6 produced in the central nervous system in viral diseases. Eur J Immunol. 1989;19:689–694. doi: 10.1002/eji.1830190418. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393:7–20. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry M, Smith PM, Hoyda TD, Duncan M, Ahima RS, Sharkey KA, Ferguson AV. Area postrema neurons are modulated by the adipocyte hormone adiponectin. J Neurosci. 2006;26:9695–9702. doi: 10.1523/JNEUROSCI.2014-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Gadient RA, Otten UH. Interleukin-6 (IL-6)—a molecule with both beneficial and destructive potentials. Prog Neurobiol. 1997;52:379–390. doi: 10.1016/s0301-0082(97)00021-x. [DOI] [PubMed] [Google Scholar]
- Garden GA, Moller T. Microglia biology in health and disease. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- Gardes J, Bouhnik J, Clauser E, Corvol P, Menard J. Role of angiotensinogen in blood pressure homeostasis. Hypertension. 1982;4:185–189. doi: 10.1161/01.hyp.4.2.185. [DOI] [PubMed] [Google Scholar]
- Gibertini M, Newton C, Friedman H, Klein TW. Spatial learning impairment in mice infected with Legionella pneumophila or administered exogenous interleukin-1-beta. Brain Behav Immun. 1995;9:113–128. doi: 10.1006/brbi.1995.1012. [DOI] [PubMed] [Google Scholar]
- Giordano R, Picu A, Marinazzo E, D'Angelo V, Berardelli R, Karamouzis I, Forno D, Zinna D, Maccario M, Ghigo E, Arvat E. Metabolic and cardiovascular outcomes in patients with Cushing's syndrome of different aetiologies during active disease and 1 year after remission. Clin Endocrinol (Oxf) 2011;75:354–360. doi: 10.1111/j.1365-2265.2011.04055.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Villalobos RA, Shen XZ, Bernstein EA, Janjulia T, Taylor B, Giani JF, Blackwell WL, Shah KH, Shi PD, Fuchs S, Bernstein KE. Rediscovering ACE: novel insights into the many roles of the angiotensin-converting enzyme. J Mol Med (Berl) 2013 doi: 10.1007/s00109-013-1051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens GH, Jocken JW, Blaak EE, Schiffers PM, Saris WH, van Baak MA. Endocrine role of the renin-angiotensin system in human adipose tissue and muscle: effect of beta-adrenergic stimulation. Hypertension. 2007;49:542–547. doi: 10.1161/01.HYP.0000256091.55393.92. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2010;119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143:239–246. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, de Lange WJ, Li H, Sakai K, Thedens DR, Cassis LA, Rahmouni K, Mark AL, Johnson AK, Sigmund CD. The brain Renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab. 2010;12:431–442. doi: 10.1016/j.cmet.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan XM, Hess JF, Yu H, Hey PJ, van der Ploeg LH. Differential expression of mRNA for leptin receptor isoforms in the rat brain. Mol Cell Endocrinol. 1997;133:1–7. doi: 10.1016/s0303-7207(97)00138-x. [DOI] [PubMed] [Google Scholar]
- Guerre-Millo M. Adipose tissue and adipokines: for better or worse. Diabetes Metab. 2004;30:13–19. doi: 10.1016/s1262-3636(07)70084-8. [DOI] [PubMed] [Google Scholar]
- Guo L, Li Q, Wang W, Yu P, Pan H, Li P, Sun Y, Zhang J. Apelin inhibits insulin secretion in pancreatic beta-cells by activation of PI3-kinase-phosphodiesterase 3B. Endocr Res. 2009;34:142–154. doi: 10.3109/07435800903287079. [DOI] [PubMed] [Google Scholar]
- Gustafson D. Adiposity indices and dementia. Lancet Neurol. 2006;5:713–720. doi: 10.1016/S1474-4422(06)70526-9. [DOI] [PubMed] [Google Scholar]
- Gustafson D, Bäckman K, Joas E, Waern M, Östling S, Guo X, Skoog I. A 37-year longitudinal follow-up of body mass index and dementia in women. J Alzheimers Dis. 2012;28:162–171. [Google Scholar]
- Gustafson DR. A life course of adiposity and dementia. Eur J Pharmacol. 2008;585:163–175. doi: 10.1016/j.ejphar.2008.01.052. [DOI] [PubMed] [Google Scholar]
- Gustafson DR. Adiposity hormones and dementia. J Neurol Sci. 2010;299:30–34. doi: 10.1016/j.jns.2010.08.036. [DOI] [PubMed] [Google Scholar]
- Gustafson DR. Positive correlation between leptin and body weight or BMI. In: Communication, P, editor. Also, elderly women and men show the similar positive correlation between leptin and body weight or BMI ed. 2012. [Google Scholar]
- Gustafson DR, Bäckman K, Waern M, Östling S, Guo XX, Zandi P, Mielke MM, Bengtsson C, Skoog I. Adiposity indicators and dementia over 32 years in Sweden. Neurology. 2009;73:1559–1566. doi: 10.1212/WNL.0b013e3181c0d4b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson DR, Karlsson C, Skoog I, Rosengren L, Lissner L, Blennow K. Mid-life adiposity factors relate to blood-brain barrier integrity in late life. J Intern Med. 2007;262:643–650. doi: 10.1111/j.1365-2796.2007.01869.x. [DOI] [PubMed] [Google Scholar]
- Gustafson DR, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow up of overweight and risk for Alzheimer's disease. Arch Intern Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- Gustafson DR, Steen B, Skoog I. Body mass index and white matter lesions in elderly women. An 18-year longitudinal study. Int Psychogeriatr. 2004;16:327–336. doi: 10.1017/s1041610204000353. [DOI] [PubMed] [Google Scholar]
- Haltia LT, Viljanen A, Parkkola R, Kemppainen N, Rinne JO, Nuutila P, Kaasinen V. Brain white matter expansion in human obesity and the recovering effect of dieting. J Clin Endocrinol Metab. 2007;92:3278–3284. doi: 10.1210/jc.2006-2495. [DOI] [PubMed] [Google Scholar]
- Harrison N. Inflammation and mental illness. J Neurol Neurosurg Psychiatry. 2013;84:e1. [Google Scholar]
- Harvey J. Leptin regulation of neuronal excitability and cognitive function. Current opinion in pharmacology. 2007;7:643–647. doi: 10.1016/j.coph.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassenstab JJ, Sweet LH, Del Parigi A, McCaffery JM, Haley AP, Demos KE, Cohen RA, Wing RR. Cortical thickness of the cognitive control network in obesity and successful weight loss maintenance: a preliminary MRI study. Psychiatry Res. 2012;202:77–79. doi: 10.1016/j.pscychresns.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden KM, Zandi PP, Lyketsos CG, Khachaturian AS, Bastian LA, Charoonruk G, Tschanz JT, Norton MC, Pieper CF, Munger RG, Breitner JC, Welsh-Bohmer KA, Cache County I. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis Assoc Disord. 2006;20:93–100. doi: 10.1097/01.wad.0000213814.43047.86. [DOI] [PubMed] [Google Scholar]
- Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, Grill HJ. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 2010;11:77–83. doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann GE, Rogers RC, Bresnahan JC, Beattie MS. Tumor necrosis factor-alpha induces cFOS and strongly potentiates glutamate-mediated cell death in the rat spinal cord. Neurobiol Dis. 2001;8:590–599. doi: 10.1006/nbdi.2001.0414. [DOI] [PubMed] [Google Scholar]
- Hileman SM, Pierroz DD, Masuzaki H, Bjorbaek C, El-Haschimi K, Banks WA, Flier JS. Characterizaton of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology. 2002;143:775–783. doi: 10.1210/endo.143.3.8669. [DOI] [PubMed] [Google Scholar]
- Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, Bauer SM, Wade M, Singhal E, Cheng CC, Volk K, Kuo MS, Gordillo R, Kharitonenkov A, Scherer PE. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17:790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Hsueh WA, Goldstone R, Carlson EJ, Horton R. Evidence that the beta-adrenergic system and prostaglandins stimulate renin release through different mechanisms. J Clin Endocrinol Metab. 1985;61:399–403. doi: 10.1210/jcem-61-3-399. [DOI] [PubMed] [Google Scholar]
- Huang XF, Koutcherov I, Lin S, Wang HQ, Storlien L. Localization of leptin receptor mRNA expression in mouse brain. Neuroreport. 1996;7:2635–2638. doi: 10.1097/00001756-199611040-00045. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7:476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Jensen LL, Harding JW, Wright JW. Role of paraventricular nucleus in control of blood pressure and drinking in rats. Am J Physiol. 1992;262:F1068–1075. doi: 10.1152/ajprenal.1992.262.6.F1068. [DOI] [PubMed] [Google Scholar]
- Jeon BT, Shin HJ, Kim JB, Kim YK, Lee DH, Kim KH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Adiponectin protects hippocampal neurons against kainic acid-induced excitotoxicity. Brain research reviews. 2009;61:81–88. doi: 10.1016/j.brainresrev.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Jeon H, Kim JH, Kim JH, Lee WH, Lee MS, Suk K. Plasminogen activator inhibitor type 1 regulates microglial motility and phagocytic activity. J Neuroinflammation. 2012;9:149. doi: 10.1186/1742-2094-9-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jequier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci. 2002;967:379–388. doi: 10.1111/j.1749-6632.2002.tb04293.x. [DOI] [PubMed] [Google Scholar]
- Kakinuma Y, Hama H, Sugiyama F, Yagami K, Goto K, Murakami K, Fukamizu A. Impaired blood-brain barrier function in angiotensinogen-deficient mice. Nat Med. 1998;4:1078–1080. doi: 10.1038/2070. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Pan W, Maness LM, Banks WA. Peptides crossing the blood-brain barrier: some unusual observations. Brain Res. 1999a;848:96–100. doi: 10.1016/s0006-8993(99)01961-7. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Pan W, Maness LM, Koletsky RJ, Ernsberger P. Decreased transport of leptin across the blood-brain barrier in rats lacking the short form of the leptin receptor. Peptides. 1999b;20:1449–1453. doi: 10.1016/s0196-9781(99)00156-4. [DOI] [PubMed] [Google Scholar]
- Kim HS, Krege JH, Kluckman KD, Hagaman JR, Hodgin JB, Best CF, Jennette JC, Coffman TM, Maeda N, Smithies O. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci U S A. 1995;92:2735–2739. doi: 10.1073/pnas.92.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- Kos K, Harte AL, da Silva NF, Tonchev A, Chaldakov G, James S, Snead DR, Hoggart B, O'Hare JP, McTernan PG, Kumar S. Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. J Clin Endocrinol Metab. 2007;92:1129–1136. doi: 10.1210/jc.2006-1841. [DOI] [PubMed] [Google Scholar]
- Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Kusminski CM, McTernan PG, Schraw T, Kos K, O'Hare JP, Ahima R, Kumar S, Scherer PE. Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia. 2007;50:634–642. doi: 10.1007/s00125-006-0577-9. [DOI] [PubMed] [Google Scholar]
- Laywell ED, Dorries U, Bartsch U, Faissner A, Schachner M, Steindler DA. Enhanced expression of the developmentally regulated extracellular matrix molecule tenascin following adult brain injury. Proc Natl Acad Sci U S A. 1992;89:2634–2638. doi: 10.1073/pnas.89.7.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehr S, Hartwig S, Lamers D, Famulla S, Muller S, Hanisch FG, Cuvelier C, Ruige J, Eckardt K, Ouwens DM, Sell H, Eckel J. Identification and validation of novel adipokines released from primary human adipocytes. Molecular & cellular proteomics: MCP. 2012;11:M111 010504. doi: 10.1074/mcp.M111.010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivo-Korpela S, Lehtimaki L, Vuolteenaho K, Nieminen R, Kankaanranta H, Saarelainen S, Moilanen E. Adipokine resistin predicts anti-inflammatory effect of glucocorticoids in asthma. Journal of inflammation. 2011;8:12. doi: 10.1186/1476-9255-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman AP, Pitha PM, Shin HS, Shin ML. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci U S A. 1989;86:6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loskutoff DJ, Sawdey M, Mimuro J. Type 1 plasminogen activator inhibitor. Prog Hemost Thromb. 1989;9:87–115. [PubMed] [Google Scholar]
- Lu XY, Kim CS, Frazer A, Zhang W. Leptin: a potential novel antidepressant. Proc Natl Acad Sci U S A. 2006;103:1593–1598. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwin SK. Reaction of oligodendrocytes and astrocytes to trauma and implantation. A combined autoradiographic and immunohistochemical study. Lab Invest. 1985;52:20–30. [PubMed] [Google Scholar]
- MacDougald OA, Burant CF. The rapidly expanding family of adipokines. Cell Metab. 2007;6:159–161. doi: 10.1016/j.cmet.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- Makni E, Moalla W, Lac G, Aouichaoui C, Cannon D, Elloumi M, Tabka Z. The Homeostasis Model Assessment-adiponectin (HOMA-AD) is the most sensitive predictor of insulin resistance in obese children. Ann Endocrinol (Paris) 2012;73:26–33. doi: 10.1016/j.ando.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Mallat M, Marin-Teva JL, Cheret C. Phagocytosis in the developing CNS: more than clearing the corpses. Current opinion in neurobiology. 2005;15:101–107. doi: 10.1016/j.conb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Mantovani RM, Rios DR, Moura LC, Oliveira JM, Carvalho FF, Cunha SB, Viana Mde F, Lamounier JA, Castro JC, Dusse LM, Simoes e Silva AC. Childhood obesity: evidence of an association between plasminogen activator inhibitor-1 levels and visceral adiposity. J Pediatr Endocrinol Metab. 2011;24:361–367. doi: 10.1515/jpem.2011.015. [DOI] [PubMed] [Google Scholar]
- Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J Biol Chem. 2004;279:32869–32881. doi: 10.1074/jbc.M311766200. [DOI] [PubMed] [Google Scholar]
- Maresh GA, Maness LM, Zadina JE, Kastin AJ. In vitro demonstration of a saturable transport system for leptin across the blood-brain barrier. Life Sci. 2001;69:67–73. doi: 10.1016/s0024-3205(01)01093-1. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 2008;64:484–490. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masos T, Miskin R. mRNAs encoding urokinase-type plasminogen activator and plasminogen activator inhibitor-1 are elevated in the mouse brain following kainate-mediated excitation. Brain Res Mol Brain Res. 1997;47:157–169. doi: 10.1016/s0169-328x(97)00040-5. [DOI] [PubMed] [Google Scholar]
- Massiera F, Bloch-Faure M, Ceiler D, Murakami K, Fukamizu A, Gasc JM, Quignard-Boulange A, Negrel R, Ailhaud G, Seydoux J, Meneton P, Teboul M. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001;15:2727–2729. doi: 10.1096/fj.01-0457fje. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, Kumada M, Okamoto Y, Nagaretani H, Nishizawa H, Kishida K, Komuro R, Ouchi N, Kihara S, Nagai R, Funahashi T, Matsuzawa Y. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–37491. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FA, Chai SY. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol. 2003;35:901–918. doi: 10.1016/s1357-2725(02)00306-0. [DOI] [PubMed] [Google Scholar]
- Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME, Takahashi HK, Curi R, Oliveira HC, Carvalheira JB, Bordin S, Saad MJ, Velloso LA. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29:359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milsted A, Barna BP, Ransohoff RM, Brosnihan KB, Ferrario CM. Astrocyte cultures derived from human brain tissue express angiotensinogen mRNA. Proc Natl Acad Sci U S A. 1990;87:5720–5723. doi: 10.1073/pnas.87.15.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskin R, Masos T. Transgenic mice overexpressing urokinase-type plasminogen activator in the brain exhibit reduced food consumption, body weight and size, and increased longevity. J Gerontol A Biol Sci Med Sci. 1997;52:B118–124. doi: 10.1093/gerona/52a.2.b118. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Montgomery SL, Bowers WJ. Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2012;7:42–59. doi: 10.1007/s11481-011-9287-2. [DOI] [PubMed] [Google Scholar]
- Morash B, Li A, Murphy PR, Wilkinson M, Ur E. Leptin gene expression in the brain and pituitary gland. Endocrinology. 1999;140:5995–5998. doi: 10.1210/endo.140.12.7288. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossman MC, Lenzlinger PM, Hans V, Stahel P, Csuka E, Ammann E, Stocker R, Trentz O, Kossmann T. Production of cytokines following brain injury: beneficial and deleterious for the damaged tissue. Mol Psychiatry. 1997;2:133–136. doi: 10.1038/sj.mp.4000227. [DOI] [PubMed] [Google Scholar]
- Morrison CD. Leptin signaling in brain: A link between nutrition and cognition? Biochim Biophys Acta. 2009;1792:401–408. doi: 10.1016/j.bbadis.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousa SA. Elevation of plasma von Willebrand factor and tumor necrosis factor-a in obese subjects and their reduction by the low molecular weight heparin tinzaparin. Int Angiol. 2005;24:278–281. [PubMed] [Google Scholar]
- Mueller K, Anwander A, Moller HE, Horstmann A, Lepsien J, Busse F, Mohammadi S, Schroeter ML, Stumvoll M, Villringer A, Pleger B. Sex-dependent influences of obesity on cerebral white matter investigated by diffusion-tensor imaging. PLoS One. 2011;6:e18544. doi: 10.1371/journal.pone.0018544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzberg H, Myers MG., Jr Molecular and anatomical determinants of central leptin resistance. Nat Neurosci. 2005;8:566–570. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Izumiya Y, Higuchi A, Shibata R, Qiu J, Kudo C, Shin HK, Moskowitz MA, Ouchi N. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation. 2008;117:216–223. doi: 10.1161/CIRCULATIONAHA.107.725044. [DOI] [PubMed] [Google Scholar]
- Novak V, Chowdhary A, Farrar B, Nagaraja H, Braun J, Kanard R, Novak P, Slivka A. Altered cerebral vasoregulation in hypertension and stroke. Neurology. 2003;60:1657–1663. doi: 10.1212/01.wnl.0000068023.14587.06. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Hirvonen J, Hannukainen JC, Immonen H, Lindroos MM, Salminen P, Nuutila P. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PLoS One. 2012;7:e31089. doi: 10.1371/journal.pone.0031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1960-1962 through 2007-2008. Health E-Stats. 2010 Jun [Google Scholar]
- Ohkubo H, Kawakami H, Kakehi Y, Takumi T, Arai H, Yokota Y, Iwai M, Tanabe Y, Masu M, Hata J, et al. Generation of transgenic mice with elevated blood pressure by introduction of the rat renin and angiotensinogen genes. Proc Natl Acad Sci U S A. 1990;87:5153–5157. doi: 10.1073/pnas.87.13.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olde Dubbelink KT, Felius A, Verbunt JP, van Dijk BW, Berendse HW, Stam CJ, Delemarre-van de Waal HA. Increased resting-state functional connectivity in obese adolescents; a magnetoencephalographic pilot study. PLoS One. 2008;3:e2827. doi: 10.1371/journal.pone.0002827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, Matsumiya T, Ishibashi M, Aou S, Li XL, Kohno D, Uramura K, Sougawa H, Yada T, Wayner MJ, Sasaki K. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;27:2738–2749. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003a;14:561–566. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, Okamoto Y, Ohashi K, Nagaretani H, Kishida K, Nishizawa H, Maeda N, Kobayashi H, Hiraoka H, Matsuzawa Y. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003b;107:671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Diurnal variation of leptin entry from blood to brain involving partial saturation of the transport system. Life Sci. 2001;68:2705–2714. doi: 10.1016/s0024-3205(01)01085-2. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Adipokines and the blood-brain barrier. Peptides. 2007;28:1317–1330. doi: 10.1016/j.peptides.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006;31:1419–1425. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Neuropeptides and the blood-brain barrier. Annu Rev Physiol. 1983;45:73–82. doi: 10.1146/annurev.ph.45.030183.000445. [DOI] [PubMed] [Google Scholar]
- Paz-Filho G, Wong ML, Licinio J. The procognitive effects of leptin in the brain and their clinical implications. Int J Clin Pract. 2010;64:1808–1812. doi: 10.1111/j.1742-1241.2010.02536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiser C, McGregor GP, Lang RE. Binding and internalization of leptin by porcine choroid plexus cells in culture. Neurosci Lett. 2000;283:209–212. doi: 10.1016/s0304-3940(00)00942-3. [DOI] [PubMed] [Google Scholar]
- Pickering M, Cumiskey D, O'Connor JJ. Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Experimental physiology. 2005;90:663–670. doi: 10.1113/expphysiol.2005.030734. [DOI] [PubMed] [Google Scholar]
- Poluektova L, Meyer V, Walters L, Paez X, Gendelman HE. Macrophage-induced inflammation affects hippocampal plasticity and neuronal development in a murine model of HIV-1 encephalitis. Glia. 2005;52:344–353. doi: 10.1002/glia.20253. [DOI] [PubMed] [Google Scholar]
- Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- Qiu G, Wan R, Hu J, Mattson MP, Spangler E, Liu S, Yau SY, Lee TM, Gleichmann M, Ingram DK, So KF, Zou S. Adiponectin protects rat hippocampal neurons against excitotoxicity. Age (Dordr) 2011;33:155–165. doi: 10.1007/s11357-010-9173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Hua X, Leow AD, Toga AW, Thompson PM. Brain structure and obesity. Hum Brain Mapp. 2010;31:353–364. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid IA. The brain renin-angiotensin system: a critical analysis. Fed Proc. 1979;38:2255–2259. [PubMed] [Google Scholar]
- Rocha VZ, Folco EJ. Inflammatory concepts of obesity. Int J Inflam. 2011;2011:529061. doi: 10.4061/2011/529061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanatto T, Cesquini M, Amaral ME, Roman EA, Moraes JC, Torsoni MA, Cruz-Neto AP, Velloso LA. TNF-alpha acts in the hypothalamus inhibiting food intake and increasing the respiratory quotient-effects on leptin and insulin signaling pathways. Peptides. 2007;28:1050–1058. doi: 10.1016/j.peptides.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Ropelle ER, Flores MB, Cintra DE, Rocha GZ, Pauli JR, Morari J, de Souza CT, Moraes JC, Prada PO, Guadagnini D, Marin RM, Oliveira AG, Augusto TM, Carvalho HF, Velloso LA, Saad MJ, Carvalheira JB. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKbeta and ER stress inhibition. PLoS biology. 2010;8 doi: 10.1371/journal.pbio.1000465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Salbe AD, Weyer C, Lindsay RS, Ravussin E, Tataranni PA. Assessing risk factors for obesity between childhood and adolescence: I. Birth weight, childhood adiposity, parental obesity, insulin, and leptin. Pediatrics. 2002;110:299–306. doi: 10.1542/peds.110.2.299. [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Jennische E, Hansson HA, Holmang A. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1345–1356. doi: 10.1152/ajpregu.00268.2005. [DOI] [PubMed] [Google Scholar]
- Schipper HS, Nuboer R, Prop S, van den Ham HJ, de Boer FK, Kesmir C, Mombers IM, van Bekkum KA, Woudstra J, Kieft JH, Hoefer IE, de Jager W, Prakken B, van Summeren M, Kalkhoven E. Systemic inflammation in childhood obesity: circulating inflammatory mediators and activated CD14(++) monocytes. Diabetologia. 2012 doi: 10.1007/s00125-012-2641-y. [DOI] [PubMed] [Google Scholar]
- Schulz C, Paulus K, Lehnert H. Adipocyte-brain: crosstalk. Results and problems in cell differentiation. 2010;52:189–201. doi: 10.1007/978-3-642-14426-4_16. [DOI] [PubMed] [Google Scholar]
- Selmaj K, Raine CS. Tumor necrosis factor mediates myelin damage in organotypic cultures of nervous tissue. Ann N Y Acad Sci. 1988;540:568–570. doi: 10.1111/j.1749-6632.1988.tb27175.x. [DOI] [PubMed] [Google Scholar]
- Severs WB, Changaris DG, Keil LC, Summy-Long JY, Klase PA, Kapsha JM. Pharmacology of angiotensin-induced drinking behavior. Fed Proc. 1978;37:2699–2703. [PubMed] [Google Scholar]
- Severs WB, Daniels-Severs AE. Effects of angiotensin on the central nervous system. Pharmacological reviews. 1973;25:415–449. [PubMed] [Google Scholar]
- Shioda S, Funahashi H, Nakajo S, Yada T, Maruta O, Nakai Y. Immunohistochemical localization of leptin receptor in the rat brain. Neurosci Lett. 1998;243:41–44. doi: 10.1016/s0304-3940(98)00082-2. [DOI] [PubMed] [Google Scholar]
- Simpson JB. The circumventricular organs and the central actions of angiotensin. Neuroendocrinology. 1981;32:248–256. doi: 10.1159/000123167. [DOI] [PubMed] [Google Scholar]
- Singh A, Foster GD, Gunawardana J, McCoy TA, Nguyen T, Vander Veur S, Komaroff E, Rao AK. Elevated circulating tissue factor procoagulant activity, factor VII, and plasminogen activator inhibitor-1 in childhood obesity: evidence of a procoagulant state. Br J Haematol. 2012;158:523–527. doi: 10.1111/j.1365-2141.2012.09160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitcheran R, Gupta P, Fisher PB, Baldwin AS. Positive and negative regulation of EAAT2 by NF-kappaB: a role for N-myc in TNFalpha-controlled repression. EMBO J. 2005;24:510–520. doi: 10.1038/sj.emboj.7600555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurk T, Hauner H. Obesity and impaired fibrinolysis: role of adipose production of plasminogen activator inhibitor-1. Int J Obes Relat Metab Disord. 2004;28:1357–1364. doi: 10.1038/sj.ijo.0802778. [DOI] [PubMed] [Google Scholar]
- Soeda S, Koyanagi S, Kuramoto Y, Kimura M, Oda M, Kozako T, Hayashida S, Shimeno H. Anti-apoptotic roles of plasminogen activator inhibitor-1 as a neurotrophic factor in the central nervous system. Thromb Haemost. 2008;100:1014–1020. doi: 10.1160/th08-04-0259. [DOI] [PubMed] [Google Scholar]
- Soeda S, Oda M, Ochiai T, Shimeno H. Deficient release of plasminogen activator inhibitor-1 from astrocytes triggers apoptosis in neuronal cells. Brain Res Mol Brain Res. 2001;91:96–103. doi: 10.1016/s0169-328x(01)00133-4. [DOI] [PubMed] [Google Scholar]
- Spranger J, Verma S, Gohring I, Bobbert T, Seifert J, Sindler AL, Pfeiffer A, Hileman SM, Tschop M, Banks WA. Adiponectin does not cross the blood-brain barrier but modifies cytokine expression of brain endothelial cells. Diabetes. 2006;55:141–147. [PubMed] [Google Scholar]
- Stanek KM, Grieve SM, Brickman AM, Korgaonkar MS, Paul RH, Cohen RA, Gunstad JJ. Obesity is associated with reduced white matter integrity in otherwise healthy adults. Obesity (Silver Spring) 2011;19:500–504. doi: 10.1038/oby.2010.312. [DOI] [PubMed] [Google Scholar]
- Stelzer I, Zelzer S, Raggam RB, Pruller F, Truschnig-Wilders M, Meinitzer A, Schnedl WJ, Horejsi R, Moller R, Weghuber D, Reeves G, Postolache TT, Mangge H. Link between leptin and interleukin-6 levels in the initial phase of obesity related inflammation. Transl Res. 2012;159:118–124. doi: 10.1016/j.trsl.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Kim J, Weller RE, Cox JE, Cook EW, Horwitz B., 3rd Effective connectivity of a reward network in obese women. Brain Res Bull. 2009;79:388–395. doi: 10.1016/j.brainresbull.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Hawelu-Johnson CL, Guyenet PG, Lynch KR. Astrocytes synthesize angiotensinogen in brain. Science. 1988;242:1444–1446. doi: 10.1126/science.3201232. [DOI] [PubMed] [Google Scholar]
- Tam CS, Garnett SP, Cowell CT, Heilbronn LK, Lee JW, Wong M, Baur LA. IL-6, IL-8 and IL-10 levels in healthy weight and overweight children. Horm Res Paediatr. 2010;73:128–134. doi: 10.1159/000277632. [DOI] [PubMed] [Google Scholar]
- Tancredi V, D'Arcangelo G, Grassi F, Tarroni P, Palmieri G, Santoni A, Eusebi F. Tumor necrosis factor alters synaptic transmission in rat hippocampal slices. Neurosci Lett. 1992;146:176–178. doi: 10.1016/0304-3940(92)90071-e. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton SN, Nicolaidis S. Blood pressure effects of iontophoretically applied bioactive hormones in the anterior forebrain of the rat. Am J Physiol. 1993;265:R826–833. doi: 10.1152/ajpregu.1993.265.4.R826. [DOI] [PubMed] [Google Scholar]
- Ukkola O, Santaniemi M. Adiponectin: a link between excess adiposity and associated comorbidities? J Mol Med (Berl) 2002;80:696–702. doi: 10.1007/s00109-002-0378-7. [DOI] [PubMed] [Google Scholar]
- Valerio A, Ghisi V, Dossena M, Tonello C, Giordano A, Frontini A, Ferrario M, Pizzi M, Spano P, Carruba MO, Nisoli E. Leptin increases axonal growth cone size in developing mouse cortical neurons by convergent signals inactivating glycogen synthase kinase-3beta. J Biol Chem. 2006;281:12950–12958. doi: 10.1074/jbc.M508691200. [DOI] [PubMed] [Google Scholar]
- Van Harmelen V, Ariapart P, Hoffstedt J, Lundkvist I, Bringman S, Arner P. Increased adipose angiotensinogen gene expression in human obesity. Obes Res. 2000a;8:337–341. doi: 10.1038/oby.2000.40. [DOI] [PubMed] [Google Scholar]
- van Harmelen V, Elizalde M, Ariapart P, Bergstedt-Lindqvist S, Reynisdottir S, Hoffstedt J, Lundkvist I, Bringman S, Arner P. The association of human adipose angiotensinogen gene expression with abdominal fat distribution in obesity. Int J Obes Relat Metab Disord. 2000b;24:673–678. doi: 10.1038/sj.ijo.0801217. [DOI] [PubMed] [Google Scholar]
- van Rossum CT, Hoebee B, van Baak MA, Mars M, Saris WH, Seidell JC. Genetic variation in the leptin receptor gene, leptin, and weight gain in young Dutch adults. Obes Res. 2003;11:377–386. doi: 10.1038/oby.2003.51. [DOI] [PubMed] [Google Scholar]
- Villarreal-Molina MT, Antuna-Puente B. Adiponectin: Anti-inflammatory and cardioprotective effects. Biochimie. 2012 doi: 10.1016/j.biochi.2012.06.030. [DOI] [PubMed] [Google Scholar]
- Visser M. Higher levels of inflammation in obese children. Nutrition. 2001;17:480–481. doi: 10.1016/s0899-9007(01)00509-3. [DOI] [PubMed] [Google Scholar]
- Walker WG, Whelton PK, Saito H, Russell RP, Hermann J. Relation between blood pressure and renin, renin substrate, angiotensin II, aldosterone and urinary sodium and potassium in 574 ambulatory subjects. Hypertension. 1979;1:287–291. doi: 10.1161/01.hyp.1.3.287. [DOI] [PubMed] [Google Scholar]
- Watt GC, Harrap SB, Foy CJ, Holton DW, Edwards HV, Davidson HR, Connor JM, Lever AF, Fraser R. Abnormalities of glucocorticoid metabolism and the renin-angiotensin system: a four-corners approach to the identification of genetic determinants of blood pressure. J Hypertens. 1992;10:473–482. doi: 10.1097/00004872-199205000-00011. [DOI] [PubMed] [Google Scholar]
- Wei H, Chadman KK, McCloskey DP, Sheikh AM, Malik M, Brown WT, Li X. Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochim Biophys Acta. 2012;1822:831–842. doi: 10.1016/j.bbadis.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Wei H, Zou H, Sheikh AM, Malik M, Dobkin C, Brown WT, Li X. IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J Neuroinflammation. 2011;8:52. doi: 10.1186/1742-2094-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland F, Verspohl EJ. Variety of angiotensin receptors in 3T3-L1 preadipose cells and differentiated adipocytes. Horm Metab Res. 2008;40:760–766. doi: 10.1055/s-0028-1082041. [DOI] [PubMed] [Google Scholar]
- Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- White DW, Kuropatwinski KK, Devos R, Baumann H, Tartaglia LA. Leptin receptor (OB-R) signaling. Cytoplasmic domain mutational analysis and evidence for receptor homo-oligomerization. J Biol Chem. 1997;272:4065–4071. doi: 10.1074/jbc.272.7.4065. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Gunderson EP, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4:103–109. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- WHO WHO. Obesity and Overweight 2012 [Google Scholar]
- Widya RL, de Roos A, Trompet S, de Craen AJ, Westendorp RG, Smit JW, van Buchem MA, van der Grond J. Increased amygdalar and hippocampal volumes in elderly obese individuals with or at risk of cardiovascular disease. Am J Clin Nutr. 2011;93:1190–1195. doi: 10.3945/ajcn.110.006304. [DOI] [PubMed] [Google Scholar]
- Wiesner G, Vaz M, Collier G, Seals D, Kaye D, Jennings G, Lambert G, Wilkinson D, Esler M. Leptin is released from the human brain: influence of adiposity and gender. J Clin Endocrinol Metab. 1999;84:2270–2274. doi: 10.1210/jcem.84.7.5854. [DOI] [PubMed] [Google Scholar]
- Wilkinson M, Brown R, Imran SA, Ur E. Adipokine gene expression in brain and pituitary gland. Neuroendocrinology. 2007;86:191–209. doi: 10.1159/000108635. [DOI] [PubMed] [Google Scholar]
- Wilsey J, Zolotukhin S, Prima V, Scarpace PJ. Central leptin gene therapy fails to overcome leptin resistance associated with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1011–1020. doi: 10.1152/ajpregu.00193.2003. [DOI] [PubMed] [Google Scholar]
- Woo MS, Park JS, Choi IY, Kim WK, Kim HS. Inhibition of MMP-3 or -9 suppresses lipopolysaccharide-induced expression of proinflammatory cytokines and iNOS in microglia. J Neurochem. 2008;106:770–780. doi: 10.1111/j.1471-4159.2008.05430.x. [DOI] [PubMed] [Google Scholar]
- Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology. 2011;76:1568–1574. doi: 10.1212/WNL.0b013e3182190d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Haan M, Blackwell T, Cherkasova E, Whitmer RA, West N. Metabolic syndrome and cognitive decline in elderly Latinos: findings from the Sacramento Area Latino Study of Aging study. J Am Geriatr Soc. 2007;55:758–762. doi: 10.1111/j.1532-5415.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Murakami T, Iida M, Kuwajima M, Shima K. Leptin receptor of Zucker fatty rat performs reduced signal transduction. Diabetes. 1997;46:1077–1080. doi: 10.2337/diab.46.6.1077. [DOI] [PubMed] [Google Scholar]
- Yanai K, Saito T, Kakinuma Y, Kon Y, Hirota K, Taniguchi-Yanai K, Nishijo N, Shigematsu Y, Horiguchi H, Kasuya Y, Sugiyama F, Yagami K, Murakami K, Fukamizu A. Renin-dependent cardiovascular functions and renin-independent blood-brain barrier functions revealed by renin-deficient mice. J Biol Chem. 2000;275:5–8. doi: 10.1074/jbc.275.1.5. [DOI] [PubMed] [Google Scholar]
- Yiannikouris F, Gupte M, Putnam K, Thatcher S, Charnigo R, Rateri DL, Daugherty A, Cassis LA. Adipocyte deficiency of angiotensinogen prevents obesity-induced hypertension in male mice. Hypertension. 2012a;60:1524–1530. doi: 10.1161/HYPERTENSIONAHA.112.192690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiannikouris F, Karounos M, Charnigo R, English VL, Rateri DL, Daugherty A, Cassis LA. Adipocyte-specific deficiency of angiotensinogen decreases plasma angiotensinogen concentration and systolic blood pressure in mice. Am J Physiol Regul Integr Comp Physiol. 2012b;302:R244–251. doi: 10.1152/ajpregu.00323.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, Kihara S, Funahashi T, Tenner AJ, Tomiyama Y, Matsuzawa Y. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–1732. [PubMed] [Google Scholar]
- Youssef AM, Hamidian Jahromi A, Vijay CG, Granger DN, Alexander JS. Intra-abdominal hypertension causes reversible blood-brain barrier disruption. The journal of trauma and acute care surgery. 2012;72:183–188. doi: 10.1097/TA.0b013e31822a3254. [DOI] [PubMed] [Google Scholar]
- Yvan-Charvet L, Quignard-Boulange A. Role of adipose tissue renin-angiotensin system in metabolic and inflammatory diseases associated with obesity. Kidney Int. 2011;79:162–168. doi: 10.1038/ki.2010.391. [DOI] [PubMed] [Google Scholar]
- Zeki Al, Hazzouri A, Stone KL, Haan MN, Yaffe K. Leptin, Mild Cognitive Impairment, and Dementia Among Elderly Women. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang S, Signore AP, Chen J. Neuroprotective effects of leptin against ischemic injury induced by oxygen-glucose deprivation and transient cerebral ischemia. Stroke. 2007;38:2329–2336. doi: 10.1161/STROKEAHA.107.482786. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Jovanovic S, Miao W, Samara S, Verma S, Farrell CL. Differential regulation of leptin transport by the choroid plexus and blood-brain barrier and high affinity transport systems for entry into hypothalamus and across the blood-cerebrospinal fluid barrier. Endocrinology. 2000;141:1434–1441. doi: 10.1210/endo.141.4.7435. [DOI] [PubMed] [Google Scholar]