Significance
Molecular chaperones are a group of proteins that are essential for avoiding the aggregation of other proteins in the crowded cellular environment. Chaperones function by interacting with these substrate proteins in different ways. However, it has remained a challenge to measure the changes that occur in the substrate proteins and understand how these changes prevent misfolding or aggregation. Here we investigate a chaperone system that keeps the substrate protein denatured by clamping the polypeptide chain. We observe an expansion of the substrate protein chain up to 30-fold in volume owing to steric repulsion between multiple copies of the chaperone bound to a single substrate protein. In this way, unwanted interactions within or between substrate proteins can be prevented.
Keywords: Hsp70, Hsp40, Förster resonance energy transfer, protein folding, FRET
Abstract
Molecular chaperones are an essential part of the machinery that avoids protein aggregation and misfolding in vivo. However, understanding the molecular basis of how chaperones prevent such undesirable interactions requires the conformational changes within substrate proteins to be probed during chaperone action. Here we use single-molecule fluorescence spectroscopy to investigate how the DnaJ–DnaK chaperone system alters the conformational distribution of the denatured substrate protein rhodanese. We find that in a first step the ATP-independent binding of DnaJ to denatured rhodanese results in a compact denatured ensemble of the substrate protein. The following ATP-dependent binding of multiple DnaK molecules, however, leads to a surprisingly large expansion of denatured rhodanese. Molecular simulations indicate that hard-core repulsion between the multiple DnaK molecules provides the underlying mechanism for disrupting even strong interactions within the substrate protein and preparing it for processing by downstream chaperone systems.
Maintaining protein homeostasis in vivo requires a tight regulation of protein folding to prevent misfolding and aggregation. Molecular chaperones have evolved as an essential part of the cellular machinery that facilitates such processes in the complex and crowded environment of a living cell (1, 2). To assist protein folding, many chaperones proceed through complex conformational cycles in an ATP-dependent manner (3–5). For several chaperone systems, these cycles have been investigated in great detail by experiment and simulation (6–8). A remarkable example are the heat shock protein (Hsp) 70 chaperones, which are essential in prokaryotes and eukaryotes and are involved in co-translational folding, refolding of misfolded and aggregated proteins, protein translocation, and protein degradation (9). The Hsp70 chaperone DnaK from Escherichia coli together with its co-chaperone DnaJ and the nucleotide exchange factor GrpE form an ATP-driven catalytic reaction cycle (7) (Fig. 1A). Many denatured or misfolded substrate proteins are first captured by DnaJ and subsequently transferred to the DnaK–ATP complex, with DnaK in an open conformation. Substrate and DnaJ synergistically trigger DnaK’s ATPase activity, which leads to locking of the substrate in the DnaK–ADP complex, with DnaK in the closed conformation. Driven by the following GrpE-catalyzed ADP–ATP exchange, the DnaK–substrate complex dissociates (10). Since this ATP-driven cycle can even solubilize protein aggregates (11, 12), substantial forces must be transduced to the substrate protein (13–15). However, as for other chaperone systems (16), surprisingly little is known about how these forces and the resulting constraints of the underlying free energy surfaces affect the conformations of the denatured or misfolded substrate proteins. To better understand this important link between chaperone action and function, we probed the conformation of a substrate protein along the different stages of the chaperone cycle of DnaK with single-molecule Förster resonance energy transfer (smFRET), correlation spectroscopy, and microfluidic mixing.
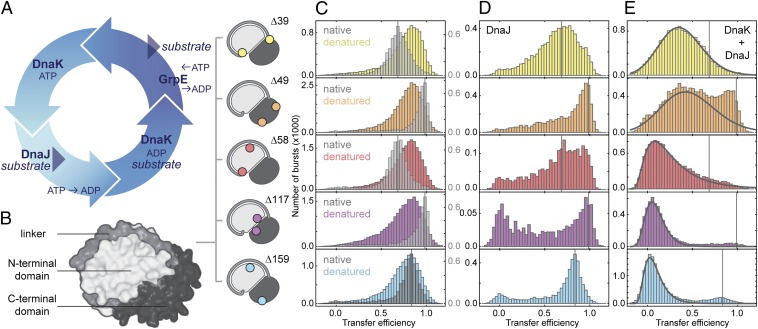
Fig. 1.
DnaK expands the denatured substrate protein. (A) Illustration of the DnaK–ATPase cycle. (B) Surface representation of rhodanese (PDB ID code 1RHS) with the subdomains indicated in different gray levels and the label positions of fluorescent dyes for single-molecule FRET measurements shown schematically. (C) FRET efficiency histograms of native rhodanese (gray) and denatured rhodanese under native conditions transiently populated in the microfluidic mixer (colored, measured 125 ms after dilution of rhodanese into native conditions). (D) FRET efficiency histograms of DnaJ–rhodanese complexes (0.5 µM DnaJ). (E) FRET efficiency histograms of DnaK–rhodanese complexes (0.5 µM DnaJ, 10 µM DnaK, and 1 mM ATP; DnaK and DnaJ were added simultaneously to rhodanese). Black lines indicate the DnaK–rhodanese complex population resulting from a fit that takes into account the residual population of refolded and DnaJ-bound rhodanese. The vertical lines in C–E indicate the positions of the FRET efficiency peaks of the native population of the respective rhodanese variants. The small populations at zero transfer efficiency in D (note the axis scaling and the small amplitudes of this population compared with E) originate from incomplete elimination of molecules with inactive acceptor fluorophores by pulsed interleaved excitation.
Results and Discussion
Single-Molecule Spectroscopy of Substrate–Chaperone Complexes.
A key strength of smFRET is to resolve subpopulations of molecules by their intramolecular distances, which is especially advantageous for investigating the conformational heterogeneity of denatured proteins and chaperone–substrate complexes (17, 18). The combination with microfluidic mixing devices enables the time-resolved observation of transient aggregation-prone species, such as denatured chaperone substrates at near-physiological conditions (19, 20). To obtain detailed information about the substrate conformation for each step in the Hsp70 chaperone cycle, we mapped several segments within the classic chaperone substrate protein rhodanese. Several protein variants were designed to report on different aspects of the structure of rhodanese (Fig. 1B): the conformation of the N-terminal domain (Δ58: E77C/K135C), the C-terminal domain (Δ49: K236C/E285C), the linker connecting both domains (Δ39: K135C/K174C), and the relative arrangement of the two domains, with the fluorophores attached close to the domain interface (Δ117: D102C/D219C) or on opposite sides of the domains (Δ159: E77C/K236C). Alexa Fluor 488 as a FRET donor and Alexa Fluor 594 as an acceptor were attached to each variant to obtain information on the distance between the corresponding amino acid residues. Fig. 1C shows transfer efficiency histograms for the five variants of rhodanese, determined from fluorescence bursts of individual molecules freely diffusing through the observation volume of the confocal instrument. The folded proteins (Fig. 1C, gray histograms) exhibit characteristic transfer efficiency distributions with averages close to the values expected from the crystal structure of rhodanese. Here, however, our focus is on the conformation of denatured rhodanese (Fig. 1C, colored histograms). As a reference state in the absence of chaperones and denaturant, we thus transiently populate denatured rhodanese in a microfluidic mixing device by rapid dilution of guanidinium chloride (GdmCl) (19, 21–23). In contrast to native rhodanese, all denatured rhodanese variants exhibit a similar and high transfer efficiency of ∼0.8, which suggests the presence of a compact denatured state ensemble under these conditions (24). Denatured rhodanese is highly prone to aggregation during refolding (25), but at the extremely low protein concentrations used here (50 pM) aggregation does not occur, and all variants fold to the native state spontaneously on a timescale of minutes, even in the absence of chaperones (19) (Fig. S1C).
When GdmCl-denatured rhodanese is diluted into buffer in the presence of DnaJ in the physiological concentration range (0.5 µM) (26), the resulting transfer efficiency histograms (Fig. 1D) are remarkably different from those of folded and denatured rhodanese (Fig. 1C). Very broad transfer efficiency distributions are obtained for all variants of rhodanese in complex with DnaJ (Fig. 1D), suggestive of a heterogeneous distribution of conformations. Only a fraction of rhodanese molecules (∼10%) fold to their native structure (Fig. 1D, Fig. S1, and Table S1), as indicated by the subpopulations at the same transfer efficiency as the folded state. Given the rate constant of ∼3 × 10−3 s−1 for the spontaneous refolding of rhodanese in the absence of chaperones (Fig. S1 and Table S1) under these conditions (19, 27), the association rate constant of DnaJ to denatured rhodanese can be estimated to be ∼4 × 104 M−1 s−1 (Fig. S1 and Table S1). In addition to the broad transfer efficiency distributions, DnaJ binding of denatured rhodanese results in a loss in brightness of the attached fluorophores (Table S2), indicative of quenching by solvent-exposed aromatic amino acid residues in a relatively compact denatured state of rhodanese (28). Time-resolved single-molecule anisotropy experiments reveal only a moderately increased fluorescence anisotropy of the attached fluorophores in the DnaJ–rhodanese complex (Table S2); a dominant influence of orientational heterogeneity on the transfer efficiency histograms (20) is thus unlikely. The quenching of the attached fluorophores together with the broad transfer efficiency distributions thus suggest that DnaJ blocks rhodanese refolding by holding it in a heterogeneous ensemble of nonnative conformations that are substantially different from those of denatured rhodanese in the absence of chaperones (Fig. 1 C, gray histograms, and D).
To obtain independent information on the size of the rhodanese–DnaJ complexes, we used two-focus fluorescence correlation spectroscopy (2f-FCS), where the cross-correlation between the fluorescence intensities from two partially overlapping foci is used to determine the translational diffusion time and the corresponding Stokes radius, RS, of the complex (29). For both folded and denatured rhodanese in the absence of chaperones, we observe RS = 2.6 ± 0.1 nm, which confirms the compactness of denatured rhodanese indicated by the transfer efficiencies (Fig. 1C). Binding of rhodanese to DnaJ at 0.5 µM results in an increase in RS to 3.1 ± 0.2 nm. Even if we assume a very compact configuration of this complex and the contribution of a small fraction of refolded rhodanese (Fig. S1E), this value of RS is incompatible with more than one DnaJ dimer (30) bound per rhodanese molecule (Table S3). Nevertheless, the rhodanese–DnaJ-bound state is very stable and persists for more than 24 h under our experimental conditions (Fig. S2). In summary, our results suggest that DnaJ arrests rhodanese in a compact, but denatured and folding-incompetent, state. The next step in the chaperone cycle is the interaction of this complex with DnaK.
Expansion of Rhodanese by DnaK.
The addition of 10 µM DnaK and 1 mM ATP to preformed rhodanese–DnaJ complexes leads to surprisingly large changes in the transfer efficiency histograms (Fig. 1E). A pronounced shift of the distributions to lower transfer efficiencies is observed, indicating that DnaK binding leads to the formation of highly expanded conformations of rhodanese. At the same time, the brightness of the molecules increases (Table S2), as expected from the increased distances between the FRET dyes and aromatic amino acids in a more expanded chain (28). The resulting conformational ensembles of rhodanese, which are even more expanded than rhodanese unfolded in 6 M GdmCl (Fig. S1G) (18, 31), are observed independent of whether DnaK and ATP are added to preformed DnaJ complexes or whether DnaJ, DnaK, and ATP are added at the same time (Fig. S3A). Since the collapse of unfolded proteins upon transfer into nondenaturing conditions occurs on timescales in the range of ∼100 ns to ∼100 µs (32–36), much faster than the association rate of DnaJ and DnaK to rhodanese (∼1 min−1, discussed below), the chaperones bind rhodanese in its compact denatured state (Fig. 1C).
The formation of rhodanese–DnaK complexes is strictly dependent on the presence of DnaJ and ATP. Neither the nonhydrolyzable ATP analog ATPγS nor ADP can trigger the expansion of rhodanese (Fig. S3C), suggesting that the ATP-bound conformation of DnaK is crucial for binding of protein substrates such as rhodanese. In the absence of nucleotide, no DnaK-driven expansion of rhodanese was observed even after several hours (Fig. S3B). With an excess of ATP (1 mM in Fig. 1E), however, the DnaK-bound complex remains stable for hours. The expansion of denatured rhodanese also depends on the relative concentrations of DnaJ and DnaK (Fig. S3 D–F). The greatest substrate expansion by DnaK is reached in the presence of ∼0.5 µM DnaJ and 10 µM DnaK (Fig. S3E), which is in the physiological concentration range (26) and was thus used as the DnaJ and DnaK concentrations in the present study. This result suggests that the substoichiometric amounts of DnaJ compared with DnaK observed in vivo are optimized in order that DnaJ keeps substrate proteins denatured but does not saturate the polypeptide, which would interfere with DnaK binding, because both chaperones recognize similar amino acid patterns in their substrates (37). Correspondingly, high concentrations of DnaJ (10 µM) inhibit the DnaK-mediated expansion of rhodanese (Fig. S3 D–F) by occupying binding sites on rhodanese, as indicated by the larger complex size of DnaJ–rhodanese at 10 µM DnaJ (RS determined with 2f-FCS: 5.4 ± 0.2 nm) compared with the complex at 0.5 µM DnaJ. Although the substrate-like binding of DnaJ to DnaK (38, 39) may contribute to a reduction of the DnaK concentration available for rhodanese binding, the affinity between the chaperones is not high enough (38) to explain the absence of the expansion of rhodanese at high DnaJ concentrations. Notably, denatured rhodanese cannot be expanded by DnaK and ATP in the absence of DnaJ (Fig. S3C), in agreement with the previously suggested sequential action of DnaJ and DnaK (13). The reason underlying the lack of DnaK-substrate binding in the absence of DnaJ was identified in previous studies that showed that a functionally impaired J domain of DnaJ is not able to couple DnaK–substrate binding and ATP hydrolysis for efficient DnaK–substrate complex formation (38, 39).
In contrast to the compact denatured state of rhodanese alone (Fig. 1C, colored histograms), the mean transfer efficiency of the five rhodanese variants bound to DnaK shows a clear decrease in efficiency with increasing sequence separation between the dyes (Fig. 2C, circles). In terms of intramolecular distances, this observation translates into a pronounced increase of the interdye distance with increasing sequence separation. This behavior is expected for rather uniformly expanded denatured proteins (40, 41) and indicates that persistent tertiary structure is absent in DnaK-bound denatured rhodanese. In comparison with denatured rhodanese in the absence of chaperones (Fig. 1C, colored histograms), the transfer efficiency distributions obtained in complex with DnaK and ATP are significantly broadened. Time-resolved fluorescence anisotropy measurements do not reveal a pronounced lack of rotational averaging of the attached fluorophores (Table S2); the broad transfer efficiency distributions are thus most likely to originate from a heterogeneous ensemble of very expanded rhodanese conformations in complex with DnaK that interconvert slowly compared with the millisecond observation time (42).
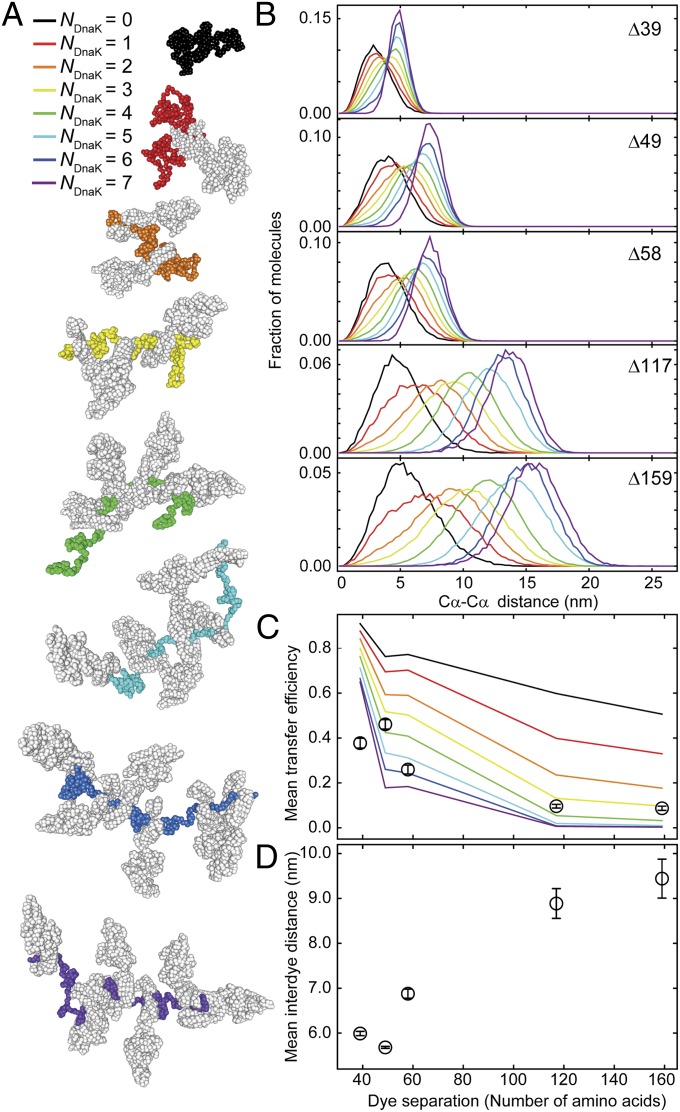
Fig. 2.
Substrate expansion can be explained by excluded volume effects from binding of several DnaK molecules per substrate molecule. (A) Representative structures from molecular dynamics (MD) simulations of rhodanese bound by a varying number of DnaK molecules (NDnaK) to all combinations of possible DnaK binding sites in the rhodanese sequence predicted by LIMBO (44). The color code from black to purple indicates the number of DnaK molecules (depicted in gray) bound per rhodanese (color code as indicated). (B) Interdye distance distributions from MD simulations for the dye positions of the different rhodanese variants used in the smFRET experiments (Fig. 1) for different NDnaK bound (colors as in A). (C) Comparison of the mean transfer efficiencies calculated from the distance distributions in the MD simulations in B as a function of NDnaK (colored lines) and mean transfer efficiencies from single-molecule data (Fig. 1E, black circles). Error bars indicate the uncertainty due to variability in instrument calibration. (D) Mean interdye distances from the FRET efficiency histograms (Fig. 1E) as a function of the sequence separation of the labeling positions. Mean transfer efficiencies from experimental data were converted to mean interdye distances assuming Gaussian distributions with the shapes of the simulated distributions for NDnaK = 6 and adjustable mean. Error bars indicate the variation of the average interdye distance upon variation of the width of the Gaussian distribution for different NDnaK.
Simulations Provide the Molecular Picture.
To obtain a more detailed picture of the underlying structural ensembles, we performed molecular simulations of the DnaK–rhodanese complexes. Owing to the complexity of the system, we used a coarse-grained model (43) where denatured rhodanese is represented as a disordered Cα chain with weak intramolecular interactions. A structure-based model of DnaK is then bound to rhodanese in one to seven copies at positions corresponding to DnaK binding sites identified using the predictor of van Durme et al. (44) (Fig. S4). With increasing numbers of DnaK molecules bound to denatured rhodanese, the simulations clearly show an increasing expansion of the complex (Fig. 2). If we compare the mean transfer efficiencies from the smFRET experiments (Fig. 1E) with the corresponding values calculated from the different simulated ensembles (Fig. 2C), the best overall agreement is observed in a range of four to seven DnaK molecules bound to rhodanese. The lowest individual mean-square deviation is obtained for six DnaK molecules, but a distribution of stoichiometries in this range is likely to contribute to the width of the smFRET efficiency distributions. By combining the experimental transfer efficiencies with the shape of the distance distributions obtained from the simulations (Fig. 2B), we estimate the average radius of gyration of the rhodanese–DnaK complexes to be ∼7.2 ± 0.3 nm (Table S4).
We can again test the molecular dimensions independently with 2f-FCS, which allows the size of the complex to be quantified in terms of the RS from its translational diffusivity. We obtain RS = 9.2 ± 0.2 nm for the rhodanese–DnaK complex, about a factor of three greater than the value for rhodanese bound to DnaJ, corroborating the strong DnaK-mediated expansion of the denatured protein. This value of RS agrees well with the Stokes radii calculated from the simulations for five to six DnaK molecules bound to rhodanese (Table S4), in agreement with the analysis of the FRET measurements. Both the experiments and the simulations thus support the picture of a substrate protein whose chain is almost saturated with DnaK. The volume exclusion between the bulky DnaK molecules underlies the pronounced substrate protein expansion we observe and results in an increase of the chain volume by a factor of ∼30–40. DnaK thus strongly shifts the conformation of the substrate protein toward a fully unfolded state where intramolecular interactions are expected to be minimized.
Dynamics of Complex Formation from Microfluidic Mixing.
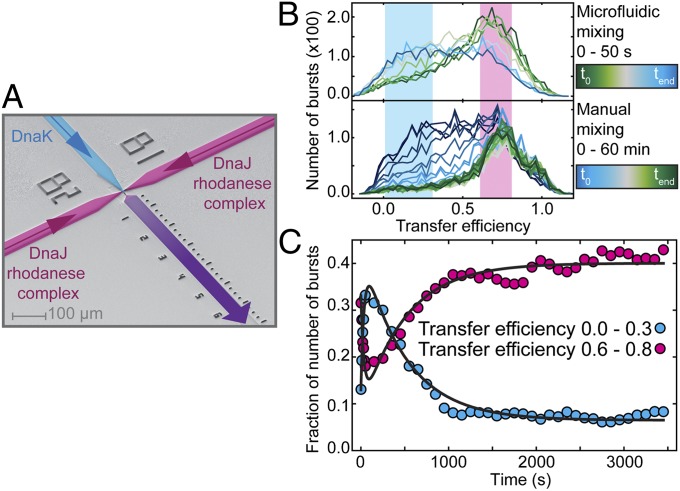
In contrast to DnaJ, the action of DnaK on the substrate protein requires continuous binding and release driven by ATP hydrolysis (45). To quantify the dynamics underlying this nonequilibrium process, we use a combination of manual and microfluidic mixing experiments, which allows us to monitor the process from milliseconds to hours. For measurements in the microfluidic device (19, 21, 23), preformed rhodanese–DnaJ complexes and ATP entering from the two side channels are rapidly mixed with DnaK in the main channel (Fig. 3A). Rapid mixing is achieved by lateral diffusion of the components in the narrow mixing neck, and transfer efficiency histograms at different times after mixing are obtained by placing the confocal volume at different positions in the following observation channel. As expected, the expanded rhodanese–DnaK population at low transfer efficiencies increases after mixing (Fig. 3B), whereas the rhodanese–DnaJ population decreases. A global fit yields a rate constant of k = 0.028 s−1 (Fig. 3C). This result is in accord with previously reported values for the conformational transition of DnaK between the open and the closed conformation triggered by DnaJ (0.04 s−1) (46), suggesting that the conformational switch in DnaK induced by substrate binding concurs with the conformational shift in the substrate ensemble.
Fig. 3.
Dynamics of association and dissociation of DnaK to DnaJ–rhodanese complexes. (A) Electron micrograph of the microfluidic mixing device. DnaK enters from the central channel (blue), and preformed DnaJ–rhodanese (Δ39) complexes and ATP enter from the side channels (magenta). Rapid mixing is achieved in the narrow mixing neck, and data are recorded with a confocal setup for 30 min at different positions in the observation channel (purple). FRET efficiency histograms were obtained at different times up to 50 s after mixing in the microfluidic mixing device (B, Upper) and up to 1 h after manual mixing (B, Lower). The starting concentrations were the same in both cases: 0.5 µM DnaJ, 10 µM DnaK, and 20 µM ATP. FRET efficiency histograms from manual mixing measurements were constructed by moving window analysis (19) using 5-min intervals starting every 100 s. To quantify the kinetics of DnaK– and DnaJ–rhodanese complex formation over time, the fractions of bursts with transfer efficiencies ranging from 0.0 to 0.3 (blue) and from 0.6 to 0.8 (magenta) determined for each histogram are represented (C). The data were fitted globally to double exponential functions with shared rate constants for expansion and release (black lines).
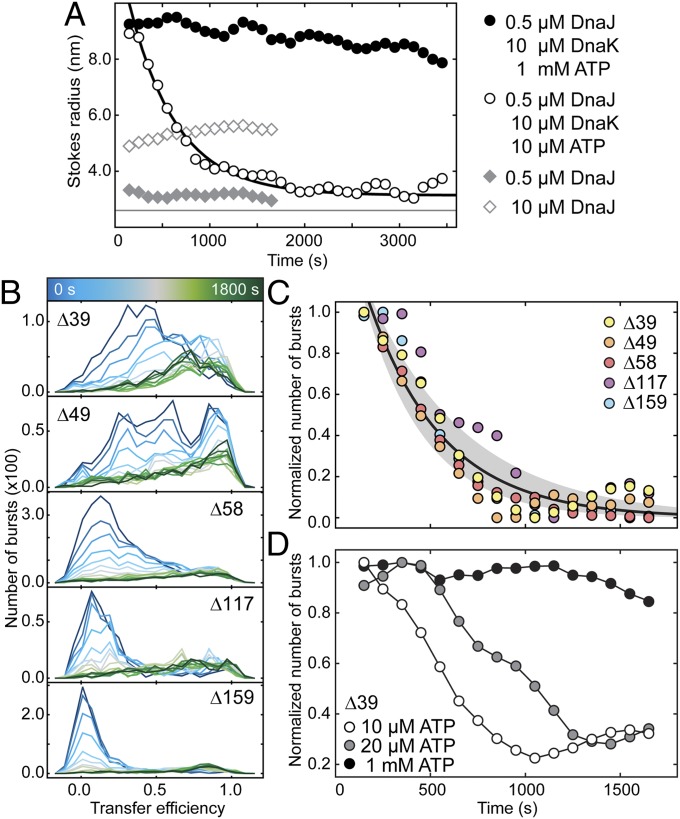
If the expansion of denatured rhodanese depends on the continuous hydrolysis of ATP by DnaK, we expect the system to relax back to its thermodynamic equilibrium when the ATP is consumed (Fig. 4D and Fig. S5A). Indeed, under conditions of limiting ATP concentrations (Fig. 4 B–D), the extended conformation of rhodanese disappears with a rate constant of (2.5 ± 0.4) 10−3 s−1 (Table S1). This rate is in a similar range as previously determined off-rates for substrates from ADP-bound DnaK (38, 47, 48). After reaching equilibrium, the transfer efficiency histograms are indistinguishable from those of the DnaJ–rhodanese complex, suggesting complete dissociation of DnaK (compare Fig. 4B, final histogram, and Fig. 1D). Interestingly, within experimental error, the dissociation rate constants are identical for all rhodanese variants (Fig. 4C and Table S1). This observation suggests that the dissociation rates of DnaK are rather independent of the position on the rhodanese chain and lack binding cooperativity, also confirmed by the independence of the dissociation rate of the DnaK concentration (Fig. S6), which justifies using only excluded volume interactions between DnaK molecules in the simulations. When the dissociation of DnaK is observed with 2f-FCS, a decrease of the RS with a rate constant of (1.7 ± 0.4) 10−3 s−1 is observed (Fig. 4A, white circles), in agreement with the decay of the expanded conformation observed in the smFRET experiments (Fig. 4 B and C). After completion of the dissociation reaction, the RS of the rhodanese–DnaJ complex is recovered, supporting our conclusion that DnaK dissociates completely once all ATP is consumed (Fig. 4A). Our observation that rhodanese does not remain stably bound to DnaK at limiting ATP concentrations contrasts with studies on other substrates (e.g., luciferase or peptides) (49, 50), indicating that the stability of the complex with DnaK depends on the substrate protein or peptide (51). We note, however, that the dependence of the expansion of rhodanese on the availability of ATP is in agreement with a recent theoretical study suggesting that the effective affinity of DnaK under nonequilibrium conditions can greatly exceed the one observed for ADP-bound DnaK (52). Whether DnaJ remains bound during the DnaK-driven expansion or rebinds after DnaK release is currently unclear (but this aspect has no bearing on our conclusions).
Fig. 4.
DnaK release kinetics are independent of the binding site on rhodanese. (A) Stokes radii of rhodanese (K174C Alexa 488) obtained from 2f-FCS measurements as a function of time after dilution of denatured rhodanese into native buffer in the presence of 0.5 µM DnaJ (gray diamonds); 10 µM DnaJ (white diamonds), 10 µM DnaK, 0.5 µM DnaJ, and 1 mM ATP (black circles); or 10 µM DnaK, 0.5 µM DnaJ, and 10 µM ATP (white circles). For comparison, the RS of native rhodanese is indicated as a horizontal line. The black line is a single-exponential fit. (B) Time-resolved FRET efficiency histograms for the five rhodanese variants after manual dilution of denatured rhodanese into native buffer in the presence of 10 µM DnaK, 0.5 µM DnaJ, and 10 µM ATP. After formation of the DnaK–rhodanese complexes during the dead time of manual mixing, we observe complex dissociation. (C) Decay of the DnaK–rhodanese complex population from the single-molecule FRET measurements shown in B represented by the change of the total number of bursts observed in each time interval, which decreases owing to the brightness difference between rhodanese with and without DnaK bound (Fig. 1 D and E and Table S2). All decays are well described with a single rate constant (black line, with confidence interval). (D) Dissociation of the DnaK–rhodanese complexes (variant Δ39) formed at 10 μM DnaK and 0.5 μM DnaJ were measured for different starting concentrations of ATP: 1 mM ATP (black), 20 µM ATP (gray), and 10 µM ATP (white).
Closing the Cycle.
The third component acting together with DnaJ and DnaK has not been considered so far: the nucleotide exchange factor GrpE (53). GrpE catalyzes the exchange of ADP with ATP in DnaK (54) and thus accelerates the nonequilibrium association/dissociation cycle of DnaK with the substrate protein (47, 55). As a result, the addition of GrpE to the rhodanese–DnaK complexes leads to a faster disappearance of the expanded rhodanese population (Fig. S5 C and D), reflecting the accelerated ATP hydrolysis by DnaK in the presence of GrpE and confirming the functionality of the entire DnaK/DnaJ/GrpE chaperone system used in our single-molecule experiments. The degree of rhodanese expansion, however, is not reduced by GrpE, indicating that the more rapid ATP turnover does not alter the average number of DnaK molecules bound per rhodanese so long as sufficient ATP is still available (Fig. S5B). As for the uncatalyzed dissociation of DnaK (Fig. 4B), the transfer efficiency histograms of all rhodanese variants after DnaK release are reminiscent of those of the DnaJ–rhodanese complexes. Notably, even with the accelerated DnaK dissociation triggered by GrpE, refolding of rhodanese is thus not observed under our experimental conditions. Whereas substrate proteins such as luciferase have been shown to refold in the presence of the ternary chaperone system DnaJ/DnaK/GrpE under comparable conditions (49), our results suggest that for rhodanese the DnaJ–rhodanese complex is the thermodynamically most stable state. Indeed, earlier studies (13) suggested that additional chaperones, such as the chaperonin system GroEL/GroES, are required to refold denatured rhodanese. Our results therefore support the model that the DnaK/DnaJ/GrpE system passes substrate proteins on to downstream chaperones (11, 56). We note, however, that with an excess of substrate present, the cellular DnaJ and DnaK concentrations may become limiting, resulting in a less pronounced expansion or even a bypassing of the DnaK/DnaJ/GrpE system (51). Another aspect that can modulate the cellular situation is that GrpE is reversibly denatured under heat-shock conditions (57), which will increase the residence time of the individual DnaK molecules on the substrate protein and thus keep the substrate protein expanded with reduced energy consumption until the conditions are again more favorable for refolding.
In summary, we find that the binding of DnaK to rhodanese–DnaJ complexes leads to a pronounced ATP-driven expansion of the denatured substrate protein. The resulting disruption of intramolecular contacts in the substrate protein may represent an efficient way of rescuing misfolded proteins from kinetic traps that would prevent folding or could lead to aggregation. DnaK-induced protein expansion also illustrates a generic mechanism underlying the action of this versatile chaperone system: Although ATP is required for the binding of DnaK to the DnaJ–rhodanese complex, the observed expansion itself is well accounted for by an entropic effect, caused by the steric exclusion of the large DnaK molecules bound to the polypeptide chain. This “entropic pulling” (58) has been suggested to also play a dominant role in the Hsp70-mediated translocation of denatured substrate proteins into mitochondria and in protein disaggregation (59). The resulting expanded, DnaK-bound proteins are not only expected to confer solubility in the crowded cellular environment, but they also provide an ideal starting point for presenting the substrate protein to the downstream chaperone machinery.
Methods
Cysteine variants of rhodanese were expressed, purified, and labeled with Alexa Fluor 488 and Alexa Fluor 594 as described previously (60). Chaperones DnaK, DnaJ, and GrpE were gifts from H.-J. Schönfeld (Hoffmann-La Roche Ltd., Basel). All experiments were carried out in buffer A [50 mM Tris⋅HCl (pH 7.5), 10 mM MgCl2, 5 mM KCl, 200 mM β-mercaptoethanol, and 0.001% (vol/vol) Tween 20 (Pierce)]. Rhodanese–chaperone complexes were formed by denaturing rhodanese in 4 M GdmCl and diluting it 100× into buffer A containing chaperones and nucleotides as indicated. Single-molecule FRET measurements were performed at 22 °C using pulsed interleaved excitation (61) on either an adapted MicroTime 200 confocal microscope (PicoQuant) or a custom-built confocal microscope. Dual-focus fluorescence correlation spectroscopy measurements were performed at 22 °C on a MicroTime 200 confocal microscope equipped with a differential interference contrast prism as described previously (29). For rapid mixing experiments, microfluidic mixers were fabricated and used as described previously (23). Molecular dynamics simulations of DnaK–rhodanese complexes were performed with CafeMol 2.0 (43) based on a Cα representation of the rhodanese chain and a structure-based model of DnaK bound to the binding sites predicted by LIMBO (44). Independent simulations of all 127 possible stoichiometry and permutations of occupied binding sites were carried out and average distance distributions and Stokes radii recovered from simulations with identical stoichiometry. For further details on experiments, instrumentation, data analysis, and simulations see Supporting Information.
Supplementary Material
Acknowledgments
We thank Paolo De Los Rios and Philipp Christen for helpful discussion and Hans-Joachim Schönfeld for the generous gift of chaperone proteins and expression plasmids. We thank Frank Hillger and Daniel Streich for the design and characterization of the rhodanese variants. This work was supported by a Starting Independent Researcher grant of the European Research Council and the Swiss National Science Foundation (to B.S. and Ambizione Grant PZ00P2_136856 to A.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1407086111/-/DCSupplemental.
References
- 1.Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125(3):443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475(7356):324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 3.Mapa K, et al. The conformational dynamics of the mitochondrial Hsp70 chaperone. Mol Cell. 2010;38(1):89–100. doi: 10.1016/j.molcel.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Schlecht R, Erbse AH, Bukau B, Mayer MP. Mechanics of Hsp70 chaperones enables differential interaction with client proteins. Nat Struct Mol Biol. 2011;18(3):345–351. doi: 10.1038/nsmb.2006. [DOI] [PubMed] [Google Scholar]
- 5.Mayer MP. Gymnastics of molecular chaperones. Mol Cell. 2010;39(3):321–331. doi: 10.1016/j.molcel.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Powers ET, Powers DL, Gierasch LM. FoldEco: A model for proteostasis in E. coli. Cell Reports. 2012;1(3):265–276. doi: 10.1016/j.celrep.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer MP, Bukau B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62(6):670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Soroka J, Buchner J. The Hsp90 chaperone machinery: Conformational dynamics and regulation by co-chaperones. Biochim Biophys Acta Mol Cell Res. 2012;1823(3):624–635. doi: 10.1016/j.bbamcr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Mayer MP. Hsp70 chaperone dynamics and molecular mechanism. Trends Biochem Sci. 2013;38(10):507–514. doi: 10.1016/j.tibs.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Schröder H, Langer T, Hartl FU, Bukau B. DnaK, DnaJ and GrpE form a cellular chaperone machinery capable of repairing heat-induced protein damage. EMBO J. 1993;12(11):4137–4144. doi: 10.1002/j.1460-2075.1993.tb06097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calloni G, et al. DnaK functions as a central hub in the E. coli chaperone network. Cell Reports. 2012;1(3):251–264. doi: 10.1016/j.celrep.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Diamant S, Ben-Zvi AP, Bukau B, Goloubinoff P. Size-dependent disaggregation of stable protein aggregates by the DnaK chaperone machinery. J Biol Chem. 2000;275(28):21107–21113. doi: 10.1074/jbc.M001293200. [DOI] [PubMed] [Google Scholar]
- 13.Langer T, et al. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992;356(6371):683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Zvi A, De Los Rios P, Dietler G, Goloubinoff P. Active solubilization and refolding of stable protein aggregates by cooperative unfolding action of individual hsp70 chaperones. J Biol Chem. 2004;279(36):37298–37303. doi: 10.1074/jbc.M405627200. [DOI] [PubMed] [Google Scholar]
- 15.Skowyra D, Georgopoulos C, Zylicz M. The E. coli dnaK gene product, the hsp70 homolog, can reactivate heat-inactivated RNA polymerase in an ATP hydrolysis-dependent manner. Cell. 1990;62(5):939–944. doi: 10.1016/0092-8674(90)90268-j. [DOI] [PubMed] [Google Scholar]
- 16.Fenton WA, Horwich AL. Chaperonin-mediated protein folding: fate of substrate polypeptide. Q Rev Biophys. 2003;36(2):229–256. doi: 10.1017/s0033583503003883. [DOI] [PubMed] [Google Scholar]
- 17.Borgia MB, et al. Single-molecule fluorescence reveals sequence-specific misfolding in multidomain proteins. Nature. 2011;474(7353):662–665. doi: 10.1038/nature10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haran G. How, when and why proteins collapse: The relation to folding. Curr Opin Struct Biol. 2012;22(1):14–20. doi: 10.1016/j.sbi.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmann H, et al. Single-molecule spectroscopy of protein folding in a chaperonin cage. Proc Natl Acad Sci USA. 2010;107(26):11793–11798. doi: 10.1073/pnas.1002356107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hillger F, et al. Probing protein-chaperone interactions with single-molecule fluorescence spectroscopy. Angew Chem Int Ed Engl. 2008;47(33):6184–6188. doi: 10.1002/anie.200800298. [DOI] [PubMed] [Google Scholar]
- 21.Pfeil SH, Wickersham CE, Hoffmann A, Lipman EA. A microfluidic mixing system for single-molecule measurements. Rev Sci Instrum. 2009;80(5):055105. doi: 10.1063/1.3125643. [DOI] [PubMed] [Google Scholar]
- 22.Soranno A, et al. Quantifying internal friction in unfolded and intrinsically disordered proteins with single-molecule spectroscopy. Proc Natl Acad Sci USA. 2012;109(44):17800–17806. doi: 10.1073/pnas.1117368109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wunderlich B, et al. Microfluidic mixer designed for performing single-molecule kinetics with confocal detection on timescales from milliseconds to minutes. Nat Protoc. 2013;8(8):1459–1474. doi: 10.1038/nprot.2013.082. [DOI] [PubMed] [Google Scholar]
- 24.Tran HT, Mao A, Pappu RV. Role of backbone-solvent interactions in determining conformational equilibria of intrinsically disordered proteins. J Am Chem Soc. 2008;130(23):7380–7392. doi: 10.1021/ja710446s. [DOI] [PubMed] [Google Scholar]
- 25.Mendoza JA, Rogers E, Lorimer GH, Horowitz PM. Unassisted refolding of urea unfolded rhodanese. J Biol Chem. 1991;266(21):13587–13591. [PubMed] [Google Scholar]
- 26.Diamant S, Goloubinoff P. Temperature-controlled activity of DnaK-DnaJ-GrpE chaperones: Protein-folding arrest and recovery during and after heat shock depends on the substrate protein and the GrpE concentration. Biochemistry. 1998;37(27):9688–9694. doi: 10.1021/bi980338u. [DOI] [PubMed] [Google Scholar]
- 27.Brinker A, et al. Dual function of protein confinement in chaperonin-assisted protein folding. Cell. 2001;107(2):223–233. doi: 10.1016/s0092-8674(01)00517-7. [DOI] [PubMed] [Google Scholar]
- 28.Haenni D, Zosel F, Reymond L, Nettels D, Schuler B. Intramolecular distances and dynamics from the combined photon statistics of single-molecule FRET and photoinduced electron transfer. J Phys Chem B. 2013;117(42):13015–13028. doi: 10.1021/jp402352s. [DOI] [PubMed] [Google Scholar]
- 29.Dertinger T, et al. Two-focus fluorescence correlation spectroscopy: A new tool for accurate and absolute diffusion measurements. ChemPhysChem. 2007;8(3):433–443. doi: 10.1002/cphc.200600638. [DOI] [PubMed] [Google Scholar]
- 30.Shi YY, Hong XG, Wang CC. The C-terminal (331-376) sequence of Escherichia coli DnaJ is essential for dimerization and chaperone activity: A small angle X-ray scattering study in solution. J Biol Chem. 2005;280(24):22761–22768. doi: 10.1074/jbc.M503643200. [DOI] [PubMed] [Google Scholar]
- 31.Schuler B. Single-molecule fluorescence spectroscopy of protein folding. ChemPhysChem. 2005;6(7):1206–1220. doi: 10.1002/cphc.200400609. [DOI] [PubMed] [Google Scholar]
- 32.Roder H, Colón W. Kinetic role of early intermediates in protein folding. Curr Opin Struct Biol. 1997;7(1):15–28. doi: 10.1016/s0959-440x(97)80004-8. [DOI] [PubMed] [Google Scholar]
- 33.Pollack L, et al. Compactness of the denatured state of a fast-folding protein measured by submillisecond small-angle x-ray scattering. Proc Natl Acad Sci USA. 1999;96(18):10115–10117. doi: 10.1073/pnas.96.18.10115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bilsel O, Matthews CR. Molecular dimensions and their distributions in early folding intermediates. Curr Opin Struct Biol. 2006;16(1):86–93. doi: 10.1016/j.sbi.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Nettels D, Gopich IV, Hoffmann A, Schuler B. Ultrafast dynamics of protein collapse from single-molecule photon statistics. Proc Natl Acad Sci USA. 2007;104(8):2655–2660. doi: 10.1073/pnas.0611093104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuler B, Hofmann H. Single-molecule spectroscopy of protein folding dynamics—expanding scope and timescales. Curr Opin Struct Biol. 2013;23(1):36–47. doi: 10.1016/j.sbi.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Rüdiger S, Schneider-Mergener J, Bukau B. Its substrate specificity characterizes the DnaJ co-chaperone as a scanning factor for the DnaK chaperone. EMBO J. 2001;20(5):1042–1050. doi: 10.1093/emboj/20.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayer MP, Laufen T, Paal K, McCarty JS, Bukau B. Investigation of the interaction between DnaK and DnaJ by surface plasmon resonance spectroscopy. J Mol Biol. 1999;289(4):1131–1144. doi: 10.1006/jmbi.1999.2844. [DOI] [PubMed] [Google Scholar]
- 39.Laufen T, et al. Mechanism of regulation of hsp70 chaperones by DnaJ cochaperones. Proc Natl Acad Sci USA. 1999;96(10):5452–5457. doi: 10.1073/pnas.96.10.5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohn JE, et al. Random-coil behavior and the dimensions of chemically unfolded proteins. Proc Natl Acad Sci USA. 2004;101(34):12491–12496. doi: 10.1073/pnas.0403643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofmann H, et al. Polymer scaling laws of unfolded and intrinsically disordered proteins quantified with single-molecule spectroscopy. Proc Natl Acad Sci USA. 2012;109(40):16155–16160. doi: 10.1073/pnas.1207719109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gopich IV, Szabo A. FRET efficiency distributions of multistate single molecules. J Phys Chem B. 2010;114(46):15221–15226. doi: 10.1021/jp105359z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kenzaki H, et al. CafeMol: A coarse-grained biomolecular simulator for simulating proteins at work. J Chem Theory Comput. 2011;7(6):1979–1989. doi: 10.1021/ct2001045. [DOI] [PubMed] [Google Scholar]
- 44.Van Durme J, et al. Accurate prediction of DnaK-peptide binding via homology modelling and experimental data. PLOS Comput Biol. 2009;5(8):e1000475. doi: 10.1371/journal.pcbi.1000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buchberger A, Valencia A, McMacken R, Sander C, Bukau B. The chaperone function of DnaK requires the coupling of ATPase activity with substrate binding through residue E171. EMBO J. 1994;13(7):1687–1695. doi: 10.1002/j.1460-2075.1994.tb06433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pierpaoli EV, et al. The power stroke of the DnaK/DnaJ/GrpE molecular chaperone system. J Mol Biol. 1997;269(5):757–768. doi: 10.1006/jmbi.1997.1072. [DOI] [PubMed] [Google Scholar]
- 47.Theyssen H, Schuster HP, Packschies L, Bukau B, Reinstein J. The second step of ATP binding to DnaK induces peptide release. J Mol Biol. 1996;263(5):657–670. doi: 10.1006/jmbi.1996.0606. [DOI] [PubMed] [Google Scholar]
- 48.Schmid D, Baici A, Gehring H, Christen P. Kinetics of molecular chaperone action. Science. 1994;263(5149):971–973. doi: 10.1126/science.8310296. [DOI] [PubMed] [Google Scholar]
- 49.Sharma SK, De los Rios P, Christen P, Lustig A, Goloubinoff P. The kinetic parameters and energy cost of the Hsp70 chaperone as a polypeptide unfoldase. Nat Chem Biol. 2010;6(12):914–920. doi: 10.1038/nchembio.455. [DOI] [PubMed] [Google Scholar]
- 50.Pierpaoli EV, Sandmeier E, Schönfeld HJ, Christen P. Control of the DnaK chaperone cycle by substoichiometric concentrations of the co-chaperones DnaJ and GrpE. J Biol Chem. 1998;273(12):6643–6649. doi: 10.1074/jbc.273.12.6643. [DOI] [PubMed] [Google Scholar]
- 51.Natalello A, et al. Biophysical characterization of two different stable misfolded monomeric polypeptides that are chaperone-amenable substrates. J Mol Biol. 2013;425(7):1158–1171. doi: 10.1016/j.jmb.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 52.De Los Rios P, Barducci A. Hsp70 chaperones are non-equilibrium machines that achieve ultra-affinity by energy consumption. eLife (Cambridge) 2014;3:e02218. doi: 10.7554/eLife.02218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zylicz M, Ang D, Georgopoulos C. The grpE protein of Escherichia coli. Purification and properties. J Biol Chem. 1987;262(36):17437–17442. [PubMed] [Google Scholar]
- 54.Liberek K, Marszalek J, Ang D, Georgopoulos C, Zylicz M. Escherichia coli DnaJ and GrpE heat shock proteins jointly stimulate ATPase activity of DnaK. Proc Natl Acad Sci USA. 1991;88(7):2874–2878. doi: 10.1073/pnas.88.7.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palleros DR, Reid KL, Shi L, Welch WJ, Fink AL. ATP-induced protein-Hsp70 complex dissociation requires K+ but not ATP hydrolysis. Nature. 1993;365(6447):664–666. doi: 10.1038/365664a0. [DOI] [PubMed] [Google Scholar]
- 56.Seyffer F, et al. Hsp70 proteins bind Hsp100 regulatory M domains to activate AAA+ disaggregase at aggregate surfaces. Nat Struct Mol Biol. 2012;19(12):1347–1355. doi: 10.1038/nsmb.2442. [DOI] [PubMed] [Google Scholar]
- 57.Grimshaw JPA, Jelesarov I, Siegenthaler RK, Christen P. Thermosensor action of GrpE. The DnaK chaperone system at heat shock temperatures. J Biol Chem. 2003;278(21):19048–19053. doi: 10.1074/jbc.M300924200. [DOI] [PubMed] [Google Scholar]
- 58.De Los Rios P, Ben-Zvi A, Slutsky O, Azem A, Goloubinoff P. Hsp70 chaperones accelerate protein translocation and the unfolding of stable protein aggregates by entropic pulling. Proc Natl Acad Sci USA. 2006;103(16):6166–6171. doi: 10.1073/pnas.0510496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goloubinoff P, De Los Rios P. The mechanism of Hsp70 chaperones: (Entropic) pulling the models together. Trends Biochem Sci. 2007;32(8):372–380. doi: 10.1016/j.tibs.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 60.Hillger F, Nettels D, Dorsch S, Schuler B. Detection and analysis of protein aggregation with confocal single molecule fluorescence spectroscopy. J Fluoresc. 2007;17(6):759–765. doi: 10.1007/s10895-007-0187-z. [DOI] [PubMed] [Google Scholar]
- 61.Müller BK, Zaychikov E, Bräuchle C, Lamb DC. Pulsed interleaved excitation. Biophys J. 2005;89(5):3508–3522. doi: 10.1529/biophysj.105.064766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.