Abstract
Tuberculosis, caused by the intracellular bacterium Mycobacterium tuberculosis, currently causes ∼1.4 million deaths per year, and it therefore remains a leading global health problem. The immune response during tuberculosis remains incompletely understood, particularly regarding immune factors that are harmful rather than protective to the host. Overproduction of the type I IFN family of cytokines is associated with exacerbated tuberculosis in both mouse models and in humans, although the mechanisms by which type I IFN promotes disease are not well understood. We have investigated the effect of type I IFN on M. tuberculosis–infected macrophages and found that production of host-protective cytokines such as TNF-α, IL-12, and IL-1β is inhibited by exogenous type I IFN, whereas production of immunosuppressive IL-10 is promoted in an IL-27–independent manner. Furthermore, much of the ability of type I IFN to inhibit cytokine production was mediated by IL-10. Additionally, type I IFN compromised macrophage activation by the lymphoid immune response through severely disrupting responsiveness to IFN-γ, including M. tuberculosis killing. These findings describe important mechanisms by which type I IFN inhibits the immune response during tuberculosis.
Introduction
Myeloid cells such as macrophages are the predominant targets of Mycobacterium tuberculosis infection (1–3). Effector functions elicited in these cells upon infection are crucial to the establishment of an adaptive immune response to M. tuberculosis, restriction of bacterial growth, and ultimately to host resistance (1–3). Key effectors linked to host protection produced by these cells include the cytokines IL-12, TNF-α, and IL-1α/β (1–3). Production of NO and other molecules that restrict intracellular bacterial growth is also of importance (4–6). IL-12 is critical for activation of CD4+ T cells, leading to the protective Th1 response and induction of IFN-γ from a variety of cellular sources (7, 8). IFN-γ is in turn crucial for the full activation of macrophage bactericidal functions (such as NO production and restriction of mycobacterial growth) and further enhancement of innate cytokine production (5, 6, 9–14).
Whereas immune mechanisms that lead to host resistance have been intensively studied, less is understood about potentially damaging or inhibitory immune responses leading to activation or exacerbation of tuberculosis (TB). There is a growing appreciation that the type I IFN family of cytokines plays a detrimental as opposed to protective role during TB, particularly when such cytokines are present in excess amounts, with evidence in both mice and humans largely supporting this hypothesis (15–25). Studies of M. tuberculosis infection of mice that lack the receptor common to all type I IFN (Ifnar1−/− mice) found reduced bacterial load and/or increased survival compared with wild-type (WT) mice (18, 19, 22, 24), pointing to a negative role of type I IFN in TB. However, results have not been unequivocal, with some suggestion that type I IFN may have protective activities under certain conditions (26, 27).
Studies where excess levels of type I IFN are induced during TB, either through direct instillation of IFN-α/β into the lung (18), administration of a polyinosinic-polycytidylic acid derivative (15), or abrogation of a negative regulator of type I IFN signaling (28) support a detrimental role for type I IFN during TB, as they all resulted in exacerbated disease. In accordance with these results, hypervirulent M. tuberculosis strains reportedly induce high levels of type I IFN (18, 19).
Recent reports have also revealed a potential role for type I IFN in the human immune response during TB, because cohorts of active TB patients in London and South Africa showed a prominent type I IFN–inducible gene signature in their blood, which cell separation experiments found was present predominantly in myeloid cells (16). Furthermore, the signature was correlated with the extent of radiographic disease and resolved upon successful treatment (16). These findings have now been verified by other groups using patient cohorts from Africa (17, 29) and Indonesia (23).
The mechanisms by which type I IFN exacerbates disease during M. tuberculosis infection are only partially understood. Recently, studies have described a role for type I IFN in suppressing production of the protective cytokine IL-1 in both in vivo mouse models (20) and human monocytes (21). This inhibition of IL-1 was partially dependent on IL-10 (20), which is known to be induced by type I IFN (30–32). IL-10 is also induced during infection with another intracellular bacteria, Listeria monocytogenes, where type I IFNs are similarly detrimental to the host (33–36). Furthermore, IL-10 is known to inhibit the immune response during TB, particularly the Th1 cell response (37–41). Earlier studies of infection with hypervirulent M. tuberculosis strains that induce high levels of type I IFN also point to a reduction in the protective Th1 response, with reduced IL-12 and IFN-γ during infection (18). In human infections with the leprosy agent Mycobacterium leprae, type I IFN has also been shown to induce IL-10 and restrict IFN-γ–mediated antibacterial responses (42). Additionally, type I IFNs have been shown to downregulate IFN-γ receptor expression on myeloid cells during L. monocytogenes infection (43).
We have investigated the effects of type I IFN on macrophage function during M. tuberculosis infection. We found that type I IFN inhibits production of multiple protective cytokines by M. tuberculosis–infected macrophages and induces the immunosuppressive cytokine IL-10. Notably, type I IFN also robustly suppressed macrophage responsiveness to IFN-γ, preventing IFN-γ–dependent enhancement of cytokine production and inhibiting IFN-γ–mediated macrophage bacterial growth inhibition and killing. These results suggest an important role for type I IFN in inhibiting the immune response to M. tuberculosis at the level of the macrophage, potentially impairing both macrophage induction of, and response to, adaptive immunity.
Materials and Methods
Mice
C57BL/6 (B6), B6 Ifnar1−/−, B6 Il10−/−, and B6 Tccr−/− (IL-27R α-chain–deficient) mice were bred and housed under specific pathogen-free conditions at the Medical Research Council National Institute for Medical Research, B6 Nos2−/− mice (and B6 WT controls) were bred and housed under specific pathogen-free conditions at the Instituto de Biologia Molecular e Celular (Porto, Portugal), and bones from these mice were shipped overnight on ice to the National Institute for Medical Research. All protocols for breeding and experiments were performed in accordance with either Home Office (U.K.) requirements and the Animal Scientific Procedures Act, 1986 or according to the European Union directive 86/609/EEC and approved by the Portugese national authority for animal health, Direcção Geral de Veterinária (Portugal). Mice were sex and age matched for use in experiments.
Reagents
Cell culture medium was RPMI 1640 (Lonza) supplemented with 5% heat-inactivated FCS (Biosera), 0.05 mM 2-ME (Sigma-Aldrich), 2 mM l-glutamine (Lonza), 1 mM sodium pyruvate (Lonza), and 10 mM HEPES (Lonza). rIFN-β was purchased from PBL Assay Science and rIFN-γ was purchased from R&D Systems. IFN-β was used at 2 ng/ml unless otherwise indicated, and IFN-γ was used at 5 ng/ml. Anti–IL-10R (clone 1B1.3A) and anti–IL-10 (clone TC40.11D8) mAbs and their isotype controls (GL113 and TC31.2F11, respectively) were gifts from DNAX Research Institute (now Merck, Palo Alto, CA) and were used at 10 μg/ml.
Generation and infection of murine bone marrow–derived macrophages and enrichment and infection of bone marrow and lung myeloid cells
Bone marrow (BM) cells were flushed from the femurs and tibias of mice and plated at 0.5 × 106 cells/ml on bacterial plates (Sterilin) in culture medium containing 10% FCS and 20% L929 cell–conditioned medium. At day 6, macrophages were harvested and seeded into 24-well tissue culture plates (Corning) at 1 × 106 cells/ml. Cells were rested overnight, washed once with PBS, and infected at a multiplicity of infection of 2:1 with M. tuberculosis H37Rv and treated where indicated with recombinant cytokines and/or mAbs. All experiments using M. tuberculosis were carried out under biosafety containment level 3 conditions. M. tuberculosis H37Rv was grown as previously described (39). M. tuberculosis was left in the wells until the supernatant or cells were harvested, unless otherwise stated. The number of bacteria in the inoculum was determined by serial dilutions on 7H11 plates supplemented with 10% OADC. For mRNA stability experiments cells were treated at 1 h after M. tuberculosis infection with 10 μg/ml actinomycin D (ActD) and then harvested for RNA at indicated time points after ActD treatment.
BM and lung myeloid cells were enriched from whole-organ cell suspensions using EasySep (StemCell Technologies) magnetic separation protocols. Cell suspensions were first enriched using the mouse monocyte enrichment kit (StemCell Technologies, catalog no. 19761A), followed by staining with anti-Ly6c FITC Ab (BD Pharmingen, clone AL-21) and further enrichment with the mouse FITC selection kit (StemCell Technologies, catalog no. 18515). Cells were then plated at 0.5 × 106 cells/ml and infected with H37Rv at a multiplicity of infection of 2:1 and treated where indicated with rIFN-β and/or rIFN-γ.
Enumeration of intracellular M. tuberculosis in macrophages following infection
To determine the number of intracellular M. tuberculosis CFUs present in macrophages following infection, supernatants were harvested, macrophages were washed once with PBS to remove extracellular bacteria, and 1 ml 0.2% saponin (Sigma-Aldrich) was added for 1 h at 37°C to lyse the cells. This suspension was then serially diluted and plated onto 7H11 plates supplemented with OADC, and colonies were counted after 14–16 d at 37°C.
Cytokine quantification by ELISA and bead array
Cytokine concentrations in the supernatants of infected cells were determined by ELISA or Luminex bead array at 24 h after M. tuberculosis infection. This time point was chosen based on pilot experiments to determine the optimal postinfection time point for analysis. Commercially available kits were used for TNF-α, IL-12p70, IL-27 (all eBioscience), and IL-1β (R&D Systems) and were used according to the manufacturers’ instructions. Matched Ab pairs were used for IL-12p40 and IL-10. IL-12p40 was detected using Ab clone C15.6.7 for capture and biotinylated Ab clone C17.8 for detection. IL-10 was detected using Ab clone JES5-2A5 for capture and biotinylated anti–IL-10 for detection (BD Biosciences, clone SXC-1). Custom magnetic bead arrays to measure cytokines in supernatant of primary ex vivo cells were purchased from Millipore (Merck Millipore, Billerica, MA) and used according to the manufacturer’s instructions. Samples were run on a Bio-Rad Luminex 200 machine (Bio-Rad, Hercules, CA). Cytokine levels from uninfected cells were below the assay level of detection unless otherwise shown (20 pg/ml for ELISA, 5 pg/ml for bead array) (data not shown).
Processing of macrophage RNA and quantitative PCR analysis
At indicated times postinfection, supernatants were removed and cells were washed once with PBS. RNA was harvested in 350 μl RLT buffer (Qiagen) and stored at −80°C before processing. RNA was processed using RNeasy Mini kits (Qiagen). RNA was reverse transcribed with a high-capacity reverse transcription kit (Applied Biosystems) to cDNA. The expression of indicated genes was quantified by real-time PCR (ABI Prism 7900 from Applied Biosystems) and normalized against Hprt mRNA levels. Murine primers were all purchased from Applied Biosystems.
Protein analysis and Western blotting
Spleens were homogenized by passing through a 70-μm sieve, and cell suspensions were then RBC lysed and cultured in media containing 1% FCS for 5 h before treatment with rIL-27 (50 ng/ml), rIFN-γ (10 ng/ml), or rIL-10 (10 ng/ml) for the indicated times. Where indicated cells were treated with 200 ng/ml Pam3CSK4 (Invivogen). BM-derived macrophages (BMDMs) were grown as described above but rested overnight in 1% FCS prior to treatment with recombinant cytokines and stimuli, as indicated. Cells were then harvested, lysed in RIPA buffer, and immunoblotting was carried out as previously described (44). Anti–phospho-STAT1 (Y701), anti–total STAT1, anti–phospho-STAT3 (Y705), anti–total STAT3 (all Cell Signaling Technology), and anti-actin (Calbiochem) primary Abs, followed by HRP-conjugated goat anti-rabbit IgG (SouthernBiotech) or goat anti-mouse IgM (Calbiochem) secondary Abs, were used to probe membranes.
Statistical analysis
Statistical analysis was carried out using Prism software version 6 (GraphPad Software). Statistical tests used to determine significance are described in the figure legends with values as follows: *p < 0.05, **p < 0.01, and ***p < 0.001.
Results
Type I IFN regulates IL-10 production in M. tuberculosis–infected macrophages
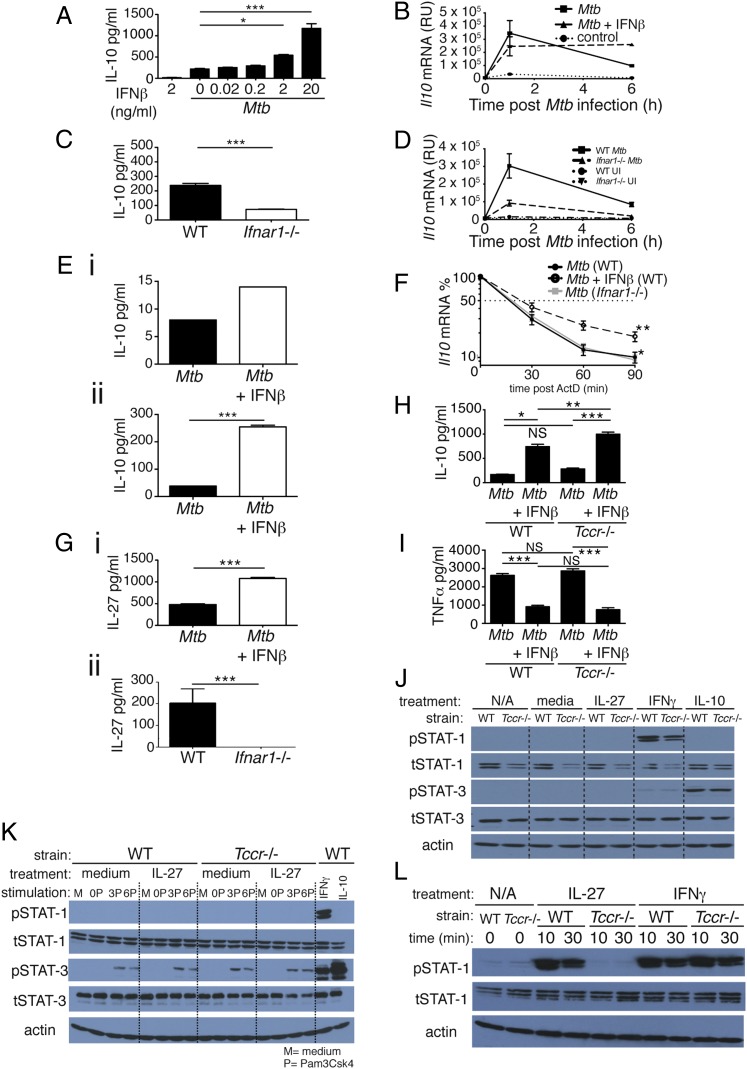
Although type I IFN has been implicated in exacerbation of TB, it remains unclear how it manifests its effects at the molecular level. We investigated whether type I IFN regulated production of IL-10, an immunosuppressive cytokine generally linked to TB exacerbation (37–41). Initially, we assessed whether type I IFN could enhance IL-10 production by infecting WT macrophages with M. tuberculosis and concomitantly adding rIFN-β at varying concentrations from 0.02 to 20 ng/ml (Fig. 1A). Addition of IFN-β significantly enhanced IL-10 production by M. tuberculosis–infected macrophages when added at 2 and 20 ng/ml (Fig. 1A). Notably, type I IFN on its own was not sufficient to induce IL-10 production by macrophages, in support of a role for this cytokine as an enhancer of IL-10 production (Fig. 1A). Additionally, we assessed the effects of IFN-β addition on Il10 mRNA transcription (Fig. 1B). Addition of 2 ng/ml IFN-β to M. tuberculosis–infected macrophages resulted in enhanced Il10 mRNA levels at 6 h postinfection (Fig. 1B).
FIGURE 1.
Type I IFN regulates IL-10 production in M. tuberculosis–infected macrophages independently of IL-27 signaling. (A) WT macrophages were infected with M. tuberculosis in the presence of increasing concentrations of IFN-β, added at the time of infection, and levels of IL-10 in culture supernatant were determined by ELISA at 24 h postinfection. (B) WT macrophages were infected with M. tuberculosis in the presence or absence of 2 ng/ml IFN-β, added at the time of infection, and levels of Il10 mRNA were determined by quantitative RT-PCR (qRT-PCR) at the time points indicated after infection. (C) WT and Ifnar1−/− macrophages were infected with M. tuberculosis and levels of IL-10 in culture supernatant were determined by ELISA at 24 h postinfection. (D) WT and Ifnar1−/− macrophages were infected with M. tuberculosis and levels of Il10 mRNA determined by qRT-PCR at the time points indicated after infection. (E) WT myeloid cells taken ex vivo from lungs (i) and BM (ii) were infected with M. tuberculosis in the presence or absence of 2 ng/ml IFN-β, added at the time of infection, and levels of IL-10 in culture supernatant were determined by Luminex bead array at 24 h postinfection. (F) WT, Ifnar1−/−, and WT treated with IFN-β macrophages were infected with M. tuberculosis, and at 1 h postinfection ActD was added. mRNA was then taken at the time points indicated and Il10 mRNA levels were determined by qRT-PCR. (G) WT macrophages treated (or not) with 2 ng/ml IFN-β at the time of infection (i) and WT and Ifnar1−/− macrophages (ii) were infected with M. tuberculosis, and levels of IL-27 in culture supernatant were determined by ELISA at 24 h postinfection. (H and I) WT and Tccr−/− (IL-27Rα−/−) macrophages were infected with M. tuberculosis in the presence or absence of 2 ng/ml IFN-β, added at the time of infection, and levels of IL-10 (H) or TNF-α (I) in culture supernatant were determined by ELISA at 24 h postinfection. (J) WT and Tccr−/− (IL-27Rα−/−) macrophages were treated for 20 min with rIL-27 (50 ng/ml), rIFN-γ (10 ng/ml), or rIL-10 (10 ng/ml) and whole-cell extracts were then analyzed by immunoblotting with the indicated Abs. (K) WT and Tccr−/− (IL-27Rα−/−) macrophages were stimulated for 0, 3, or 6 h with Pam3CSK4 (200 ng/ml) and then treated (or not) for 20 min with rIL-27 (50 ng/ml). WT macrophages treated with IFN-γ (10 ng/ml) or IL-10 (10 ng/ml) were included as positive controls for STAT-1 and STAT-3 phosphorylation, respectively. Whole-cell extracts were then analyzed by immunoblotting with the indicated Abs. (L) WT and Tccr−/− splenocytes were treated for the indicated times with rIL-27 (50 ng/ml) or rIFN-γ (10 ng/ml). Whole-cell extracts were then analyzed by immunoblotting with the indicated Abs. Graphs show means ± SEM of triplicate samples, except for (E), which shows duplicates. For ELISA and Luminex bead array results, uninfected control samples were below the detection limit (20 and 5 pg/ml, respectively) for the cytokines measured (data not shown). Significance was determined using an unpaired t test (A, C, E, and G), a one-way ANOVA with a Bonferroni post hoc test (H and I), or a two-way ANOVA, with significance relative to WT (F). Data are representative of at least two independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
We also investigated the effect of autocrine type I IFN on macrophage IL-10 production in response to M. tuberculosis by infecting WT and Ifnar1−/− macrophages with M. tuberculosis H37Rv (Fig. 1C). Ifnar1−/− macrophages produced significantly less IL-10 protein compared with WT macrophages following M. tuberculosis infection (Fig. 1C), and Il10 mRNA transcription was greatly reduced at 1 and 6 h postinfection in the Ifnar1−/− macrophages (Fig. 1D). We then examined myeloid cells taken directly ex vivo from lungs and BM of mice for their ability to produce IL-10 following M. tuberculosis infection and IFN-β treatment (Fig. 1E). As with BMDMs, IFN-β increased the levels of IL-10 produced by M. tuberculosis–infected ex vivo myeloid cells from lung (Fig. 1Ei) and BM (Fig. 1Eii). Thus, our data support a role for type I IFN signaling in inducing IL-10 production by M. tuberculosis–infected macrophages, likely through transcriptional regulation. Addition of IFN-β to M. tuberculosis–infected macrophages led to stable Il10 mRNA between 1 and 6 h (Fig. 1B), suggestive of some effect on mRNA stability. To investigate this further we performed experiments to assess Il10 mRNA stability in the presence or absence of type I IFN signaling (Fig. 1F). WT macrophages, with or without addition of rIFN-β, and Ifnar1−/− macrophages were infected with M. tuberculosis and 1 h after infection ActD was added to the cultures to inhibit further transcription. Il10 mRNA was then measured at 0, 30, 60, and 90 min after ActD addition and plotted as a percentage of the Il10 mRNA level in the 0 min ActD-treated samples (Fig. 1F). Loss of type I IFN signaling did not greatly affect the decay of Il10 mRNA levels over time, as WT and Ifnar1−/− macrophages had a similar percentage of Il10 mRNA over time after ActD treatment (Fig. 1F). However, addition of rIFN-β to infected WT macrophages increased Il10 mRNA stability, as the percentage of Il10 mRNA remaining in IFN-β–treated macrophages was significantly increased compared with M. tuberculosis–infected WT macrophages alone and Ifnar1−/− macrophages (Fig. 1F). Thus, type I IFN increases Il10 mRNA stability but is not absolutely required for Il10 mRNA stability. Taken together, these data suggest that type I IFN likely regulates IL-10 levels through a combination of transcriptional control and modulation of Il10 mRNA stability.
IL-10 production, as well as enhancement of IL-10 production by type I IFN, is independent of IL-27 signaling in M. tuberculosis–infected macrophages
IL-27 is known to induce IL-10 production by T cells (45–47) and has been suggested to act as an intermediate between LPS-induced type I IFN and IL-10 production by macrophages (32). However, in human monocytes (48) and some dendritic cells (49) it has been reported that IL-27 inhibits IL-10 production. Furthermore, it has been suggested that murine BMDMs are minimally responsive to IL-27 (48). We therefore investigated a role for IL-27 in IL-10 induction by type I IFN during M. tuberculosis infection of macrophages. Initially we determined whether type I IFN was required for IL-27 production by M. tuberculosis–infected macrophages. Addition of IFN-β to WT macrophages upon M. tuberculosis infection led to enhanced IL-27 production (Fig. 1Gi), in agreement with previous studies with LPS-stimulated macrophages (32). Furthermore, infection of WT and Ifnar1−/− macrophages revealed that type I IFN signaling was required for IL-27 production upon M. tuberculosis infection (Fig. 1Gii).
We then investigated whether IL-27 was required for IL-10 production in M. tuberculosis–infected macrophages, and also for promotion of IL-10 production by type I IFN. This was done by infecting WT and Tccr−/− macrophages (that lack the IL-27R α-chain and hence a functional IL-27 receptor) (50) with M. tuberculosis in the presence or absence of IFN-β (Fig. 1H). As expected, M. tuberculosis–infected WT macrophages produced IL-10 and the levels of IL-10 were increased by addition of IFN-β (Fig. 1H). Tccr−/− macrophages also produced IL-10 in response to M. tuberculosis infection, and levels were also increased upon addition of IFN-β (Fig. 1H). Levels of TNF-α and other proinflammatory cytokines were not reproducibly different between WT and Tccr−/− macrophages infected with M. tuberculosis (Fig. 1I and data not shown). Resting BMDMs expressed only low levels of the transcripts for both IL-27R subunits compared with naive T cells (Supplemental Fig. 1A). LPS- or CpG-treated BMDMs (Supplemental Fig. 1B), BM-derived dendritic cells, and BM monocytes (data not shown) did not produce different levels of IL-10 following treatment with rIL-27. These data suggest that IL-27R signaling is not required for IL-10 production by M. tuberculosis–infected macrophages or for IFN-β–mediated enhancement of IL-10 production by M. tuberculosis–infected macrophages.
It has previously been suggested that IL-27 induces IL-10 downstream of type I IFN in BMDMs in a STAT-1– and STAT-3–dependent manner (32). To investigate whether WT BMDMs activated STAT-1 and STAT-3 following IL-27 treatment and to confirm that Tccr−/− cells did not transduce any signal from IL-27, we treated BMDMs from WT and Tccr−/− mice with rIL-27 for 20 min and then probed by Western blot for phosphorylation of STAT-1 and STAT-3 (Fig. 1J). Neither STAT-1 nor STAT-3 was phosphorylated in response to IL-27 in both WT and Tccr−/− macrophages (Fig. 1J). BMDMs of both genotypes were capable of phosphorylating STAT-1 and STAT-3, because p–STAT-1 and p–STAT-3 were detected in both in response to IFN-γ or IL-10 addition, which led to phosphorylation of STAT-1 and STAT-3, respectively (Fig. 1J).
To control for the possibility that WT BMDMs might upregulate IL-27R following TLR stimulation and thereby activate STAT-1 and/or STAT-3 in response to IL-27 treatment in the presence of TLR activation, we stimulated WT and Tccr−/− macrophages with the TLR2 agonist Pam3CSK4 for 0, 3, or 6 h, treated them with IL-27 for 20 min, and then assayed for phosphorylation of STAT-1 and STAT-3 using Western blot (Fig. 1K). We specifically chose Pam3CSK4 as a stimulus, as it does not activate STAT-1 and only activates low levels of STAT-3 (data not shown) and hence would minimally confound any effects of IL-27 that other stimuli that activate these STATs would. STAT-3 was phosphorylated in both WT and Tccr−/− macrophages 3 and 6 h after stimulation with Pam3CSK4, but this was not enhanced further by treatment with IL-27 or reduced in Tccr−/− cells (Fig. 1K). STAT-1 phosphorylation was not induced under these conditions but could be induced by addition of IFN-γ (Fig. 1K).
However, rIL-27 was capable of activating signaling in other WT cells, because STAT-1 was phosphorylated in whole splenocytes treated with IL-27 for 10 or 30 min but not in Tccr−/− splenocytes, demonstrating both the activity of the recombinant cytokine and the specificity of the knockout (Fig. 1L). These data confirm that the IL-27R in the Tccr−/− mice used is nonfunctional.
Collectively, our data demonstrate that although IL-27 is induced by type I IFN, IL-27 is not required for type I IFN–mediated IL-10 upregulation by M. tuberculosis–infected macrophages.
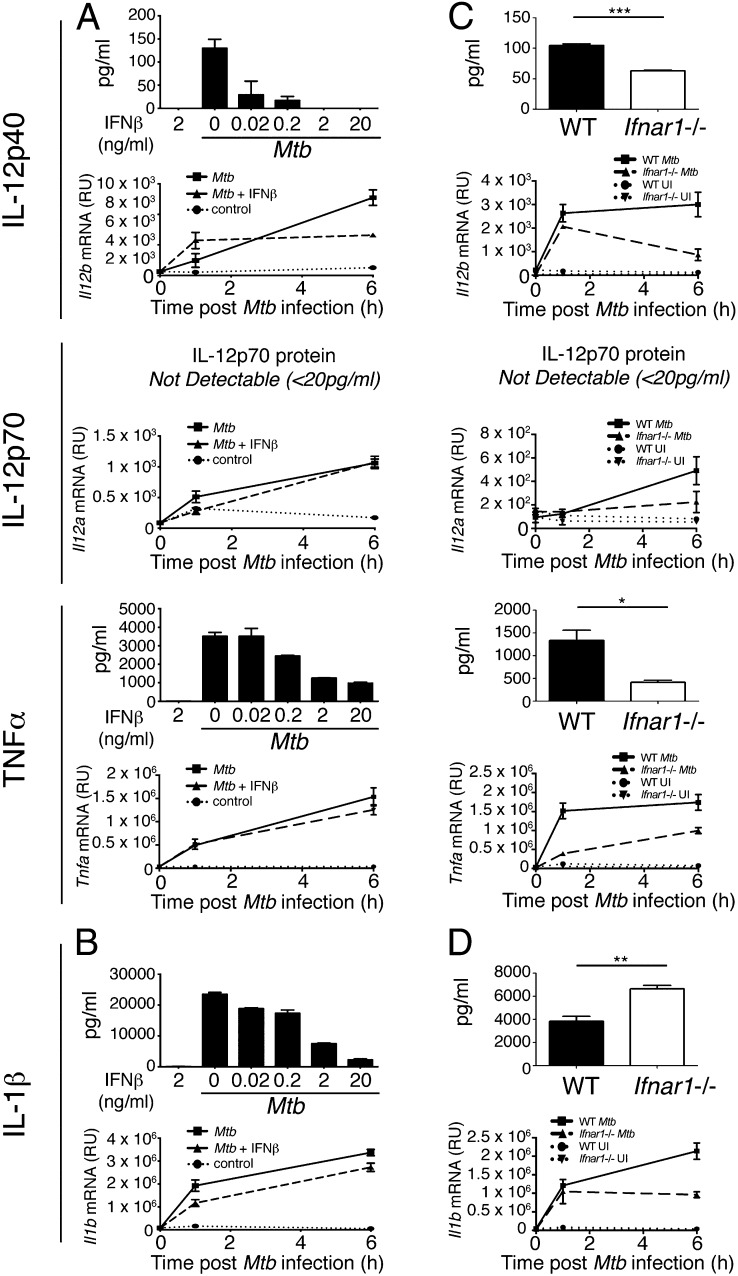
Exogenous IFN-β inhibits IL-12, TNF, and IL-1β production in M. tuberculosis–infected macrophages despite type I IFN signaling being required for optimal IL-12p40 and TNF-α production
We next wanted to examine how type I IFN might affect production by macrophages of proinflammatory cytokines important in host protection against M. tuberculosis. During in vivo M. tuberculosis infection a number of diverse cell types may produce type I IFN to influence macrophage function. We therefore initially sought to assess the effects of exogenous sources of type I IFN on proinflammatory cytokine production by M. tuberculosis–infected macrophages by infecting WT macrophages with M. tuberculosis and concomitantly adding rIFN-β at varying doses (Fig. 2A, 2B). Addition of IFN-β to M. tuberculosis–infected macrophages inhibited both IL-12p40 and TNF-α production (Fig. 2A). IL12p40 production appeared to be more sensitive than TNF-α to IFN-β treatment, being inhibited greatly at doses as low as 0.02 ng/ml, whereas TNF-α production was only inhibited at doses of 0.2 ng/ml or higher (Fig. 2A). IL-12p70 protein was not detected in any group (limit of detection [LOD], 20 pg/ml) (Fig. 2A). Levels of gene transcription for these cytokines were also assessed following addition of 2 ng/ml IFN-β to M. tuberculosis–infected macrophages. Il12b mRNA was not significantly affected at 1 h postinfection by addition of IFN-β but was greatly decreased in IFN-β–treated, M. tuberculosis–infected macrophages at 6 h compared with M. tuberculosis–infected alone macrophages (Fig. 2A). Similarly, Tnfa mRNA levels were lower at 6 h postinfection in IFN-β–treated macrophages but not at 1 h (Fig. 2A). Conversely, IFN-β treatment reduced Il12a mRNA in infected macrophages compared with M. tuberculosis–infected alone macrophages at 1 h but not at 6 h postinfection (Fig. 2A). Levels of IL-1β production were also assessed in M. tuberculosis–infected macrophages treated with IFN-β (Fig. 2B). Similar to the findings for IL-12p40 and TNF-α, IL-1β production was inhibited by IFN-β addition, with IL-1β protein levels being reduced at doses of 0.02 ng/ml or higher (Fig. 2B). Il1b mRNA levels were also reduced following IFN-β treatment of M. tuberculosis–infected macrophages, being reduced at 1 and 6 h postinfection compared with M. tuberculosis–infected alone macrophages (Fig. 2B).
FIGURE 2.
Opposing effects of exogenous IFN-β treatment and autocrine type I IFN signaling on IL-12 and TNF-α production in M. tuberculosis–infected macrophages. (A) WT macrophages were infected with M. tuberculosis in the presence of increasing concentrations of IFN-β (for protein) or 2 ng/ml IFN-β (for mRNA), added at the time of infection, and levels of IL-12p40, IL-12p70, and TNF-α protein in supernatant and Il12b, Il12a, and Tnfa mRNA from cells were determined by ELISA at 24 h postinfection and qRT-PCR at indicated times after infection. (B) IL-1β protein and Il1b mRNA from macrophages in (A) were measured by ELISA at 24 h postinfection and qRT-PCR at indicated times after infection. (C) WT and Ifnar1−/− macrophages were infected with M. tuberculosis and IL-12p40, IL-12p70, and TNF-α protein in supernatant and Il12b, Il12a, and Tnfa mRNA from cells were determined by ELISA at 24 h postinfection and qRT-PCR at indicated times after infection. (D) IL-1β protein and Il1b mRNA from macrophages in (C) were measured by ELISA at 24 h postinfection and qRT-PCR at indicated times after infection. Graphs show means ± SEM of triplicate samples. For ELISA results, uninfected control samples were below the detection limit (20 pg/ml) for the cytokines measured (data not shown). Significance was determined using an unpaired t test. Data are representative of at least three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
We next examined what effects autocrine type I IFN signaling had on proinflammatory cytokine production upon infection of WT and Ifnar1−/− macrophages with M. tuberculosis. Unexpectedly, given the previous results with addition of IFN-β, the amount of IL-12p40 secreted was reduced in M. tuberculosis–infected Ifnar1−/− macrophages compared with WT cells, as were the levels of Il12b mRNA at 1 and 6 h postinfection (Fig. 2C). IL-12p70 was not detectable from either WT or Ifnar1−/− macrophages infected with M. tuberculosis (LOD, 20 pg/ml). However, Il12a mRNA levels were reduced in Ifnar1−/− macrophages compared with WT at 6 h postinfection (Fig. 2C). Additionally, TNF-α protein production and Tnfa mRNA levels were also reduced in Ifnar1−/− compared with WT macrophages following M. tuberculosis infection (Fig. 2C). In contrast to these cytokines, the secretion of IL-1β was increased in Ifnar1−/− macrophages in response to M. tuberculosis infection as compared with WT cells, despite Il1b mRNA levels also being reduced at 6 h (Fig. 2D). Collectively, these results suggest that although high levels of IFN-β inhibit the ability of macrophages to make proinflammatory cytokines, paradoxically, autocrine type I IFN signaling is required in macrophages for optimal production of IL-12p40 and TNF-α following M. tuberculosis infection but still inhibits macrophage production of IL-1β.
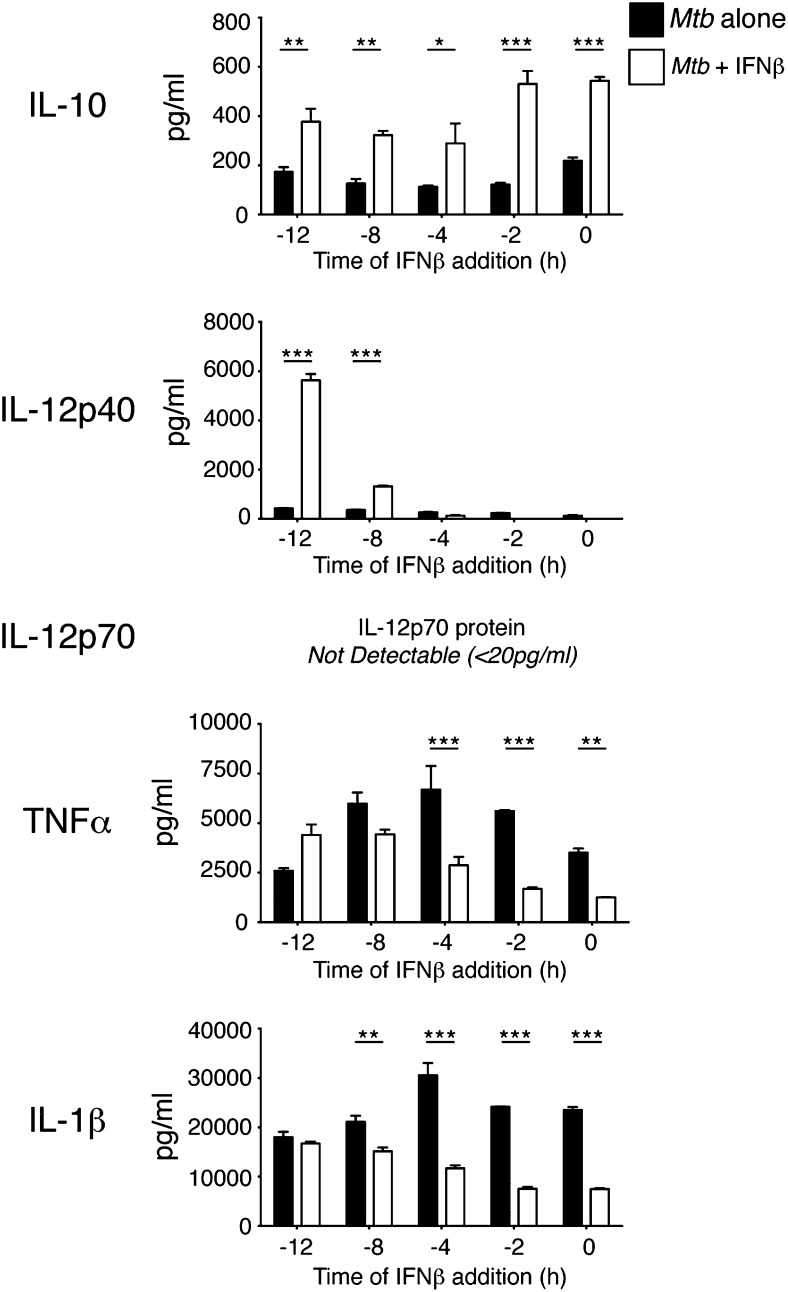
Timing of IFN signaling determines effects on macrophage cytokine production upon M. tuberculosis infection
To investigate whether timing of type I IFN signaling relative to M. tuberculosis infection might explain the differences in cytokine production observed between addition of recombinant type I IFN and autocrine type I IFN signaling, we pretreated macrophages for varying times prior to M. tuberculosis infection. Pretreatment of macrophages with IFN-β for 8 or 12 h prior to M. tuberculosis infection enhanced IL-12p40, and to a lesser extent TNF-α production, indicating that timing of IFN signaling relative to signaling through pattern recognition receptors is important in determining the effects of type I IFN in regulating the secretion of these proinflammatory cytokines (Fig. 3). IL-12p70 protein was not detected in any group (LOD, 20 pg/ml). IL-10 levels were enhanced by pre-addition of IFN-β at all time points tested but had the greatest effect when added at or shortly before M. tuberculosis infection (Fig. 3). IL-1β production by macrophages upon M. tuberculosis infection was inhibited by pre-addition of type I IFN, at most pretreatment time points investigated, although this did not significantly affect IL-1β levels at 12 h of pretreatment (Fig. 3). Again, the greatest effect was seen when IFN-β was added close to the time of infection.
FIGURE 3.
IFN-β pretreatment of M. tuberculosis–infected macrophages enhances IL-12 and TNF-α production. WT macrophages were infected with M. tuberculosis alone or in the presence of IFN-β (2 ng/ml), added at the indicated times prior to infection. Cytokine levels in culture supernatants were determined by ELISA at 24 h postinfection. Uninfected control samples were below the detection limit (20 pg/ml) for the cytokines measured (data not shown). Graphs show means ± SEM of triplicate samples. Data are representative of two independent experiments. Significance was determined using a two-way ANOVA with a Bonferroni post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001.
Exogenous IFN-β inhibits macrophage responsiveness to concomitant IFN-γ addition
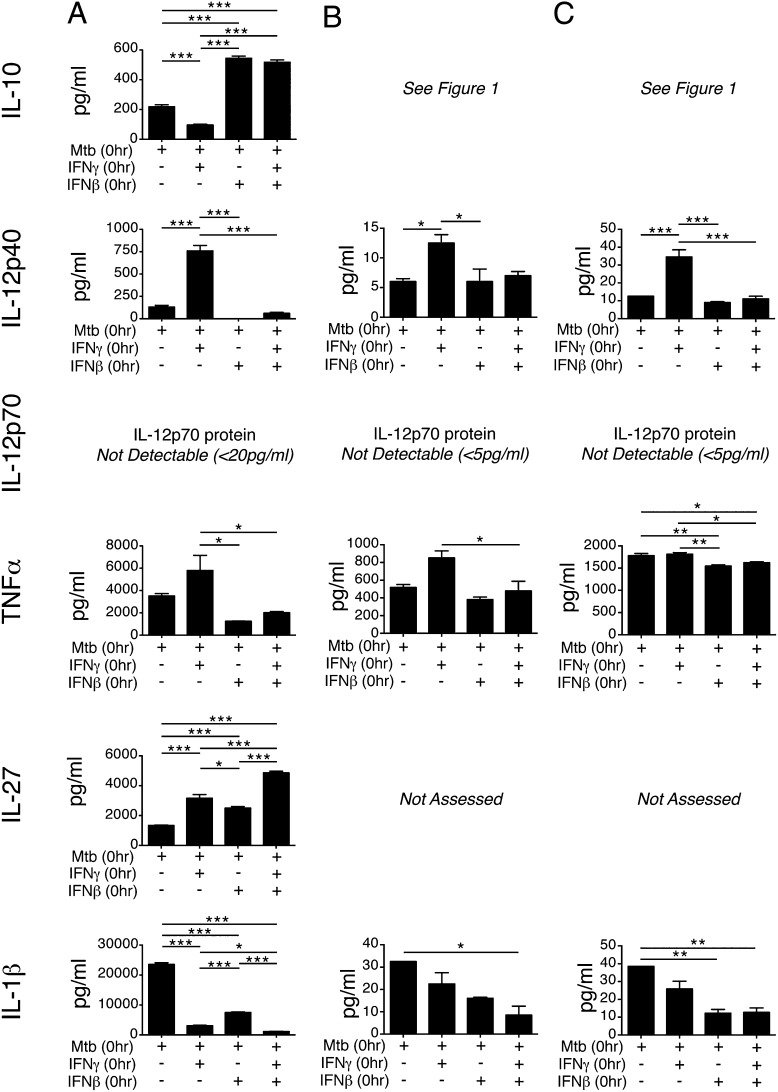
IFN-γ is crucial to the host response against M. tuberculosis infection (10, 14) and is known to induce or enhance production of important host-protective cytokines such as IL-12 and TNF-α by macrophages and other myeloid cells while inhibiting IL-10 production (5, 9, 13, 14, 51–53). Because exogenous type I IFN and exogenous IFN-γ appear to have opposing effects on macrophages, we investigated which IFN type would have a dominant effect in influencing macrophage cytokine production following M. tuberculosis infection. To test this we infected WT macrophages with M. tuberculosis, also adding IFN-γ and IFN-β at the time of infection (Fig. 4). M. tuberculosis–infected macrophages treated singly with IFN-γ or IFN-β or left untreated were included as controls.
FIGURE 4.
Exogenous IFN-β inhibits M. tuberculosis–infected macrophage responsiveness to concomitant IFN-γ addition. (A) WT macrophages were infected with M. tuberculosis alone or with M. tuberculosis and IFN-β (2 ng/ml), M. tuberculosis and IFN-γ (5 ng/ml), or M. tuberculosis and both IFN-β (2 ng/ml) and IFN-γ (5 ng/ml) together, added at the time of infection. Cytokine levels in culture supernatants were determined at 24 h postinfection. Uninfected control samples were below the detection limit (20 pg/ml) for the cytokines measured (data not shown). Graphs show means ± SEM. Significance was determined using a one-way ANOVA with a Bonferroni post hoc test. Data are representative of three independent experiments. (B) Myeloid cells (Lin−Ly6c+Ly6G−) were sorted from the lungs of WT mice and infected with M. tuberculosis and treated with IFN as in (A). Cytokine levels in culture supernatant were determined at 24 h postinfection using a Luminex bead array. Uninfected control samples were below the detection limit (5 pg/ml) for the cytokines measured (data not shown). Graphs show means ± SEM. Data are representative of two independent experiments. (C) Myeloid cells (Lin−Ly6c+Ly6G−) were sorted from the BM of WT mice and infected with M. tuberculosis and treated with IFN as in (A) and (B). Cytokine levels in culture supernatant were determined at 24 h postinfection using a Luminex bead array. Uninfected control samples were below the detection limit for the cytokines measured (data not shown). Graphs show means ± SEM. Data are representative of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
As expected, IL-10 production was inhibited in M. tuberculosis–infected macrophages treated with IFN-γ alone and enhanced in M. tuberculosis–infected macrophages treated with IFN-β alone (Fig. 4A). When both IFN-γ and IFN-β were added to macrophages infected with M. tuberculosis, IL-10 production was enhanced to a level at least equal to that seen with IFN-β alone, suggesting that IFN-β was overriding IFN-γ–mediated inhibition of IL-10 (Fig. 4A). Il10 mRNA levels at 6 h postinfection were similarly affected by IFN treatment (Supplemental Fig. 2). A similar trend was seen with IL-12p40 production by M. tuberculosis–infected macrophages; that is, whereas IFN-γ alone greatly promoted IL-12p40 production, IFN-β alone and IFN-β together with IFN-γ resulted in markedly reduced levels of IL-12p40 (Fig. 4A). Similar results were found at the transcriptional level with Il12a and Il12b mRNA (Supplemental Fig. 2). TNF-α production by macrophages infected with M. tuberculosis was similarly affected, with IFN-γ alone promoting TNF-α, whereas IFN-β alone and IFN-β/IFN-γ together resulted in inhibition of TNF-α production (Fig. 4A). Tnfa mRNA, however, did not follow this trend, being downregulated by both IFN-γ and IFN-β (Supplemental Fig. 2). Effects on IL-27 and IL-1β were the exception to the trend of IFN-β overriding IFN-γ. IL-27 production was upregulated by both IFN-γ and IFN-β and further upregulated when both were added concomitantly (Fig. 4A). IFN-γ alone and IFN-β alone treatment both inhibited IL-1β production from M. tuberculosis–infected macrophages, although IFN-γ was slightly more potent than IFN-β (Fig. 4A). When both IFN-β and IFN-γ were added to M. tuberculosis–infected macrophages, IL-1β levels were reduced to a level below the single cytokine treatment groups (Fig. 4A). mRNA levels were similarly affected (Supplemental Fig. 2). In addition to adding both IFN to macrophages at the time of infection, we also repeated the experiment with 2 h of IFN-β pretreatment, prior to infection and IFN-γ addition, or 2 h of IFN-γ pretreatment, followed by infection and IFN-β addition, and we found the same results in both cases as adding both IFN at the time of infection that type I IFN effects are dominant (data not shown). Type I IFN has been shown to inhibit IFN-γ responsiveness during L. monocytogenes infection through inhibiting transcription of the gene encoding IFN-γ receptor subunit 1 (43, 54). We therefore examined mRNA levels of Ifngr1 during M. tuberculosis infection of macrophages. Downregulation of Ifngr1 mRNA levels upon M. tuberculosis infection could be observed, but this was only partially dependent on type I IFN signaling (data not shown), illustrating the complexity of this system.
Finally, to confirm that similar responses to IFN-β and IFN-γ were seen in ex vivo–derived cells, primary myeloid cells were sorted from the lungs and BM of WT mice, M. tuberculosis infected, and IFN treated as for experiments with BMDMs (Fig. 4B, 4C). Although overall levels of IL-12p40 were low in supernatant from M. tuberculosis–infected lung and BM myeloid cells, these cells responded similarly to BMDMs upon IFN treatment, with IFN-γ enhancing IL12p40 levels and IFN-β treatment inhibiting IFN-γ–mediated IL-12p40 production (Fig. 4B, 4C). IL-12p70 was not detected under any condition (Fig. 4). For TNF-α production, lung myeloid cells also showed a similar pattern of response to M. tuberculosis infection and IFN treatment as for IL-12p40, with the upregulation induced by IFN-γ being repressed by IFN-β treatment (Fig. 4B). In BM myeloid cells levels of TNF-α were not affected by IFN-γ compared with M. tuberculosis infection alone, whereas IFN-β treatment resulted in a modest, but significant reduction compared with M. tuberculosis infection alone and M. tuberculosis infection plus IFN-γ (Fig. 4C). Similar to findings in BMDMs, IL-1β production by M. tuberculosis–infected lung and BM myeloid cells was inhibited by both IFN-γ and IFN-β (Fig. 4B, 4C).
Collectively, these results suggest that exogenous type I IFN powerfully overcomes the macrophage response to IFN-γ during M. tuberculosis infection. However, this effect is not universal, as seen with IL-1β production, where both cytokines are inhibitory.
Exogenous IFN-β inhibits IL-12, TNF-α, and IL-1β production in M. tuberculosis–infected macrophages through IL-10–dependent and –independent mechanisms
IL-10 is a prominent inhibitor of myeloid cell functions (55). Given our previous results, we hypothesized that type I IFN was mediating its suppressive effects on proinflammatory cytokine production by M. tuberculosis–infected macrophages through induction of IL-10. To test this we infected IL-10–deficient macrophages with M. tuberculosis with and without addition of IFN-β (Fig. 5A). Il10−/− macrophages produced greatly increased levels of IL-12p40 compared with WT cells upon M. tuberculosis infection (Fig. 5A). Addition of IFN-β to Il10−/− macrophages reduced IL-12p40 levels, but not to the same extent as IFN-β added to WT macrophages infected with M. tuberculosis (∼25% reduction in Il10−/− cells versus ∼70% in WT cells) (Fig. 5A). These results suggested that the inhibitory effect of IFN-β on IL-12p40 production is largely dependent on IL-10. IL-12p70 was not detectable in any group. TNF-α production was similarly increased in Il10−/− M. tuberculosis–infected macrophages compared with WT cells (Fig. 5A). IFN-β treatment did not result in reduced TNF-α in Il10−/− macrophages, indicating that the inhibitory effects of IFN-β on TNF-α production are totally dependent on IL-10 (Fig. 5A). Although IL-10 reduced the ability of macrophages to produce IL-27 following M. tuberculosis infection, it did not greatly affect the ability of IFN-β treatment to enhance IL-27 production (Fig. 5A). IL-1β production was only increased by 30% in M. tuberculosis–infected Il10−/− macrophages compared with WT controls. IFN-β treatment was still able to significantly inhibit IL-1β production by IL-10–deficient macrophages infected with M. tuberculosis (Fig. 5A). This suggested that type I IFN had a dominant inhibitory effect on IL-1β that was minimally dependent on IL-10 during M. tuberculosis infection of macrophages.
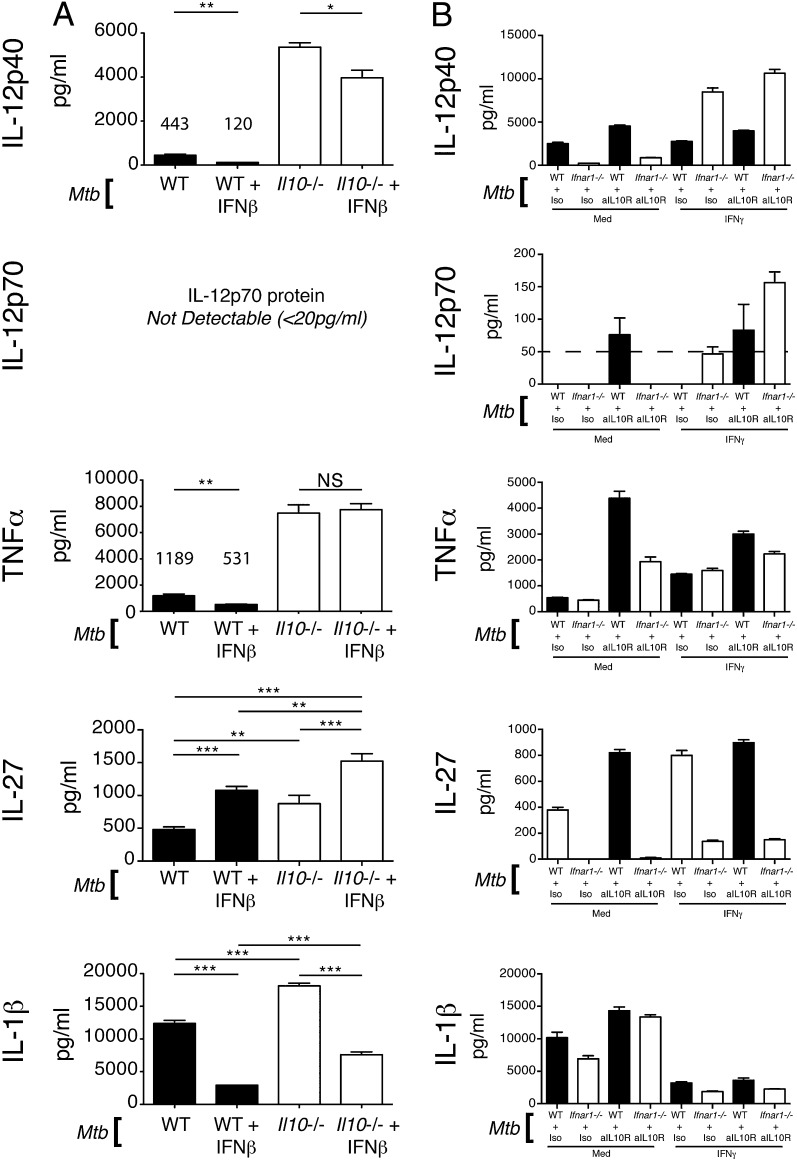
FIGURE 5.
Exogenous IFN-β and endogenous type I IFN signaling affect proinflammatory cytokine production and responsiveness to IFN-γ in M. tuberculosis–infected macrophages through IL-10–dependent and –independent mechanisms. (A) WT and Il10−/− macrophages were infected with M. tuberculosis or with M. tuberculosis and IFN-β (2 ng/ml), added at the time of infection. Levels of the indicated cytokines in culture supernatant were determined at 24 h postinfection by ELISA. Graphs show means ± SEM of triplicate samples. Significance was determined using a one-way ANOVA with a Bonferroni post hoc test. (B) WT and Ifnar1−/− macrophages were infected with M. tuberculosis, treated with anti–IL-10R or isotype control Abs and with IFN-γ (5 ng/ml) or not, added at the time of infection. Levels of the indicated cytokines in culture supernatant were determined at 24 h postinfection by ELISA. Uninfected control samples were below the detection limit (20 pg/ml) for the cytokines measured (data not shown). Graphs show means ± SEM of triplicate samples. Data are representative of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
Type I IFN signaling impairs IFN-γ effects on M. tuberculosis–infected macrophage cytokine production via IL-10–dependent and –independent mechanisms
As described above, IFN-γ is crucial to the host response against M. tuberculosis infection (10, 14) and is known to induce or enhance production of important host-protective cytokines such as IL-12 (5, 9, 13, 14, 51–53). We noted that even in the absence of type I IFN or IL-10 signaling macrophages could not be induced to consistently make significant amounts of IL-12p70, the biologically active form of IL-12, upon M. tuberculosis infection. This indicated that removal of inhibitory signals alone might not be sufficient for induction of robust levels of this cytokine and that IFN-γ may be required.
To confirm that IFN-γ was required for IL-12p70 production in M. tuberculosis–infected macrophages and to test whether type I IFN together with IL-10 might exert negative regulatory effects on IFN-γ action, we infected WT or Ifnar1−/− macrophages with M. tuberculosis in the presence or absence of rIFN-γ and anti–IL-10R or anti–IL-10 Abs (Fig. 5B and data not shown). IL-12p40 production could be enhanced in M. tuberculosis–infected WT macrophages either treated with IFN-γ or anti–IL-10R and was further enhanced in M. tuberculosis–infected Ifnar1−/− macrophages treated with IFN-γ or IFN-γ and anti–IL-10R (Fig. 5B). Addition of IFN-γ, a known inducer of IL-12p70 in concert with TLR signals (51, 53, 56), induced little to no IL-12p70 in M. tuberculosis–infected WT macrophages (Fig. 5B). However, in the absence of type I IFN signaling or upon Ab blockade of the IL-10R, IFN-γ was able to induce robustly detectable levels of IL-12p70 production (Fig. 5B). TNF-α production by M. tuberculosis–infected macrophages was also enhanced upon IFN-γ treatment, and this was not further increased by abrogation of the type I IFN receptor but was increased upon anti–IL-10R blockade (Fig. 5B). As expected, IL-27 levels were greatly impaired in Ifnar1−/− BMDMs compared with WT, and although anti–IL-10R blockade and IFN-γ treatment increased IL-27 in both WT and Ifnar1−/−, levels in the type I IFN receptor–deficient BMDMs could not be rescued to those of the WT controls (Fig. 5B).
IL-1β production by both WT and Ifnar1−/− M. tuberculosis–infected macrophages was greatly inhibited by IFN-γ addition (Fig. 5B). The relative level of inhibition was similar between WT and Ifnar1−/− macrophages, although there was a trend to greater inhibition in the Ifnar1−/− macrophages (Fig. 5B).
Altogether, these results are in agreement with previous studies reporting that IFN-γ is an important inducer and/or enhancer of IL-12 and TNF-α production. However, we now show that during M. tuberculosis infection of macrophages the ability of IFN-γ to positively regulate production of these cytokines is significantly impaired by both autocrine type I IFN and IL-10. Production of bioactive IL-12p70, in particular, is strongly suppressed by these two negative regulatory factors.
Type I IFN regulation of IL-1β production is dependent on inducible NO synthase and IL-10
In contrast to type I IFN effects on TNF-α, we found that inhibition of IL-1β (and IL-12) production by IFN-β was only partially dependent on IL-10. Additionally, we observed co-operative inhibition of IL-1β by IFN-γ and IFN-β, in contrast to their usually cross-regulatory effects on each other’s action on cytokine production. IFN-γ has recently been described to negatively regulate IL-1β production during M. tuberculosis infection in an inducible NO synthase (iNOS, gene name Nos2)–dependent manner (57). Because type I IFN can also induce iNOS, we hypothesized that IFN-β was also inhibiting IL-1β production through upregulation of iNOS. To test this we infected WT and Nos2−/− BMDMs with M. tuberculosis and concomitantly added IFN-γ or IFN-β (Fig. 6). We also included groups treated with anti–IL-10R blocking Abs to compare IL-10 effects versus iNOS effects (Fig. 6).
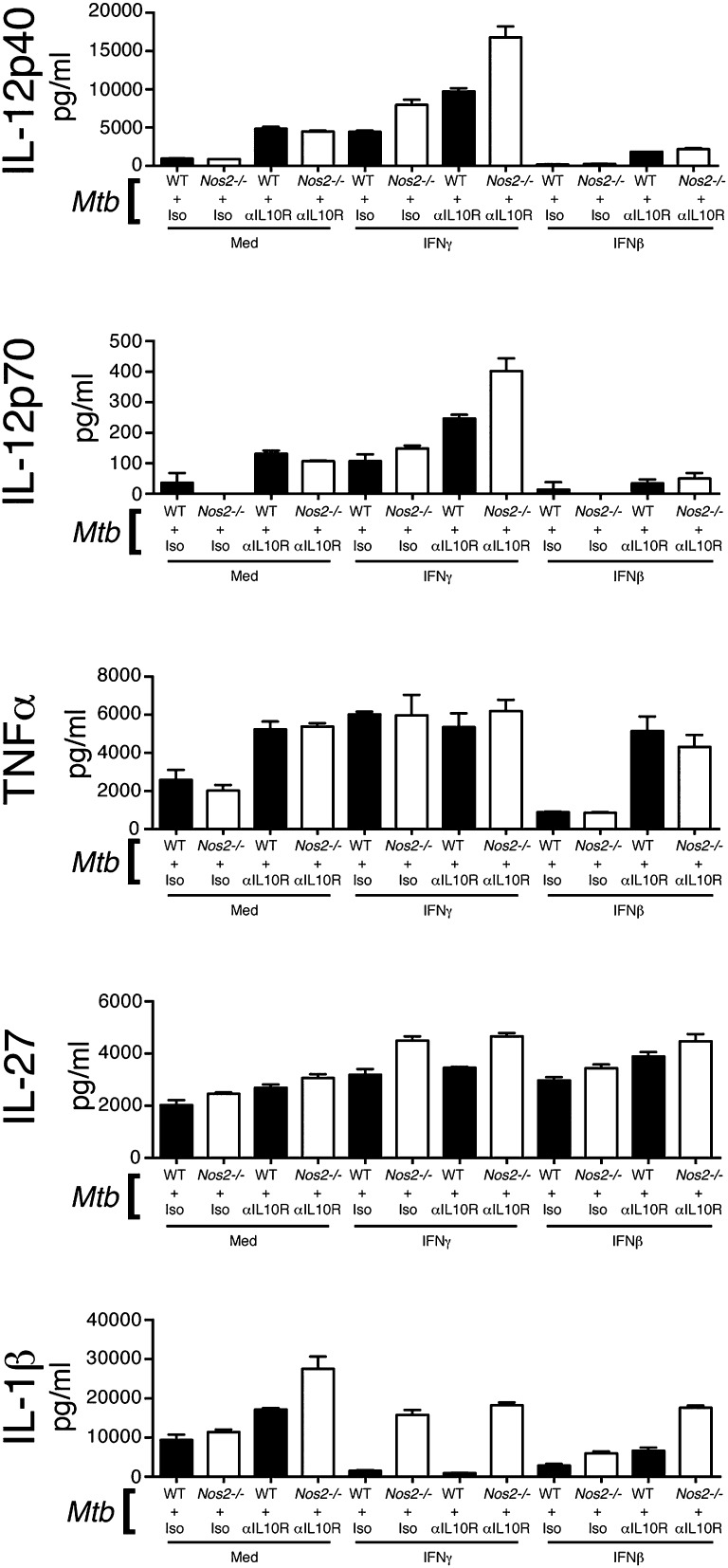
FIGURE 6.
Type I IFN regulation of IL-1β production is dependent on iNOS and IL-10. WT and Nos2−/− macrophages were infected with M. tuberculosis, treated with anti–IL-10R or isotype control Abs and with either IFN-γ (5 ng/ml) or IFN-β (2 ng/ml) or media, added at the time of infection. Levels of the indicated cytokines in culture supernatant were determined at 24 h postinfection by ELISA. Uninfected control samples were below the detection limit (20 pg/ml) for the cytokines measured (data not shown). Graphs show means ± SEM of triplicate samples. Data are representative of at least three independent experiments.
In macrophages infected with M. tuberculosis alone, abrogation of Nos2 had only minor effects on IL-1β production (Fig. 6). However, in M. tuberculosis–infected Nos2−/− BMDMs treated with anti–IL-10R Abs levels of IL-1β were elevated over levels in infected WT, anti–IL-10R-treated BMDMs, suggestive of IL-10 regulation of iNOS and/or its function (Fig. 6). When M. tuberculosis–infected WT macrophages were treated with IFN-γ, IL-1β production was almost entirely abrogated and this abrogation was completely relieved in infected Nos2−/− BMDMs treated with IFN-γ (Fig. 6). This suggests that iNOS mediates suppression of IL-1β production by IFN-γ, in agreement with the findings of Mishra et al. (57). IL-10R blockade had little effect on macrophages treated with IFN-γ, likely due to IFN-γ suppressing IL-10 production (Fig. 6; see Fig. 4). As expected, IL-1β production by M. tuberculosis–infected macrophages was also suppressed by treatment with IFN-β (Fig. 6). This was partially relieved by the abrogation of iNOS, but not to the same extent as seen in IFN-γ–treated groups (Fig. 6). Ab blockade of IL-10R in IFN-β–treated, M. tuberculosis–infected macrophages lacking Nos2 did restore IL-1β levels to those of WT infected, untreated macrophages, suggesting that IFN-β inhibits IL-1β production through the dual mechanisms of IL-10 and iNOS (Fig. 6).
Interestingly, we also noted that although iNOS did not greatly affect production of the other cytokines measured in M. tuberculosis–infected alone macrophages or M. tuberculosis–infected and IFN-β–treated macrophages, in macrophages infected with M. tuberculosis and treated with IFN-γ, levels of IL-12 and IL-27 were elevated in the absence of Nos2 (Fig. 6). This suggests that iNOS also acts as a feedback negative regulator of IFN-γ function.
Type I IFN signaling inhibits macrophage restriction of M. tuberculosis growth and killing in response to IFN-γ
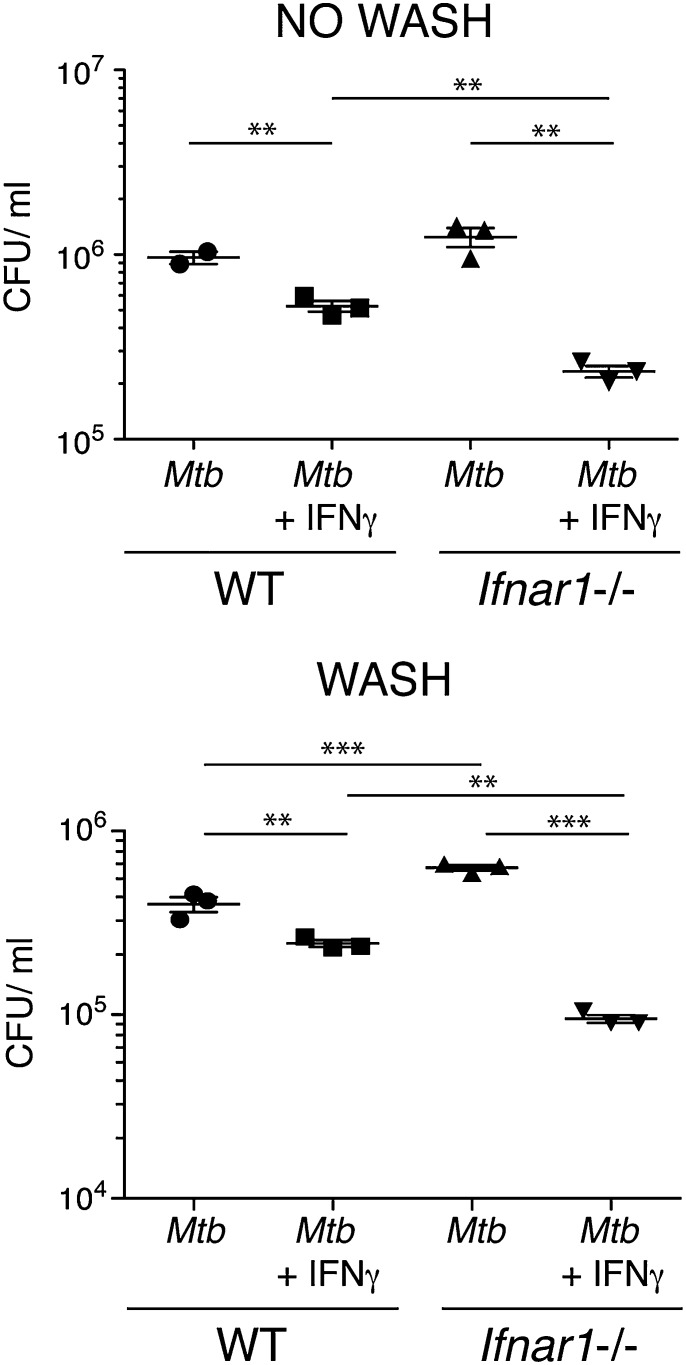
In addition to induction of crucial host-protective cytokines, IFN-γ is known to be important in activating macrophages to restrict intracellular bacterial growth, including that of M. tuberculosis (5, 6, 11, 12, 58, 59). Indeed, IFN-γ has been shown to be the crucial cytokine for induction of the reactive nitrogen and oxygen species that are necessary for antimicrobial activity in murine macrophages (60). In view of our previous results showing that IFN-β interferes with the IFN-γ–mediated regulation of several cytokines, we next investigated whether type I IFN signaling could also affect IFN-γ–mediated restriction of M. tuberculosis in infected macrophages. To study this, WT and Ifnar1−/− macrophages were infected with M. tuberculosis in the presence or absence of IFN-γ, and at 96 h postinfection bacterial loads were assessed (Fig. 7). We employed two methods of infection for these experiments: either leaving bacteria in the well for the full 96 h postinfection (Fig. 7, top) or washing out after 4 h of infection (Fig. 7, bottom), with matching results being observed between the two. Additionally, we controlled for differences in bacterial uptake between WT and Ifnar1−/− macrophages by enumerating CFUs at 4 h postinfection, and we found no difference between the two cell types (data not shown). Bacterial loads were not significantly different between WT and Ifnar1−/− macrophages infected with M. tuberculosis alone with no wash but were slightly increased in Ifnar1−/− cells versus WT when the M. tuberculosis inoculum was washed out (Fig. 7). IFN-γ treatment induced a reduction in bacterial load in WT macrophages of ∼2-fold compared with M. tuberculosis infection alone, but treatment of Ifnar1−/− macrophages with IFN-γ led to a reduction in bacterial load of >5-fold compared with Ifnar1−/− macrophages infected with M. tuberculosis alone (Fig. 7). Addition of IFN-β to M. tuberculosis–infected WT macrophages did not alter bacterial loads compared with M. tuberculosis infection alone (data not shown). Treatment of M. tuberculosis–infected macrophages with IFN-γ and IFN-β together did not alter bacterial loads compared with M. tuberculosis–infected macrophages treated with IFN-γ alone (data not shown). These results suggest that endogenous levels of type I IFN induced by M. tuberculosis infection can inhibit the ability of IFN-γ to restrict bacterial levels in M. tuberculosis–infected macrophages, in addition to inhibiting IFN-γ effects on macrophage cytokine production during M. tuberculosis infection.
FIGURE 7.
Type I IFN signaling inhibits macrophage restriction of M. tuberculosis growth and killing in response to IFN-γ. WT and Ifnar1−/− macrophages were infected with M. tuberculosis in the presence or absence of IFN-γ (5 ng/ml), added at the time of infection. At 4 h postinfection media containing M. tuberculosis was removed, cells were washed in PBS, and fresh media (with no M. tuberculosis) were replaced (bottom panel) or not (top panel). At 96 h postinfection cells were washed in PBS, lysed in 0.2% saponin, and bacterial loads were enumerated via serial dilution and plating. Line and bars show means ± SEM. Significance was determined using an unpaired t test. Data are representative of at least two independent experiments for each of the two systems (i.e., wash or no wash). **p < 0.01, ***p < 0.001.
Discussion
There is growing evidence in both mice and humans that type I IFNs are detrimental to host resistance during TB (15, 16, 18–25, 29). However, the mechanisms by which type I IFN negatively impacts the host response to M. tuberculosis are not well understood. Several recent studies have highlighted the importance of type I IFN in the myeloid cell response to M. tuberculosis (16, 20, 21, 25). In this study, we show that type I IFN signaling is required for macrophage induction of the immunosuppressive cytokine IL-10 during M. tuberculosis infection. Type I IFN is also responsible for suppressing production of several proinflammatory cytokines by macrophages, particularly IL-12, TNF-α, and IL-1β, which are considered crucial to host protection against M. tuberculosis (7, 8, 20, 61–64). IL-10 is an important mediator of the suppressive effect on IL-12 and TNF-α production. IFN-γ is a key cytokine for activation of myeloid cells to both produce increased levels of protective cytokines, particularly IL-12, and to restrict intracellular bacterial growth (5, 6, 9, 11–13, 58, 65). Importantly, we now show that type I IFN dramatically suppresses the macrophage response to IFN-γ for induction of IL-12p40 and IL-12p70, as well as for M. tuberculosis growth restriction and killing.
We first investigated the effect of type I IFN on IL-10 production by macrophages infected with M. tuberculosis and found both a requirement for type I IFN signaling in induction of IL-10 and an increase in IL-10 production following addition of exogenous IFN-β to M. tuberculosis–infected macrophages, in agreement with several other studies in different systems, such as LPS stimulation (30–32). Analysis of Il10 mRNA levels suggested that type I IFN mediated its effects on IL-10 production through regulating initial transcription of the Il10 gene but also through stabilization of Il10 transcripts. IL-27 has previously been reported as a mediator of IL-10 induction downstream of type I IFN following LPS stimulation of BMDMs (32). This induction of IL-10 by IL-27 was reported to be dependent on STAT-1 and STAT-3 activation (32). We found that type I IFN was required for induction of IL-27 in M. tuberculosis–infected macrophages and that addition of IFN-β enhanced IL-27 production, in agreement with this study and others (32, 66). However, we found no requirement for IL-27 signaling in IL-10 induction or enhancement of IL-10 production by IFN-β. IL-10 production by BM-derived dendritic cells and BM monocytes was also unaffected by IL-27. Furthermore, our BMDMs did not activate STAT-1 and STAT-3 following IL-27 treatment, in keeping with another study (48) that also did not detect STAT-1 and STAT-3 phosphorylation in BMDMs following IL-27 treatment. In this study the authors could not detect induction of STAT-1–dependent genes in BMDMs following IL-27 treatment and reported low levels of IL-27R expression, suggesting that these cells were “minimally responsive” to IL-27 (48). We also found low relative levels of IL-27R expression in our BMDMs, in accordance with this study (48) and others who found low relative levels of IL-27R expression on F4/80+ splenic macrophages (67) and plastic adherent splenic macrophages (50), whereas we show that T cells do express the IL-27R, as others report (50, 67). Although this lack of STAT-1 and STAT-3 activation is in contrast to Iyer et al. (32), the reasons for the difference between our findings and those of Iyer et al. are presently unclear, but may be due to laboratory differences in generation and culture conditions of BMDMs. Note that our findings do not exclude IL-27 responsiveness by other types of macrophage or myeloid cell that may also respond in a manner not assessed in this study (68, 69).
We also investigated the effects of type I IFN on proinflammatory cytokine production. Addition of IFN-β to M. tuberculosis–infected macrophages potently suppressed IL-12p40 production, in agreement with a number of studies in viral and some bacterial models showing suppression of IL-12 by type I IFN (24, 70–73). TNF-α and IL-1β production were also suppressed by type I IFN addition. Interestingly, and in contrast to these findings, autocrine type I IFN was also partially required for optimal IL-12p40 and TNF-α production by macrophages following M. tuberculosis infection, in agreement with a previous study where type I IFN signaling was required for IL-12 production following TLR stimulation (74). The discrepancy between our results with addition of IFN-β versus deficiency in type I IFN signaling appears to be due to the timing of type I IFN signaling relative to signaling through pattern recognition receptors, because 12 h of pretreatment with IFN-β resulted in increased levels of IL-12 and TNF-α upon M. tuberculosis infection, whereas IFN-β treatment proximal to M. tuberculosis infection consistently resulted in suppression of IL-12p40 and TNF-α production. This may indicate that distinct mechanistic programs of action of type I IFN exist, dependent on proximity to other stimuli (such as infection).
In contrast to IL-12p40 and TNF-α, type I IFN signaling inhibited macrophage production of IL-1β protein following M. tuberculosis infection under all conditions tested. This is in clear agreement with recent studies in both mouse models (20) and human cells (21) and illustrates an important mechanism of type I IFN negative regulation of the host response, because IL-1 is crucial to protection during TB (20, 62–64).
Given that type I IFN induced IL-10 in M. tuberculosis–infected macrophages, we assessed whether IL-10 was mediating the inhibitory effects of type I IFN on IL-12p40, TNF-α, and IL-1β. IFN-β treatment of IL-10–deficient macrophages infected with M. tuberculosis revealed that IL-10 did indeed mediate much of the suppressive action of type I IFN on IL-12p40 production and was totally responsible for inhibition of TNF-α production. Low levels of TNF-α and IL-12 in the lungs of M. tuberculosis–infected mice have previously been associated with high type I IFN levels, although a role for IL-10 was not formally investigated (18). However, IL-10 appears to limit IL-12 production during mycobacterial infection (38) and also limits the Th1 response and associated IFN-γ production during M. tuberculosis infection in mice (39). These results suggest that IL-10 may be an important intermediary in type I IFN–mediated disruption of the Th1 response during M. tuberculosis infection through suppression of macrophage production of cytokines such as IL-12. IL-10 also partially mediated suppression of IL-1β production by M. tuberculosis–infected macrophages, in agreement with a recent study (20), but suggests that most of inhibition of IL-1β production by type I IFN in M. tuberculosis–infected macrophages is through IL-10–independent mechanisms. Analysis of Nos2-deficient macrophages suggested that iNOS is a significant mechanism additional to IL-10 through which type I IFN mediates its suppressive effects on IL-1β synthesis. Interestingly, in the context of IFN-γ treatment, iNOS was also found to negatively regulate production of other cytokines, including IL-12 and IL-27.
We noted that even in the absence of the negative regulation of type I IFN or IL-10, M. tuberculosis–infected macrophages rarely produced robustly detectable levels of the biologically active form of IL-12, IL-12p70. This suggested that IL-12p70 is under tight regulation by other factors and/or might require additional promoting signals for its induction in M. tuberculosis–infected macrophages. IFN-γ is known as an inducer of IL-12p70 (51, 53, 56) and as a crucial activator of macrophages during M. tuberculosis infection (5, 11, 12, 14). We therefore assessed the effects of IFN-γ stimulation on M. tuberculosis–infected macrophages and whether type I IFN (either autocrine or exogenous) could perturb macrophage responsiveness to IFN-γ. IFN-γ treatment enhanced IL-12p40 and TNF-α production while suppressing IL-10 and IL-1β in M. tuberculosis–infected macrophages. However, little to no IL-12p70 was induced by IFN-γ in WT macrophages infected with M. tuberculosis. Only in the absence of either type I IFN signaling or IL-10 could IFN-γ addition induce robustly detectable amounts of IL-12p70. This indicates that these two pathways act as important regulators of the macrophage response to IFN-γ during M. tuberculosis infection. In particular, the ability of IFN-γ to drive macrophage production of biologically active IL-12p70 in response to M. tuberculosis remains compromised when the type I IFN or IL-10 pathways are intact.
To mimic a situation in which an M. tuberculosis–infected macrophage responds to both type I IFN and IFN-γ present in the microenvironment, we infected macrophages with M. tuberculosis and concomitantly added type I IFN and IFN-γ. Strikingly, type I IFN completely abrogated the ability of IFN-γ to downregulate IL-10 production and enhance IL-12p40 and TNF-α production. Type I IFN therefore seems to play a dominant regulatory role in M. tuberculosis–infected macrophages, which IFN-γ is unable to overcome. How type I IFN blocks macrophage responsiveness to IFN-γ remains unclear. Type I IFN can downregulate IFN-γ receptor expression (43, 54) and inhibit signaling (75) to limit macrophage activation. In our system this mechanism is likely to be only partially active, because type I IFN did not block all effects of IFN-γ on macrophages and only partially mediated effects on Ifngr1 transcript levels. In particular, the ability of IFN-γ to suppress IL-1β production was largely unaffected by IFN-β treatment, and if anything was actually increased by the presence of IFN-β. This suggests that the effects of type I IFN on macrophage IFN-γ responsiveness are specific, rather than global.
Myeloid cells such as macrophages are known to be primary reservoirs of M. tuberculosis bacteria during TB (1–3). A critical role of IFN-γ is therefore to activate these cells to restrict intracellular bacterial growth through induction of antimicrobial effectors such as iNOS (5, 6, 12, 14). Our data suggest that type I IFN signaling has inhibitory effects on IFN-γ–induced macrophage restriction of M. tuberculosis. The mechanism for this remains unclear but may involve inhibition of reactive oxygen species induction (76).
In summary, we show in the present study that type I IFN is a powerful negative regulator of the immune response to M. tuberculosis at the level of the infected macrophage. Type I IFN suppresses both initial proinflammatory cytokine production by infected macrophages, in large part through induction of high levels of IL-10, and also strongly inhibits the macrophage’s ability to respond to IFN-γ stimulation. Type I IFN may therefore act to interrupt the Th1 immune response, which is crucial to host resistance to M. tuberculosis, through suppressing production of the proinflammatory cytokines that prime this response but also through making macrophages unresponsive to subsequent IFN-γ feedback from Th1 cells and other IFN-γ sources. Additionally, type I IFN may act directly to prevent IFN-γ–mediated M. tuberculosis growth restriction by macrophages, allowing a niche of M. tuberculosis permissive cells to develop.
Supplementary Material
Acknowledgments
We are indebted to the Medical Research Council National Institute for Medical Research Biological Services for animal husbandry and technical support. We thank S. Caidan and the Medical Research Council National Institute for Medical Research safety section. We thank L. Gabrysova and C. Taubert for assistance with data analysis. We thank Rui Appelberg (Instituto de Biologia Molecular e Celular, Porto, Portugal) for the gift of Nos2−/− mice.
This work was funded by Medical Research Council, U.K. Grant U117565642 and European Research Council Grant 294682-TB-PATH. M.S. and L.M.-T. were funded by the Fundação para a Ciência e Tecnologia, Portugal. M.S. is a Fundação para a Ciência e Tecnologia, Portugal investigator. L.M.T. was supported by Fundação para a Ciência e Tecnologia, Portugal Grant SFRH/BPD/77399/2011.
The online version of this article contains supplemental material.
- ActD
- actinomycin D
- B6
- C57BL/6
- BM
- bone marrow
- BMDM
- bone marrow–derived macrophage
- iNOS
- inducible NO synthase
- LOD
- limit of detection
- qRT-PCR
- quantitative RT-PCR
- TB
- tuberculosis
- WT
- wild-type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Cooper A. M. 2009. Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol. 27: 393–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flynn J. L., Chan J.. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19: 93–129 [DOI] [PubMed] [Google Scholar]
- 3.North R. J., Jung Y. J.. 2004. Immunity to tuberculosis. Annu. Rev. Immunol. 22: 599–623 [DOI] [PubMed] [Google Scholar]
- 4.Chan J., Xing Y., Magliozzo R. S., Bloom B. R.. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175: 1111–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacMicking J. D., North R. J., LaCourse R., Mudgett J. S., Shah S. K., Nathan C. F.. 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 94: 5243–5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacMicking J. D., Taylor G. A., McKinney J. D.. 2003. Immune control of tuberculosis by IFN-γ-inducible LRG-47. Science 302: 654–659 [DOI] [PubMed] [Google Scholar]
- 7.Cooper A. M., Magram J., Ferrante J., Orme I. M.. 1997. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J. Exp. Med. 186: 39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn J. L., Goldstein M. M., Triebold K. J., Sypek J., Wolf S., Bloom B. R.. 1995. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J. Immunol. 155: 2515–2524 [PubMed] [Google Scholar]
- 9.Bundschuh D. S., Barsig J., Hartung T., Randow F., Döcke W. D., Volk H. D., Wendel A.. 1997. Granulocyte-macrophage colony-stimulating factor and IFN-γ restore the systemic TNF-α response to endotoxin in lipopolysaccharide-desensitized mice. J. Immunol. 158: 2862–2871 [PubMed] [Google Scholar]
- 10.Cooper A. M., Dalton D. K., Stewart T. A., Griffin J. P., Russell D. G., Orme I. M.. 1993. Disseminated tuberculosis in interferon γ gene-disrupted mice. J. Exp. Med. 178: 2243–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denis M. 1991. Involvement of cytokines in determining resistance and acquired immunity in murine tuberculosis. J. Leukoc. Biol. 50: 495–501 [DOI] [PubMed] [Google Scholar]
- 12.Denis M. 1991. Interferon-γ-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell. Immunol. 132: 150–157 [DOI] [PubMed] [Google Scholar]
- 13.Déry R. E., Bissonnette E. Y.. 1999. IFN-γ potentiates the release of TNF-α and MIP-1α by alveolar macrophages during allergic reactions. Am. J. Respir. Cell Mol. Biol. 20: 407–412 [DOI] [PubMed] [Google Scholar]
- 14.Flynn J. L., Chan J., Triebold K. J., Dalton D. K., Stewart T. A., Bloom B. R.. 1993. An essential role for interferon γ in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178: 2249–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonelli L. R., Gigliotti Rothfuchs A., Gonçalves R., Roffê E., Cheever A. W., Bafica A., Salazar A. M., Feng C. G., Sher A.. 2010. Intranasal poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J. Clin. Invest. 120: 1674–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berry M. P., Graham C. M., McNab F. W., Xu Z., Bloch S. A., Oni T., Wilkinson K. A., Banchereau R., Skinner J., Wilkinson R. J., et al. 2010. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466: 973–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maertzdorf J., Repsilber D., Parida S. K., Stanley K., Roberts T., Black G., Walzl G., Kaufmann S. H.. 2011. Human gene expression profiles of susceptibility and resistance in tuberculosis. Genes Immun. 12: 15–22 [DOI] [PubMed] [Google Scholar]
- 18.Manca C., Tsenova L., Bergtold A., Freeman S., Tovey M., Musser J. M., Barry C. E., III., Freedman V. H., Kaplan G.. 2001. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-α/β. Proc. Natl. Acad. Sci. USA 98: 5752–5757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manca C., Tsenova L., Freeman S., Barczak A. K., Tovey M., Murray P. J., Barry C., Kaplan G.. 2005. Hypervirulent M. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J. Interferon Cytokine Res. 25: 694–701 [DOI] [PubMed] [Google Scholar]
- 20.Mayer-Barber K. D., Andrade B. B., Barber D. L., Hieny S., Feng C. G., Caspar P., Oland S., Gordon S., Sher A.. 2011. Innate and adaptive interferons suppress IL-1α and IL-1β production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity 35: 1023–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novikov A., Cardone M., Thompson R., Shenderov K., Kirschman K. D., Mayer-Barber K. D., Myers T. G., Rabin R. L., Trinchieri G., Sher A., Feng C. G.. 2011. Mycobacterium tuberculosis triggers host type I IFN signaling to regulate IL-1β production in human macrophages. J. Immunol. 187: 2540–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ordway D., Henao-Tamayo M., Harton M., Palanisamy G., Troudt J., Shanley C., Basaraba R. J., Orme I. M.. 2007. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J. Immunol. 179: 522–531 [DOI] [PubMed] [Google Scholar]
- 23.Ottenhoff T. H., Dass R. H., Yang N., Zhang M. M., Wong H. E., Sahiratmadja E., Khor C. C., Alisjahbana B., van Crevel R., Marzuki S., et al. 2012. Genome-wide expression profiling identifies type 1 interferon response pathways in active tuberculosis. PLoS ONE 7: e45839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanley S. A., Johndrow J. E., Manzanillo P., Cox J. S.. 2007. The type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J. Immunol. 178: 3143–3152 [DOI] [PubMed] [Google Scholar]
- 25.Wu K., Dong D., Fang H., Levillain F., Jin W., Mei J., Gicquel B., Du Y., Wang K., Gao Q., et al. 2012. An interferon-related signature in the transcriptional core response of human macrophages to Mycobacterium tuberculosis infection. PLoS ONE 7: e38367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper A. M., Pearl J. E., Brooks J. V., Ehlers S., Orme I. M.. 2000. Expression of the nitric oxide synthase 2 gene is not essential for early control of Mycobacterium tuberculosis in the murine lung. Infect. Immun. 68: 6879–6882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desvignes L., Wolf A. J., Ernst J. D.. 2012. Dynamic roles of type I and type II IFNs in early infection with Mycobacterium tuberculosis. J. Immunol. 188: 6205–6215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNab F. W., Ewbank J., Rajsbaum R., Stavropoulos E., Martirosyan A., Redford P. S., Wu X., Graham C. M., Saraiva M., Tsichlis P., et al. 2013. TPL-2-ERK1/2 signaling promotes host resistance against intracellular bacterial infection by negative regulation of type I IFN production. J. Immunol. 191: 1732–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cliff J. M., Lee J. S., Constantinou N., Cho J. E., Clark T. G., Ronacher K., King E. C., Lukey P. T., Duncan K., Van Helden P. D., et al. 2013. Distinct phases of blood gene expression pattern through tuberculosis treatment reflect modulation of the humoral immune response. J. Infect. Dis. 207: 18–29 [DOI] [PubMed] [Google Scholar]
- 30.Chang E. Y., Guo B., Doyle S. E., Cheng G.. 2007. Cutting edge: involvement of the type I IFN production and signaling pathway in lipopolysaccharide-induced IL-10 production. J. Immunol. 178: 6705–6709 [DOI] [PubMed] [Google Scholar]
- 31.Guarda G., Braun M., Staehli F., Tardivel A., Mattmann C., Förster I., Farlik M., Decker T., Du Pasquier R. A., Romero P., Tschopp J.. 2011. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34: 213–223 [DOI] [PubMed] [Google Scholar]
- 32.Iyer S. S., Ghaffari A. A., Cheng G.. 2010. Lipopolysaccharide-mediated IL-10 transcriptional regulation requires sequential induction of type I IFNs and IL-27 in macrophages. J. Immunol. 185: 6599–6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auerbuch V., Brockstedt D. G., Meyer-Morse N., O’Riordan M., Portnoy D. A.. 2004. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J. Exp. Med. 200: 527–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carrero J. A., Calderon B., Unanue E. R.. 2004. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J. Exp. Med. 200: 535–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connell R. M., Saha S. K., Vaidya S. A., Bruhn K. W., Miranda G. A., Zarnegar B., Perry A. K., Nguyen B. O., Lane T. F., Taniguchi T., et al. 2004. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J. Exp. Med. 200: 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stockinger S., Kastner R., Kernbauer E., Pilz A., Westermayer S., Reutterer B., Soulat D., Stengl G., Vogl C., Frenz T., et al. 2009. Characterization of the interferon-producing cell in mice infected with Listeria monocytogenes. PLoS Pathog. 5: e1000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beamer G. L., Flaherty D. K., Assogba B. D., Stromberg P., Gonzalez-Juarrero M., de Waal Malefyt R., Vesosky B., Turner J.. 2008. Interleukin-10 promotes Mycobacterium tuberculosis disease progression in CBA/J mice. J. Immunol. 181: 5545–5550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demangel C., Bertolino P., Britton W. J.. 2002. Autocrine IL-10 impairs dendritic cell (DC)-derived immune responses to mycobacterial infection by suppressing DC trafficking to draining lymph nodes and local IL-12 production. Eur. J. Immunol. 32: 994–1002 [DOI] [PubMed] [Google Scholar]
- 39.Redford P. S., Boonstra A., Read S., Pitt J., Graham C., Stavropoulos E., Bancroft G. J., O’Garra A.. 2010. Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. Eur. J. Immunol. 40: 2200–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Redford P. S., Murray P. J., O’Garra A.. 2011. The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol. 4: 261–270 [DOI] [PubMed] [Google Scholar]
- 41.Turner J., Gonzalez-Juarrero M., Ellis D. L., Basaraba R. J., Kipnis A., Orme I. M., Cooper A. M.. 2002. In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J. Immunol. 169: 6343–6351 [DOI] [PubMed] [Google Scholar]
- 42.Teles R. M., Graeber T. G., Krutzik S. R., Montoya D., Schenk M., Lee D. J., Komisopoulou E., Kelly-Scumpia K., Chun R., Iyer S. S., et al. 2013. Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science 339: 1448–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rayamajhi M., Humann J., Penheiter K., Andreasen K., Lenz L. L.. 2010. Induction of IFN-αβ enables Listeria monocytogenes to suppress macrophage activation by IFN-γ. J. Exp. Med. 207: 327–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaiser F., Cook D., Papoutsopoulou S., Rajsbaum R., Wu X., Yang H. T., Grant S., Ricciardi-Castagnoli P., Tsichlis P. N., Ley S. C., O’Garra A.. 2009. TPL-2 negatively regulates interferon-β production in macrophages and myeloid dendritic cells. J. Exp. Med. 206: 1863–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Awasthi A., Carrier Y., Peron J. P., Bettelli E., Kamanaka M., Flavell R. A., Kuchroo V. K., Oukka M., Weiner H. L.. 2007. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 8: 1380–1389 [DOI] [PubMed] [Google Scholar]
- 46.Fitzgerald D. C., Zhang G. X., El-Behi M., Fonseca-Kelly Z., Li H., Yu S., Saris C. J., Gran B., Ciric B., Rostami A.. 2007. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat. Immunol. 8: 1372–1379 [DOI] [PubMed] [Google Scholar]
- 47.Stumhofer J. S., Silver J. S., Laurence A., Porrett P. M., Harris T. H., Turka L. A., Ernst M., Saris C. J., O’Shea J. J., Hunter C. A.. 2007. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 8: 1363–1371 [DOI] [PubMed] [Google Scholar]
- 48.Kalliolias G. D., Ivashkiv L. B.. 2008. IL-27 activates human monocytes via STAT1 and suppresses IL-10 production but the inflammatory functions of IL-27 are abrogated by TLRs and p38. J. Immunol. 180: 6325–6333 [DOI] [PubMed] [Google Scholar]
- 49.Zeitvogel J., Werfel T., Wittmann M.. 2012. IL-27 acts as a priming signal for IL-23 but not IL-12 production on human antigen-presenting cells. Exp. Dermatol. 21: 426–430 [DOI] [PubMed] [Google Scholar]
- 50.Yoshida H., Hamano S., Senaldi G., Covey T., Faggioni R., Mu S., Xia M., Wakeham A. C., Nishina H., Potter J., et al. 2001. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity 15: 569–578 [DOI] [PubMed] [Google Scholar]
- 51.Abdi K., Singh N., Matzinger P.. 2006. T-cell control of IL-12p75 production. Scand. J. Immunol. 64: 83–92 [DOI] [PubMed] [Google Scholar]
- 52.Hayes M. P., Freeman S. L., Donnelly R. P.. 1995. IFN-γ priming of monocytes enhances LPS-induced TNF production by augmenting both transcription and mRNA stability. Cytokine 7: 427–435 [DOI] [PubMed] [Google Scholar]
- 53.Hayes M. P., Wang J., Norcross M. A.. 1995. Regulation of interleukin-12 expression in human monocytes: selective priming by interferon-γ of lipopolysaccharide-inducible p35 and p40 genes. Blood 86: 646–650 [PubMed] [Google Scholar]
- 54.Kearney S. J., Delgado C., Eshleman E. M., Hill K. K., O’Connor B. P., Lenz L. L.. 2013. Type I IFNs downregulate myeloid cell IFN-γ receptor by inducing recruitment of an early growth response 3/NGFI-A binding protein 1 complex that silences ifngr1 transcription. J. Immunol. 191: 3384–3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore K. W., de Waal Malefyt R., Coffman R. L., O’Garra A.. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19: 683–765 [DOI] [PubMed] [Google Scholar]
- 56.Trinchieri G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3: 133–146 [DOI] [PubMed] [Google Scholar]
- 57.Mishra B. B., Rathinam V. A., Martens G. W., Martinot A. J., Kornfeld H., Fitzgerald K. A., Sassetti C. M.. 2013. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1β. Nat. Immunol. 14: 52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flesch I. E., Kaufmann S. H.. 1991. Mechanisms involved in mycobacterial growth inhibition by γ interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect. Immun. 59: 3213–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jung Y. J., LaCourse R., Ryan L., North R. J.. 2002. Virulent but not avirulent Mycobacterium tuberculosis can evade the growth inhibitory action of a T helper 1-dependent, nitric oxide synthase 2-independent defense in mice. J. Exp. Med. 196: 991–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ding A. H., Nathan C. F., Stuehr D. J.. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 141: 2407–2412 [PubMed] [Google Scholar]
- 61.Flynn J. L., Goldstein M. M., Chan J., Triebold K. J., Pfeffer K., Lowenstein C. J., Schreiber R., Mak T. W., Bloom B. R.. 1995. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2: 561–572 [DOI] [PubMed] [Google Scholar]
- 62.Fremond C. M., Togbe D., Doz E., Rose S., Vasseur V., Maillet I., Jacobs M., Ryffel B., Quesniaux V. F.. 2007. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J. Immunol. 179: 1178–1189 [DOI] [PubMed] [Google Scholar]
- 63.Mayer-Barber K. D., Barber D. L., Shenderov K., White S. D., Wilson M. S., Cheever A., Kugler D., Hieny S., Caspar P., Núñez G., et al. 2010. Caspase-1 independent IL-1β production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 184: 3326–3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamada H., Mizumo S., Horai R., Iwakura Y., Sugawara I.. 2000. Protective role of interleukin-1 in mycobacterial infection in IL-1α/β double-knockout mice. Lab. Invest. 80: 759–767 [DOI] [PubMed] [Google Scholar]
- 65.Ma X., Chow J. M., Gri G., Carra G., Gerosa F., Wolf S. F., Dzialo R., Trinchieri G.. 1996. The interleukin 12 p40 gene promoter is primed by interferon γ in monocytic cells. J. Exp. Med. 183: 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Molle C., Goldman M., Goriely S.. 2010. Critical role of the IFN-stimulated gene factor 3 complex in TLR-mediated IL-27p28 gene expression revealing a two-step activation process. J. Immunol. 184: 1784–1792 [DOI] [PubMed] [Google Scholar]
- 67.Chen Q., Ghilardi N., Wang H., Baker T., Xie M. H., Gurney A., Grewal I. S., de Sauvage F. J.. 2000. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature 407: 916–920 [DOI] [PubMed] [Google Scholar]
- 68.Hölscher C., Hölscher A., Rückerl D., Yoshimoto T., Yoshida H., Mak T., Saris C., Ehlers S.. 2005. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J. Immunol. 174: 3534–3544 [DOI] [PubMed] [Google Scholar]
- 69.Mascanfroni I. D., Yeste A., Vieira S. M., Burns E. J., Patel B., Sloma I., Wu Y., Mayo L., Ben-Hamo R., Efroni S., et al. 2013. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat. Immunol. 14: 1054–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Byrnes A. A., Ma X., Cuomo P., Park K., Wahl L., Wolf S. F., Zhou H., Trinchieri G., Karp C. L.. 2001. Type I interferons and IL-12: convergence and cross-regulation among mediators of cellular immunity. Eur. J. Immunol. 31: 2026–2034 [DOI] [PubMed] [Google Scholar]
- 71.Cousens L. P., Orange J. S., Su H. C., Biron C. A.. 1997. Interferon-α/β inhibition of interleukin 12 and interferon-γ production in vitro and endogenously during viral infection. Proc. Natl. Acad. Sci. USA 94: 634–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cousens L. P., Peterson R., Hsu S., Dorner A., Altman J. D., Ahmed R., Biron C. A.. 1999. Two roads diverged: interferon α/β- and interleukin 12-mediated pathways in promoting T cell interferon γ responses during viral infection. J. Exp. Med. 189: 1315–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dalod M., Hamilton T., Salomon R., Salazar-Mather T. P., Henry S. C., Hamilton J. D., Biron C. A.. 2003. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon α/β. J. Exp. Med. 197: 885–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gautier G., Humbert M., Deauvieau F., Scuiller M., Hiscott J., Bates E. E., Trinchieri G., Caux C., Garrone P.. 2005. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J. Exp. Med. 201: 1435–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoshida R., Murray H. W., Nathan C. F.. 1988. Agonist and antagonist effects of interferon α and β on activation of human macrophages. Two classes of interferon γ receptors and blockade of the high-affinity sites by interferon α or β. J. Exp. Med. 167: 1171–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deguchi M., Sakuta H., Uno K., Inaba K., Muramatsu S.. 1995. Exogenous and endogenous type I interferons inhibit interferon-γ-induced nitric oxide production and nitric oxide synthase expression in murine peritoneal macrophages. J. Interferon Cytokine Res. 15: 977–984 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.