Abstract
Patients with chronic HBV infection are at risk of reactivation of HBV should they require immunosuppressive therapies for a variety of clinical settings, including chemotherapy for patients with cancer, immunosuppression for solid organ and stem cell transplant recipients, and use of anti-CD20 antibodies, TNF inhibitors, or corticosteroids in patients with oncological, gastrointestinal, rheumatological or dermatological conditions. The key to preventing HBV reactivation is the identification of patients with HBV infection prior to immunosuppressive therapy, initiation of prophylactic antiviral therapy in patients at moderate or high risk of HBV reactivation, and close monitoring of other patients so that antiviral therapy can be initiated at the first sign of HBV reactivation. Unfortunately, many patients infected with HBV are unaware of their infection or risk factors, and physicians often do not have sufficient time to systematically assess patients for risk factors for HBV prior to starting immunosuppressive therapy. In this article, we review the incidence, risk factors and outcomes of HBV reactivation, and the efficacy of antiviral therapy in preventing its occurrence. We also propose an algorithm for managing patients with HBV infection who require immunosuppressive therapy.
Introduction
Patients infected with HBV are at risk of reactivation of the virus should they require immunosuppressive therapy. Reactivation of HBV replication can occur in patients with chronic or past HBV infection. This reactivation is most commonly reported in patients receiving cancer chemotherapy for haematological malignancies and those receiving bone marrow or stem cell transplant ation.1 Reactivation can also occur in a wide variety of clinical settings, including patients receiving chemotherapy for solid tumours, recipients of solid organ transplants, and patients with oncological, gastrointestinal, rheumatological or dermatological conditions who are receiving treatment with anti-CD20 antibodies, TNF inhibitors, corticosteroids or other immunosuppressive agents.1–4
Reactivation of HBV replication can be mild and asymptomatic, or severe and, potentially, result in hepatocellular injury, liver failure and death.5,6 Prophylactic antiviral therapy is effective at preventing HBV reactivation,6 but the lack of awareness among physicians prescribing immunosuppressive therapy7,8 and the inconsistency in guideline recommendations9–14 have resulted in continued reports of fatal HBV reactivation. In this article, we review the incidence, risk factors and outcomes of HBV reactivation, and the efficacy of antiviral therapy at preventing its occurrence. An algorithm for the management of patients with HBV infection who require immunosuppressive therapy is also proposed.
Basis for HBV reactivation
In individuals with chronic HBV infection—that is, hepatitis B surface antigen (HBsAg)-positive and hepatitis B core antibody IgG (anti-HBc)-positive—the serum HBV DNA levels can vary from undetectable (<20 international units [IU]/ml) to >1,000,000,000 (>9 log10) IU/ml depending on the balance between HBV replication and immune control.15 The vast majority of people who have serological recovery from HBV infection (HBsAg-negative, hepatitis B surface antibody [anti-HBs]-positive and anti-HBc-positive) have undetectable HBV DNA in serum, but HBV persists in the liver16 and its replication is controlled by the immune system.17 The delicate balance between viral replication and immune control explains why immunosuppressive therapy can augment HBV replication in chronically infected persons and reactivate ‘dormant’ HBV in individuals regarded as ‘recovered’. Some persons have so-called isolated anti-HBc status—presence of anti-HBc antibodies without HBsAg or anti-HBs antibodies (antibodies against the HBsAg)—and most of them had past HBV infection and are at risk of HBV reactivation.18,19
Immune control of HBV infection is largely mediated through HBV-specific cytotoxic T cells,17 but B cells also have a role in antigen presentation and viral clearance.20 Reactivation of HBV replication during immunosuppressive therapy can occur indirectly via suppression of immune control,5 but also directly via glucocorticoid stimulation of a glucocorticoid-responsive element in the HBV genome, leading to upregulation of HBV gene expression.21 TNF has been shown in some studies to promote HBV clearance and to decrease HBV transcription;22 thus, inhibition of TNF might also have a direct effect on enhancing HBV replication.
Clinical manifestations
The course of HBV reactivation has been described as comprising three phases (Figure 1).5 During the first phase, HBV reactivation is increased, as manifested by an increase in levels of HBV DNA in the serum of an HBsAg-positive person or a reappearance of HBsAg or HBV DNA in serum in a person who was previously HBsAg-negative or had undetectable serum HBV DNA, respectively.5 Symptoms of hepatitis are usually absent and alanine aminotransferase (ALT) levels are not elevated.
Figure 1.
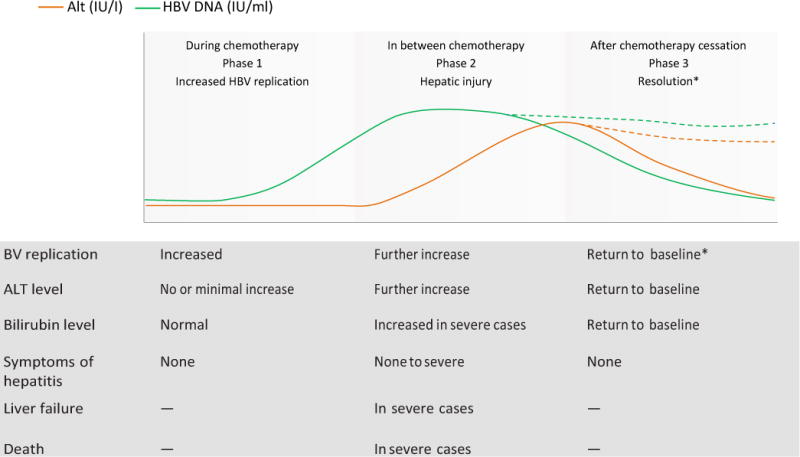
Phases of HBV reactivation. Generally, three phases of HBV reactivation occur.5 Phase 1: HBV DNA levels increase, patients are typically asymptomatic, and ALT levels might not be increased. Phase 2: HBV DNA and ALT levels are increased, and in severe cases there might be symptoms of hepatitis, jaundice and liver failure. Phase 3: Resolution occurs in most, but not all, patients. Some HBsAg-positive patients might continue to have higher HBV DNA levels than at baseline, and some HBsAg-negative patients might remain HBsAg-positive. Solid lines represent the majority of patients who have resolution, whereas dashed lines represent the minority of patients in whom hepatitis B might not fully resolve. Abbreviations: ALT, alanine aminotransferase; HBsAg, hepatitis B surface antigen; IU, international unit.
During the second phase, serum HBV DNA levels continue to increase, accompanied by elevations in ALT levels with or without symptoms of acute hepatitis; in some patients, hepatic injury progresses, resulting in liver failure and death.5 These changes in the second phase can occur in between chemotherapy administrations or after cessation of chemotherapy, and result from reconsti tution of the host immune response.23 In the third phase, hepatic injury resolves either spontaneously or as a result of withholding immunosuppressive therapy or initiation of antiviral therapy.5
Studies of patients who received chemotherapy for lymphoma showed that hepatitis related to HBV reactivation typically occurred after the second or third courses of chemotherapy;24 however, HBV reactivation can occur at any time during or after immunosuppressive therapy. In patients receiving a monoclonal antibody to CD20 antigen—such as rituximab, which causes B-cell depletion—HBV reactivation has been reported to occur after a median of six doses and up to 12 months after the last dose in patients with lymphoproliferative disorders.25 In patients who require long-term or lifelong immunosuppressive treatment (for example, patients with rheumatoid arthritis, IBD or who have undergone solid organ transplantation) HBV reactivation can occur at any time during immunosuppressive therapy. Studies of HBsAg-negative-anti-HBc-positive persons who received stem cell transplantation showed that HBV reactivation can occur up to several years after transplantation because of the long delay in reconstituting the recipient’s immune response to HBV.26
Incidence and outcomes
The incidence of HBV reactivation in patients receiving immunosuppressive therapy is poorly defined and can depend on many factors including host characteristics, underlying disease, type of immunosuppressive therapy received and baseline HBV status. Much of the confusion regarding the incidence of HBV reactivation relates to the lack of standardized nomenclature and definitions. In addition, most studies have been retrospective in design with incomplete characterization of baseline HBV status and inconsistent follow-up testing.
Nomenclature
Hepatitis was initially defined in 1991 as a ≥3-fold increase in ALT level exceeding 100 U/l and attributed to reactivation of hepatitis B when there was an associated >10-fold increase in HBV DNA level or appearance of HBV DNA in serum in the absence of other causes of liver injury.24 Since then, the definitions of hepatitis and reactivation have evolved.27–31
At a conference in 2013 on “Reactivation of Hepatitis B” organized by the American Association for the Study of Liver Diseases, standardized nomenclature was proposed.32 Reactivation of HBV replication should be defined as a marked increase in HBV replication (≥2 log increase from baseline levels or a new appearance of HBV DNA to a level of ≥100 IU/ml) in a person with previously stable or undetectable levels. The types of reactivation should be described as follows: exacerbation of chronic hepatitis B or reactivation of past hepatitis B. The latter can be further defined as reverse HBsAg seroconversion (reappearance of HBsAg), or appearance of HBV DNA in serum in the absence of HBsAg (Figure 2). The severity of reactivation (presence or absence of jaundice and liver failure) and its outcome (return to baseline status or persistence in an activated state, that is, higher levels of HBV DNA than at baseline or continued HBsAg-positivity in the case of reverse seroconversion), need for liver transplantation or death, should also be reported. Adoption of standardized nomenclature (Box 1) and reporting will require collaboration across many specialties including, but not limited to, hepatology, oncology, rheumatology, gastroenterology, dermatology and infectious diseases. These efforts will be critical in clarifying the true incidence and outcome of HBV reactivation in each clinical setting and guide evidence-based recommendations on the need for HBV screening and prophylactic antiviral therapy.
Figure 2.

Types of HBV reactivation. Patients with chronic HBV infection (HBsAg+ and anti-HBc+ test results) can have either an increase in HBV DNA level or an appearance of HBV DNA, depending on whether they did or did not have detectable HBV DNA before immunosuppression, respectively. Patients with past HBV infection (HBsAg− and anti-HBc+) can be diagnosed as having HBV reactivation upon the appearance of HBsAg or HBV DNA. Abbreviations: +, positive; −, negative; anti-HBc, anti-hepatitis B core antibody IgG; HBsAg, hepatitis B surface antigen.
Box 1. Nomenclature for HBV reactivation.
Reactivation of HBV: demonstrated by either an exacerbation of chronic HBV infection or reactivation of past HBV infection after the start of immunosuppressive therapy
Exacerbation of chronic HBV: marked increase in HBV DNA levels, which can include any of the following in a person with HBsAg-positive test, ≥2 log10 increase in HBV DNA levels from baseline levels, detection of HBV DNA with level >100 IU/ml in a person with undetectable HBV DNA at baseline, or detection of HBV DNA with level ≥100,000 IU/ml in a person with no baseline HBV DNA
Reactivation of past HBV: reverse HBsAg seroconversion, HBsAg-negative becomes HBsAg-positive; or appearance of HBV DNA in absence of HBsAg, HBV DNA-undetectable becomes HBV DNA-detectable
Outcomes of reactivation: resolution, return to baseline; cure, loss of HBsAg- positivity; persistence, HBV DNA level is higher or HBsAg remains positive
(in HBsAg-negative patient at baseline) ≥6 months after onset of reactivation; death or liver transplantation
Preventative antiviral therapy: use of antiviral therapy to prevent reactivation of HBV infection, includes both prophylactic and pre-emptive antiviral therapies
Prophylactic antiviral therapy: antiviral therapy started for all patients with HBV before immunosuppressive therapy, ALT level is usually normal, and HBV DNA level is usually undetectable at the time antiviral therapy is started
Pre-emptive antiviral therapy: whilst monitoring patients with HBV on immunosuppressive therapy, antiviral therapy started when ALT and/or HBV DNA levels increase; patients are without signs of jaundice or liver failure at the time antiviral therapy is started
Therapeutic antiviral therapy: use of antiviral therapy to treat reactivation of HBV infection; typically, this step occurs in patients who were not screened for HBV infection prior to start of immunosuppressive therapy or were not monitored during immunosuppressive therapy, and antiviral therapy is initiated when patients are noted to have high HBV DNA level and marked increase in ALT levels with accompanying jaundice or liver failure
Risk factors for reactivation: host,24,27 male sex; disease,35 lymphoma, breast cancer; immunosuppressive therapy,58,71 rituximab, steroids; viral,12,35 HBsAg-positive, high HBV DNA level before immunosuppressive therapy
Systemic cancer chemotherapy
HBV reactivation has been studied most extensively in patients receiving chemotherapy for lymphoma. In one of the earliest prospective studies, 100 patients in Hong Kong had serum HBV DNA levels tested at baseline and before each cycle of chemotherapy.24 Prior to chemo therapy, 27 patients were HBsAg-positive, 51 were HBsAg-negative and positive for anti-HBc and/or positive for anti-HBs, and 22 were negative for all three HBV serological markers. Hepatitis attributed to reactivation of HBV replication occurred in 48%, 4% and 0% of patients, respectively, and reactivation-related liver failure occurred in 7%, 2% and 0% of patients, respectively. In another prospective study of 244 HBsAg-negative patients who received chemotherapy for lymphoma, eight (3%) developed reactivation, of whom three progressed to liver failure and one of these patients died of liver failure.30
HBV reactivation can also occur in patients receiving chemotherapy for solid tumours. Many reports have come from patients treated for breast cancer,33,34 but HBV reactivation has been reported in patients treated for a wide variety of solid tumours including colon, lung, and head and neck cancers.27,35 The incidence of HBV reactivation has been reported to be 10–38% in a study that included 63 HBsAg-positive patients with solid tumours.27 A higher incidence of reactivation has been reported in patients with breast cancer (41%) than in patients with other solid tumours (7–29%).35
In a systematic review of 14 studies of prophylactic lamivudine in HBsAg-positive patients receiving chemotherapy for haematological or solid malignancies, among the control participants who received no lamivudine prophylaxis or received lamivudine therapy when reactivation occurred, the pooled (range) incidence of HBV reactivation, HBV-related hepatitis, HBV-related liver failure and HBV-related death was reported to be 37% (24–88%), 33% (24–88%), 13% (5–33%) and 7% (0–63%), respectively.6 In comparison, among the participants who received prophylactic lamivudine, the pooled (range) incidence of HBV reactivation, HBV-related hepatitis, HBV-related liver failure and HBV-related death was 4% (0–13%), 4% (0–13%), 0% (all 0%), and 2% (0–13%).6
Transarterial chemoembolization for HCC
Transarterial chemoembolization (TACE) has been shown to improve survival of patients with intermediate-stage hepatocellular carcinoma (HCC) as compared with supportive care,36 and is also widely used for down staging HCC prior to liver transplantation.37,38 Chemotherapy is administered into a branch of the hepatic artery that supplies the tumour to maximize efficacy and to minimize systemic adverse effects. Nevertheless, reactivation of HBV replication (including the end results of liver failure and death) has been reported in patients who have received TACE but no other systemic chemotherapy.29,39 Although the TACE procedure itself can contribute to elevations in ALT levels, in a randomized control study of HBsAg-positive patients with HCC who received TACE with or without antiviral prophylaxis, the proportion of patients who had HBV reactivation (10-fold increase in DNA levels compared with baseline) and severe increase in ALT levels (>5 times upper limit of normal) was substantially higher in the control group who did not receive antiviral prophylaxis: 41% versus 3% experienced HBV reactivation and 30% versus 8% had a severe increase in ALT levels (>5 times upper limit of normal). These findings indicate that ALT level elevation in patients treated with TACE could be caused by HBV reactivation and not just tumour necrosis.29
Stem cell transplantation
HBV reactivation is of particular concern in patients receiving bone marrow or stem cell transplantation. These patients typically receive intense chemotherapy for the underlying malignancy to induce remission, followed by additional chemotherapy and radiation therapy to ablate bone marrow.40,41 It is therefore not surprising that the incidence of HBV-related hepatitis is high, not only in HBsAg-positive patients (32–50%),31,42 but also in HBsAg-negative–anti-HBc-positive patients (3–50%).26,43–46 In the latter group, low titre anti-HBs (<10 mIU/ml) is a predictor of reverse HBsAg sero conversion (reappearance of HBsAg).26 The risk of reverse seroconversion can persist for many years after stem cell transplantation because of the long delay in reconstitution of the recipient’s immune response to HBV. In one retrospective study of 61 HBsAg-negative–anti-HBc-positive stem cell transplant recipients, the cumulative probability of reverse seroconversion was reported to have increased from 9% at the end of year 1 to 43% at the end of year 4.26
Solid organ transplantation
Rates of HBV reactivation have been reported to be 50–90% in HBsAg-positive patients who have undergone kidney transplantation, and reactivation has been shown to lead to liver failure, accelerated progression to cirrhosis and HCC, and increased liver-related mortality.3,47–50 Reactivation of HBV replication with reappearance of HBsAg has also been reported in HBsAg-negative–anti-HBc-positive recipients of kidney transplants, although the incidence is low (0.9–5%);51,52 however, reverse seroconversion can occur as late as 15 years after transplantation, and in some instances, correlate with a decrease in the anti-HBs titre.51 Similar findings have been reported in heart and lung transplant recipients.53–56
Monoclonal antibodies to B cells or T cells
Monoclonal antibodies (such as rituximab) are used to treat a wide variety of medical conditions, including non-Hodgkin lymphoma, chronic lymphocytic leukaemia, refractory rheumatoid arthritis, autoimmune haemolytic anaemia, idiopathic thrombocytopaenic purpura, and vasculitis associated with cryoglobulinaemia, among others.57 A meta-analysis found that rituximab-containing regimens for haematological malignancies were associated with a sixfold higher odds of HBV reactivation than identical regimens without rituximab in HBsAg-negative–anti-HBc-positive patients.25 In one study of46 HBsAg-negative patients with lymphoma, 5 of 21 who received cyclophosphamide, hydroxydaunorubicin, oncovin and prednisolone (CHOP) and rituximab had HBV reactivation and one died of acute liver failure compared with no patients (n = 25) who received CHOP without rituximab.58 A preliminary analysis of the postmarketing data from the FDA Adverse Event Reporting System found 109 cases of rituximab or of atumumab-associated fatal HBV-related acute liver failure between market approval for rituximab (November 1997)59 and for ofatumumab (October 2009)60 and August 2012; of these patients, more than half had either not been screened or been inadequately screened (testing for HBsAg but not anti-HBc) for HBV, and most had not received prophylactic antiviral therapy.61 These findings have prompted the FDA to revise the product labels of all antibodies directed against CD20 to add HBV reactivation to the boxed warning, recommending anti-HBc in addition to HBsAg screening tests before initiation of therapy, and antiviral therapy in those who test positive to prevent reactivation.62–64
Antibodies directed against other antigens on immune cells might have similar effects as anti-CD20 antibodies. One of these, alemtuzumab—a monoclonal antibody directed against CD52, which is expressed on B cells and T cells, natural killer cells and macrophages—has been approved for refractory chronic lymphocytic leukaemia and used in recipients of stem cell as well as solid organ transplantation. Reactivation of HBV replication has been reported in association with alemtuzumab.65,66
TNF antagonists
Anti-TNF agents are used to treat a broad spectrum of medical conditions including rheumatoid arthritis, IBD and psoriasis. Reactivation of HBV replication, HBV-related liver failure and death has been reported in HBsAg-positive as well as in HBsAg-negative–anti-HBc-positive patients receiving anti-TNF agents. One study identified 89 HBsAg-positive and 168 HBsAg-negative–anti-HBc positive patients from a literature search of HBV and anti-TNF agent use.67 35 (39%) HBsAg-positive patients experienced HBV reactivation; five of these patients had acute liver failure, of whom four died. Among the HBsAg-negative–anti-HBc-positive patients, 9 (5%) experienced HBV reactivation, and one had acute liver failure and later died. The authors noted that the risk of HBV reactivation was higher with infliximab (compared with other anti-TNF agents) and with concomitant use of other immunosuppressive drugs, and the risk was substantially lower with prophylactic antiviral therapy.
Corticosteroids and immunosuppressive agents
Reactivation of HBV replication might be mediated through suppression of immune control or direct stimulation of a glucocorticoid-responsive element in the HBV genome.21 Reactivation has been reported in patients receiving corticosteroids alone;68–70 however, the incidence of HBV reactivation in this setting remains unclear as steroids are commonly given in combination with other immunosuppressive therapies. It is also not known whether there is a threshold dose or duration of corticosteroid below which the risk of HBV reactivation is absent or negligible. When used in combination with other immunosuppressive agents, corticosteroids have been shown to increase the risk of HBV reactivation. In one study,49 HBsAg-positive patients with non-Hodgkin lymphoma were randomly assigned to receive chemotherapy with or without prednisolone. HBV reactivation occurred in 72% of those who received prednisolone compared with 38% of those who did not (P = 0.02); however, the rate of complete remission of lymphoma was lower in the prednisolone-free group than in the group who received prednisolone.71
HBV reactivation has also been reported in association with other immunosuppressive agents such as methotrexate72 and azathioprine.73 Most of these reports involved isolated cases; thus, the risk of HBV reactivation when these agents are used alone is unclear.
Risk factors
Not all patients with chronic HBV infection receiving immunosuppressive therapy will experience HBV reactivation. Why some patients do and others do not, and why some patients have a self-limiting course whereas others progress to liver failure and die, is not fully understood. Information from published studies, most of which are from Asia, suggest that host factors, underlying disease, type of immunosuppressive therapy received and baseline HBV status contribute to the risk and outcomes.
Host factors
Male sex has been the most consistent host factor shown to be associated with an increased risk of HBV reactivation.24,27 In one study of 78 HBsAg-positive patients with various cancer types, 29% of the male patients had r eactivation as compared with 10% of the female patients.27
Underlying disease
Lymphoma has been the most common underlying disease in reports of HBV reactivation. Whether lymphoma per se is associated with an increased risk of HBV reactivation is unclear because comparisons with other diseases treated with similar chemotherapy regimens have not been reported. The frequent association between lymphoma and HBV reactivation might be related to the intensity of the chemotherapy regimen, resulting in marked immunosuppression. Alternatively, it might be related to a higher prevalence of HBV infection among patients with lymphoma compared with population controls.74 Fewer reports exist of HBV reactivation in patients receiving chemotherapy for solid tumours than haematological malignancies; whether this finding reflects a lower incidence, less awareness of the treating physicians, or less intense immunosuppression with the treatment regimens used is not clear. Of the solid tumours, breast cancer is most commonly associated with HBV reactivation,35 and both retrospective and prospective studies have shown that the rate of reactivation in HBsAg-positive patients with breast cancer is 25–40%.33,35 The high incidence of HBV reactivation in patients with breast cancer has been attributed to the concomitant use of anthracyclines and corticosteroids;75 both drugs have been shown to increase HBV DNA expression.21,76
The treatment for the underlying disease might also influence the timing of HBV reactivation. In patients with lymphoma, HBV reactivation typically occurs after the second or third course of chemotherapy,5 and the risk is usually limited to the period between start of chemotherapy and 4–6 months following completion, but might be as late as 11 months after the cessation of therapy if rituximab was administered.18 By contrast, HBV reactivation can occur at any time in patients with rheumatoid arthritis or IBD and in solid organ transplant recipients who can require many years or even lifelong immunosuppressive therapy.
Immunosuppressive therapy
The intensity of immunosuppression has often been implicated to be an important risk factor for HBV reactivation, but no consensus has been reached on how this aspect can be measured and compared across different treatment regimens. Studies that compared identical chemotherapy regimens for lymphoma with and without rituximab, and with and without corticosteroids clearly showed that the addition of rituximab or corticosteroids increased the risk of HBV reactivation. As mentioned earlier, in one study of 46 HBsAg-negative–anti-HBc-positive patients with lymphoma, 24% of patients treated with rituximab and CHOP had HBV reactivation compared with none of those treated with CHOP without rituximab.58 In another study, 49 HBsAg-positive patients with lymphoma were randomly assigned to receive epirubicin, cyclophosphamide and etoposide with or without prednisolone.71 The risk of reactivation was twofold higher in the group that received prednisolone than the group that did not (RR 1.9, P = 0.021). The cumulative incidence of HBV reactivation 9 months after starting chemotherapy was 72% and 38% in the groups with and without prednisolone, respectively (P = 0.02). Patients in the prednisolone group also had more severe hepatitis than those who did not receive prednisolone.
Viral factors
Baseline HBV replication status has been demonstrated to be an important risk factor in all clinical settings, with HBsAg-positive patients having a higher risk of HBV reactivation than HBsAg-negative–anti-HBc-positive patients. In HBsAg-positive patients, those with detect-able or high levels of serum HBV DNA prior to start of immunosuppressive therapy have a higher risk of HBV reactivation than those with undetectable or low levels of HBV DNA.35,77 Most HBsAg-negative–anti-HBc-positive patients have undetectable serum HBV DNA levels, and the risk of HBV reactivation is higher in the minority with detectable serum HBV DNA at baseline.30,78 In HBsAg-negative–anti-HBc-positive patients, those who have an undetectable anti-HBs level at the onset of immunosuppressive therapy or have a loss of anti-HBs during immunosuppressive therapy have an increased risk of HBV reactivation.26,43,79,80
Prevention
HBV screening
The key to prevention of HBV reactivation is the identification of patients with HBV infection prior to initiation of immunosuppressive therapy. Unfortunately, only 35% of people in the USA who are chronically infected with HBV are aware of their infection.81 In the European Union, it is estimated that up to 90% of those infected with HBV are not aware of their infection.82 Although risk factors for HBV infection are well known, no validated risk tools are available that can be easily applied in clinical practice. Most physicians do not have time to systematically screen patients who are about to start immunosuppressive therapy for risk factors of HBV, and many patients are not aware that they are at risk or might not acknowledge they have had risk behaviours. The US Centers for Disease Control and Prevention,9 European Association for the Study of the Liver,10 and Asian–Pacific Association for the Study of the Liver11 recommends universal HBV screening prior to initiation of immunosuppressive therapy whilst societies such as the American Association for the Study of Liver Diseases,12 American Society of Clinical Oncology13 and the National Comprehensive Cancer Network14 recommend screening of patients with risk factors. A retrospective study has shown that screening occurred in <20% of patients with risk factors for HBV infection in the USA.83 Risk-based screening might be more cost-effective than universal screening if it is sensitive and logistically easy to implement; however, a study of pregnant women in the USA showed that only 60% of the women who tested positive for HBsAg would have been identified by a risk-based screening approach,84 and it is unknown whether the risk-based screening tool used in that study can be implemented in everyday practice owing to time constraints.
Preventative antiviral therapy
The purpose of HBV screening is to identify patients who are infected and who might benefit from preventative antiviral therapy or close monitoring. We propose to use the term ‘preventative antiviral therapy’ to include both prophylactic therapy—in which antiviral therapy is started prior to or at the same time as the initiation of immunosuppressive therapy and before any elevations in ALT or HBV DNA levels—and pre-emptive therapy, in which antiviral therapy is initiated when serum HBV DNA or ALT levels are elevated but before symptomatic manifestation of hepatitis or liver failure. Prophylactic antiviral therapy has been demonstrated to be effective in prevention of HBV reactivation and its sequelae (Table 1). In a systematic review of 14 studies that included 275 HBsAg-positive patients who received lamivudine prophylaxis and 475 control participants (no lamivudine prophylaxis or lamivudine therapy when reactivation occurred) who received chemotherapy,6 the risk of both HBV reactivation and HBV-related hepatitis in the lamivudine prophylaxis group was decreased by 79–100%, and none of the patients had HBV-related liver failure. Patients in the lamivudine prophylaxis group also had lower cancer-related and all-cause mortality, probably related to less frequent interruptions of chemo therapy. Only two of 14 studies were randomized controlled trials, and the types of malignancies and chemotherapy regimens were highly variable; nevertheless, although the effect size varied, the direction of the effect was consistently in favour of a risk reduction in the lamivudine prophylaxis group.
Table 1.
Randomized controlled trials supporting the benefit of prophylactic antiviral therapy in preventing HBV reactivation*
| Reference) | HBV status | Cancer diagnosis | Chemotherapy drugs | Antivirals vs controls (n) | Antiviral duration | Reactivation rates | Reactivation definition |
|---|---|---|---|---|---|---|---|
| Lau et al. (2003)85 | HBsAg+ | Lymphoma | CEOP,ABVD, CHOP,COPP | 15 lamivudine vs 15 controls | 1 week prior-6 weeks after | 0% vs 53% P <0.01 | HBV DNA levels 10× baseline |
| Jang et al. (2006)29 | HBsAg+ | Hepatocellular carcinoma | TACE | 38 lamivudine vs 38 controls | Beginning-12 months after | 3% vs 41% P <0.001 | HBV DNA levels 10× baseline |
| Hsu et al. (2008)86 | HBsAg+ | Non-Hodgkin lymphoma | CHOP | 26 lamivudine vs 26 controls | Beginning-2 months after | 12% vs 56% P <0.01 | HBV DNA levels 10× baseline |
| Huang et al. (2013)18 | HBsAg- anti-HBc+ | CD20+ non-Hodgkin lymphoma | R-CHOP | 41 entecavir vs 39 controls | 1 week prior-3 month after | 2% vs 18% P <0.05 | HBV DNA 2,000 IU/ml Reverse HBsAg seroconversion |
All controls received antiviral therapy if reactivation occurred.
in HBsAg-positive and in HBsAg-negative–anti-HBc-positive patients receiving cancer chemotherapy.
Abbreviations: +, positive, −, negative; ABVD, adriamycin, bleomycin, vinblastine, dacarbazine; anti-HBc, anti-hepatitis B core antibody IgG; CEOP, cyclophosphamide, epirubicin, vincristine, prednisolone; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisolone; COPP, cyclophosphamide, vincristine, procarbazine, prednisolone; HBsAg, hepatitis B antigen; R-CHOP, rituximab-CHOP; TACE, transarterial chemoembolization with epirubicin and cisplatin.
Three randomized controlled trials compared the efficacy of prophylactic versus pre-emptive antiviral therapy in preventing HBV reactivation. In one study,85,30 consecutive HBsAg-positive patients with lymphoma undergoing chemotherapy were randomly assigned to receive lamivudine prior to chemotherapy or when there was a >10-fold increase in serum HBV DNA level or the appearance of HBV DNA in patients with previously undetectable serum HBV DNA. None of the patients who received prophylactic lamivudine, versus 53% of those who received pre-emptive lamivudine, experienced HBV reactivation (P = 0.002). The median onset of HBV reactivation in the latter patients was 16 weeks (range, 4–36 weeks). In another study,86,51 HBsAg-positive patients who were receiving chemotherapy for non-Hodgkin lymphoma were randomly assigned to either prophylactic lamivudine or pre-emptive therapy when ALT levels increased to >1.5-fold the upper normal limit. Compared with those who received pre-emptive lamivudine, fewer patients receiving prophylactic lamivudine had HBV reactivation (12% versus 56%, P = 0.001). In a third study,18,80 HBsAg-negative–anti-HBc-positive patients with lymphoma receiving chemotherapy regimens that included anti-CD20 agents were randomly assigned to receive entecavir prophylactically or pre-emptively when reactivation occurred. The rate of HBV reactivation was lower in the prophylactic group than the pre-emptive group (2% versus 18%, P <0.05).
These three studies show that prophylactic antiviral therapy is more effective than pre-emptive antiviral therapy in preventing HBV reactivation in a high-risk setting. Whether pre-emptive antiviral therapy is more cost-effective in clinical settings associated with moderate risk of HBV reactivation (for example, HBsAg-negative–anti-HBc-positive patients receiving chemotherapy for lymphoma that does not include anti-CD20, or anti-TNF agents or steroids for other disease conditions) is unclear (Table 1). A major limitation of pre-emptive anti-viral therapy is its reliance on close monitoring of HBV DNA levels and coordination of care between physicians prescribing the immunosuppressive therapy and those knowledgeable in the management of patients with hepatitis B such that antiviral therapy can be initiated at the earliest sign of HBV reactivation.
The benefit of preventative antiviral therapy has also been demonstrated in patients receiving immunosuppressive therapy for nonmalignant diseases. One study showed that pre-emptive lamivudine improved survival of HBsAg-positive kidney transplant recipients compared with historical controls who did not receive antiviral treatment.3 Preventative antiviral therapy has also been found to decrease the incidence of HBV reactivation in HBsAg-positive patients with rheumatological diseases (such as rheumatoid arthritis or ankylosing spondylitis) treated with TNF inhibitors.67,87
Most studies of preventative antiviral therapy have used lamivudine because it was the first nucleos(t)ide analogue approved for treatment of hepatitis B, but lamivudine has been shown to have a low barrier to resistance with rates of antiviral resistance of 14–32% after 1 year of treatment and 60–70% after 5 years of treatment in clinical trials in patients with chronic hepatitis B.12 Emergence of antiviral drug resistance mutations negates the benefits of preventative antiviral therapy and can lead to hepatitis flares, liver failure and death.12 Although most patients have low or undetectable serum HBV DNA levels at the start of immunosuppressive therapy, lamivudine resistance and hepatitis flares has been reported in patients receiving preventative lamivudine during immunosuppressive therapy.3,88
Entecavir and tenofovir have more potent antiviral activity and higher barrier to resistance, and studies in immunocompetent patients with chronic hepatitis B showed rates of antiviral resistance in nucleos(t)ide-naïve patients of 1.2% and 0%, respectively, after 5 years of continued therapy.89,90 Both entecavir and tenofovir have an excellent safety profile without myelosuppressive adverse effects or interactions with cytochrome P450 or P-glycoprotein,91,92 indicating that interactions with pharmacokinetics of other drugs are unlikely to occur. Tenofovir has been reported to be associated with a low risk of nephrotoxicity.89,93 Emerging data have shown that entecavir is safe in patients with cancer receiving chemotherapy and in recipients of solid organ or stem cell transplantation. A nonrandomized study of HBsAg-positive patients with lymphoma showed that prophylactic therapy with entecavir (n = 34) was associated with markedly lower rates of hepatitis (6% versus 27%), HBV reactivation (0% versus 12%), and chemotherapy interruptions (6% versus 20%) compared with lamivudine (n = 89).94 The only adverse events in the entecavir group were fatigue and anorexia in two patients. A randomized controlled trial of HBsAg-negative–anti-HBc-positive patients receiving chemotherapy that included an anti-CD20 agent found lower rates of HBV reactivation in the entecavir prophylaxis group than the control group who received therapeutic entecavir once HBV reactivation was noted (2% versus 18%, P <0.05).18 Other smaller studies have confirmed the safety and efficacy of entecavir as preventative therapy for HBV reactivation in HBsAg-negative–anti-HBc-positive patients with haematological malignancies.26,95 Entecavir has also been extensively studied and shown to be safe and effective as prophylaxis in HBsAg-positive patients waiting for or after liver transplantation.96
Although strong data support that antiviral therapy is more efficacious when initiated prior to or at the start of immunosuppressive therapy, fewer data are available to inform when antiviral therapy can be stopped. In one study,46 HBsAg-positive patients with haematological malignancies received prophylactic lamivudine until at least 3 months after completion of chemotherapy;11 patients experienced HBV reactivation after antiviral therapy was stopped.97 The cumulative probability of HBV reactivation after lamivudine withdrawal at 3, 6, 12, 18, and 36 months was 0%, 2%, 5%, 13%, 16%, and 33%, respectively.97 High levels of serum HBV DNA (≥4 log10 copies/ml) before chemotherapy was the most important predictor of HBV reactivation after withdrawal of prophylactic antiviral therapy.97 Patients receiving rituximab have an increased risk of delayed reactivation after cessation of chemotherapy. In one study of 80 HBsAg-negative–anti-HBc-positive patients with lymphoma randomly assigned to entecavir prophylaxis or no prophylaxis (control), patients in the control group developed reactivation as late as 17 months after the start of rituximab, or ~11 months after the cessation of rituximab therapy.18 In a smaller study of four HBsAg-positive patients with lymphoma who received lamivudine prophylaxis before and until 1 month after cessation of CHOP plus rituximab, delayed reactivation occurred ~6–8 months after chemotherapy in three patients.98 These data suggest that prophylactic antiviral therapy should be continued for at least 6 months and preferably 12 months after the cessation of chemotherapy in both HBsAg-positive and HBsAg-negative–anti-HBc-positive patients. Prophylactic antiviral therapy should be continued until the therapeutic end point for chronic hepatitis B is achieved in patients who are HBsAg-positive with high viral load prior to start of chemotherapy.
Treatment
Studies comparing prophylactic versus pre-emptive antiviral therapy showed that deferring antiviral therapy until after elevation of ALT or HBV DNA levels has occurred is less effective in preventing progression to liver failure.18,85,86 Clinical signs of poor prognosis include jaundice, encephalopathy, ascites, elevated bilirubin levels or prolonged prothrombin time. Although a few cases of successful liver transplantation have been reported, many of these patients died because their underlying diseases often precluded them from being considered for liver transplantation.99 Referral to a liver transplantation centre should be considered for all patients with clinical signs of liver failure, but the benefits of liver transplantation must be weighed against the prognosis of the underlying cancer or other medical condition for which immunosuppressive therapy was prescribed.
Recommendations
HBV reactivation can occur in a variety of clinical settings. Although potentially fatal, HBV reactivation is highly preventable through screening with a simple blood test and administration of one pill a day as preventative antiviral therapy for patients with moderate or high risk of HBV reactivation. On the basis of varying levels of risk, we propose a stratification framework (Table 2) and an algorithm for the management of patients with hepatitis B who require immunosuppressive therapy (Figure 3). Because of the difficulty in identifying patients with risk factors for HBV infection and until stronger data support otherwise, we recommend screening all patients about to start immunosuppressive therapy with HBsAg and anti-HBc tests. We recommend prophylactic antiviral therapy for all patients who are deemed to be at high risk of HBV reactivation. Patients considered to be at moderate risk can receive prophylactic antiviral therapy, or they can be closely monitored and pre-emptive antiviral therapy initiated if there is evidence of HBV reactivation. Patients at low risk of HBV reactivation can receive usual medical care, and pre-emptive antiviral therapy initiated if there is evidence of HBV reactivation. We prefer entecavir over tenofovir because of the lack of nephrotoxicity. Lamivudine could be used in patients with undetectable serum HBV DNA before the start of immunosuppressive therapy and if the anticipated duration of use is short (for example, <12 months) to avoid resistance. We recommend starting prophylactic antiviral therapy as soon as possible, preferably before the start of immunosuppressive therapy. We do not believe that it is necessary to delay the start of immunosuppressive therapy except in patients with high baseline serum HBV DNA levels (for example, >4 log10 IU/ml); for these patients, the benefit of delaying immunosuppressive therapy until HBV DNA level is suppressed must be weighed against the risk of progression of the underlying medical condition. We recommend continuing preventative antiviral therapy for at least 6 months after the completion of immunosuppressive therapy and even longer for those who receive rituximab or who had high serum HBV DNA levels before the start of immunosuppressive therapy. Our recommendations are based on evidence in the literature supplemented with our clinical experience. We recognize there are many grey areas, in particular, risk stratification, management of moderate-risk patients and frequency of monitoring. Collaboration across multiple disciplines is needed to conduct properly designed, adequately powered studies to clarify these issues such that evidence-based guidelines will be accepted by all professional societies and adopted by physicians managing patients infected with HBV who require immunosuppressive therapy.
Table 2.
Risk stratification for HBV reactivation
| HBsAg+ | HBsAg− and anti-HBc+* | Antiviral therapy | |
|---|---|---|---|
| High risk | Chemotherapy Anti-CD20 and/or anti-CD52 agents IST for transplantation (stem cell, solid organ) Steroids in combination with other IST |
Chemotherapy for haematological malignancies Anti-CD20 and/or anti-CD52 agents |
Prophylaxis |
| Moderate risk | Anti-TNF agents; Maintenance low dose steroids alone‡; Other IST without steroids‡ |
Chemotherapy for solid tumours‡; IST for transplantation (stem cell, solid organ)‡; Steroids in combination with other IST‡ |
Prophylaxis or pre-emptive |
| Low risk | Steroids alone for a few days‡ | Anti-TNF agent‡; maintenance on low-dose steroids alone‡; other immunosuppressive therapy without steroids‡ |
No prophylaxis |
Risk of HBV reactivation in HBsAg-/anti-HBc+ patients with detectable serum HBV DNA at baseline should be considered same as HBsAg+ patients.
HBV reactivation has been reported in these settings but there is limited data to classify risk.
Abbreviations: +, positive; −, negative; anti-HBc, anti-hepatitis B core antibody IgG; HBsAg, hepatitis B surface antigen; IST, immunosuppressive therapy.
Figure 3.

A management algorithm for patients with HBV infection prior to starting immunosuppressive therapy. Algorithm based on scientific literature when available and opinions of the authors when literature lacked data. *Risk: stratify patients by risk of reactivation (see Table 2). ‡Prophylactic antiviral therapy: patients at high or moderate risk of reactivation (see Table 2) should start antiviral therapy and be assessed for virologic response through HBV DNA testing every 3 months. §Monitoring: check HBV DNA and ALT every 3 months; check HBsAg in patients who were HBsAg−/anti-HBc+ before immunosuppressive therapy. II Pre-emptive antiviral therapy: initiate antiviral therapy if ALT levels are elevated, HBV DNA levels are elevated, or appearance of HBV DNA or HBsAg in patients who had undetectable HBV DNA or HBsAg before immunosuppressive therapy, respectively. Abbreviations: +, positive; −, negative; anti-HBc, anti-hepatitis B core antibody IgG; HBsAg, hepatitis B surface antigen.
Conclusions
Imprecision surrounding the nomenclature of HBV reactivation has contributed to confusion regarding its incidence. Standardization of terminology and definitions is needed to provide precise estimates of the incidence and risk factors for HBV reactivation in different clinical settings. Screening for HBV prior to the start of immunosuppressive therapy is the key to preventing reactivation of HBV infection, and large, collaborative population-based studies are needed to determine efficient methods of screening and subsequent antiviral prophylaxis in order to prevent HBV reactivation.
Key points.
Imprecision surrounding the nomenclature of HBV reactivation has contributed to confusion regarding its incidence; standardization of terminology and definitions are needed
Patients positive for hepatitis B surface antigen (HBsAg) and those who are HBsAg-negative and anti-HBc (hepatitis B core antibody IgG)-positive are at risk of HBV reactivation during immunosuppressive therapies
Level of risk of HBV reactivation depends not only on the serological profile of the patient, but also their underlying medical conditions and the immunosuppressive therapies that will be used
Strong evidence exists to support antiviral prophylaxis to prevent reactivation of HBV infection; in general, HBsAg-positive patients should be started on antiviral prophylaxis before immunosuppression
HBsAg-negative–anti-HBc-positive patients receiving immunosuppressive therapy might be monitored unless they are considered for certain therapies (such as anti-CD20 antibodies), in which case, these patients should receive antiviral prophylaxis
Screening for HBV prior to the start of immunosuppressive therapy is the key to preventing reactivation of HBV infection
Abbreviations
- ALT
alanine aminotransferase
- HBsAg
hepatitis B surface antigen
Footnotes
Review criteria
Relevant articles for this review were identified by searching PubMed, Embase, Ovid Medline and Scopus using the following terms, alone and in combination: “immunosuppression”, “immunocompromised host”, “immunosuppressive agents”, “hepatitis B”, “hepatitis B virus”, “HBV”, “reactivation”, and “management”. The authors also manually reviewed the bibliographies to identify additional relevant articles.
Competing interests
S.-F. Lok declares an association with the following companies: Bristol–Myers Squibb, Gilead, GlaxoSmithKline, Novartis. Please see the article online for full details of the relationships. J. P. Hwang declares no competing interests.
Contributor Information
Jessica P. Hwang, Department of General Internal Medicine, Division of Internal Medicine, The University of Texas MD Anderson Cancer Center, 1400 Pressler Street, Houston, TX 77030, USA
Anna S.-F. Lok, Department of Internal Medicine and Gastroenterology, A. Alfred Taubman Health Care Center, University of Michigan, 1500 East Medical Center Drive, Room 3912, Ann Arbor, MI 48109–5362, USA
References
- 1.Keam B, Lee JH, Im SA, Yoon JH. Why, when, and how to prevent hepatitis B virus reactivation in cancer patients undergoing chemotherapy. J Natl Compr Canc Netw. 2011;9:465–77. doi: 10.6004/jnccn.2011.0045. [DOI] [PubMed] [Google Scholar]
- 2.Vassilopoulos D, Calabrese LH. Management of rheumatic disease with comorbid HBV or HCV infection. Nature Reviews Rheumatology. 2012;8:348–57. doi: 10.1038/nrrheum.2012.63. [DOI] [PubMed] [Google Scholar]
- 3.Chan TM, et al. Preemptive lamivudine therapy based on HBV DNA level in HBsAg-positive kidney allograft recipients. Hepatology. 2002;36:1246–52. doi: 10.1053/jhep.2002.36156. [DOI] [PubMed] [Google Scholar]
- 4.Vigano M, Degasperi E, Aghemo A, Lampertico P, Colombo M. Anti-TNF drugs in patients with hepatitis B or C virus infection: Safety and clinical management. Expert Opinion on Biological Therapy. 2012;12:193–207. doi: 10.1517/14712598.2012.646986. [DOI] [PubMed] [Google Scholar]
- 5.Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009;49:S156–65. doi: 10.1002/hep.22945. [DOI] [PubMed] [Google Scholar]
- 6.Loomba R, et al. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med. 2008;148:519–28. doi: 10.7326/0003-4819-148-7-200804010-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran TT, Rakoski MO, Martin P, Poordad F. Screening for hepatitis B in chemotherapy patients: survey of current oncology practices. Aliment Pharmacol Ther. 2010;31:240–6. doi: 10.1111/j.1365-2036.2009.04158.x. [DOI] [PubMed] [Google Scholar]
- 8.Day FL, Link E, Thursky K, Rischin D. Current hepatitis B screening practices and clinical experience of reactivation in patients undergoing chemotherapy for solid tumors: a nationwide survey of medical oncologists. J Oncol Pract. 2011;7:141–7. doi: 10.1200/JOP.2010.000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinbaum CM, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1–20. [PubMed] [Google Scholar]
- 10.EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–85. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Liaw YF, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: A 2012 update. Hepatology International. 2012;6:531–561. doi: 10.1007/s12072-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 12.Lok ASF, McMahon BJ. AASLD Practice Guideline Update: Chronic Hepatitis B: Update 2009. 2009 Available at: http://www.aasld.org/practiceguidelines/Documents/Bookmarked%20Practice%20Guidelines/Chronic_Hep_B_Update_2009%208_24_2009.pdf Accessed May 4, 2013.
- 13.Artz AS, et al. American Society of Clinical Oncology provisional clinical opinion: chronic hepatitis B virus infection screening in patients receiving cytotoxic chemotherapy for treatment of malignant diseases. J Clin Oncol. 2010;28:3199–202. doi: 10.1200/JCO.2010.30.0673. [DOI] [PubMed] [Google Scholar]
- 14.Baden LR. Prevention and treatment of cancer-related infections. J Natl Compr Canc Netw. 2012;10:1412–45. doi: 10.6004/jnccn.2012.0146. [DOI] [PubMed] [Google Scholar]
- 15.Chu CJ, Hussain M, Lok AS. Quantitative serum HBV DNA levels during different stages of chronic hepatitis B infection. Hepatology. 2002;36:1408–15. doi: 10.1053/jhep.2002.36949. [DOI] [PubMed] [Google Scholar]
- 16.Fong TL, Di Bisceglie AM, Gerber MA, Waggoner JG, Hoofnagle JH. Persistence of hepatitis B virus DNA in the liver after loss of HBsAg in chronic hepatitis B. Hepatology. 1993;18:1313–8. [PubMed] [Google Scholar]
- 17.Rehermann B, Ferrari C, Pasquinelli C, Chisari FV. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996;2:1104–8. doi: 10.1038/nm1096-1104. [DOI] [PubMed] [Google Scholar]
- 18.Huang YH, et al. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol. 2013;31:2765–72. doi: 10.1200/JCO.2012.48.5938. [DOI] [PubMed] [Google Scholar]
- 19.Hsu C, et al. Chemotherapy-induced hepatitis B reactivation in lymphoma patients with resolved HBV infection: A prospective study. Hepatology. 2013 doi: 10.1002/hep.26718. [DOI] [PubMed] [Google Scholar]
- 20.Chang JJ, Lewin SR. Immunopathogenesis of hepatitis B virus infection. Immunol Cell Biol. 2007;85:16–23. doi: 10.1038/sj.icb.7100009. [DOI] [PubMed] [Google Scholar]
- 21.Tur-Kaspa R, Burk RD, Shaul Y, Shafritz DA. Hepatitis B virus DNA contains a glucocorticoid-responsive element. Proc Natl Acad Sci U S A. 1986;83:1627–31. doi: 10.1073/pnas.83.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carroll MB, Forgione MA. Use of tumor necrosis factor alpha inhibitors in hepatitis B surface antigen-positive patients: a literature review and potential mechanisms of action. Clin Rheumatol. 2010;29:1021–9. doi: 10.1007/s10067-010-1523-2. [DOI] [PubMed] [Google Scholar]
- 23.Lalazar G, Rund D, Shouval D. Screening, prevention and treatment of viral hepatitis B reactivation in patients with haematological malignancies. Br J Haematol. 2007;136:699–712. doi: 10.1111/j.1365-2141.2006.06465.x. [DOI] [PubMed] [Google Scholar]
- 24.Lok AS, et al. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology. 1991;100:182–8. doi: 10.1016/0016-5085(91)90599-g. [DOI] [PubMed] [Google Scholar]
- 25.Evens AM, et al. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: meta-analysis and examination of FDA safety reports. Ann Oncol. 2011;22:1170–80. doi: 10.1093/annonc/mdq583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammond SP, et al. Hepatitis B virus reactivation following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:1049–59. doi: 10.1016/j.bbmt.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Yeo W, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol. 2000;62:299–307. doi: 10.1002/1096-9071(200011)62:3<299::aid-jmv1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 28.Yeo W, et al. Hepatitis B reactivation in patients with hepatocellular carcinoma undergoing systemic chemotherapy. Ann Oncol. 2004;15:1661–6. doi: 10.1093/annonc/mdh430. [DOI] [PubMed] [Google Scholar]
- 29.Jang JW, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology. 2006;43:233–40. doi: 10.1002/hep.21024. [DOI] [PubMed] [Google Scholar]
- 30.Hui CK, et al. Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytotoxic chemotherapy. Gastroenterology. 2006;131:59–68. doi: 10.1053/j.gastro.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Hsiao L-T, et al. Extended lamivudine therapy against hepatitis B virus infection in hematopoietic stem cell transplant recipients. Biology of Blood and Marrow Transplantation. 2006;12:84–94. doi: 10.1016/j.bbmt.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 32.American Association for the Study of Liver Diseases Emerging Trends Conference, Reactivation of Hepatitis B; March 21–22, 2013; Arlington, VA. 2013. [Google Scholar]
- 33.Yeo W, et al. Hepatitis B virus reactivation in breast cancer patients receiving cytotoxic chemotherapy: a prospective study. J Med Virol. 2003;70:553–61. doi: 10.1002/jmv.10430. [DOI] [PubMed] [Google Scholar]
- 34.Yun J, et al. Prophylactic use of lamivudine for hepatitis B exacerbation in post-operative breast cancer patients receiving anthracycline-based adjuvant chemotherapy. Br J Cancer. 2011;104:559–63. doi: 10.1038/bjc.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeo W, et al. Comprehensive analysis of risk factors associating with hepatitis B virus (HBV) reactivation in cancer patients undergoing cytotoxic chemotherapy. Br J Cancer. 2004;90:1306–11. doi: 10.1038/sj.bjc.6601699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–42. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 37.Cescon M, Cucchetti A, Ravaioli M, Pinna AD. Hepatocellular carcinoma locoregional therapies for patients in the waiting list. Impact on transplantability and recurrence rate. J Hepatol. 2013;58:609–18. doi: 10.1016/j.jhep.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 38.Golfieri R, et al. Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (<5 cm) hepatocellular carcinomas. Hepatology. 2011;53:1580–1589. doi: 10.1002/hep.24246. [DOI] [PubMed] [Google Scholar]
- 39.Lao X-M, et al. Changes in hepatitis B virus DNA levels and liver function after transcatheter arterial chemoembolization of hepatocellular carcinoma. Hepatology Research. 2011;41:553–563. doi: 10.1111/j.1872-034X.2011.00796.x. [DOI] [PubMed] [Google Scholar]
- 40.Gupta V, Lazarus HM, Keating A. Myeloablative conditioning regimens for AML allografts: 30 years later. Bone Marrow Transplant. 2003;32:969–78. doi: 10.1038/sj.bmt.1704285. [DOI] [PubMed] [Google Scholar]
- 41.Wahid SF. Indications and outcomes of reduced-toxicity hematopoietic stem cell transplantation in adult patients with hematological malignancies. Int J Hematol. 2013;97:581–98. doi: 10.1007/s12185-013-1313-0. [DOI] [PubMed] [Google Scholar]
- 42.Lau GK, Liang R, Chiu EK, Lee CK, Lam SK. Hepatic events after bone marrow transplantation in patients with hepatitis B infection: a case controlled study. Bone Marrow Transplant. 1997;19:795–9. doi: 10.1038/sj.bmt.1700744. [DOI] [PubMed] [Google Scholar]
- 43.Park S, et al. Changes of hepatitis B virus serologic status after allogeneic hematopoietic stem cell transplantation and impact of donor immunity on hepatitis B virus. Biol Blood Marrow Transplant. 2011;17:1630–7. doi: 10.1016/j.bbmt.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Seth P, et al. Hepatitis B virus reactivation with clinical flare in allogeneic stem cell transplants with chronic graft-versus-host disease. Bone Marrow Transplant. 2002;30:189–94. doi: 10.1038/sj.bmt.1703614. [DOI] [PubMed] [Google Scholar]
- 45.Vigano M, et al. Risk of hepatitis B surface antigen seroreversion after allogeneic hematopoietic SCT. Bone Marrow Transplant. 2011;46:125–31. doi: 10.1038/bmt.2010.70. [DOI] [PubMed] [Google Scholar]
- 46.Ramos CA, et al. Resolved hepatitis B virus infection is not associated with worse outcome after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:686–94. doi: 10.1016/j.bbmt.2009.12.532. [DOI] [PubMed] [Google Scholar]
- 47.Liu CJ, et al. Lamivudine treatment for hepatitis B reactivation in HBsAg carriers after organ transplantation: a 4-year experience. J Gastroenterol Hepatol. 2001;16:1001–8. doi: 10.1046/j.1440-1746.2001.02532.x. [DOI] [PubMed] [Google Scholar]
- 48.Dusheiko G, et al. Natural history of hepatitis B virus infection in renal transplant recipients–a fifteen-year follow-up. Hepatology. 1983;3:330–6. doi: 10.1002/hep.1840030309. [DOI] [PubMed] [Google Scholar]
- 49.Mathurin P, et al. Impact of hepatitis B and C virus on kidney transplantation outcome. Hepatology. 1999;29:257–63. doi: 10.1002/hep.510290123. [DOI] [PubMed] [Google Scholar]
- 50.Kalia H, Fabrizi F, Martin P. Hepatitis B virus and renal transplantation. Transplant Rev (Orlando) 2011;25:102–9. doi: 10.1016/j.trre.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 51.Berger A, Preiser W, Kachel HG, Sturmer M, Doerr HW. HBV reactivation after kidney transplantation. J Clin Virol. 2005;32:162–5. doi: 10.1016/j.jcv.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Chen GD, Gu JL, Qiu J, Chen LZ. Outcomes and risk factors for hepatitis B virus (HBV) reactivation after kidney transplantation in occult HBV carriers. Transpl Infect Dis. 2013;15:300–5. doi: 10.1111/tid.12065. [DOI] [PubMed] [Google Scholar]
- 53.Grossi P, et al. Prevalence and outcome of hepatitis B virus (HBV) infection following thoracic organ transplantation. J Heart Lung Transplant. 2001;20:179. doi: 10.1016/s1053-2498(00)00362-4. [DOI] [PubMed] [Google Scholar]
- 54.Lunel F, et al. Hepatitis virus infections in heart transplant recipients: epidemiology, natural history, characteristics, and impact on survival. Gastroenterology. 2000;119:1064–74. doi: 10.1053/gast.2000.17951. [DOI] [PubMed] [Google Scholar]
- 55.Shitrit AB, et al. Lamivudine prophylaxis for hepatitis B virus infection after lung transplantation. Ann Thorac Surg. 2006;81:1851–2. doi: 10.1016/j.athoracsur.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 56.Zampino R, et al. Heart transplantation in patients with chronic hepatitis B: clinical evolution, molecular analysis, and effect of treatment. Transplantation. 2005;80:1340–3. doi: 10.1097/01.tp.0000176941.21438.95. [DOI] [PubMed] [Google Scholar]
- 57.Gea-Banacloche JC. Rituximab-associated infections. Seminars in Hematology. 2010;47:187–98. doi: 10.1053/j.seminhematol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 58.Yeo W, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009;27:605–11. doi: 10.1200/JCO.2008.18.0182. [DOI] [PubMed] [Google Scholar]
- 59.U.S. Food and Drug Administration. Approval History for Rituximab. 1997 Available at: http://www.accessdata.fda.gov/drugsatfda_docs/appletter/1997/ritugen112697L.htm. Accessed October 2, 2013.
- 60.U.S. Food and Drug Administration. Approval History for Arzerra. 2009 Available at: http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2009/125326s000ltr.pdf. Accessed October 2, 2013.
- 61.Nayernama A, et al. Hepatitis B virus reactivation in CD20 antibody-treated patients: evaluation of post-market data from the FDA adverse event reporting system (abst. #8). American Association for the Study of Liver Diseases Emerging Trends Conference, Reactivation of Hepatitis B; March 21–22, 2013; Arlington, VA. 2013. [Google Scholar]
- 62.FDA Drug Safety Communication: Boxed Warning and new recommendations to decrease risk of hepatitis B reactivation with the immune-suppressing and anti-cancer drugs Arzerra (ofatumumab) and Rituxan (rituximab) 2013. [Google Scholar]
- 63.U.S. Food and Drug Administration. Label Information for RITUXIMAB. 2013 Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Overview&amo;DrugName=RITUXAN&CFID=16109442&CFTOKEN=7198ecbe25123dfd-C527B8E7-D68E-3DF9-679D44F79131A718.
- 64.U.S. Food and Drug Administration. Label Information for ARZERRA. 2013 Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#labelinfo. Accessed October 2, 2013.
- 65.Iannitto E, et al. Hepatitis B virus reactivation and alemtuzumab therapy. Eur J Haematol. 2005;74:254–8. doi: 10.1111/j.1600-0609.2004.00375.x. [DOI] [PubMed] [Google Scholar]
- 66.Moses SE. Lamivudine prophylaxis and treatment of hepatitis B Virus-exposed recipients receiving reduced intensity conditioning hematopoietic stem cell transplants with alemtuzumab. J Med Virol. 2006;78:1560–3. doi: 10.1002/jmv.20705. [DOI] [PubMed] [Google Scholar]
- 67.Perez-Alvarez R, et al. Hepatitis B virus (HBV) reactivation in patients receiving tumor necrosis factor (TNF)-targeted therapy: analysis of 257 cases. Medicine (Baltimore) 2011;90:359–71. doi: 10.1097/MD.0b013e3182380a76. [DOI] [PubMed] [Google Scholar]
- 68.Scullard GH, Smith CI, Merigan TC, Robinson WS, Gregory PB. Effects of immunosuppressive therapy on viral markers in chronic active hepatitis B. Gastroenterology. 1981;81:987–91. [PubMed] [Google Scholar]
- 69.Hoofnagle JH, et al. A short course of prednisolone in chronic type B hepatitis. Report of a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1986;104:12–7. doi: 10.7326/0003-4819-104-1-12. [DOI] [PubMed] [Google Scholar]
- 70.Sheen IS, Liaw YF, Lin SM, Chu CM. Severe clinical rebound upon withdrawal of corticosteroid before interferon therapy: incidence and risk factors. J Gastroenterol Hepatol. 1996;11:143–7. doi: 10.1111/j.1440-1746.1996.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 71.Cheng AL, et al. Steroid-free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV-carriers with lymphoma. Hepatology. 2003;37:1320–8. doi: 10.1053/jhep.2003.50220. [DOI] [PubMed] [Google Scholar]
- 72.Flowers MA, et al. Fulminant hepatitis as a consequence of reactivation of hepatitis B virus infection after discontinuation of low-dose methotrexate therapy. Ann Intern Med. 1990;112:381–2. doi: 10.7326/0003-4819-112-5-381. [DOI] [PubMed] [Google Scholar]
- 73.Droz N, et al. Kinetic profiles and management of hepatitis B virus reactivation in patients with immune-mediated inflammatory diseases. Arthritis Care Res (Hoboken) 2013 doi: 10.1002/acr.21990. [DOI] [PubMed] [Google Scholar]
- 74.Liang RH, et al. Hepatitis B infection in patients with lymphomas. Hematol Oncol. 1990;8:261–70. doi: 10.1002/hon.2900080504. [DOI] [PubMed] [Google Scholar]
- 75.Yeo W, Chan HL. Hepatitis B virus reactivation associated with anti-neoplastic therapy. J Gastroenterol Hepatol. 2013;28:31–7. doi: 10.1111/j.1440-1746.2012.07280.x. [DOI] [PubMed] [Google Scholar]
- 76.Hsu CH, et al. Doxorubicin activates hepatitis B virus (HBV) replication in HBV-harboring hepatoblastoma cells. A possible novel mechanism of HBV reactivation in HBV carriers receiving systemic chemotherapy. Anticancer Res. 2004;24:3035–40. [PubMed] [Google Scholar]
- 77.Lau GK, et al. High hepatitis B virus (HBV) DNA viral load as the most important risk factor for HBV reactivation in patients positive for HBV surface antigen undergoing autologous hematopoietic cell transplantation. Blood. 2002;99:2324–30. doi: 10.1182/blood.v99.7.2324. [DOI] [PubMed] [Google Scholar]
- 78.Ferraro D, et al. Evaluating the risk of hepatitis B reactivation in patients with haematological malignancies: is the serum hepatitis B virus profile reliable? Liver Int. 2009;29:1171–7. doi: 10.1111/j.1478-3231.2009.02071.x. [DOI] [PubMed] [Google Scholar]
- 79.Kanaan N, et al. Significant rate of hepatitis B reactivation following kidney transplantation in patients with resolved infection. Journal of Clinical Virology. 2012;55:233–238. doi: 10.1016/j.jcv.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 80.Onozawa M, et al. Progressive disappearance of anti-hepatitis B surface antigen antibody and reverse seroconversion after allogeneic hematopoietic stem cell transplantation in patients with previous hepatitis B virus infection. Transplantation. 2005;79:616–9. doi: 10.1097/01.tp.0000151661.52601.fb. [DOI] [PubMed] [Google Scholar]
- 81.Colvin HM, Mitchel AE. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. Institute of Medicine; 2010. [PubMed] [Google Scholar]
- 82.Hatzakis A, et al. The state of hepatitis B and C in Europe: report from the hepatitis B and C summit conference. J Viral Hepat. 2011;18(Suppl 1):1–16. doi: 10.1111/j.1365-2893.2011.01499.x. [DOI] [PubMed] [Google Scholar]
- 83.Hwang JP, et al. Low rates of hepatitis B virus screening at the onset of chemotherapy. J Oncol Pract. 2012;8:e32–9. doi: 10.1200/JOP.2011.000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McQuillan GM, et al. Prevention of perinatal transmission of hepatitis B virus: the sensitivity, specificity, and predictive value of the recommended screening questions to detect high-risk women in an obstetric population. Am J Epidemiol. 1987;126:484–91. doi: 10.1093/oxfordjournals.aje.a114680. [DOI] [PubMed] [Google Scholar]
- 85.Lau GK, et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125:1742–9. doi: 10.1053/j.gastro.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 86.Hsu C, et al. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin’s lymphoma: a randomized trial. Hepatology. 2008;47:844–53. doi: 10.1002/hep.22106. [DOI] [PubMed] [Google Scholar]
- 87.Lan JL, et al. Kinetics of viral loads and risk of hepatitis B virus reactivation in hepatitis B core antibody-positive rheumatoid arthritis patients undergoing anti-tumour necrosis factor alpha therapy. Ann Rheum Dis. 2011;70:1719–25. doi: 10.1136/ard.2010.148783. [DOI] [PubMed] [Google Scholar]
- 88.Vassilopoulos D, et al. Long-term safety of anti-TNF treatment in patients with rheumatic diseases and chronic or resolved hepatitis B virus infection. Ann Rheum Dis. 2010;69:1352–5. doi: 10.1136/ard.2009.127233. [DOI] [PubMed] [Google Scholar]
- 89.Marcellin P, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–75. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 90.Tenney DJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology. 2009;49:1503–14. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 91.Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet. 2004;43:595–612. doi: 10.2165/00003088-200443090-00003. [DOI] [PubMed] [Google Scholar]
- 92.Weiss J, Weis N, Ketabi-Kiyanvash N, Storch CH, Haefeli WE. Comparison of the induction of P-glycoprotein activity by nucleotide, nucleoside, and non-nucleoside reverse transcriptase inhibitors. Eur J Pharmacol. 2008;579:104–9. doi: 10.1016/j.ejphar.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 93.Cooper RD, et al. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51:496–505. doi: 10.1086/655681. [DOI] [PubMed] [Google Scholar]
- 94.Li HR, et al. Comparison of entecavir and lamivudine in preventing hepatitis B reactivation in lymphoma patients during chemotherapy. J Viral Hepat. 2011;18:877–83. doi: 10.1111/j.1365-2893.2010.01386.x. [DOI] [PubMed] [Google Scholar]
- 95.Matsue K, et al. Reactivation of hepatitis B virus after rituximab-containing treatment in patients with CD20-positive B-cell lymphoma. Cancer. 2010;116:4769–76. doi: 10.1002/cncr.25253. [DOI] [PubMed] [Google Scholar]
- 96.Fung J, et al. Entecavir monotherapy is effective in suppressing hepatitis B virus after liver transplantation. Gastroenterology. 2011;141:1212–9. doi: 10.1053/j.gastro.2011.06.083. [DOI] [PubMed] [Google Scholar]
- 97.Hui CK, et al. Hepatitis B reactivation after withdrawal of pre-emptive lamivudine in patients with haematological malignancy on completion of cytotoxic chemotherapy. Gut. 2005;54:1597–603. doi: 10.1136/gut.2005.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dai MS, Chao TY, Kao WY, Shyu RY, Liu TM. Delayed hepatitis B virus reactivation after cessation of preemptive lamivudine in lymphoma patients treated with rituximab plus CHOP. Ann Hematol. 2004;83:769–74. doi: 10.1007/s00277-004-0899-y. [DOI] [PubMed] [Google Scholar]
- 99.Noterdaeme T, et al. Liver transplantation for acute hepatic failure due to chemotherapy-induced HBV reactivation in lymphoma patients. World J Gastroenterol. 2011;17:3069–72. doi: 10.3748/wjg.v17.i25.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]


