Abstract
Objectives
Lymph node counts are a proposed measure of quality assurance for numerous malignancies. Investigation of patient factors associated with lymph node counts are lacking. We sought to determine if body mass index (BMI) is associated with lymph node counts in patients treated with a primary retroperitoneal lymph node dissection (RPLND).
Methods
Using the Memorial Sloan-Kettering Testis Cancer Database, we identified 255 patients treated with a primary RPLND for nonseminomatous germ cell tumors (NSGCT) from 1999–2008. The associations between BMI and node counts were evaluated using linear regression models in univariate and multivariable models adjusting for features reported to predict higher node counts (year of surgery, stage, and surgeon volume).
Results
Median BMI (IQR) was 26.1 (23.4 – 28.7) and median (IQR) total node count was 38 (27–53). Median total node count for patients with a BMI <25, 25–<30, and >30 was 35, 42, and 44 nodes, respectively. In a univariate analysis, higher BMI was significantly associated with higher total node counts (coefficient 0.7 nodes for each 1 unit increase in BMI; p=0.026). Features associated with higher node count on multivariate analysis included high volume surgeon (p=0.047), pathologic stage (p=0.017), more recent year of surgery (p<0.001), and higher BMI (p=0.009).
Conclusion
Our results suggest for the first time that BMI is independently associated with higher lymph node counts during a lymph node dissection. If confirmed by others, these results may be important when using lymph node counts as a surrogate for adequacy of a lymph node dissection.
Keywords: Testicular neoplasms, Lymph nodes, Lymph node excision, Neoplasm staging, Body mass index
Introduction
There is a substantial volume of literature demonstrating that the number of lymph nodes removed is both prognostic and predictive of oncologic outcome for numerous malignancies including bladder,1 lung,2 esophageal,3 pancreatic,4 breast,5 gastric,6 and colon cancers.7,8 For example, it is recommended that at least 10–14 nodes be counted in patients treated without neoadjuvant therapy for proper staging of patients with colorectal cancer.9 In regards to testis cancer, we recently reported our contemporary experience with primary retroperitoneal lymph node dissection (RPLND) for patients with non-seminomatous germ cell tumors (NSGCT).10,11 Our results demonstrated that high volume surgeon, higher clinical stage, and more recent year of surgery were each independently associated with higher lymph node counts.10
Previous observations suggest that higher body mass index (BMI) reduces the efficacy of sentinal lymph node biopsies in the setting of breast cancer.12 In the setting of colorectal cancer, Linebarger et al recently observed no association between BMI and node count after hypothesizing that node count would be less with increasing BMI secondary to difficulty in node identification with a thickened mesentery and restricted access to the origin of the mesentery.13 However, to our knowledge, no previous report has investigated the potential relationship between BMI and total node count following lymphadenectomy for a urologic malignancy. Thus, we sought to determine if BMI is associated with total lymph node counts in patients treated with a primary RPLND. To accomplish this, we identified a previous cohort of patients treated with a primary RPLND for NSGCT over a 10-year period and performed a multivariable analysis adjusting for features known to predict for higher lymph node counts.
Materials and Methods
Patient Selection
After obtaining Institutional Review Board approval, we queried the Memorial Sloan-Kettering Cancer Center (MSKCC) Testis Cancer Registry and identified 273 patients treated with primary RPLND for NSGCT between the years 1999 and 2008. Eighteen patients who did not have a discernible lymph node count (i.e. “multiple benign lymph nodes”) were excluded, leaving 255 patients available for analysis. The MSKCC Testis Cancer Registry is prospectively maintained with >100 variables collected and recorded for all patients who undergo RPLND at MSKCC. For this project, charts were reviewed for all patients to ensure that the location and number of lymph nodes recorded in the registry was accurate.
Operative Boundaries and Pathologic Sectioning
The upper limits of dissection included the skeletonized renal vessels and crus of the diaphragm while lower limits included the external iliac vessels on the ipsilateral side and the bifurcation of the great vessel on the contralateral side. Packets consisting of the paracaval, interaortocaval, paraortic, and ipsilateral iliac lymph nodes were routinely submitted for pathologic examination. Rarely, interiliac nodes or contralateral iliac were removed and submitted separately. At MSKCC, it was routine practice over the study time frame to perform a bilateral template primary RPLND with nerve sparing for patients interested in preserved ejaculation.[13, 14] It should be noted that this practice of a full bilateral template with nerve sparing is distinctly different than other institutions where right- and left- sided templates are frequently utilized to reduce the incidence of ejaculatory dysfunction.
The nodal packets are received in pathology from the operating room in a fresh state. Using visual inspection, palpation and blunt dissection with a scalpel blade, the adipose tissue is dissected free and all firm, rubbery areas suspicious for a lymph node are then separated and submitted for microscopic examination. If no nodes are palpated, the tissue is submitted for microscopic examination in its entirety. Pathologic specimen preparation did not change and fat dissolution techniques were not utilized during the time frame of this study. The nodal count was performed by the Pathology Fellow and Attending Pathologist.
Clinical and Pathologic Features
The clinical and pathologic features studied included BMI, total lymph node count, surgeon, pathologic stage, primary tumor histology, and year of surgery. A high volume surgeon was defined as performing >10 primary RPLND’s over the study time period.
Statistical Analysis
The clinical and pathologic features are summarized with median and interquartile rage (IQR) or frequency and percentage as appropriate. Associations between BMI and lymph node counts were evaluated using linear regression models both in a univariate and a multivariable model adjusting for features previously reported to be independently associated with total node counts10 (year of surgery, surgeon (dichotomized as high volume vs low volume), and pathologic stage (stage I vs II). While multiple different pathologists evaluated node counts over the time frame of this study, we chose not to adjust for pathologist since we have previously demonstrated that pathologist was not significantly associated with node counts in this same data set.10 Statistical analyses were performed with Stata v8.2, all tests were two-sided, and p-values <0.05 were considered statistically significant.
Results
A summary of baseline features for the 255 patients studied is detailed in Table 1. Fifty-two % of patients had right sided primary tumors (n=133) and 47% had left sided primary tumors (n=119), while 3 (1%) patients had bilateral NSGCT. The number of primary RPLND performed per year ranged from 16 to 36. Median (IQR) BMI was 26 (23 – 37) and median (IQR) total node count was 38 (27 – 53). Positive lymph nodes were found in 75 patients, including 56 (75%) with viable tumor only, 10 (13%) with viable tumor and teratoma, and 9 (12%) with teratoma only.
Table 1.
Summary of baseline features for 255 patients treated with primary RPLND for NSGCT.
| Feature | Median | IQR |
|---|---|---|
|
| ||
| Age | 30 | 23–37 |
|
| ||
| BMI | 26 | 23–29 |
|
| ||
| Total node count | 38 | 27–53 |
| Feature | No. | % |
|---|---|---|
|
| ||
| Primary Histology | ||
| Mixed | 210 | 82.4 |
| Pure Embryonal | 41 | 16.0 |
| Pure Teratoma | 3 | 1.2 |
| Pure Malignant Transformation | 1 | 0.4 |
|
| ||
| Clinical Stage | ||
| IA | 26 | 10 |
| IB | 147 | 58 |
| IIA | 82 | 32 |
|
| ||
| pN Stage | ||
| N0 | 180 | 70 |
| N1 | 50 | 20 |
| N2 | 25 | 10 |
|
| ||
| RPLND Histology | ||
| Benign | 180 | 70 |
| Viable GCT (only) | 56 | 22 |
| Viable GCT and Teratoma | 10 | 4 |
| Teratoma (only) | 9 | 4 |
Median total node count for patients with a BMI <25, 25–<30, and >30 was 35, 42, and 44 nodes, respectively. In a univariate analysis, higher BMI was significantly associated with higher total node counts (coefficient 0.7 nodes for each 1 unit increase in BMI; 95% confidence interval 0.1 – 1.2; p=0.026; Figure 1). Year of surgery (p<0.001), high vs. low volume surgeon (p<0.001), and pathologic stage (p=0.027) were also significantly associated with higher total node counts univariately (Table 2). On average, 3.0 additional nodes were counted with each increasing year during the study time frame, 15.0 additional nodes were counted if a high volume surgeon performed the RPLND, and 6.4 additional nodes were counted if positive nodes were found at time of RPLND. In a multivariate analysis, year of surgery (p<0.001), surgeon volume (p=0.047), and pathologic stage (p=0.017) were each independently associated with higher lymph node counts (Table 2). Adjusting for these three features in a multivariate analysis, higher BMI remained significantly associated with higher lymph node counts (p=0.009, Table 2). There was not a significant difference in node count among patients with left vs. right sided primary tumors (coefficient −1.5; 95% confidence interval −6.7 – 3.8; p=0.0.6)
Figure 1.
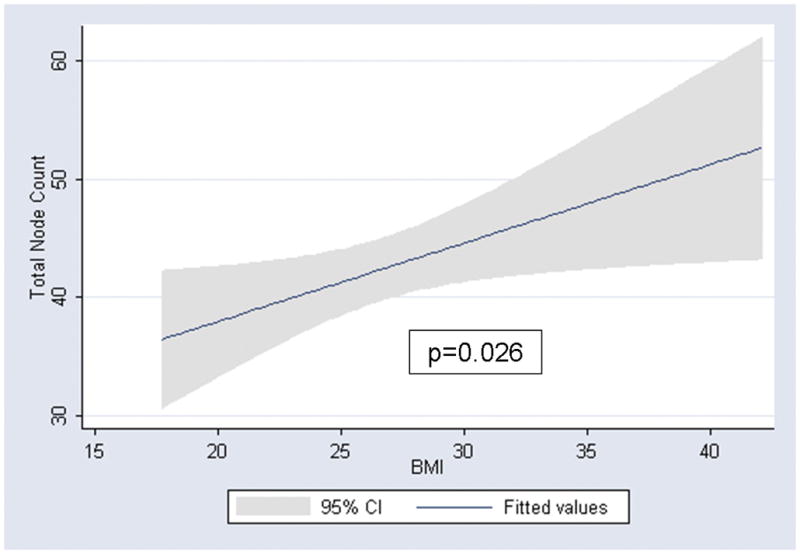
Linear regression association between BMI and total node count
Table 2.
Univariate and multivariate features predictive of higher lymph node counts in 255 patients treated with primary RPLND
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Feature | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value |
| Year of Surgery | 3.0 (2.2 – 3.8) | <0.001 | 2.9 (2.0 – 3.7) | <0.001 |
| Surgeon (High vs Low Volume) | 15.0 (7.0 – 23.0) | <0.001 | 7.6 (0.1 – 15.1) | 0.047 |
| Pathologic stage (pN+ vs pN0) | 6.4 (0.7 – 12.1) | 0.027 | 6.2 (1.1 – 11.2) | 0.017 |
| BMI | 0.7 (0.1 – 1.2) | 0.026 | 0.7 (0.2 – 1.2) | 0.009 |
Discussion
These results, to our knowledge, are the first to demonstrate an association between BMI and lymph node counts for any malignancy. We observed that a higher BMI is significantly associated with higher lymph node counts even after adjusting for known features predictive of node counts in a multivariable analysis (including surgeon volume, year of surgery, and pathologic stage). A strength of this study is that patients were treated with a lymphadenectomy that included well-defined boundaries of dissection including the paracaval, interaortocaval, paraaortic, and ipsilateral iliac lymph nodes. In addition, patients were relatively young and healthy, had not received previous chemotherapy or radiation, and represented an ideal population to test the hypothesis that higher BMI is associated with higher lymph node counts. These results may have important implications if number of lymph nodes are incorporated into future staging systems, are used to establish adequacy of a lymph node dissection, or become a requirement for financial reimbursement.
Colorectal adenocarcinoma is perhaps the malignancy most intimately associated with lymph node counts. As sponsored by the National Cancer Institute in 2000, a panel of experts recommended that a minimum of 12 lymph nodes be evaluated for adequate staging of colon cancer.14 In 2005, the 12-node minimum standard was adopted by the National Comprehensive Cancer Network (NCCN) and 10 – 14 nodes are the reference minimum for the AJCC TNM staging system for patients who have not received neoadjuvant chemotherapy.9 By current guidelines, identification of fewer than 10–14 nodes for patients with colon cancer is regarded as inadequate, considered a risk factor for systemic recurrence, and is an indication for consideration of upstaging for systemic therapy.13 Furthermore, in 2007, the National Quality Forum officially endorsed the first recognized hospital-based measure of lymphadenectomy quality in colorectal cancer care.13
There is scant literature comparing lymph node counts and BMI. In fact, we found only one peer-reviewed manuscript in the literature that specifically addresses the potential association of BMI and lymph node counts.13 In a recent evaluation of lymph node retrieval during colon cancer resection, Linebarger et al studied 401 patients in a community-based health system.13 They hypothesized that extent of lymph node harvest would decrease as BMI increased due to the notion that a thickened mesentery restricts access to the origin of the mesentery and a thick body wall complicates retraction and exposure of the surgical field.13 While their observations did not demonstrate an association between node retrieval and BMI, this group from La Crosse, WI did report a higher node count for right sided colon tumors as compared with the left.13 Additionally, they observed a higher node count among more experienced surgeons which is consistent with our findings.13 A multivariable analysis, accounting for these potentially confounding features however, was not reported by Linebarger et al.13 Despite the obvious differences in biology between colon and testis cancer, we submit that the disparate findings between our observations and those of Linebarger et al are multi-factorial. First, the boundaries of lymphadenectomy for testis cancer patients are well defined and are not altered based on side of primary tumor at our institution. In the report by Linebarger et al, eight different surgical procedures were included in their colon cancer resection (i.e. right hemicolectomy, sigmoid resection, extended right hemicolectomy, transverse resection, low anterior resection, left hemicolectomy, ileocecectomy, and a combination).13 Second, all cases presented herein were performed with an open surgical approach whereas Linebarger et al included open, pure laparoscopic, and hand assisted laparoscopic approaches. Third, the median age of our testis cancer patients was 30, including many patients who have never had a prior abdominal operation, as compared with a mean age of 73 years for the patients with colon cancer. Thus, we submit that our observations represent a near ideal patient population to study the potential association between BMI and node count.
This report is not without limitations. While the data was collected in a prospective fashion, it was analyzed retrospectively and is subject to the many inherent biases associated with this approach. For example, surgical decisions such as performing a more extensive dissection if suspicious nodes are found intra-operatively cannot be accounted for in this analysis. Nevertheless, as part of a quality assurance, the pathology reports for all 273 patients initially identified were reviewed to ensure the accuracy regarding the number and location of lymph node counts. Furthermore, while a majority of RPLND’s at our institution were performed by a single surgeon who utilized similar boundaries of dissection over the entire study time period, node counts clearly increased over the last decade. We believe this observation likely reflects increased emphasis on nodal counts by all pathologists for many malignancies that occurred during the last 10 years.1–8
In conclusion, our results suggest that BMI is independently associated with higher lymph node counts found during a lymph node dissection. These results may be important when using lymph node counts as a surrogate for adequacy of a lymph node dissection and may have important implications if number of lymph nodes are incorporated into future staging systems or become a requirement for financial reimbursement.
Acknowledgments
Support: Sidney Kimmel Foundation for Urologic Cancer and the NIH T32 CA082088
Footnotes
There are no financial disclosures associated with this project
References
- 1.Koppie TM, Vickers AJ, Vora K, et al. Standardization of pelvic lymphadenectomy performed at radical cystectomy: can we establish a minimum number of lymph nodes that should be removed? Cancer. 2006;107:2368–2374. doi: 10.1002/cncr.22250. [DOI] [PubMed] [Google Scholar]
- 2.Ludwig MS, Goodman M, Miller DL, et al. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest. 2005;128:1545–1550. doi: 10.1378/chest.128.3.1545. [DOI] [PubMed] [Google Scholar]
- 3.Bollschweiler E, Baldus SE, Schroder W, et al. Staging of esophageal carcinoma: length of tumor and number of involved regional lymph nodes. Are these independent prognostic factors? J Surg Oncol. 2006;94:355–363. doi: 10.1002/jso.20569. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Ann Surg Oncol. 2006;13:1189–1200. doi: 10.1245/s10434-006-9016-x. [DOI] [PubMed] [Google Scholar]
- 5.Woodward WA, Vinh-Hung V, Ueno NT, et al. Prognostic value of nodal ratios in node-positive breast cancer. J Clin Oncol. 2006;24:2910–2916. doi: 10.1200/JCO.2005.03.1526. [DOI] [PubMed] [Google Scholar]
- 6.Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114–7124. doi: 10.1200/JCO.2005.14.621. [DOI] [PubMed] [Google Scholar]
- 7.Chang GJ, Rodriguez-Bigas MA, Skibber JM, et al. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433–441. doi: 10.1093/jnci/djk092. [DOI] [PubMed] [Google Scholar]
- 8.Ricciardi R, Baxter NN. Association versus causation versus quality improvement: setting benchmarks for lymph node evaluation in colon cancer. J Natl Cancer Inst. 2007;99:414–415. doi: 10.1093/jnci/djk106. [DOI] [PubMed] [Google Scholar]
- 9.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. 7. New York: Springer Science + Business Media LLC; 2010. [Google Scholar]
- 10.Thompson RH, Carver BS, Bosl GJ, et al. Evaluation of lymph node counts in primary retroperitoneal lymph node dissection. Cancer. 2010;116:5243–5250. doi: 10.1002/cncr.25266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson RH, Carver BS, Bosl GJ, et al. Contemporary lymph node counts during primary retroperitoneal lymph node dissection. Urology. 2011;77:368–372. doi: 10.1016/j.urology.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derossis AM, Fey JV, Cody HS, 3rd, et al. Obesity influences outcome of sentinel lymph node biopsy in early-stage breast cancer. J Am Coll Surg. 2003;197:896–901. doi: 10.1016/j.jamcollsurg.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Linebarger JH, Mathiason MA, Kallies KJ, et al. Does obesity impact lymph node retrieval in colon cancer surgery? Am J Surg. 2010;200:478–482. doi: 10.1016/j.amjsurg.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583–596. doi: 10.1093/jnci/93.8.583. [DOI] [PubMed] [Google Scholar]


