Abstract
Custom-designed zinc finger nucleases (ZFNs) are becoming powerful tools in gene targeting—the process of replacing a gene within a genome by homologous recombination. Here, we have studied the DNA cleavage by one such ZFN, ΔQNK-FN, in order to gain insight into how ZFNs cleave DNA and how two inverted sites promote double-strand cleavage. DNA cleavage by ΔQNK-FN is greatly facilitated when two ΔQNK-binding sites are close together in an inverted orientation. Substrate cleavage was not first order with respect to the concentration of ΔQNK-FN, indicating that double-strand cleavage requires dimerization of the FokI cleavage domain. Rates of DNA cleavage decrease as the substrate concentrations increase, suggesting that the ΔQNK-FN molecules are effectively “trapped” in a 1:1 complex on DNA when the DNA is in excess. The physical association of two ZFN monomers on DNA was monitored by using the biotin-pull-down assay, which showed that the formation of ΔQNK-FN active complex required both binding of the two ΔQNK-FN molecules to specific DNA sites and divalent metal ions.
Keywords: Chimeric nucleases, Gene targeting, Gene therapy, Homologous recombination, Sequence-specific cleavage, Zinc finger nucleases, Non-homologous end joining, Protein engineering, Directed mutagenesis, Homology-directed repair
FokI, the archetypical type IIs restriction enzyme, was previously shown to have separable N-terminal DNA-binding and C-terminal endonuclease domains[1-5], and the crystal structures of FokI and FokI complexed with DNA confirm the domain architecture [6,7]. The endonuclease domain by itself has no cleavage specificity. By fusing a DNA-binding domain to the endonuclease domain of FokI, new sequence specificities can be created. Fusion proteins that have been successfully created include the three common eukaryotic DNA-binding motifs: namely, the helix–turn–helix motif, the zinc finger (ZF) motif, and the basic helix–loop–helix protein containing a leucine zipper motif [8-10]. FokI cleavage domain fusions to transcription factors have helped to identify their recruitment to various promoter sites within cells [11-14].
The most extensively studied group of chimeric nucleases is based on the ZF motifs because ZF can be designed or selected to bind a large range of DNA sites[15-18]. The modular structure of FokI endonuclease [2] and the modular nature of ZF DNA-binding motifs [19] have made it possible to design zinc finger nucleases (ZFNs) with tailor-made sequence specificities [20-22]. Previously, we have suggested that binding of two ZFN monomers, each recognizing a 9 bp inverted site, is necessary for the efficient DNA cleavage reaction because dimerization of the FokI catalytic domain is required to produce a DSB. Thus, ZFNs effectively have an 18 bp recognition site [23] long enough to specify a unique genomic address in plants and mammals.
Experiments from several laboratories indicate that ZFNs find and cleave their chromosomal targets in cells; and as expected, they induce local homologous recombination (HR) at the site of cleavage [20,22,24-27] in the presence of an exogenously added donor DNA. ZFNs have also been used to induce “directed” mutagenesis in the absence of donor DNA in Drosophila [28] and in Arabidopsis [29]. These studies provide a glimpse at the potential future applications of ZFNs in modifying and rewiring the plant and mammalian genomes.
Even though ZFN designs based on our original construct have been successfully used to target cleavage of specific sites in vivo, they also appear to be somewhat cytotoxic to cells. Further improvements in sequence specificity and selectivity of ZFNs will likely reduce their cytotoxicity and make them an even more versatile tool for widespread use in gene targeting experiments, particularly for therapeutic applications. A clear understanding of the mechanism of double-strand cleavage by ZFNs is essential for refining and improving their sequence-specific cleavage properties. Here, we have studied the mechanism of double-strand cleavage by one such ZFN, ΔQNK-FN [30], in order to gain insight into how ZFNs cleave DNA and how two inverted sites facilitate double-strand cleavage. We report that DNA cleavage by ΔQNK-FN is not first order with respect to the ΔQNK-FN, indicating that double-strand cleavage by ZFNs requires dimerization of the cleavage domain. This is consistent with a similar higher than first order dependency that was seen for DNA cleavage by FokI restriction enzyme supporting dimerization of the cleavage domain [31]. Biotin-pull-down assay [32] was then used to show physical association of two ZFN monomers in the ΔQNK-FN active complex; this required both binding of the two ZFN molecules to specific DNA sites and divalent metal ions.
Materials and methods
Construction of the substrates pKT8, pKT28, and pKS is described elsewhere [23]. pKT8 has two inverted binding sites separated by 8 bp. pKT28 has two inverted binding sites like pKT8 but 28 bp separating the binding sites. pKS has only a single binding site. The same binding site, d(GGGGCGGAA), was included in all three substrates. The protocol for biotin pull-down assay using ZFN is similar to the one described elsewhere [32]. ΔQNK-FN was purified to homogeneity as described elsewhere [30].
Determining kinetics of DNA cleavage by ΔQNK-FN
Substrates for the kinetics, namely pTK8, pTK28, and pKS, were linearized with Cfr101 so that cleavage at the inserted binding site by ΔQNK-FN would result in two fragments of approximately equal size. For instance, pKT8 would yield 1337 and 1349 bp fragments. On a 1% agarose gel, the two cleavage products run together to provide a strong signal. The 5′ phosphates of the linear plasmids were removed by calf intestinal alkaline phosphatase (CIP). The linear plasmids were radioactively labeled using [α-32P]ATP and T4 polynucleotide kinase. Since the cleavage products are radioactively labeled at only one end of the DNA while the substrate is labeled at both ends, the overlap of cleavage products on the gel also allows a direct comparison of the substrate and product band intensities.
The kinetic experiments were performed using purified ΔQNK-FN. Digests with ΔQNK-FN were performed in 120 μl containing 10 mM Tris, pH 8.5, 1 mM DTT, 100 μM ZnCl2, 50 μg/ml BSA, 100 μg/ml tRNA, 10 mM MgCl2, and 50 mM NaCl. The reactions were pre-incubated at 37 °C before adding the ΔQNK-FN. The protein was added to the reaction, mixed by pipetting up and down, and incubated in a 37 °C water bath. Time points were collected by transferring 18 μl of the digestion reaction to a tube containing 100 μl phenol/chloroform, which was used to quench the reaction at the appropriate time. The quenched reactions from each time point was extracted once with 100 μl chloroform to remove the phenol. Loading dye was added and the samples were run on a 1% agarose gel. The gel was dried, placed on a phosphoimaging screen, and scanned by Molecular Dynamics Storm 860. The computer programs Imagequant, Excel, and KaleidaGraph were used to quantitate and analyze the data. The amount of product produced in each time point was calculated by dividing the intensity of the product band by the sum of the intensities of the substrate band and the product band. The resulting fraction was then multiplied by the amount of substrate initially added to the digest to give a product concentration in nM. Two types of kinetic experiments were run with ΔQNK-FN: the first varied ΔQNK-FN concentration (4, 8, 16, 32, and 64 nM) with a constant concentration of substrate (8 nM pKT28); the second varied the amount of substrate (2, 4, 8, 12, 16, and 24 nM) with a constant concentration of ΔQNK-FN (16 nM). The concentration of product in a fraction was plotted versus the time it was taken in order to determine the rates of the cleavage reactions. The plots were analyzed using two different methods, which gave equivalent results. The first method fit a straight line fixed at zero to the initial time points and the slope of the line was recorded as the rate. In the second method, all of the time points were fit to the following exponential equation: Product = limit * (1 − exp(−k * t)); where t is time and k is the rate. Rates determined by the second method are reported.
Biotin pull-down assay
The protocol for biotin pull-down assay using ZFN is similar to the one described elsewhere [32]. A double-stranded 31 bp oligodeoxyribonucleotide, [d(Biotin-5′-ATCTTCCGCCCCTAGTACTAGAATCAAATAC-3′: 3′-TAGAAGGCGGGGATCATGATCTTAGTTTATG-5′)] containing a single ΔQNK-FN site with a biotin molecule at one end was used to co-precipitate a second 32P-labeled double-stranded 31-mer that contained either a single ΔQNK-FN site [d(32P-5′-ATCAGAATCAAATAGTACTGGGGCGGAATAC-3′: 3′-TAGTCTTAGTTTATCATGACCCCGCCTTATG-5′)] or an entirely random sequence [d(32P-5′-ATCAGAATCAAATAGTACTAGAATCAAATAC-3′: 3′-TAGTCTTAGTTTATCATGATCTTAGTTTATG-5′)] in the presence or absence of ΔQNK-FN and magnesium ions. The 32P-labeled oligonucleotides had a sequence different from that of the biotinylated DNA to avoid annealing between oligonucletide substrates. ΔQNK-FN construct was transcribed and translated in a rabbit reticulocyte lysate (TnT T7 Quick Coupled Transcription/Translation system from Promega-L1170) as recommended by the manufacturer and the generated fusion protein was used in the biotin pull-down assay without further purification. The ΔQNK-FN active complex was isolated using strepavidin-coated beads and the recovered DNA was resolved by denaturing PAGE.
Results
Rates versus ΔQNK-FN concentration support requirement for dimerization
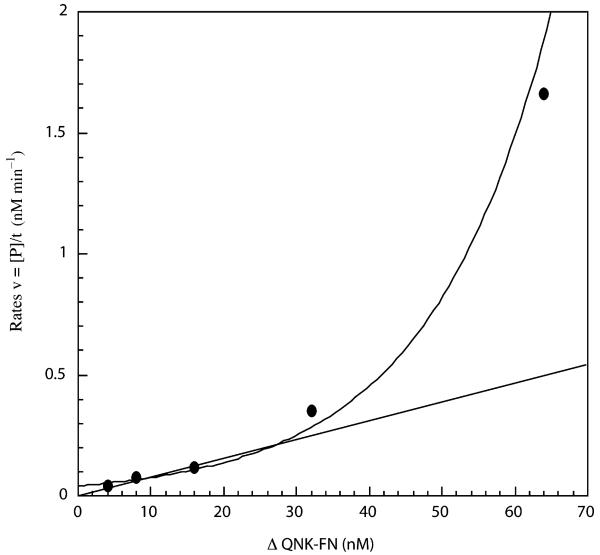
Rates were determined for the cleavage of pKT28 by using 4, 8, 16, 32, and 64 nM ΔQNK-FN. The rates were plotted versus the corresponding ΔQNK-FN concentration (Fig. 1). Rates increase exponentially rather than linearly with increasing protein concentration. The plot indicates that the reaction is higher than first order with respect to ΔQNK-FN, which is consistent with similar findings for FokI endonuclease [31]. The non-linear correlation between double-strand cleavage rates and ΔQNK-FN concentrations provides evidence that, as in the case of FokI endonuclease, ZFN must dimerize through the cleavage domain in order to cut the DNA substrate.
Fig. 1.
Rates of DNA cleavage with various ΔQNK-FN concentrations. A range of ΔQNK-FN concentrations (4, 8, 16, 32, and 64 nM) were used to cleave a fixed concentration of Cfr101-linearized pKT28 (8 nM). The reaction was performed in buffer containing 50 mM NaCl. The rates (v) for the cleavage, determined from the generation of product (P) over time (t), are plotted versus the corresponding ΔQNK-FN concentrations, which indicate the reaction is higher than first order. The line indicates the predicted rates if the reaction was first order with respect to ΔQNK-FN concentrations.
Rates versus substrate concentration
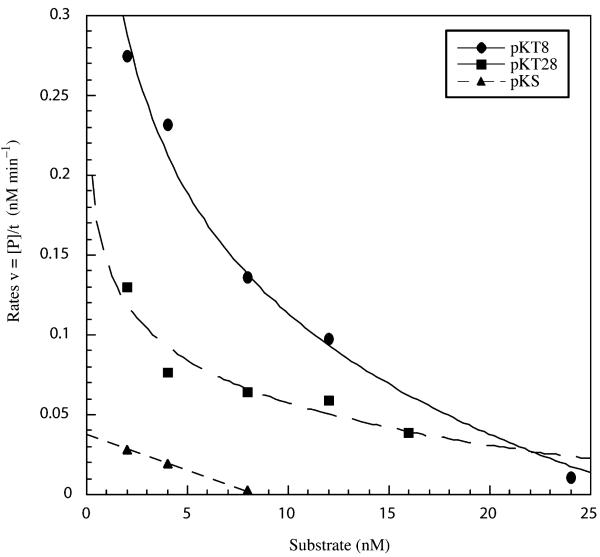
Rates were also determined for cleavage reactions using a constant concentration of ΔQNK-FN (16 nM) and by varying substrate concentrations from 2 to 24 nM. Three different substrates were used: pKT8, pKT28, and pKS. A logarithmic decay was observed for substrates pTK8 and pTK28 when rates were plotted versus the corresponding substrate concentrations (Fig. 2). The rates of cleavage for both pKT8 and pKT28 are much higher than that for pKS, the substrate with a single binding site. The cleavage of the single site is observed only under unusually high concentrations of ΔQNK-FN and even then the cleavage rate is very low probably because there is no second binding site nearby to facilitate dimerization of the cleavage domains. Thus, a pair of binding sites on the same molecule of DNA in an inverted orientation increases double-strand cleavage by facilitating dimerization of the nuclease domains. The rates of cleavage for pKT8 are higher than those for pKT28 (Fig. 2). The faster rate of cleavage for pKT8 versus pKT28 may be due to a closer proximity of the binding sites in pKT8 versus pKT28, which facilitate better dimerization.
Fig. 2.
Rates of DNA cleavage with various concentrations of three different substrates. The ΔQNK-FN concentration in the digests was fixed at 16 nM. The substrate concentrations were varied depending on the substrate: pKT8 (2, 4, 8, 12, and 24 nM); pKT28 (2, 4, 8, 12, and 16 nM); and pKS (2, 4, and 8 nM). The reaction was performed in buffer containing 50 mM NaCl. The rates are plotted versus the substrate concentrations for each substrate: pKT8 (circles), pKT28 (squares), and pKS (triangles).
Biotin pull-down assay
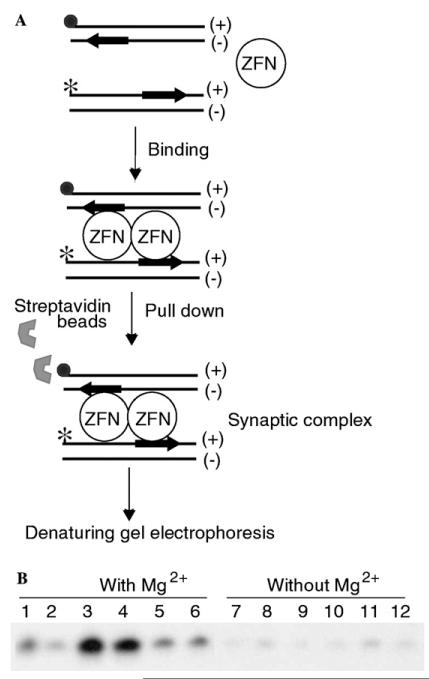
As an independent test for the physical association of two ZFN molecules in the active complex, and as a test of whether they are bound specifically or non-specifically to DNA, we monitored ΔQNK-FN-binding to DNA using the biotin pull-down assay [32]. A double-stranded 31 bp oligodeoxyribonucleotide containing a single ΔQNK site with a biotin molecule at one end was used to co-precipitate a second 32P-labeled double-stranded 31-mer that contained either a single ZFN site or an entirely random sequence in the presence or absence of ΔQNK-FN and magnesium ions. We reasoned that with ZFNs, as seen in the case of FokI endonuclease [32], only the 32P-labeled DNA containing the specific ΔQNK-binding site would be present in the pull-down complex (Fig. 3A). In the absence of ΔQNK-FN, only background levels of co-precipitated DNA will be found. Biotin pull-down assay indicates that the formation of the ΔQNK-FN active complex (Fig. 3B; lanes 3 and 4) requires both specific DNA and magnesium ions. However, the precipitated DNA was not cleaved because the ZFN sites were located on different DNA molecules and the geometry of dimer formation by the nuclease domain likely did not facilitate double-strand cleavage unlike the adjacent inverted repeat ZFN sites on the same molecule of DNA.
Fig. 3.
Biotin pull-down assay. (A) Schematic representation of the biotin pull-down assay. The filled circle represents the biotin tag and the star represents the 32P-label. The substrate encodes the ΔQNK site near the 3′ end of the (+) strand. (B) PAGE profile of the biotin pull-down assay. The biotin-tagged DNA was recovered from the reaction using streptavidin coated magnetic beads. The 32P-labeled DNA from the complex was analyzed by using PAGE. Lanes 1–6 with Mg2+ions; 1, control IVTT (without ZFN plasmid) + specific DNA; 2, control IVTT + non-specific DNA; 3, ΔQNK-FN (1 μl) + specific DNA; 4, ΔQNK-FN (4 μl) + specific DNA; 5, ΔQNK-FN (1 μl) + non-specific DNA; 6, ΔQNK-FN (4 μl) + non-specific DNA; and 7–12, same as lanes 1–6, but without Mg2+ions.
Effect of salt concentration on ΔQNK-FN cleavage activity
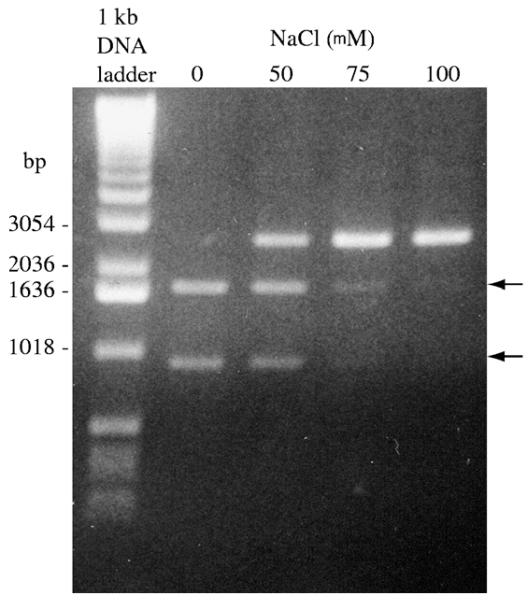
We also investigated the effect of salt concentration on pKT8 cleavage by ΔQNK-FN. Salt concentration appears to have a dramatic effect on the cleavage activity of the fusion protein (Fig. 4). Complete digestion of the substrate was seen when no salt was present in the reaction mixture. DNA cleavage decreases with increasing concentration of NaCl. This is probably because the salt affects the ΔQNK-FNÕs binding affinity for DNA.
Fig. 4.
Factors that affect cleavage activity of ΔQNK-FN. Cleavage of pKT8 using a 4-fold excess of ΔQNK-FN at 37 °C. The concentration of NaCl was varied in the ΔQNK-FN digests of pKT8 as indicated above the lanes. The expected fragments from cleavage reaction are indicated by arrow.
Discussion
Mechanism of double-strand DNA cleavage by ZFNs
In ZFNs, there are no protein–protein interactions between the ZF motifs and the FokI cleavage domain. The ZF motifs of the ZFN monomer interact with the entire recognition sequence and the catalytic domain has the function of cleaving just one strand of DNA. This necessitates dimerization of the cleavage domain to produce a double-strand break. The kinetics of double-strand cleavage by ΔQNK-FN further support the fact that dimerization of the nuclease domains is required for DNA cleavage. The cleavage reactions are higher than first order with respect to the concentration of the ZFN (Fig. 1). A similar result was reported for FokI endonuclease as evidence that it formed dimers to cleave DNA [31]. The authors have suggested that this non-linear relationship is due to the dimerization of two molecules of FokI through their nuclease domains in order to cut DNA.
Two inverted binding sites facilitate double-strand cleavage by ZFNs presumably because the sites orient both nuclease domains towards each other making, the formation of the dimer easier [23]. The logarithmic decay that is observed when rates are plotted against substrate concentration (Fig. 2) provides some insight into the cleavage mechanism and how it is facilitated. Increasing the substrate concentration decreases the chance that two ZFN monomers will bind to the same molecule of DNA, essentially ‘trapping’ ZFN molecules in a 1:1 complex when DNA is in excess. This would not affect the cleavage rate of ZFN if they were active as monomers. The decay in rates occurs because more than one ZFN must bind to the same molecule of DNA in order to cleave. Increasing the substrate concentration does not reduce the chance that a protein will interact with another protein on a different molecule of DNA or an unbound protein molecule in solution. The decay in rates suggests that ZFNs do not cleave double-strand DNA when they are bound to two different molecules of DNA. This may help explain why the double-strand cleavage by ZFNs is so greatly facilitated by two inverted sites that are in close proximity on the same molecule of DNA.
A comparison of the ΔQNK-FN cleavage of pKT8 and pKT28 (Fig. 2) indicates that inverted sites appear to facilitate cleavage better when they are positioned closer. A shorter distance between sites may increase the likelihood that both nuclease domains will interact and form a dimer. An optimal spacer length between ZFN sites is preferred because it will necessitate less strain on the glycine-serine linker connecting the ZF to the nuclease domain, and hence, making the dimer more stable. These observations for ZFN cleavage mechanism of DNA are consistent with the reports from other laboratories on the mechanism of double-strand cleavage by FokI restriction enzyme [31-35].
This is also consistent with the results observed in the biotin pull-down assay: a 31 bp biotinylated DNA and 32P-labeled DNA each containing a single ΔQNK site showed the formation of a ΔQNK-FN active complex (Fig. 3A); but this required both specific DNA and divalent ions (Fig. 3B). Our result suggests that binding of divalent metal is critical for the dimerization of ZFNs on DNA, and that dimerization can be achieved only when both ZFN molecules are bound to their cognate sites and not on the non-cognate sequence. However, as in the case of FokI [32], double-strand cleavage of the 32P-labeled DNA by ZFN was not observed. Neither specific DNA nor metal ion alone was sufficient to trigger dimer formation. Furthermore, in order for a single binding site to be cleaved, the ZFN bound to the site must dimerize with another ZFN that is non-specifically bound to the same molecule of DNA. This is likely to be a very inefficient process. This implies single sites will be cut very slowly, if at all. The ZFN concentration must be unusually high over the number of binding sites for this to occur; otherwise, each ZFN will be bound specifically as a monomer on different molecules of DNA and no cleavage will occur. Two inverted sites on the same DNA molecule bind two ZFN monomers specifically and help to orient the nuclease domains for dimerization. The ZFN concentration does not have to be in excess over the substrate to observe DNA cleavage, provided both specific sites are occupied by ZFN monomers on the same DNA molecule.
Complete cleavage of pKT8 substrate containing two neighboring inverted ΔQNK sites is observed when ΔQNK-FN is in excess over the DNA sites. The concentration of NaCl in the reaction mixture shows a dramatic effect on the cleavage activity of ΔQNK-FN (Fig. 4). An accurate determination of the turnover rate of ΔQNK-FN was not possible at this time because of various complicating factors such as the stability of the cleavage domain during prolonged incubation at 37 °C, protein aggregation and precipitation at low salt concentrations, low cleavage activity at higher salt concentrations, etc., even though the DNA-binding domain of the ZFN is very stable in the presence of zinc ion. We measured the turnover rate of ZF ΔQNK using gel-shift assays, which was in the expected range reported for other three-finger proteins in the literature [36].
Two-site model for the mechanism of double-strand cleavage by ZFNs
The two-site model proposed for the mechanism of DNA cleavage by FokI restriction enzyme [32] best explains the double-strand cleavage by ZFNs as well. First, the ZF domains of each ZFN monomer bind separately to adjacent cognate sites on DNA. This brings the free cleavage domains in close proximity, enabling dimer formation. Second, the ZFN monomers associate through the cleavage domains to form the dimer required for the DNA cleavage reaction. Furthermore, dimerization of ZFNs has to occur on adjacent cognate sites on the same DNA molecule to properly align the two catalytic domains and activate them for double-strand cleavage. If this were true, ZFNs should cleave a two-site substrate faster than the one-site one when the ZFNs are present at a higher concentration than the DNA. The data presented above suggest this to be the case (Fig. 2).
Further support for the two-site model comes from the previously reported study [23], which showed that the adjacent cognate sites separated over 35 bp are not cleaved by ZFNs. The DNA substrates with adjacent cognate sites separated over 35 bp essentially behave like substrates containing a single binding site. The bridging interaction between two ZFN monomers bound to adjacent recognition sites needs to be within the 35 bp, which in turn is determined by the length of the (Gly4Ser)3 linker that connects the ZF motifs to the FokI cleavage domain. Furthermore, mutations of two residues (Asp483 and Arg487 identified in FokI restriction enzyme) that form a salt bridge between the cleavage domains completely abolished the cleavage activity of ZFN [23]. An alternate scheme is that a ZFN monomer bound to its recognition site recruits a second free ZFN monomer from solution, rather than association between two ZFN monomers that are each bound to a separate recognition site. The small dimer interface between the cleavage domains likely prevents this from happening. The complex containing two ZFN monomers at a single recognition site may therefore not be stable and it is likely to fall apart often before cleaving DNA. As in the case of FokI, the small dimer interface between the cleavage domains limits the chance of their dimerization in solution, preventing non-specific cleavage by ZFNs when they are not bound to their adjacent cognate sites.
Incidentally, this DNA cleavage mechanism also explains the observed exquisite sequence specificity of ZFNs and their successful application in gene targeting experiments in frog oocytes [24], in Drosophila [26,28], plants [29] and human cells [25]. Otherwise, recombinant cells from these experiments would not have proven to be viable. The study outlined here provides us with a better understanding of the mechanism of double-strand cleavage by ZFNs: how they cleave DNA and how two inverted sites facilitate double-strand cleavage. Based on these studies, we speculate that the cytotoxicity observed as a result of continued expression of three-finger ZFNs in cells is probably due to ZFN cleavage at secondary degenerate sites positioned in an inverted orientation and not likely due to cleavage at single sites. We expect that improvements in sequence specificity and selectivity of ZFNs will make them less toxic to cells. It appears that this is borne out in the recent study by Urnov et al. [27], where four-finger ZFNs have been shown to promote a highly sequence-specific cleavage in human cells while exhibiting very little or no cytotoxicity.
Acknowledgments
We thank Dr. Neil Clarke for helpful suggestions and comments on the kinetics experiments. This work was funded by a grant from National Institutes of Health (GM 53923). We also thank Environmental Health Sciences Center Core Facility (supported by Grant ES 03819) for the synthesis of oligonucleotides. We thank Dr. Sundar Durai for help with figures. K.K. was supported for a year by the Johns Hopkins University Center for AIDS Research (CFAR; Grant # P30 AI42855) grant.
References
- [1].Szybalski W, Kim SC, Hasan N, Podhajska AJ. Class-IIS restriction enzymes—a review. Gene. 1991;100:13–26. doi: 10.1016/0378-1119(91)90345-c. [DOI] [PubMed] [Google Scholar]
- [2].Li L, Wu LP, Chandrasegaran S. Functional domains in FokI restriction endonuclease. Proc. Natl. Acad. Sci. USA. 1992;89:4275–4279. doi: 10.1073/pnas.89.10.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Li L, Chandrasegaran S. Alteration of the cleavage distance of FokI restriction endonuclease by insertion mutagenesis. Proc. Natl. Acad. Sci. USA. 1993;90:2764–2768. doi: 10.1073/pnas.90.7.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li L, Wu LP, Clarke R, Chandrasegaran S. C-terminal deletion mutants of the FokI restriction endonuclease. Gene. 1993;133:79–84. doi: 10.1016/0378-1119(93)90227-t. [DOI] [PubMed] [Google Scholar]
- [5].Kim YG, Li L, Chandrasegaran S. Insertion and deletion mutants of FokI restriction endonuclease. J. Biol. Chem. 1994;269:31978–31982. [PubMed] [Google Scholar]
- [6].Wah DA, Bitinaite J, Schildkraut I, Aggarwal AK. Structure of FokI has implications for DNA cleavage. Proc. Natl. Acad. Sci. USA. 1998;95:10564–10569. doi: 10.1073/pnas.95.18.10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wah DA, Hirsch JA, Dorner LF, Schildkraut I, Aggarwal AK. Structure of the multimodular endonuclease FokI bound to DNA. Nature. 1997;388:97–100. doi: 10.1038/40446. [DOI] [PubMed] [Google Scholar]
- [8].Kim YG, Chandrasegaran S. Chimeric restriction endonuclease. Proc. Natl. Acad. Sci. USA. 1994;91:883–887. doi: 10.1073/pnas.91.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim YG, Cha J, Chandrasegaran S. Hybrid restriction enzymes: zinc finger fusions to FokI cleavage domain. Proc. Natl. Acad. Sci. USA. 1996;93:1156–1160. doi: 10.1073/pnas.93.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kim YG, Smith J, Durgesha M, Chandrasegaran S. Chimeric restriction enzyme: Gal4 fusion to FokI cleavage domain. Biol. Chem. 1998;379:489–495. doi: 10.1515/bchm.1998.379.4-5.489. [DOI] [PubMed] [Google Scholar]
- [11].Ruminy P, Derambure C, Chandrasegaran S, Salier JP. Long-range identification of hepatocyte nuclear factor-3 (FoxA) high and low-affinity binding sites with a chimeric nuclease. J. Mol. Biol. 2001;310:523–535. doi: 10.1006/jmbi.2001.4788. [DOI] [PubMed] [Google Scholar]
- [12].Kim MK, Lee JS, Chung JH. In vivo transcription factor recruitment during thyroid hormone receptor-mediated activation. Proc. Natl. Acad. Sci. USA. 1999;96:10092–10097. doi: 10.1073/pnas.96.18.10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lee JS, Lee CH, Chung JH. Studying the recruitment of Sp1 to the beta-globin promoter with an in vivo method: protein position identification with nuclease tail (PIN*POINT) Proc. Natl. Acad. Sci. USA. 1998;95:969–974. doi: 10.1073/pnas.95.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kim YG, Kim PS, Herbert A, Rich A. Construction of a Z-DNA-specific restriction endonuclease. Proc. Natl. Acad. Sci. USA. 1997;94:12875–12879. doi: 10.1073/pnas.94.24.12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Greisman HA, Pabo CO. A general strategy for selecting high-affinity zinc finger proteins for diverse DNA target sites. Science. 1997;275:657–661. doi: 10.1126/science.275.5300.657. [DOI] [PubMed] [Google Scholar]
- [16].Rebar EJ, Greisman HA, Pabo CO. Phage display methods for selecting zinc finger proteins with novel DNA-binding specificities. Methods Enzymol. 1996;267:129–149. doi: 10.1016/s0076-6879(96)67010-4. [DOI] [PubMed] [Google Scholar]
- [17].Jamieson AC, Wang H, Kim SH. A zinc finger directory for high-affinity DNA recognition. Proc. Natl. Acad. Sci. USA. 1996;93:12834–12839. doi: 10.1073/pnas.93.23.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Desjarlais JR, Berg JM. Redesigning the DNA-binding specficity of a zinc finger protein: a data base-guided approach. Proteins. 1992;12:101–104. doi: 10.1002/prot.340120202. [DOI] [PubMed] [Google Scholar]
- [19].Pavletich NP, Pabo CO. Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1Å. Science. 1991;252:809–817. doi: 10.1126/science.2028256. [DOI] [PubMed] [Google Scholar]
- [20].Kandavelou K, Mani M, Durai S, Chandrasegaran S. Engineering and applications of chimeric nucleases. In: Pingoud AM, editor. Nucleic Acids and Molecular Biology. Springer-Verlag, Berlin: 2004. pp. 413–434. [Google Scholar]
- [21].Chandrasegaran S, Smith J. Chimeric restriction enzymes: What is next? Biol. Chem. 1999;380:841–848. doi: 10.1515/BC.1999.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kandavelou K, Mani M, Durai S, Chandrasegaran S. ‘Magic’ scissors for genome surgery. Nat. Biotechnol. 2005;23:686–687. doi: 10.1038/nbt0605-686. [DOI] [PubMed] [Google Scholar]
- [23].Smith J, Bibikova M, Whitby FG, Reddy AR, Chandrasegaran S, Carroll D. Requirements for double-strand cleavage by chimeric restriction enzymes with zinc finger DNA-recognition domains. Nucleic Acids Res. 2000;28:3361–3369. doi: 10.1093/nar/28.17.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bibikova M, Carroll D, Segal DJ, Trautman JK, Smith J, Kim YG, Chandrasegaran S. Stimulation of homologous recombination through targeted cleavage by chimeric nucleases. Mol. Cell. Biol. 2001;21:289–297. doi: 10.1128/MCB.21.1.289-297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Porteus MH, Baltimore D. Chimeric nucleases stimulate gene targeting in human cells. Science. 2003;300:763. doi: 10.1126/science.1078395. [DOI] [PubMed] [Google Scholar]
- [26].Bibikova M, Beumer K, Trautman JK, Carroll D. Enhancing gene targeting with designed zinc finger nucleases. Science. 2003;300:764. doi: 10.1126/science.1079512. [DOI] [PubMed] [Google Scholar]
- [27].Urnov FD, Miller JC, Lee YL, Beausejour CM, Rock JM, Augustus S, Jamieson AC, Porteus MH, Gregory PD, Holmes MC. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature. 2005;435:646–651. doi: 10.1038/nature03556. [DOI] [PubMed] [Google Scholar]
- [28].Bibikova M, Golic M, Golic KG, Carroll D. Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics. 2002;161:1169–1175. doi: 10.1093/genetics/161.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lloyd A, Plaisier CL, Carroll D, Drews GN. Targeted mutagenesis using zinc-finger nucleases in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2005;102:2232–2237. doi: 10.1073/pnas.0409339102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Smith J, Berg JM, Chandrasegaran S. A detailed study of the substrate specificity of a chimeric restriction enzyme. Nucleic Acids Res. 1999;27:674–681. doi: 10.1093/nar/27.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bitinaite J, Wah DA, Aggarwal AK, Schildkraut I. FokI dimerization is required for DNA cleavage. Proc. Natl. Acad. Sci. USA. 1998;95:10570–10575. doi: 10.1073/pnas.95.18.10570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vanamee ES, Santagata S, Aggarwal AK. FokI requires two specific DNA sites for cleavage. J. Mol. Biol. 2001;309:69–78. doi: 10.1006/jmbi.2001.4635. [DOI] [PubMed] [Google Scholar]
- [33].Bath AJ, Milsom SE, Gormley NA, Halford SE. Many type IIs restriction endonucleases interact with two recognition sites before cleaving DNA. J. Biol. Chem. 2002;277:4024–4033. doi: 10.1074/jbc.M108441200. [DOI] [PubMed] [Google Scholar]
- [34].Soundararajan M, Chang Z, Morgan RD, Heslop P, Connolly BA. DNA binding and recognition by the IIs restriction endonuclease MboII. J. Biol. Chem. 2002;277:887–895. doi: 10.1074/jbc.M109100200. [DOI] [PubMed] [Google Scholar]
- [35].Pingoud A, Fuxreiter M, Pingoud V, Wende W. Type II restriction endonucleases: structure and mechanism. Cell. Mol. Life Sci. 2005;62:685–707. doi: 10.1007/s00018-004-4513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Young ET, Kacherovsky N, Cheng C. An accessory DNA binding motif in the zinc finger protein Adr1 assists stable binding to DNA and can be replaced by a third finger. Biochemistry. 2000;39:567–574. doi: 10.1021/bi992049r. [DOI] [PubMed] [Google Scholar]