Abstract
Objectives:
To determine the correlation of skeletal bone mineral density (BMD) with mandibular density and mandibular radiographic indices estimated on digital panoramic radiographs.
Methods:
Study comprised 112 female subjects older than 45 years. Digital panoramic radiographs were taken, and patients were referred to densitometric measuring (dual energy X-ray absorptiometry) of BMD in the hip bones and lumbar spine regions (L1–L4). On the radiographs, mandibular bone density was estimated and the following indices were measured by the DIGORA® software (Soredex, Tuusula, Finland): mental index (MI), gonial index (GI), antegonial index (AI), panoramic mandibular index (PMI) and alveolar crest resorption degree (M/M). Mandibular cortical index (MCI) was visually estimated.
Results:
Mandibular density and visual index MCI are significant predictors of hip and spine BMD. Mandibular density was marked by a significant square trend: it decreased until the age of 54 years and remained constant until the age of 64 years when it started to increase. Significant correlations were found between MI, AI and PMI values and BMD in the hip but not in the lumbar spine region. The GI and M/M values did not show statistically significant correlations with BMD of either region.
Conclusions:
Mandibular bone density and mandibular radiographic indices are useful in detecting patients with decreased BMD. The applicability of orthopantomograms in diagnosing osteoporosis/osteopenia should be recognized as the potential greatest benefit of this everyday diagnostic method in dental practice.
Keywords: panoramic radiography, mandible, bone density, osteoporosis, radiographic indices
Introduction
Osteoporosis is a condition of the skeletal system characterized by a decrease in bone mass and density that can result in potential trauma with minimal strain, including routine everyday activities. Overall bone strength, that is the ability of the bone to resist trauma, depends on bone mass, bone morphology and microarchitecture, as well as on the characteristics of bone tissues.1
Osteoporosis is the most common metabolic disease affecting bones. Owing to its high frequency and indiscernible symptomology, it is known as “the silent epidemic”. Bone fractures are the most serious complication of osteoporosis, with fractures most commonly affecting the vertebrae, femur and forearm.2 Early diagnostics and timely treatment are of the utmost importance to prevent complications.
Since dentists use radiographs in everyday practice, it is necessary to evaluate the possibility of recognizing decreased bone mineral density (BMD) on panoramic radiographs, particularly in menopausal and post-menopausal females.3,4 There are several radiomorphometric indices that use measurements in orthopantomograms, as well as visual indices, which have been suggested as possible indicators of the decreased value of BMD.5–10 Results of previous studies are inconsistent, but most were carried out using conventional radiographs.3
The purpose of this study is to evaluate the correlation between skeletal BMD and mandibular density, mandibular cortical index (MCI), mandibular panoramic indices and age in menopausal and post-menopausal females. The main advantage of this study is the use of digital panoramic radiographs and specific computer software, which provided more reliable results.
Methods and materials
A sample of 112 females older than 45 years of age participated in this cross-sectional study. Of that number, 69 were dentate (at least one of the teeth, either the second pre-molar or the first molar) in the region of interest (ROI), 38 were edentulous in the ROI and 5 were totally edentulous in the mandible. The sample was derived from patients of the Department of General Dentistry, Dental Clinic, University Hospital Centre Zagreb, Zagreb, Croatia. Excluded from study were patients who: (1) were taking medications affecting bone metabolism; (2) had existing bone metabolic diseases; (3) had medical problems, including carcinoma with bone metastasis, hyperparathyroidism, leukaemia, multiple myeloma, renal insufficiency or liver disease; (4) exhibited secondary osteoporosis; or (5) were using glucocorticoids. The study was approved by the Ethics Committee of the School of Dental Medicine, University of Zagreb, Zagreb, Croatia (05-PA-26-24/06); informed consent was obtained from all participants.
Radiological analysis
Digital panoramic radiographs were taken by CRANEX® D Ceph (Soredex, Tuusula, Finland). BMD was determined for the lumbar spine region (L1–L4) and the proximal femur by dual energy X-ray absorptiometry technology (Hologic QDR 4500; Hologic Inc., Bedford, MA) at the Clinic for Nuclear Medicine and Oncology at the Clinical Hospital Center “Sestre milosrdnice”, Zagreb, Croatia.
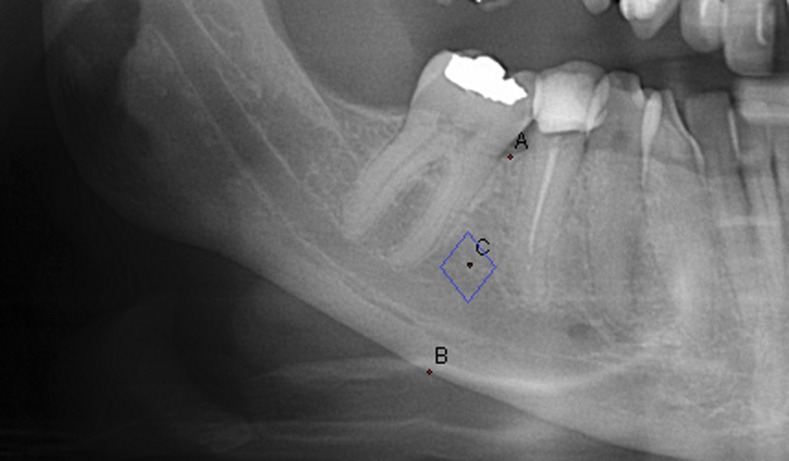
Digital panoramic images were analysed by the software DIGORA® for Windows v. 2.8 (Soredex), designed for this purpose. The images had a ratio of 1:1, and there was no need for magnification correction. One examiner (ISP) performed all the measurements on the right side of the mandible. Mandibular mineral bone density was estimated by DIGORA. The software provided measurements of grey intensity in the area between the second pre-molar and the first molar at half distance between the upper and lower edges of the mandibular bone (ROI) (Figure 1).
Figure 1.
Mandibular density was measured as grey intensity in the area between second pre-molar and first molar at half distance (c) between upper (a) and lower edge (b) of the mandibular bone (region of interest).
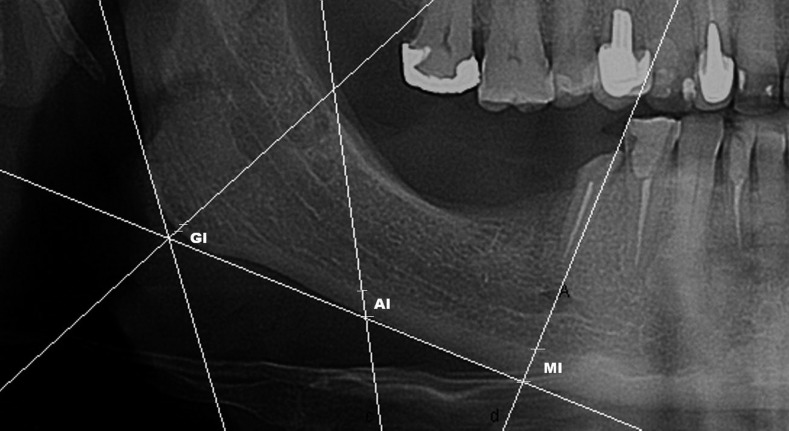
Mental index (MI), gonial index (GI) and antegonial index (AI) were measured on digital panoramic images by software (Figure 2), while upper panoramic mandibular index (uPMI) and lower panoramic mandibular indices were calculated. MI is the thickness of the mandibular lower cortex measured on the line passing through the middle of mental foramen and perpendicular to the tangent to the lower border.6 GI is the cortical thickness at the gonial angle measured on the bisector of the angle between the tangent to the posterior border of the ramus and another line tangent to the lower border of the mandible.11 The AI is a measurement of the mandibular lower cortex thickness measured on the best fitting line on the anterior border of mandibular ramus passing through the mandibular lower border.5 Panoramic mandibular index (PMI) is the ratio of the thickness of the mandibular cortex to the distance between the mental foramen and the inferior mandibular cortex.6 It is measured along the same line as the MI. PMI is calculated as upper mandibular indices and lower panoramic mandibular indices. The uPMI is derived using the upper border of the mental foramen to measure the distance between the mental foramen and the inferior mandibular cortex. The lower PMI is calculated when the measurement is from the lower border of the mental foramen. Additionally, the MCI was evaluated12 according to Klemetti et al.7 Subjects whose panoramic images show a straight and clear cortical edge (Figure 3) belong to category C1, patients whose cortical edge was fragmented with semilunar defects belong to category C2 (Figure 4), whereas patients with porosity of the cortical bone are classified as category C3 (Figure 5) of the MCI. The extent of alveolar ridge resorption was also measured (M/M). It was calculated by dividing the mandibular height by the distance from the middle of the mental foramen to the lower mandibular edge.13 Density of the mandibular bone was evaluated in a ROI.
Figure 2.
Measurements of the cortical thickness on the panoramic radiograph. Mental index (MI), gonial index (GI) and antegonial index (AI) of cortical thickness were measured according to the method described by Ledgerton et al.5
Figure 3.
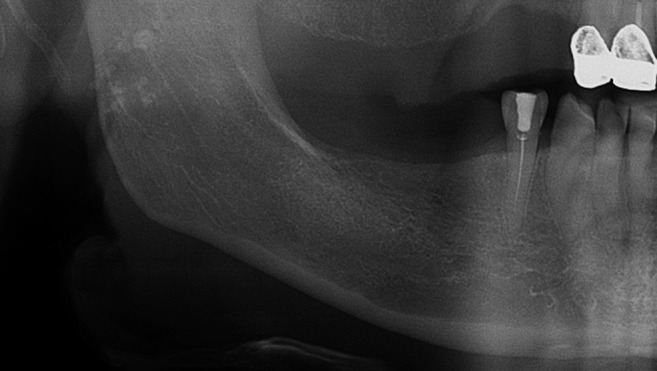
Mandibular cortical index—category C1; the endosteal margin of the cortex is even and sharp.
Figure 4.
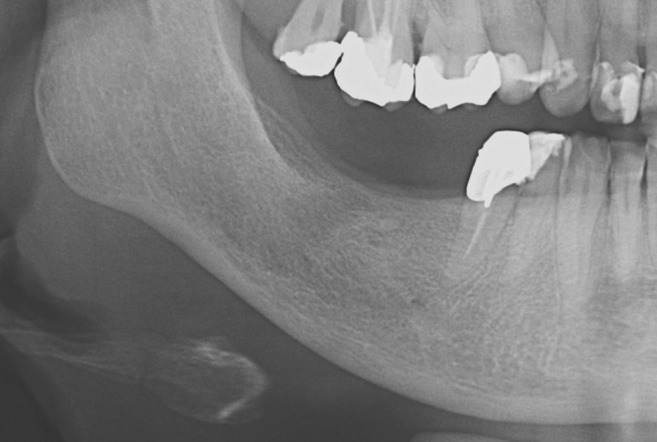
Mandibular cortical index—category C2; the endosteal margin shows semilunar defects, signs of lacunar resorption, on one or both sides.
Figure 5.
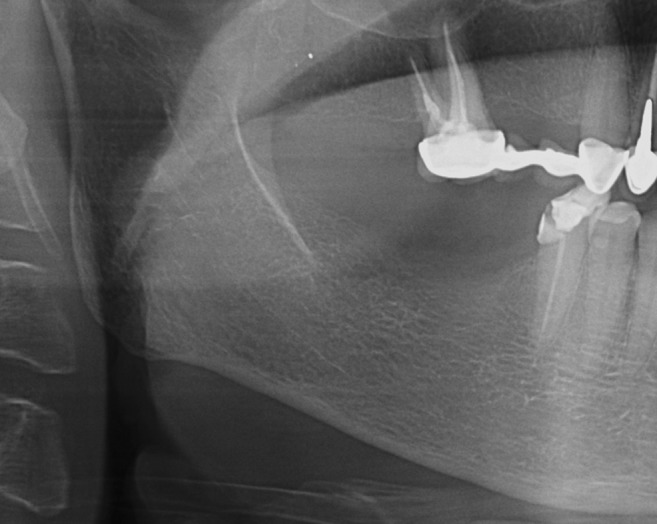
Mandibular cortical index—category C3; the cortical layer shows pronounced porosity.
To calculate intra-observer reliability, measurements were repeated after 2 weeks on 20 randomly selected images. A t-test for related samples revealed no statistically significant difference (p > 0.05).
Statistical analysis
Pearson and Spearman correlation coefficients were used to assess the relationship of the two numerical (ordinal) variables. The analysis of numerical data divided into two target groups was carried out by a t-test for independent samples. Univariate and multivariate linear regression models were used for the analysis of BMD in the hip and spine regions as the dependant parameter with age, body mass index, mandibular density and MCI as predictor variables. Apart from Epanechnikov non-parametric kernel density estimation, locally weighted scatterplot smoothing was used to analyse the age at which the change in mandibular density was most pronounced. The analysis was carried out by the statistical software SPSS® v. 13 (SPSS Inc, Chicago, IL), with p < 0.05 set as the level of statistical significance.
Results
The study included a total of 112 female subjects, 89 of whom (79.5%) were menopausal. The basic features of the sample are shown in Table 1.
Table 1.
Basic group parameters of the analysed sample (n = 112)
| Parameter | Mean ± standard deviation/median (minimum–maximum) |
|---|---|
| Age (years) | 58 (45–80) |
| Weight (kg) | 71.16 ± 12.73 |
| Height (cm) | 163.67 ± 5.75 |
| Body mass index (kg m−2) | 26.6 ± 4.85 |
| Menarche (years) | 13 (10–18) |
| Menopause (years) | 50 (35–64) |
| BMD—hip (g cm−2) | 0.90 ± 0.14 |
| BMD—lumbar (g cm−2) | 0.94 ± 0.17 |
| Bone density—mandible | 131.11 ± 21.19 |
| Mental index (mm) | 3.58 ± 0.63 |
| Antegonial index (mm) | 2.99 ± 0.77 |
| Gonial index (mm) | 1.58 ± 0.65 |
| Upper panoramic mandibular index | 0.3 ± 0.07 |
| Lower panoramic mandibular index | 0.38 ± 0.09 |
| Mandibular cortical index, n (%) | |
| 1 | 54 (48.2) |
| 2 | 53 (47.3) |
| 3 | 5 (4.5) |
| M/Ma | 2.27 ± 0.36 |
BMD, bone mineral density.
Alveolar ridge resorption degree.
There was no significant difference in the mandibular density between edentulous and dentate patients in ROI (p > 0.05). The group of patients totally edentulous in the mandible was too small to analyse the difference in bone density between them and dentate patients.
The analysis of mandibular density pointed to a significant correlation with MCI (p < 0.001) as well as with both indicators of BMD (p < 0.001, Table 2). General linear modelling in the analysis of the hip BMD pointed to body mass index, mandibular density and visual index MCI as significant predictors, whereas age was not a significant predictor. In the analysis of the spine, BMD results were the same except for body mass index, which was not a significant predictor (Table 3).
Table 2.
Correlation between radiographic indicators of lower jaw condition and bone mineral density (BMD) in the regions of hip bone and lumbar spine
| Correlation coefficient; r (p-value) | BMD—hip | BMD—lumbar spine |
|---|---|---|
| Bone density—mandible | 0.37 (0.001) | 0.57 (<0.001) |
| Mandibular cortical index | −0.39 (<0.001) | −0.47 (<0.001) |
| Mental index | 0.32 (0.004) | 0.08 (0.488) |
| Antegonial index | 0.28 (0.009) | 0.11 (0.323) |
| Gonial index | 0.15 (0.180) | 0.04 (0.742) |
| Upper panoramic mandibular index | 0.28 (0.011) | 0.07 (0.537) |
| Lower panoramic mandibular index | 0.26 (0.017) | 0.04 (0.711) |
Table 3.
Univariate and multivariate regression with bone mineral density (BMD) of the hip and lumbar spine as dependant parameters (n = 83)
| Independent variables | Dependant variable: BMD hip |
Dependant variable: BMD lumbar spine |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
|||||||||
| b ± SE (b) | t | p-value | b ± SE (b) | t | p-value | b ± SE (b) | t | p-value | b ± SE (b) | t | p-value | |
| Age | −0.001 ± 0.001 | −0.586 | 0.559 | – | – | – | 0.001 ± 0.001 | −0.549 | 0.585 | – | – | – |
| Body mass index | 0.009 ± 0.003 | 2.585 | 0.012 | 0.008 ± 0.003 | 2.557 | 0.012 | 0.005 ± 0.003 | 1.257 | 0.212 | – | – | – |
| Bone density—mandible | 0.002 ± 0.001 | 2.375 | 0.020 | 0.002 ± 0.001 | 2.364 | 0.020 | 0.004 ± 0.001 | 4.598 | <0.001 | 0.004 ± 0.001 | 4.620 | <0.001 |
| Mandibular cortical index | −0.064 ± 0.028 | −2.244 | 0.028 | −0.066 ± 0.027 | −2.349 | 0.021 | −0.068 ± 0.031 | −2.140 | 0.035 | −0.075 ± 0.031 | −2.386 | 0.019 |
SE (b), standard error of the regression coefficient b.
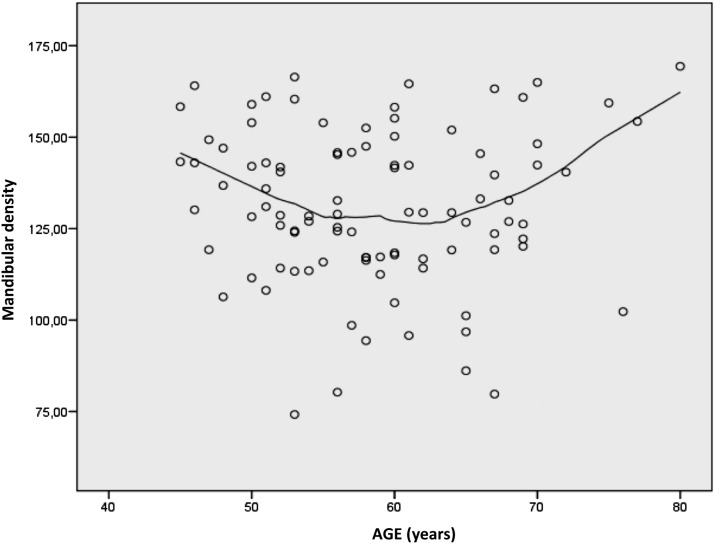
The use of regression for the curve interpolation describing the correlation between age and mandibular density pointed to significant square trend (β = −3.91; p = 0.003, Figure 6). Two points of inflection were recorded where the changes were pronounced: at the ages of 54 years (p = 0.012) and 64 years (p < 0.001). The first point marked the age at which the decrease in mandibular density slowed down and became constant with the increase in age, whereas at the age of 64 years, mandibular density increased.
Figure 6.
Change of mandibular density with age. The line of regression for the curve interpolation is marked by a significant square trend (β = −3.91; p = 0.003) showing two points of inflection with pronounced changes in density, at the age of 54 years (p = 0.012) and the age of 64 years (p < 0.001).
A comparison of BMD to mandibular radiographic indices resulted in significant positive correlations between MI, AI and both PMI values and BMD in the hip region but not in the lumbar spine region. Subjects with a MI <3 mm had a statistically significantly decreased BMD in the hip region (0.84 ± 0.12) compared with subjects with a MI >3 mm (0.92 ± 0.15; p = 0.034). The GI value did not show a statistically significant correlation with either BMD in the hip region or in the lumbar spine region (Table 2). The M/M ratio did not show a significant correlation with BMD of the hip bone and lumbar spine nor with mandibular density.
Discussion
Condition of the oral cavity and jaws is examined radiographically more often than any other part of the body.14,15 Since dentists in their everyday practice use panoramic radiographs, they may be the first clinicians in a position to recognize signs indicative of osteoporosis. The present study evaluated mandibular bone quality indicators on digital orthopantomograms. All the orthopantomograms were taken on the same apparatus, CRANEX D Ceph, and measurements were made by a single investigator (ISP) in the Soredex software DIGORA. This approach ensured high-quality radiographs and maximum quality measurements on a representative sample of 112 females, which is the advantage of our investigation.
Measuring mineral density of the jaw can be useful in the detection of oral or systemic diseases, planning and performing implant surgery, evaluation of treatment success and further monitoring of results.16 So far, microdensitometry, with the use of aluminium or copper step wedge as a referent value, has been used most frequently in studies of jaw density.7,17–19 However, inexpensive and readily available tools are needed for everyday clinical work. In our study, mandibular density was estimated by software that used level of greyness at the ROI, which proved to be, along with the MCI, a significant predictor of BMD of both hip and spine regions (Table 3). These results were ensured by using standardized digital orthopantomograms and software supplied by the same manufacturer. Results might possibly be different if orthopantomograms were taken using another manufacturer's device. Other investigators also found a correlation between mandibular density and skeletal BMD.20–22
Another interesting finding of our study was that mandibular density showed a significant square trend in relation to age. The density decreased until the age of 54 years and became constant until age of 64 years. After 64 years, mandibular density increased with age. Kingsmill and Boyde23 investigated mandibular density on sectioned slices of individuals aged 19–96 years and had similar findings. They found that the mandible shows an increase in density with age. The possible explanation would be the constant loading during mastication in association with muscular activity. Another factor might be tooth loss with ageing, for which some authors found a positive correlation with mandibular BMD.24
Drozdzowska et al20 evaluated mandibular radiographic indices in post-menopausal edentulous females and found that the MCI was not suitable to distinguish normal from osteopenic/osteoporotic patients. This finding is in contrast with ours but may reflect the use of different samples.
After instruction on using MCI, it was successfully applied in osteoporosis screening by dental students.25 It is a simple method that does not demand specific software and is applicable on digital orthopantomograms produced on different devices, including analogue orthopantomograms.
Our study found a statistically significant positive correlation between MI (p = 0.004), AI (p = 0.009) and PMI (uPMI, p = 0.011; lower PMI, p = 0.017) with BMD of the hip bone. Contrary to this, BMD of the lumbar vertebrae did not demonstrate a significant correlation with those indices. The explanation may be in the similar proportion of the trabecular and cortical bone of the proximal femur and the mandible, while the spine consists mostly of the trabecular bone with a thin cortical layer, or in the different loading on the bones.26
Because of differences in structure, there are significant deviations of BMD in different parts of the body.27 Subjects with a MI <3 mm had significantly decreased BMD in the hip region, compared with subjects with a MI >3 mm (p = 0.034). The GI and M/M indices were not useful indicators of BMD, since they did not show a significant correlation with either the BMD value of the hip bone or lumbar spine.
Devlin and Horner11 measured a MI on panoramic radiographs but without magnification correction, which amounted to a difference of 25%. The magnification issue should be considered when proposing the border value of MI and when results are compared with similar studies. Horner and Devlin6 suggested a mandibular cortex thickness of 3 mm as the border value beyond which the female patients should be referred to densitometry, whereas White16 believed that the limit should be 4 mm. Klemetti and Kolmakow28 consider the limit of 4 mm of cortical thickness to be optimal yet insufficient as a separate criterion indicating osteoporosis.
Duncea et al10 researched the possible correlation between PMI and the presence of osteoporosis in 97 post-menopausal females. According to their results, which parallel our findings, they concluded that the determination of a low PMI value represents a warning sign for possible osteoporosis in a patient.
Hastar et al29 analysed MI, PMI and MCI on 487 panoramic images of elderly male and female patients. They found statistically significant differences between MI and PMI values in patients with osteoporosis and without osteoporosis. Also, the C1 category of MCI was more frequently seen in patients without osteoporosis (62.6%), whereas the C2 category was more common in patients with osteoporosis (71.4%). These observations are consistent with our findings.
Vlasiadis et al30 analysed the panoramic radiographs of 133 female subjects of post-menopausal age, ranging from 38 years to 80 years. They found that by reducing the thickness of the mandibular cortex (MI) by 1 mm, the likelihood of osteopenia or osteoporosis increases by as much as 43% and the likelihood of moderate or severe cortical erosion by as much as 96%, taking into account the impact of the number of years passed since the onset of menopause.30 This is supported by the study of Zlatarić and Čelebić,17 who investigated the mandibular cortex of 136 subjects and found a significant correlation with mineral density of the mandible measured by microdensitometry using a copper calibrating step wedge.
Kribbs31 found that gonial cortical thickness was significantly greater in a normal group than in an osteoporotic group. In our study, as well as in similar studies, the correlation of GI and BMD has not been found.27,32,33 A possible reason for the deviation of GI from other indicators of mandibular cortex thickness is the anatomic origin of the masseter and the medial pterygoid muscle in the region of the mandibular angle. Owing to the functional load of this part of the bone, the effects of osteoporosis can be concealed.34
Jonasson et al26 investigated the possible availability of alveolar trabecular pattern in the estimation of skeletal BMD. The trabecular bone pattern was assessed using a visual index proposed by Lindh et al35 and modified by Jonasson et al.18,36 They used three reference radiographs with sparse, alternating sparse and dense or dense trabeculation. Assessment was made on initial periapical radiographs and on a second one taken 10 years later. Dense trabeculation was a strong indicator of high skeletal BMD, whereas sparse trabeculation was a strong indicator of osteopenia.36 Trabeculation was a significantly better predictor of forearm (r = 0.57; p = 0.001) than hip (r = 0.36; p = 0.02) and spine (r = 0.20; p = 0.19) BMD. The possible explanation is the difference in structural characteristics and loading on the bones. The forearms are not under as much loading as the hip and spine and in that segment are more similar to the mandible that is loaded by teeth and masticatory muscles.26 According to this result, it would be necessary to investigate the correlation of panoramic indices used in our study with forearm BMD.
Conclusion
Using mandibular density, morphometric and visual mandibular radiographic indices, except for GI and M/M, it is possible to recognize patients who should be referred for osteoporosis evaluation. While morphometric indices demand certain procedures, mandibular density estimation and MCI are simple and useful methods valuable in the detection of decreased skeletal BMD. Dentists should be aware of these parameters, which can be the greatest benefit of the panoramic radiograph. Early recognition of the signs of decreased BMD could lead to early diagnostics, timely treatment and prevention of complications. This would help maintain quality of life, especially in menopausal and post-menopausal females, and reduce healthcare costs. The applicability of the orthopantomograms in diagnosing osteoporosis should be included in undergraduate and continuing dental education, and also in osteoporosis detection guidelines.37 This would ensure that orthopantomograms will be used not only in the evaluation of oral conditions but also for general health status.
References
- 1.Bouxsein M. Biomechanics of age-related fractures. In: Marcus R, Feldman D, Kelsey J, eds. Osteoporosis. 2nd edn. San Diego, CA: Academic Press; 2001. pp. 509–34. [Google Scholar]
- 2.Cvijetić S, Grazio S, Kaštelan D, Koršić M. Epidemiology of osteoporosis. [In Croatian.] Arh Hig Rada Toksikol 2007; 58: 13–18. [DOI] [PubMed] [Google Scholar]
- 3.López-López J, Estrugo-Devesa A, Jane-Salas E, Ayuso-Montero R, Gómez-Vaquero C. Early diagnosis of osteoporosis by means of orthopantomograms and oral x-rays: a systematic review. Med Oral Patol Oral Cir Bucal 2011; 16: e905–13. [DOI] [PubMed] [Google Scholar]
- 4.White SC. Oral radiographic predictors of osteoporosis. Dentomaxillofac Radiol 2002; 31: 84–92. doi: 10.1038/sj.dmfr.4600674 [DOI] [PubMed] [Google Scholar]
- 5.Ledgerton D, Horner K, Devlin H, Worthington H. Radiomorphometric indices of the mandible in a British female population. Dentomaxillofac Radiol 1999; 28: 173–81. doi: 10.1038/sj/dmfr/4600435 [DOI] [PubMed] [Google Scholar]
- 6.Horner K, Devlin H. The relationship between mandibular bone mineral density and panoramic radiographic measurements. J Dent 1998; 26: 337–43. [DOI] [PubMed] [Google Scholar]
- 7.Klemetti E, Vainio P, Lassila V, Alhava E. Cortical bone mineral density in the mandible and osteoporosis status in postmenopausal women. Scand J Dent Res 1993; 101: 219–23. [DOI] [PubMed] [Google Scholar]
- 8.Devlin H, Karayianni K, Mitsea A, Jacobs R, Lindh C, van der Stelt P, et al. Diagnosing osteoporosis by using dental panoramic radiographs: the OSTEODENT project. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 104: 821–8. doi: 10.1016/j.tripleo.2006.12.027 [DOI] [PubMed] [Google Scholar]
- 9.Yasar F, Akgünlü F. The differences in panoramic mandibular indices and fractal dimension between patients with and without spinal osteoporosis. Dentomaxillofac Radiol 2006; 35: 1–9. doi: 10.1259/dmfr/97652136 [DOI] [PubMed] [Google Scholar]
- 10.Duncea I, Pop A, Georgescu CE. The relationship between osteoporosis and the panoramic mandibular index. HVM Bioflux 2013; 5: 14–18. [Google Scholar]
- 11.Devlin H, Horner A. Mandibular radiomorphometric indices in the diagnosis of reduced skeletal bone mineral density. Osteoporosis Int 2002; 13: 373–8. doi: 10.1007/s001980200042 [DOI] [PubMed] [Google Scholar]
- 12.Klemetti E, Kolmakov S, Kröger H. Pantomography in assessment of the osteoporosis risk group. Scand J Dent Res 1994; 102: 68–72. [DOI] [PubMed] [Google Scholar]
- 13.Taguchi A, Tanimoto K, Suei Y, Otani K, Wada T. Oral signs as indicators of possible osteoporosis in elderly women. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995; 80: 612–16. [DOI] [PubMed] [Google Scholar]
- 14.United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and effects of ionizing radiation: UNSCEAR report. New York, NY: United Nations; 2000. [Google Scholar]
- 15.International Atomic Energy Agency. Radiological protection for medical exposure to ionizing radiation, safety guide, RS-G-1.5. Vienna, Austria: IAEA; 2002. [Google Scholar]
- 16.White SC, Taguchi A, Kao D, Wu S, Service SK, Yoon D, et al. Clinical and panoramic predictors of femur bone mineral density. Osteoporos Int 2005; 16: 339–46. doi: 10.1007/s00198-004-1692-4 [DOI] [PubMed] [Google Scholar]
- 17.Zlatarić DK, Celebić A. Clinical bone densitometric evaluation of the mandible in removable denture wearers dependent on the morphology of the mandibular cortex. J Prosthet Dent 2003; 90: 86–91. [DOI] [PubMed] [Google Scholar]
- 18.Jonasson G, Jonasson L, Kiliaridis S. Changes in the radiographic characteristics of the mandibular alveolar process in dentate women with varying bone mineral density: a 5-year prospective study. Bone 2006; 38: 714–21. doi: 10.1016/j.bone.2005.10.008 [DOI] [PubMed] [Google Scholar]
- 19.Pavicin IS, Ivosević-Magdalenić N, Badel T, Basić K, Keros J. Analysis of dental supportive structures in orthodontic therapy. Coll Antropol 2012; 36: 779–83. [PubMed] [Google Scholar]
- 20.Drozdzowska B, Pluskiewicz W, Tarnawska B. Panoranic based mandibular indices in relation to mandibular bone mineral density and skeletal status assessed by dual energy X-ray absortiptiometry and quantitative ultrasound. Dentomaxillofac Radiol 2002; 31: 361–7. doi: 10.1038/sj.dmfr/4600729 [DOI] [PubMed] [Google Scholar]
- 21.Makker A, Singh MM, Mishra G, Singh BP, Jain GK, Jadhav S. Relationship between bone turnover biomarkers, mandibular bone mineral density, and systemic skeletal bone mineral density in premenopausal and postmenopausal Indian women. Menopause 2012; 19: 642–9. doi: 10.1097/gme.0b013e31823dbbf7 [DOI] [PubMed] [Google Scholar]
- 22.Hedström L, Baigi A, Bergh H. The relation between bone mineral density in the heel and pixel intensity in the mandibular jaw bone among elderly women. Dentomaxillofac Radiol 2010; 39: 409–13. doi: 10.1259/dmfr/50171873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kingsmill VJ, Boyde A. Variation in apparent density of human mandibular bone with age and dental status. J Anat 1998; 192: 233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buyukkaplan US, Tonguc MO, Guldag MU, Yildiz M, Gumus BA. Comparison of mandibular bone mineral densities in dentate and edentulous patients. J Prosthodont 2013; 22: 23–7. doi: 10.1111/j.1532-849X.2012.00908.x [DOI] [PubMed] [Google Scholar]
- 25.Shintaku WH, Enciso R, Covington JS, Migliorati CA. Can dental students be taught to use dental radiographs for osteoporosis screening? J Dent Educ 2013; 77: 598–603. [PubMed] [Google Scholar]
- 26.Jonasson G. Bone mass and trabecular pattern in the mandible as an indicator of skeletal osteopenia: a 10-year follow-up study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 108: 284–91. doi: 10.1016/j.tripleo.2009.01.014 [DOI] [PubMed] [Google Scholar]
- 27.Bonnick SL, Nichols DL, Sanborn CF, Lloyd K, Payne SG, Lewis L, et al. Dissimilar spine and femoral Z-scores in premenopausal women. Calcif Tissue Int 1997; 61: 263–5. [DOI] [PubMed] [Google Scholar]
- 28.Klemetti E, Kolmakow S. Morphology of the mandibular cortex on panoramic radiographs as an indicator of bone quality. Dentomaxillofac Radiol 1997; 26: 22–5. [DOI] [PubMed] [Google Scholar]
- 29.Hastar E, Yilmaz HH, Orhan H. Evaluation of mental index, mandibular cortical index and panoramic mandibular index on dental panoramic radiographs in the elderly. Eur J Dent 2011; 5: 60–7. [PMC free article] [PubMed] [Google Scholar]
- 30.Vlasiadis KZ, Skouteris CA, Velegrakis GA, Fragouli I, Neratzoulakis JM, Damilakis J, et al. Mandibular radiomorphometric measurements as indicators of possible osteoporosis in postmenopausal women. Maturitas 2007; 58: 226–35. doi: 10.1016/j.maturitas.2007.08.014 [DOI] [PubMed] [Google Scholar]
- 31.Kribbs P. Comparison of mandibular bone in normal and osteoporotic women. J Prosthet Dent 1990; 63: 218–22. [DOI] [PubMed] [Google Scholar]
- 32.Leite AF, Figueiredo PT, Guia CM, Melo NS, de Paula AP. Correlations between seven panoramic radiomorphometric indices and bone mineral density in postmenopausal women. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 109: 449–56. doi: 10.1016/j.tripleo.2009.02.028 [DOI] [PubMed] [Google Scholar]
- 33.Dutra V, Devlin H, Susin C, Yang J, Horner K, Fernandes AR. Mandibular morphological changes in low bone mass edentulous females: evaluation of panoramic radiographs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006; 102: 663–8. doi: 10.1016/j.tripleo.2006.02.023 [DOI] [PubMed] [Google Scholar]
- 34.Dervis E. Oral implications of osteoporosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005; 100: 349–56. doi: 10.1016/j.tripleo.2005.04.010 [DOI] [PubMed] [Google Scholar]
- 35.Lindh C, Petersson A, Rohlin M. Assessment of the trabecular pattern before endosseous implant treatment: diagnostic outcome of periapical radiography in the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996; 82: 335–43. [DOI] [PubMed] [Google Scholar]
- 36.Jonasson G, Bankvall G, Kiliaridis S. Estimation of bone mineral density by means of the trabecular pattern of the alveolar bone, its interdental thickness and the bone mass of the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001; 92: 346–52. [DOI] [PubMed] [Google Scholar]
- 37.Compston J, Bowring C, Cooper A, Cooper C, Davies C, Francis R, et al. Diagnosis and management of osteoporosis in postmenopausal women and older men in the UK: National Osteoporosis Guideline Group (NOGG) update 2013. Maturitas 2013; 75: 392–6. doi: 10.1016/j.maturitas.2013.05.013 [DOI] [PubMed] [Google Scholar]