The recognition that adipose tissue is an endocrine organ marked a watershed in understanding the regulatory control of energy metabolism, and raised new prospects for harnessing adipose-derived hormones for the treatment of metabolic disorders and their cardiovascular complications (1). The promise of one such adipokine, the 30 kDa protein adiponectin, has loomed especially large in the wake of multiple studies demonstrating its insulin-sensitizing, anti-inflammatory, anti-atherogenic, and cardiomyocyte-protective properties in experimental settings (2; 3). These findings notwithstanding, the study of adiponectin in the clinical and epidemiological spheres has produced a far more complicated picture of this adipokine’s role as a biomarker and putative mediator of cardiometabolic disease in human populations.
Unlike most other adipokines, circulating levels of adiponectin fall with increased adiposity (4). Consistent with this relationship, prospective studies have consistently documented that higher circulating adiponectin is associated with lower risk of incident diabetes (5; 6). This has not been the case, however, for cardiovascular outcomes. Despite an initial report that related higher plasma adiponectin to lower incidence of coronary heart disease (CHD) in predominantly healthy middle-aged men (6), subsequent prospective studies often failed to replicate the association (7). Moreover, when studied in patients with kidney disease (8), heart failure (HF) (9), cardiovascular disease (CVD) (10) or general elderly cohorts (11), increasing levels of the adipokine were instead associated with higher mortality. This so-called adiponectin “paradox” (12) has been at the crux of disentangling adiponectin’s (patho)physiologic properties in humans.
In this issue of Metabolism, two reports bring the disparate associations of this adipokine into focus. In the first, Wu and colleagues (ref) investigated the association between total adiponectin and mortality in a meta-analysis of 16 prospective studies of patients with prevalent CVD. There was moderate-to-large heterogeneity across study findings, but pooling of the maximally adjusted effect estimates from individual studies yielded 45% (17%–79%) and 69% (35%–110%) risk increases for all-cause and cardiovascular mortality, respectively, for the upper versus lower tertile of the adipokine’s distribution.
The second report, by Kuwashiro and colleagues (ref), examined the association of total adiponectin measured at 0, 3, 7, 14, and 90 days in patients with acute ischemic stroke in comparison to individuals free of CVD who had a one-time measurement. Cases (n=171) were chosen from the Fukuoka Stroke Registry, whereas controls were age- and sex-matched participants (1:1) selected from a second prospective cohort, the Hiyasama Study. The authors found that initial plasma adiponectin did not differ between cases and controls, but levels were lower for the atherothrombotic, and higher for the cardioembolic, stroke subtype as compared with controls. After multivariable adjustment, each 1-ug/mL increment in plasma adiponectin was associated with a 25% (9%–42%) lower odds of atherothrombotic stroke; there was no significant association for cardioembolic stroke, although exclusion of cases related to atrial fibrillation (AF) showed a 17% (2%–35%) increased risk. During the post-stroke course, adiponectin levels declined from their initial values, reaching their nadir at day 7, after which they returned to their original levels. This sequence paralleled that of total and LDL cholesterol, but was reciprocal to C-reactive protein levels. Furthermore, in unadjusted analyses, higher plasma adiponectin was related to greater stroke severity acutely, and to increased disability at 90 days.
Together, these two studies underscore that adiponectin’s contrary associations are closely dependent on context. As strengths, the report by Wu and colleagues included a larger number of studies than heretofore possible, while that by Kuwashiro and colleagues can be credited with its characterization of ischemic stroke subtypes, and its serial measures of adiponectin and other biomarkers post-stroke. But the two studies also have substantial limitations, which highlight the multiple key factors that can influence the relations of adiponectin with outcomes, and the importance of appropriately accounting for these in different clinical settings.
The systematic review by Wu and colleagues follows four recent metaanalyses that evaluated the relationship of circulating adiponectin with CHD, stroke, and/or fatal events. These earlier meta-analyses did not detect a significant association of higher adiponectin levels with new-onset CHD (13–15), but did find a significantly increased risk of recurrent CHD (14). Higher adiponectin levels were linked to greater mortality, but this was particularly true for studies involving participants with pre-existing CHD (14), a finding confirmed by the current meta-analysis (ref). Yet in such studies, Wu and colleagues’ included, fully adjusted risk estimates from the individual studies were used to calculate the pooled risk estimate, and such individual risk estimates often included covariates favorably associated with the adipokine – glycemic, lipid, and inflammatory markers – and potentially in the causal pathway to CVD events and mortality. That adjustment for such putative intermediates can mask protective associations of adiponectin with outcome in the absence of prevalent CVD, and accentuate untoward associations in its presence, has been documented (16; 17). Indeed, pooling of risk estimates adjusting for both potential confounders and mediators revealed a positive association between adionectin and stroke that proved null when the adjustment was limited only to potential confounders (18). Because adjustment for potential mediators may remove effects inherent to an exposure variable – and can additionally introduce bias (19) – the true measure of an exposure-outcome relationship is one that accounts only for the aggregate of confounding, that is, only for factors related to the exposure and the outcome but not in the causal pathway between them. Since, as the authors acknowledge, separate risk estimates adjusting only for potential confounders were not reported in many of the component studies, the pooled risk estimates account away for the beneficial impact of the adipokine’s associations with metabolic and inflammatory risk factors, leading to likely overestimation.
If the principal limitation of the study by Wu and colleagues is over-adjustment for potential mediators, the chief limitation of Kuwashiro and colleagues’ study may be under-adjustment for potential confounders. The lower adiponectin levels associated with atherothrombotic stroke are consistent with previous observations relating the adipokine to prevalent CHD, whereas the tendency to higher adiponectin levels in patients with cardioembolic stroke likely reflects the higher prevalence of HF and AF in this subgroup than in controls, as the authors state. In this regard, since natriuretic peptides stimulate adipocyte secretion of adiponectin (20), it is likely that higher natriuretic peptide levels are a major factor accounting for the differences observed. As such, any attempt to assess the value of circulating adiponectin for classification of stroke subtypes needs to show its incremental value over natriuretic peptide levels, a more direct measure of heart disease already in use in clinical practice (21). Indeed, higher natriuretic peptide levels have been linked to anticoagulation response in noncardioembolic stroke, attesting to the value of this cardiac biomarker (22). Thus, lack of adjustment for this important upstream factor leaves the true value of the adipokine for ischemic stroke classification uncertain. Beyond natriuretic peptides, adjustment for central measures of adiposity and kidney function would have strengthened the validity of the differences observed as attributable to adiponectin itself.
Apart from these considerations, the analyses as presented do not address adiponectin’s contribution to model discrimination between different stroke subtypes, for which the study’s modest sample size is an impediment, and which is essential to judging the biomarker’s actual usefulness for ischemic stroke classification. Furthermore, the relationship between higher initial adiponectin in stroke patients and greater stroke severity and disability at 90 days was unadjusted. It is uncertain whether this association would have persisted had key determinants of both adiponectin levels and infarct size and location been considered.
Despite these limitations, the two studies in question place a spotlight on the adipokine’s contrasting clinical associations. The basis for the adiponectin paradox remains poorly defined, but an improved understanding of its features is emerging. As relates to aging, which is associated with longitudinal increases in adiponectin levels (23), both high and low concentrations of total adiponectin and its high-molecular-weight isoform were linked to adverse outcomes in elders free of prevalent CVD, but adjustment for metabolic and inflammatory factors abolished the association at the low range of concentrations (16). Similar adjustment uncovered or accentuated a positive monotonic relationship with these outcomes in elders with prevalent CVD, suggesting that associations with unfavorable factors drive the positive associations of adiponectin with adverse outcomes.
Regarding such unfavorable factors, a common thread running through chronic disorders or advanced age is weight loss/cachexia, which could explain both the higher adipokine levels and the poorer survival, although the latter has persisted even after adjustment for measures of body size (11; 24; 25). Recently, however, computed tomography-determined adipose-tissue density in the visceral and subcutaneous depots, which correlates with smaller adipocyte size and higher adiponectin concentrations, was shown to be an independent predictor of mortality in two older cohorts (26). Whether the positive association of adiponectin with mortality is independent of adipose-tissue density has not been reported, but would address the role of changes in adipocyte size and function as an underpinning in aging, and perhaps other disorders such as HF. Interestingly, critical illness is associated with preservation of fat mass but development of newly differentiated, small adipocytes, which would account for a rise in adiponectin levels in this high-risk setting (27).
Furthermore, although adiponectin derives primarily from adipocytes, it is also produced by other cells types, including cardiomyocytes, skeletal myocytes, and endothelial cells (28). A study of HF-associated cachexia documented upregulated expression of adiponectin in diseased skeletal muscle, in conjunction with downregulation of its AdipoR1 receptor and decreased downstream signaling, consistent with adiponectin resistance (29). The degree to which non-adipose tissue sources contribute to elevated plasma adiponectin in chronic diseases, however, has not been determined. Nor has the impact of adiponectin resistance on adiponectin production, or on the efficacy of the proposed counter-regulatory response to disease for which elevated adiponectin levels may be intended, been delineated.
As relates to health-promoting functions, the high concentrations of adiponectin found in the circulation may serve to facilitate phagocytic clearance of apoptotic cells through low-affinity binding of the macrophage calreticulin receptor (30). This role accords with adiponectin’s broader anti-inflammatory properties (4), and would make hyperadiponectinemia in response to underlying disease a marker of illness severity and worse prognosis. Interestingly, although low-grade obesity-related inflammation suppresses adiponectin production, levels of the adipokine are elevated in high-grade inflammatory conditions such as collagen vascular diseases, although the basis for these elevations is unclear (31). Another possibility still is that adiponectin could also have direct proinflammatory effects through its demonstrated ability to activate complement, but the relevance of this in vitro finding to the clinical setting is uncertain (32).
Another emerging factor that appears pivotal in the regulation of adiponectin levels is the T-cadherin receptor, which is predominantly expressed in the heart and vasculature, and binding to which has been shown to mediate adiponectin’s cardiovascular effects (33). Mouse experiments have shown that T-cadherin deficiency raises adiponectin levels, leading to the proposition that T-cadherin binding of adiponectin acts as a reservoir for the adipokine, which is released into the bloodstream with loss of the receptor (34). Consistent with these findings, genome-wide association studies have linked the T-cadherin gene (cdh13) to circulating adiponectin levels in humans (35). It remains to be determined, however, how increased vascular expression of T-cadherin in the context of atherosclerosis, which would lower adiponectin levels, may account for the adipokine’s positive association with mortality in the setting of prevalent CVD.
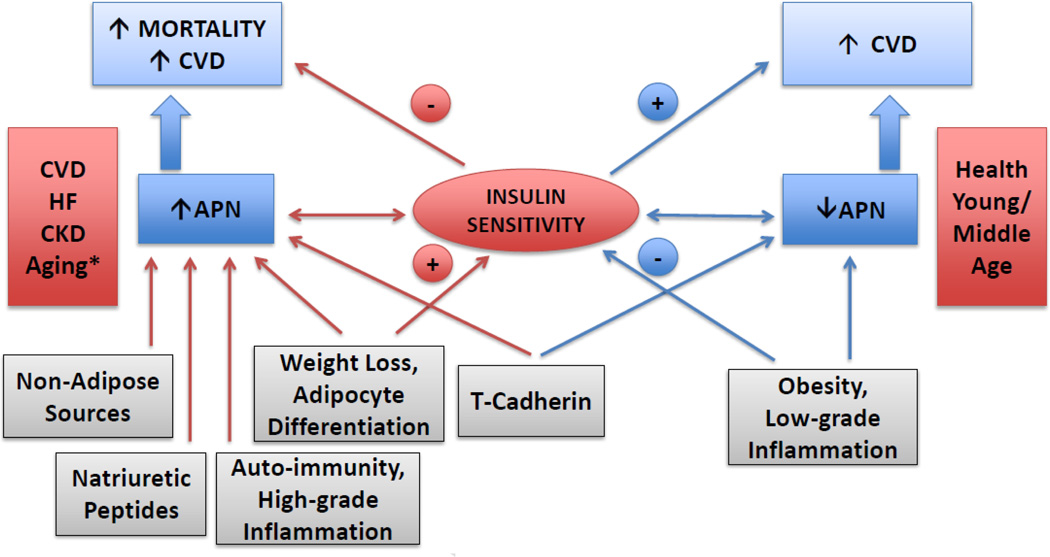
Hence, available evidence concerning the adipokine’s associations supports the notion that opposing factors influencing, or influenced by, adiponectin levels determine its association with chronic diseases and disease outcomes (Figure). In health, the association of lower adiponectin with obesity, low-grade inflammation and insulin resistance drives its inverse association with adverse events, whereas in chronic disease, weight loss, sarcopenia, and natriuretic peptide elevations, among other factors, may combine with insufficient/ineffective counter-regulation to account instead for its positive association.
Figure 1.
Proposed conceptual model for the adiponectin paradox. Among healthy young and middle-aged adults, obesity and low-grade inflammation are associated with hypoadiponectinemia, and each is associated with reduced insulin sensitivity. In such individuals, low circulating adiponectin portends a greater risk of cardiovascular disease. By contrast, in the setting of prevalent cardiovascular disease, heart failure, chronic kidney disease and advanced age, leanness more generally reflects involuntary weight loss / cachexia – which may be accompanied by smaller, differentiated adipocytes – and this is associated with higher plasma adiponectin. Increased production from non-adipose tissues or through direct stimulation by natriuretic peptides, decreased elimination, or other mechanisms associated with high-grade inflammation, may also contribute to elevation in circulating adiponectin levels. Such increased adiponectin levels, whether as markers of underlying illness severity, decreased signaling efficacy (adiponectin resistance) or, possibly, direct pro-inflammatory or other adverse actions, are related to a heightened risk of cardiovascular complications and mortality. Last, heart and vascular expression of T-cadherin also appears to regulate adiponectin levels, apart from mediating its actions. *In healthy elders, the association has been shown to be bidirectional, that is, both low and high adiponectin levels are associated with increased risk.
Further complicating matters, however, the once uncontested notion that adiponectin promotes insulin sensitivity in humans, as has been convincingly demonstrated in mice, has been called into question. Evidence from hereditary disorders of insulin signaling has been cited to support the concept that the inverse association between adiponectin and insulin resistance in humans may reflect hyperinsulinemia-driven suppression of adiponectin production through selective preservation of yet undefined insulin-signaling pathways (36). Moreover, the causal basis for the association between adiponectin and insulin resistance has been lately explored using Mendelian randomization approaches. A cohort study documented an association between variants in AdipoQ and insulin sensitivity by euglycemic clamp (37), although attenuation by adiposity raises questions about whether the relationship can be considered causal (38). In turn, a subsequent meta-analysis found no evidence that genetically lower adiponectin levels were causally associated with higher fasting insulin or diabetes, although there was suggestive evidence of a causal association with lower insulin sensitivity (39). Notably, the same study did find that genetic variants associated with higher fasting insulin were associated with lower adiponectin levels. These observations raise the possibility that insulin, rather than adiponectin, could be the primary causal factor underlying the reciprocal relationship between adiponectin and insulin resistance or that a bidirectional association may exist, but this will require further study.
In summary, the extensive laboratory, clinical, and epidemiologic investigation of adiponectin to date has revealed a multi-faceted molecule that bears complex relationships with cardiovascular, metabolic, and immune/inflammatory pathways across a range of tissues. The two newly published studies in this issue showcase these complex relationships, and the opposite associations that result in health and disease. Although the initial promise of this adipokine as an insulin sensitizer and atheroprotective molecule has not been realized, unraveling the basis for the context-specific prognostic implications of this adipokine is a foremost concern, and may yet lead to potentially useful applications of this mystifying molecule for therapeutic, diagnostic, and prognostic ends.
Acknowledgements
This work was supported by R01 HL-094555 from the National Heart, Lung and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The author has no relevant financial relationship or conflict of interest to disclose.
References
- 1.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 2.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 3.Szmitko PE, Teoh H, Stewart DJ, Verma S. Adiponectin and cardiovascular disease: state of the art? Am J Physiol Heart Circ Physiol. 2007;292:H1655–H1663. doi: 10.1152/ajpheart.01072.2006. [DOI] [PubMed] [Google Scholar]
- 4.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. Jama. 2009;302:179–188. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 6.Kizer JR, Arnold AM, Benkeser D, Ix JH, Djousse L, Zieman SJ, Barzilay JI, Tracy RP, Mantzoros CS, Siscovick DS, Mukamal KJ. Total and high-molecular-weight adiponectin and risk of incident diabetes in older people. Diabetes Care. 2012;35:415–423. doi: 10.2337/dc11-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM, Danesh J, Whincup PH. Adiponectin and coronary heart disease. A prospective study and meta-analysis. Circulation. 2006;114:623–629. doi: 10.1161/CIRCULATIONAHA.106.618918. [DOI] [PubMed] [Google Scholar]
- 8.Menon V, Li L, Wang X, Greene T, Balakrishnan V, Madero M, Pereira AA, Beck GJ, Kusek JW, Collins AJ, Levey AS, Sarnak MJ. Adiponectin and mortality in patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2599–2606. doi: 10.1681/ASN.2006040331. [DOI] [PubMed] [Google Scholar]
- 9.Kistorp C, Faber J, Galatius S, Gustafsson F, Frystyk J, Flyvbjerg A, Hildebrandt P. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 10.Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Clark LT, Pinsky DJ, Marmur JD. Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. Eur Heart J. 2006;27:2300–2309. doi: 10.1093/eurheartj/ehl153. [DOI] [PubMed] [Google Scholar]
- 11.Poehls J, Wassel CL, Harris TB, Havel PJ, Swarbrick MM, Cummings SR, Newman AB, Satterfield S, Kanaya AM. Association of adiponectin with mortality in older adults: the Health, Aging, and Body Composition Study. Diabetologia. 2009;52:591–595. doi: 10.1007/s00125-009-1261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teoh H, Strauss MH, Szmitko PE, Verma S. Adiponectin and myocardial infarction: A paradox or a paradigm? Eur Heart J. 2006;27:2266–2268. doi: 10.1093/eurheartj/ehl248. [DOI] [PubMed] [Google Scholar]
- 13.Hao G, Li W, Guo R, Yang JG, Wang Y, Tian Y, Liu MY, Peng YG, Wang ZW. Serum total adiponectin level and the risk of cardiovascular disease in general population: a meta-analysis of 17 prospective studies. Atherosclerosis. 2013;228:29–35. doi: 10.1016/j.atherosclerosis.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Sook Lee E, Park SS, Kim E, Sook Yoon Y, Ahn HY, Park CY, Ho Yun Y, Woo Oh S. Association between adiponectin levels and coronary heart disease and mortality: a systematic review and meta-analysis. Int J Epidemiol. 2013;42:1029–1039. doi: 10.1093/ije/dyt087. [DOI] [PubMed] [Google Scholar]
- 15.Kanhai DA, Kranendonk ME, Uiterwaal CS, van der Graaf Y, Kappelle LJ, Visseren FL. Adiponectin and incident coronary heart disease and stroke. A systematic review and meta-analysis of prospective studies. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2013;14:555–567. doi: 10.1111/obr.12027. [DOI] [PubMed] [Google Scholar]
- 16.Kizer JR, Benkeser D, Arnold AM, Mukamal KJ, Ix JH, Zieman SJ, Siscovick DS, Tracy RP, Mantzoros CS, Defilippi CR, Newman AB, Djousse L. Associations of total and high-molecular-weight adiponectin with all-cause and cardiovascular mortality in older persons: the Cardiovascular Health Study. Circulation. 2012;126:2951–2961. doi: 10.1161/CIRCULATIONAHA.112.135202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kizer JR, Benkeser D, Arnold AM, Djousse L, Zieman SJ, Mukamal KJ, Tracy RP, Mantzoros CS, Siscovick DS, Gottdiener JS, Ix JH. Total and high-molecular-weight adiponectin and risk of coronary heart disease and ischemic stroke in older adults. J Clin Endocrinol Metab. 2013;98:255–263. doi: 10.1210/jc.2012-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arregui M, Buijsse B, Fritsche A, di Giuseppe R, Schulze MB, Westphal S, Isermann B, Boeing H, Weikert C. Adiponectin and risk of stroke: prospective study and meta-analysis. Stroke. 2014;45:10–17. doi: 10.1161/STROKEAHA.113.001851. [DOI] [PubMed] [Google Scholar]
- 19.Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiology. 1992;3:143–155. doi: 10.1097/00001648-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Tsukamoto O, Fujita M, Kato M, Yamazaki S, Asano Y, Ogai A, Okazaki H, Asai M, Nagamachi Y, Maeda N, Shintani Y, Minamino T, Asakura M, Kishimoto I, Funahashi T, Tomoike H, Kitakaze M. Natriuretic peptides enhance the production of adiponectin in human adipocytes and in patients with chronic heart failure. J Am Coll Cardiol. 2009;53:2070–2077. doi: 10.1016/j.jacc.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 21.Maisel AS, Daniels LB. Breathing not properly 10 years later: what we have learned and what we still need to learn. J Am Coll Cardiol. 2012;60:277–282. doi: 10.1016/j.jacc.2012.03.057. [DOI] [PubMed] [Google Scholar]
- 22.Longstreth WT, Jr, Kronmal RA, Thompson JL, Christenson RH, Levine SR, Gross R, Brey RL, Buchsbaum R, Elkind MS, Tirschwell DL, Seliger SL, Mohr JP, deFilippi CR. Amino terminal pro-B-type natriuretic peptide, secondary stroke prevention, and choice of antithrombotic therapy. Stroke. 2013;44:714–719. doi: 10.1161/STROKEAHA.112.675942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kizer JR, Arnold AM, Jenny NS, Cushman M, Strotmeyer ES, Ives DG, Ding J, Kritchevsky SB, Chaves PH, Hirsch CH, Newman AB. Longitudinal changes in adiponectin and inflammatory markers and relation to survival in the oldest old: the Cardiovascular Health Study All Stars study. J Gerontol A Biol Sci Med Sci. 2011;66:1100–1107. doi: 10.1093/gerona/glr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kizer JR, Barzilay JI, Kuller LH, Gottdiener JS. Adiponectin and risk of coronary heart disease in older men and women. J Clin Endocrinol Metab. 2008;93:3357–3364. doi: 10.1210/jc.2008-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wannamethee SG, Whincup PH, Lennon L, Sattar N. Circulating adiponectin levels and mortality in elderly men with and without cardiovascular disease and heart failure. Arch Intern Med. 2007;167:1510–1517. doi: 10.1001/archinte.167.14.1510. [DOI] [PubMed] [Google Scholar]
- 26.Murphy RA, Register TC, Shively CA, Carr JJ, Ge Y, Heilbrun ME, Cummings SR, Koster A, Nevitt MC, Satterfield S, Tylvasky FA, Strotmeyer ES, Newman AB, Simonsick EM, Scherzinger A, Goodpaster BH, Launer LJ, Eiriksdottir G, Sigurdsson S, Sigurdsson G, Gudnason V, Lang TF, Kritchevsky SB, Harris TB. Adipose tissue density, a novel biomarker predicting mortality risk in older adults. J Gerontol A Biol Sci Med Sci. 2014;69:109–117. doi: 10.1093/gerona/glt070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marques MB, Langouche L. Endocrine, metabolic, and morphologic alterations of adipose tissue during critical illness. Critical care medicine. 2013;41:317–325. doi: 10.1097/CCM.0b013e318265f21c. [DOI] [PubMed] [Google Scholar]
- 28.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 29.Van Berendoncks AM, Garnier A, Beckers P, Hoymans VY, Possemiers N, Fortin D, Martinet W, Van Hoof V, Vrints CJ, Ventura-Clapier R, Conraads VM. Functional adiponectin resistance at the level of the skeletal muscle in mild to moderate chronic heart failure. Circ Heart Fail. 2010;3:185–194. doi: 10.1161/CIRCHEARTFAILURE.109.885525. [DOI] [PubMed] [Google Scholar]
- 30.Ouchi N, Walsh K. A novel role for adiponectin in the regulation of inflammation. Arterioscler Thromb Vasc Biol. 2008;28:1219–1221. doi: 10.1161/ATVBAHA.108.165068. [DOI] [PubMed] [Google Scholar]
- 31.Fantuzzi G. Adiponectin in inflammatory and immune-mediated diseases. Cytokine. 2013;64:1–10. doi: 10.1016/j.cyto.2013.06.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peake PW, Shen Y, Walther A, Charlesworth JA. Adiponectin binds C1q and activates the classical pathway of complement. Biochem Biophys Res Commun. 2008;367:560–565. doi: 10.1016/j.bbrc.2007.12.161. [DOI] [PubMed] [Google Scholar]
- 33.Parker-Duffen JL, Walsh K. Cardiometabolic effects of adiponectin. Best practice & research Clinical endocrinology & metabolism. 2014;28:81–91. doi: 10.1016/j.beem.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denzel MS, Scimia MC, Zumstein PM, Walsh K, Ruiz-Lozano P, Ranscht B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest. 2010;120:4342–4352. doi: 10.1172/JCI43464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dastani Z, Hivert MF, Timpson N, Perry JR, Yuan X, Scott RA, Henneman P, Heid IM, Kizer JR, Lyytikainen LP, Fuchsberger C, Tanaka T, Morris AP, Small K, Isaacs A, Beekman M, Coassin S, Lohman K, Qi L, Kanoni S, Pankow JS, Uh HW, Wu Y, Bidulescu A, Rasmussen-Torvik LJ, Greenwood CM, Ladouceur M, Grimsby J, Manning AK, Liu CT, Kooner J, Mooser VE, Vollenweider P, Kapur KA, Chambers J, Wareham NJ, Langenberg C, Frants R, Willems-Vandijk K, Oostra BA, Willems SM, Lamina C, Winkler TW, Psaty BM, Tracy RP, Brody J, Chen I, Viikari J, Kahonen M, Pramstaller PP, Evans DM, St Pourcain B, Sattar N, Wood AR, Bandinelli S, Carlson OD, Egan JM, Bohringer S, van Heemst D, Kedenko L, Kristiansson K, Nuotio ML, Loo BM, Harris T, Garcia M, Kanaya A, Haun M, Klopp N, Wichmann HE, Deloukas P, Katsareli E, Couper DJ, Duncan BB, Kloppenburg M, Adair LS, Borja JB, Wilson JG, Musani S, Guo X, Johnson T, Semple R, Teslovich TM, Allison MA, Redline S, Buxbaum SG, Mohlke KL, Meulenbelt I, Ballantyne CM, Dedoussis GV, Hu FB, Liu Y, Paulweber B, Spector TD, Slagboom PE, Ferrucci L, Jula A, Perola M, Raitakari O, Florez JC, Salomaa V, Eriksson JG, Frayling TM, Hicks AA, Lehtimaki T, Smith GD, Siscovick DS, Kronenberg F, van Duijn C, Loos RJ, Waterworth DM, Meigs JB, Dupuis J, Richards JB, Voight BF, Scott LJ, Steinthorsdottir V, Dina C, Welch RP, Zeggini E, Huth C, Aulchenko YS, Thorleifsson G, McCulloch LJ, Ferreira T, Grallert H, Amin N, Wu G, Willer CJ, Raychaudhuri S, McCarroll SA, Hofmann OM, Segre AV, van Hoek M, Navarro P, Ardlie K, Balkau B, Benediktsson R, Bennett AJ, Blagieva R, Boerwinkle E, Bonnycastle LL, Bostrom KB, Bravenboer B, Bumpstead S, Burtt NP, Charpentier G, Chines PS, Cornelis M, Crawford G, Doney AS, Elliott KS, Elliott AL, Erdos MR, Fox CS, Franklin CS, Ganser M, Gieger C, Grarup N, Green T, Griffin S, Groves CJ, Guiducci C, Hadjadj S, Hassanali N, Herder C, Isomaa B, Jackson AU, Johnson PR, Jorgensen T, Kao WH, Kong A, Kraft P, Kuusisto J, Lauritzen T, Li M, Lieverse A, Lindgren CM, Lyssenko V, Marre M, Meitinger T, Midthjell K, Morken MA, Narisu N, Nilsson P, Owen KR, Payne F, Petersen AK, Platou C, Proenca C, Prokopenko I, Rathmann W, Rayner NW, Robertson NR, Rocheleau G, Roden M, Sampson MJ, Saxena R, Shields BM, Shrader P, Sigurdsson G, Sparso T, Strassburger K, Stringham HM, Sun Q, Swift AJ, Thorand B, Tichet J, Tuomi T, van Dam RM, van Haeften TW, van Herpt T, van Vliet-Ostaptchouk JV, Walters GB, Weedon MN, Wijmenga C, Witteman J, Bergman RN, Cauchi S, Collins FS, Gloyn AL, Gyllensten U, Hansen T, Hide WA, Hitman GA, Hofman A, Hunter DJ, Hveem K, Laakso M, Morris AD, Palmer CN, Rudan I, Sijbrands E, Stein LD, Tuomilehto J, Uitterlinden A, Walker M, Watanabe RM, Abecasis GR, Boehm BO, Campbell H, Daly MJ, Hattersley AT, Pedersen O, Barroso I, Groop L, Sladek R, Thorsteinsdottir U, Wilson JF, Illig T, Froguel P, van Duijn CM, Stefansson K, Altshuler D, Boehnke M, McCarthy MI, Soranzo N, Wheeler E, Glazer NL, Bouatia-Naji N, Magi R, Randall J, Elliott P, Rybin D, Dehghan A, Hottenga JJ, Song K, Goel A, Lajunen T, Doney A, Cavalcanti-Proenca C, Kumari M, Timpson NJ, Zabena C, Ingelsson E, An P, O'Connell J, Luan J, Elliott A, McCarroll SA, Roccasecca RM, Pattou F, Sethupathy P, Ariyurek Y, Barter P, Beilby JP, Ben-Shlomo Y, Bergmann S, Bochud M, Bonnefond A, Borch-Johnsen K, Bottcher Y, Brunner E, Bumpstead SJ, Chen YD, Chines P, Clarke R, Coin LJ, Cooper MN, Crisponi L, Day IN, de Geus EJ, Delplanque J, Fedson AC, Fischer-Rosinsky A, Forouhi NG, Franzosi MG, Galan P, Goodarzi MO, Graessler J, Grundy S, Gwilliam R, Hallmans G, Hammond N, Han X, Hartikainen AL, Hayward C, Heath SC, Hercberg S, Hillman DR, Hingorani AD, Hui J, Hung J, Kaakinen M, Kaprio J, Kesaniemi YA, Kivimaki M, Knight B, Koskinen S, Kovacs P, Kyvik KO, Lathrop GM, Lawlor DA, Le Bacquer O, Lecoeur C, Li Y, Mahley R, Mangino M, Martinez-Larrad MT, McAteer JB, McPherson R, Meisinger C, Melzer D, Meyre D, Mitchell BD, Mukherjee S, Naitza S, Neville MJ, Orru M, Pakyz R, Paolisso G, Pattaro C, Pearson D, Peden JF, Pedersen NL, Pfeiffer AF, Pichler I, Polasek O, Posthuma D, Potter SC, Pouta A, Province MA, Rayner NW, Rice K, Ripatti S, Rivadeneira F, Rolandsson O, Sandbaek A, Sandhu M, Sanna S, Sayer AA, Scheet P, Seedorf U, Sharp SJ, Shields B, Sigurethsson G, Sijbrands EJ, Silveira A, Simpson L, Singleton A, Smith NL, Sovio U, Swift A, Syddall H, Syvanen AC, Tonjes A, Uitterlinden AG, van Dijk KW, Varma D, Visvikis-Siest S, Vitart V, Vogelzangs N, Waeber G, Wagner PJ, Walley A, Ward KL, Watkins H, Wild SH, Willemsen G, Witteman JC, Yarnell JW, Zelenika D, Zethelius B, Zhai G, Zhao JH, Zillikens MC, Borecki IB, Meneton P, Magnusson PK, Nathan DM, Williams GH, Silander K, Bornstein SR, Schwarz P, Spranger J, Karpe F, Shuldiner AR, Cooper C, Serrano-Rios M, Lind L, Palmer LJ, Hu FBs, Franks PW, Ebrahim S, Marmot M, Kao WH, Pramstaller PP, Wright AF, Stumvoll M, Hamsten A, Buchanan TA, Valle TT, Rotter JI, Penninx BW, Boomsma DI, Cao A, Scuteri A, Schlessinger D, Uda M, Ruokonen A, Jarvelin MR, Peltonen L, Mooser V, Sladek R, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Chasman DI, Johansen CT, Fouchier SW, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Feitosa MF, Orho-Melander M, Melander O, Li X, Li M, Cho YS, Go MJ, Kim YJ, Lee JY, Park T, Kim K, Sim X, Ong RT, Croteau-Chonka DC, Lange LA, Smith JD, Ziegler A, Zhang W, Zee RY, Whitfield JB, Thompson JR, Surakka I, Spector TD, Smit JH, Sinisalo J, Scott J, Saharinen J, Sabatti C, Rose LM, Roberts R, Rieder M, Parker AN, Pare G, O'Donnell CJ, Nieminen MS, Nickerson DA, Montgomery GW, McArdle W, Masson D, Martin NG, Marroni F, Lucas G, Luben R, Lokki ML, Lettre G, Launer LJ, Lakatta EG, Laaksonen R, Kyvik KO, Konig IR, Khaw KT, Kaplan LM, Johansson A, Janssens AC, Igl W, Hovingh GK, Hengstenberg C, Havulinna AS, Hastie ND, Harris TB, Haritunians T, Hall AS, Groop LC, Gonzalez E, Freimer NB, Erdmann J, Ejebe KG, Doring A, Dominiczak AF, Demissie S, Deloukas P, de Faire U, Crawford G, Chen YD, Caulfield MJ, Boekholdt SM, Assimes TL, Quertermous T, Seielstad M, Wong TY, Tai ES, Feranil AB, Kuzawa CW, Taylor HA, Jr, Gabriel SB, Holm H, Gudnason V, Krauss RM, Ordovas JM, Munroe PB, Kooner JS, Tall AR, Hegele RA, Kastelein JJ, Schadt EE, Strachan DP, Reilly MP, Samani NJ, Schunkert H, Cupples LA, Sandhu MS, Ridker PM, Rader DJ, Kathiresan S. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS genetics. 2012;8:e1002607. doi: 10.1371/journal.pgen.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cook JR, Semple RK. Hypoadiponectinemia--cause or consequence of human "insulin resistance"? J Clin Endocrinol Metab. 2010;95:1544–1554. doi: 10.1210/jc.2009-2286. [DOI] [PubMed] [Google Scholar]
- 37.Gao H, Fall T, Dam RMv, Flyvbjerg A, Zethelius B, Ingelsson E, Hagg S. Evidence of a causal relationship between adiponectin levels and insulin sensitivity: A Mendelian randomization study. Diabetes. 2012 doi: 10.2337/db12-0935. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kizer JR. A tangled threesome: adiponectin, insulin sensitivity, and adiposity: can Mendelian randomization sort out causality? Diabetes. 2013;62:1007–1009. doi: 10.2337/db12-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yaghootkar H, Lamina C, Scott RA, Dastani Z, Hivert MF, Warren LL, Stancakova A, Buxbaum SG, Lyytikainen LP, Henneman P, Wu Y, Cheung CY, Pankow JS, Jackson AU, Gustafsson S, Zhao JH, Ballantyne CM, Xie W, Bergman RN, Boehnke M, el Bouazzaoui F, Collins FS, Dunn SH, Dupuis J, Forouhi NG, Gillson C, Hattersley AT, Hong J, Kahonen M, Kuusisto J, Kedenko L, Kronenberg F, Doria A, Assimes TL, Ferrannini E, Hansen T, Hao K, Haring H, Knowles JW, Lindgren CM, Nolan JJ, Paananen J, Pedersen O, Quertermous T, Smith U, Consortium G, Consortium R, Lehtimaki T, Liu CT, Loos RJ, McCarthy MI, Morris AD, Vasan RS, Spector TD, Teslovich TM, Tuomilehto J, van Dijk KW, Viikari JS, Zhu N, Langenberg C, Ingelsson E, Semple RK, Sinaiko AR, Palmer CN, Walker M, Lam KS, Paulweber B, Mohlke KL, van Duijn C, Raitakari OT, Bidulescu A, Wareham NJ, Laakso M, Waterworth DM, Lawlor DA, Meigs JB, Richards JB, Frayling TM. Mendelian randomization studies do not support a causal role for reduced circulating adiponectin levels in insulin resistance and type 2 diabetes. Diabetes. 2013;62:3589–3598. doi: 10.2337/db13-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]