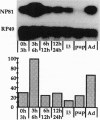
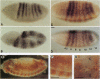
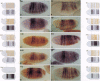
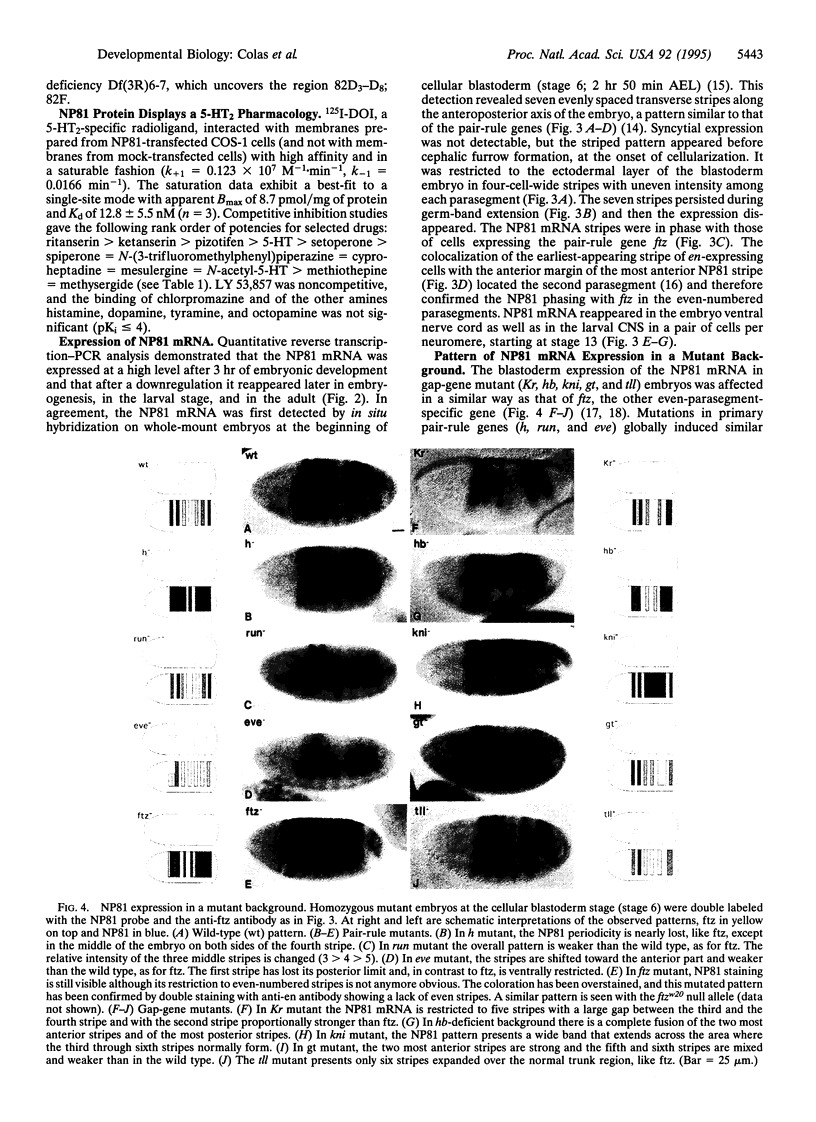
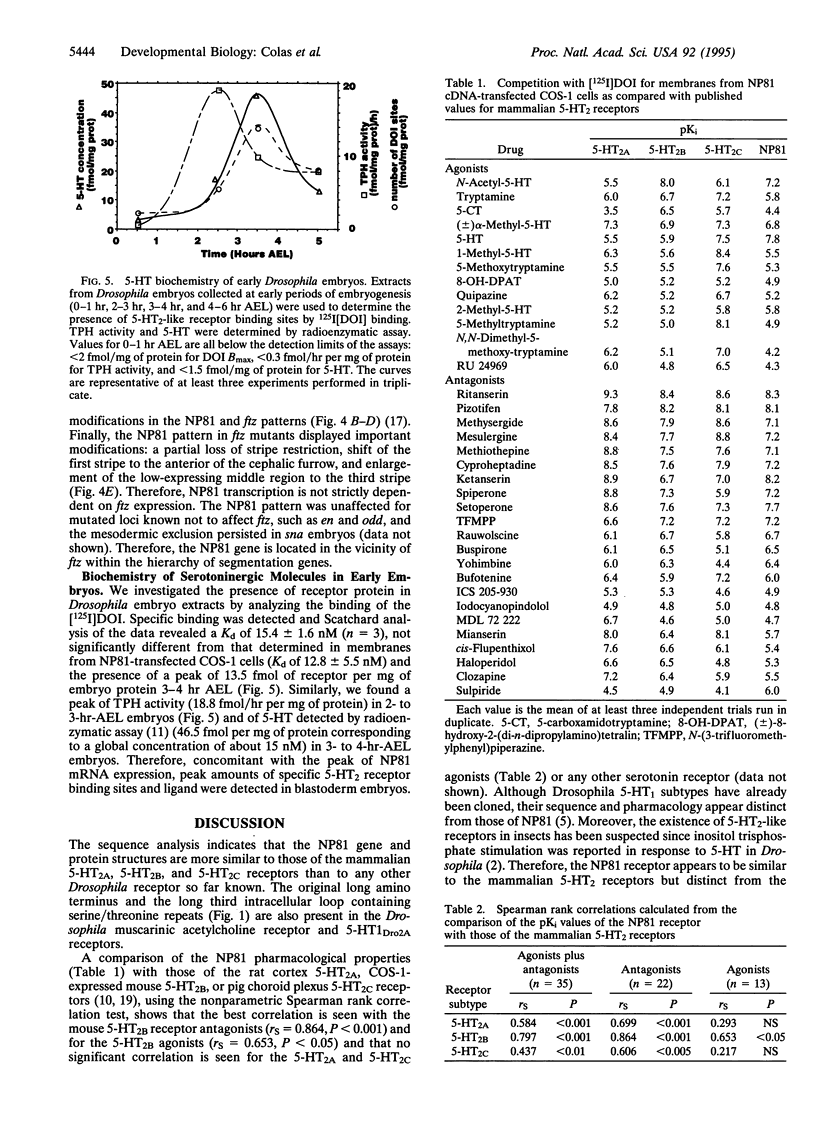
Abstract
Serotonin, first described as a neurotransmitter in invertebrates, has been investigated mostly for its functions in the mature central nervous system of higher vertebrates. Serotonin receptor diversity has been described in the mammalian brain and in insects. We report the isolation of a cDNA coding for a Drosophila melanogaster serotonin receptor that displays a sequence, a gene organization, and pharmacological properties typical of the mammalian 5-HT2 serotonin receptor subtype. Its mRNA can be detected in the adult fly; moreover, a high level of expression occurs at 3 hr of Drosophila embryogenesis. This early embryonic expression is surprisingly organized in a seven-stripe pattern that appears at the cellular blastoderm stage. In addition, this pattern is in phase with that of the even-parasegment-expressed pair-rule gene fushi-tarazu and is similarly modified by mutations affecting segmentation genes. Simultaneously with this pair-rule expression, the complete machinery of serotonin synthesis is present and leads to a peak of ligand concomitant with a peak of 5-HT2-specific receptor sites in blastoderm embryos.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akam M. The molecular basis for metameric pattern in the Drosophila embryo. Development. 1987 Sep;101(1):1–22. [PubMed] [Google Scholar]
- Baumgartner S., Martin D., Hagios C., Chiquet-Ehrismann R. Tenm, a Drosophila gene related to tenascin, is a new pair-rule gene. EMBO J. 1994 Aug 15;13(16):3728–3740. doi: 10.1002/j.1460-2075.1994.tb06682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buc-Caron M. H., Launay J. M., Lamblin D., Kellermann O. Serotonin uptake, storage, and synthesis in an immortalized committed cell line derived from mouse teratocarcinoma. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1922–1926. doi: 10.1073/pnas.87.5.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V., Wu C. F., White K. Altered branching of serotonin-containing neurons in Drosophila mutants unable to synthesize serotonin and dopamine. J Neurosci. 1989 Aug;9(8):2866–2877. doi: 10.1523/JNEUROSCI.09-08-02866.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. B., Scott M. P. Zygotically active genes that affect the spatial expression of the fushi tarazu segmentation gene during early Drosophila embryogenesis. Cell. 1986 Apr 11;45(1):113–126. doi: 10.1016/0092-8674(86)90543-x. [DOI] [PubMed] [Google Scholar]
- Doe C. Q. Molecular markers for identified neuroblasts and ganglion mother cells in the Drosophila central nervous system. Development. 1992 Dec;116(4):855–863. doi: 10.1242/dev.116.4.855. [DOI] [PubMed] [Google Scholar]
- Gietz R. D., Hodgetts R. B. An analysis of dopa decarboxylase expression during embryogenesis in Drosophila melanogaster. Dev Biol. 1985 Jan;107(1):142–155. doi: 10.1016/0012-1606(85)90383-5. [DOI] [PubMed] [Google Scholar]
- Glennon R. A., Seggel M. R., Soine W. H., Herrick-Davis K., Lyon R. A., Titeler M. [125I]-1-(2,5-dimethoxy-4-iodophenyl)-2-amino-propane: an iodinated radioligand that specifically labels the agonist high-affinity state of 5-HT2 serotonin receptors. J Med Chem. 1988 Jan;31(1):5–7. doi: 10.1021/jm00396a003. [DOI] [PubMed] [Google Scholar]
- Hen R. Of mice and flies: commonalities among 5-HT receptors. Trends Pharmacol Sci. 1992 Apr;13(4):160–165. doi: 10.1016/0165-6147(92)90054-a. [DOI] [PubMed] [Google Scholar]
- Hoyer D., Clarke D. E., Fozard J. R., Hartig P. R., Martin G. R., Mylecharane E. J., Saxena P. R., Humphrey P. P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev. 1994 Jun;46(2):157–203. [PubMed] [Google Scholar]
- Krause H. M., Gehring W. J. Stage-specific phosphorylation of the fushi tarazu protein during Drosophila development. EMBO J. 1989 Apr;8(4):1197–1204. doi: 10.1002/j.1460-2075.1989.tb03492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A., Bashan-Ahrend A., Budai-Hadrian O., Gartenberg D., Menasherow S., Wides R. Odd Oz: a novel Drosophila pair rule gene. Cell. 1994 May 20;77(4):587–598. doi: 10.1016/0092-8674(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Loric S., Maroteaux L., Kellermann O., Launay J. M. Functional serotonin-2B receptors are expressed by a teratocarcinoma-derived cell line during serotoninergic differentiation. Mol Pharmacol. 1995 Mar;47(3):458–466. [PubMed] [Google Scholar]
- Lundell M. J., Hirsh J. Temporal and spatial development of serotonin and dopamine neurons in the Drosophila CNS. Dev Biol. 1994 Oct;165(2):385–396. doi: 10.1006/dbio.1994.1261. [DOI] [PubMed] [Google Scholar]
- Mahoney P. A., Lengyel J. A. The zygotic segmentation mutant tailless alters the blastoderm fate map of the Drosophila embryo. Dev Biol. 1987 Aug;122(2):464–470. doi: 10.1016/0012-1606(87)90310-1. [DOI] [PubMed] [Google Scholar]
- Monnier D., Colas J. F., Rosay P., Hen R., Borrelli E., Maroteaux L. NKD, a developmentally regulated tachykinin receptor in Drosophila. J Biol Chem. 1992 Jan 15;267(2):1298–1302. [PubMed] [Google Scholar]
- Neckameyer W. S., White K. A single locus encodes both phenylalanine hydroxylase and tryptophan hydroxylase activities in Drosophila. J Biol Chem. 1992 Feb 25;267(6):4199–4206. [PubMed] [Google Scholar]
- Peroutka S. J. 5-HT receptors: past, present and future. Trends Neurosci. 1995 Feb;18(2):68–69. [PubMed] [Google Scholar]
- Probst W. C., Snyder L. A., Schuster D. I., Brosius J., Sealfon S. C. Sequence alignment of the G-protein coupled receptor superfamily. DNA Cell Biol. 1992 Jan-Feb;11(1):1–20. doi: 10.1089/dna.1992.11.1. [DOI] [PubMed] [Google Scholar]
- Taghert P. H., Goodman C. S. Cell determination and differentiation of identified serotonin-immunoreactive neurons in the grasshopper embryo. J Neurosci. 1984 Apr;4(4):989–1000. doi: 10.1523/JNEUROSCI.04-04-00989.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. F., Friedman D. W., Jimenez A. A modified enzymatic-isotopic microassay for serotonin (5HT) using 5HT-N-acetyltransferase partially purified from Drosophila. Life Sci. 1983 Nov 7;33(19):1915–1924. doi: 10.1016/0024-3205(83)90676-8. [DOI] [PubMed] [Google Scholar]
- Wright T. R. The genetics of biogenic amine metabolism, sclerotization, and melanization in Drosophila melanogaster. Adv Genet. 1987;24:127–222. [PubMed] [Google Scholar]