Abstract
The study of CNS glial cell function requires experimental methods to detect, purify, and manipulate each cell population with fidelity and specificity. With the identification and cloning of cell- and stage-specific markers, glial cell analysis techniques have grown beyond physical methods of tissue dissociation and cell culture, and become highly specific with immunoselection of cell cultures in vitro and genetic targeting in vivo. The unique plasticity of glial cells offers the potential for cell replacement therapies in neurological disease that utilize neural cells derived from transplanted neural stem and progenitor cells. In this mini-review, we outline general physical and genetic approaches for macroglial cell generation. We summarize cell culture methods to obtain astrocytes and oligodendrocytes and their precursors, from developing and adult tissue, as well as approaches to obtain human neural progenitor cells through the establishment of stem cells. We discuss popular targeting rodent strains designed for cell-specific detection, selection and manipulation of neuroglial cell progenitors and their committed progeny. Based on shared markers between astrocytes and stem cells, we discuss genetically modified mouse strains with overlapping expression, and highlight SOX-expressing strains available for targeting of stem and progenitor cell populations. We also include recently established mouse strains for detection, and tag-assisted RNA and miRNA analysis. This discussion aims to provide a brief overview of the rapidly expanding collection of experimental approaches and genetic resources for the isolation and targeting of macroglial cells, their sources, progeny and gene products to facilitate our understanding of their properties and potential application in pathology.
Keywords: neural precursor, astrocytes, oligodendroglia, cell purification, genetic targeting, transgenic mice
1. INTRODUCTION
Since their initial discovery, the perception of glia as being mere supportive “glue” for neurons has been transformed into an unexpectedly complex role as valuable partners in cell-cell communication and homeostasis. Diverse glial functions in neuronal conduction, synaptic development and repair have now overshadowed the previous concepts of glial cells as passive structural and, at best, trophic support for neurons. Aside from radial glia, ependymal cells, and cells of the neurovasculature, at least three major subtypes of glial cells: astrocytes, oligodendrocytes and microglia are present in the central nervous system (CNS). A fourth type, NG2 (nerve/glial antigen 2) cells or polydendrocytes, although widely recognized as oligodendrocyte progenitor cells (OPCs) in white matter (WM) structures, show expression patterns and electrical properties that defy conventional glial cell classification. Astrocytes are important regulators of neurotransmitter function, metabolic support, neuronal migration and development, synaptic processing, cell plasticity, excitotoxicity, immune function and blood-brain barrier integrity. Oligodendrocytes are myelin-producing cells of the CNS, and whose layers of compacted cell membranes ensheath axons with lipid-rich insulation that is critical for rapid salutatory conduction by axon fibers. In addition to myelination, oligodendroglia have been found to provide axons with direct metabolic support via lactate, a process that prevents axon degeneration and death (Lee et al., 2012). Both astrocytes and oligodendrocytes are described to develop from common progenitor populations. While many brain tumors consist predominantly of precursors of astrocytic nature, each of the individual glial cell types is now recognized as a viable therapeutic candidate in neurological disease, in addition to neurons. The growing importance of glial cells has led to the development of a variety of techniques for their purification and analysis. An important aspect of studying cellular function in the CNS lies in the ability to target individual cell populations, based on identity, developmental stage, and cellular lineage or fate. For their analysis in vitro and in vivo, specific glial populations may be collected by tissue isolation and culture methods, as well as through the use of genetic animal models. In separate sections of this paper, we will: A) review culture techniques for the production of astrocytes and oligodendrocyte progenitor cells primarily from rodent brain, and stem cell-based approaches to generate these cells, and B) describe driver mouse strains for glial-specific cell labeling and gene manipulation. In the second section, we will also highlight new reporter and effector mouse strains for driver-assisted gene ablation, cell identification and in vivo molecular capture that are now available for gene expression analysis.
2. Cell purification and primary glial cell culture
Glial cells may be acutely isolated from dissociated brain and spinal cord tissue either as a mixed population or with further purification via immunolabeling or reporter fluorescence in recombinant mouse strains. The first step of tissue dissociation consists of subjecting dissected tissue pieces to enzymatic digestion with papain and DNase I, followed by mechanical trituration using a series of needles of decreasing gauge size (e.g. 19, 21 then 23 G) (Belachew et al., 2002) and subsequent removal of aggregates by passing the suspension through a cell strainer (Belachew et al., 2002). For mature CNS white matter tissue with high myelin content, an additional purification step prior to cell selection is often beneficial to cell yield (Jiho Sohn, Univ California, Davis, personal communication) (Sohn et al., 2006). This involves layering the dissociated cell suspension onto a pre-formed density gradient of Percoll™, followed by high speed centrifugation, to separate neural cells from lipid-rich myelin, debris (Avellana-Adalid et al., 1996; Lubetzki et al., 1991) and blood cells. These purified cells, once cleared of Percoll™, may be maintained in culture (Zhang et al., 2004). Acutely isolated cells may also be selected by immunolabeling before collection by fluorescence-activated cell sorting (FACS) (Nielsen et al., 2006) (Figure 1). Alternatively, cells from fluorescent reporter mouse strains may be directly collected by single-channel FACS (Belachew et al., 2002) or doubly selected by a combination of immunolabeling and dual-channel FACS collection (Belachew et al., 2003).
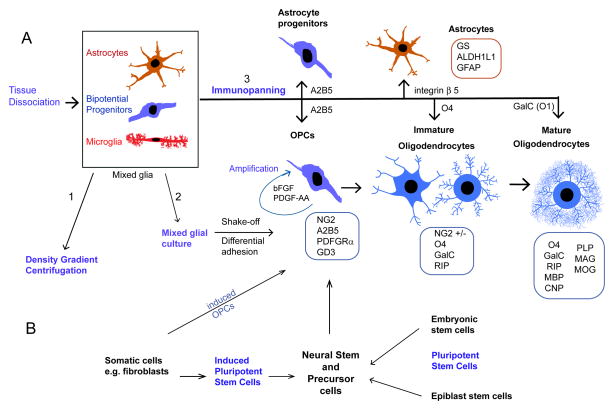
Figure 1.
Overview of strategies to produce astrocytes and oligodendrocytes from brain tissue or stem cells. A. CNS tissue-derived approach begins with tissue dissociation. The resulting cell suspension may be subjected to: 1. Density gradient centrifugation to remove myelin debris and blood cells, and cell fractions are isolated according to buoyant density. 2. Culture as mixed glia. At high plating density which promotes progenitor cell generation, when the bed monolayer of astrocytes reaches confluency and progenitor clusters loosely attached, bipotential progenitors and microglia are shaken off. OPC clusters are enriched after microglial removal by preferential adhesion to culture dishes, and may be subsequently amplified with mitogens PDGF-AA and bFGF. 3. Immunopanning sequentially on culture dishes coated with antibodies for positive and negative selection, depending on desired cell population. O4 and O1(GalC) have been used for the selection of committed oligodendroglial cells, while A2B5 has been used for selection of bipotential progenitor cells, namely from the optic nerve. GalC is a sphingolipid of myelin. A2B5 cells can generate both oligodendrocytes and type-2 astrocytes, which is believed to be an in vitro phenomenon (de Castro and Bribian, 2005). Boxes represent commonly used markers for the identification of the various glial cell stages over development. Immature oligodendrocytes which express the O4 antigen consist of NG2-expressing cells as well as those that have lost NG2 expression (NG2+/-).
B. Stem cell derived approach. Neural stem and precursor cells are obtained from pluripotent stem cells (iPS) which are cultured from embryonic or non-embryonic sources, including somatic cells such as adult or embryonic mouse fibroblasts. Both human and rodent iPS cells have been successfully differentiated into functional oligodendrocytes (Wang et al., 2013) through generating neural-restricted precursor cells. Mouse embryonic and lung fibroblasts have also produced induced OPCs by direct reprogramming with transcription factors (Najm et al., 2013).
Despite the limitation that primary cultured cells in isolation are not morphological and functional duplicates of their in vivo counterparts, cell cultures still hold an important and special place in current methodologies. Indeed it was in cultures developed by McCarthy and de Vellis (McCarthy and de Vellis, 1980) that astrocytes and oligodendrocyte progenitor cells (OPCs) were prepared from the neonatal rat and characterized in exhaustive detail, forming the foundation of current knowledge of glial cell characteristics, physiology and development. As summarized in Figure 1 and Table 1, astrocytes and oligodendrocytes are frequently obtained by the establishment of mixed glial cultures from dissociated CNS tissue of neonatal rodents, isolation of their common progenitor by shaking (Levine, 1989; Saneto and de Vellis, 1985) and positive immune-selection –‘panning’- with monoclonal antibody A2B5 against the surface antigen (Stallcup and Beasley, 1987). With the advantages of sensitivity, ease and cost (relative to whole animal models), applications such as high throughput pharmacological analysis (James et al., 2011) rely on cultures for reasons of volume and scalability. Importantly, the establishment of co-cultures between neurons and astrocytes or oligodendrocytes (Jones et al., 2012; Kunze et al., 2013; Pang et al., 2012; Yang et al., 2009), and between distinct glial cell types (Afshari and Fawcett, 2012; John, 2012; Schmitz et al., 2011), provides useful reconstructive evidence to corroborate tissue explant and whole animal observations.
Table 1.
Macroglial culture from CNS tissue
| Method | Tissue source | Yield, Purity, culture conditions | Cellular characteristics General Remarks | References |
|---|---|---|---|---|
| ASTROCYTES | ||||
| Shaking and removal of contaminating cells | Neonatal rat cerebral hemisphere | Defined media with serum, 10 days in culture, withstands multiple passages | Proliferative, plastic cells lacking processes, differences in glutamate receptor expression, and more activated than in-vivo adult astrocytes | (Cole and de Vellis, 1997; Morrison and de Vellis, 1981; Morrison et al., 1985) (Nakagawa and Schwartz, 2004) (Sun and Dietrich, 2013) |
| Adult cells Mechanically dissociated and plated | P90 adult rat Cerebral Cortex | 15×106 cells/cortex. With serum in DMEM/F12, Maintained for over 5 weeks | Reportedly more similar to adult astrocytes than prenatal counterparts | (Souza et al., 2013) |
| Immuno-panning, Sequential negative selection | E17 and P1 neonatal Rat Optic nerve | 5000 purified astrocyte precursor cells per E17 rat, 4000 astrocytes per P1 optic nerve (90%) | E17: GFAP-, S100b-, A2B5+, Pax2+, Unlike P1 astrocytes, precursor survival requires bFGF and GGF, and differentiate with CNTF or LIF. | (Mi and Barres, 1999) |
| Immuno-panning, Negative selection | Adult human optic nerve heads | Based on neonatal rat method of Mi et al (Mi and Barres, 1999) | Further purification with cloning rings to eliminate smooth muscle and fibroblasts | (Yang and Hernandez, 2003) |
| Immuno-panning, Negative and Positive selection (Integrin beta5) | P1 – P18 rat cortex | 98% GFAP+ astrocytes, Maintained with HB-EGFF or Wnt7a without serum | Slowly dividing, more similar to acutely isolated astrocytes in vivo | (Foo et al., 2011) |
| Rat OPCs | ||||
| Shake-off | Newborn rat cerebral hemispheres | Mixed glial cultures established in serum-containing media for 7–9 days, then shaken overnight. 95% oligodendroglia | Economical, high yield. Same mixed glial cultures used with different purification techniques after shake-off to generate astrocytes and oligodendroglia | (McCarthy and de Vellis, 1980) |
| A2B5-FACS or Shake-off and differential-adhesion | Newborn rat cerebral hemispheres | Up to 95% purity of OPCs. Utilized differential adhesion properties between microglia and oligodendroglia | Amplification of purified OPCs shown to occur by incubating with PDGF and insulin. | (Behar et al., 1988) |
| Dual passaging to eliminate astrocytes and neurons | E17 rat cortices | 2 weekly passages with trypsin in serum media, then serum-free media with PDGF-AA for 2 days to expand OPCs. | Further differentiated with T3, T4 and NT3 without PDGF. OLs formed compact myelin in neuron/astrocyte co-culture | (Itoh, 2002) |
| Shake-off and differential adhesion removal of astrocytes and microglia | Neonatal rat cerebral cortices | Purification by differential adhesion: astrocytes and microglia attach to untreated plastic more efficiently than OPCs. | Economical and high yield. PDGF and bFGF used to amplify purified OPCs for 7–10 days. | (Chen et al., 2007) |
| Shake-off and differential adhesion | P2-P4 rat cerebral cortices | Mixed glia maintained for 10 days before shake-off. | OPCs amplified in N1/DMEM with PDGF Successful for OPC/hippocampal neuron co-cultures | (Yang et al., 2005) |
| Immunopanni ng using negative and positive selection | Neonatal rat cortices and optic nerve; adult optic nerve | Ran-2 and GalC-based removal of astrocytes and OLs followed by positive selection with A2B5. >99.5% purity of OPCs. | Higher purity than shake-off, less damaging than FACS. Highly specific, has capacity to distinguish developmental stages. | (Dugas and Emery, 2013; Gard et al., 1993; Gard et al., 1995; Mayer-Proschel, 2001; Shi et al., 1998) |
| OPCs from NPCs | Embryonic rat spinal cord | NPCs in bFGF/EGF were gradually switched to PDGF and bFGF to generate oligospheres and then passaged at 7 days. Yields 95% pure OPCs | PDGF/bFGF more efficient than B104CM/bFGF, and preference for Accutase over trypsin to dissociate oligospheres | (Fu et al., 2007) |
| Preamplification before chemical modification of shake-off method | P1 rat cerebral cortices | 15% B104 conditioned medium to expand mixed glia, isolation media with EDTA and DNase1 to detach OPCs by aspiration, further purified by gentle shaking. 85–95% OPC purity | Avoids costly purified growth factors; gentler than overnight shake-off. Reported higher yield of OPCs than shake-off method when compared directly. | (Niu et al., 2012) |
| Mouse OPCs | ||||
| Generation of Oligospheres | P0-3 mouse cortices | Percoll purification of dissociated cells and differential adhesion to deplete astrocytes. Plating in uncoated flasks generates floating spheres in N1/B104. Oligospheres obtained in 7 days. | Oligospheres expressed GAP43 and GD3. Spheres expandable for 2 months, myelinated shiverer mouse after transplantation. | (Vitry et al., 1999) |
| Generation of Oligospheres | E14.5-E17.5 multipotent progenitors | 4 day-old embryonic neurospheres (10 days from neonate) in EGF/bFGF replaced by B104 medium to form oligospheres. Yields 95% OPCs | Circumvents difficulty with shake-off. Economical, high yield. Free floating oligospheres amenable to passaging. | (Chen et al., 2007) |
| Clonal expansion with B104 media, passaged repeatedly. | P5 mouse neocortex | Culture for 18 days, re-plated at low density in uncoated dish for 2–3 week to capture single cell-derived colonies, then passaged 50 generations to generate mOPCs. >99% NG2+, A2B5+ | After capture, expanded clones are stored frozen. Aggregates removed during 2 weeks differentiation. Implantation into adult mouse brain produces OLs | (Lin et al., 2006) |
| FACS with NG2 | Embryonic/Neonatal cortices | Mixed glia mildly trypsinized before FACS >95% OPCs Require cAMP for growth and survival | Unlike overlapping A2B5 and NG2 expression in rat OPCs, 25% NG2+ mouse OPCs expressed A2B5. | (Horiuchi et al., 2010) |
| Magnetic activated cell sorting with O4 | P5-7 mouse cerebral cortex | Dissociated cells cleared with anti- mouse IgM magnetic beads before O4 antibody. Yields 3.68 × 105 OPCs per brain, >90% NG2+, A2B5+ after passage. | O4+ cells declined after passage. Bipotentiality reduced in DRG co-culture. In vitro OPC differentiation led to cell death; required CNTF for survival and maturation. | (Dincman et al., 2012) |
| Immunopanning Negative and Positive selection | P7 mouse cortex (OPCs); P12-16 (OLs) | Papain dissociation. PDGFRa (mouse CD140a), GalC or MOG selection, trypsinized and re- plated after panning. Yields 0.5 × 106 OPCs per brain | High purity and specificity. Consecutive positive panning for multiple stages possible. PDGF + NT3 or T3 for proliferation and maturation respectively | (Emery and Dugas, 2013) |
| Shake-off and differential adhesion | P0-P2 mouse cortices | Papain and mechanical dissociation, mixed glia supplemented with insulin, shake-off at 9 days. 50–60% OPCs | Technical considerations challenging, and lower OPC enrichment, but economical and avoids using mitogens. Convenient postnatal age for dorsal root ganglion co-cultures. | (O’Meara et al., 2011) |
| Shake-off and selective adhesion | Postnatal (P0, P15) and adult (P60, P180) cerebral cortex | Mechanical dissociation and various papain digestion conditions. Mixed glia cultured 10–25 days (postnatal) or 25–35 days (adult) before shake-off, enriched by 2 rounds of adhesion. PDGF-AA in growth medium. | Economical method with enhanced efficiency especially for adult OPCs, with 100,000 OPCs per gram weight adult CD1 tissue. OPC yield of 80,000 cells/animal higher than with FACS or optic nerve immunopanning and magnetic sorting. Reports mouse strain differences in numbers of adult OPCs isolated. | (Medina-Rodriguez et al., 2013) |
| Shake-off and selective adhesion | Human adult tumor margins and non-tumor biopsies | Same technique as above, except T25 flasks instead of T75. Confluency in 30–40 days before shake-off at lower speed than murine cells. | First reported protocol demonstrating OPCs isolation from biopsies. and use of PDGF-AA in growth medium | (Medina-Rodriguez et al., 2013) |
2.1 Astrocytes
Astrocytes are responsible for the homeostasis of extracellular glutamate, therefore the expression of the glutamate transporters GLAST (EAAT1), GLT1 (EAAT2), and glutamine synthetase (GS) are unique identifying features (Bak et al., 2006). In addition, astrocyte differentiation is characterized by induction of intermediate filament proteins glial fibrillary acidic protein (GFAP) and vimentin (Desclaux et al., 2009; Menet et al., 2001), along with S100B and aldehyde dehydrogenase family 1 member L1 (ALDH1L1) (Brozzi et al., 2009; Yang et al., 2011) (Figure 1). In essence, the glial culture protocol is based on the inability of neurons to survive in cultures derived from newborn rats. The basic protocol consists of preparing dissociated cells from cerebral hemispheres, plating in flasks to obtain mixed glia in serum-containing medium, followed by mechanical separation of astrocytes, oligodendrocyte precursors and microglia, using physical properties of these cells (Figure 1). Purified astrocytes in serum-containing or defined media (Morrison and de Vellis, 1981; Morrison et al., 1985) are obtained through constant shaking to remove contaminating microglia and OPCs over the course of the 10-day procedure (Cole and de Vellis, 1997) (Table 1). These isolated neonatal cells lack processes and are highly plastic and proliferative, with a capacity to withstand multiple passages. Although widely used in many contexts including aging, these neonatal cells are known to possess distinct gene expression properties, and are considered more ‘activated’ than normal adult, mature astrocytes (Nakagawa and Schwartz, 2004), including gene changes in response to beta-amyloid exposure (Kurronen et al., 2012). Other differences, such as the differential expression of metabotropic glutamate receptors, have revealed underlying developmental changes in neuroglial communication (Sun et al., 2013). Recently, adult rat astrocytes obtained from postnatal day 90 rats, maintained over 5 weeks in serum-containing media, showed many characteristics of normal adult astrocytes (Souza et al., 2013), i.e. expression of GFAP, GS, ALDH1L1, and S100B, as well as the ability to metabolize glutamate and glucose. Based on the duration of the incubation, it is likely these astrocytes were derived from adult progenitor cells, and their susceptibility to oxidative stress (Lin et al., 2007; Pertusa et al., 2007) suggested an effect of aging in these cultures (Souza et al., 2013). However, clarification of adult characteristics await further direct comparison of these cells with neonatal astrocytes.
In addition to physical methods of cell enrichment, the versatile immunopanning procedure is widely used to select for specific glial populations. This technique uses antibody-based capture of cells via the cell- or stage-specific expression of cell surface antigens (Barres, 1993) (Figure 1, Table 1). Neonatal astrocytes and their precursors are purified using negative selection by passing cell suspensions from P1 rat optic nerves over sequential ‘panning’ dishes coated with antibodies – e.g. MRC-OX7 anti-Thy1.1 to deplete microglia and meningeal cells, followed by either an A2B5 or O4 dish to deplete OPCs (Mi and Barres, 1999). To meet the demand for directly isolated mature astrocytes, an immunopanning protocol was recently developed for tissue from rodents at the postnatal age of day 1 through 18 (P1-P18) (Foo et al., 2011), which until now had not been successful because of the lack of astrocyte-specific surface antigens for positive selection. These cells were obtained through a succession of panning plates for the sequential removal of endothelial cells and microglia, followed by microglia/macrophages (CD45), and O4+ OPCs before positively selecting for integrin beta 5, based on gene profiling data (Cahoy et al., 2008). This acute purification procedure yielded astrocytes which were dependent on trophic factors for survival, such as heparin-binding epidermal growth factor, and may thus be maintained in defined media without serum, as serum was found to irreversibly alter gene expression (Foo et al., 2011). Comparison with astrocytes obtained by the McCarthy and de Vellis protocol showed that the acutely isolated astrocytes showed more mature characteristics, namely slower cell division, and vascular dependence, as well as greater similarity in gene expression profile with cortical astrocytes (Foo et al., 2011). These would now be useful for studies of glial reactivity in degeneration and brain injury.
2.2 Oligodendrocyte progenitor cells (OPCs)
Oligodendrocytes are the myelin-producing cells of the CNS which not only facilitate the conduction of action potentials but also support axonal integrity and metabolic activity (Funfschilling et al., 2012; Lee et al., 2012; Nave, 2010). Oligodendrocytes develop from oligodendrocyte progenitor cells (OPCs) which express cell surface antigens such as A2B5, platelet derived growth factor receptor alpha (PDGFRα), GD3 and NG2 (Figure 1). In addition, the lineage can be identified by the expression of transcription factors Sox10, Olig1 and Olig2. These transcription factors are not restricted to progenitors, and are also expressed in mature myelinating oligodendrocytes. Myelin proteins are widely used indicators of OPC maturation; these generally do not colocalize with OPC markers, and include: myelin basic protein (MBP), myelin-associated glycoprotein (MAG), myelin oligodendrocyte glycoprotein (MOG), myelin oligodendrocyte basic protein (MOBP), Connexin 47, proteolipid protein (PLP) and 2′,3′-cyclic nucleotide 3′ phosphodiesterase (CNP) (de Castro and Bribian, 2005).
OPC cultures from rat cortices or optic nerve had been the mainstay of in vitro methods to produce oligodendrocytes, because of the high yield of cells needed for histological and biochemical studies. Methods for the isolation of rat OPCs include the ‘shake-off’ method of McCarthy and de Vellis (McCarthy and de Vellis, 1980) (Table 1), exploiting differential adherence properties (Szuchet and Yim, 1984), immunopanning of dissociated neonatal rat cortices or adult optic nerve (Dugas and Emery, 2013; Gard et al., 1993; Gard et al., 1995; Mayer-Proschel, 2001; Shi et al., 1998), FACS with surface antigens (Behar et al., 1988), and density gradient centrifugation (e.g. Percoll™) (Colello and Sato-Bigbee, 2001; Vitry et al., 2001) (Figure 1), or combinations thereof. However, the demand for OPC cultures from mice has grown dramatically, largely due to the popularity of mouse genetic models and availability of sensitive assay technologies. Nevertheless, there are still major obstacles to overcome in obtaining mouse OPC cultures in adequate quantities using established rat techniques, because of innate species-specific differences in: antigen expression (Fanarraga et al., 1995), adhesion and cell proliferation/differentiation properties (Chen et al., 2007).
Typically, the shake-off method in combination with selective adhesion for astrocyte and microglial cell removal (Table 1) allows the economical enrichment of rat OPCs expressing A2B5 or NG2 antigens (Chen et al., 2007). The cells may be amplified and passaged post-shake with the addition of mitogens PDGF-AA and bFGF. The choice of medium affects cell survival, particularly under a mitogen-free differentiation paradigm, i.e. a N1-supplemented DMEM medium showed more cell death than B27/Neurobasal medium (Yang et al., 2005). This combination of B27 with Neurobasal medium also supported co-cultures of OPCs with hippocampal neurons (Yang et al., 2005). An alternative to the shake-off method involves enriching OPCs from dissociated E17 rat embryonic cortices by two sequential passages with trypsin, followed by culture in media containing PDGF-AA (Itoh, 2002). In addition to amplification of purified OPCs, the combination of PDGF and bFGF (Figure 1) may also be used to induce the formation of OPCs from neurosphere cultures from embryonic spinal cord (Fu et al., 2007). Yet another alternative for rat OPCs consists of pre-amplification in growth medium containing B104 neuroblastoma cell-conditioned medium which serves as an economical source of mitogens to amplify OPCs, followed by an isolation medium of DMEM/F12 containing EDTA, DNase I and insulin (Niu et al., 2012) to detach OPCs from the underlying bed of astrocytes with gentle aspiration.
Given the known differences in cell properties between rat and mouse OPCs, novel solutions for mouse OPC isolation have been developed to improve the efficiency and purity of isolation (Table 1). Approaches for mouse OPCs include: generation of oligospheres (Vitry et al., 1999) from neurospheres (Chen et al., 2007); serial passaging in mitogen-containing growth medium and selection and expansion of OP cell colonies from single cells (Lin et al., 2006), or enrichment through immuno-selection of NG2 cells by FACS (Horiuchi et al., 2010) or O4 antigen-based magnetic activated cell sorting (Dincman et al., 2012). An immunopanning procedure for mouse OPCs has also been developed which uses positive selection by PDGFRα instead of A2B5 (Emery and Dugas, 2013). These procedures primarily used embryonic or neonatal mouse cortices. Interestingly, a recent revival of the shake-off/selective adhesion method was described for mouse OPCs not only from neonatal but also adult cerebral cortex (Medina-Rodriguez et al., 2013) as well as human adult biopsies. This protocol uses papain to aid the removal of meninges and choroid plexus, and to facilitate mechanical dissociation. The papain concentration, incubation time and cell plating density are increased with age of the animal (up to P180). Unlike the McCarthy and deVellis protocol, PDGF-AA is included in the growth medium for plating of mixed glia of postnatal and adult mice, and the time in culture before shake-off varies from a minimum of 15 days for P15 cortices to 25 days for P180 (Medina-Rodriguez et al., 2013) (Table 1). When compared directly, the yield of cells with this method was reportedly an order of magnitude higher than with FACS (Medina-Rodriguez et al., 2013). Some minor modifications for human biopsy tissue, which require at least 30 days in culture, include reducing the size of the plating flask, increasing starting tissue mass and adjusting digestion conditions (Medina-Rodriguez et al., 2013). Continued improvements in the yield of OPCs are expected to enable investigators to conduct a wider range of cellular, molecular and biochemical assays with greater resolution.
2.3 Macroglial cells from pluripotent stem cells
The field of regenerative medicine has seen dramatic expansion and great promise with the discovery and generation of pluripotent stem cells. These not only provide a renewable source of genomically stable cells endowed with fate plasticity for transplantation therapies, but can also be used to help instruct investigators about the complexities of organ formation and cortical patterning (van den Ameele et al., 2014). Application of this technology to cell-based neurological repair strategies requires the conversion to functional cells over several stages, i.e. pluripotent to neural stem cell resembling neurogenic radial glia (Conti et al., 2005; Pollard et al., 2006), to neural precursor and then conversion to committed glia. Multi- or pluripotent stem cells include, and are not limited to, embryonic stem cells (ES), epiblast stem cells, and induced pluripotent stem cells (iPS) which do not have an embryonic origin (Figure 1). In addition to embryonic stem cells, neural stem cells can also be derived from fetal and adult mouse CNS (Conti et al., 2005; Pollard et al., 2006), or established from human fetal neural tissue (Sun et al., 2008). Conversely, neural stem cells (NSCs) can also serve as a source of pluripotent stem cells by reprogramming with factors (Kim et al., 2008).
Pluripotent cells exist during different stages of embryonic development, offering a range of pluripotent states. Embryonic stem cells have been proposed as sources of neural precursor cells (NPCs) (Table 2) for transplantation and oligodendrocyte repair in demyelinating disease (Brustle et al., 1999) and as gene therapy vector in brain tumors (Benveniste et al., 2005; Uzzaman et al., 2005). Epiblast stem cells have also been used to generate NSCs (Jang et al., 2014). Several differences are notable between mouse EpiSCs and ES, in cell cycle characteristics, gene expression and signaling mechanisms for self-renewal and differentiation (Brons et al., 2007; Tesar et al., 2007). Both represent distinct pluripotent states (Jang et al., 2014), and these differences may underlie their differential abilities in chimera formation in the post-implantation embryo (Huang et al., 2012). Mouse epiblast stem cells have been isolated from early postimplantation rodent blastocysts (Brons et al., 2007; Tesar et al., 2007). However, recently, mouse epiblast stem cells isolated from preimplantation mouse embryos have been directed to produce NSCs under PDGF, FGF and Sonic Hedgehog (Najm et al., 2011b), which subsequently transition to expandable OPCs for the purposes of drug screening to promote myelinating oligodendrocytes (Najm et al., 2011b).
Table 2.
Generation of macroglia cells from pluripotent stem cells
| Origin | Method | Cell Products | Remarks & Application | References |
|---|---|---|---|---|
| Embryonic Stem cells (ESC) | ||||
| Mouse embryonic stem cell line previously established (Li et al., 1992) | ESC aggregated to embryoid bodies without LIF in 4 days, expanded in propagation media (ITSFn) for 5 days + 5 days in media with insulin, transferrin, selenium chloride, putrescine, progesterone, FGF2, and laminin. Glial precursor cell proliferation was induced in media with FGF2, EGF, and PDGF. | A2B5+ glial precursors were obtained. 4 days of growth factor withdrawal induces formation of 38% immature OLs (O4+) and 35% astrocytes (GFAP+) |
Transplantation in myelin-deficient rat model | (Brustle et al., 1999) |
| Mouse embryonic stem cells previously established (Kyba et al., 2002) | Embryoid bodies formed without serum were transferred to media containing serum replacement (4 days), and then into serum replacement, EGF, FGF-2 and laminin (5 days), followed by serum replacement with FGF2 and PDGF. | Differentiation for 10 days: 98% astrocytes (GFAP+), further selected by FACS depletion of CD31. | ESC-derived astrocytes as gene therapy vector for transplantation into mice | (Benveniste et al., 2005; Uzzaman et al., 2005) |
| hESCs previously established (Thomson et al., 1998) | Retinoic acid and SHH agonist, purmorphamine, and T3 were used to induce oligodendroglial differentiation (110- 150 days in-vitro) | OPC production efficiency: 45% (OLIG2+/NKX2.2+ cell clusters) Generates: 83% OL lineage (OLIG2+) 37% OPCs (CD140α) |
Generates OPCs but at lower efficiency than from hiPSCs (See iPS section for same study comparison.) | (Wang et al., 2013) |
| Epiblast stem cells | ||||
| Mouse epiblast stem cells with Oct-4 GFP transgene (post-implantation) | NSC induced by: 2 days in Activin A+ bFGF, then 2 days in neurosphere medium, then 3 days in expansion medium (EGF+bFGF) modified from: (Conti et al., 2005) (high bFGF) | 3 weeks growth factor withdrawal induced: 50% astrocytes (GFAP+), 10% neurons (MAP2+), and 40% SOX-2, reportedly comparable with brain- derived NSCs. | Both epiblast and ESC-derived NSCs are multipotent but express different gene patterns. When transplanted into neonatal mouse brains both differentiated into neurons and astrocytes. | (Jang et al., 2014) |
| Mouse epiblast stem cells from pre- and post-implantation mouse embryos | Modulation of Activin and BMP signaling to NSC; then passaged in PDGF, FGF and SHH. | Neurons (not sustained) and primarily Olig2+/Nkx2.2+ OPCs. 64% OPCs subsequently expressed O4 and myelin proteins. | OPCs showed myelination in-vitro, in-vivo and in slice cultures. Useful for drug screening | (Najm et al., 2011a; Najm et al., 2011b) |
| iPS | ||||
| Induced epiblast stem cells from adult and embryonic mouse fibroblasts | 4 iPS factors used as reported (Takahashi and Yamanaka, 2006), but chemically defined media allowed development of induced epiblast stem cell without an embryonic stem cell intermediate. | Induced epiblast stem cells formed tetratomas, but not chimeras. | iEpiblast stem cells take longer to derive (3–5 week) than iPS cells (1–2 week). Overexpression of Klf4 allows for reversion to ESC. | (Han et al., 2011) |
| iPS cells derived from mouse embryonic fibroblast or adult tail tip fibroblasts | Retroviral transduction with Oct3/4, Sox2, Klf4 and c-Myc then cultured with ES media and feeder cells | Most iPS clones exhibited pluripotency and were genetically more similar to ES than fibroblasts. Differed from ESC in gene expression profile (Chin et al., 2009) |
Low conversion efficiency to iPS cells. Teratoma formation decreased without c-Myc (Nakagawa et al., 2008; Nakagawa et al., 2010) | (Takahashi et al., 2007a; Takahashi and Yamanaka, 2006) |
| iPS cells derived from mouse embryonic fibroblast | Retroviral transduction with Oct3/4, Sox2, Klf4 and cMyc (Takahashi and Yamanaka, 2006); Produced NSCs with N2 medium, FGF, and EGF Produced NPCs with N2, PDGF, EGF, bFGF, then PDGF | OPCs differentiated in T3, and NT3. Astrocytes, a few neurons, and 18% MBP+ OL (80% OL purity after PDGFRα FACS) | OL myelinated neurons in culture and after transplantation in a cuprizone model without teratoma formation. | (Czepiel et al., 2011) |
| iPS cell line: MEF-Ng-20D-17 from embryonic mouse fibroblasts previously established (Okita et al., 2007) | Conversion of embryoid bodies to Nestin+ cells and then in N2, bFGF, bFGF+EGF and PDGF+bFGF OPCs differentiated with T3; modified from (Brustle et al., 1999) |
14.1% A2B5+ OPCs were obtained from iPSCs compared to 12.6% from ESC. Differentiation to O4+ was lower in iPSC than ESCs. (Tokumoto et al., 2010) Immuno-panning increased efficiency of differentiation to O4+ from 2.3% to 43.5%. 62% of O4+ became MBP+ (21 days). (Ogawa et al., 2011a) |
Similar efficiency of iPS and ESC to differentiation into A2B5+ OPCs. Lower efficiency of iPS to O4+ cells may be due to inhibitors of terminal differentiation of OPCs. |
(Tokumoto et al., 2010) |
| iPS cells derived from mouse fibroblasts previously established (Moretti et al., 2010) | NSC induction with FGF and EGF are based on (Conti et al., 2005). NPC commitment involved decreasing bFGF and using valproic acid and BDNF (Spiliotopoulos et al., 2009). For Oligodendroglial commitment: 4 days with N2, forskolin, FGF-2, and PDGF and 4 days with T3 and ascorbic acid (Glaser et al., 2007) For astrocyte differentiation: 5 days with serum without EGF or FGF2 | 22 day neuronal protocol generated: 62% betaIII tubulin, 53% MAP2, 8% GFAP+ and some Nestin+/betaIIItubulin-. Oligodendroglial commitment protocol: All cells were NG2+ or O4+ and 25 % were committed through expressing NG2+/O4+. Astrocyte differentiation protocol: 100% GFAP+ cells |
NSC from iPS have a reported similar differentiation efficiency to neurons as NSC derived from ESC. | (Onorati et al., 2010) |
| iPS cells derived from reprogramming adult mouse neural stem cells cultured from adult Oct-4-GFP mice | iPS cells produced with Oct-4 and Klf4 (2–3 weeks), or Oct-4 alone (4–5 weeks) if cells endogenously express Sox-2, Klf-4 and C-Myc | NSCs differentiate into neurons, astrocytes and oligodendrocytes after 20 passages and in-vivo and form teratomas. (Kim et al., 2009) | Two factors, depending on endogenous expression, may be sufficient for reprogramming NSCs into iPS cells. | (Kim et al., 2009; Kim et al., 2008) |
| iPS cells derived from mouse embryonic and lung fibroblasts | Doxycycline-inducible lentiviral vectors for Olig2, Nkx6.2, and Sox10 were used. OPCs produced with FGF2, PDGF-AA and SHH | Induced OPCs (iOPCs) produced in 14–21 days. | OPCs transplanted into slice cultures of Shiverer mice formed compact myelin. | (Najm et al., 2013) |
| hiPS from adult human dermal fibroblasts | Retroviral transduction of iPS factors: Oct3/4, Sox2, Klf4 and cMyc (Takahashi and Yamanaka, 2006) | iPS cells formed embryoid bodies in vitro and teratomas (made of ectoderm, mesoderm and endoderm) in vivo suggesting pluripotency. | hiPS could differentiate into neural cells. | (Takahashi et al., 2007b) |
| hiPS from adult human skin biopsy from a patient with Multiple Sclerosis | Dermal fibroblast expansion (14 days) (Park et al., 2008) followed by transduction with Oct3/4, Sox2, Klf4 and cMyc (Takahashi et al., 2007b). Cells were re-plated and cultured with feeder cells, hbFGF and butyrate and mechanically disrupted 18 days post-transduction | NSC were induced and differentiated into mixed culture containing astrocytes (50.5% GFAP+) and neurons (72.7% βIII-tubulin+ and 56.9% MAP2+) after 4 weeks in NS medium. Modified culture conditions enriched for immature and mature oligodendrocytes (47.2% O4+ and 88.9% MBP+) | Butyrate increased efficiency for derivation of iPS from expanded human dermal fibroblasts from 0.033–0.066% to 0.24–0.67%. iPS generated from a patient with MS has pluripotency. | (Song et al., 2012) |
| hiPS clones from adult human dermal fibroblasts | Feeder and serum free method using retroviral vectors for transfection with Oct3/4, Sox2, Klf4 and cMyc (Totonchi et al., 2010) A 9 week oligodendrocyte differentiation protocol included hESC medium/glial restriction medium, retinoic acid bFGF, and EGF at specified time points based on a method for hESC (Hatch et al., 2009). | Immunocytochemical analysis was used to determine 94% of cells at the final differentiation stage were of OL lineage (OLIG +). 95.7% A2B5+ 19.5% of the cells were mature OL (MBP+). 73.7% of cells expressed GalC+ suggesting an immature OL phenotype. 52.4% of cells were NG2+ and 42.9% were α+ PDGFR suggesting an OPC phenotype. Non-oligodendrocyte cells included 1% astrocytes (GFAP+) and 5% neurons (MAP2+) |
OPCs transplanted into focal demyelinating optic nerve differentiated into myelinating OL (MBP+ and PLP +) and some astrocytes and neurons but not teratomas or tumors. | (Pouya et al., 2011) |
| hiPSC lines (fibroblast and keratinocyte origin) previously established (Chambers et al., 2009; Maherali et al., 2008) | Retinoic acid and SHH agonist, purmorphamine, and T3 were used to induce oligodendroglial differentiation. (110- 150 days in-vitro) GFAP + cells were found at 70 DIV. Withdrawal of growth factors for 2 weeks was used to induce maturation of OPCs (186–205 DIV) |
OPC production efficiency: 73–80% (OLIG2+/NKX2.2+ cell clusters) Generates: 87–95% OL lineage (OLIG2+) 32–41% OPCs (CD140α) 40–50% astrocytes (GFAP+) 4–12% cells matured from OPCs to immature or mature OLs (O4+) FACS selection of O4+, CD140α, or A2B5 further purifies OPCs for transplant studies |
Higher OPC derivation efficiency than from hESC (See ESC section for same study comparison.) Method favored OPC and immature OL differentiation for transplant. OLs myelinate neurons in-vitro; 4–10% of donor OPCs in Shiverer brains became MBP+ cells (78% OLIG2+ and the rest were GFAP+). | (Wang et al., 2013) |
iPS cells are a major breakthrough in innovative clinical approaches (Inoue et al., 2014). These are generated by reprogramming differentiated somatic cells, such as fibroblasts, with a set of four transcription factors: Octamer-binding transcription factor 4 (Oct4), SRY-Box gene 2 (Sox2), Kruppel-like factor 4 (Klf4), and c-Myc (Takahashi et al., 2007b; Takahashi and Yamanaka, 2006). To minimize the risk of tumorigenicity from c-Myc reactivation, it was later found that c-Myc could be omitted (Nakagawa et al., 2008) or substituted with a non-transforming Myc family member (Nakagawa et al., 2010). As with epiblasts, iPS cells are not identical to ES cells, differing not only in origin, but also gene expression profile (Chin et al., 2009), and efficiency of oligodendrocyte generation (Tokumoto et al., 2010). Epiblast stem cells have also been derived from fibroblasts through reprogramming (Han et al., 2011). In addition to their potential for therapeutic cell replacement applications, iPS cell-derived astrocytes and neurons generated from patient somatic cells have been shown to offer novel approaches to studying the pathophysiology of neurological diseases such as Huntington’s and multiple sclerosis (Juopperi et al., 2012; Song et al., 2012).
Both mouse (Czepiel et al., 2011) and human (Ogawa et al., 2011b; Pouya et al., 2011; Wang et al., 2013) iPS cells have been successfully differentiated into precursors of oligodendrocytes (Table 2). The iPS-derived OPCs were transplanted into different demyelination models: a rat model of focal demyelination in the optic chiasm, (Pouya et al., 2011), as well as a cuprizone-induced paradigm (Czepiel et al., 2011). These were found to differentiate into MBP-expressing oligodendrocytes in vivo (Czepiel et al., 2011; Pouya et al., 2011). A recent study showed that iPS cells are not the only source of induced OPCs. The reprogramming of mouse embryonic fibroblasts with oligodendrocyte-specific genes, including Olig1, Olig2, Nkx2.2, Sox10, Myrf and Myt1, has resulted in the generation of induced OPCs which were capable of myelination following transplantation into slice cultures (Najm et al., 2013) (Figure 1) (Table 2). Because of the nature of multipotency, improvements in the directed differentiation of iPS-derived precursors are constantly being sought. It was found that the additional step of OPC purification or enrichment, by immunopanning from these iPS-derived cultures improved the efficiency of differentiation in vitro (Ogawa et al., 2011a), although transplant experiments using sorted and unsorted iPS-derived OPCs showed that terminal differentiation appeared more important than purity of cell stage for the prevention of teratoma formation in vivo (Czepiel et al., 2011). Despite losing the majority of the differentiated, transplanted OPCs to apoptosis, those that survived became mature and integrated functionally with axons (Czepiel et al., 2011). In contrast, transplantation of GFAP+ astrocytes for spinal cord injury has produced mixed results between glial-restricted precursors or iPS-derived cells (Hayashi et al., 2011), suggesting that for this type of injury, replacement of a single differentiated cell type may be less effective than NSCs or acutely isolated NPCs (Karimi-Abdolrezaee et al., 2010).
Recently, the reprogramming of astrocytes using hGFAP-driven Sox2 expression generated ectopic neuroblasts in the adult mouse striatum in vivo, which eventually produced mature neurons (Niu et al., 2013). Although glia have not been shown to be generated this way, it is possible that the cellular outcome of Sox2 overexpression is region-specific.
3. Genetic models for glial-specific targeting
The analysis and manipulation of glial cell function in vivo or ex vivo requires methods to detect and label the cells by lineage with consistency and fidelity. Many glial marker genes whose products have been identified previously as specific for a particular cell type or developmental stage have now become widely used as targeting tools, both as transgenic or knock-in mouse strains. These are listed in http://www.networkglia.eu/en/animal_models as well as under the GenSat project http://www.gensat.org/MMRC_report.jsp. For a comprehensive treatise on the background of glial markers and genetic targeting strains for macroglial, microglial and ependymal cells, the reader is directed to this recent review article (Pfrieger and Slezak, 2012). The following section instead aims to summarize, with the aid of Tables 3 and 4, the range of cell types found to be labeled and targeted by existing and new transgenic and knock-in mouse strains. New mouse lines developed for Cre-dependent or glial-specific molecular tagging are also described.
Table 3.
Mouse linesa for targeting Astroglia
| Line | Construct | Cell Type | References |
|---|---|---|---|
| Aldh1L1-eGFP | BAC | Astrocytes, Neural Stem Cells, Muller Glia, Bergmann Glia, Radial Glia | GenSat* (Gong et al., 2003; Heintz, 2004) (Anthony and Heintz, 2007) (Bardehle et al., 2013; Cahoy et al., 2008; Doyle et al., 2008; Foo and Dougherty, 2013; Tsai et al., 2012; Yang et al., 2011) |
| Prism 1.0 and 2.0 (Aldh1L1-cerulean, SNAP25-mCherry, MOBP-YFP) | BAC | Astrocytes, Neurons, Oligodendrocytes | *(Dougherty et al., 2012) |
| Aldh1L1-Cre | BAC | Astrocytes, Radial Glia, Neural Stem Cells | *(Tien et al., 2012) (Foo and Dougherty, 2013) |
| Apoe4-EGFP | Knock-in | Astrocytes, Neuroepithelial Cells, Vascular Smooth Muscle Cells, Choroid Plexus Cells | *(Xu et al., 2006) |
| Apoe4-CreERT2 | BAC | Astrocytes | *(Slezak et al., 2007) |
| Aqp4- CreERT2 | BAC | Astrocytes | *(Slezak et al., 2007) |
| Blbp-GFP | Mouse 1.6 kb | Neural Stem Cells, Neural Progenitor Cells, Radial Glia | *(Anthony et al., 2004) |
| Blbp-EGFP | BAC | Astrocytes, Bergmann glia, radial glia, Neural Progenitor Cells | *(Schmid et al., 2006) (Howard et al., 2008; Mo et al., 2007) |
| Blbp-EYFP | BAC | Astrocytes, Neural Stem Cells, Radial Glia | *(Schmid et al., 2006) |
| Blbp-dsRed2 | BAC | Astrocytes, Neural Stem Cells, Radial Glia | *(Schmid et al., 2006) |
| Blbp-Cre | Mouse 1.6 kb | Astrocytes, Radial Glial, Neural Stem Cells, Neural Progenitor Cells | *(Anthony et al., 2004) (Gibson et al., 2010; Hegedus et al., 2007) |
| Cx30-LacZ | Knock-in | Astrocytes, Ependymal Cells, Leptomeningeal Cells, Neurovascular Cells | *(Teubner et al., 2003) (Gosejacob et al., 2011) |
| Cx30-CreERT2 | BAC | Astrocytes, Neurovascular Cells | *(Slezak et al., 2007) (Barnabe-Heider et al., 2010; Gosejacob et al., 2011; Nomura et al., 2010) |
| Cx43-LacZ | Knock-in | Astrocytes, Neural Stem cells | *(Theis et al., 2001) (Contreras et al., 2002; Mori et al., 2006) |
| Cx43-CreERT | Knock-in | Astrocytes, Neural Stem cells | *(Eckardt et al., 2004) (Gosejacob et al., 2011) |
| FGFR3-EGFP | BAC | Astrocytes | *(Heintz, 2004) |
| FGFR3-iCreERT2 | PAC | Astrocytes, Radial Glia, Neural Stem Cells | *(Bansal et al., 2003) (Cox et al., 2012; Rivers et al., 2008; Young et al., 2010; Zawadzka et al., 2010) |
| hGFAP-S65T-GFP | Human 2.2 kb | Astroyctes, Muller Glia, Radial Glia, Neural Stem Cells, Cortical Neurons | *(Zhuo et al., 2001) (Silbereis et al., 2010) |
| hGFAP-EGFP | Human 2.2 kb | Astrocytes, Radial Glia, Neural Stem Cells, Bergmann Glia | *(Nolte et al., 2001) (Benesova et al., 2009; Hirrlinger et al., 2005; Matthias et al., 2003; Wehner et al., 2003) |
| GFAP-GFP | Mouse 2.0 kb | Astrocytes, Neural Stem Cells, Radial Glia | *(Platel et al., 2009) |
| GFAP-EGFP | Mouse 2.6 kb | Astrocytes, Non-myelinating Schwann Cells | *(Suzuki et al., 2003) (Yamazaki et al., 2011) |
| GFAP-EGFP | Mouse 2.5 kb | Astrocytes, Muller Glia | *(Kuzmanovic et al., 2003) |
| hGFAP-AsRed2, -AmCyan1, -mRFP1 | Human 2.2 kb | Astrocytes, Bergmann Glia | *(Hirrlinger et al., 2005) |
| hGFAP-dsRed | Human 2.2 kb | Astrocytes, Cerebellar Granule Neuron Precursors, Bergmann Glia, Radial Glia | *(Noraberg et al., 2007) (Silbereis et al., 2010) |
| hGFAP-LacZ | Human 2.2 kb | Astrocytes, Schwann Cells, Bergmann Glia, Muller Glia | *(Mucke et al., 1991) (Brenner et al., 1994; Johnson et al., 1995; Rio et al., 2002; Verderber et al., 1995) |
| hGFAP-LacZ | Human 1.8 kb | Astrocytes, Radial Glia, Neuroepithelial Precursor Cells | *(Andrae et al., 2001) |
| GFAP-LacZ | Mouse + 2.0 kb 5′ | Astrocytes, Schwann Cells, Bergmann Glia | *(Mucke et al., 1991) (Campbell et al., 1993) |
| GFAP-LacZ | Mouse 2.0 kb | Radial Glia, Bergmann Glia, Tanycytes | *(Galou et al., 1994) |
| GFAP-LacZ | Mouse gene (ATG/TTG mutant) | Schwann Cells, Bergmann Glia | *(Johnson et al., 1995) |
| GFAP-Cre | Mouse gene (ATG/TTG mutant) | Neural Progenitor Cells, Ependymal Cells, Granule Neurons | *(Marino et al., 2000) (Garcia et al., 2004; Kwon et al., 2001) |
| GFAP-Cre | Mouse 2.0 kb | Astrocytes, Radial Glia | *(Casper and McCarthy, 2006) |
| hGFAP-Cre | Human 2.2 kb with insulators | Astrocytes, Radial Glia, Oligodendrocytes | *(Casper and McCarthy, 2006) |
| hGFAP-Cre | Human 2.2 kb | Astrocytes, Ependymal Cells, Radial Glia | *(Zhuo et al., 2001) (Bajenaru et al., 2002; McCarty et al., 2005; Peng et al., 2010) |
| hGFAP-CreERTM | Human 2.2 kb | Astrocytes, Bergmann Glia | *(Chow et al., 2008) |
| hGFAP-CreERT2 | Human 2.2 kb | Astrocytes, Radial Glia, Bergmann Glia, Neural Stem Cells, Olfactory Bulb Neurons | *(Ganat et al., 2006) (Bruchas et al., 2011; Casper et al., 2007; Hirrlinger et al., 2006; Silbereis et al., 2010; Song et al., 2013) |
| GFAP-Luciferase | Mouse 12.0 kb | Astrocytes | *(Zhu et al., 2004) (Jany et al., 2013) |
| GFAP-Luciferase | Human 2.2 kb + 0.5 kb GAPDH with Renilla Luciferase | Astrocytes | *(Cho et al., 2009) |
| hGFAP-tTA | Human 2.2 kb | Astrocytes, Bergmann Glia | *(Lin et al., 2004; Wang et al., 2004) (Lopez et al., 2011) |
| hGFAP-tTA -mP1 | Human 2kb, Gfa2 | Astrocytes | *(Pascual et al., 2005) |
| hGFAP-tetO1-tTA Transgenic rat | Human 2.2 kb | Astrocytes | *(Barton et al., 2002) |
| GFAP-tet-ON | Mouse gene (ATG/TTG mutant) | Astrocytes, Muller Glia | *(Kim et al., 2003) |
| GLAST-EGFP | BAC | Astrocytes, Radial Glia | GenSat |
| GLAST-dsRed | BAC | Astrocytes, Radial Glia, Neural Stem Cells, Oligodendrocytes | *(Glowatzki et al., 2006) *(Regan et al., 2007) |
| GLAST-Cre-GFP | Knock-in | Astrocytes, Radial Glia, Muller glia, Bergmann glia | *(Mori et al., 2006) |
| GLAST-EMTB-GFP | BAC | Astrocytes, Radial Glia | *(Eom et al., 2011) |
| GLAST-Cre | BAC | Astrocytes, Radial Glia | *(Anthony and Heintz, 2008) |
| GLAST-CreERT2 | Knock-in | Astrocytes, Neural Progenitor Cells, Radial Glia, Tanycytes | *(Mori et al., 2006) (Bardehle et al., 2013; Bergami et al., 2008; Bonilla et al., 2008; Ninkovic et al., 2007; Robins et al., 2013; Snapyan et al., 2009) |
| GLAST-CreERT2 | BAC | Astrocytes, Bergmann Glia, Muller Glia, Neural Stem Cells, Radial Glia | *(Slezak et al., 2007) (Ehm et al., 2010; Fauquier et al., 2014; Sabelstrom et al., 2013) |
| Gli1-EGFP | Knock-in | Astrocytes, Radial Glia, Neural Stem Cells | (Garcia et al., 2010; Mesman et al., 2014) |
| Gli1-CreERT2 | Knock-in | Astrocytes, Radial Glia, Neural Stem Cells, Neural Progenitor Cells, OPCs | *(Ahn and Joyner, 2005) |
| Glt1-EGFP | BAC | Astrocytes, Radial Glia, Neural Stem Cells, Hippocampal and Somatosensory Cortical Neurons, Bergmann Glia | *(Regan et al., 2007) (de Vivo et al., 2010) |
| Glt1/EAAT2-tdTomato | 8.3 kb transgenic | Astrocytes | *(Yang et al., 2011) |
| Kir4.1-EGFP | BAC | Astrocytes, NG2 Cells | *(Hibino et al., 2004) (Tang et al., 2009) |
| Nestin-eGFP | BAC | Astrocytes, Neural Stem Cells, Neural Progenitor Cells, Ependymal Cells | *(Roy et al., 2000) (Kawaguchi et al., 2001; Keyoung et al., 2001; Mignone et al., 2004) |
| Nestin-LacZ | Knock-in | Astrocytes, Neural Stem Cells, Neuroepithelial Cells | *(Zimmerman et al., 1994) (Frisen et al., 1995; Lin et al., 1995; Shan et al., 2006; Zhuo et al., 2001) |
| Nestin-rtTA | Knock-in | Astrocytes, Neural Stem Cells, Neural Progenitor Cells | *(Mitsuhashi et al., 2001) (Caviness et al., 2003; Farioli-Vecchioli et al., 2008; Suter et al., 2007) |
| Nestin-Cre | Rat 1.8 kb | Astrocytes, Neural Stem Cells, Neural Progenitor Cells | *(Zimmerman et al., 1994) (Betz et al., 1996; Hara et al., 2006; Imai et al., 2006) |
| Nestin-CreERTM | BAC | Astrocytes, Neural Stem Cells, Neural Progenitor Cells, Ependymal Cells, Radial Glia | *(Kuo et al., 2006) (Pan et al., 2012) |
| Nestin-CreERT2 | Rat 5.3 kb | Astrocytes, Neural Stem Cells, Neural Progenitor Cells, Cerebellar Granule Neurons, Neurovascular Cells | *(Dranovsky et al., 2011) (Sahay et al., 2011; Sun et al., 2014) |
| Nestin-CreERT2 | Mouse 5.6 kb | Astrocytes, Radial Glia, Neural Stem Cells | *(Lagace et al., 2007) (Chen et al., 2009; DeCarolis et al., 2013) |
| Nestin-CreERT2 | Rat 5.8 kb | Astrocytes, Neural Stem Cells, Neural Progenitor Cells, Cerebellar Granule Neurons, Neurovascular Cells, Pericytes | *(Imayoshi et al., 2006) (Sun et al., 2014) |
| S100b-EGFP | Mouse 4.8 kb | Astrocytes, Oligodendrocytes, Ependymal Cells, Neurons, Enteric Glia, NG2 Cells | *(Vives et al., 2003) (Gulbransen and Sharkey, 2009; Hachem et al., 2005; Hachem et al., 2007; Raponi et al., 2007) |
| S100b-EGFP | Rat 9.0 kb | Astrocytes, Pituitary Folliculo-Stellate Cells | *(Itakura et al., 2007) |
| S100b-EGFP | Human 9.4 kb | Astrocytes, Schwann Cells | *(Zuo et al., 2004) |
| S100b-EYFP | Human 9.4 kb | Astrocytes, Schwann cells, Microglia, Bergmann Glia | *(Zuo et al., 2004) (Hayashi et al., 2007) |
| S100b-dsRed | Human 9.4 kb (9.6kb in the actual paper) | Astrocytes, Olfactory Ensheathing Cells | *(Windus et al., 2007; Windus et al., 2010) |
| S100b-YC 3.60 cameleon | Human 9.4 kb | Astrocytes, Schwann Cells, NG2 Cells, Mature Oligodendrocytes | *(Atkin et al., 2009) |
| S100b-Cre | Mouse 11.7 kb | Astrocytes, Bergmann Glia | *(Tanaka et al., 2008) |
| S100b-CreERT2 | BAC | Astrocytes, NG2 cells, Chondrocytes, Neural Dorsal Root Ganglion Cells, | *(McMahon and Zhang, 2010) |
| Sox2-EGFP | Knock-in | Embryonic, Trophoblasts, Neural Stem Cells | *(Ellis et al., 2004) |
| Sox2-Cre | Transgenic | Epiblast Cells, Neural Stem Cells | *(Hayashi et al., 2002) |
| Sox2-CreERTM | BAC | Adult Neural Stem Cells, Some astrocytes and neurons | *(Kang and Hebert, 2012) |
| Sox2-iCreERT2 | Knock-in | Radial Glial Cells, Adult Neural Stem Cells, Neural Progenitor Cells, Neuroepithelial Cells, Ependymal Cells | *(Arnold et al., 2011) |
| Sox2-B geo G418 | Transgenic | Neural Stem Cells | *(Zappone et al., 2000) |
| Sox2-HSTK | Knock-in | Neural Stem Cells | *(Arnold et al., 2011) |
Note:
denotes originator of the line. Findings in some cells are the result of fate-mapping analyses.
Most lines listed are mouse except where otherwise indicated e.g. hGFAP-TetO1-tTA.
Table 4.
Mouse lines for targeting Oligodendroglia
| Line | Construct | Cell Type | References |
|---|---|---|---|
| Cx47-EGFP | Knock-in | Mature Oligodendrocytes | *(Odermatt et al., 2003) |
| CNP-lacZ | Mouse 4.0 kb | OPCs and myelinating OLs | *(Gravel et al., 1998) *(Chandross et al., 1999) |
| CNP-lacZ neo | |||
| CNP-EGFP | Mouse 4.0 kb | OPCs, myelinating OLs, Multipotent SVZ cells, Schwann cells, and neuronal precursors | *(Yuan et al., 2002) (Belachew et al., 2003) (Aguirre and Gallo, 2004; Belachew et al., 2001) |
| CNP-Cre | Knock-in | OPCs, myelinating OLs, PNS Neurons, and Schwann Cell Precursors | *(Lappe-Seifke et al., 2003) (Genoud et al., 2002) |
| MBP-lacZ | Mouse 1.3 kb Mouse 1.34 kb Mouse 1.9 kb Mouse 3.1 kb Mouse 9 kb |
Mature Oligodendrocytes; Schwann cells (in 9 kb) | *(Turnley et al., 1991) *(Asipu et al., 2001) *(Gow et al., 1992) *(Foran and Peterson, 1992) |
| MBP-lacZ | Mouse 1.34–1.9 kb Mouse 6 kb |
Mature oligodendrocytes, non- neural epithelial cells, astrocytic shaped oligodendrocytes, compact perivascular oligodendrocyte lineage cells; Schwann cells (in 6 kb) |
*(Asipu et al., 2001) (Schiff et al., 2002) *(Forghani et al., 2001) |
| MBP-Cre | Mouse 1.3 kb Mouse 5.0 kb |
Mature Oligodendrocytes | *(Niwa-Kawakita et al., 2000) *(Hisahara S et al., 2000) |
| MBP-CreERT2 | Mouse 1.9 kb | Mature Myelinating Oligodendrocytes | *(Gow, 2011) |
| MOBP-EGFP | BAC | Mature oligodendrocytes | * GENSAT, 2008 |
| Prism 2.0: Snap25-DsRedMax/MOBP-YFP/Aldh1L1-Cerulean |
BAC | Neurons, Oligodendrocytes, and Astrocytes | *(Dougherty et al., 2012) |
| MOG-iCre | Knock-in | Mature Oligodendrocytes | *(Hovelmeyer et al., 2005) |
| PLP-lacZ | Mouse 2.4 kb –exon 2 | OPCs, myelinating OLs, and Schwann Cells | *(Wight et al., 1993) |
| PLP-sh ble-lacZ | Mouse 2.4 kb –exon 2 | OPCs, myelinating OLs, and Schwann Cells | *(Spassky et al., 1998) |
| PLP-sh ble EGFP | Mouse 2.4 kb –exon 2 | OPCs, myelinating OLs, and Schwann Cells | *(Sobottka et al., 2011) |
| PLP-EGFP | Mouse 2.4 kb –exon 2 | OPCs, myelinating OLs, and Schwann Cells; OLs, spinal cord stem cells, neuronal progenitors, and non-myelinating satellite cells in sympathetic glia |
*(Mallon et al., 2002) (Harlow et al., 2014) |
| PLP-GFP- S65T | Mouse 2.4 kb-intron 1 | OPCs, myelinating OLs, and Schwann Cells | *(Fuss et al., 2000b) |
| PLP-dsRed | Mouse 2.4 kb-intron 1 | OPCs, myelinating OLs, and Schwann Cells | *(Hirrlinger et al., 2005) |
| PLP-mtTA | Mouse 2.4 kb-intron 1 | Myelinating OLs, Bergmann glia, Schwann cells in adult sciatic nerve | *(Inamura et al., 2012) |
| PLP-Cre | Mouse 3.7 kb Mouse 2.4 kb |
OPCs, myelinating OLs, and Schwann Cells Embryonic Progenitors (neuroblasts and glioblasts) neuroepithelial and radial glial cells | *(Delaunay et al., 2008) *(Michalski et al., 2011) (Delaunay et al., 2009) |
| PLP-Cre ERT | Mouse 2.4 kb | OPCs, myelinating OLs, Schwann Cells, Bergmann glia; Neurons and immature astrocytes in ventral forebrain, dorsal cerebral cortex, and hippocampus | *(Doerflinger et al., 2003) (Guo et al., 2009) (Chung et al., 2013) (Guo et al., 2010) |
| PLP-Cre ERT2 | Mouse 2.4 kb | OPCs, myelinating OLs and Schwann Cells | *(Leone et al., 2003) |
| Ascl1/Mash1-Cre ERTM | BAC | OPCs, and Embryonic neuronal and oligodendrocyte-restricted precursors | *(Battiste et al., 2007) |
| Ascl1/Mash1-Cre ERT2 | Knock-in | OPCs, adult neural precursors; Hippocampal and olfactory bulb stem and progenitor cells | *(Kim et al., 2011) |
| NG2-dsRed | BAC | OPCs, pericytes, and microglia | *(Ziskin et al., 2007) *(Zhu et al., 2008) (Hall et al., 2014) |
| NG2-EYFP | Knock-in | OPCs, astrocytes | *(Karram et al., 2008) |
| NG2-Cre | BAC | OPCs, Astrocytes; neurons | *(Zhu et al., 2008) (Nishiyama et al., 2014) |
| NG2-Cre ERTM | BAC | OPCs, pericytes, astrocytes | *(Zhu et al., 2011) |
| NG2-CreER T2 | Knock-in | OPCs, gray matter astrocytes, and neurons | *(Huang et al., 2014) |
| Olig1-lacZ | Knock-in | OPCs | *(Zhou and Anderson, 2002) |
| Olig1-Cre | Knock-in | Motoneurons and OPCs | *(Lu et al., 2002) |
| Olig2-GFP | Knock-in | OPCs | *(Zhou and Anderson, 2002) |
| Olig2-Cre ERTM | Knock-in | Motoneuron, OPCs and astrocytes | *(Takebayashi et al., 2002) (Dimou et al., 2008) |
| Olig2-tva-Cre | Knock-in | OPCs | *(Schuller et al., 2008) |
| PDGFRα-GFP | Knock-in | OPCs | *(Hamilton et al., 2003) |
| PDGFRα-CreER T2 | PAC | OPCs, neurons, and astrocytes | *(Rivers et al., 2008) (Tripathi et al., 2010) |
| PDGFRα–CreERTM | BAC | OPCs and some ventral neurons | *(Kang et al., 2010) |
| Sox10-Venus | BAC | OPCs, Schwann cells, and neural crest cells | *(Shibata et al., 2010) |
| Sox10-Cre | PAC | OPCs, Oligodendrocytes, Neural Crest Cells, Enteric Glia | *(Matsuoka et al., 2005) (Laranjeira et al., 2011; Potzner et al., 2007; Stolt et al., 2006) |
| Sox10-fos-Cre (S4F:Cre) | Transgenic | OPCs, myelinating OLs, and neural crest cells | *(Stine et al., 2009) |
| Sox10-rtTA | Knock-in | OPCs, myelinating OLs, and neural crest cells | *(Ludwig et al., 2004) (Kanaykina et al., 2010; Wahlbuhl et al., 2012) |
| Sox10-iCreER T2 | BAC | OPCs, myelinating OLs, neural crest cells, and ependymal NG2 cells | *(Simon et al., 2012) (Ortega et al., 2013) |
Note:
denotes originator of the line. OPCs, oligodendrocyte progenitor cells; OLs, oligodendrocytes. mtTA, mammalian-optimized tetracycline-controlled transcriptional activator. Findings in some cells are the result of fate-mapping analyses.
3.1 Astrocytes / Stem cells
In addition to the inherent heterogeneity of astrocytes, which can be classified into many subtypes, astrocytes express many of the same markers as NSCs, likely due to a shared astroglial lineage (Ihrie and Alvarez-Buylla, 2008). Therefore, the ability to selectively target the entire astrocyte population, or distinct subpopulations without also labeling or affecting neural stem and progenitor cells, remains an important and largely unresolved issue in the field.
For the experimental targeting of astroglial cells, an extensive collection of genetic mouse lines has been generated that drive transgene expression under a variety of astrocytic promoter elements, leading to strain-specific expression dynamics in populations of astrocytes and neural stem/progenitors, with varying levels of overlap between these and other cell types (Table 3).
In spite of this, we make a cautionary note that specific expression patterns for all mouse lines outlined here, continue to lack thorough investigation and await more detailed analysis during development, adulthood, and aging. Transgene expression dynamics for every strain should be experimentally determined or validated by the researcher, whenever possible.
Among the available driver lines, the canonical and most-widely used astrocytic gene is GFAP. In particular, the 2.2 kb human GFAP promoter (gfa2), has been used to visualize and manipulate astrocytes with higher specificity and broader CNS expression compared to other GFAP promoter elements (Lee et al., 2008; Lee et al., 2006). Interestingly, using selective deletion of GFAP promoter regions, it has been determined that gfa2 expresses throughout the brain while expression from a 488 bp gfa28 promoter fragment is limited, thereby underlying regional astrocyte heterogeneity (Lee et al., 2006). Additionally, gfa28 also expresses in neurons as well as astrocytes, while gfa2 does not, indicating that a specific base-pair sequence is required for silencing neuronal expression, and suggests that expression in other cell types such as NSCs and neural progenitors is controlled by different GFAP promoter elements that may be silenced to narrow or expand expression in astrocytes and stem/progenitor cells (Lee et al., 2008).
In addition to being used to understand the behavior of astroglia per se, GFAP reporter transgenic mice have been developed for bioluminescence studies (Badr and Tannaus, 2011). Whereas luciferase activities have long been in use to define and quantify molecular interactions at promoter and enhancer regions in vitro, the imaging of luciferase activity in situ in transgenic mice offers a quantifiable measurement of gliosis in vivo, and can detect graded changes in astrocytic reactivity, for example after physiological stress, injury or neoplastic conditions (Burda and Sofroniew, 2014; Rivera-Zengotita and Yachnis, 2012). The first GFAP-luciferase transgenic mouse with about 12 kb of the mouse GFAP promoter has been used to monitor gliosis after kainate injury (Zhu et al., 2004), traumatic brain injury (Luo et al., 2014), experimentally induced inflammation (Luo et al., 2008), prion infection (Tamguney et al., 2009), and in a mouse model of Alzheimer’s disease (Watts et al., 2011). A double transgenic strain was more recently generated which not only expresses firefly luciferase under 2.2kb of the human GFAP promoter but also the Renilla Luciferase driven by 0.5kb of the human GAPDH promoter (Cho et al., 2009). Determination of dual reporter ratios was reported to reduce variability between samples (Cho et al., 2010; Cho et al., 2009). These strains allow sensitive, non-invasive measurement of cellular response, a technique compatible with longitudinal studies of function and behavior (Luo et al., 2014).
While there is clear potential for the use of the human GFAP promoter to target astroglia, recent characterization of hGFAP-derived GFP expression suggests that it does not fully recapitulate endogenous GFAP activity in mice (Moon et al., 2011), perhaps indicating that upstream GFAP transcription-control mechanisms differ for (largely undefined) different subtypes of astrocytes. Further reflecting the functional diversity of astrocytes, endogenous GFAP activity is only detectable in a small proportion of protoplasmic (grey matter) astrocytes, compared to fibrous (white matter) astrocytes (Molofsky et al., 2012). Thus, the use of the GFAP promoter to label astrocytes in grey matter brain structures may be problematic.
Probing how the heterogeneity of astroglia is defined at the molecular level is integral to increasing our understanding of the functional differences between astrocytes and other cell types. To this end, useful mouse lines for more effectively targeting CNS regions have been developed based on the different established astrocyte promoter genes (Table 3).
For example, a mouse line in which EGFP expression was driven by the astroglial promoter S100b (Vives et al., 2003) has been used in experiments to dissect the molecular underpinnings defining the identity of astrocytes and neural stem cells of the subventricular zone (SVZ). It was found that the expression of S100b defines a late developmental stage in astroglial cells, after which cells expressing GFAP lose their NSC multipotency and commit to mature into cortical astrocytes (Raponi et al., 2007). Furthermore, grafting experiments suggest that S100B expression is repressed in the adult SVZ microenvironment (Raponi et al., 2007).
One particular astroglial promoter, worth highlighting, that has received increased support as a pan-astrocytic marker is the folate metabolism enzyme, aldehyde dehydrogenase 1 family, member L1 (Aldh1L1) (Cahoy et al., 2008). The Aldh1L1-eGFP mouse line (Anthony and Heintz, 2007) was shown to label more astrocytes in the CNS than the glutamate transporter GLT1, which only co-localized with a subset of eGFP+ cells (Yang et al., 2011). Genetic fate mapping analysis using this line, along with newly generated Aldh1L1 BAC Cre animals, also revealed that adult-born neuroblasts in some areas of the brain are derived from Aldh1L1+ precursors, suggesting that these lines also target adult neural stem cells (Foo and Dougherty, 2013).
Although an inducible Cre mouse driven by the Aldh1L1 promoter has not yet been developed, recent inducible lines such as hGFAP-CreERT2 (Ganat et al., 2006), GLAST-CreERT2 (Mori et al., 2006), S100b-EGFP/CreERT2 (McMahon and Zhang, 2010) as well as Tet-On/Off systems, which are both inducible and reversible, have been successful in achieving relatively tight temporal control over astroglial gene expression (Table 3).
In an interesting study to determine whether subpopulations of astrocytes are actually committed to specific functions, or whether all astrocytes can take on many functions over time, the researchers crossed GLAST-CreERT2 (Mori et al., 2006) mice with an inducible EGFP reporter strain, and used the progeny (GLAST/eGFP) in comparison with Aldh1L1-eGFP (Anthony and Heintz, 2007) and other lines, to reveal significant heterogeneity in the reaction of astrocytes to injury (Bardehle et al., 2013). With live 2-photon imaging to visualize individual astrocytes, the authors found different astrocyte subtypes with unique injury responses, (either retaining their baseline morphology, directing their processes toward a lesion, or proliferating at juxtavascular sites), and concluded that astrocyte recruitment after injury relies on specific niche proliferation, which is markedly reduced after knock out of astrocytic RhoGTPase Cdc42 (Bardehle et al., 2013).
Another marker which is expressed in neural stem cells as well as differentiated neurons and astrocytes is the transcription factor Sox2. Sox2, a member of the SRY-related, high mobility group box family of transcription factors, is known to regulate self-renewal and multipotency (Avilion et al., 2003; Fong et al., 2008) while restricting differentiation (Boyer et al., 2005; Rodda et al., 2005), and is one of the four factors for establishing iPS cells (Takahashi et al., 2007a). However, although it is largely downregulated after fate commitment and maturation, Sox2 protein is still found in many differentiated astrocytes and some neurons in the neocortex, striatum and thalamus, indicating functions in these mature cells (Jinno, 2011; Komitova and Eriksson, 2004). Consistent with a role in astrogliogenesis, its overexpression promoted precursor differentiation into astroglia in favor of neurons (Bani-Yaghoub et al., 2006). The developmental relationship between Sox2 expression and the formation of astrocytes and even oligodendrocytes (Hoffmann et al., 2014), is being closely investigated.
The generation of a Sox2-EGFP knock-in mouse, which facilitates the isolation of these cells (Ellis et al., 2004) in vivo and in vitro demonstrated the reliability of Sox2 as a bona fide stem cell marker. Characterization of this knock-in strain has shown that Sox2 is expressed during embryonic development, and that its expression persists in neurogenic zones of adult tissues, such as brain, retina, tongue, testis, cervix lens epithelium and squamous epithelium of esophagus (Arnold et al., 2011). In the same study, lineage ablation with Sox2-Herpes Simplex Thymidine Kinase (HSTK) knock-in mice demonstrated a role in tissue replenishment and homeostasis (Arnold et al., 2011). A Sox2 transgenic mouse strain carrying 5.7 kb 5′ flanking sequences placed upstream of the (beta) geo G418 resistance gene facilitated the selection of cells for clonogenic assays (Zappone et al., 2000). A Sox2-Cre transgenic strain carrying 12.5 kb of upstream sequences (Hayashi et al., 2002; Vincent et al., 2003) has been demonstrated to be highly effective for epiblast-specific gene ablation. Interestingly, because of its early expression, an unexpected finding of maternal inheritance conferring transgene-independent recombination turned out to be a convenient advantage in complex breeding strategies (Hayashi et al., 2003; Vincent and Robertson, 2003). In order to target stem/progenitor cells temporally, however, a more recently established inducible Sox2-Cre BAC-based mouse strain (Kang and Hebert, 2012) should prove useful for modulating floxed genes in adult neural stem cells of neurogenic regions including the subventricular zone (SVZ) and dentate subgranular zone (SGZ). This study also showed Sox2-driven reporter expression in some adult astrocytes and neurons (Kang and Hebert, 2012).
3.2 Oligodendroglia
Many genes are specifically expressed by cells of the oligodendrocyte lineage, and these are widely used as reliable markers for different stages of the developing oligodendrocyte cell. For the majority of markers in this cell lineage, transgenic/knock-in reporter activity largely reflects the developmental expression of the endogenous protein. These genetic ‘driver’ strains may be loosely divided into three categories based on gene promoter activity: a) mature oligodendrocytes (MBP, MAG, MOBP, Cx47), b) progenitor cells (NG2, PDGFRa) and c) multiple developmental stages (CNP, PLP, Olig2, Olig1, Sox10) (Table 4).
With the exception of MBP, transgene expression driven by genes specific for mature myelinating oligodendrocytes, such as MAG, MOG, MOBP are largely germ-line. A Tamoxifen-inducible MBP line with 1.9kb of the mouse promoter (MCreERT2G) was generated for the temporal manipulation of the unfolded protein response in mature myelinating oligodendrocytes (Gow, 2011), which reportedly did not suffer from leakiness or premature suppression as in previous constructs (Foran and Peterson, 1992; Turnley et al., 1991). In contrast, markers for OPCs are less restricted spatially and temporally, and their targeting strains are understandably designed with tamoxifen inducibility (ER-T2 or Tm), for use in studies of postnatal and adult progenitor cells. The chondroitin sulfate proteoglycan CSPG4, also known by its acronym NG2, is notably expressed in OPCs, but is also found in pericytes - perivascular cells immunopositive for PDGFRb, that regulate development and remodeling of blood vessels - and microglia (Gao et al., 2010; Zhu et al., 2012). Collectively, given their broad range of cell fates and function atypical of glia (Kukley et al., 2008; Sun and Dietrich, 2013), NG2+ polydendrocytes may be considered to constitute an independent category of cells (Butt et al., 2005; Nishiyama et al., 2009; Nishiyama et al., 2005). For this reason, labeling with NG2 to identify OPCs often involves the use of a second marker, like Olig2, to determine fate commitment (Ligon et al., 2006).
In addition to other OPC markers, like PDGFRα, and Ascl1/MASH1, the prominent oligodendrocyte and Schwann cell transcription factor of myelin, Sox10, is now beginning to be recognized as a viable option to label OPCs. Mouse lines have been generated to label Sox10-expressing cells of the neural crest and oligodendroglial lineage, and their progeny using fluorescent reporters (Shibata et al., 2010). Because Sox10 is expressed in both OPCs as well as in myelinating oligodendrocytes (Kuhlbrodt et al., 1998), as a driver of transgene and Cre expression, it may function in a manner akin to the CNP promoter, which is active over several stages of OPC lineage progression (see below). In lineage characterization studies with the Sox10-Cre or S4F:Cre transgenic mouse line that bears 28.5 kb upstream sequences placed in front of the mouse c-Fos promoter, the neural crest-derived cells and oligodendroglial populations were labeled, similar to endogenous expression of Sox10 (Stine et al., 2009). Tetracycline-inducible Sox10-directed transgene expression was attained with the establishment of Sox10-rtTA knockin (Ludwig et al., 2004). To enable temporal control of Sox10-mediated Cre expression, a Sox10-iCreERT2 BAC mouse strain was generated (Simon et al., 2012). Analysis of this strain using the floxed R26R reporter showed labeling of NG2+ OPCs and GSTpi oligodendrocytes, including an intermediate stage presumed to be GPR17+ (Simon et al., 2012).
Although both 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP) and proteolipid protein (PLP) are recognized as protein indicators of mature cells capable of producing myelin, their transcripts and hence promoter activities are detected early in development (Kanfer et al., 1989; Lubetzki et al., 1991; Yuan et al., 2002), in OPCs (Mallon et al., 2002; Yuan et al., 2002) and beyond (Fuss et al., 2000a; Wight et al., 1993), allowing targeting of reporter or transgene expression in neural precursor cells and throughout the postnatal oligodendrocyte lineage – from OPCs through to myelinating oligodendrocyte (Ming et al., 2013; Yuan et al., 2002). Both are also active in the Schwann cell lineage (Mallon et al., 2002; Yuan et al., 2002). It is worth noting that both CNP- and PLP-promoter activities have been detected in neurons in gray matter structures of the medulla and hippocampus (Belachew et al., 2003; Miller et al., 2009) (Table 4). However, unlike the CNP promoter, the PLP promoter was recently found to be active in stem cells of the spinal cord (Harlow et al., 2014) (Table 4). The CNP-Cre knock-in (Lappe-Seifke et al., 2003) also shows early activity in Schwann cell precursors at E12 (Genoud et al., 2002), and has been used for gene ablation in OPCs (Genoud et al., 2002), myelinating oligodendrocytes (Brockschnieder et al., 2004; Kaga et al., 2006), Schwann cells (Grigoryan et al., 2013), and even enteric neurons and glia (Viader et al., 2011). The PLP-Cre is available as several tamoxifen-inducible strains (Doerflinger et al., 2003; Leone et al., 2003) for Cre targeting to oligodendroglia and Schwann cells. Interestingly, in addition to these lineages, fate-mapping of NG2+ cells using the PLP-Cre-ERT2 and Olig2-Cre-ERT2 lines have demonstrated multipotentiality of their targeted cells, with cerebellar Bergmann glia being labeled in addition to oligodendroglia and astrocytes (Chung et al., 2013).
3.3 Other reporter and effector mouse lines
BAC-based Prism transgenic animals are reporter lines which carry 3 transgenic alleles (Dougherty et al., 2012). Now available through Jackson Laboratories, these were generated to circumvent the combination of several alleles through extensive and costly breeding paradigms, and to determine the feasibility of producing a mouse line with multiple independently regulated transgenes at a single locus. The combination of three BACs – SNAP25, MOBP and Aldh1L1 - allows the simultaneous visualization of neurons, oligodendrocytes and astrocytes using 3 distinct fluorophores: mCherry, YFP and Cerulean respectively. In a second generation line, Prism 2.0, some toxicity issues were addressed by replacing mCherry with DsRedMax, and confining the YFP to the cytoplasm (Dougherty et al., 2012) (Table 3). These could be useful for FACS- cell isolation or immunodetection.
Recent developments in monitoring Cre-mediated recombination include the generation of dual fluorescent reporter Cre-dependent mouse strains. These fluorescent reporters express either red fluorescent protein or green fluorescent protein before recombination, and then either green fluorescent or red fluorescence respectively, after recombination (De Gasperi et al., 2008; Hasegawa et al., 2013). The interesting discovery of a pink mouse among colonies of another newly established strain expressing mCherry/EGFP dual reporters, further adds convenience during animal husbandry with the strong emission of mCherry that is visible to the naked eye (Hartwich et al., 2012). These dual fluorescent strains represent a departure from the previous dual reporters consisting of the combination of a fluorophore with an enzymatic reporter like beta-galactosidase or alkaline phosphatase (Lobe et al., 1999; Novak et al., 2000). Other dual fluorescent reporter strains, like the mT/mG, label fine projections by targeting reporter protein to membranes (Muzumdar et al., 2007). Another membrane-targeted reporter strain was recently described with mGFP that is uniquely expressed from the Tau locus (Hippenmeyer et al., 2005) as a knock-in, instead of the more commonly used chicken actin promoter. In this Tau-mGFP strain, the targeting cassette contains LoxP-STOP-LoxP-mGFP-IRES-NLS-LacZ (Hippenmeyer et al., 2005), allowing Cre-mediated control of mGFP expression from the Tau locus. Tau is expressed specifically in neurons and oligodendrocytes (Richter-Landsberg and Gorath, 1999). The Tau-mGFP strain was used with a PDGFRa-CreERT2 strain in a recent study to label the processes of newly generated oligodendrocytes in myelin internode analysis without occupying two fluorescent channels (Young et al., 2013).
While the isolation of targeted cells, either with transgene-reporter strains or using Cre/LoxP combinations enables transcriptome analysis, the process of cell isolation is laborious and notoriously low-yield, especially where low abundance RNA is being analyzed in rare cell populations such as adult progenitor cells. These types of studies have now been made more efficient and specific by removing the dependence on physical methods of cell isolation. Molecular tagging of polyribosome-bound RNA from the oligodendroglial lineage, the BAC-TRAP transgenic mice – Translating ribosome affinity purification (TRAP) – not only allows one to forego tissue dissociation and FACS, and disruption-associated artifacts, but also facilitates the isolation of RNA subpopulations that are actively translated (Doyle et al., 2008). A number of mouse strains expressing core ribosomal protein subunits have been developed for this purpose. For oligodendroglial analysis, L10a, a protein component of the 60S large ribosomal subunit, is expressed as a fusion gene with GFP under the control of the mouse CNP gene (Heiman et al., 2008). This allows subsequent GFP-mediated affinity purification of polysomal mRNAs from CNP-expressing oligodendroglial cells, as well as tracking oligodendrocytes from intact animals by fluorescence (Heiman et al., 2008; Lee et al., 2012). To overcome the limitation that different transgenic animals would be needed for different cell types, recently a conditional TRAP line (Rosa26fsTRAP) bearing GFP-RPL10a was established (Zhou et al., 2013), which is more widely accessible through the use of available Cre strains. Another Cre-dependent mouse line, called RiboTag, bears an RPL22 allele with a haemagglutinin epitope tag (Sanz et al., 2009). The RiboTag strain was elegantly designed with a duplicated exon 4 to ensure adequate expression of the wild type RPL22 allele prior to recombination (Sanz et al., 2009). Such polyribosome isolation systems are useful for analyzing oligodendroglial transcriptomes (Lee et al., 2012), ribosome binding of RNA populations and translational activity. A Rosa26-mCherry-RPL10a (mCherryTRAP) strain has also been generated (Hupe et al., 2014) for these applications.
Other RNA species, such as microRNA or miRNA, can now be immunoprecipitated through the expression of Argonaute (AGO) proteins. AGO proteins are central to the RNA-induced silencing complex (RISC) and bind miRNAs directly. AGO immunoprecipitation has been used to isolate miRNAs and their targets (Beitzinger et al., 2007; Easgow et al., 2007; Hendrickson et al., 2008; Karginov et al., 2007). In a recently developed AGO2 BAC strain, GFP and Myc are fused to the N-terminus of (tAGO2) of AGO2, and the transgene is knocked into the Rosa26 allele for Cre/LoxP-dependent recombination (R26-LSL-tAGO2) (He et al., 2012). tAGO2 may be immunoprecipitated with AGO2, Myc or GFP, allowing many options for studies of gene regulation involving miRNA.
4. Concluding remarks
This article provides a summary of in vitro and in vivo approaches to study astrocytes and oligodendrocytes by the selective isolation from, and cell-specific targeting within rodent brain. Because of their common origins (Zhu et al., 2008), strategies for generating cultures of these individual cell types from stem and progenitor cells are based on distinguishing physical properties, antigen or protein expression for cell selection, or directed maturation of stem cell populations. The advent of iPS cells has made human neural cells far more accessible, and although much work is still needed to implement the use of iPS cells for replacement therapy, these cells are useful to shed light on fundamental developmental processes and human disease mechanisms. With the ongoing characterization of more stem- and progenitor cell markers, a growing collection of glial-specific transgenic, Cre-based or inducible systems will continue to provide increasingly precise reporter-mediated detection and targeting of these cells and their gene products from brain tissue. These tools and the information gleaned from them may offer novel avenues vital for the diagnosis and treatment of brain disease.
Highlights.
Astrocytes and oligodendrocytes express stage-specific, cell surface markers useful for cell identification and selection
Astrocytes and oligodendrocytes can be acutely isolated from CNS tissue, cultured from progenitors or derived from stem cells
Genetic models offer a variety of marker genes with which to target stem cells, astrocytes and oligodendrocytes in vivo, with overlapping expression across cell types
Novel genetic models offer molecular tagging in vivo to facilitate gene expression analysis
Acknowledgments
We thank Drs Joseph Scafidi, Lina Chakrabarti and Beata Jablonska at Children’s National Medical Center, Dr Jiho Sohn at UC Davis, and Aaron Friedman at UC Berkeley for discussion. The authors receive support from the following agencies: National Multiple Sclerosis Society RG3954A1/2 (L-J C), partial support for L-J C from RG4706A4/2, R01NS056427 and R21NS078731 (Vittorio Gallo); Clare Boothe Luce Foundation (C.A.D), and National Science Foundation GRFP (VV S, Jr).
Abbreviations
- GalC
galactocerebroside
- FACS
Fluorescence-Activated Cell Sorting
- BAC
bacterial artificial chromosome
- PAC
P1 artificial chromosome
- EGFP
enhanced green fluorescent protein
- Aqp4
Aquoporin4
- Apoe4
Apolipoprotein E4
- Blbp
brain lipid binding protein
- Cx
Connexin
- FGFR
fibroblast growth factor receptor
- GFAP
glial fibrillary acidic protein
- GLAST
Glutamate-Aspartate transporter, also known as EAAT1
- EAAT
Excitatory amino acid transporter
- GLT1
glutamate transporter 1, also known as EAAT2
- Kir
Inwardly-rectifying potassium channel
- CNP
2′,3′-cyclic nucleotide 3′-phosphodiesterase
- MBP
myelin basic protein
- MOBP
myelin-associated oligodendrocyte basic protein
- MAG
myelin-associated glycoprotein
- MOG
myelin oligodendrocyte glycoprotein
- PLP
proteolipid protein
- NG2
neuron-glia antigen 2
- PDGFRα
platelet derived growth factor receptor alpha
- PDGF
platelet derived growth factor
- bFGF
basic fibroblast growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afshari FT, Fawcett JW. An in vitro assay to examine oligodendrocyte precursor cell migration on astrocytes. Methods Mol Biol. 2012;814:393–9. doi: 10.1007/978-1-61779-452-0_26. [DOI] [PubMed] [Google Scholar]
- Aguirre A, Gallo V. Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J Neurosci. 2004;24:10530–41. doi: 10.1523/JNEUROSCI.3572-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–7. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- Andrae J, Bongcam-Rudloff E, Hansson I, Lendahl U, Westermark B, Nister M. A 1. 8kb GFAP-promoter fragment is active in specific regions of the embryonic. CNS Mech Dev. 2001;107:181–5. doi: 10.1016/s0925-4773(01)00460-9. [DOI] [PubMed] [Google Scholar]
- Anthony TE, Heintz N. The folate metabolic enzyme ALDH1L1 is restricted to the midline of the early CNS, suggesting a role in human neural tube defects. J Comp Neurol. 2007;500:368–83. doi: 10.1002/cne.21179. [DOI] [PubMed] [Google Scholar]
- Anthony TE, Heintz N. Genetic lineage tracing defines distinct neurogenic and gliogenic stages of ventral telencephalic radial glial development. Neural Dev. 2008;3:30. doi: 10.1186/1749-8104-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–90. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- Arnold K, Sarkar A, Yram M, Polo J, Bronson R, Sengupta S, Seandel M, Geijsen N, Hochedlinger K. Sox2+ adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–29. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asipu A, Mellor AL, Blair GE. The specificity of the myelin basic protein gene promoter studied in transgenic mice. Biochemical and biophysical research communications. 2001;288:809–18. doi: 10.1006/bbrc.2001.5837. [DOI] [PubMed] [Google Scholar]
- Atkin SD, Patel S, Kocharyan A, Holtzclaw LA, Weerth SH, Schram V, Pickel J, Russell JT. Transgenic mice expressing a cameleon fluorescent Ca2+ indicator in astrocytes and Schwann cells allow study of glial cell Ca2+ signals in situ and in vivo. J Neurosci Methods. 2009;181:212–26. doi: 10.1016/j.jneumeth.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avellana-Adalid V, Nait-Oumesmar B, Lachapelle F, Baron-Van Evercooren A. Expansion of rat oligodendrocyte progenitors into proliferative “oligospheres” that retain differentiation potential. Journal of neuroscience research. 1996;45:558–70. doi: 10.1002/(SICI)1097-4547(19960901)45:5<558::AID-JNR6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on Sox2 function. Genes and Development. 2003;17:126–40. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr CE, Tannaus BA. Bioluminescence imaging: progress and applications. Trends in Biotechnology. 2011;29:624–33. doi: 10.1016/j.tibtech.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajenaru ML, Zhu Y, Hedrick NM, Donahoe J, Parada LF, Gutmann DH. Astrocyte-specific inactivation of the neurofibromatosis 1 gene (NF1) is insufficient for astrocytoma formation. Mol Cell Biol. 2002;22:5100–13. doi: 10.1128/MCB.22.14.5100-5113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98:641–53. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- Bani-Yaghoub M, Tremblay RG, Lei JX, Zhang D, Zurakowski B, Sandhu JK, Smith B, Ribecco-Lutkiewicz M, Kennedy J, Walker PR, Sikorska M. Role of Sox2 in the development of the mouse neocortex. Dev Biol. 2006;295:52–66. doi: 10.1016/j.ydbio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Bansal R, Lakhina V, Remedios R, Tole S. Expression of FGF receptors 1, 2, 3 in the embryonic and postnatal mouse brain compared with Pdgfralpha, Olig2 and Plp/dm20: implications for oligodendrocyte development. Dev Neurosci. 2003;25:83–95. doi: 10.1159/000072258. [DOI] [PubMed] [Google Scholar]
- Bardehle S, Kruger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, Dimou L, Gotz M. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci. 2013;16:580–6. doi: 10.1038/nn.3371. [DOI] [PubMed] [Google Scholar]
- Barnabe-Heider F, Goritz C, Sabelstrom H, Takebayashi H, Pfrieger FW, Meletis K, Frisen J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7:470–82. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Barres BA. Purification of rat optic nerve cell types by immunopanning. Neuroprotocols. 1993;2:205–8. [Google Scholar]
- Barton MD, Dunlop JW, Psaltis G, Kulik J, DeGennaro L, Kwak SP. Modified GFAP promoter auto-regulates tet-activator expression for increased transactivation and reduced tTA-associated toxicity. Brain research Molecular brain research. 2002;101:71–81. doi: 10.1016/s0169-328x(02)00170-5. [DOI] [PubMed] [Google Scholar]
- Battiste J, Helms AW, Kim EJ, Savage TK, Lagace DC, Mandyam CD, Eisch AJ, Miyoshi G, Johnson JE. Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development. 2007;134:285–93. doi: 10.1242/dev.02727. [DOI] [PubMed] [Google Scholar]
- Behar T, McMorris FA, Novotny EA, Barker JL, Dubois-Dalcq M. Growth and differentiation properties of O-2A progenitors purified from rat cerebral hemispheres. Journal of neuroscience research. 1988;21:168–80. doi: 10.1002/jnr.490210209. [DOI] [PubMed] [Google Scholar]
- Beitzinger M, Peters L, Zhu JY, Kremmer E, Meister G. Identification of human microRNA targets from isolated argonaute protein complexes. RNA Biol. 2007;4:76–84. doi: 10.4161/rna.4.2.4640. [DOI] [PubMed] [Google Scholar]
- Belachew S, Aguirre AA, Wang H, Vautier F, Yuan X, Anderson S, Kirby M, Gallo V. Cyclin-dependent kinase-2 controls oligodendrocyte progenitor cell cycle progression and is downregulated in adult oligodendrocyte progenitors. J Neurosci. 2002;22:8553–62. doi: 10.1523/JNEUROSCI.22-19-08553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. The Journal of cell biology. 2003;161:169–86. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belachew S, Yuan X, Gallo V. Unraveling oligodendrocyte origin and function by cell-specific transgenesis. Dev Neurosci. 2001;23:287–98. doi: 10.1159/000048712. [DOI] [PubMed] [Google Scholar]
- Benesova J, Hock M, Butenko O, Prajerova I, Anderova M, Chvatal A. Quantification of astrocyte volume changes during ischemia in situ reveals two populations of astrocytes in the cortex of GFAP/EGFP mice. Journal of neuroscience research. 2009;87:96–111. doi: 10.1002/jnr.21828. [DOI] [PubMed] [Google Scholar]
- Benveniste RJ, Keller G, Germano I. Embryonic stem cell-derived astrocytes expressing drug-inducible transgenes: differentiation and transplantation into the mouse brain. J Neurosurg. 2005;103:115–23. doi: 10.3171/jns.2005.103.1.0115. [DOI] [PubMed] [Google Scholar]
- Bergami M, Rimondini R, Santi S, Blum R, Gotz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15570–5. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz UA, Vosshenrich CA, Rajewsky K, Muller W. Bypass of lethality with mosaic mice generated by Cre-loxP-mediated recombination. Curr Biol. 1996;6:1307–16. doi: 10.1016/s0960-9822(02)70717-3. [DOI] [PubMed] [Google Scholar]
- Bonilla S, Hall AC, Pinto L, Attardo A, Gotz M, Huttner WB, Arenas E. Identification of midbrain floor plate radial glia-like cells as dopaminergic progenitors. Glia. 2008;56:809–20. doi: 10.1002/glia.20654. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–56. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M, Kisseberth WC, Su Y, Besnard F, Messing A. GFAP promoter directs astrocyte-specific expression in transgenic mice. J Neurosci. 1994;14:1030–7. doi: 10.1523/JNEUROSCI.14-03-01030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockschnieder D, Lappe-Siefke C, Goebbels S, Boesl MR, Nave KA, Riethmacher D. Cell depletion due to diphtheria toxin fragment A after Cre-mediated recombination. Mol Cell Biol. 2004;24:7636–42. doi: 10.1128/MCB.24.17.7636-7642.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, Vallier L. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–5. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- Brozzi F, Arcuri C, Giambanco I, Donato R. S100B Protein Regulates Astrocyte Shape and Migration via Interaction with Src Kinase: IMPLICATIONS FOR ASTROCYTE DEVELOPMENT, ACTIVATION, AND TUMOR GROWTH. J Biol Chem. 2009;284:8797–811. doi: 10.1074/jbc.M805897200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, Lemos JC, Hagan CE, Neumaier JF, Quintana A, Palmiter RD, Chavkin C. Selective p38alpha MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron. 2011;71:498–511. doi: 10.1016/j.neuron.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RDG. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754–6. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–48. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM, Hamilton N, Hubbard P, Pugh M, Ibrahim M. Synantocytes: the fifth element. J Anat. 2005;207:695–706. doi: 10.1111/j.1469-7580.2005.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–78. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:10061–5. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper KB, Jones K, McCarthy KD. Characterization of astrocyte-specific conditional knockouts. Genesis. 2007;45:292–9. doi: 10.1002/dvg.20287. [DOI] [PubMed] [Google Scholar]
- Casper KB, McCarthy KD. GFAP-positive progenitor cells produce neurons and oligodendrocytes throughout the CNS. Mol Cell Neurosci. 2006;31:676–84. doi: 10.1016/j.mcn.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Goto T, Tarui T, Takahashi T, Bhide PG, Nowakowski RS. Cell output, cell cycle duration and neuronal specification: a model of integrated mechanisms of the neocortical proliferative process. Cereb Cortex. 2003;13:592–8. doi: 10.1093/cercor/13.6.592. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–80. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandross KJ, Cohen RI, Paras PJ, Gravel M, Braun PE, Hudson LD. Identification and characterization of early glial progenitors using a transgenic selection strategy. J Neurosci. 1999;19:759–74. doi: 10.1523/JNEUROSCI.19-02-00759.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kwon CH, Lin L, Li Y, Parada LF. Inducible site-specific recombination in neural stem/progenitor cells. Genesis. 2009;47:122–31. doi: 10.1002/dvg.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Balasubramaniyan V, Peng J, Hurlock EC, Tallquist M, Li J, Lu QR. Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nature Protocols. 2007;2:1044–51. doi: 10.1038/nprot.2007.149. [DOI] [PubMed] [Google Scholar]
- Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, Ambartsumyan G, Aimiuwu O, Richter L, Zhang J, Khvorostov I, Ott V, Grunstein M, Lavon N, Benvenisty N, Croce CM, Clark AT, Baxter T, Pyle AD, Teitell MA, Pelegrini M, Plath K, Lowry WE. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5:111–23. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho W, Brenner M, Peters N, Messing A. Drug screening to identify suppressors of GFAP expression. Human Molecular Genetics. 2010;19:3169–78. doi: 10.1093/hmg/ddq227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho W, Hagemann TL, Johnson DA, Johnson JA, Messing A. Dual transgenic reporter mice as a tool for monitoring expression of GFAP. J Neurochem. 2009;110:343–51. doi: 10.1111/j.1471-4159.2009.06146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow LM, Zhang J, Baker SJ. Inducible Cre recombinase activity in mouse mature astrocytes and adult neural precursor cells. Transgenic Res. 2008;17:919–28. doi: 10.1007/s11248-008-9185-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SH, Guo F, Jiang P, Pleasure DE, Deng W. Olig2/Plp-positive progenitor cells give rise to Bergmann glia in the cerebellum. Cell death & disease. 2013;4:e546. doi: 10.1038/cddis.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R, de Vellis J. Astrocyte and oligodendrocyte cultures. In: Federoff S, Richardson A, editors. Protocols for Neural Cell Culture. 1997. pp. 117–30. [Google Scholar]
- Colello RJ, Sato-Bigbee C. Purification of oligodendrocytes and their progenitors using immunomagnetic separation and Percoll gradient centrifugation. Curr Protoc Neurosci. 2001 doi: 10.1002/0471142301.ns0312s03. [DOI] [PubMed] [Google Scholar]
- Conti L, Pollard SM, Gorba T, Reitano E, Toselli M, Biella G, Sun Y, Sanzone S. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. Plos Biol. 2005;3:e283. doi: 10.1371/journal.pbio.0030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras JE, Sanchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV, Saez JC. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BC, Liu Z, Lagarde MM, Zuo J. Conditional gene expression in the mouse inner ear using Cre-loxP. Journal of the Association for Research in Otolaryngology: JARO. 2012;13:295–322. doi: 10.1007/s10162-012-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czepiel M, Balasubramaniyan V, Schaafsma W, Stancic M, Mikkers H, Huisman C, Boddeke E, Copray S. Differentiation of induced pluripotent stem cells into functional oligodendrocytes. Glia. 2011;59:882–92. doi: 10.1002/glia.21159. [DOI] [PubMed] [Google Scholar]
- de Castro F, Bribian A. The molecular orchestra of the migration of oligodendrocyte precursors during development. Brain research Brain research reviews. 2005;49:227–41. doi: 10.1016/j.brainresrev.2004.12.034. [DOI] [PubMed] [Google Scholar]
- De Gasperi R, Rocher AB, Gama Sosa MA, Wearne SL, Perez GM, Friedrich VLJ, Hof PR, Elder GA. The IRG mouse: a two-color fluorescent reporter for assessing Cre-Mediated recombination and imaging complex cellular relationships in situ. Genesis. 2008;46:308–17. doi: 10.1002/dvg.20400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vivo L, Melone M, Rothstein JD, Conti F. GLT-1 Promoter Activity in Astrocytes and Neurons of Mouse Hippocampus and Somatic Sensory Cortex. Front Neuroanat. 2010;3:31. doi: 10.3389/neuro.05.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarolis NA, Mechanic M, Petrik D, Carlton A, Ables JL, Malhotra S, Bachoo R, Gotz M, Lagace DC, Eisch AJ. In vivo contribution of nestin- and GLAST-lineage cells to adult hippocampal neurogenesis. Hippocampus. 2013;23:708–19. doi: 10.1002/hipo.22130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaunay D, Heydon K, Cumano A, Schwab MH, Thomas JL, Suter U, Nave KA, Zalc B, Spassky N. Early neuronal and glial fate restriction of embryonic neural stem cells. J Neurosci. 2008;28:2551–62. doi: 10.1523/JNEUROSCI.5497-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaunay D, Heydon K, Miguez A, Schwab M, Nave KA, Thomas JL, Spassky N, Martinez S, Zalc B. Genetic tracing of subpopulation neurons in the prethalamus of mice (Mus musculus) J Comp Neurol. 2009;512:74–83. doi: 10.1002/cne.21904. [DOI] [PubMed] [Google Scholar]
- Desclaux M, Teigell M, Amar L, Vogel R, Gimenez YRM, Privat A, Mallet J. A novel and efficient gene transfer strategy reduces glial reactivity and improves neuronal survival and axonal growth in vitro. PloS One. 2009;4:e6227. doi: 10.1371/journal.pone.0006227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou L, Simon C, Kirchhoff F, Takebayashi H, Gotz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci. 2008;28:10434–42. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dincman TA, Beare JE, Ohri SS, Whittemore SR. Isolation of cortical mouse oligodendrocyte precursor cells. J Neurosci Methods. 2012;209:219–26. doi: 10.1016/j.jneumeth.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerflinger NH, Macklin WB, Popko B. Inducible site-specific recombination in myelinating cells. Genesis. 2003;35:63–72. doi: 10.1002/gene.10154. [DOI] [PubMed] [Google Scholar]
- Dougherty JD, Zhang J, Feng H, Gong S, Heintz N. Mouse transgenesis in a single locus with independent regulation for multiple fluorosphores. Plos One. 2012;7:e40511. doi: 10.1371/journal.pone.0040511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, Bupp S, Shrestha P, Shah RD, Doughty ML, Gong S, Greengard P, Heintz N. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135:749–62. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranovsky A, Picchini AM, Moadel T, Sisti AC, Yamada A, Kimura S, Leonardo ED, Hen R. Experience dictates stem cell fate in the adult hippocampus. Neuron. 2011;70:908–23. doi: 10.1016/j.neuron.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas J, Emery B. Purification and culture of oligodendrocyte lineage cells. Cold Spring harb Protoc. 2013;2013:810–4. doi: 10.1101/pdb.top074898. [DOI] [PubMed] [Google Scholar]
- Easgow G, Teleman AA, Cohen SM. Isolation of microRNA targets by miRNP immunopurification. RNA. 2007;13:1198–204. doi: 10.1261/rna.563707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt D, Theis M, Degen J, Ott T, van Rijen HV, Kirchhoff S, Kim JS, de Bakker JM, Willecke K. Functional role of connexin43 gap junction channels in adult mouse heart assessed by inducible gene deletion. J Mol Cell Cardiol. 2004;36:101–10. doi: 10.1016/j.yjmcc.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Ehm O, Goritz C, Covic M, Schaffner I, Schwarz TJ, Karaca E, Kempkes B, Kremmer E, Pfrieger FW, Espinosa L, Bigas A, Giachino C, Taylor V, Frisen J, Lie DC. RBPJkappa-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J Neurosci. 2010;30:13794–807. doi: 10.1523/JNEUROSCI.1567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, McMahon A, Rao M, Pevny L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26:148–65. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- Emery B, Dugas JC. Purification of Oligodendrocyte lineage cells from Mouse cortices by Immunopanning. Cold Spring harb Protoc. 2013;13:854–68. doi: 10.1101/pdb.prot073973. [DOI] [PubMed] [Google Scholar]
- Eom TY, Stanco A, Weimer J, Stabingas K, Sibrack E, Gukassyan V, Cheng J, Anton ES. Direct visualization of microtubules using a genetic tool to analyse radial progenitor-astrocyte continuum in brain. Nat Commun. 2011;2:446. doi: 10.1038/ncomms1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanarraga ML, Sommer I, Griffiths IR. O-2A progenitors of the mouse optic nerve exhibit a developmental pattern of antigen expression different from the rat. Glia. 1995;15:95–104. doi: 10.1002/glia.440150202. [DOI] [PubMed] [Google Scholar]
- Farioli-Vecchioli S, Saraulli D, Costanzi M, Pacioni S, Cina I, Aceti M, Micheli L, Bacci A, Cestari V, Tirone F. The timing of differentiation of adult hippocampal neurons is crucial for spatial memory. Plos Biol. 2008;6:e246. doi: 10.1371/journal.pbio.0060246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauquier T, Chatonnet F, Picou F, Richard S, Fossat N, Aguilera N, Lamonerie T, Flamant F. Purkinje cells and Bergmann glia are primary targets of the TRalpha1 thyroid hormone receptor during mouse cerebellum postnatal development. Development. 2014;141:166–75. doi: 10.1242/dev.103226. [DOI] [PubMed] [Google Scholar]
- Fong H, Hohenstein KA, Donovan PJ. Regulation of sel-renewal and pluripotency by Sox2 in human embryonic stem cells. Stem Cells. 2008;26:1931–8. doi: 10.1634/stemcells.2007-1002. [DOI] [PubMed] [Google Scholar]
- Foo LC, Allen NJ, Bushong EA, Ventura PB, Chung WS, Zhou L, Cahoy JD, Daneman R, Zong H, Ellisman MH, Barres BA. Development of a method for the purification and culture of rodent astrocytes. Neuron. 2011;71:799–811. doi: 10.1016/j.neuron.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo LC, Dougherty JD. Aldh1L1 is expressed by postnatal neural stem cells in vivo. Glia. 2013;61:1533–41. doi: 10.1002/glia.22539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foran DR, Peterson AC. Myelin acquisition in the central nervous system of the mouse revealed by an MBP-Lac Z transgene. J Neurosci. 1992;12:4890–7. doi: 10.1523/JNEUROSCI.12-12-04890.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forghani R, Garofalo L, Foran DR, Farhadi HF, Lepage P, Hudson TJ, Tretjakoff I, Valera P, Peterson A. A distal upstream enhancer from the myelin basic protein gene regulates expression in myelinforming schwann cells. J Neurosci. 2001;21:3780–7. doi: 10.1523/JNEUROSCI.21-11-03780.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisen J, Johansson CB, Torok C, Risling M, Lendahl U. Rapid, widespread, and longlasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. The Journal of cell biology. 1995;131:453–64. doi: 10.1083/jcb.131.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S-L, Hu J-G, Li Y, Wang Y-X, Jin J-Q, Xu X-M, Lu P-H. A simplified method for generating oligodendrocyte progenitor cells from neural precursor cells isolated from the E16 rat spinal cord. Acta Neurobiol Exp. 2007;67:367–77. doi: 10.55782/ane-2007-1654. [DOI] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–21. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss B, Mallon B, Phan T, Ohlemeyer C, Kirchhoff F, Nishiyama A, Macklin WB. Purification and analysis of in vivo-differentiated oligodendrocytes expressing the green fluorescent protein. Dev Biol. 2000a;218:259–74. doi: 10.1006/dbio.1999.9574. [DOI] [PubMed] [Google Scholar]
- Fuss B, Mallon B, Phan T, Ohlemeyer C, Kirchhoff F, Nishiyama A, Macklin WB. Purification and analysis of in vivo-differentiated oligodendrocytes expressing the green fluorescent protein. Dev Biol. 2000b;218:259–74. doi: 10.1006/dbio.1999.9574. [DOI] [PubMed] [Google Scholar]
- Galou M, Pournin S, Ensergueix D, Ridet JL, Tchelingerian JL, Lossouarn L, Privat A, Babinet C, Dupouey P. Normal and pathological expression of GFAP promoter elements in transgenic mice. Glia. 1994;12:281–93. doi: 10.1002/glia.440120405. [DOI] [PubMed] [Google Scholar]
- Ganat YM, Silbereis J, Cave C, Ngu H, Anderson GM, Ohkubo Y, Ment LR, Vaccarino FM. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J Neurosci. 2006;26:8609–21. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Lu J, Huo Y, Baby N, Ling EA, Dheen ST. NG2, a member of chondroitin sulfate proteoglycans family mediates the inflammatory response of activated microglia. Neuroscience. 2010;165:386–94. doi: 10.1016/j.neuroscience.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–41. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Garcia AD, Petrova R, Eng L, Joyner AL. Sonic hedgehog regulates discrete populations of astrocytes in the adult mouse forebrain. J Neurosci. 2010;30:13597–608. doi: 10.1523/JNEUROSCI.0830-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard AL, Pfeiffer SE, Williams WCI. Immunopanning and developmental stage-specific primary culture of oligodendrocyte progenitors (O4+ GalC-) directly from postnatal rodent cerebrum. Neuroprotocols. 1993;2:209–18. [Google Scholar]
- Gard AL, Williams WCI, Burrell MR. Oligodendroblasts distinbuished from O-2A glial progenitors by surface phenotype (O4+GalC-) and response to cytokines using signal transducer LIFR beta. Dev Biol. 1995;167:596–608. doi: 10.1006/dbio.1995.1051. [DOI] [PubMed] [Google Scholar]
- Genoud S, Lappe-Seifke C, Goebbels S, Radtke F, Aguet M, Scherer SS, Suter U, Nave KA, Mantei N. Notch1 control of oligodendrocyte diffferentiation in the spinal cord. The Journal of cell biology. 2002;158:709–18. doi: 10.1083/jcb.200202002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C, Kranenburg TA, Hogg T, Poppleton H, Martin J, Finkelstein D, Pounds S, Weiss A, Patay Z, Scoggins M, Ogg R, Pei Y, Yang ZJ, Brun S, Lee Y, Zindy F, Lindsey JC, Taketo MM, Boop FA, Sanford RA, Gajjar A, Clifford SC, Roussel MF, McKinnon PJ, Gutmann DH, Ellison DW, Wechsler-Reya R, Gilbertson RJ. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010;468:1095–9. doi: 10.1038/nature09587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser T, Pollard SM, Smith A, Brustle O. Tripotential differentiation of adherently expandable neural stem (NS) cells. PLoS One. 2007;2:e298. doi: 10.1371/journal.pone.0000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowatzki E, Cheng N, Hiel H, Yi E, Tanaka K, Ellis-Davies GC, Rothstein JD, Bergles DE. The glutamate-aspartate transporter GLAST mediates glutamate uptake at inner hair cell afferent synapses in the mammalian cochlea. J Neurosci. 2006;26:7659–64. doi: 10.1523/JNEUROSCI.1545-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–25. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Gosejacob D, Dublin P, Bedner P, Huttmann K, Zhang J, Tress O, Willecke K, Pfrieger F, Steinhauser C, Theis M. Role of astroglial connexin30 in hippocampal gap junction coupling. Glia. 2011;59:511–9. doi: 10.1002/glia.21120. [DOI] [PubMed] [Google Scholar]
- Gow A. Using temporal genetic switches to synchronize the unfolded protein response in cell populations in vivo. Methods Enzymol. 2011;491:143–61. doi: 10.1016/B978-0-12-385928-0.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow A, Friedrich VLJ, Lazzarini RA. Myelin basic protein gene contains separate enhancers for oligodendrocyte and Schwann cell expression. The Journal of cell biology. 1992;119:605–16. doi: 10.1083/jcb.119.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravel M, Di Polo A, Valera PB, Braun PE. Four-kilobase sequence of the mouse CNP gene directs spatial and temporal expression of lacZ in transgenic mice. Journal of neuroscience research. 1998;53:393–404. doi: 10.1002/(SICI)1097-4547(19980815)53:4<393::AID-JNR1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Grigoryan T, Stein S, Qi J, Wende H, Garratt AN, Nave KA, Birchmeier C, Birchmeier W. Wnt/Rspondin/beta-catenin signals control axonal sorting and lineage progression in Schwann cell development. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18174–9. doi: 10.1073/pnas.1310490110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology. 2009;136:1349–58. doi: 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- Guo F, Ma J, McCauley E, Bannerman P, Pleasure D. Early postnatal proteolipid promoter-expressing progenitors produce multilineage cells in vivo. J Neurosci. 2009;29:7256–70. doi: 10.1523/JNEUROSCI.5653-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Maeda Y, Ma J, Xu J, Horiuchi M, Miers L, Vaccarino F, Pleasure D. Pyramidal neurons are generated from oligodendroglial progenitor cells in adult piriform cortex. J Neurosci. 2010;30:12036–49. doi: 10.1523/JNEUROSCI.1360-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachem S, Aguirre A, Vives V, Marks A, Gallo V, Legraverend C. Spatial and temporal expression of S100B in cells of oligodendrocyte lineage. Glia. 2005;51:81–97. doi: 10.1002/glia.20184. [DOI] [PubMed] [Google Scholar]
- Hachem S, Laurenson AS, Hugnot JP, Legraverend C. Expression of S100B during embryonic development of the mouse cerebellum. BMC developmental biology. 2007;7:17. doi: 10.1186/1471-213X-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JM, Vetreno RP, Savage LM. Differential cortical neurotrophin and cytogenetic adaptation after voluntary exercise in normal and amnestic rats. Neuroscience. 2014;258:131–46. doi: 10.1016/j.neuroscience.2013.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton TG, Klinghoffer RA, Corrin PD, Soriano P. Evolutionary divergence of platelet-derived growth factor alpha receptor signaling mechanisms. Mol Cell Biol. 2003;23:4013–25. doi: 10.1128/MCB.23.11.4013-4025.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DW, Greber B, Wu G, Tapia N, Arauzo-Bravo MJ, Ko K, Bernemann C, Stehling M, Scholer HR. Direct reprogramming of fibroblasts into epiblast stem cells. Nat Cell Biol. 2011;13:66–71. doi: 10.1038/ncb2136. [DOI] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–9. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Harlow DE, Saul KE, Culp CM, Vesely EM, Macklin WB. Expression of proteolipid protein gene in spinal cord stem cells and early oligodendrocyte progenitor cells is dispensable for normal cell migration and myelination. J Neurosci. 2014;34:1333–43. doi: 10.1523/JNEUROSCI.2477-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwich H, Satheesh SV, Nothwang HG. A pink mouse reports the switch from red to green fluorescence upon Cre-mediated recombination. BMC Research Notes. 2012;5:296. doi: 10.1186/1756-0500-5-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Daitoku Y, Sekiguchi K, Tanimoto Y, Mizuno-Iijima S, Mizuno S, Kajiwara N, Ema M, MIwa Y, Mekada K, Yoshiki A, Takahashi S, Sugiyama F, Yagami K. Novel ROSA26 Cre-reporter knock-in C57BL/6N mice exhibiting green emission before and red emission after Cre-mediated recombination. Exp Anim. 2013;62:295–304. doi: 10.1538/expanim.62.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch MN, Nistor G, Keirstead HS. Derivation of high-purity oligodendroglial progenitors. Methods Mol Biol. 2009;549:59–75. doi: 10.1007/978-1-60327-931-4_5. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Koob JW, Liu DZ, Tong AY, Hunter DA, Parsadanian A, Mackinnon SE, Myckatyn TM. A double-transgenic mouse used to track migrating Schwann cells and regenerating axons following engraftment of injured nerves. Experimental neurology. 2007;207:128–38. doi: 10.1016/j.expneurol.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Hashimoto M, Koda M, Naito AT, Murata A, Okawa A, Takahashi K, Yamazaki M. Increase of sensitivity to mechanical stimulus after transplantation of murine induced pluripotent stem cell-derived astrocytes in a rat spinal cord injury model. J Neurosurg Spine. 2011;15:582–93. doi: 10.3171/2011.7.SPINE10775. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre mouse strain. Gene Expr Patterns. 2002;2:93–7. doi: 10.1016/s0925-4773(02)00292-7. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Tenzen T, McMahon AP. Maternal inheritance of Cre activity in a Sox2Cre deleter strain. Genesis. 2003;37:51–3. doi: 10.1002/gene.10225. [DOI] [PubMed] [Google Scholar]
- He M, Liu Y, Wang X, Zhang MQ, Hannon G, Huang ZJ. Cell-type based analysis of microRNA profiles in the mouse brain. Neuron. 2012;73:35–48. doi: 10.1016/j.neuron.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedus B, Dasgupta B, Shin JE, Emnett RJ, Hart-Mahon EK, Elghazi L, Bernal-Mizrachi E, Gutmann DH. Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP-and Ras-dependent mechanisms. Cell Stem Cell. 2007;1:443–57. doi: 10.1016/j.stem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, Suarez-Farinas M, Schwarz C, Stephan DA, Surmeier DJ, Greengard P, Heintz N. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–48. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N. Gene expression nervous system atlas (GENSAT) Nat Neurosci. 2004;7:483. doi: 10.1038/nn0504-483. [DOI] [PubMed] [Google Scholar]
- Hendrickson DG, Hogan DJ, Herschlag D, Ferrell JE, Brown PO. Systematic identification of mRNAs recruited to argonaute 2 by specific microRNAs and corresponding changes in transcript abundance. Plos One. 2008;3:e2126. doi: 10.1371/journal.pone.0002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H, Fujita A, Iwai K, Yamada M, Kurachi Y. Differential assembly of inwardly rectifying K+ channel subunits, Kir4.1 and Kir5. 1, in brain astrocytes. J Biol Chem. 2004;279:44065–73. doi: 10.1074/jbc.M405985200. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. Plos Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirrlinger PG, Scheller A, Braun C, Hirrlinger J, Kirchhoff F. Temporal control of gene recombination in astrocytes by transgenic expression of the tamoxifen-inducible DNA recombinase variant CreERT2. Glia. 2006;54:11–20. doi: 10.1002/glia.20342. [DOI] [PubMed] [Google Scholar]
- Hirrlinger PG, Scheller A, Braun C, Quintela-Schneider M, Fuss B, Hirrlinger J, Kirchhoff F. Expression of reef coral fluorescent proteins in the central nervous system of transgenic mice. Mol Cell Neurosci Lett. 2005;30:291–303. doi: 10.1016/j.mcn.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Hisahara SAT, Sugiyama F, Yagami K, Suzuki M, Abe K, Yamamura K, MJ, Momoi T, Saruta T, Bernard CC, Okano HMM. Targeted expression of baculovirus p35 caspase inhibitor in oligodendrocytes protects mice against autoimmunemediated demyelination. EMBO J. 2000;19:341–8. doi: 10.1093/emboj/19.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann SA, Hos D, Kuspert M, Lang RA, Lovell-Badge R, Wegner M, Reiprich S. Stem cell factor Sox2 and its close relative Sox3 have differentiation functions in oligodendrocytes. Development. 2014;141:39–50. doi: 10.1242/dev.098418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi M, Lindsten T, Pleasure D, Itoh T. Differing in vitro survival dependency of mouse and rat NG2+ oligodendroglial progenitor cells. Journal of neuroscience research. 2010;88:957–70. doi: 10.1002/jnr.22262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovelmeyer N, Hao Z, Kranidioti K, Kassiotis G, Buch T, Frommer F, Von Hoch L, Kramer D, Minichiello L, Kollias G, Lassmann H, Waisman A. Apoptosis of oligodendrocytes via Fas and TNF-R1 is a key event in the induction of experimental autoimmune encephalomyelitis. Journal of immunology. 2005;175:5875–84. doi: 10.4049/jimmunol.175.9.5875. [DOI] [PubMed] [Google Scholar]
- Howard BM, Zhicheng M, Filipovic R, Moore AR, Antic SD, Zecevic N. Radial glia cells in the developing human brain. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2008;14:459–73. doi: 10.1177/1073858407313512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Zhao N, Bai X, Karram K, Trotter J, Goebbels S, Scheller A, Kirchhoff F. Novel NG2-CreERT2 knock-in mice demonstrate heterogeneous differentiation potential of NG2 glia during development. Glia. 2014;62:896–913. doi: 10.1002/glia.22648. [DOI] [PubMed] [Google Scholar]
- Huang Y, Osorno R, Tsakiridis A, Wilson V. In vivo differentiation potential of epiblast stem cells revealed by chineric embryo formation. Cell Reports. 2012;2:1571–8. doi: 10.1016/j.celrep.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Hupe M, Li MX, Gertow Gillner K, Adams RH, Stenman JM. Evaluation of TRAP-sequencing technology with a versatile conditional mouse model. Nucleic acids research. 2014;42:e14. doi: 10.1093/nar/gkt995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrie RA, Alvarez-Buylla A. Cells in the astroglial lineage are neural stem cells. Cell Tissue Res. 2008;331:179–91. doi: 10.1007/s00441-007-0461-z. [DOI] [PubMed] [Google Scholar]
- Imai F, Hirai S, Akimoto K, Koyama H, Miyata T, Ogawa M, Noguchi S, Sasaoka T, Noda T, Ohno S. Inactivation of aPKClambda results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development. 2006;133:1735–44. doi: 10.1242/dev.02330. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Ohtsuka T, Metzger D, Chambon P, Kageyama R. Temporal regulation of Cre recombinase activity in neural stem cells. Genesis. 2006;44:233–8. doi: 10.1002/dvg.20212. [DOI] [PubMed] [Google Scholar]
- Inamura N, Sugio S, Macklin WB, Tomita K, Tanaka KF, Ikenaka K. Gene induction in mature oligodendrocytes with a PLP-tTA mouse line. Genesis. 2012;50:424–8. doi: 10.1002/dvg.20808. [DOI] [PubMed] [Google Scholar]
- Inoue H, Nagata N, Kurokawa H, Yamanaka S. iPS cells: a game changer for future medicine. EMBO J. 2014;33:409–17. doi: 10.1002/embj.201387098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura E, Odaira K, Yokoyama K, Osuna M, Hara T, Inoue K. Generation of transgenic rats expressing green fluorescent protein in S-100beta-producing pituitary folliculo-stellate cells and brain astrocytes. Endocrinology. 2007;148:1518–23. doi: 10.1210/en.2006-1390. [DOI] [PubMed] [Google Scholar]
- Itoh K. Culture of oligodendrocyte precursor cells (NG2+/O1-) and oligodendrocytes (NG2-/O1+) from embryonic rat cerebrum. Brain Research Protocols. 2002;10:23–30. doi: 10.1016/s1385-299x(02)00177-0. [DOI] [PubMed] [Google Scholar]
- James LR, Andrews S, Walker S, de Sousa PRS, Ray A, Russell NA, Bellamy TC. High-throughput analysis of calcium signalling kinetics in astrocytes stimulated with different neurotransmitters. PloS One. 2011;6:e26889. doi: 10.1371/journal.pone.0026889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HJ, Kim JS, Choi HW, Jeon I, Choi S, Kim MJ, Song J, Do JT. Neural stem cells derived from epiblast stem cells display distinctive properties. Stem Cell Res. 2014;12:506–16. doi: 10.1016/j.scr.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Jany PL, Hagemann TL, Messing A. GFAP expression as an indicator of disease severity in mouse models of Alexander disease. ASN neuro. 2013;5:e00109. doi: 10.1042/AN20130003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno S. Regional and laminar differences in antigen profiles and spatial distributions of astrocytes in the mouse hippocampus, with reference to aging. Neuroscience. 2011;180:41–52. doi: 10.1016/j.neuroscience.2011.02.013. [DOI] [PubMed] [Google Scholar]
- John GR. Investigation of astrocyte-oligodendrocyte interactions in human cultures. Methods Mol Biol. 2012;814:401–14. doi: 10.1007/978-1-61779-452-0_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WB, Ruppe MD, Rockenstein EM, Price J, Sarthy VP, Verderber LC, Mucke L. Indicator expression directed by regulatory sequences of the glial fibrillary acidic protein (GFAP) gene: in vivo comparison of distinct GFAP-lacZ transgenes. Glia. 1995;13:174–84. doi: 10.1002/glia.440130304. [DOI] [PubMed] [Google Scholar]
- Jones EV, Cook D, Murai KK. A neuron-astrocyte co-culture system to investigate astrocyte-secreted factors in mouse neuronal development. Methods Mol Biol. 2012;814:341–52. doi: 10.1007/978-1-61779-452-0_22. [DOI] [PubMed] [Google Scholar]
- Juopperi TA, Kim WR, Chiang CH, Yu H, Margolis RL, Ross CA, Ming GL, Song H. Astrocytes generated from patient induced pluripotent stem cells recapitulate features of Huntington’s disease patient cells. Mol Brain. 2012;5:17. doi: 10.1186/1756-6606-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaga Y, Shoemaker WJ, Furusho M, Bryant M, Rosenbluth J, Pfeiffer SE, Oh L, Rasband M, Lappe-Siefke C, Yu K, Ornitz DM, Nave KA, Bansal R. Mice with conditional inactivation of fibroblast growth factor receptor-2 signaling in oligodendrocytes have normal myelin but display dramatic hyperactivity when combined with CNP1 inactivation. J Neurosci. 2006;26:12339–50. doi: 10.1523/JNEUROSCI.3573-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaykina N, Abelson K, King D, Liakhovitskaia A, Schreiner S, Wegner M, Kozlova EN. In vitro and in vivo effects on neural crest stem cell differentiation by conditional activation of Runx1 short isoform and its effect on neuropathic pain behavior. Upsala journal of medical sciences. 2010;115:56–64. doi: 10.3109/03009730903572065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanfer J, Parenty M, Goujet-Zalc C, Monge M, Bernier L, Campagnoni AT, Dautigny A, Zalc B. Developmental expression of myelin proteolipid, basic protein, and 2′,3′-cyclic nucleotide 3′-phosphodiesterase transcripts in different rat brain regions. Journal of molecular neuroscience: MN. 1989;1:39–46. doi: 10.1007/BF02896855. [DOI] [PubMed] [Google Scholar]
- Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–81. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang W, Hebert JM. A Sox2 BAC transgenic approach for targeting adult neural stem cells. PloS One. 2012;7:e49038. doi: 10.1371/journal.pone.0049038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karginov FV, Conaco C, Xuan Z, Schmidt BH, Parker JS, Mandel G, Hannon GJ. A biochemical approach to identifying microRNA targets. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19291–6. doi: 10.1073/pnas.0709971104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Schut D, Fehlings MG. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci. 2010;30:1657–76. doi: 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karram K, Goebbels S, Schwab M, Jennissen K, Seifert G, Steinhauser C, Nave KA, Trotter J. NG2-expressing cells in the nervous system revealed by the NG2-EYFP-knockin mouse. Genesis. 2008;46:743–57. doi: 10.1002/dvg.20440. [DOI] [PubMed] [Google Scholar]
- Kawaguchi A, Miyata T, Sawamoto K, Takashita N, Murayama A, Akamatsu W, Ogawa M, Okabe M, Tano Y, Goldman SA, Okano H. Nestin-EGFP transgenic mice: visualization of the self-renewal and multipotency of CNS stem cells. Mol Cell Neurosci. 2001;17:259–73. doi: 10.1006/mcne.2000.0925. [DOI] [PubMed] [Google Scholar]
- Keyoung HM, Roy NS, Benraiss A, Louissaint A, Jr, Suzuki A, Hashimoto M, Rashbaum WK, Okano H, Goldman SA. High-yield selection and extraction of two promoter-defined phenotypes of neural stem cells from the fetal human brain. Nat Biotechnol. 2001;19:843–50. doi: 10.1038/nbt0901-843. [DOI] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. The American journal of pathology. 2003;162:1693–707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Ables JL, Dickel LK, Eisch AJ, Johnson JE. Ascl1(Mash1) defines cells with long-term neurogenic potential in subgranular and subventricular zones in adult mouse brain. PLoS ONE. 2011;6:e18472. doi: 10.1371/journal.pone.0018472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB, Sebastiano V, Wu G, Arauzo-Bravo MJ, Sasse P, Gentile L, Ko K, Ruau D, Ehrich M, van den Boom D, Meyer J, Hubner K, Bernemann C, Ortmeier C, Zenke M, Fleischmann BK, Zaehres H, Scholer HR. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136:411–9. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Kim JB, Zaehres H, Wu G, Gentile L, Ko K, Sebastiano V, Arauzo-Bravo MJ, Ruau D, Han DW, Zenke M, Scholer HR. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454:646–50. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- Komitova M, Eriksson PS. Sox-2 is expressed by neural progenitors and astroglia in the adult rat brain. Neurosci Lett. 2004;369:24–7. doi: 10.1016/j.neulet.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M. Sox10, a novel transcriptional modulator in Glial cells. Journal of Neuroscience. 1998;18:237–50. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M, Kiladze M, Tognatta R, Hans M, Swandulla D, Schramm J, Dietrich D. Glial cells are borh with synapses. FASEB J. 2008;22:2957–69. doi: 10.1096/fj.07-090985. [DOI] [PubMed] [Google Scholar]
- Kunze A, Lengacher S, Dirren E, Aebischer P, Magistretti PJ, Renaud P. Astrocyte-neuron co-culture on microchips based on the model of SOD mutation to mimic ALS. Integr Biol. 2013;5:964–75. doi: 10.1039/c3ib40022k. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Mirzadeh Z, Soriano-Navarro M, Rasin M, Wang D, Shen J, Sestan N, Garcia-Verdugo J, Alvarez-Buylla A, Jan LY, Jan YN. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell. 2006;127:1253–64. doi: 10.1016/j.cell.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurronen A, Pihlaja R, Pollari E, Kanninen K, Storvik M, Wong G, Koistinaho M, Koistinaho J. Adult and neonatal astrocytes exhibit diverse gene expression profiles in response to beta amyloid ex vivo. World Journal of Neuroscience. 2012;2:57–67. [Google Scholar]
- Kuzmanovic M, Dudley VJ, Sarthy VP. GFAP promoter drives Muller cell-specific expression in transgenic mice. Invest Ophthalmol Vis Sci. 2003;44:3606–13. doi: 10.1167/iovs.02-1265. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Zhu X, Zhang J, Knoop LL, Tharp R, Smeyne RJ, Eberhart CG, Burger PC, Baker SJ. Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nat Genet. 2001;29:404–11. doi: 10.1038/ng781. [DOI] [PubMed] [Google Scholar]
- Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, Donovan MH, Fischer SJ, Farnbauch LA, Beech RD, DiLeone RJ, Greer CA, Mandyam CD, Eisch AJ. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27:12623–9. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe-Seifke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of CNP1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–74. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Laranjeira C, Sandgren K, Kessaris N, Richardson W, Potocnik A, Vanden Berghe P, Pachnis V. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. The Journal of clinical investigation. 2011;121:3412–24. doi: 10.1172/JCI58200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Messing A, Su M, Brenner M. GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia. 2008;56:481–93. doi: 10.1002/glia.20622. [DOI] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang P-W, Pellerin L, Magistretti PJ, Rothstein JD. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–50. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Su M, Messing A, Brenner M. Astrocyte heterogeneity revealed by expression of a GFAP-LacZ transgene. Glia. 2006;53:677–87. doi: 10.1002/glia.20320. [DOI] [PubMed] [Google Scholar]
- Leone DP, Genoud S, Atanasoski S, Grausenburger R, Berger P, Metzger D, Macklin WB, Chambon P, Suter U. Tamoxifen-inducible glia-specific Cre mice for somatic mutagenesis in oligodendrocytes and Schwann cells. Mol Cell Neurosci. 2003;22:430–40. doi: 10.1016/s1044-7431(03)00029-0. [DOI] [PubMed] [Google Scholar]
- Levine JM. Neuronal influences on glial progenitor cell development. Neuron. 1989;3:103–13. doi: 10.1016/0896-6273(89)90119-0. [DOI] [PubMed] [Google Scholar]
- Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–26. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Kesari S, Kitada M, Sun T, Arnett HA, Alberta JA, Anderson DJ, Stiles CD, Rowitch DH. Development of NG2 neural progenitor cells requires Olig2 gene function. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7853–8. doi: 10.1073/pnas.0511001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DT, Wu J, Holstein D, Upadhyay G, Rourk W, Muller E, Lechleiter JD. Ca2+ signaling, mitochondria and sensitivity to oxidative stress in aging astrocytes. Neurobiol Aging. 2007;28:99–111. doi: 10.1016/j.neurobiolaging.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Lin RC, Matesic DF, Marvin M, McKay RD, Brustle O. Re-expression of the intermediate filament nestin in reactive astrocytes. Neurobiol Dis. 1995;2:79–85. doi: 10.1006/nbdi.1995.0008. [DOI] [PubMed] [Google Scholar]
- Lin T, Xiang Z, Cui L, Stallcup W, Reeves SA. New mouse oligodendrocyte precursor (mOP) cells for studies on oligodendrocyte maturation and function. J Neurosci Methods. 2006;157:187–94. doi: 10.1016/j.jneumeth.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Lin W, Kemper A, McCarthy KD, Pytel P, Wang JP, Campbell IL, Utset MF, Popko B. Interferon-gamma induced medulloblastoma in the developing cerebellum. J Neurosci. 2004;24:10074–83. doi: 10.1523/JNEUROSCI.2604-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A. Z/AP, a double repoerter for Cre-mediated recombination. Dev Biol. 1999;208:281–92. doi: 10.1006/dbio.1999.9209. [DOI] [PubMed] [Google Scholar]
- Lopez ME, Klein AD, Dimbil UJ, Scott MP. Anatomically defined neuron-based rescue of neurodegenerative Niemann-Pick type C disorder. J Neurosci. 2011;31:4367–78. doi: 10.1523/JNEUROSCI.5981-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Lubetzki C, Goujet-Zalc C, Gansmuller A, Monge M, Brillat A, Zalc B. Morphological, biochemical, and functional characterization of bulk isolated glial progenitor cells. J Neurochem. 1991;56:671–80. doi: 10.1111/j.1471-4159.1991.tb08202.x. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Schlierf B, Schardt A, Nave KA, Wegner M. Sox10-rtTA mouse line for tetracycline-inducible expression of transgenes in neural crest cells and oligodendrocytes. Genesis. 2004;40:171–5. doi: 10.1002/gene.20083. [DOI] [PubMed] [Google Scholar]
- Luo J, Ho P, Steinman L, Wyss-Coray T. Bioluminescence in vivo imaging of autoimmune encephalomyelitis predicts disease. J Neuroinflammation. 2008;5:1–6. doi: 10.1186/1742-2094-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Nguyen A, Villeda S, Zhang H, Ding Z, Lindsey D, Bieri G, Castellano JM, Beaupre GS, Wyss-Coray T. Long-term cognitive impairments and pathological alterations in a mouse model of repetitive mild traumatic brain injury. Frontiers in Neurology. 2014;5:1–13. doi: 10.3389/fneur.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali N, Ahfeldt T, Rigamonti A, Utikal J, Cowan C, Hochedlinger K. A high-efficiency system for the generation and study of human induced pluripotent stem cells. Cell Stem Cell. 2008;3:340–5. doi: 10.1016/j.stem.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallon BS, Shick HE, Kidd GJ, Macklin WB. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J Neurosci. 2002;22:876–85. doi: 10.1523/JNEUROSCI.22-03-00876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 2000;14:994–1004. [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T, Ahlberg PE, Kessaris N, Iannarelli P, Dennehy U, Richardson WD, McMahon AP, Koentges G. Neural crest origins of the neck and shoulder. Nature. 2005;436:347–55. doi: 10.1038/nature03837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias K, Kirchhoff F, Seifert G, Huttmann K, Matyash M, Kettenmann H, Steinhauser C. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23:1750–8. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Proschel M. Isolation and generation of oligodendrocytes by immunopanning. Curr Protoc Neurosci. 2001 doi: 10.1002/0471142301.ns0313s00. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. The Journal of cell biology. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, Savill J, Roes J, Hynes RO. Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–76. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- McMahon A, Zhang P. The GenitoUrinary Development Molecular Anatomy Project (GUDMAP) 2010. [Google Scholar]
- Medina-Rodriguez EM, Arenzana FJ, Bribian A, de Castro F. Protocol to isolate a large amount of functional oligodendrocyte precursor cells from the cerebral cortex of adult mice and humans. PloS One. 2013;8:e81620. doi: 10.1371/journal.pone.0081620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menet V, Gimenez y Ribotta M, Chauvet N, Drian MJ, Lannoy J, Colucci-Guyon E, Privat A. Inactivation of the glial fibrillary acidic protein gene, but not that of vimentin, improves neuronal survival and neurite growth by modifying adhesion molecule expression. J Neurosci. 2001;21:6147–58. doi: 10.1523/JNEUROSCI.21-16-06147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesman S, von Oerthel L, Smidt MP. Mesodiencephalic Dopaminergic Neuronal Differentiation Does Not Involve GLI2A-Mediated SHH-Signaling and Is under the Direct Influence of Canonical WNT Signaling. PLoS One. 2014;9:e97926. doi: 10.1371/journal.pone.0097926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Barres BA. Purification and characterization of astrocyte precursor cells in the developing rat optic nerve. J Neurosci. 1999;19:1049–61. doi: 10.1523/JNEUROSCI.19-03-01049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalski JP, Anderson C, Beauvais A, De Repentigny Y, Kothary R. The proteolipid protein promoter drives expression outside of the oligodendrocyte lineage during embryonic and early postnatal development. PLoS ONE. 2011;6:e19772. doi: 10.1371/journal.pone.0019772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311–24. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Kangas CD, Macklin WB. Neuronal expression of the proteolipid protein gene in the medulla of the mouse. Journal of neuroscience research. 2009;87:2842–53. doi: 10.1002/jnr.22121. [DOI] [PubMed] [Google Scholar]
- Ming X, Chew LJ, Gallo V. Transgenic overexpression of Sox17 promotes oligodendrocyte development and attenuates demyelination. J Neurosci. 2013;33:12528–42. doi: 10.1523/JNEUROSCI.0536-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi T, Aoki Y, Eksioglu YZ, Takahashi T, Bhide PG, Reeves SA, Caviness VS., Jr Overexpression of p27Kip1 lengthens the G1 phase in a mouse model that targets inducible gene expression to central nervous system progenitor cells. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6435–40. doi: 10.1073/pnas.111051398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Z, Moore AR, Filipovic R, Ogawa Y, Kazuhiro I, Antic SD, Zecevic N. Human cortical neurons originate from radial glia and neuron-restricted progenitors. J Neurosci. 2007;27:4132–45. doi: 10.1523/JNEUROSCI.0111-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Krenick R, Ullian E, Tsai H-h, Deneen B, Richardson WD, Barres BA, Rowitch DH. Astrocytes and disease: a neurodevelopmental perspective. Genes and Development. 2012;26:891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon Y, Kim HJ, Kim JY, Kim H, Kim WR, Sun W. Different expression of human GFAP promoter-derived GFP in different subsets of astrocytes in the mouse brain. Animal Cells and Systems. 2011;15:268–73. [Google Scholar]
- Moretti A, Bellin M, Jung CB, Thies TM, Takashima Y, Bernshausen A, Schiemann M, Fischer S, Moosmang S, Smith AG, Lam JT, Laugwitz KL. Mouse and human induced pluripotent stem cells as a source for multipotent Isl1+ cardiovascular progenitors. FASEB J. 2010;24:700–11. doi: 10.1096/fj.09-139477. [DOI] [PubMed] [Google Scholar]
- Mori T, Tanaka K, Buffo A, Wurst W, Kuhn R, Gotz M. Inducible gene deletion in astroglia and radial glia--a valuable tool for functional and lineage analysis. Glia. 2006;54:21–34. doi: 10.1002/glia.20350. [DOI] [PubMed] [Google Scholar]
- Morrison RS, de Vellis J. Growth of purified astrocytes in a chemically defined medium. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:7205–9. doi: 10.1073/pnas.78.11.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison RS, De Vellis J, Lee YL, Bradshaw RA, Eng LF. Hormones and growth factors induce the synthesis of glial fibrillary acidic protein in rat brain astrocytes. Journal of neuroscience research. 1985;14:167–76. doi: 10.1002/jnr.490140202. [DOI] [PubMed] [Google Scholar]
- Mucke L, Oldstone MB, Morris JC, Nerenberg MI. Rapid activation of astrocyte-specific expression of GFAP-lacZ transgene by focal injury. New Biol. 1991;3:465–74. [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A Global Double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Najm FJ, Chenoweth JG, Anderson PD, Nadeau JH, Redline RW, McKay RDG, Tesar PJ. Isolation of Epiblast Stem Cells from preimplantation mouse embryos. Cell Stem Cell. 2011a;8:318–25. doi: 10.1016/j.stem.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm FJ, Lager AM, Zaremba A, Wyatt K, Caprariello AV, Factor DC, Karl RT, Maeda T, Miller RH, Tesar PJ. Transcription factor-mediated reprogramming of fibroblasts to expandable, myelinogenic oligodendrocyte progenitor cells. Nature Biotechnology. 2013;31:426–33. doi: 10.1038/nbt.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm FJ, Zaremba A, Caprariello AV, Nayak S, Freundt EC, Scacheri PC, MIller RH, Tesar PJ. Rapid and robust generation of functional oligodendrocyte progenitor cells from epiblast stem cells. Nature Methods. 2011b;8:957–62. doi: 10.1038/nmeth.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–6. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Takizawa N, Narita M, Ichisaka T, Yamanaka S. Promotion of direct reprogramming by transformation-deficient Myc. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14152–7. doi: 10.1073/pnas.1009374107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Schwartz JP. Gene expression patterns in in vivo normal adult astrocytes compared with cultured neonatal and normal adult astrocytes. Neurochem Int. 2004:45. doi: 10.1016/j.neuint.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Nave KA. Myelination and the trophic support of long axons. Nat Rev Neurosci. 2010;11:275–83. doi: 10.1038/nrn2797. [DOI] [PubMed] [Google Scholar]
- Nielsen JA, Maric D, Lau P, Barker JL, Hudson LD. Identification of a novel oligodendrocyte cell adhesion protein using gene expression profiling. J Neurosci. 2006;26:9881–91. doi: 10.1523/JNEUROSCI.2246-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic J, Mori T, Gotz M. Distinct modes of neuron addition in adult mouse neurogenesis. J Neurosci. 2007;27:10906–11. doi: 10.1523/JNEUROSCI.2572-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nature Reviews Neuroscience. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Suzuki R, Zhu X. NG2 cells (polydendrocytes) in brain physiology and repair. Frontiers in Neuroscience. 2014:8. doi: 10.3389/fnins.2014.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Yang Z, Butt A. Astrocytes and NG2-glia: what’s in a name. J Anat. 2005;207:587–93. doi: 10.1111/j.1469-7580.2005.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J, Wang L, Liu S, Li C, Kong J, Shen H-Y, Xiao L. An efficient and economical culture approach for the enrichment of purified oligodendrocyte progenitor cells. J Neurosci Methods. 2012;209:241–9. doi: 10.1016/j.jneumeth.2012.05.032. [DOI] [PubMed] [Google Scholar]
- Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, Zhang C-L. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol. 2013;15:1164–76. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa-Kawakita M, Abramowski V, Kalamarides M, Thomas G, Giovannini M. Targeted expression of Cre recombinase to myelinating cells of the central nervous system in transgenic mice. Genesis. 2000;26:127–9. doi: 10.1002/(sici)1526-968x(200002)26:2<127::aid-gene8>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Nolte C, Matyash M, Pivneva T, Schipke CG, Ohlemeyer C, Hanisch UK, Kirchhoff F, Kettenmann H. GFAP promoter-controlled EGFP-expressing transgenic mice: a tool to visualize astrocytes and astrogliosis in living brain tissue. Glia. 2001;33:72–86. [PubMed] [Google Scholar]
- Nomura T, Goritz C, Catchpole T, Henkemeyer M, Frisen J. EphB signaling controls lineage plasticity of adult neural stem cell niche cells. Cell Stem Cell. 2010;7:730–43. doi: 10.1016/j.stem.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noraberg J, Jensen CV, Bonde C, Montero M, Nielsen JV, Jensen NA, Zimmer J. The developmental expression of fluorescent proteins in organotypic hippocampal slice cultures from transgenic mice and its use in the determination of excitotoxic neurodegeneration. Altern Lab Anim. 2007;35:61–70. doi: 10.1177/026119290703500121. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–55. [PubMed] [Google Scholar]
- O’Meara RW, Ryan SD, Colognato H, Kothary R. Derivation of enriched oligodendrocyte cultures and oligodendrocyte/neuron myelinating co-cultures from post-natal murine tissues. J Vis Exp. 2011 doi: 10.3791/3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt B, Wellershaus K, Wallraff A, Seifert G, Degen J, Euwens C, Fuss B, Bussow H, Schilling K, Steinhauser C, Willecke K. Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J Neurosci. 2003;23:4549–59. doi: 10.1523/JNEUROSCI.23-11-04549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Tokumoto Y, Miyake J, Nagamune T. Immunopanning selection of A2B5-positive cells increased the differentiation efficiency of induced pluripotent stem cells into oligodendrocytes. Neurosci Lett. 2011a;489:79–83. doi: 10.1016/j.neulet.2010.11.070. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Tokumoto Y, Miyake J, Nagamune T. Induction of oligodendrocyte differentiation from adult human fibroblast-derived induced pluripotent stem cells. In Vitro Cell Dev Biol Anim. 2011b;47:464–9. doi: 10.1007/s11626-011-9435-2. [DOI] [PubMed] [Google Scholar]
- Okita K, OIchisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Onorati M, Camnasio S, Binetti M, Jung CB, Moretti A, Cattaneo E. Neuropotent self-renewing neural stem (NS) cells derived from mouse induced pluripotent stem (iPS) cells. Mol Cell Neurosci. 2010;43:287–95. doi: 10.1016/j.mcn.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Ortega F, Gascon S, Masserdotti G, Deshpande A, Simon C, Fischer J, Dimou L, Chichung Lie D, Schroeder T, Berninger B. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nat Cell Biol. 2013;15:602–13. doi: 10.1038/ncb2736. [DOI] [PubMed] [Google Scholar]
- Pan YW, Zou J, Wang W, Sakagami H, Garelick MG, Abel G, Kuo CT, Storm DR, Xia Z. Inducible and conditional deletion of extracellular signal-regulated kinase 5 disrupts adult hippocampal neurogenesis. J Biol Chem. 2012;287:23306–17. doi: 10.1074/jbc.M112.344762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Zheng B, Kimberly SL, Cai Z, Rhodes PG, Lin RCS. Neuron-oligodendrocyte myelination co-culture derived from embryonic spinal cord and cerebral cortex. Brain and Behavior. 2012;2:53–67. doi: 10.1002/brb3.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–6. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–6. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Peng YJ, He WQ, Tang J, Tao T, Chen C, Gao YQ, Zhang WC, He XY, Dai YY, Zhu NC, Lv N, Zhang CH, Qiao YN, Zhao LP, Gao X, Zhu MS. Trio is a key guanine nucleotide exchange factor coordinating regulation of the migration and morphogenesis of granule cells in the developing cerebellum. J Biol Chem. 2010;285:24834–44. doi: 10.1074/jbc.M109.096537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertusa M, Garcia-Matas S, Rodriguez-Farre E, Sanfeliu C, Cristofol R. Astrocytes aged in vitro show a decreased neuroprotective capacity. J Neurochem. 2007;101:794–805. doi: 10.1111/j.1471-4159.2006.04369.x. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW, Slezak M. Genetic approaches to study glial cells in the rodent brain. Glia. 2012;60:681–701. doi: 10.1002/glia.22283. [DOI] [PubMed] [Google Scholar]
- Platel JC, Gordon V, Heintz T, Bordey A. GFAP-GFP neural progenitors are antigenically homogeneous and anchored in their enclosed mosaic niche. Glia. 2009;57:66–78. doi: 10.1002/glia.20735. [DOI] [PubMed] [Google Scholar]
- Pollard SM, Conti L, Sun Y, Goffredo D, Smith A. Adherent neural stem (NS) cells from fetal and adult forebrain. Cereb Cortex. 2006;16:i112–i20. doi: 10.1093/cercor/bhj167. [DOI] [PubMed] [Google Scholar]
- Potzner MR, Griffel C, Lutjen-Drecoll E, Bosl MR, Wegner M, Sock E. Prolonged Sox4 expression in oligodendrocytes interferes with normal myelination in the central nervous system. Mol Cell Biol. 2007;27:5316–26. doi: 10.1128/MCB.00339-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouya A, Satarian L, Kiani S, Javan M, Baharvand H. Human induced pluripotent stem cells differentiation into oligodendrocyte progenitors and transplantation in a rat model of optic chiasm demyelination. PloS One. 2011;6:e27925. doi: 10.1371/journal.pone.0027925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raponi E, Agenes F, Delphin C, Assard N, Baudier J, Legraverend C, Deloulme JC. S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage. Glia. 2007;55:165–77. doi: 10.1002/glia.20445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan MR, Huang YH, Kim YS, Dykes-Hoberg MI, Jin L, Watkins AM, Bergles DE, Rothstein JD. Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J Neurosci. 2007;27:6607–19. doi: 10.1523/JNEUROSCI.0790-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Landsberg C, Gorath M. Developmental regulation of alternatively spliced isoforms of mRNA encoding MAP2 and tau in rat brain oligodendrocytes during culture maturation. Journal of neuroscience research. 1999;56:259–70. doi: 10.1002/(SICI)1097-4547(19990501)56:3<259::AID-JNR5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Rio C, Dikkes P, Liberman MC, Corfas G. Glial fibrillary acidic protein expression and promoter activity in the inner ear of developing and adult mice. J Comp Neurol. 2002;442:156–62. doi: 10.1002/cne.10085. [DOI] [PubMed] [Google Scholar]
- Rivera-Zengotita M, Yachnis AT. Gliosis versus glioma?: don’t grade until you know. Advances in anatomic pathology. 2012;19:239–49. doi: 10.1097/PAP.0b013e31825c6a04. [DOI] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins SC, Stewart I, McNay DE, Taylor V, Giachino C, Goetz M, Ninkovic J, Briancon N, Maratos-Flier E, Flier JS, Kokoeva MV, Placzek M. alpha-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat Commun. 2013;4:2049. doi: 10.1038/ncomms3049. [DOI] [PubMed] [Google Scholar]
- Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, Robson P. Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem. 2005;280:24731–7. doi: 10.1074/jbc.M502573200. [DOI] [PubMed] [Google Scholar]
- Roy NS, Wang S, Jiang L, Kang J, Benraiss A, Harrison-Restelli C, Fraser RA, Couldwell WT, Kawaguchi A, Okano H, Nedergaard M, Goldman SA. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat Med. 2000;6:271–7. doi: 10.1038/73119. [DOI] [PubMed] [Google Scholar]
- Sabelstrom H, Stenudd M, Frisen J. Neural stem cells in the adult spinal cord. Experimental neurology. 2013 doi: 10.1016/j.expneurol.2013.01.026. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–70. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneto RP, de Vellis J. Characterization of cultured rat oligodendrocytes proliferating in a serum-free, chemically defined medium. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:3509–13. doi: 10.1073/pnas.82.10.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13939–44. doi: 10.1073/pnas.0907143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff R, Rosenbluth J, Dou WK, Liang WL, Moon D. Distribution and morphology of transgenic mouse oligodendroglial-lineage cells following transplantation into normal and myelin-deficient rat CNS. J Comp Neurol. 2002;446:46–57. doi: 10.1002/cne.10192. [DOI] [PubMed] [Google Scholar]
- Schmid RS, Yokota Y, Anton ES. Generation and characterization of brain lipid-binding protein promoter-based transgenic mouse models for the study of radial glia. Glia. 2006;53:345–51. doi: 10.1002/glia.20274. [DOI] [PubMed] [Google Scholar]
- Schmitz T, Ritter J, Mueller S, Felderhoff-Mueser U, Chew LJ, Gallo V. Cellular changes underlying hyperoxia-induced delay of white matter development. Journal of Neuroscience. 2011;31:4327–44. doi: 10.1523/JNEUROSCI.3942-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller U, Heine VM, Mao J, Kho AT, Dillon AK, Han YG, Huillard E, Sun T, Ligon AH, Qian Y, Ma Q, Alvarez-Buylla A, McMahon AP, Rowitch DH, Ligon KL. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell. 2008;14:123–34. doi: 10.1016/j.ccr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan X, Chi L, Bishop M, Luo C, Lien L, Zhang Z, Liu R. Enhanced de novo neurogenesis and dopaminergic neurogenesis in the substantia nigra of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease-like mice. Stem Cells. 2006;24:1280–7. doi: 10.1634/stemcells.2005-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Marinovich A, Barres BA. Purification and characterization of adult oligodendrocyte precursor cells from the rat optic nerve. J Neurosci. 1998;18:4627–36. doi: 10.1523/JNEUROSCI.18-12-04627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Yasuda A, Renault-Mihara F, Suyama S, Katoh H, Inoue T, Inoue Y, Nagoshi N, Sato M, Nakamura M, Akazawa C, Okano H. Sox10-Venus mice: a new tool for real-time labeling of neural crest lineage cells and oligodendrocytes. Mol Brain. 2010;3:31. doi: 10.1186/1756-6606-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbereis J, Heintz T, Taylor MM, Ganat Y, Ment LR, Bordey A, Vaccarino F. Astroglial cells in the external granular layer are precursors of cerebellar granule neurons in neonates. Mol Cell Neurosci. 2010;44:362–73. doi: 10.1016/j.mcn.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Lickert H, Gotz M, Dimou L. Sox10-iCreERT2: a mouse line to inducibly trace the neural crest and oligodendrocyte lineage. Genesis. 2012;50:506–15. doi: 10.1002/dvg.22003. [DOI] [PubMed] [Google Scholar]
- Slezak M, Goritz C, Niemiec A, Frisen J, Chambon P, Metzger D, Pfrieger FW. Transgenic mice for conditional gene manipulation in astroglial cells. Glia. 2007;55:1565–76. doi: 10.1002/glia.20570. [DOI] [PubMed] [Google Scholar]
- Snapyan M, Lemasson M, Brill MS, Blais M, Massouh M, Ninkovic J, Gravel C, Berthod F, Gotz M, Barker PA, Parent A, Saghatelyan A. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29:4172–88. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobottka B, Ziegler U, Kaech A, Becher B, Goebels N. CNS live imaging reveals a new mechanism of myelination: The liquid croissant model. GLIA. 2011;59:1841–9. doi: 10.1002/glia.21228. [DOI] [PubMed] [Google Scholar]
- Sohn J, Natale J, Chew LJ, Belachew S, Cheng Y, Aguirre A, Lytle J, Nait-Oumesmar B, Kerninon C, Kanai-Azuma M, Kanai Y, Gallo V. Identification of Sox17 as a transcription factor that regulates oligodendrocyte development. J Neurosci. 2006;26:9722–35. doi: 10.1523/JNEUROSCI.1716-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Sun G, Herszfeld D, Sylvain A, Campanale NV, Hirst CE, Caine S, Parkington HC, Tonta MA, Coleman HA, Short M, Ricardo SD, Reubinoff B, Bernard CC. Neural differentiation of patient specific iPS cells as a novel approach to study the pathophysiology of multiple sclerosis. Stem Cell Res. 2012;8:259–73. doi: 10.1016/j.scr.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Song Y, Zhang Q, Kutlu B, Difilippantonio S, Bash R, Gilbert D, Yin C, O’Sullivan TN, Yang C, Kozlov S, Bullitt E, McCarthy KD, Kafri T, Louis DN, Miller CR, Hood L, Van Dyke T. Evolutionary etiology of high-grade astrocytomas. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17933–8. doi: 10.1073/pnas.1317026110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza DG, Bellaver B, Souza DO, Quincozes-Santos A. Characterization of Adult rat astrocyte cultures. Plos One. 2013 doi: 10.1371/journal.pone.0060282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N, Goujet-Zalc C, Parmantier E, Olivier C, Martinez S, Ivanova A, Ikenaka K, Macklin W, Cerruti I, Zalc B, Thomas JL. Multiple restricted origin of oligodendrocytes. J Neurosci. 1998;18:8331–43. doi: 10.1523/JNEUROSCI.18-20-08331.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliotopoulos D, Goffredo D, Conti L, Di Febo F, Biella G, Toselli M, Cattaneo E. An optimized experimental strategy for efficient conversion of embryonic stem (ES)-derived mouse neural stem (NS) cells into a nearly homogeneous mature neuronal population. Neurobiol Dis. 2009;34:320–31. doi: 10.1016/j.nbd.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Stallcup WB, Beasley L. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci. 1987;7:2737–44. doi: 10.1523/JNEUROSCI.07-09-02737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine ZE, Huynh JL, Softus SK, Gorkin DU, Salmasi AH, Novak T, Purves T, Miller RA, Antonellis A, Gearhart JP, Pavan WJ, McCallion AS. Oligodendroglial and pan-neural crest expression of Cre recombinase directed by Sox10 enhancer. Genesis. 2009;47:765–70. doi: 10.1002/dvg.20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt CC, Schlierf A, Lommes P, Hillgartner S, Werner T, Kosian T, Sock E, Kessaris N, Richardson WD, Lefebvre V, Wegner M. SoxD proteins influence multiple stages of oligodendrocyte development and modulate SoxE protein function. Developmental cell. 2006;11:697–709. doi: 10.1016/j.devcel.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Sun MY, Yetman MJ, Lee TC, Chen Y, Jankowsky JL. Specificity and efficiency of reporter expression in adult neural progenitors vary substantially among nestin-CreER(T2) lines. J Comp Neurol. 2014;522:1191–208. doi: 10.1002/cne.23497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Dietrich D. Synaptic integration by NG2 cells. Front Cell Neurosci. 2013;7:255. doi: 10.3389/fncel.2013.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, Lovatt D, Han X, Smith Y, Nedergaard M. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science. 2013;339:197–200. doi: 10.1126/science.1226740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Pollard SM, Conti L, Toselli M, Biella G, Parkin G, Willatt L, Falk A, Cattaneo E, Smith A. Long-term tripotent differentiation capacity of human neural stem (NS) cells in adherent culture. Mol Cell Neurosci. 2008;38:245–58. doi: 10.1016/j.mcn.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Suter B, Nowakowski RS, Bhide PG, Caviness VS. Navigating neocortical neurogenesis and neuronal specification: a positional information system encoded by neurogenetic gradients. J Neurosci. 2007;27:10777–84. doi: 10.1523/JNEUROSCI.3091-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Watanabe J, Arata S, Funahashi H, Kikuyama S, Shioda S. A transgenic mouse model for the detailed morphological study of astrocytes. Neurosci Res. 2003;47:451–4. doi: 10.1016/j.neures.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Szuchet S, Yim SH. Characterization of a subset of oligodendrocytes separated on the basis of selective adherence properties. Journal of neuroscience research. 1984;11:131–44. doi: 10.1002/jnr.490110203. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nature Protocols. 2007a;2:3081–9. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007b;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Nabeshima Y, Yoshida S, Chisaka O, Ikenaka K, Nabeshima Y. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol. 2002;12:1157–63. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Tamguney G, Francis KP, Giles K, Lemus A, DeArmond SJ, Prusiner SB. Measuring prions by bioluminescence imaging. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15002–6. doi: 10.1073/pnas.0907339106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Yamaguchi K, Tatsukawa T, Nishioka C, Nishiyama H, Theis M, Willecke K, Itohara S. Lack of Connexin43-mediated bergmann glial gap junctional coupling does not affect cerebellar long-term depression, motor coordination, or eyeblink conditioning. Front Behav Neurosci. 2008;2:1. doi: 10.3389/neuro.08.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Taniguchi K, Kofuji P. Heterogeneity of Kir4. 1 channel expression in glia revealed by mouse transgenesis. Glia. 2009;57:1706–15. doi: 10.1002/glia.20882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–9. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- Teubner B, Michel V, Pesch J, Lautermann J, Cohen-Salmon M, Sohl G, Jahnke K, Winterhager E, Herberhold C, Hardelin JP, Petit C, Willecke K. Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential. Hum Mol Genet. 2003;12:13–21. doi: 10.1093/hmg/ddg001. [DOI] [PubMed] [Google Scholar]
- Theis M, de Wit C, Schlaeger TM, Eckardt D, Kruger O, Doring B, Risau W, Deutsch U, Pohl U, Willecke K. Endothelium-specific replacement of the connexin43 coding region by a lacZ reporter gene. Genesis. 2001;29:1–13. doi: 10.1002/1526-968x(200101)29:1<1::aid-gene1000>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tien AC, Tsai HH, Molofsky AV, McMahon M, Foo LC, Kaul A, Dougherty JD, Heintz N, Gutmann DH, Barres BA, Rowitch DH. Regulated temporal-spatial astrocyte precursor cell proliferation involves BRAF signalling in mammalian spinal cord. Development. 2012;139:2477–87. doi: 10.1242/dev.077214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumoto Y, Ogawa S, Nagamune T, Miyake J. Comparison of efficiency of terminal differentiation of oligodendrocytes from induced pluripotent stem cells versus embryonic stem cells in vitro. J Biosci Bioeng. 2010;109:622–8. doi: 10.1016/j.jbiosc.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Totonchi M, Taei A, Seifinejad A, Tabebordbar M, Rassouli H, Farrokhi A, Gourabi H, Aghdami N, Hosseini-Salekdeh G, Baharvand H. Feeder- and serum-free establishment and expansion of human induced pluripotent stem cells. Int J Dev Biol. 2010;54:877–86. doi: 10.1387/ijdb.092903mt. [DOI] [PubMed] [Google Scholar]
- Tripathi RB, Rivers LE, Young KM, Jamen F, Richardson WD. NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease. J Neurosci. 2010;30:16383–90. doi: 10.1523/JNEUROSCI.3411-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT, Fancy SP, Merkle F, Kessaris N, Alvarez-Buylla A, Richardson WD, Rowitch DH. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337:358–62. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnley AM, Morahan G, Okano H, Bernard O, Mikoshiba K, Allison J, Bartlett PF, Miller JF. Dysmyelination in transgenic mice resulting from expression of class I histocompatibility molecules in oligodendrocytes. Nature. 1991;353:566–9. doi: 10.1038/353566a0. [DOI] [PubMed] [Google Scholar]
- Uzzaman M, Benveniste RJ, Keller G, Germano IM. Embryonic stem cell-derived astrocytes: a novel gene therapy vector for brain tumors. Neurosurg Focus. 2005;19:E6. doi: 10.3171/foc.2005.19.3.7. [DOI] [PubMed] [Google Scholar]
- van den Ameele J, Tiberi L, Vanderhaeghen P, Espuny-Camacho I. Thinking out of the dish: what to learn about cortical development using pluripotent stem cells. Trends Neurosci. 2014 doi: 10.1016/j.tins.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Verderber L, Johnson W, Mucke L, Sarthy V. Differential regulation of a glial fibrillary acidic protein-LacZ transgene in retinal astrocytes and Muller cells. Invest Ophthalmol Vis Sci. 1995;36:1137–43. [PubMed] [Google Scholar]
- Viader A, Wright-Jin EC, Vohra BPS, Heuckeroth RO, Milbrandt J. Differential regional and subtype-specific vulnerability of enteric neurons to mitochondrial dysfunction. Plos One. 2011 doi: 10.1371/journal.pone.0027727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SD, Dunn NR, Hayashi S, Norris DP, Robertson EJ. Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes and Development. 2003;17:1646–62. doi: 10.1101/gad.1100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SD, Robertson EJ. Highly efficient transgene-independent recombination directed by a maternally derived SOX2Cre transgene. Genesis. 2003;37:54–6. doi: 10.1002/gene.10226. [DOI] [PubMed] [Google Scholar]
- Vitry S, Avellana-Adalid V, Hardy R, Lachapelle F, Baron-Van Evercooren A. Mouse oligospheres: from pre-progenitors to functional oligodendrocytes. Journal of neuroscience research. 1999;1999:735–51. [PubMed] [Google Scholar]
- Vitry S, Avellana-Adalid V, Lachapelle F, Baron-Van Evercooren A. Migration and multipotentiality of PSA-NCAM+ neural precursors transplanted in the developing brain. Mol Cell Neurosci. 2001;17:983–1000. doi: 10.1006/mcne.2001.0987. [DOI] [PubMed] [Google Scholar]
- Vives V, Alonso G, Solal AC, Joubert D, Legraverend C. Visualization of S100B-positive neurons and glia in the central nervous system of EGFP transgenic mice. J Comp Neurol. 2003;457:404–19. doi: 10.1002/cne.10552. [DOI] [PubMed] [Google Scholar]
- Wahlbuhl M, Reiprich S, Vogl MR, Bosl MR, Wegner M. Transcription factor Sox10 orchestrates activity of a neural crest-specific enhancer in the vicinity of its gene. Nucleic acids research. 2012;40:88–101. doi: 10.1093/nar/gkr734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lin W, Popko B, Campbell IL. Inducible production of interferon-gamma in the developing brain causes cerebellar dysplasia with activation of the Sonic hedgehog pathway. Mol Cell Neurosci. 2004;27:489–96. doi: 10.1016/j.mcn.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, Maherali N, Studer L, Hochedlinger K, Windrem M, Goldman SA. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12:252–64. doi: 10.1016/j.stem.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JC, Giles K, Grillo SK, Lemus A, DeArmond SJ, Prusiner SB. Bioluminescence imaging of Abeta deposition in bigenic mouse models of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2528–33. doi: 10.1073/pnas.1019034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner T, Bontert M, Eyupoglu I, Prass K, Prinz M, Klett FF, Heinze M, Bechmann I, Nitsch R, Kirchhoff F, Kettenmann H, Dirnagl U, Priller J. Bone marrow-derived cells expressing green fluorescent protein under the control of the glial fibrillary acidic protein promoter do not differentiate into astrocytes in vitro and in vivo. J Neurosci. 2003;23:5004–11. doi: 10.1523/JNEUROSCI.23-12-05004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight PA, Duchala CS, Readhead C, Macklin WB. A myelin proteolipid protein-LacZ fusion protein is developmentally regulated and targeted to the myelin membrane in transgenic mice. The Journal of cell biology. 1993;123:443–54. doi: 10.1083/jcb.123.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windus LC, Claxton C, Allen CL, Key B, St John JA. Motile membrane protrusions regulate cell-cell adhesion and migration of olfactory ensheathing glia. Glia. 2007;55:1708–19. doi: 10.1002/glia.20586. [DOI] [PubMed] [Google Scholar]
- Windus LC, Lineburg KE, Scott SE, Claxton C, Mackay-Sim A, Key B, St John JA. Lamellipodia mediate the heterogeneity of central olfactory ensheathing cell interactions. Cellular and molecular life sciences: CMLS. 2010;67:1735–50. doi: 10.1007/s00018-010-0280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci. 2006;26:4985–94. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147:1146–58. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- Yang P, Hernandez MR. Purification of astrocytes from adult human optic nerve heads by immunopanning. Brain research Brain research protocols. 2003;12:67–76. doi: 10.1016/s1385-299x(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gozen O, Watkins A, Lorenzini I, Lepore A, Gao Y, Vidensky S, Brennan J, Poulsen D, Won Park J, Li Jeon N, Robinson MB, Rothstein JD. Presynaptic regulation of astroglial excitatory neurotransmitter transporter GLT1. Neuron. 2009;61:880–94. doi: 10.1016/j.neuron.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Vidensky S, Jin L, Jie C, Lorenzini I, Frankl M, Rothstein JD. Molecular comparison of GLT1+ and ALDH1L1+ astrocytes in vivo in astroglial reporter mice. Glia. 2011;59:200–7. doi: 10.1002/glia.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Watanabe M, Nishiyama A. Optimization of oligodendrocyte progenitor cell culture method for enhanced survival. J Neurosci Methods. 2005;149:50–6. doi: 10.1016/j.jneumeth.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Young KM, Mitsumori T, Pringle N, Grist M, Kessaris N, Richardson WD. An Fgfr3-iCreER(T2) transgenic mouse line for studies of neural stem cells and astrocytes. Glia. 2010;58:943–53. doi: 10.1002/glia.20976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, Tohyama K, Richardson WD. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77:873–85. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Chittajallu R, Belachew S, Anderson S, McBain CJ, Gallo V. Expression of the green fluorescent protein in the oligodendrocyte lineage: a transgenic mouse for developmental and physiological studies. Journal of neuroscience research. 2002;70:529–45. doi: 10.1002/jnr.10368. [DOI] [PubMed] [Google Scholar]
- Zappone MV, Galli R, Catena R, Meani N, De Biasi S, Mattei E, Tiveron C, Vescovi AL, Lovell-Badge R, Ottolenghi S, Nicolis SK. Sox2 regulatory sequences direct expression of a (beta)-geo transgene to telencephalic neural stem cells and precursors of the mouse embryo, revealing regionalization of gene expression in CNS stem cells. Development. 2000;127:2367–82. doi: 10.1242/dev.127.11.2367. [DOI] [PubMed] [Google Scholar]
- Zawadzka M, Rivers LE, Fancy SP, Zhao C, Tripathi R, Jamen F, Young K, Goncharevich A, Pohl H, Rizzi M, Rowitch DH, Kessaris N, Suter U, Richardson WD, Franklin RJ. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6:578–90. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Vutskits L, Calaora V, Durbec P, Kiss JZ. A role for the polysialic acid-neural cell adhesion molecule in PDGF-induced chemotaxis of oligodendrocyte precursor cells. Journal of cell science. 2004;117:93–103. doi: 10.1242/jcs.00827. [DOI] [PubMed] [Google Scholar]
- Zhou P, Zhang Y, Ma Q, Gu F, Day DS, He A, Zhou B, Li J, Stevens SM, Romo D, Pu WT. Interrogating translational efficiency and lineage-specific transcriptomes using ribosome affinity purificaton. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15395–400. doi: 10.1073/pnas.1304124110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Zhu L, Ramboz S, Hewitt D, Boring L, Grass DS, Purchio AF. Non-invasive imaging of GFAP expression after neuronal damage in mice. Neurosci Lett. 2004;367:210–2. doi: 10.1016/j.neulet.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Zhu L, Xiang P, Guo K, Wang A, Lu J, Tay SS, Jiang H, He BP. Microglia/monocytes with NG2 expression have no phagocytic function in the cortex after LPS focal injection into the rat brain. Glia. 2012;60:1417–26. doi: 10.1002/glia.22362. [DOI] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–57. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- Zhu X, Hill RA, Dietrich D, Komitova M, Suzuki R, Nishiyama A. Age-dependent fate and lineage restriction of single NG2 cells. Development. 2011;138:745–53. doi: 10.1242/dev.047951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]
- Zimmerman L, Parr B, Lendahl U, Cunningham M, McKay R, Gavin B, Mann J, Vassileva G, McMahon A. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12:11–24. doi: 10.1016/0896-6273(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–30. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Lubischer JL, Kang H, Tian L, Mikesh M, Marks A, Scofield VL, Maika S, Newman C, Krieg P, Thompson WJ. Fluorescent proteins expressed in mouse transgenic lines mark subsets of glia, neurons, macrophages, and dendritic cells for vital examination. J Neurosci. 2004;24:10999–1009. doi: 10.1523/JNEUROSCI.3934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]