Abstract
Despite 50 years of pharmacological and psychosocial interventions, schizophrenia remains one of the leading causes of disability. Schizophrenia is also a life-shortening illness, caused mainly by poor physical health and its complications. The end result is a considerably reduced lifespan that is marred by reduced levels of independence, with few novel treatment options available.
Disability is a multidimensional construct that results from different, and often interacting, factors associated with specific types and levels of impairment. In schizophrenia, the most poignant and well characterized determinants of disability are symptoms, cognitive and related skills deficits, but there is limited understanding of other relevant factors that contribute to disability. Here we conceptualize how reduced physical performance interacts with aging, neurobiological, treatment-emergent, and cognitive and skills deficits to exacerbate ADL disability and worsen physical health. We argue that clearly defined physical performance components represent underappreciated variables that, as in mentally healthy people, offer accessible targets for exercise interventions to improve ADLs in schizophrenia, alone or in combination with improvements in cognition and health. And, finally, due to the accelerated aging pattern inherent in this disease – lifespans are reduced by 25 years on average – we present a training model based on proven training interventions successfully used in older persons. This model is designed to target the physical and psychological declines associated with decreased independence, coupled with the cardiovascular risk factors and components of the metabolic syndrome seen in schizophrenia due to their excess prevalence of obesity and low fitness levels.
Keywords: Obesity, Disability, Cognition, Functional skills, Schizophrenia, Exercise
1. Background
Schizophrenia-spectrum disorders are among the world’s most disabling illnesses Murray and Lopez, 1997. Despite the striking nature of psychotic symptoms in schizophrenia, and partially related to cognitive deficits, the most debilitating problem in these conditions is impairments in everyday functioning, which spans major functional domains of independence in residence, productive activities, and social interactions Harvey, 2010. An increasingly complex society has increased the level of everyday functional demands, including life and health maintenance, perception–cognition, physical self-maintenance, purposeful activity resulting from the motivation to explore, and social behaviors, requiring evolving skill sets and effective deployment thereof. In schizophrenia, the breadth of resultant impairments across activities of daily living (ADLs) from the most basic (i.e., bathing, brushing teeth) to the more involved (instrumental ADLs; for example, financial and medication management, community mobility, shopping, housework; job skills) requires targeted assessments that can quantify the level of dysfunction and reveal, when relevant, the physical parameters associated with specific physical and psychological skills, and how they interact with each other to produce disability. Our conceptual framework defines a successful endpoint in the treatment of schizophrenia as adequate functioning in the community, including residential, vocational and social settings. Effective participation in each of these settings requires the ability to function successfully in the physical environment, which includes physical capacity and motor abilities, as well as translation of those skills into goal-directed physical activity behavior in each of these settings. This has been a highly neglected topic in the past.
Embedded in the discussion of physical performance as it relates to independence is a larger ecological factor, the unprecedented obesity epidemic throughout developed nations, with the US showing the highest prevalence. Poor physical health, associated with excess body fat and related cardiometabolic derangements, is pervasive in schizophrenia, even more so than in the society at large, and is underpinned by the patients’ excessively low levels of physical fitness. A vicious cycle of physical impairment and low activity levels, exacerbated by symptomatic and cognitive deficits, may represent a major impediment to effective everyday functioning in schizophrenia. We argue that improving physical performance in schizophrenia is a neglected topic that, if effectively addressed, can lead to meaningful reductions in disability, including reduced symptoms and improved cognition, beyond those which can be achieved using current approaches.
This is because everyday activities require complex environment-bound interactions among health-related, cognitive- and physical performance variables. Without adequate physical performance levels, everyday functioning suffers on every level, as do cognition and skills deployment. The aggregate of the components of physical performance, including endurance, strength, power, flexibility, balance, mobility, flexibility, motor coordination, muscle mechanics, and gait efficiency, strongly correlates with everyday outcome in the Western population as a whole. Underlying neurobiological and treatment-emergent motor deficits of schizophrenia aside, physical performance components represent variables that, just as in mentally healthy people, offer accessible targets for interventions to improve ADLs, while also improving cognition and health. We believe that improving functioning of people with schizophrenia is possible using training programs which specifically target the patterns, skills and levels of physical decline inherent to this disease. We present in this paper, training options which provide the greatest potential for enhancing independence and improving metabolic health in these patients, and the rationales supporting their use.
2. Physical performance factors in mentally healthy people
Physical performance, defined as the ability to perform muscular work satisfactorily Brouchard et al., 1990, is imperative for all activities of daily living (ADLs) including basic and instrumental ADLs, and mobility tasks important for independent living Guralnik and Simonsick, 1993; Lawton, 1988. Understanding the differential contribution of various physical performance factors facilitates the development effective interventions aimed at preserving and improving independence.
In the general population, psychiatric symptoms, cognitive performance, number of physical health limitations and age all explain variance in disability. These factors, however, do not confound the robust relationship between physical performance and disability, making physical performance the strongest predictor of ADL limitations Van Heuvelen et al., 2000. These limitations reduce independence and the quality of life on an individual, and subsequently increase disability levels and commensurate healthcare costs to society.
2.1. Sarcopenic obesity
The US is facing an obesity epidemic of unprecedented magnitude. Two thirds of the population (68.8%) is overweight Flegal et al., 2010. Obesity rates (BMI > 30) exceed one-third in most sex–age groups Flegal et al., 2012. Recent projections by the CDC estimate an additional 33% increase in obesity and a 130% increase in severe obesity (BMI > 40) over the next 2 decades Finkelstein et al., 2012. Excess body weight and attendant poor metabolic health limit everyday functioning Kyrou et al., 2011; Houston et al., 2007, especially when combined with other behaviors such as smoking or a sedentary lifestyle Seidell, 1995. A shift in body composition towards increased adiposity and reduced muscle mass due to inactivity reduces all factors associated with physical performance. Termed sarcopenic obesity (literally ‘fat with little flesh’), increased adiposity and reduced muscle mass when combined with gradual age-related losses in muscle quality, mass, and strength, often reduce ADL performance. Sarcopenic obesity is associated with a three- to fourfold increase in the likelihood of disability, moderated by lifestyle factors, health behaviors, and socioeconomic status Baumgartner et al., 1998, 2004; Castaneda and Janssen, 2005. Sarcopenia increases mobility limitations Dufour et al., 2013; Morley et al., 2011; Waters et al., 2010 and worsens CVD (cardiovascular disease) risk Chung et al., 2013; Clark and Manini, 2012; Cruz-Jentoft et al., 2010; Dominguez and Barbagallo, 2007; Kaess et al., 2010; Mitchell et al., 2012; Prado et al., 2012; Senechal et al., 2012.
2.2. Obesity-related health status and comorbidities
Adverse cardiometabolic changes, such as elevated fasting blood triglycerides, low levels of high-density lipoprotein, high fasting blood glucose, and elevated blood pressure, are all markedly accentuated by weight gain. The resultant micro- and macrovascular end-organ damage worsens existing patterns of decline in musculoskeletal, respiratory and cardiovascular system functions. Once manifest, these comorbidities and their sequelae, (i.e., coronary artery disease, angina pectoris, diabetes mellitus and peripheral neuropathy, hypertension, stroke) carry overt functional implications by themselves Peeters et al., 2004, which are amplified by dynapenia Chung et al., 2013; Dominguez and Barbagallo, 2007; Kaess et al., 2010; Prado et al., 2012; Stephen and Janssen, 2009.
2.3. Muscle strength and power
In healthy individuals, bone and muscle grow in harmony with changes in weight. This adaptive physiological mechanism is impaired in obesity. A progressive mismatch between body mass and strength occurs because of a progressive decline in muscle quality including: decreased fiber size and number, reduced intrinsic contractility; fat infiltration; motor unit restructuring, and impaired neurological modulation of contraction Stenholm et al., 2008; Visser et al., 2005. Obese persons with their higher body mass have reduced relative strength levels creating limitations in body-weight dependent daily activities such as walking, climbing stairs, rising from a chair or transferring from a lying to a standing position.
Muscle power (or work rate) is the product of the force produced by a muscle and its movement velocity Newton and Kraemer, 1994. Reduced muscular power and movement velocity are associated with a reduction in the ability to perform activities of daily living (ADLs), including stair climbing, rising from a chair and walking without assistance Bassey et al., 1992; Foldvari et al., 2000; Grabiner et al., 1993; Whipple et al., 1987. The relationship between gait speed and independence is well established Friedman et al., 1988; Gibbs et al., 1996; Guralnik et al., 1994; Judge et al., 1996; and many ADLs, such as stopping abruptly to avoid a car at a crosswalk, recovering from a stumble, or grabbing a handrail and entering a bus depend on speed Cummings and Nevitt, 1989; Dutta et al., 1997; Smeesters et al., 2001.
2.4. Flexibility
Flexibility, defined as “an intrinsic property of the body tissues that determines the range of motion achievable without injury at a joint or group of joints”, improves the compliancy of connective tissues and muscle length (sarcomerogenesis), increasing stored elastic energy, antagonist compliance and the velocity at which peak power is produced, respectively Edgerton et al., 1986; Greg et al., 1994; Holt et al., 1996; Hunter and Marshall, 2002; Wilson et al., 1992; Worrell et al., 1994. These mechanical changes increase in importance as age Allander et al., 1974; Jette et al., 1990, inactivity Caspersen et al., 1985 and obesity Park et al., 2010 further interfere with flexibility.
2.5. Balance
Balance can be defined using a number of criteria Spirduso, 1995. The role of balance in performing ADLs is complex. Balance, if only mildly impaired, such as with normal aging, may be an independent predictor of vigorous ADLs only Van Heuvelen et al., 2000; whereas more pronounced balance impairments affect both basic and instrumental ADLs Ensrud et al., 1994; Laukkanen et al., 1994; Lord et al., 2001. With the exceptions of visual and vestibular problems, balance can be improved through exercise training.
2.6. Mobility
Functional mobility is the capacity to use movements such standing, bending, walking and climbing to move within an environment in order to perform ADLs Forhan and Gill, 2013. The most common risk factors for mobility impairment are older age, low physical activity, obesity, impairments in muscle power, strength and balance, and chronic diseases such as diabetes or arthritis Brown and Flood, 2013; Kidde et al., 2009. Dual task conditions (divided attention) also affect mobility Hausdorff et al., 2008; as do factors such as weak social networks and limited social activities Yeom et al., 2008.
2.7. Cardiovascular fitness
Maximal oxygen uptake (VO2max), or the maximal capacity of an individual to use oxygen; declines with age, further complicated by a sedentary lifestyle. VO2max declines between 5% and 15% per decade beginning at 25–30 years of age due to reduced maximal cardiac output and declining maximal arterio-venous oxygen difference (a-vO2diff) American College of Sports Medicine Current Comment. Limitations in physical capacity predict the severity of disability in mentally healthy subjects Anderson-Hanley et al., 2010 and compromise the ability to perform lifestyle-relevant physical activities that are necessary for social, community, and work-related functioning in diverse non-psychiatric Miller et al., 2000 and psychiatric patient samples Semkovska et al., 2004. Moreover, low cardiorespiratory fitness has been recognized as a prominent behavioral risk factor for cardiovascular disease (CVD) and morbidity, and an independent risk factor for all-cause mortality in adults Blair et al., 1989; Kampert et al., 1996. Higher cardiorespiratory fitness decreases overall mortality rates, and morbidity and mortality due to CVD in a dose–response fashion Centers for Disease Control and Prevention, 1996. These associations are quite robust and have been demonstrated to be largely independent from other major risk factors Blair et al., 1989, 1996; Elekund et al., 1988.
3. Physical performance factors in schizophrenia
The physical health status of patients with schizophrenia is extremely poor. They have higher rates of obesity and related metabolic comorbidities than the mentally healthy Hennekens et al., 2005; Homel et al., 2002; Jin et al., 2011; McEvoy et al., 2005, Dyslipidemia, insulin resistance and hyperglycemia are all more common in this population Newcomer, 2004, with similarly increased prevalence of CVD, type 2 diabetes, hypertension Casey et al., 2004, and metabolic syndrome Newcomer, 2007. Health status-related mortality shortens the lifespan of people with schizophrenia by 25 years on average Hennekens et al., 2005. Reasons for excess body weight and attendant medical comorbidities are manifold, extending beyond genetic vulnerability Newcomer and Hennekens, 2007. In the context of socioeconomic challenges, schizophrenia leads to particularly unhealthy lifestyles that include poor diets, little exercise, marked sedentary behavior and high rates of smoking, with commensurately low physical activity levels Brown et al., 1999; Strassnig et al., 2006. Antipsychotic medications add deleterious adipogenic and cardio-metabolic risks Newcomer, 2004; Wheeler et al., 2008. Cognitive, symptomatic and functional capacity limitations worsen lifestyle-relevant ADLs and IADLs (i.e., diet, exercise, shopping for food, medical care), creating a vicious health cycle, with health risk amplified by low rates of medical screening, monitoring, and intervention.
3.1. Neurobiological limitations to motor performance
The neurodevelopmental hypothesis of schizophrenia suggests an unequivocal neurodevelopmental basis Keshavan and Murray, 1997; Lotspeich and Ciaranello, 1995; Murray and Lewis, 1987; Waddington and Buckley, 1996; Weinberger, 1987. There is compelling literature illustrating premorbid abnormalities in patients who later go on to develop schizophrenia, including abnormalities in functional motor performance Foerster et al., 1991; Murray, 1994. The impact of these well-established motor abnormalities on ADLs and IADLs remains unexplored.
Abnormal involuntary movements such as spontaneous dyskinesias and Parkinsonian symptoms have been observed in neuroleptic-naive patients with schizophrenia Waddington and Youssef, 1986. Moreover, neurological ‘soft signs’ imply deficits in complex motor tasks (voluntary motor control and integrative motor function, reduced motor steadiness and excessive force) such as coordination, sensory integration and motor sequencing have been observed Schroder et al., 1992, and can impair motor performance. Some of those deficits are also found in first-degree relatives or offspring of patients Wolff and O'Driscoll, 1999. Catatonic Symptoms have received little attention over the past few decades, but have historically been considered core aspects of schizophrenia. Pure motor signs of catatonia, including posturing, mannerisms, immobility, rigor, stereotypies, catalepsy, grimacing and waxy flexibility, are seen less and less frequently in clinical practice, but if present even on a sub threshold level can have profound impact on ADLs. Volitional aspects, including negativism, refusal to eat, withdrawal, and ambitendency, are seen more frequently than pure motor signs and can unquestionably interfere with ADLs and IADLs.
3.2. Symptomatic limitations
Psychotic symptoms are important predictors of the long-term course of disability in schizophrenia Mason et al., 1995. Negative symptoms reduce the likelihood of patients engaging in goal-directed behavior, including physical activity. The negative symptoms that appear to have the greatest correlation with functional outcomes tend to be from the domain of motivational deficits, since they reduce the likelihood that motor skills will be effectively applied. Positive symptoms can have distracting effects, making it difficult for patients to focus on any given ADL. Symptoms, such as paranoia, decrease the likelihood for patients to participate mentally and physically in any particular fear-inducing situation or circumstance. Depressive disorders are common in schizophrenia, and often go undiagnosed Hasan et al., 2011. The contribution of depressive symptoms to disability and functional capacity in schizophrenia merits greater attention Abramowitz et al., 2014. Motor retardation factors, including psychomotor and motor slowing observed in 40%–60% of depressive episodes, are common features of depressive disorders Caligiuri and Ellwanger, 2000 and reduce motor performance. Characteristic eye movements of patients with psychomotor retardation are fixed gaze and poor maintenance of eye contact, which reduce interpersonal functioning. Gross psychomotor slowing, including diminished movements of the hands, legs, torso and head and poor posture, carry overt functional implications Buyukdura et al., 2011.
3.3. Cognitive limitations
Cognitive deficits interfere with motor-driven ADLs in a cascade-like manner. Lifestyle-relevant IADLs (i.e., diet, exercise, medical care) worsen with cognitive impairment, as do motor skills, reducing physical performance. In turn, the physical capacity to carry out these ADLs suffers. Finally, limited capacity reduces goal-directed physical activity. For example, observable slowing and reduction of various motor processes such as gait, fine motor movements, speech, facial expression, motor activity and prosody, are common in schizophrenia. Psychomotor slowing, resulting in part from reduced processing speed and interacting with other motor deficits, can exacerbate ADL disability caused by other factors (i.e., by obesity, symptoms). On a neurobiological level, lack of anticipatory reward expectation reduces the desire to plan healthy and pleasurable activities, at the cost of immediate gratification. Healthy behaviors are never learned because the benefits are intangible. While these relationships are inherent to schizophrenia, treatment options currently available fail to address these deficits and may, in fact, worsen them.
Cognitive deficits can not only worsen physical health and increase risk factors, such as obesity or metabolic syndrome, they can also worsen functional skills, further reducing ADLs. In mentally healthy individuals, elevated BMI is associated with reduced cognitive performance Boeka and Lokken, 2008 changes in brain structure and function Willeumier et al., 2011 and reduced decision making capacity Brogan et al., 2011. Sequelae of chronic hyperlipidemia and of manifest hypertension and diabetes, likely worsen and cause deficits across a variety of cognitive domains Beeri et al., 2009; Panza et al., 2010 including attention, processing speed, memory and executive functioning McCrimmon et al., 2012; Novak and Hajjar, 2010. Obesity reduces regional cerebral blood flow, specifically to the prefrontal cortex, negatively impacting behaviors associated with that area thereby reducing executive functioning Pannacciulli et al., 2006; Walther et al., 2010; Willeumier et al., 2011. Reduced frontal lobe baseline metabolism correlates with body mass index (BMI), and negatively correlates with cognitive performance Mozley et al., 2001; Volkow et al., 2008. The extent of cognitive dysfunction seen in obese adults is highly variable Kanoski, 2012, perhaps caused by interactions or additive effects with related metabolic comorbidities. Despite the substantial quantity of developing literature on obesity and metabolic complications, and the equally large volume of literature on cognitive impairments in schizophrenia, there has been little research on the direct contributions of obesity and metabolic complications to cognitive deficits in these patients. Preliminary evidence suggests that obesity and hypertension worsen cognitive performance not only in the mentally healthy, but also in patients with schizophrenia Friedman et al., 2010 and bipolar disorder Yim et al., 2012. In the pertinent studies, it was determined that select metabolic parameters exerted an adverse impact on several domains of cognitive impairment in people with schizophrenia. Further, individuals with BMI scores over 25 showed a trend toward declining cognitive performance. Diabetes has also been shown to worsen cognition in schizophrenia Dickinson et al., 2008.
3.4. Treatment-emergent motor limitations
Motor side effects of antipsychotic medication are well known. Patients taking clozapine, for example, show significantly lower accuracy (greater variability) of force control. The observed force control deficit may be the result of an increase in myoclonus and generally lower levels of overall motor activity Vrtunski et al., 1996. Sedative properties of various antipsychotics, sometimes mistaken for negative or cognitive symptoms, can impair a person’s ability to function normally in the long term Miller, 2004. Anticholinergic medications impair cognitive and psychomotor performance Nebes et al., 2007. Adaptive motor learning, that is, learning of new voluntary movement patterns, may also be impaired by antipsychotic and anticholinergic medication, interfering with dopaminergic activity and perhaps muscarinic cholinergic activity used to associate temporally related motor movements Kelley et al., 2003.
3.5. Aging and schizophrenia
Schizophrenia has been described as a syndrome of accelerated aging Tang et al., 2009. Physiological capacities, such as functional declines in work capacity, strength, endurance, muscle mass, flexibility, bone density, muscle/fat ratio, and cardiac output all decline with age, and these declines are exacerbated by a sedentary lifestyle Carter et al., 1993. It has been postulated that physiological changes in body structures and functions that are associated with normal aging occur approximately 25 years earlier in people with schizophrenia Kirkpatrick et al., 2008. Normal age-emergent factors that influence disability, such as impairments in one or multiple organs or physical domains that reach a certain level of severity, common on schizophrenia, may transition patients from non-disabled to disabled states much more quickly than empirically anticipated in the healthy population, and help explain the high rates of disability seen in this population. This faster decline requires much earlier intervention to address the resultant physical limitations and ADL disability.
4. Implications for schizophrenia
Despite the ubiquitously poor fitness levels of patients with schizophrenia and the recognized impact of fitness on health status and physical and cognitive functioning reported in non-psychiatric samples, little information is available concerning how different physical performance factors can impact health and daily activities in schizophrenia patients. Without this information targeted interventions to improve health and reduce disability are not possible Capodaglio et al., 2010; Fleg et al., 2000; Levinger et al., 2009.
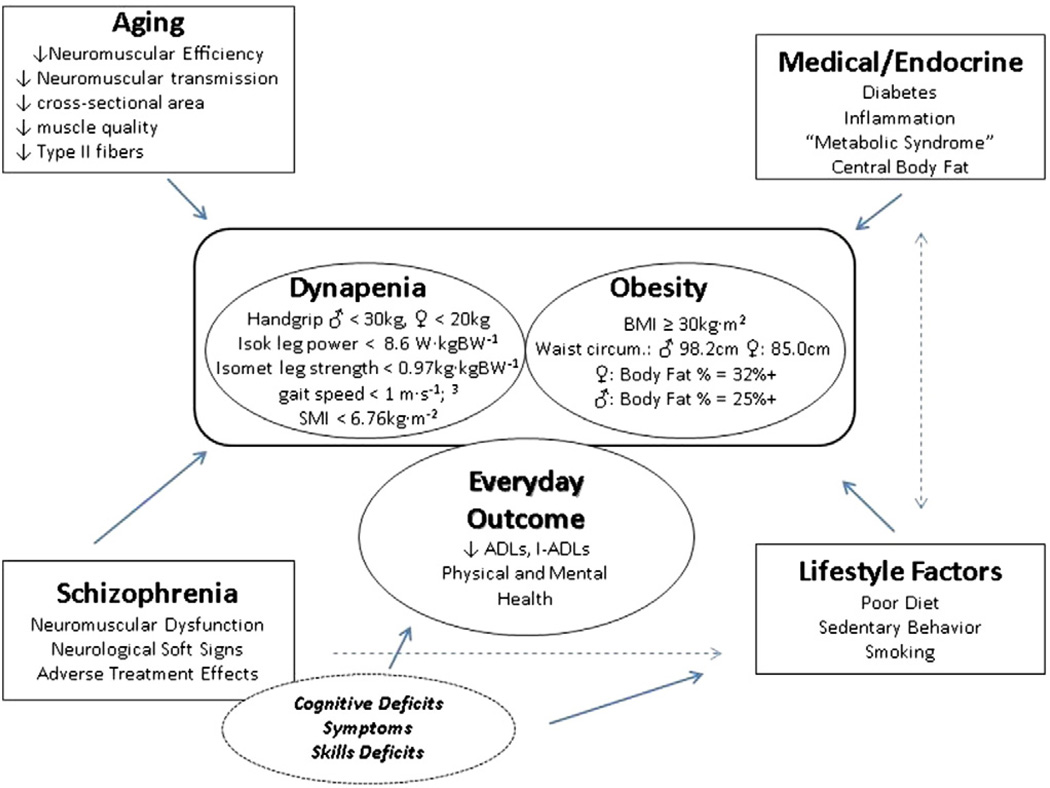
We have developed a comprehensive model (Fig. 1) to conceptualize the multimodal relationships between the known and novel factors presented above that may predict ADL disability in schizophrenia. We Strassnig et al., 2011a and others Vancampfort et al., 2011 have already identified severe limitations in physical capacity amongst patients with schizophrenia, and their potential interactions with obesity and cardio-metabolic risk. These physical capacity limitations interfere with routine activities of daily living, leaving patients in a state of self-perpetuating physical deconditioning Harvey and Strassnig, 2012, akin to deconditioning states observed in other chronic, medical illness. The clear link between physical capacity and outcome in schizophrenia has already spurred novel intervention approaches designed to improve endurance, one of the many fitness parameters mentioned above Fogarty et al., 2004. We have also determined that pronounced body composition shifts are present early in the course of schizophrenia Strassnig et al., 2011a, indicating emerging levels of sarcopenic obesity at an early age. These findings are supported by those of other authors who have reported excess body fat in relation to body mass Thiels, 2005. Moreover, we are beginning to understand the cognitive benefits of cardiovascular fitness training in healthy and schizophrenia populations. It is, in fact, becoming increasingly evident that physical capacity improvements have much broader functional benefits than simply improving fitness since they can induce neurobiological, functional and structural changes associated with cognition and psychiatric symptoms. The literature, for example relates reduced executive function to reduced capacity to perform ADL Johnson et al., 2007 and argues that exercise, especially exercise related to improving or learning motor tasks (a form of procedural learning), can improve executive function. Additionally, other extrinsic and intrinsic factors, such as reducing reliance on pharmacological interventions and decreasing levels of depression, can be addressed by the increases in self-efficacy and self-image associated with exercise training Behrman and Ebmeier, 2014, which we and others have shown in preliminary studies.
Fig. 1.
Determinants of disability in schizophrenia.
Measuring objectively other physical performance factors isolates the individual’s movement-related or motor performance-related capacities and has the potential to define clearly targets for selective interventions. Assessment can be made within the context of socio-cultural roles and environmental constraints Greene et al., 1993, and provides quantification of wellness variables (i.e., sarcopenic obesity, mobility) that are well known in epidemiology research, easily obtained, but not commonly assessed in diagnostic batteries evaluating schizophrenia Strassnig et al., 2012.
Altogether, physical performance factors, physical capacity and physical activity, comprehensively measured, add highly meaningful variability to ADL performance independent of health status or physical disability Strassnig et al., 2011b; Vancampfort et al., 2012 and potentially have an even greater adverse impact in schizophrenia than in the general population. Domains of physical performance, neuromuscular and psychomotor deficits are all eminently modifiable in the general population, and as we have shown, in schizophrenia Strassnig et al., 2012. We believe that an accurately defined ‘holistic approach’ to disability reduction in schizophrenia, accounting for these domains, offers tremendous opportunity to move the disability burden in a positive direction. The salutatory effect of physical exercise on cognitive improvements, consistently shown in mentally healthy participants across all age-groups, is one of many opportunities to start, and has already shown efficacy in other mental and neurodegenerative disorders as well Tetlie et al., 2008. Physical health benefits are a welcome addition to standard care that may well initiate a feedback cycle to improve ADLs, underlying neurobiological and treatment-emergent motor deficits of schizophrenia aside. If properly implemented, improving physical performance in schizophrenia may lead to meaningful reductions in disability, including changes in difference factors like symptoms and cognition, beyond what can be achieved with current approaches.
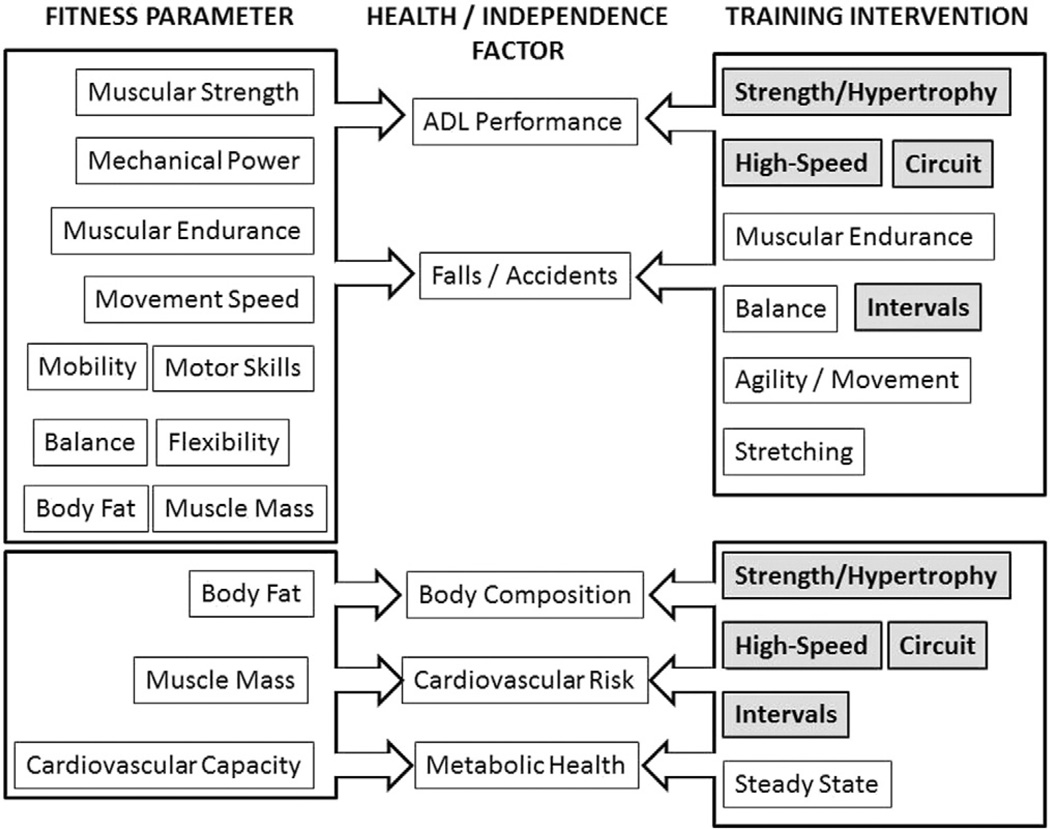
Given the accelerated patterns of aging and declines in physical capacity due to inactivity, as well as cognitive and skills decline, and social disengagement, the intervention model developed for aging provides a working template for schizophrenia. A flow chart, showing the interactions among fitness parameters, independence and health-related factors, and training interventions, is presented in Fig. 2. The unique model represents the novel approach we have taken to exercise intervention in these patients. Four training interventions, strength and hypertrophy-based resistance training, high-speed training, circuit training (with or without resistance) and interval training, all have the distinction of addressing both independence-oriented (ADL performance) and health-related (body composition, cardiovascular risk, and metabolic health) factors. The majority of the exercise interventions in schizophrenia have concentrated on standard steady-state aerobic exercises; often coupled with progressive resistance and body relaxation elements Cramer et al., 2013; Hjorth et al., 2014; Papanastasiou, 2012; Vancampfort et al., 2014.
Fig. 2.
Multimodal skills-based performance intervention.
When considering improvements in functional limitations and physical disability in individuals' strength and hypertrophy training programs, employing the typical progressive resistance training patterns has been shown to have a modest and selective impacts Latham et al., 2004; Liu and Latham, 2009. Additionally, the consensus is that the limitations associated with ADL performance are predominantly neuromuscular, and not cardiovascular in nature Pendergast et al., 1993, although over time declines in aerobic capacity may eventually contribute to losses of independence, increased incidence of disability and reduced quality of life Mazzeo and Tanaka, 2001. Studies comparing power and strength training have shown that power training has a greater capacity to improve function in older persons Katula et al., 2008; Miszko et al., 2003. Our laboratory and others have shown that power is best increased using moderate load, high-speed training Bean et al., 2004; Fielding et al., 2002; Mignardot et al., 2013; Mow et al., 2009; Signorile et al., 2002, 2005; Thomas et al., 2007.
Although the classic exercise intervention used to increase cardiovascular fitness, produce positive changes in body composition and address factors associated with metabolic syndrome is steady-state training using a target heart rate, other training methods, classically categorized as “anaerobic” training often prove as effective, if not more effective than this technique. Notably, in populations from athletes to heart failure patients, interval training has produced greater increases in VO2max using a lower training volume Daussin et al., 2007; Gibala and McGee, 2008; Helgerud et al., 2007; Wisløff et al., 2007.
Circuit resistance training constitutes a very special instance of interval training where intensity is increased by applying an external load using free weight or weight stack, pneumatic, hydraulic or elastic resistance. Circuit resistance training can improve cardiovascular condition through a combination of increased capillary and mitochondrial density, increased concentrations of oxidative enzymes and enhanced triglyceride use Romero-Arenas et al., 2013; Williams et al., 2007. Further indications of the effective cardiovascular overload offered by circuit resistance training are offered by the results of a number of studies that have shown significant increases in excess post-exercise oxygen consumption (EPOC) that rival, and in the early stages of recovery exceed, those produced during longer duration, steady state, aerobic training Boutcher, 2011; Braun et al., 2005; Da Silva et al., 2010; Haltom et al., 1999. For example, Trapp et al. (2008) found that high-speed, short duration intervals have a more dramatic effect on central obesity and fasting insulin levels than traditional steady state aerobic training. Other researchers have reported the benefits of interval training on body weight Drigny et al., 2013; Haram et al., 2009; Tjønna et al., 2008, BMI Drigny et al., 2013, waist circumference Drigny et al., 2013; Mora-Rodriguez et al., 2014, blood pressure Haram et al., 2009; Mora-Rodriguez et al., 2014; Tjønna et al., 2008, homeostatic model of insulin sensitivity (HOMA) Mora-Rodriguez et al., 2014; Tjønna et al., 2008, blood lipoproteins Drigny et al., 2013; Haram et al., 2009; Mora-Rodriguez et al., 2014, myocardial function Drigny et al., 2013, endothelial function Tjønna et al., 2008, and the muscle and systemic adaptations in oxygen consumption and fat oxidation Haram et al., 2009; Mora-Rodriguez et al., 2014; Tjønna et al., 2008, and in many cases reported changes superior to those seen with continuous steady-state training Haram et al., 2009; Tjønna et al., 2008. In addition to these physiological changes noted above, positive transcriptional changes have also been reported with high-speed interval training that can reduce cardiovascular risk factors in patients with metabolic syndrome Bye et al., 2009; Stensvold et al., 2010.
5. Schizophrenia, brain function and physical exercise
There are clear cognitive benefits of physical exercise in healthy and diverse mentally ill populations (Cotman et al., 2007; Hillman et al., 2008), reaching beyond improvements in health and physical functioning alone (Fabel et al., 2003; Kronenberg et al., 2005; van Praag et al., 1999). Regular physical exercise induces neurobiological, functional and structural brain changes associated with cognition (and by inference, functional capacity) and psychiatric symptoms. fMRI data show that regular physical activity disproportionately improves tasks that necessitate greater amounts of executive control, located within the PFC (Ekkekakis, 2009), and, very well documented, memory, with compelling growth effects on hippocampal structures (Li et al., 2014; Thomas and Baker, 2013). Hippocampal and temporal lobe volumes are larger in higher-fit adults (Erickson et al., 2009), and hippocampal volumes mediate improvements in memory. Exercise training increases cerebral blood flow Burdette et al., 2010 and neurogenesis in the dentate gyrus (Pereira et al., 2007), improving pattern separation (Sahay et al., 2007). Changes in serum BDNF are associated with changes in hippocampal volume (Lipsky and Marini, 2007). The hippocampus is rich in BDNF, and BDNF levels increase with exercise in rodents and humans (Cotman and Bertchtold, 2002). BDNF, a putative mediator of neurogenesis (Vaynman et al., 2004), contributes to dendritic expansion, and in conjunction with VEGF, is critical for memory formation (Figurov et al., 1996; Kang and Schuman, 1996; Pang et al., 2004). Moreover, physical exercise produces antidepressant effects via the peptide precursor of neutrotropic factors, VGF or serotonergic 5-HT1a receptors (Blumenthal, 2011; Malberg and Monteggia, 2008) and via neurogenesis (Jun et al., 2012); with commensurately increased production of neurotrophic factors, including insulin-like growth factor 1, BDNF and VEGF (Chen and Russo-Neustadt, 2007; Coelho et al., 2013; Russo-Neustadt and Chen, 2005).
Several small trials in chronic schizophrenia, using mostly low to moderate intensity cardiovascular exercise, show improvements in both positive and negative psychotic symptoms with only one exception (Heggelund et al., 2011), and in quality of life. Effects of physical exercise on cognition in schizophrenia have rarely been measured, and have never been measured comprehensively; effects on functional capacity have never been measured. The one available study Pajonk et al., 2010 showed improvement in memory after a routine 12-week cardiovascular exercise program in chronic schizophrenia, increasing relative hippocampal volume by 12%, with no change in a non-exercise group (−1%). Changes in hippocampal volume in the exercise group were correlated with improvements in aerobic fitness (VO2max) (r= 0.71;P = .003). Improvements in short-term memory were correlated with changes in hippocampal volume (r = 0.51; P < .05). Other, scarcely available studies show mixed results on brain volume, owing to small samples and perhaps, low exercise intensity (Falkai et al., 2013; Takahashi et al., 2012). We have recently completed a pilot study using the multimodal exercise model depicted in Fig. 2, and have achieved significant improvements in cognition after 12 weeks of training (Strassnig et al., in preparation).
Considerable data support neuronal restructuring of the motor system specific to motor pattern training. These changes have been reported in the motor cortex (Hlustík et al., 2004; Jackson et al., 2003; Karni et al., 1995, 1998), basal ganglia (Doyon et al., 2003; Kreitzer and Malenka, 2008), and cerebellum (Morton and Bastian, 2006; Thach and Bastian, 2004). Increasing evidence suggests that interventions promoting more involvement in activities that are both cognitively and physically stimulating optimize brain structure and function (Boyke et al., 2008; Erickson et al., 2011; Pieramico et al., 2012) if the trained skills are challenging to master, but successfully acquired (Curlik and Shors, 2011). The types of training shown to be effective involve processes related to associative learning, spatial learning, and even new physical skills (Gould et al., 1999; Kempermann et al., 2010; Shors et al., 2012; Wurm et al., 2007), all of which are emphasized in more complex exercise interventions as opposed to steady state cardiovascular exercise. This is because newly generated neurons will be integrated with the coded restructuring of the motor system to further increase physical capacity, especially if the patterns of movement (kinetic chains) are imitative of ADL and IADL performance.
5.1. Adherence
Adherence rates of schizophrenia patients to physical exercise may not differ much from those in other groups, but have not been formally established in controlled trials of sufficient size and quality (Martinsen, 2003; Roberts and Bailey, 2011). Individuals with schizophrenia and ‘normal’ sedentary members of the population do not differ widely in their attitudes to exercise Faulkner and Biddle, 1999 In fact, a survey we completed in 143 patients with chronic schizophrenia showed a desire to exercise, but limited resources and know-how (Strassnig et al., 2005). Our own preliminary exercise studies identify the availability of transportation as an important factor for adherence; another important factor appears be limited know-how about physical exercise which can be addressed through thorough on-site explanation of procedures, assessments, and training modules. Moreover, physical training must be started slowly, so that participants can get comfortable with exercise, without experiencing side effects such as protracted muscle soreness or shortness of breath that could be experienced as deterrents from further exercise. We have observed in our pilot studies that schizophrenia patients are very appreciative of the general supportive atmosphere, the camaraderie and peer support, and the availability of an exercise facility for their enjoyment, and availability of knowledgeable support staff (for many participants, our exercise pilot studies were their first opportunities to work out in a gym setting). Motivational aspects should be addressed prior to commencing exercise. Motivational interventions have been piloted to facilitate exercise interventions in schizophrenia (Beebe et al., 2011).
6. Summary and direction
Given the patterns of premature aging, increased obesity, reduced daily function and increased metabolic and cardiovascular risk, and the information detailed above concerning the specific changes induced by selected training modalities, we are currently examining the application of a unique multimodal skills-specific high-speed circuit training program as a multifaceted exercise intervention for schizophrenia patients. Aside from clear benefits of multimodal exercise on physical health and ADLs, potentially the most important argument in favor of our current research is the fact that these training programs may improve cognitive parameters and skills more so than traditional steady state cardiovascular training, thereby potentially providing synergistic or even interactive benefits for disability reduction, setting aside health benefits.
Acknowledgments
Role of funding source
Carolina Gonzales was supported through the Howard Hughes Medical Institute Undergraduate Research Program at the University of Miami.
Footnotes
Contributors
All authors contributed to the manuscript writing.
Conflict of interest
Dr. Harvey has served as a consultant to Abbvie, Boeheringer Ingelheim, En Vivo, Genentech, Otsuka America, Sunovion Pharma, and Takeda Pharma. This consultation work was on phase 2 or 3 drug development and is not related to the content of this paper. Drs. Strassnig and Signorile, and Ms. Gonzalez report no conflicts of interest.
References
- Abramowitz A, Ginger E, Gollan J, Smith M. Empathy, depressive symptoms, and social functioning among individuals with schizophrenia. Psychiatry Res. 2014;216(2):161–290. doi: 10.1016/j.psychres.2014.02.028. [DOI] [PubMed] [Google Scholar]
- Allander E, Bjornsson OJ, Olafsson O, Sigfusson N, Thorsteinsson J. Normal range of joint movements in shoulder, hip, wrist and thumb with special reference to side: a comparison between two populations. Int J Epidemiol. 1974;3:253–261. doi: 10.1093/ije/3.3.253. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine Current Comment, d. Exercise and the Older Adult. http://www.acsm.org/docs/current-comments/exerciseandtheolderadult.pdf.
- Anderson-Hanley C, Nimon JP, Westen SC. Cognitive health benefits of strengthening exercise for community-dwelling older adults. J Clin Exp Neuropsychol. 2010;32(9) doi: 10.1080/13803391003662702. http://dx.doi.org/10.1080/13803391003662702. [DOI] [PubMed] [Google Scholar]
- Bassey EJ, Fiatarone MA, O’Neill EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci (Colch) 1992;82(3):321–327. doi: 10.1042/cs0820321. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Wayne SJ, Waters DL, et al. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12:1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- Bean JF, Herman S, Kiely DK, Frey IC, Leveille SG, Fielding RA, Frontera WR. Increased Velocity Exercise Specific to Task (InVEST) training: a pilot study exploring effects on leg power, balance, and mobility in community-dwelling older women. J Am Geriatr Soc. 2004;52(5):799–804. doi: 10.1111/j.1532-5415.2004.52222.x. [DOI] [PubMed] [Google Scholar]
- Beebe LH, Smith K, Burk R, et al. Effect of a motivational intervention on exercise behavior in persons with schizophrenia spectrum disorders. Community Ment Health J. 2011;47:629–636. doi: 10.1007/s10597-010-9363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeri MS, Ravona-Springer R, Silverman JM, Haroutunian V. The effects of cardiovascular risk factors on cognitive compromise. Dialogues Clin Neurosci. 2009;11(2):201–212. doi: 10.31887/DCNS.2009.11.2/msbeeri. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrman S, Ebmeier KP. Can exercise prevent cognitive decline? Practitioner. 2014;258(1767):17–21. (2–3) [PubMed] [Google Scholar]
- Blair SN, Kohl HW, III, Paffenbarger RS, et al. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262(17):2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- Blair SN, Kampert JB, Kohl HW, III, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276(3):205–210. [PubMed] [Google Scholar]
- Blumenthal JA. New frontiers in cardiovascular behavioral medicine: comparative effectiveness of exercise and medication in treating depression. Cleve Clin J Med. 2011;78(Suppl 1):S35–S39. doi: 10.3949/ccjm.78.s1.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeka AG, Lokken KL. Neuropsychological performance of a clinical sample of extremely obese individuals. Arch Clin Neuropsychol. 2008;23(4):467–474. doi: 10.1016/j.acn.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Boutcher SH. High-intensity intermittent exercise and fat loss: review article. J Obes 868305. 2011 doi: 10.1155/2011/868305. http://dx.doi.org/10.1155/2011/868305 (Epub 2010 Nov 24) [DOI] [PMC free article] [PubMed]
- Boyke J, Driemeyer J, Gaser C, Buechl C, May A. Training induced brain structure changes in the elderly. J Neurosci. 2008;28:7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun WA, Hawthorne WE, Markofski MM. Acute EPOC response in women to circuit training and treadmill exercise of matched oxygen consumption. Eur J Appl Physiol. 2005;94(5–6):500–504. doi: 10.1007/s00421-005-1383-7. [DOI] [PubMed] [Google Scholar]
- Brogan A, Hevey D, O’Callaghan G, Yoder R, O’Shea D. Impaired decision making among morbidly obese adults. J Psychosom Res. 2011;70:189–196. doi: 10.1016/j.jpsychores.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Brouchard C, Shephard RJ, Stephens T, Sutton JR, McPherson BD, editors. Exercise, Fitness, and Health. A Consensus of Current Knowledge. Champaign: Human Kinetics; 1990. p. 6. [Google Scholar]
- Brown CJ, Flood KL. Mobility limitation in the older patient: a clinical review. JAMA. 2013;310(11):1168–1177. doi: 10.1001/jama.2013.276566. [DOI] [PubMed] [Google Scholar]
- Brown S, Birtwistle J, Roe L, Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychol Med. 1999;29(3):697–701. doi: 10.1017/s0033291798008186. [DOI] [PubMed] [Google Scholar]
- Burdette JH, et al. Using network science to evaluate exercise-associated brain changes in older adults. Front Aging Neurosci. 2010;2:23. doi: 10.3389/fnagi.2010.00023. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyukdura JS, McClintock SM, Croarkin PE. Psychomotor retardation in depression: biological underpinnings, measurement, and treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:395–409. doi: 10.1016/j.pnpbp.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bye A, Tjønna AE, Stølen TO, Røsbjørgen RE, Wisløff U. Transcriptional changes in blood after aerobic interval training in patients with the metabolic syndrome. Eur J Cardiovasc Prev Rehabil. 2009;16(1):47–52. doi: 10.1097/HJR.0b013e32831c13a0. [DOI] [PubMed] [Google Scholar]
- Caligiuri M, Ellwanger J. Motor and cognitive aspects of motor retardation in depression. J Affect Disord. 2000;57:83–93. doi: 10.1016/s0165-0327(99)00068-3. [DOI] [PubMed] [Google Scholar]
- Capodaglio P, Castelnuovo G, Brunani A, Vismara L, Villa V, Capodaglio EM. Functional limitations and occupational issues in obesity: a review. Int J Occup Saf Ergon. 2010;16(4):507–523. doi: 10.1080/10803548.2010.11076863. [DOI] [PubMed] [Google Scholar]
- Carter JS, Williams HG, Macera CA. Relationships between physical activity habits and functional neuromuscular capacities in healthy older adults. J Appl Gerontol. 1993;12:283–293. [Google Scholar]
- Casey DE, Haupt DW, Newcomer JW, Henderson DC, Sernyak MJ, Davidson M, Lindenmayer JP, Manoukian SV, Banerji MA, Lebovitz HE, Hennekens CH. Antipsychotic-induced weight gain and metabolic abnormalities: implications for increased mortality in patients with schizophrenia. J Clin Psychiatry. 2004;65(Suppl 7):4–18. [PubMed] [Google Scholar]
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- Castaneda C, Janssen I. Ethnic comparisons of sarcopenia and obesity in diabetes. Ethn Dis. 2005;15(4):664–670. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. US Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Atlanta, Ga: US Dept of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. Centers for Disease Control and Prevention, 1996. [Google Scholar]
- Chen MJ, Russo-Neustadt AA. Running exercise- and antidepressant-induced increases in growth and survival-associated signaling molecules are IGF-dependent. Growth Factors. 2007;25(2):118–131. doi: 10.1080/08977190701602329. [DOI] [PubMed] [Google Scholar]
- Chung JY, Kang HT, Lee DC, et al. Body composition and its association with cardiometabolic risk factors in the elderly: a focus on sarcopenic obesity. Arch Gerontol Geriatr. 2013;56:270–278. doi: 10.1016/j.archger.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Clark BC, Manini TM. What is dynapenia? Nutrition. 2012;28:495–503. doi: 10.1016/j.nut.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho FG, Gobbi S, Andreatto CA, et al. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): a systematic review of experimental studies in the elderly. Arch Gerontol Geriatr. 2013;56(1):10–15. doi: 10.1016/j.archger.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Cotman CV, Bertchtold NC. Exercise a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Cramer H, Lauche R, Klose P, Langhorst J, Dobos G. Yoga for schizophrenia: a systematic review and meta-analysis. BMC Psychiatry. 2013;13:32. doi: 10.1186/1471-244X-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings SR, Nevitt MC. A hypothesis: the causes of hip fractures. J Gerontol. 1989;44:M107–M111. doi: 10.1093/geronj/44.4.m107. [DOI] [PubMed] [Google Scholar]
- Curlik DM, II, Shors TJ. Learning increases the survival of newborn neurons provided that learning is difficult to achieve and successful. J Cogn Neurosci. 2011;23(9):2159–2170. doi: 10.1162/jocn.2010.21597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva RL, Brentano MA, Kruel LF. Effects of different strength training methods on postexercise energetic expenditure. J Strength Cond Res. 2010;24(8):2255–2260. doi: 10.1519/JSC.0b013e3181aff2ba. [DOI] [PubMed] [Google Scholar]
- Daussin FN, Ponsot E, Dufour SP, Lonsdorfer-Wolf E, Doutreleau S, Geny B, Piquard F, Richard R. Improvement of VO 2 max; by cardiac output and oxygen extraction adaptation during intermittent versus continuous endurance training. Eur J Appl Physiol. 2007;101:377–383. doi: 10.1007/s00421-007-0499-3. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Gold JM, Dickerson FB, et al. Evidenceof exacerbated cognitive deficits in schizophrenia patients with comorbid diabetes. Psychosomatics. 2008;49:123–131. doi: 10.1176/appi.psy.49.2.123. [DOI] [PubMed] [Google Scholar]
- Dominguez LJ, Barbagallo M. The cardiometabolic syndrome and sarcopenic obesity in older persons. J Cardiometab Syndr. 2007;2:183–189. doi: 10.1111/j.1559-4564.2007.06673.x. [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the corticostriatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41(3):252–262. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Drigny J, Gremeaux V, Guiraud T, Gayda M, Juneau M, Nigam A. Long-term high-intensity interval training associated with lifestyle modifications improves QT dispersion parameters in metabolic syndrome patients. Ann Phys Rehabil Med. 2013;56(5):356–370. doi: 10.1016/j.rehab.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Dufour AB, Hannan MT, Murabito JM, et al. Sarcopenia definitions considering body size and fat mass are associated with mobility limitations: the Framingham Study. J Gerontol A Biol Sci Med Sci. 2013;68:168–174. doi: 10.1093/gerona/gls109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta C, Hadley EC, Lexell J. Sarcopenia and physical performance in old age: overview. Muscle Nerve. 1997;5(Suppl.):S5–S9. [PubMed] [Google Scholar]
- Edgerton VR, Roy RR, Gregor RJ, Rugg S. Morphological basis of skeletal muscle power output. Hum Muscle Power. 1986:43–64. [Google Scholar]
- Ekkekakis P. Illuminating the black box: investigating prefrontal cortical hemodynamics during exercise with near-infrared spectroscopy. J Sport Exerc Psychol. 2009;31(4):505–553. doi: 10.1123/jsep.31.4.505. [DOI] [PubMed] [Google Scholar]
- Elekund LG, Haskell WL, Johnson JL, et al. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men. The Lipid Research Clinics Mortality Follow-up Study. N Engl J Med. 1988;319(21):1379–1384. doi: 10.1056/NEJM198811243192104. [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Nevitt MC, Yunis C, et al. Correlates of impaired function in older women. J Am Geriatr Soc. 1994;42:481–489. doi: 10.1111/j.1532-5415.1994.tb04968.x. [DOI] [PubMed] [Google Scholar]
- Erickson KL, et al. Aerobic fitness isassociated with hippocampal volume inelderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss M, Prakash RS, Basak C, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;10:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Toda H, et al. Copernican stem cells: regulatory constellations in adult hippocampal neurogenesis. J Cell Biochem. 2003;88(1):41–50. doi: 10.1002/jcb.10377. [DOI] [PubMed] [Google Scholar]
- Falkai P, Malchow B, Wobrock T, et al. The effect of aerobic exercise on cortical architecture in patients with chronic schizophrenia: a randomized controlled MRI study. Eur Arch Psychiatry Clin Neurosci. 2013;263(6):469–473. doi: 10.1007/s00406-012-0383-y. [DOI] [PubMed] [Google Scholar]
- Faulkner G, Biddle S. Exercise as an adjunct treatment for schizophrenia: a review of the literature. J Ment Health. 1999;8:441–457. [Google Scholar]
- Fielding RA, LeBrasseur NK, Cuoco A, et al. High-velocity resistance training increases skeletal muscle peak power in older women. J Am Geriatr Soc. 2002;50:555–662. doi: 10.1046/j.1532-5415.2002.50159.x. [DOI] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Want T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LLTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Finkelstein EA, Khavjou OA, Thompson H, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42(6):563–570. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Fleg JL, Pina IL, Balady GJ, et al. Assessment of functional capacity in clinical and research applications. Circulation. 2000;102:1591–1597. doi: 10.1161/01.cir.102.13.1591. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Foerster A, Lewis S, Owen M, et al. Pre-morbid adjustment and personality in psychosis: effects of sex and diagnosis. Br J Psychiatry. 1991;158:171–176. doi: 10.1192/bjp.158.2.171. [DOI] [PubMed] [Google Scholar]
- Fogarty M, Happell B, Pinikahana J. The benefits of an exercise program for people with schizophrenia: a pilot study. Psychiatr Rehabil J. 2004;28(2):173–176. doi: 10.2975/28.2004.173.176. http://dx.doi.org/10.2975/28.2004.173.176. [DOI] [PubMed] [Google Scholar]
- Foldvari M, Clark M, Laviolette LC, et al. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol A Biol Sci Med Sci. 2000;55A:M192–M199. doi: 10.1093/gerona/55.4.m192. [DOI] [PubMed] [Google Scholar]
- Forhan M, Gill SV. Obesity, functional mobility and quality of life. Best Pract Res Clin Endocrinol Metab. 2013;27(2):129–137. doi: 10.1016/j.beem.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Friedman PJ, Richmond DE, Baskett JJ. A prospective trial of serial gait speed as a measure of rehabilitation in the elderly. Age Ageing. 1988;17(4):227–235. doi: 10.1093/ageing/17.4.227. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Wallenstein S, Moshier E, et al. The effects of hypertension and body mass index on cognition in schizophrenia. Am J Psychiatry. 2010;167(10):1232–1239. doi: 10.1176/appi.ajp.2010.09091328. [DOI] [PubMed] [Google Scholar]
- Gibala MJ, McGee SL. Metabolic adaptations to short-term high-intensity interval training: a little pain for a lot of gain? Exerc Sport Sci Rev. 2008;36(2):58–63. doi: 10.1097/JES.0b013e318168ec1f. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Hughes S, Dunlop D, Singer R, Chang RW. Predictors of change in walking velocity in older adults. J Am Geriatr Soc. 1996;44:126–132. doi: 10.1111/j.1532-5415.1996.tb02427.x. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2(3):260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Grabiner MD, Koh TJ, Lundin TM, Jahnigen DW. Kinematics of recovery from a stumble. J Gerontol. 1993;48:M97–M102. doi: 10.1093/geronj/48.3.m97. [DOI] [PubMed] [Google Scholar]
- Greene LS, Williams HG, Macera CA, Carter JS. Identifying dimensions of physical (motor) functional capacity in healthy older adults. J Aging Health. 1993;5:163–178. [Google Scholar]
- Greg J, Murphy A, Pryor J. Musculotendinous stiffness: its relationship to eccentric, isometric and concentric performance. J Appl Physiol. 1994;76:2714–2719. doi: 10.1152/jappl.1994.76.6.2714. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM. Physical disability in older Americans. J Gerontol. 1993;48:3–10. doi: 10.1093/geronj/48.special_issue.3. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Haltom RW, Kraemer RR, Sloan RA, Hebert EP, Frank K, Tryniecki JL. Circuit weight training and its effects on excess postexercise oxygen consumption. Med Sci Sports Exerc. 1999;31(11):1613–1618. doi: 10.1097/00005768-199911000-00018. [DOI] [PubMed] [Google Scholar]
- Haram PM, Kemi OJ, Lee SJ, Bendheim MØ, Al-Share QY, Waldum HL, Gilligan LJ, Koch LG, Britton SL, Najjar SM, Wisløff U. Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovasc Res. 2009;81(4):723–732. doi: 10.1093/cvr/cvn332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD. Cognitive functioning and disability in schizophrenia. Curr Dir Psychol Sci. 2010;19:249–254. [Google Scholar]
- Harvey PD, Strassnig M. Predicting the severity of everyday functional disability in people with schizophrenia: cognitive deficits, functional capacity, symptoms, and health status. World Psychiatry. 2012;11(2):73–79. doi: 10.1016/j.wpsyc.2012.05.004. http://dx.doi.org/10.1016/j.wpsyc.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan A, Nitsche M, Herrmann M. Impaired long-term depression in schizophrenia: a cathodal tDCS pilot study. Brain Stimul. 2011 doi: 10.1016/j.brs.2011.08.004. http://dx.doi.org/10.1016/j.brs.2011.08.004. [DOI] [PubMed]
- Hausdorff JM, Schweiger A, Herman T, Yogev-Seligmann G, Giladi N. Dual-task decrements in gait: contributing factors among healthy older adults. J Gerontol A Biol Sci Med Sci. 2008;63(12):1335–1343. doi: 10.1093/gerona/63.12.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggelund J, Nilsberg GE, Hoff J, Morken G, Helgerud J. Effects of high aerobic intensity training in patients with schizophrenia: a controlled trial. Nord J Psychiatry. 2011;65(4):269–275. doi: 10.3109/08039488.2011.560278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgerud J, Hkydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, Simonsen T, Helgesen C, Hjorth N, Bach R, Hoff J. Aerobic high-intensity intervals improve VO2MAX more than moderate training. Med Sci Sports Exerc. 2007;39(4):665–671. doi: 10.1249/mss.0b013e3180304570. [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115–1121. doi: 10.1016/j.ahj.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9(1):58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Hjorth P, Davidsen AS, Kilian R, Skrubbeltrang C. A systematic review of controlled interventions to reduce overweight and obesity in people withschizophrenia. Acta Psychiatr Scand. 2014 doi: 10.1111/acps.12245. http://dx.doi.org/10.1111/acps.12245 (Epub ahead of print) [DOI] [PubMed]
- Hlustík P, Solodkin A, Noll DC, Small SL. Cortical plasticity during three-week motor skill learning. J Clin Neurophysiol. 2004;21(3):180–191. doi: 10.1097/00004691-200405000-00006. [DOI] [PubMed] [Google Scholar]
- Holt JL, Holt E, Pelhan TW. Flexibility redefined. XIIIth International Symposium for Biomechanics in Sport. 1996:170–174. [Google Scholar]
- Homel P, Casey D, Allison DB. Changes in body mass index for individuals with and without schizophrenia, 1987–1996. Schizophr Res. 2002;55(3):277–284. doi: 10.1016/s0920-9964(01)00256-0. [DOI] [PubMed] [Google Scholar]
- Houston DK, Ding J, Nicklas BJ, et al. The association between weight history and physical performance in the Health, Aging and Body composition Study. Int J Obes. 2007;31:1680–1687. doi: 10.1038/sj.ijo.0803652. [DOI] [PubMed] [Google Scholar]
- Hunter JP, Marshall RN. Effects of power and flexibility training on vertical jump technique. Med Sci Sports Exerc. 2002;34:478–486. doi: 10.1097/00005768-200203000-00015. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Lafleur MF, Malouin F, Richards CL, Doyon J. Functional cerebral reorganization following motor sequence learning through mental practice with motor imagery. Neuroimage. 2003;20(2):1171–1180. doi: 10.1016/S1053-8119(03)00369-0. [DOI] [PubMed] [Google Scholar]
- Jette AM, Branch LG, Berlin J. Musculoskeletal impairments and physical disablement among the aged. J Gerontol. 1990;45:M203–M208. doi: 10.1093/geronj/45.6.m203. [DOI] [PubMed] [Google Scholar]
- Jin H, Folsom D, Sasaki A, Mudaliar S, Henry R, Torres M, Golshan S, Glorioso DK, Jeste D. Increased Framingham 10-year risk of coronary heart disease in middle-aged and older patients with psychotic symptoms. Schizophr Res. 2011;125(2–3):295–299. doi: 10.1016/j.schres.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JK, Lui LY, Yaffe K. Executive function, more than global cognition, predicts functional decline and mortality in elderly women. J Gerontol A Biol Sci Med Sci. 2007;62(10):1134–1141. doi: 10.1093/gerona/62.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge JO, Schechtman K, Cress E. The relationship between physical performance measures and independence in instrumental activities of daily living. The FICSIT Group. Frailty and Injury: Cooperative Studies of Intervention Trials. J Am Geriatr Soc. 1996;44:1332–1341. doi: 10.1111/j.1532-5415.1996.tb01404.x. [DOI] [PubMed] [Google Scholar]
- Jun H, Mohammed Qasim Hussaini S, Rigby MJ, Jang MH. Functional role of adult hippocampal neurogenesis as a therapeutic strategy for mental disorders. Neural Plast 854285. 2012 doi: 10.1155/2012/854285. http://dx.doi.org/10.1155/2012/854285. [DOI] [PMC free article] [PubMed]
- Kaess BM, Jozwiak J, Mastej M, et al. Association between anthropometric obesity measures and coronary artery disease: a cross-sectional survey of 16,657 subjects from 444 Polish cities. Heart. 2010;96:131–135. doi: 10.1136/hrt.2009.171520. [DOI] [PubMed] [Google Scholar]
- Kampert JB, Blair SN, Barlow, et al. Physical activity, physical fitness, and all-cause cancer mortality: a prospective study of men and women. Ann Epidemiol. 1996;6(5):452–457. doi: 10.1016/s1047-2797(96)00059-2. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- Kanoski SE. Cognitive and neuronal systems underlying obesity. Physiol Behav. 2012;106(3):337–344. doi: 10.1016/j.physbeh.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, et al. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377(6545):155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95(3):861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katula JA, Rejeski WJ, Marsh AP. Enhancing quality of life in older adults: a comparison of muscular strength and power training. Health Qual Life Outcomes. 2008;6:45. doi: 10.1186/1477-7525-6-45. http://dx.doi.org/10.1186/1477-7525-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Andrzejewski ME, Baldwin AE, Hernandez PJ, Pratt WE. Glutamate-mediated plasticity in corticostriatal networks: role in adaptive motor learning. Ann N Y Acad Sci. 2003;1003:159–168. doi: 10.1196/annals.1300.061. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Fabel K, Ehninger D, Babu H, Leal-Galicia P, Garthe A, Wolf SA. Why and how physical activity promotes experience-induced brain plasticity. Front Neurosci. 2010;8(4):189. doi: 10.3389/fnins.2010.00189. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Murray RM. Neurodevelopment and Adult Psychopathology. NewYork: Cambridge University Press; 1997. [Google Scholar]
- Kidde J, Marcus R, Dibble L, Smith S, Lastayo P. Regional muscle and whole-body composition factors related to mobility in older individuals: a review. Physiother Can. 2009;61(4):197–209. doi: 10.3138/physio.61.4.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Messias E, Harvey PD, et al. Is schizophrenia a syndrome of accelerated aging? Schizophr Bull. 2008;34(6):1024–1032. doi: 10.1093/schbul/sbm140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60(4):543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging. 2005;10:1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Kyrou I, Osei-Assibey G, Williams N, et al. Self-reported disability in adults with severe obesity. J Obes. 2011:918402. doi: 10.1155/2011/918402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham NK, Bennett DA, Stretton CM, Anderson CS. Systematic review of progressive resistance strength training in older adults. J Gerontol A Biol Sci Med Sci. 2004;59(1):48–61. doi: 10.1093/gerona/59.1.m48. [DOI] [PubMed] [Google Scholar]
- Laukkanen P, Era P, Heikkinen RL, et al. Factors related to carrying out everyday activities among elderly people aged 80. Aging (Milano) 1994;6:433–444. doi: 10.1007/BF03324275. [DOI] [PubMed] [Google Scholar]
- Lawton MP. Scales to measure competence in everyday activities. Psychopharmacol Bull. 1988;24:609–614. [PubMed] [Google Scholar]
- Levinger C, Goodman D, Hare G, et al. Functional capacity and quality of life in middle-age men and women with high and low number of metabolic risk factors. Int J Cardiol. 2009;133(2):281–283. doi: 10.1016/j.ijcard.2007.11.046. [DOI] [PubMed] [Google Scholar]
- Li Rui, Zhu X, Yin S, Niu Y, et al. Multimodal intervention in older adults improves resting state functional connectivity between the medial prefrontal cortex and medial temporal lobe. Front Aging Neurosci. 2014;6:1–13. doi: 10.3389/fnagi.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky RH, Marini AM. Brain-derived neurotropic factor in neuronal survival and behavior-related plasticity. Ann N Y Acad Sci. 2007;1122:130–143. doi: 10.1196/annals.1403.009. [DOI] [PubMed] [Google Scholar]
- Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;3:CD002759. doi: 10.1002/14651858.CD002759.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord SR, Sherrington C, Menz HB. Falls in Older People: Risk Factors and Strategies for Prevention. 2001 [Google Scholar]
- Lotspeich LJ, Ciaranello RD. The neurobiology of early infantile autism. Neurosci-entist. 1995:361–367. [Google Scholar]
- Malberg JE, Monteggia LM. VGF, a new player in antidepressant action? Sci Signal. 2008;1(18):e19. doi: 10.1126/stke.118pe19. http://dx.doi.org/10.1126/stke.118pe19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinsen EW. The effects of exercise on mental health in clinical populations. In: Biddle SJJ, editor. European Perspectives on Exercise and Sport Psychology. Champain, IL: Human Kinetics; 2003. pp. 71–84. [Google Scholar]
- Mason P, Harrison G, Glazenbrook C, et al. Characteristics of outcome in schizophrenia at 13 years. Br J Psychiatry. 1995;167:596–603. doi: 10.1192/bjp.167.5.596. [DOI] [PubMed] [Google Scholar]
- Mazzeo RS, Tanaka H. Exercise prescription for the elderly. Sports Med. 2001;31(11):809–818. doi: 10.2165/00007256-200131110-00003. [DOI] [PubMed] [Google Scholar]
- McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379(9833):2291–2299. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, Meltzer HY, Hsiao J, Scott Stroup T, Lieberman JA. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Mignardot JB, Olivier I, Promayon E, Nougier V. Origins of balance disorders during a daily living movement in obese: can biomechanical factors explain everything? PLoS One. 2013;8(4):e60491. doi: 10.1371/journal.pone.0060491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DD. Atypical antipsychotics: sleep, sedation, and efficacy. Prim Care Companion J Clin Psychiatry. 2004;6(Suppl 2):3–7. [PMC free article] [PubMed] [Google Scholar]
- Miller ME, Rejeski WJ, Reboussin BA, Have TR, Ettinger WH. Physical activity, functional limitations, and disability in older adults. J Am Geriatr Soc. 2000;48(10):1264–1272. doi: 10.1111/j.1532-5415.2000.tb02600.x. [DOI] [PubMed] [Google Scholar]
- Miszko TA, Cress ME, Slade JM, et al. Effect of strength power training on physical function in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2003;58A:171–175. doi: 10.1093/gerona/58.2.m171. [DOI] [PubMed] [Google Scholar]
- Mitchell WK, Williams J, Atherton P, et al. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3:260. doi: 10.3389/fphys.2012.00260. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Rodriguez R, Ortega JF, Hamouti N, Fernandez-Elias VE, Cañete Garcia-Prieto J, Guadalupe-Grau A, Saborido A, Martin-Garcia M, Guio de Prada V, Ara I, Martinez-Vizcaino V. Time-course effects of aerobic interval training and detraining in patients with metabolic syndrome. Nutr Metab Cardiovasc Dis. 2014;24(7):792–798. doi: 10.1016/j.numecd.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Morley JE, Abbatecola AM, Argiles JM, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403–409. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci. 2006;26(36):9107–9116. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mow S, Roos BA, Serravite DH, Signorile JF. Optimal loading for power in older men. Med Sci Sports Exerc. 2009;41(5):370–371. [Google Scholar]
- Mozley LH, Gur RH, Mozley PD, Gur RE. Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry. 2001;158:1492–1499. doi: 10.1176/appi.ajp.158.9.1492. [DOI] [PubMed] [Google Scholar]
- Murray RM. Neurodevelopmental schizophrenia: the rediscovery of dementia praecox. Br J Psychiatry. 1994;165(Suppl 25):6–12. [PubMed] [Google Scholar]
- Murray RM, Lewis LW. Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed) 1987;295(6600):681–682. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Pollock BG, Halligan EM, Kirshner MA, Houck PR. Serum anticho-linergic activity and motor performance in elderly persons. J Gerontol. 2007;62:83–85. doi: 10.1093/gerona/62.1.83. [DOI] [PubMed] [Google Scholar]
- Newcomer JW. Metabolic risk during antipsychotic treatment. Clin Ther. 2004;26(12):1936–1946. doi: 10.1016/j.clinthera.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Newcomer JW. Metabolic syndrome and mental illness. Am J Manage Care. 2007;13(7 Suppl):S170–S177. [PubMed] [Google Scholar]
- Newcomer J, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298:1794–1796. doi: 10.1001/jama.298.15.1794. [DOI] [PubMed] [Google Scholar]
- Newton RU, Kraemer WJ. Developing explosive muscular power: implications for mixed methods training strategy. Strength Cond J. 1994;16(5):20–31. [Google Scholar]
- Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nat Rev Cardiol. 2010;7(12):686–698. doi: 10.1038/nrcardio.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajonk FG, Wobrock T, Gruber O, Scherk H, et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010;67(2):133–143. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- Pang PT, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hip-pocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Pannacciulli N, Del Parigi A, Chen K, et al. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006;31:1419–1425. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Panza F, Frisardi V, Capurso C, Imbimbo BP, Vendemiale G, et al. Metabolic syndrome and cognitive impairment: current epidemiology and possible underlying mechanisms. J Alzheimers Dis. 2010;21(3):691–724. doi: 10.3233/JAD-2010-091669. [DOI] [PubMed] [Google Scholar]
- Papanastasiou E. Interventions for the metabolic syndrome in schizophrenia: a review. Ther Adv Endocrinol Metab. 2012;3(5):141–162. doi: 10.1177/2042018812458697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W, Ramachandran J, Weisman P, Jung ES. Obesity effect on male active joint range of motion. Ergonomics. 2010;53(1):102–108. doi: 10.1080/00140130903311617. [DOI] [PubMed] [Google Scholar]
- Peeters A, Bonneux L, Nusselder WJ, et al. Adult obesity and the burden of disability throughout life. Obes Res. 2004;12(7):1145–1151. doi: 10.1038/oby.2004.143. [DOI] [PubMed] [Google Scholar]
- Pendergast DR, Fisher NM, Calkins E. Cardiovascular, neuromuscular, and metabolic alterations with age leading to frailty. J Gerontol. 1993;48:61–67. doi: 10.1093/geronj/48.special_issue.61. (Special Issue) [DOI] [PubMed] [Google Scholar]
- Pereira AC, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieramico V, Esposito R, Sensi F, Cilli F, Mantini D, Mattei PA, et al. Combination training in aging individuals modifies functional connectivity and cognition, and is potentially affected by dopamine-related genes. PLoS ONE. 2012;7:e43901. doi: 10.1371/journal.pone.0043901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado CM, Wells JC, Smith SR, et al. Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr. 2012;31:583–601. doi: 10.1016/j.clnu.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Roberts SH, Bailey JE. Incentives and barriers to lifestyle interventions for people with severe mental illness: a narrative synthesis of quantitative, qualitative and mixed methods studies. J Adv Nurs. 2011;67(4):690–708. doi: 10.1111/j.1365-2648.2010.05546.x. [DOI] [PubMed] [Google Scholar]
- Romero-Arenas S, Martínez-Pascual M, Alcaraz PE. Impact of resistance circuit training on neuromuscular, cardiorespiratory and body composition adaptations in the elderly. Aging Dis. 2013;4(5):256–263. doi: 10.14336/AD.2013.0400256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Chen MJ. Brain-derived neurotrophic factor and antidepres-sant activity. Curr Pharm Des. 2005;11(12):1495–1510. doi: 10.2174/1381612053764788. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2007;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder J, Niethammer R, Geider FJ, et al. Neurological soft signs in schizophrenia. Schizophr Res. 1992;6:25–30. doi: 10.1016/0920-9964(91)90017-l. [DOI] [PubMed] [Google Scholar]
- Seidell JC. The impact of obesity on health status. Int J Obes Relat Metab Disord. 1995;19(Suppl 6):S13–S16. [PubMed] [Google Scholar]
- Semkovska M, Bédard M, Godbout L, Limoge F, Stip E. Assessment of executive dysfunction during activities of daily living in schizophrenia. Schizophr Res. 2004;69(2–3):289–300. doi: 10.1016/j.schres.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Senechal M, Dionne IJ, Brochu M. Dynapenic abdominal obesity and metabolic risk factors in adults 50 years of age and older. J Aging Health. 2012;24:812–826. doi: 10.1177/0898264312440324. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Anderson ML, Curlik DM, II, Nokia MS. Use it or lose it: how neurogenesis keeps the brain fit for learning. Behav Brain Res. 2012;227(2):450–458. doi: 10.1016/j.bbr.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorile JF, Carmel MP, Czaja SJ, Asfour SS, Morgan RO, Khalil TM, Ma F, Roos BA. Differential increases in average isokinetic power by specific muscle groups of older women due to variations in training and testing. J Gerontol A Biol Sci Med Sci. 2002;57(10):M683–M690. doi: 10.1093/gerona/57.10.m683. [DOI] [PubMed] [Google Scholar]
- Signorile JF, Carmel MP, Lai S, Roos BA. Early plateaus of power and torque gains during high- and low-speed training in older women. J Appl Physiol. 2005;98:1213–1220. doi: 10.1152/japplphysiol.00742.2004. [DOI] [PubMed] [Google Scholar]
- Smeesters C, Hayes WC, McMahon TA. Disturbance type and gait speed affect fall direction and impact location. J Biomech. 2001;34(3):309–317. doi: 10.1016/s0021-9290(00)00200-1. [DOI] [PubMed] [Google Scholar]
- Spirduso W. Physical Dimensions of Aging. 1995:155–183. [Google Scholar]
- Stenholm S, Harris TB, Rantanen T, et al. Sarcopenic obesity - definition, etiology and consequences. Curr Opin Clin Nutr Metab Care. 2008;11(6):693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen WC, Janssen I. Sarcopenic-obesity and cardiovascular disease risk in the elderly. J Nutr Health Aging. 2009;13:460–466. doi: 10.1007/s12603-009-0084-z. [DOI] [PubMed] [Google Scholar]
- Stensvold D, Tjønna AE, Skaug EA, Aspenes S, Stølen T, Wisløff U, Slørdahl SA. Strength training versus aerobic interval training to modify risk factors of metabolic syndrome. J Appl Physiol. 2010;108(4):804–810. doi: 10.1152/japplphysiol.00996.2009. [DOI] [PubMed] [Google Scholar]
- Strassnig M, Brar JS, Ganguli R. Self-reported body weight perception and dieting practices in community-dwelling patients with schizophrenia. Schizophr Res. 2005;75(2–3):425–432. doi: 10.1016/j.schres.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Strassnig M, Brar JS, Ganguli R. Increased caffeine and nicotine consumption in community-dwelling patients with schizophrenia. Schizophr Res. 2006;86(1–3):269–275. doi: 10.1016/j.schres.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Strassnig M, Brar JS, Ganguli R. Low cardiorespiratory fitness and physical functional capacity in obese patients with schizophrenia. Schizophr Res. 2011a;126(1–3) doi: 10.1016/j.schres.2010.10.025. http://dx.doi.org/10.1016/j.schres.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassnig M, Brar JS, Ganguli R. Low cardiorespiratory fitness and physical functional capacity in obese patients with schizophrenia. Schizophr Res. 2011b;126(1–3):103–109. doi: 10.1016/j.schres.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassnig M, Caceda R, Newcomer J, Harvey PD. Cognitive deficits, obesity and disability in schizophrenia. Transl Neurosci. 2012;3(4):345–354. [Google Scholar]
- Takahashi H, Sassa T, Shibuya T, et al. Effects of sports participation on psychiatric symptoms and brain activations during sports observation in schizophrenia. Transl Psychiatry. 2012;2:e96. doi: 10.1038/tp.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B, Chang W, Lanigan C, Dean B, Sutcliffe J, Thomas E. Normal human aging and early-stage schizophrenia share common molecular profiles. Aging Cell. 2009;8(3) doi: 10.1111/j.1474-9726.2009.00468.x. http://dx.doi.org/10.1111/j.1474-9726.2009.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetlie T, Eik-Nes N, Palmstierna T, Callaghan P, Nøttestad J. The effect of exercise on psychological & physical health outcomes: preliminary results from a Norwegian forensic hospital. J Psychosoc Nurs Ment Health Serv. 2008;46(7):38–43. doi: 10.3928/02793695-20080701-08. [DOI] [PubMed] [Google Scholar]
- Thach WT, Bastian AJ. Role of the cerebellum in the control and adaptation of gait in health and disease. Prog Brain Res. 2004;43:353–366. doi: 10.1016/s0079-6123(03)43034-3. [DOI] [PubMed] [Google Scholar]
- Thiels C. Obesity and schizophrenia. Br J Psychiatry. 2005;187:489. doi: 10.1192/bjp.187.5.489. http://dx.doi.org/101192/bjp.187.5.489. [DOI] [PubMed] [Google Scholar]
- Thomas C, Baker CI. Teachinganadult brain new tricks:acritical review ofevidence for training-dependent structural plasticity in humans. Neuroimage. 2013;73:225–236. doi: 10.1016/j.neuroimage.2012.03.069. [DOI] [PubMed] [Google Scholar]
- Thomas EE, De Vito G, Macaluso A. Speed training with body weight unloading improves walking energy cost and maximal speed in 75- to 85-year-old healthy women. J Appl Physiol. 2007;103(5):1598–1603. doi: 10.1152/japplphysiol.00399.2007. [DOI] [PubMed] [Google Scholar]
- Tjønna AE, Lee SJ, Rognmo Ø, Stølen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slørdahl SA, Kemi OJ, Najjar SM, Wisløff U. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118(4):346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp EG, Chisholm DJ, Freund J, Boutcher SH. The effects of high-intensity intermittent exercise training on fat loss and fasting insulin levels of young women. Int J Obes (Lond) 2008;32:684–691. doi: 10.1038/sj.ijo.0803781. [DOI] [PubMed] [Google Scholar]
- Vancampfort D, Probst M, Sweers K, Maurissen K, Knapen J, De Hert M. Relationships between obesity, functional exercise capacity, physical activity participation and physical self-perception in people with schizophrenia. Acta Psychiatr Scand. 2011;123(6):423–430. doi: 10.1111/j.1600-0447.2010.01666.x. http://dx.doi.org/10.1111/j.1600-0447.2010.01666.x. [DOI] [PubMed] [Google Scholar]
- Vancampfort D, Probst M, Scheewe T, Knapen J, De Herdt A, De Hert M. The functional exercise capacity is correlated with global functioning in patients with schizophrenia. Acta Psychiatr Scand. 2012;125(5):382–387. doi: 10.1111/j.1600-0447.2011.01825.x. [DOI] [PubMed] [Google Scholar]
- Vancampfort D, Probst M, De Hert M, Soundy A, Stubbs B, Stroobants M, De Herdt A. Neurobiological effects of physical exercise in schizophrenia: a systematic review. Disabil Rehabil (Epub ahead of print) 2014 doi: 10.3109/09638288.2013.874505. [DOI] [PubMed] [Google Scholar]
- Van Heuvelen MJG, Kempen GIJM, Bruower WH, De Greef MHG. Physical fitness related to disability in older persons. Gerontology. 2000;46:333–341. doi: 10.1159/000022187. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltaration as predictors of incident mobility limitations in well-functionig older persons. J Gerontol A Biol Sci Med Sci. 2005;60:M324–M333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, et al. Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity. 2008;17:60–65. doi: 10.1038/oby.2008.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrtunski PB, Konicki PE, Kwon KY, Jaskiw GJ, Jaskiw GE. Effect of clozapine on motor function in schizophrenic patients. Schizophr Res. 1996;20:187–198. doi: 10.1016/0920-9964(95)00085-2. [DOI] [PubMed] [Google Scholar]
- Waddington JL, Youssef HA. Late onset involuntary movements in chronic schizophrenia: relationship of 'tardive' dyskinesia to intellectual impairment and negative symptoms. Br J Psychiatry. 1986;149:616–620. doi: 10.1192/bjp.149.5.616. [DOI] [PubMed] [Google Scholar]
- Waddington J, Buckley P. The Neurodevelopmental Basis of Schizophrenia. Austin, TX: Landes; 1996. [Google Scholar]
- Walther K, Birdsill AC, Gilsky EL, Ryan L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp. 2010;31:1052–1064. doi: 10.1002/hbm.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters DL, Hale L, Grant AM, et al. Osteoporosis and gait and balance disturbances in older sarcopenic obese New Zealanders. Osteoporos Int. 2010;21:351–357. doi: 10.1007/s00198-009-0947-5. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Wheeler A, Humberstone V, Robinson E. Ethnic comparisons of antipsychotic use in schizophrenia. Aust N Z J Psychiatry. 2008;42(10):863–873. doi: 10.1080/00048670802345482. [DOI] [PubMed] [Google Scholar]
- Whipple RH, Wolfson LI, Amerman PM. The relationship of knee and ankle weakness to falls in nursing home residents: an isokinetic study. J Am Geriatr Soc. 1987;35:13–20. doi: 10.1111/j.1532-5415.1987.tb01313.x. [DOI] [PubMed] [Google Scholar]
- Willeumier KC, Taylor DV, Amen DG. Elevated BMI is associated with decreased blood flow in the prefrontal cortex using SPECT imaging in healthy adults. Obesity. 2011;19:1095–1097. doi: 10.1038/oby.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AD, Carey MF, Selig S, Hayes A, Krum H, Patterson J, Toia D, Hare DL. Circuit resistance training in chronic heart failure improves skeletal muscle mi-tochondrial ATP production rate-a randomized controlled trial. J Card Fail. 2007;13(2):79–85. doi: 10.1016/j.cardfail.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Wilson GJ, Elliott BC, Wood GA. Stretch-shortening cycle performance enhancement through flexibility training. Med Sci Sports Exerc. 1992;24:116–123. [PubMed] [Google Scholar]
- Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen Ø, Skjærpe T. Continuous training inheart failure patients: a randomized study superior cardiovascular effectofaerobic interval training versus moderate. Circulation. 2007;115:3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- Wolff AL, O'Driscoll GA. Motor deficits and schizophrenia: the evidence from neuroleptic naive patients and populations at risk. J Psychiatry Neurosci. 1999;24(4):304–314. [PMC free article] [PubMed] [Google Scholar]
- Worrell TW, Smith TL, Winegardner J. Effect of hamstring stretching on hamstring muscle performance. J Orthop Sports Phys Ther. 1994;20:154–159. doi: 10.2519/jospt.1994.20.3.154. [DOI] [PubMed] [Google Scholar]
- Wurm F, Keiner S, Kunze A, Witte OW, Redecker C. Effects of skilled forelimb training on hippocampal neurogenesis and spatial learning after focal cortical infarcts in the adult rat brain. Stroke. 2007;38(10):2833–2840. doi: 10.1161/STROKEAHA.107.485524. [DOI] [PubMed] [Google Scholar]
- Yeom HA, Fleury J, Keller C. Risk factors for mobility limitation in community-dwelling older adults: a social ecological perspective. Geriatr Nurs. 2008;29(2):133–140. doi: 10.1016/j.gerinurse.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Yim CY, Soczynska JK, Kennedy SH, Woldeyohannes HO, Brietzke E, McIntyre RS. The effect of overweight/obesity on cognitive function in euthymic individuals with bipolar disorder. Eur Psychiatry. 2012;27:223–228. doi: 10.1016/j.eurpsy.2011.02.004. [DOI] [PubMed] [Google Scholar]