Abstract
F1-ATPase from Bacillus subtilis (BF1) is severely suppressed by the MgADP inhibition. Here, we have tested if this is due to the loss of nucleotide binding to the noncatalytic site that is required for the activation. Measurements with a tryptophan mutant of BF1 indicated that the noncatalytic sites could bind ATP normally. Furthermore, the mutant BF1 that cannot bind ATP to the noncatalytic sites showed much lower ATPase activity. It was concluded that the cause of strong MgADP inhibition of BF1 is not the weak nucleotide binding to the noncatalytic sites but the other steps required for the activation.
Introduction
FoF1-ATPase/synthase (FoF1) catalyzes ATP synthesis from ADP and inorganic phosphate coupled with the H+ flow driven by the electrochemical gradient of H+ across cellular membranes. FoF1 consists of a water-soluble F1 part (F1-ATPase) connected to a membrane-embedded H+ channel, Fo [1]–[3]. F1-ATPase consists of α3, β3, γ, δ and ε subunits and its hydrolysis of one ATP molecule at a catalytic site on the β subunit drives a discrete 120° rotation of the γε subunits relative to the α3β3δ [4]. In FoF1, rotation of the rotor subunits of F1 (γ and ε) is transferred to the c subunit ring of Fo to couple ATP synthesis/hydrolysis and H+ flow.
The catalytic mechanism of ATP synthase has been extensively studied by structural studies and single-molecular experiments and the mechanism of the regulation of ATP synthase becomes attracting more interests. Several regulatory mechanisms are known: The mitochondrial ATP synthase has specific regulatory protein called IF1, which prevent ATP hydrolysis; The chloroplast ATP synthase has a pair of cystein residues in the γ subunit and the formation of the disulfide between them inhibits the activity; The ε subunit of bacterial and chloroplast ATP synthase inhibits ATP hydrolysis: and so on. Among them, the most prominent is MgADP inhibition [5]–[7]. When the ATP hydrolysis product, MgADP, is tightly bound at a catalytic site, the F1-ATPase is stalled. It is a common mechanism among all ATP synthases examined so far. Several factors are known to affect MgADP inhibition; Sodium azide stabilizes MgADP inhibition [6]: A detergent lauryldimethylamine N-oxide (LDAO) releases MgADP inhibition [8]: Incubation with Pi reduces MgADP inhibition [9]: and so on. It is also known that nucleotide binding to the noncatalytic nucleotide binding sites on the α subunits facilitate escape from MgADP inhibition [10]. Thus, in the ATP hydrolysis reaction, initial high activity decreases with time due to the MgADP inhibition. Then F1 reaches equilibrium between active and MgADP inhibited states, resulting in lower steady-state activity compared to the initial one [5], [11].
Our recent study revealed that the ATPase activity of F1-ATPase from Bacillus subtilis (BF1) is highly suppressed by the MgADP inhibition [12]. The initial ATPase activity, which is not inhibited by the MgADP inhibition, falls down rapidly to several percent in the steady state [12]. That is very large inactivation compared to other F1-ATPases because they only fall into half, one third or so [5], [11]. LDAO activates BF1 more than a hundredfold [12] and this activation is also very large compared to those of other F1-ATPases (only several fold) [8]. Due in part to the strong MgADP inhibition, BF1 has a strange ATP concentration dependency of steady-state ATPase activity, the ATPase activity at 20∼100 µM ATP is lower than those at 1∼10 µM or 200∼5000 µM [12]. Interestingly, the ε subunit does not inhibit but activates BF1 by releasing MgADP inhibition [12]. In bacterial ATP synthases, the relationship between these two inhibitions must be very important to gain proper regulation fit for the physiological demand. Thus, studying such a characteristic behavior of BF1 will help us to understand how the regulation of ATP synthase varies depending on the environment where the source organisms live.
Studies with F1-ATPases from other species showed that the ATP binding to the noncatalytic site promotes release of inhibitory MgADP from catalytic sites and results in the substantial activation [10], [13]. A mutant F1-ATPase from thermophilic Bacillus PS3 (TF1) that cannot bind nucleotide to the noncatalytic site showed large initial inactivation that reached to essentially no steady-state activity [13]. In eubacterial V-type ATPases, which is thought to have the same origin as F1-ATPases, the noncatalytic B subunit does not bind nucleotide and V1-ATPase from Thermus thermophilus HB8 showed strong MgADP inhibition and no steady-state activity [14]. Inspired by these observations, we hypothesized that strong MgADP inhibition of BF1 is due to the inability of noncatalytic sites to bind nucleotide. To examine this hypothesis, we prepared a mutant α3β3γ complex of BF1 in which nucleotide binding to the noncatalytic nucleotide binding sites can be monitored by the changes in the fluorescence from the tryptophan residues introduced near the noncatalytic sites. The result indicated that the noncatalytic sites of BF1 could bind ATP. Thus, the cause of strong MgADP inhibition of BF1 is not the weak binding ability of the noncatalytic sites but other steps required for the recovery from the MgADP inhibition. However, the mutant α3β3γ complex of BF1 that cannot bind nucleotide to the noncatalytic sites showed lowered ATPase activity, indicating that the nucleotide binding to the noncatalytic sites has a substantial role for recovery from MgADP inhibition in BF1.
Materials and Methods
Plasmid construction and protein preparation
The mutation (αR354W), which corresponded to the same mutant of Escherichia coli F1-ATPase (EF1) [15], was introduced by overlap extension PCR method [16] with KOD-plus DNA polymerase (Toyobo) and following primers by using the expression plasmid for the wild type (WT) α3β3γ complex of BF1, pET21-BF1 [12] as a template. Mutagenic primers were 5′-CTCAGGCGTATGGCCAGCGATCAATGCCGG-3′ and 5′-TTGATCGCTGGCCATACGCCTGAGAAGAAC-3′ and the franking primers were 5′-GCCGTATTGTAAACCCGCTAGGCCAG-3′ and 5′-TCTTGTGTGATGGCTGCTTGGCGAG-3′. The resulting 2.2 kbp DNA fragment was introduced into the EcoRV site of pZero2.1 vector (Novagen). Then the 0.8 kbp DNA fragment containing mutation was excised out by cutting this plasmid with NotI and NcoI. The fragment was put back to the original site of WT pET21-BF1 by ligating with NcoI/BamHI fragment (1.2 kbp) and NotI/BamHI fragment (7.3 kbp) of WT pET21-BF1. The resulting plasmid, pET21-BF1(αR354W) was used for protein expression. The mutations (αK175A/T176A), which is known to suppress nucleotide binding to the noncatalytic site [13], [17], were introduced in addition to αR354W by overlap extension PCR method with following primers by using pET21-BF1(αR354W) as a template. Mutagenic primers were 5′-CCGTCAAACAGGTGCAGCATCTGTTG-3′ and 5′- ATCGCAACAGATGCTGCACCTGTTTG-3′ and the franking primers were 5′-GAAATTAATACGACTCACTATAGG-3′ and 5′- GATAAGCACTCCGTAAAACCGAACTG-3′. The resulting 2.0 kbp DNA fragment was introduced into the EcoRV site of pZero2.1 vector (Novagen). Then the 1.6 kbp DNA fragment containing mutations was excised out by cutting this plasmid with XbaI and NcoI. The fragment was put back to the original site of pET21-BF1(αR354W) by ligating with NcoI/BamHI fragment (1.3 kbp) and XbaI/BamHI fragment (6.4 kbp) of pET21-BF1(αR354W). The resulting plasmid, pET21-BF1(αK175A/T176A/R354W) was used for protein expression. Mutations were confirmed by DNA sequencing. WT (α3β3γWT), αR354W mutant (α3β3γRW), and αK175A/T176A/R354W mutant (α3β3γΔNC) α3β3γ complexes of BF1 were prepared as described previously [12].
Fluorescence measurement
The assay mixture consisted of 50 mM Tris-H2SO4 (pH 7.5), 50 mM K2SO4 and 2 mM MgSO4 was transferred to a quartz cuvette (1.5 ml). The cuvette was placed in a fluorescence spectrophotometer, FP-6500 (JASCO, Tokyo) and the temperature was controlled to 25°C. The α3β3γ complex of BF1 was added to 100 nM. The concentrated ATP-Mg solution (a mixture of equal molar ATP and MgSO4) was injected into the cuvette at the time indicated and the changes in the fluorescence were measured every 0.5 s or 1 s until the fluorescence reached a plateau. Excitation and emission wavelengths were set at 300 nm and 350 nm, respectively. Excitation and emission slit widths were 5 and 10 nm, respectively. The solution was stirred continuously during the measurement. Emission spectra were measured before and after the time-course measurement at a rate 50 nm/min.
Fluorescence data analysis
The time course of the fluorescence was corrected for baseline with buffer. The fluorescence change at a plateau was plotted against the ATP concentration and fitted with the simple binding equation or the Hill equation by the computer software (Origin 9.0 J, Microcal Co.). The sum of two simple binding equations did not improve fitting (data not shown).
ATPase assay
ATPase activity was measured by NADH-coupled ATP-regenerating system at 25°C as described previously [12]. Reaction rates were determined at 3–5 s (initial) and 12–13 min (steady state) after the start of the reaction. The reaction rate in the presence of 0.1% LDAO was determined 100–150 s after the addition of LDAO.
Other methods
Protein concentration was determined by the method of Bradford [18] using bovine serum albumin as a standard. Chemicals were of the highest grades available.
Results and Discussion
Tryptophan fluorescence of α3β3γRW was completely quenched by the addition of ATP
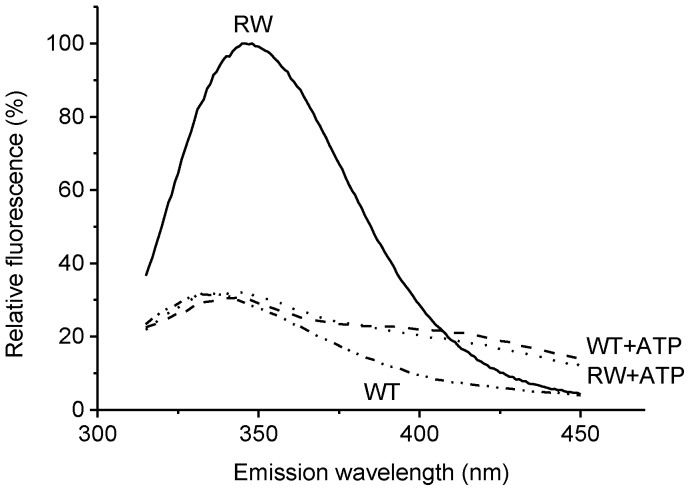
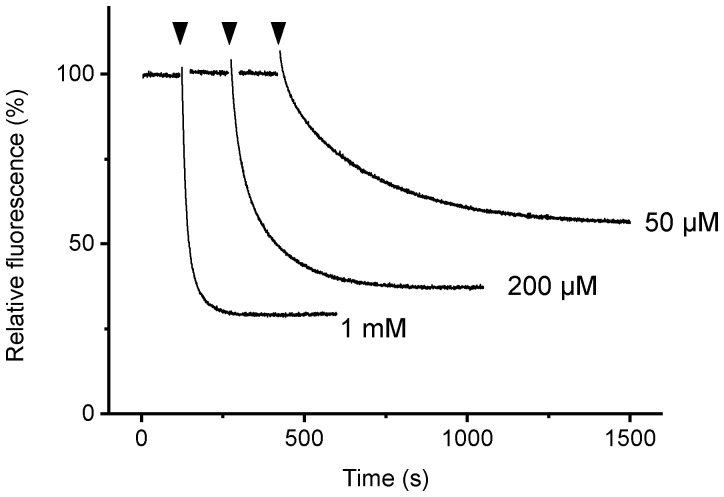
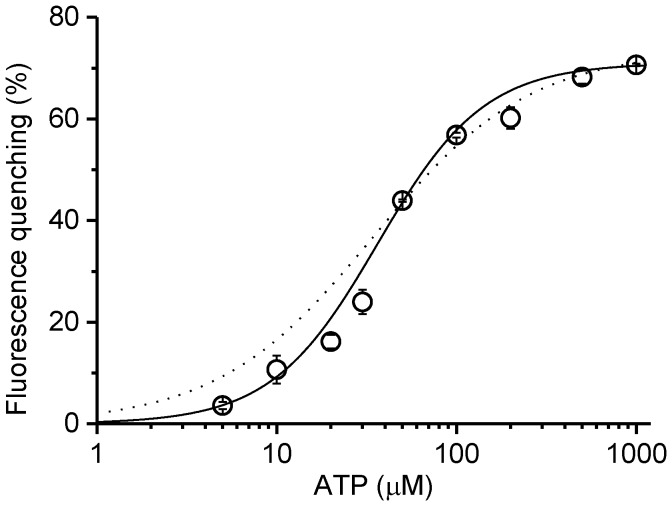
The mutant α3β3γRW showed large fluorescence compared to the WT (Fig. 1). Addition of ATP resulted in the quenching of fluorescence to the same level as the WT background, indicating that the fluorescence from tryptophan introduced near the noncatalytic sites was completely quenched by the addition of ATP. Thus, as reported on EF1 [15], tryptophan fluorescence could be used as the indicator of nucleotide binding to the noncatalytic sites of the α3β3γRW complex. The time course of fluorescence showed the ATP-concentration dependent rate and magnitude of fluorescence quenching (Fig. 2). There was a small jump in the fluorescence upon addition of ATP but this was not considered in the calculation of the degree of quenching. The titration with ATP showed an apparent K d = 34.4 µM with simple binding equation and an apparent K d = 36.5 µM and n = 1.47 with Hill equation (Fig. 3). These values were in the same range as that reported on EF1 (K d of the noncatalytic site for MgATP is 25 µM) [15]. It should be noted that the part of ATP will be hydrolyzed into ADP and Pi and the noncatalytic sites will be filled with some combination of ATP and ADP depending on initial ATP concentration since the fluorescence measurement did not include pyruvate kinase, an ATP-regenerating enzyme. Nevertheless, according to the results of ATPase measurement (Fig. 4), only a few percent of ATP was hydrolyzed before fluorescence reached the plateau (∼100 s) at high concentrations of ATP such as 1 mM.
Figure 1. Emission spectra of α3β3γWT and α3β3γRW complexes of BF1.
Fluorescence emission spectra of α3β3γWT and α3β3γRW complexes in the absence and presence of 1 mM ATP are shown. Excitation wavelength was 300 nm and the fluorescence emission spectra were recorded at 50 nm/min. Excitation and emission slit-widths were set at 5 and 10 nm, respectively. The fluorescence values are normalized to peak of α3β3γRW in the absence of ATP as 100%. Solid line, dotted line, two-dot-chain line and dashed line represent α3β3γRW-ATP, α3β3γRW+ATP, α3β3γWT-ATP and α3β3γWT+ATP, respectively.
Figure 2. Time course of fluorescence change of α3β3γRW upon addition of ATP.
MgATP was added at the times indicated by the arrowheads. Final ATP concentration in each measurement is shown on the right. Fluorescence is normalized to that before the addition of ATP.
Figure 3. Titration of fluorescence by ATP.
Changes in the fluorescence upon addition of ATP are expressed as percent of the fluorescence before the addition of ATP. Values are taken when the fluorescence reached a plateau. Error bars represent standard errors. The solid line represents the theoretical curve with the Hill equation (Fluorescence quenching = ΔFL max×[ATP]n/(Kd n+[ATP]n)) with the following parameters(± standard error); Kd = 36.5±1.2 µM, ΔFL max = 71.0±0.7%, n = 1.47±0.1. The dotted line represents the theoretical curve with simple binding equation (Fluorescence quenching = ΔFL max×[ATP]/(Kd+[ATP])) with K d = 34.4±2.9 µM, ΔFL max = 73.5±1.5%.
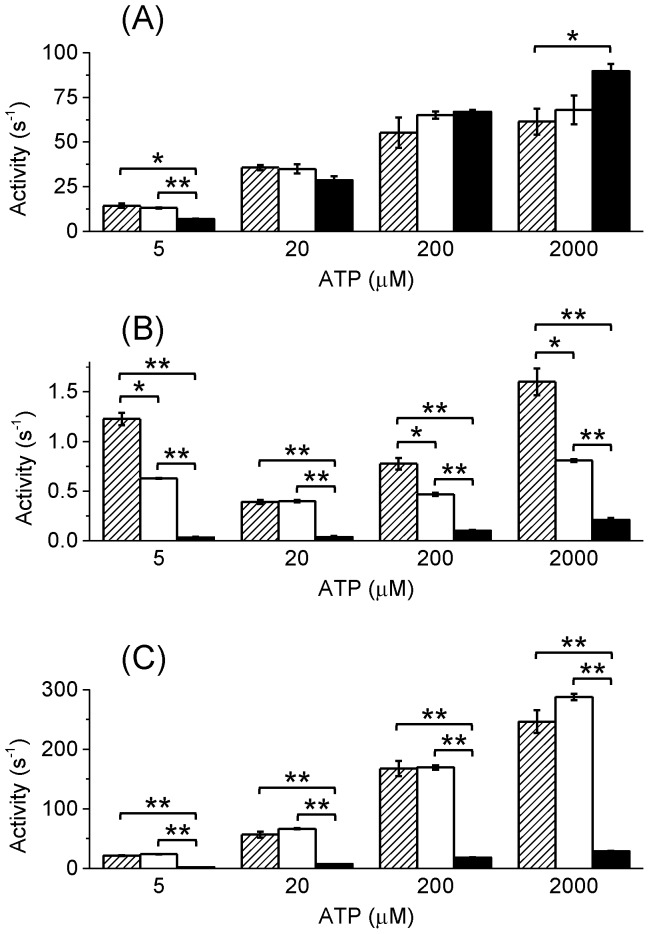
Figure 4. ATPase activities of α3β3γ complexes.
The initial (A) and steady-state (B) ATPase activities and ATPase activity in the presence of 0.1% LDAO (C) were determined. Hatched, open and solid bars represent α3β3γWT, α3β3γRW and α3β3γΔNC, respectively. Error bars represent standard errors. Asterisks represent statistically significant differences (*p<0.05 and **p<0.01, Student's t-test). Data for WT are taken from [12].
α3β3γRW Was inhibited severely even the noncatalytic sites were filled
Except for the lower steady-state activity, the mutant α3β3γRW showed similar ATPase properties to the α3β3γWT (Fig. 4); very high initial activity and strong inactivation to approximately 1% of the initial activity, and activation by LDAO more than 300-fold at 2 mM ATP for example. The lower steady-state activity may be due to the altered affinity or specificity of noncatalytic site by the mutation, although the fluorescence measurements (Fig. 3) indicate that ATP should fill noncatalytic sites in the steady-state (12–13 min after the start of the reaction) ATPase measurement at >200 µM ATP. Thus, BF1 was strongly inhibited by the MgADP inhibition even when the noncatalytic sites were filled with ATP. Although ATP and ADP could affect differently on releasing MgADP inhibition as reported on chloroplast F1 [19], [20], noncatalytic sites of BF1 must be filled with ATP during the ATPase measurement because our ATPase measurement contained ATP-regenerating system.
α3β3γΔNC Showed even lower ATPase activity
Since there are no obvious correlation between rate of very rapid inactivation [12] and that of nucleotide binding to the noncatalytic sites (Fig. 2), it was unclear that whether the nucleotide binding to the noncatalytic sites could facilitate release of inhibitory MgADP or not. To clarify this, we prepared the mutant α3β3γ complex of BF1 that contained mutations in Walker A motif (α3β3γΔNC) to test if the nucleotide binding to the noncatalytic sites of BF1 promotes recovery from MgADP inhibition, even if weak. With the α3β3γΔNC, no fluorescence quenching upon addition of ATP was observed (Fig. S1), indicating that the mutation totally abolished nucleotide binding to the noncatalytic sites as reported on TF1 [13]. The initial ATPase activity of α3β3γΔNC complex was essentially the same level as the WT (Fig. 4A). However, the steady-state ATPase activity was much lower (2.7∼13% of WT, Fig. 4B). Even in the presence of LDAO, the activity was very low compared to the WT (10∼13% of WT, Fig. 4C). These properties of the α3β3γΔNC complex were similar to those reported on the same mutant of TF1 [13], [21], suggesting that the noncatalytic site of BF1 also has the substantial role to facilitate the release of inhibitory MgADP from the catalytic sites even if low efficiency.
Conclusions
The noncatalytic nucleotide binding sites of BF1 can bind nucleotides by the affinity similar to other F1-ATPases. From the result, there rose the possibility that the nucleotide binding to noncatalytic site of BF1 does not affect release of MgADP inhibition. However, this was not the case because the α3β3γΔNC mutant had even lower steady-state activity than the WT or the RW mutant. Thus, the most part of strong MgADP inhibition of BF1 is due to the different factors. Inefficient transmission of the conformational changes from the noncatalytic sites to the catalytic sites induced by the binding of ATP to the noncatalytic sites [22] or the intrinsic propensity of catalytic β subunit, for example, are the candidates. Following results presented here, we are carrying out the experiments that may determine the part responsible for the strong MgADP inhibition of BF1 from the different point of view. Although the MgADP inhibition is common to all ATP synthases, the degree of that varied considerably. There have been no report about such strong MgADP inhibition on other F1-ATPases. To study what determines the degree of the MgADP inhibition may help us to understand the whole picture of the physiological regulation of ATP synthases from various species living in the various environment.
Supporting Information
Emission spectra of α3β3γΔNC of BF1. Fluorescence emission spectra of α3β3γΔNC in the absence and presence of 1 mM ATP were measured as Fig. 1. Solid line and dotted line represent in the absence and presence of ATP, respectively. The fluorescence values are normalized to peak in the absence of ATP as 100%.
(TIF)
Acknowledgments
We thank Dr. Takamitsu Haruyama (Kanazawa Univ.) and members of Kato-Yamada's laboratory for their help and fruitful discussion.
Funding Statement
This work was supported in parts by the Grant-in-Aid for Young Scientists (B) (No. 23770157) from the Japanese Society for the Promotion of Science, the Strategic Research Foundation Grant-aided Project for Private Universities (No. S1201003) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (www.mext.go.jp) (to Y. K.-Y.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Boyer PD (1997) The ATP synthase—a splendid molecular machine. Annu Rev Biochem 66: 717–749. [DOI] [PubMed] [Google Scholar]
- 2. Yoshida M, Muneyuki E, Hisabori T (2001) ATP synthase—a marvellous rotary engine of the cell. Nat Rev Mol Cell Biol 2: 669–677. [DOI] [PubMed] [Google Scholar]
- 3. Senior AE, Nadanaciva S, Weber J (2002) The molecular mechanism of ATP synthesis by F1Fo-ATP synthase. Biochim Biophys Acta 1553: 188–211. [DOI] [PubMed] [Google Scholar]
- 4. Noji H, Yasuda R, Yoshida M, Kinosita K Jr (1997) Direct observation of the rotation of F1-ATPase. Nature 386: 299–302. [DOI] [PubMed] [Google Scholar]
- 5. Vasilyeva EA, Minkov IB, FitinAF, Vinogradov AD (1982) Kinetic mechanism of mitochondrial adenosine triphosphatase. ADP-specific inhibition as revealed by the steady-state kinetics. Biochem J 202: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vasilyeva EA, Minkov IB, Fitin AF, Vinogradov AD (1982) Kinetic mechanism of mitochondrial adenosine triphosphatase. Inhibition by azide and activation by sulphite. Biochem J 202: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou J-M, Xue Z, Du Z, Melese T, Boyer PD (1988) Relationship of tightly bound ADP and ATP to control and catalysis by chloroplast ATP synthase. Biochemistry 27: 5129–5135. [DOI] [PubMed] [Google Scholar]
- 8. Jault J-M, Dou C, Grodsky NB, Matsui T, Yoshida M, et al. (1996) The subcomplex of the F1-ATPase from the thermophilic Bacillus PS3 with the βT165S substitution does not entrap inhibitory MgADP in a catalytic site during turnover. J Biol Chem 271: 28818–28824. [DOI] [PubMed] [Google Scholar]
- 9. Drobinskaya IY, Kozlov IA, Murataliev MB, Vulfson EN (1985) Tightly bound adenosine diphosphate, which inhibits the activity of mitochondrial F1-ATPase, is located at the catalytic site of the enzyme. FEBS lett 182: 419–424. [DOI] [PubMed] [Google Scholar]
- 10. Jault J-M, Allison WS (1993) Slow binding of ATP to noncatalytic nucleotide binding sites which accelerates catalysis is responsible for apparent negative cooperativity exhibited by the bovine mitochondrial F1-ATPase. J Biol Chem 268: 1558–1566. [PubMed] [Google Scholar]
- 11. Hirono-Hara Y, Noji H, Nishiura M, Muneyuki E, Hara KY, et al. (2001) Pause and rotation of F1-ATPase during catalysis. Proc Natl Acad Sci U S A 98: 13649–13654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mizumoto J, Kikuchi Y, Nakanishi YH, Mouri N, Cai A, et al. (2013) ε Subunit of Bacillus subtilis F1-ATPase relieves MgADP inhibition. PLoS ONE 8: e73888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matsui T, Muneyuki E, Honda M, Allison WS, Dou C, et al. (1997) Catalytic activity of the α3β3γ complex of F1-ATPase without noncatalytic nucleotide binding site. J Biol Chem 272: 8215–8221. [DOI] [PubMed] [Google Scholar]
- 14. Yokoyama K, Muneyuki E, Amano T, Mizutani S, Yoshida M, et al. (1998) V-ATPase of Thermus thermophilus is inactivated during ATP hydrolysis but can synthesize ATP. J Biol Chem 273: 20504–20510. [DOI] [PubMed] [Google Scholar]
- 15. Weber J, Wilke-Mounts S, Grell E, Senior AE (1994) Tryptophan fluorescence provides direct probe of nucleotide binding in the noncatalytic sites of Escherichia coli F1-ATPase. J Biol Chem 269: 11261–11268. [PubMed] [Google Scholar]
- 16. Higuchi R, Krummel B, Saiki RK (1988) A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res 16: 7351–7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ono S, Hara KY, Hirao J, Matsui T, Noji H, et al. (2003) Origin of apparent negative cooperativity of F1-ATPase. Biochim Biophys Acta 1607: 35–44. [DOI] [PubMed] [Google Scholar]
- 18. Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 19. Milgrom YM, Ehler LL, Boyer PD (1990) ATP binding at noncatalytic sites of soluble chloroplast F1-ATPase is required for expression of the enzyme activity. J Biol Chem 265: 18725–18728. [PubMed] [Google Scholar]
- 20. Milgrom YM, Ehler LL, Boyer PD (1991) The characteristics and effect on catalysis of nucleotide binding to noncatalytic sites of chloroplast F1-ATPase. J Biol Chem 266: 11551–11558. [PubMed] [Google Scholar]
- 21. Amano T, Matsui T, Muneyuki E, Noji H, Hara K, et al. (1999) α3β3γ Complex of F1-ATPase from thermophilic Bacillus PS3 can maintain steady-state ATP hydrolysis activity depending on the number of non-catalytic sites. Biochem J 343: 135–138. [PMC free article] [PubMed] [Google Scholar]
- 22. Jault J-M, Matsui T, Jault FM, Kaibara C, Muneyuki E, et al. (1995) α3β3γ Complex of the F1-ATPase from thermophilic Bacillus PS3 containing the αD261N substitution fails to dissociate inhibitory MgADP from a catalytic site when ATP binds to noncatalytic sites. Biochemistry 34: 16412–16418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Emission spectra of α3β3γΔNC of BF1. Fluorescence emission spectra of α3β3γΔNC in the absence and presence of 1 mM ATP were measured as Fig. 1. Solid line and dotted line represent in the absence and presence of ATP, respectively. The fluorescence values are normalized to peak in the absence of ATP as 100%.
(TIF)