Abstract
Biologics are being increasingly used for the treatment of many diseases. These treatments typically require repeated doses administered by injection. Alternate routes of administration, particularly oral, are considered favorable because of improved convenience and compliance by patients, but physiological barriers such as extreme pH, enzyme degradation, and poor intestinal epithelium permeability limit absorption. Encapsulating biologics in drug delivery systems such as polymeric nanoparticles (NPs) prevents inactivation and degradation caused by low pH and enzymes of the gastrointestinal (GI) tract. However, transport across the intestinal epithelium remains the most critical barrier to overcome for efficient oral delivery. This review focuses on recent advances in polymeric NPs being developed to overcome transport barriers and their potential for translation into clinical use.
Technology Primer
The revolution in biomedical research that has occurred over the last half-century has resulted in an unprecedented understanding of the biological processes associated with many diseases. With this knowledge, exciting new therapies are being developed that could have a significant impact on the treatment of numerous diseases. Many of these new therapies are biological in nature, such as peptide hormones, antibodies, growth factors, enzymes, vaccines, and nucleic acids, and are known as biologics1. However, the impact of biologics may be limited to a narrow set of indications because administration is currently restricted to parenteral methods. Many diseases that would benefit from biologics are chronic, requiring frequent treatments over a prolonged period of time.
Injection-based therapies can suffer from poor patient compliance and reduced efficacy due to the pain and inconvenience associated with treatment regimens2,3. Therefore, alternate routes of administration are under investigation. Of these routes, oral is considered the most desirable because of the convenience and improved compliance4.
Polymeric NPs are one technology that is currently being studied for oral delivery applications5,6. NPs are particles with a diameter on the order of 100 nanometers (nm), comparable in size to many viruses7. For drug delivery applications, most NPs are formed from biodegradable or biocompatible polymers that are approved for human use by the US Food and Drug Administration (FDA)8,9. Polymeric NPs are usually formed through a self-assembly process during which therapeutics such as small molecule drugs and biologics are encapsulated within the core of the NP10,11. The choice of polymer and self-assembly conditions provide considerable design flexibility by enabling modulation of physicochemical parameters (size, surface charge, hydrophobicity) and drug release properties (controlled or triggered)12. The surface properties of NPs can be modified by using different polymer end groups or attaching polymers to the NP surface13. Targeting ligands such as antibodies, peptides, or small molecules can also be attached to the NP surface to allow specific interactions with tissue components or cellular receptors14. Finally, because of their small size, NPs have large specific surface areas for interaction with epithelial surfaces.
Findings
Challenges of Oral Delivery
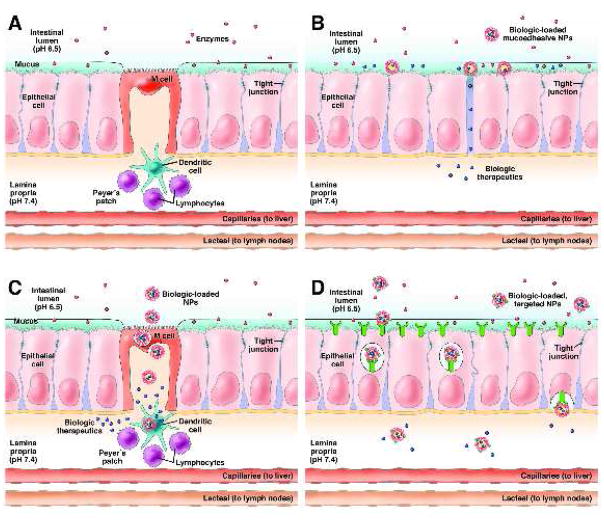
The challenges associated with oral delivery are due to the number of barriers that must be overcome in the GI tract before a therapeutic is absorbed and enters the bloodstream (Figure 1A). The first barrier is the harsh pH environment of the stomach and small intestine and enzymes secreted for digestion15. The pH environments in the GI tract can range from 1 in the stomach to 8 in parts of the intestine16, which can cause pH-induced oxidation, deamidation or hydrolysis of protein therapeutics, leading to loss of activity17. Enzymatic degradation is due to proteases, nucleases, and lipases secreted to digest biological molecules prior to absorption. GI proteases generally digest 94–98% of orally administered proteins18. From the lumen of the small intestine, a therapeutic must reach the mucosal barrier for absorption. The intestinal mucosal barrier can be divided into extrinsic and intrinsic barriers19. The extrinsic barrier consists of the microenvironment overlying the epithelial cells, in particular the mucus layer. The role of mucus is to protect epithelial surfaces by trapping pathogens and foreign particulates and rapidly clearing them20,21. Penetration of this mucus barrier is necessary in order to reach the absorptive epithelial cells of the intestine. The intrinsic barrier consists of the epithelial cell monolayer, representing the major restrictive barrier to passive diffusion of material from the lumen to the lamina propria. The cells maintain this barrier through the formation of tight junctions, which are fusions between lateral membranes of adjacent cells that regulate the permeability of the monolayer22.
Figure 1.
(A) Schematic illustration of the barriers to oral biologic delivery presented by the GI tract, including acidic pH, enzymatic degradation, a mucus layer above the epithelial cells, the epithelial monolayer with limited permeability due to tight junctions, and immune cells associated with the M cells. (B) Mucoadhesive materials adhere to the mucus layer and release drug at high concentrations near the epithelial cells as well as reversibly open tight junctions to allow for biologic transport across the epithelium through the paracellular pathway. (C) The M cell transcytosis pathway is an approach used to enable NPs to cross the epithelium, but underlying dendritic cells associated with the Peyer’s Patches may endocytose NPs in the lamina propria. (D) Receptor-mediated transcytosis pathways such as the vitamin B12 receptor and FcRn can be targeted by NPs to enable transport across the epithelium and release into the lamina propria.
There are several pathways used by molecules to cross the epithelial cell barrier, which include transcellular (transport through the cell, with crossing of the cell membranes), paracellular (transport between adjacent cells), and transcytosis transport pathways15. Because of the size and charge of biologics, most are restricted to the transcytosis pathway, which is an active transport pathway that relies on receptors to bind and guide material in endosomes through the cell without entering a degradation pathway23. Once past the mucosal barrier, the biologics must diffuse through the lamina propria and enter the bloodstream through the capillaries or the lacteals. Biologics entering the capillaries are transported through blood vessels from the intestines through the portal system where they are also susceptible to first-pass metabolism.
Polymeric NPs encapsulating biologic molecules are able to overcome several of the barriers to oral delivery. They are stable in the GI environment and can protect encapsulated biologics from the harsh pH and enzyme degradation24–26. Modification of the NP surface with poly(ethylene glycol) enables the NPs to penetrate the mucus barrier20. However, the remaining hurdle is that intestinal absorption of NPs is highly inefficient because NP properties such as particle size prevent effective transport across the intestinal epithelium. Several strategies are under investigation to overcome this intestinal transport barrier in order to make oral biologics delivery feasible.
Mucoadhesives
Mucoadhesives are materials designed to interact with the intestinal mucus layer in order to increase the residence time and contact of NPs with the epithelium, resulting in an increase in concentration of biologics at the absorption site27 (Figure 1B). Some examples of mucoadhesives include chitosan28, polyacrylic acid29, and poly(fumaric-co-sebacic) anhydride30. Many mucoadhesive materials also act as permeation enhancers, reversibly opening tight junctions to enhance paracellular transport31. While the NPs are unable to pass through the paracellular pathway because of particle size, many biologics are able to cross through this pathway32. One disadvantage of this approach is that the permeation enhancer activity is non-selective, meaning that toxins and other pathogens present in the intestines could potential cross the intestinal barrier once the tight junctions are open33.
M Cells
Other approaches to oral delivery have focused on taking advantage of natural transcytosis pathways present in the GI tract. One pathway that has been the focus of many studies is the transcytosis pathway used by M cells to transport antigens across the intestinal barrier for immune surveillance34,35 (Figure 1C). M cells are associated with Peyer’s Patches (PP), an organized component of the gut-associated lymphoid tissue (GALT). M cells have several properties that allow for adherence by NPs, such as reduced proteases, lack of mucus secretion, and a sparse glycocalyx36. A number of approaches have been used to target NPs to M cells. Studies have shown that larger NPs37,38 and more hydrophobic polymers39 improve M cell transport. In addition, targeting ligands conjugated to the NP surface, such as bacterial adhesins40, IgA antibodies41, and toxins42, increase M cell transport. One drawback to this approach is that the M cells are closely associated with immune cells in the lamina propria as part of the PP. Studies with glucan NPs showed that a majority of NPs were endocytosed by dendritic cells after M cell transcytosis, preventing them from reaching the bloodstream43. Besides limiting the effectiveness of the delivery system, uptake by dendritic cells increases the risk that the NPs or biologics induce an immune response44. This may limit the use of this approach for long-term oral delivery, but could enable this approach to be used for oral mucosal vaccinations45.
Vitamin B12 Receptor
More recent approaches have focused on receptor-mediated transcytosis pathways that are not associated with the GALT (Figure 1D). One example is the vitamin B12 receptor. When vitamin B12 is ingested, it is bound by intrinsic factor (IF) in the small intestine and the vitamin B12-IF complex interacts with an IF receptor that traffics vitamin B12 across the intestinal epithelium and allows it to reach the bloodstream46. NPs have been designed to target this pathway by conjugating vitamin B12 to the surface of biologic-loaded NPs. Studies with insulin-loaded NPs targeted to the vitamin B12 receptor have demonstrated enhanced absorption of insulin after oral administration47–49.
Neonatal Fc Receptor
Another receptor targeted by NPs is the neonatal Fc receptor (FcRn), which mediates IgG transport across the intestinal epithelium50,51. FcRn is present in the apical region of epithelial cells in the small intestine and colon52. FcRn interacts with the Fc portion of IgG in a pH-dependent manner, binding with high affinity in acidic (pH <6.5) but not physiological environments (pH ~7.4)53. Therefore, in segments of the intestine that are acidic such as the duodenum and jejunum54, targeted NPs can interact with FcRn at the apical surface of the epithelial cells and be taken up by receptor-mediated endocystosis. In segments of the intestine that are not acidic, the targeted NPs can still be taken into the cells by fluid-phase pinocytosis and interact with FcRn once inside acidic endosomes55. Targeted NPs bound to FcRn in the endosomes are trafficked across the epithelial cells through the FcRn transcytosis pathway and released from the receptor on the basolateral side of the cells at neutral pH. NPs loaded with insulin and surface-modified with IgG Fc demonstrated successful transcytosis across the epithelium and entry into the bloodstream, where the NPs were able to reach the liver, spleen, lungs, and kidneys56. The NPs were able to generate a hypoglycemic response for 15 hr with a clinically-relevant dose of only 1.1 U/kg57, lower than other oral insulin delivery systems that require 10–100 U/kg to generate a glucose response15.
Importance
Oral administration is considered a desirable alternative to intravenous administration because of the convenience and improved compliance by patients2,3,58. Oral formulations also have advantages for physicians, providing flexibility and adaptability to tune the dosing schedule to individual patients responses based on efficacy and toxicity. Without the intensive demands on staff required by intravenous administrations, oral formulations can also increase the number of patients treated59 and reductions in the number of hospital or clinic visits could reduce overall costs60–63.
Translation
Polymeric NPs are currently being studied for administration of a variety of therapeutics, including chemotherapeutics for cancer64,65, siRNA and small molecule drugs for inflammatory bowel disease66–68, and insulin for diabetes47,56. While these technologies are not yet in clinical trials, they are improving in efficiency to the point of becoming clinically relevant. Recent approaches focused on using natural transcytosis pathways to cross the intestinal barrier also avoid some of the potential safety issues associated with manipulating the integrity of the intestinal barrier through permeation enhancement. Polymeric NP-based therapeutics for other applications, such as treatments for cancer administered intravenously, are currently in clinical trials or have been approved9,69,70. This demonstrates that it is possible for polymeric NP technologies to be translated to the clinic and achieve approval by the FDA.
Roadblocks and/or Limitations
There are still challenges that must be overcome before these technologies are used in the clinic. Improved understanding of the differences in the GI tract between animal models and humans would ensure more reliable translation of performance from preclinical to clinical studies. Also, a better understanding of how patient-to-patient variability, diet, fasting states, and disease states affect absorption would help with clinical translation and prediction of performance. Finally, the effects of long-term administration of some therapeutics may need further investigation as some therapeutics can act as growth factors and induce changes in the epithelium, while other therapeutics may be toxic to the intestines71.
Conclusions
Oral delivery technologies have the potential to have a significant impact on the treatment of many diseases by enabling therapeutics currently limited to parenteral administration to be administered through the oral route. There are currently several different polymeric NP approaches being studied to overcome the barriers of the intestine. Recent studies have demonstrated that polymeric NPs can efficiently deliver therapeutics orally, but further studies are required before these technologies can be translated to the clinic.
Acknowledgments
Funding: Supported in part by a Koch-Prostate Cancer Foundation Award in Nanotherapeutics (R.L. and O.C.F.), the NCI Center of Cancer Nanotechnology Excellence at MIT-Harvard (U54-CA151884), and an NIH R01 grant (EB015419-01).
Footnotes
Competing interests: O.C.F. has financial interests in BIND Therapeutics, Selecta Biosciences, and Blend Therapeutics, which are developing NP therapeutics. O.C.F., F.A., and E.M.P are inventors on patent applications related to the FcRn technology. BIND and Selecta have an exclusive license from Brigham and Women’s Hospital (BWH) and MIT for this technology.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aggarwal S. What’s fueling the biotech engine-2012 to 2013. Nat Biotechnol. 2014;32(1):32–9. doi: 10.1038/nbt.2794. [DOI] [PubMed] [Google Scholar]
- 2.Liu G, Franssen E, Fitch MI, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15(1):110–5. doi: 10.1200/JCO.1997.15.1.110. [DOI] [PubMed] [Google Scholar]
- 3.Borner MM, Schoffski P, de Wit R, et al. Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fluorouracil and leucovorin: a randomised crossover trial in advanced colorectal cancer. Eur J Cancer Oxf Engl 1990. 2002;38(3):349–58. doi: 10.1016/s0959-8049(01)00371-9. [DOI] [PubMed] [Google Scholar]
- 4.Peppas NA, Kavimandan NJ. Nanoscale analysis of protein and peptide absorption: insulin absorption using complexation and pH-sensitive hydrogels as delivery vehicles. Eur J Pharm Sci Off J Eur Fed Pharm Sci. 2006;29(3–4):183–97. doi: 10.1016/j.ejps.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Mo R, Jiang T, Di J, Tai W, Gu Z. Emerging micro- and nanotechnology based synthetic approaches for insulin delivery. Chem Soc Rev. 2014;43(10):3595. doi: 10.1039/c3cs60436e. [DOI] [PubMed] [Google Scholar]
- 6.Galindo-Rodriguez S, Allemann E, Fessi H, Doelker E. Polymeric nanoparticles for oral delivery of drugs and vaccines: a critical evaluation of in vivo studies. Crit Rev Ther Drug Carr Syst. 2005;22(5):419–64. doi: 10.1615/critrevtherdrugcarriersyst.v22.i5.10. [DOI] [PubMed] [Google Scholar]
- 7.Pridgen E, Langer R, Farokhzad O. Biodegradable, polymeric nanoparticle delivery systems for cancer therapy. Nanomedicine Lond. 2007;2(5):669–80. doi: 10.2217/17435889.2.5.669. [DOI] [PubMed] [Google Scholar]
- 8.Mansour HM, Rhee Y-S, Wu X. Nanomedicine in pulmonary delivery. Int J Nanomed. 2009;4:299–319. doi: 10.2147/ijn.s4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hrkach J, Von Hoff D, Ali MM, et al. Preclinical Development and Clinical Translation of a PSMA-Targeted Docetaxel Nanoparticle with a Differentiated Pharmacological Profile. Sci Transl Med. 2012;4(128):128ra39–128ra39. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 10.Davis ME, Chen Z (Georgia), Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–82. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 11.Bilati U, Allémann E, Doelker E. Nanoprecipitation versus emulsion-based techniques for the encapsulation of proteins into biodegradable nanoparticles and process-related stability issues. AAPS PharmSciTech. 2005;6(4):E594–E604. doi: 10.1208/pt060474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41(7):2971. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valencia PM, Pridgen EM, Rhee M, Langer R, Farokhzad OC, Karnik R. Microfluidic Platform for Combinatorial Synthesis and Optimization of Targeted Nanoparticles for Cancer Therapy. ACS Nano. 2013;7(12):10671–80. doi: 10.1021/nn403370e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu MK, Park J, Jon S. Targeting Strategies for Multifunctional Nanoparticles in Cancer Imaging and Therapy. Theranostics. 2012;2(1):3–44. doi: 10.7150/thno.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen M-C, Sonaje K, Chen K-J, Sung H-W. A review of the prospects for polymeric nanoparticle platforms in oral insulin delivery. Biomaterials. 2011;32(36):9826–38. doi: 10.1016/j.biomaterials.2011.08.087. [DOI] [PubMed] [Google Scholar]
- 16.Kararli T. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory-animals. Biopharm Drug Dispos. 1995;16:351–80. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- 17.Sood A, Panchagnula R. Peroral route: an opportunity for protein and peptide drug delivery. Chem Rev-Columb. 2001;101(11):3275–304. doi: 10.1021/cr000700m. [DOI] [PubMed] [Google Scholar]
- 18.Ganapathy V, Gupta N, Martindale R. Physiology of the gastrointestinal tract. 4. Burlington: Academic Press; 2006. Protein digestion and absorption. [Google Scholar]
- 19.Johnson L, Christensen J, Jackson M, Jacobson E, Walsh J. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1987. [Google Scholar]
- 20.Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64(6):557–70. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol-Gastrointest Liver Physiol. 2001;280(5):G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 22.Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol-Gastrointest Liver Physiol. 2000;279(2):G250–G254. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- 23.Tuma PL, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev. 2003;83(3):871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- 24.Carino, Mathiowitz Oral insulin delivery. Adv Drug Deliv Rev. 1999;35(2–3):249–57. doi: 10.1016/s0169-409x(98)00075-1. [DOI] [PubMed] [Google Scholar]
- 25.Lowe PJ, Temple CS. Calcitonin and insulin in isobutylcyanoacrylate nanocapsules — protection against proteases and effect on intestinal-absorption in rats. J Pharm Pharmacol. 1994;46:547–552. doi: 10.1111/j.2042-7158.1994.tb03854.x. [DOI] [PubMed] [Google Scholar]
- 26.Damge C, Vranckx H, Balschmidt P, Couvreur P. Poly (alkyl cyanoacrylate) nanospheres for oral administration of insulin. J Pharm Sci. 1997;86(12):1403–9. doi: 10.1021/js970124i. [DOI] [PubMed] [Google Scholar]
- 27.Smart J. The basics and underlying mechanisms of mucoadhesion. Adv Drug Deliv Rev. 2005;57(11):1556–68. doi: 10.1016/j.addr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Chen M-C, Mi F-L, Liao Z-X, et al. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv Drug Deliv Rev. 2013;65(6):865–79. doi: 10.1016/j.addr.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Makhlof A, Werle M, Tozuka Y, Takeuchi H. A mucoadhesive nanoparticulate system for the simultaneous delivery of macromolecules and permeation enhancers to the intestinal mucosa. J Controlled Release. 2011;149(1):81–8. doi: 10.1016/j.jconrel.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Furtado S, Abramson D, Burrill R, et al. Oral delivery of insulin loaded poly(fumaric-co-sebacic) anhydride microspheres. Int J Pharm. 2008;347(1–2):149–55. doi: 10.1016/j.ijpharm.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 31.Sonaje K, Lin Y-H, Juang J-H, Wey S-P, Chen C-T, Sung H-W. In vivo evaluation of safety and efficacy of self-assembled nanoparticles for oral insulin delivery. Biomaterials. 2009;30(12):2329–39. doi: 10.1016/j.biomaterials.2008.12.066. [DOI] [PubMed] [Google Scholar]
- 32.Sonaje K, Chuang E-Y, Lin K-J, et al. Opening of Epithelial Tight Junctions and Enhancement of Paracellular Permeation by Chitosan: Microscopic, Ultrastructural, and Computed-Tomographic Observations. Mol Pharm. 2012:120413125623001. doi: 10.1021/mp200572t. [DOI] [PubMed] [Google Scholar]
- 33.Yeh T-H, Hsu L-W, Tseng MT, et al. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials. 2011;32:6164–73. doi: 10.1016/j.biomaterials.2011.03.056. [DOI] [PubMed] [Google Scholar]
- 34.Bakhru SH, Furtado S, Morello AP, Mathiowitz E. Oral delivery of proteins by biodegradable nanoparticles. Adv Drug Deliv Rev. 2013;65(6):811–21. doi: 10.1016/j.addr.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Macdonald T, Monteleone G. Immunity, Inflammation, and Allergy in the Gut. Science. 2005;307(5717):1920–5. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 36.Jang MH, Kweon M-N, Iwatani K, et al. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc Natl Acad Sci U S A. 2004;101(16):6110–5. doi: 10.1073/pnas.0400969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Des Rieux A, Fievez V, Théate I, Mast J, Préat V, Schneider Y-J. An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells. Eur J Pharm Sci. 2007;30(5):380–91. doi: 10.1016/j.ejps.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 38.Des Rieux A, Fievez V, Garinot M, Schneider Y-J, Préat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Controlled Release. 2006;116(1):1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 39.Eldridge JH, Hammond CJ, Meulbroek JA, Staas JK, Gilley RM, Tice TR. Controlled vaccine release in the gut-associated lymphoid tissues. I. Orally administered biodegradable microspheres target the Peyer’s patches. J Controlled Release. 1990;11(1):205–14. [Google Scholar]
- 40.Clark M, Jepson MA, Hirst BH. Exploiting M cells for drug and vaccine delivery. Adv Drug Deliv Rev. 2001;50(1):81–106. doi: 10.1016/s0169-409x(01)00149-1. [DOI] [PubMed] [Google Scholar]
- 41.Porta C, James PS, Phillips AD, Savidge TC, Smith MW, Cremaschi D. Confocal analysis of fluorescent bead uptake by mouse Peyer’s patch follicle-associated M cells. Exp Physiol. 1992;77(6):929–32. doi: 10.1113/expphysiol.1992.sp003662. [DOI] [PubMed] [Google Scholar]
- 42.Brayden DJ. Oral vaccination in man using antigens in particles: current status. Eur J Pharm Sci. 2001;14(3):183–9. doi: 10.1016/s0928-0987(01)00175-0. [DOI] [PubMed] [Google Scholar]
- 43.De Jesus M, Ostroff GR, Levitz SM, Bartling TR, Mantis NJ. A Population of Langerin-Positive Dendritic Cells in Murine Peyer’s Patches Involved in Sampling β-Glucan Microparticles. PLoS ONE. 2014;9(3):e91002. doi: 10.1371/journal.pone.0091002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khafagy E-S, Morishita M, Onuki Y, Takayama K. Current challenges in non-invasive insulin delivery systems: a comparative review. Adv Drug Deliv Rev. 2007;59(15):1521–46. doi: 10.1016/j.addr.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 45.Fievez V, Plapied L, des Rieux A, et al. Targeting nanoparticles to M cells with non-peptidic ligands for oral vaccination. Eur J Pharm Biopharm. 2009;73(1):16–24. doi: 10.1016/j.ejpb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Petrus AK, Fairchild TJ, Doyle RP. Traveling the Vitamin B12 Pathway: Oral Delivery of Protein and Peptide Drugs. Angew Chem Int Ed. 2009;48(6):1022–8. doi: 10.1002/anie.200800865. [DOI] [PubMed] [Google Scholar]
- 47.Chalasani KB, Russell-Jones GJ, Jain AK, Diwan PV, Jain SK. Effective oral delivery of insulin in animal models using vitamin B12-coated dextran nanoparticles. J Controlled Release. 2007;122(2):141–50. doi: 10.1016/j.jconrel.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 48.Chalasani KB, Russell-Jones GJ, Yandrapu SK, Diwan PV, Jain SK. A novel vitamin B12-nanosphere conjugate carrier system for peroral delivery of insulin. J Controlled Release. 2007;117(3):421–9. doi: 10.1016/j.jconrel.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Fowler R, Vllasaliu D, Trillo FF, et al. Nanoparticle Transport in Epithelial Cells: Pathway Switching Through Bioconjugation. Small. 2013 doi: 10.1002/smll.201202623. n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida M, Claypool SM, Wagner JS, et al. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20(6):769–83. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Dickinson BL, Badizadegan K, Wu Z, et al. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J Clin Invest. 1999;104(7):903–11. doi: 10.1172/JCI6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Israel EJ, Taylor S, Wu Z, et al. Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology. 1997;92(1):69–74. doi: 10.1046/j.1365-2567.1997.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raghavan M, Gastinel LN, Bjorkman PJ. The class I major histocompatibility complex related Fc receptor shows pH-dependent stability differences correlating with immunoglobulin binding and release. Biochemistry (Mosc) 1993;32(33):8654–60. doi: 10.1021/bi00084a037. [DOI] [PubMed] [Google Scholar]
- 54.Lalezari D. Gastrointestinal pH profile in subjects with irritable bowel syndrome. Ann Gastroenterol. 2012;25(4):333. [PMC free article] [PubMed] [Google Scholar]
- 55.Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2010;11(1):50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- 56.Pridgen EM, Alexis F, Kuo TT, et al. Transepithelial Transport of Fc-Targeted Nanoparticles by the Neonatal Fc Receptor for Oral Delivery. Sci Transl Med. 2013;5(213):213ra167–213ra167. doi: 10.1126/scitranslmed.3007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cochran E, Musso C, Gorden P. The use of U-500 in patients with extreme insulin resistance. Diabetes Care. 2005;28(5):1240–4. doi: 10.2337/diacare.28.5.1240. [DOI] [PubMed] [Google Scholar]
- 58.Von Pawel J, Gatzemeier U, Pujol JL, et al. Phase ii comparator study of oral versus intravenous topotecan in patients with chemosensitive small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2001;19(6):1743–9. doi: 10.1200/JCO.2001.19.6.1743. [DOI] [PubMed] [Google Scholar]
- 59.James R, Blanco C, Farina C. Savings in staff time as a result of switching from de Gramont to oral capecitabine for patients with advanced colorectal cancer. Eur J Cancer. 2003;1 (Suppl 5):S83. (Abstr 271) [Google Scholar]
- 60.Yabroff K, Warren J, Knopf K, Davis W, Brown M. Estimating Patient Time Costs Associated with Colorectal Cancer Care. Med Care. 2005;43:640–8. doi: 10.1097/01.mlr.0000167177.45020.4a. [DOI] [PubMed] [Google Scholar]
- 61.De Portu S, Mantovani LG, Ravaioli A, et al. Cost Analysis of Capecitabine vs 5-Fluorouracil-Based Treatment for Metastatic Colorectal Cancer Patients. J Chemother. 2010;22(2):125–8. doi: 10.1179/joc.2010.22.2.125. [DOI] [PubMed] [Google Scholar]
- 62.Jansman F, Postma M, van Hartskamp D, Willemse P, Brouwers J. Cost-benefit analysis of capecitabine versus 5-fluorouracil/leucovorin in the treatment of colorectal cancer in the Netherlands. Clin Ther. 2004;26:579–89. doi: 10.1016/s0149-2918(04)90060-4. [DOI] [PubMed] [Google Scholar]
- 63.Ward S, Kaltenthaler E, Cowan J, Marples M, Orr B, Seymour M. The clinical and economic benefits of capecitabine and tegafur with uracil in metastatic colorectal cancer. Br J Cancer. 2006;95(1):27–34. doi: 10.1038/sj.bjc.6603215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bisht S, Feldmann G, Koorstra J-BM, et al. In vivo characterization of a polymeric nanoparticle platform with potential oral drug delivery capabilities. Mol Cancer Ther. 2008;7(12):3878–88. doi: 10.1158/1535-7163.MCT-08-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Attili-Qadri S, Karra N, Nemirovski A, et al. Oral delivery system prolongs blood circulation of docetaxel nanocapsules via lymphatic absorption. Proc Natl Acad Sci. 2013;110(43):17498–503. doi: 10.1073/pnas.1313839110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lamprecht A. Nanoparticles Enhance Therapeutic Efficiency by Selectively Increased Local Drug Dose in Experimental Colitis in Rats. J Pharmacol Exp Ther. 2005;315(1):196–202. doi: 10.1124/jpet.105.088146. [DOI] [PubMed] [Google Scholar]
- 67.Wilson DS, Dalmasso G, Wang L, Sitaraman SV, Merlin D, Murthy N. Orally delivered thioketal nanoparticles loaded with TNF-α–siRNA target inflammation and inhibit gene expression in the intestines. Nat Mater. 2010;9(11):923–8. doi: 10.1038/nmat2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yin L, Song Z, Qu Q, et al. Supramolecular Self-Assembled Nanoparticles Mediate Oral Delivery of Therapeutic TNF-α siRNA against Systemic Inflammation. Angew Chem Int Ed. 2013;52(22):5757–61. doi: 10.1002/anie.201209991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davis ME, Zuckerman JE, Choi CHJ, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–70. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang AZ, Langer R, Farokhzad OC. Nanoparticle Delivery of Cancer Drugs. Annu Rev Med. 2012;63(1):185–98. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 71.Brogden RN, Heel RC. Human insulin. A review of its biological activity, pharmacokinetics and therapeutic use. Drugs. 1987;34:350–71. doi: 10.2165/00003495-198734030-00003. [DOI] [PubMed] [Google Scholar]