Abstract
Purpose
We explored whether AMH, as a surrogate for oocyte supply, varies by FMR1 genotype in women diagnosed with diminished ovarian reserve (DOR), a subset of the Primary Ovarian Insufficiency phenotype. Research is inconsistent on the relationship between AMH and FMR1 repeat length, controlling for age.
Method
Seventy-nine cycling women diagnosed with DOR, and without a family history of fragile X syndrome, provided blood for FMR1 and AMH testing. DOR was defined as elevated FSH and/or low AMH and/or low antral follicle count, with regular menses. FMR1 CGG repeats were stratified by the larger allele <35 repeats (n = 70) v. ≥35 repeats (n = 9). Quadratic and linear models were fit to predict log (AMH) controlling for age. The AMH sample used as the outcome variable was drawn at a later date than the diagnostic AMH.
Results
Serum AMH concentration median was 0.30 ng/mL; Ages ranged from 26–43 years. A quadratic model (including age2) did not show a relationship with FMR1 CGG level (p-value = 0.25). A linear model of log (AMH), corresponding to an exponential decline of AMH with increasing age, was significantly different, and had a steeper slope, for women with ≥ 35 CGG repeats than women with < 35 repeats (p = 0.035).
Conclusion
Findings suggest a greater rate of follicular loss that starts at later ages in women with DOR and ≥ 35 CGG repeats.
Keywords: FMR1, Primary ovarian insufficiency, Diminished ovarian reserve, Anti-mullerian hormone, Ovarian reserve, Female infertility, Statistical modeling
Introduction
Anti-mullerian hormome (AMH), also known as Mullerian-inhibiting substance, is a dimeric glycoprotein. It is produced by Sertoli cells of the testis in males and by ovarian granulosa cells in females. AMH is increasingly used by clinicians to measure ovarian reserve in pre-menopausal women and the volume of publications using AMH measurements has significantly increased. As reviewed by Nelson and La Marca [16], AMH has several unique characteristics: it appears that circulating AMH in females is produced solely by the ovarian granulosa cells from primary to small antral follicles (≤4-6 mm) [33,34]. In a sample of 42 ovaries obtained by oophorectomy, the age-adjusted correlation between serum AMH and ln (number of primordial follicles) was 0.48, supporting the view that AMH reflects, in part, the size of the oocyte pool [8]. The literature is inconsistent on the variability of AMH throughout a menstrual cycle [7,9], with 1 report indicating greater variability in younger women with higher AMH levels [27]. Controversy also exists as to whether AMH is influenced by current use of hormonal therapy [11,18,29].
While a reduction in oocyte quantity and quality with advanced age is a normal physiologic occurrence, some women experience declines in ovarian reserve before the mid-forties and thus become prematurely infertile. This is termed “diminished ovarian reserve” (DOR) [25], and is diagnosed in approximately 10 % of women seeking fertility assistance [15,23]. Women with DOR continue to have regular menstrual periods and their DOR diagnosis is generally a surprise as they believe they are fertile because they menstruate regularly [4].
A full mutation of the Fragile X Mental Retardation (FMR1) gene (over 200 FMR1 CGG repeats) results in silencing of the FMR1 protein and Fragile X Syndrome (FXS) in a majority of the males and a lesser proportion of females with this genetic mutation. Repeats in the range of 55–199 are termed “premutation”, repeats in the range of 45–54 are termed “gray zone” or “intermediate”, and fewer than 45 repeats are considered phenotypically normal. Research since 2000 has provided evidence that women with a premutation allele [12,26], and potentially women with a high normal [19,30] or gray zone [5,12] level repeat have an increased risk of primary ovarian insufficiency (specifically, a diagnosis of diminished ovarian reserve and/or premature ovarian failure).
Given that FMR1 is associated with early ovarian aging and AMH is a common measure of ovarian reserve, it is logical to investigate AMH levels by FMR1 CGG repeat. The scant literature (six publications) on this topic is inconsistent, with some papers reporting an inverse association of AMH and FMR1 CGG repeat length [6,21,28], no association [3,13], and a positive association [2]. Our purpose was to use mathematical modeling to explore whether AMH, as a surrogate for follicular loss, might vary by FMR1 genotype in women diagnosed with DOR, after adjustment for age.
Materials and methods
This was a cross-sectional analysis of a multi-center cohort study of infertile women diagnosed with DOR who were enrolled between March 2005 – February 2013. Seventy-nine women clinically diagnosed with DOR, and without a family history of fragile X syndrome, provided blood for FMR1 and AMH testing specifically for research purposes. As described previously [19], DOR was defined as elevated FSH and/or low AMH and/or low antral follicle count, with regular menses. Note that the outcome of interest was AMH measured at the time of study participation, and not the previous AMH measurement used for clinical assessment of ovarian reserve. This cohort was enrolled from several sites: academic Reproductive Endocrinology and Infertility clinics in California (54 %), and North Carolina (9 %), plus private fertility practices in Virginia (32 %, formerly an academic clinic) and North Carolina (5 %). This study was approved by the Human Ethics Boards at all academic sites (IRB #: 11448, 11–1535, 6208–16182).
Eligibility requirements included: diagnosis of DOR (cycle day 2–5 FSH > 10 mIU/mL, OR FSH > 12 mIU/mL after 5 days of 100 mg clomiphene citrate medication, OR fewer than 6 early follicular antral follicles sized 2–10 mm, OR low AMH for her age as detailed below), age at DOR diagnosis ≤ 42 years, and regular menstrual cycles for the past 6 months. The antral follicle count (AFC) was used for enrollment at only one site (Stanford University) where the volume of patients was quite high and, thus, interobserver variation would be minimized. The AMH criteria for study eligibility was
age ≤ 30 AMH ≤ 1.1 ng/mL
age 31–35 AMH ≤ 0.675 ng/mL
age 36–40 AMH ≤ 0.34 ng/mL
age 41–42 AMH ≤ 0.25 ng/mL, which was based on the report by Nelson [17].
The criteria for exclusion were: known cause of elevated FSH for one’s age unrelated to fragile X syndrome (e.g., surgical removal of either one or both ovaries, chemotherapy or radiation therapy, Turners Syndrome, autoimmune disease), or a family history of FXS or premutation. Knowing that FSH values can vary by assay [24,31], de-identified samples were run at each satellite site and the primary site (University of Virginia). Based on those results, the cycle day 2–5 FSH enrollment criteria was increased by 1 point (Immulite 2500 machine; bioMérieux Vidas machine) or decreased by 1.8 points (Ortho 5600 machine) to ensure consistency in the enrollment criteria across sites.
After signing an informed consent, women provided a single blood sample for FMR1 trinucleotide assessment and received pretest genetic counseling by an experienced certified genetic counselor. Questionnaires completed at the study visit and/or medical record reviews were the source of all demographic, reproductive and family medical history variables. All materials were coded with an assigned ID to maintain anonymity. Participants were paid $40 compensation.
After DNA extraction from the peripheral blood lymphocytes, FMR1 CGG repeats were determined with capillary electrophoresis by a single academic lab (University of Virginia Molecular Diagnostics Lab, Charlottesville, Virginia, USA). AMH was assayed by a single academic lab (Clinical Laboratory Research Core at Massachusetts General Hospital, Boston, MA, USA) using the AMH Gen II ELISA kit from Beckman Coulter according to the manufacturer’s protocol. The sensitivity of the assay was 0.05 ng/mL. To get the sensitivity as low as 0.02 ng/mL, the lab modified the incubation time for this study.
The FMR1 CGG repeat length was stratified by the larger allele having <35 repeats (n = 70) v. ≥35 repeats (n = 9) based on prior research [19,30]. Potential bias in the two groups was assessed for differences in age or race with t-tests and Fisher’s exact tests. Mean and median AMH levels were calculated for 4 age categories and stratified by the larger allele length. For the means and medians, the detection limit was used for any result at the detection limit, so these calculations may overestimate the true means and medians. Quadratic and linear models were fit to predict the log (AMH); all models controlled for age. The equations are shown below. The second model allowed both the slope and intercept to differ between the CGG groups. It is a common mathematical practice to include terms so that a model fits the data even if those terms are not easily interpretable. Readers will note that the quadratic model includes “age x age”; it is because of this squared term that it is called a quadratic model, and it is acknowledged that an “age x age” term is not easily interpreted in a clinical setting.
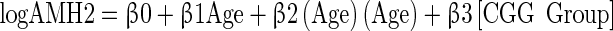 |
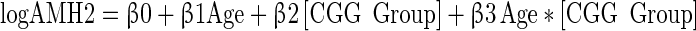 |
In the models, AMH values below the detection limit were handled by maximum likelihood assuming a Gaussian distribution. Model fitting was performed using the Survival R language software package [32]. Statistical significance was based on an alpha = 0.05.
Results
The age of the participants ranged from 26–43 years (median = 37, sd = 3.9) at the time of the blood draw (Table 1). Although the majority of participants were Caucasian (66 %), a sizeable minority were of Asian race (27 %). Approximately one-third had never been pregnant (37 %) and three-quarters had never had a live birth. Few participants had ever smoked (14 %), and only 1 participant was an active smoker at the time of the study. There were no differences between the high and low repeat groups in the age at the blood draw or race (p = 0.78 and p = 0.63, respectively).
Table 1.
Characteristics of the participants (n = 79)
| Factor | N (%) |
|---|---|
| Age at diagnosis of DOR (years) | Mean 36.4 (sd 3.9) |
| Median 37.0 | |
| Range 25 – 42 | |
| Age at blood draw (years) | Mean 37.4 (sd 3.9) |
| Median 38.0 | |
| Range 26 – 43 | |
| Age at menarche (years) | Mean 12.6 (sd 1.3) |
| Median 13.0 | |
| Range 9 – 16 | |
| Race | |
| White | 52 (66 %) |
| Black | 1 (1 %) |
| Asian | 21 (27 %) |
| Mixed race | 5 (6 %) |
| Hispanic ethnicity | 7 (9 %) |
| Nulligravid | 29 (37 %) |
| Nulliparous | 58 (75 %) |
| BMI | Mean 23.0 (sd 3.0) |
| Median 22.6 | |
| Range 17.9 – 30.9 | |
| Ever smoked | 11 (14 %) |
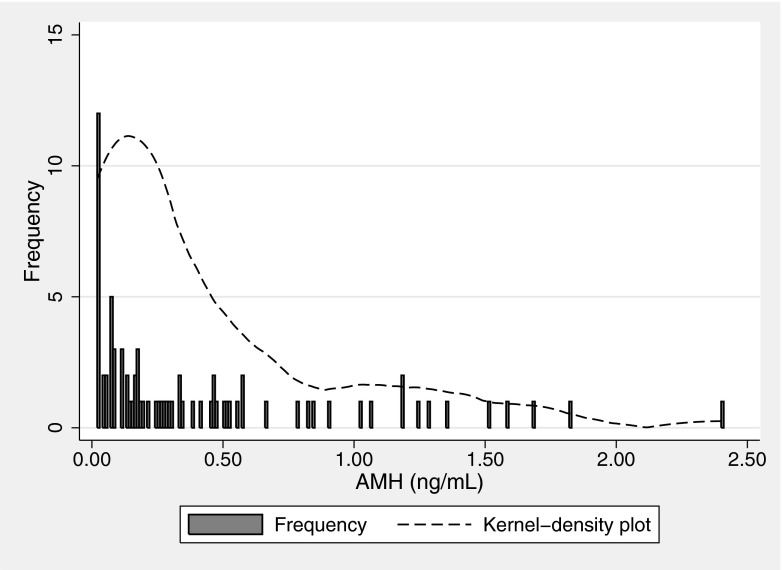
The range of AMH was from below the lower limit of detection of 0.02 to 2.4 ng/mL (median = 0.30, sd = 1.472), as shown in Fig. 1. The mean and median AMH values by four age categories are displayed in Table 2. Ten samples were below the detection limit. Readers may be surprised that a woman with DOR had an AMH level of 2.4 ng/ml. This particular individual qualified for the study with a cycle day 3 FSH of 15.8 mIU/mL, thus her results are an example of discordant diagnostic tests [14]. She additionally had AMH measured four months prior to her study participation, which was 2.5 ng/mL, thus there was no laboratory error.
Fig. 1.
Discrete AMH distribution
Table 2.
AMH levels by four age categories and stratified by the longer FMR1 repeat length
| Age | <35 CGG Repeat Mean (median) ng/mL | ≥35 CGG Repeats Mean (median) ng/mL |
|---|---|---|
| <30 years | 0.23 (0.23) | ---- |
| 30-34 years | 0.60 (0.49) | 0.59 (0.52) |
| 35-39 years | 0.45 (0.18) | 0.77 (0.58) |
| > = 40 years | 0.25 (0.17) | 0.02 (0.02) |
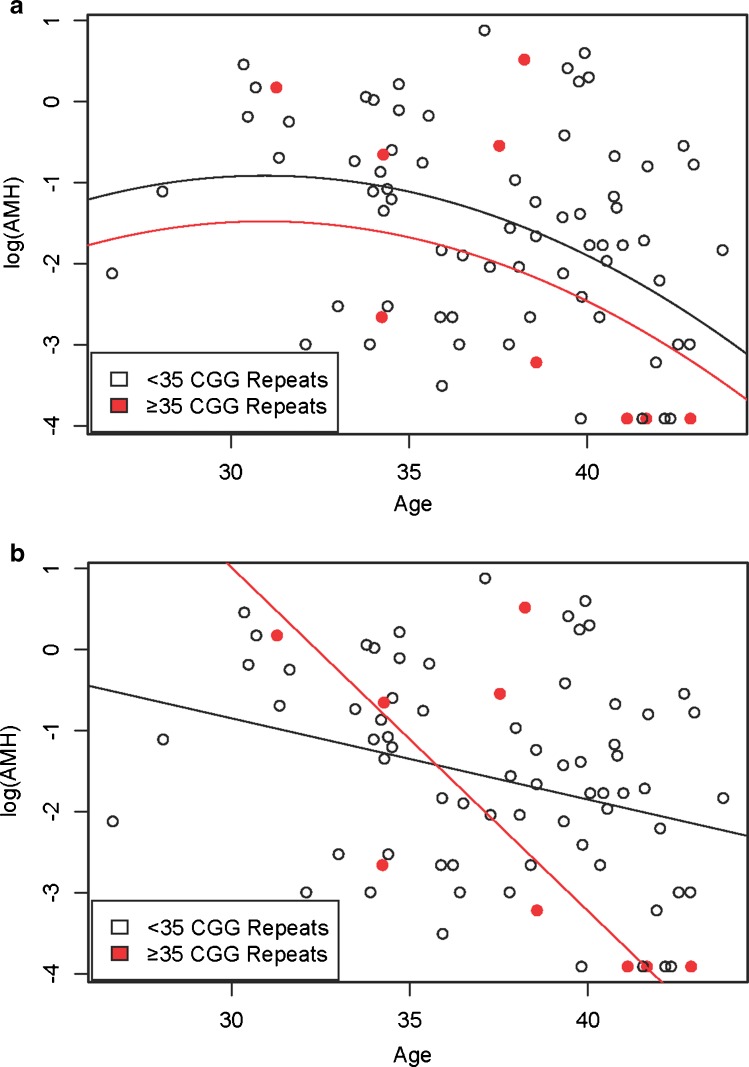
A quadratic model (including age2) of log (AMH) did not show a relationship with FMR1 CGG length (beta p-value = 0.25, Fig. 2a). The higher CGG repeat group is displayed as red solid circles and the lower CGG repeat group is displayed as black open circles. A linear model of log (AMH), corresponding to an exponential decline of AMH with increasing age, was significantly different, and had a steeper slope, for women with ≥35 CGG repeats than women with < 35 repeats (p = 0.035, Fig. 2b). The lines for each FMR1 stratum cross near age 36. The highest allele ranged from 22 to 34 (median 29) in the group with fewer than 35 repeats, and from 35 to 45 (median 37) in the group with at least 35 repeats.
Fig. 2.
a. Quadratic Model of log (AMH) by Age Stratified by FMR1 Gene (Higher Allele), b. Linear Model of log (AMH) by Age Stratified by FMR1 Gene (Higher Allele)
Exploratory analyses were conducted using the CGG repeat as a continuous variable, and no additional relationships could be gleaned.
Discussion
Our best fitting model was a linear model of the log (AMH), which corresponds to an exponential decline of AMH with increasing age. This model showed an inverse relationship between AMH and age, as expected, with a significant difference by FMR1 CGG repeat level. Specifically, among women with DOR, it appeared that the decline in AMH with increasing age occurred faster, and this decline may have commenced at older ages, for women with ≥35 CGG repeats than women with < 35 repeats. Prior research with over 9,000 infertility patients examined five different models (linear, biphasic linear, differential, power, and quadratic) for the decline in AMH with increasing age, and found that the quadratic model was optimal albeit the differences between the models were minimal [17]. That analysis, which provided a starting point for our model, did not consider any genetic factors. The results in this publication should be viewed as preliminary, due to the limited sample size in the high normal and intermediate repeat length ranges.
The scant literature (six publications) on this topic is inconsistent. Differences in the relationship between AMH and FMR1 adjusted for age may be due to the populations studied (FXS families, fertile women, or infertile women) and/or the CGG repeat lengths analyzed. Three reports have reported lower serum AMH in women with higher CGG repeats primarily in women in their 30’s (inverse relationship). Among 158 consecutive cycling infertility patients (none of whom carried the premutation) at a single center in the US (New York state), AMH was reported to be inversely correlated with CGG repeat length [6]. Specifically, in women under age 40, AMH was lower in women with 35–50 CGG repeats (n = 35) than in women with < 35 repeats (n = 122, p = 0.025). Rohr et al. [21] reported an inverse association only in women aged 31–40. We summarize their findings among the women who were not using hormone treatment as a conservative analytic approach given that some research has reported that AMH declines with combined contraceptive use irrespective of administration route [11]. With a population that combined women from the general female population and women with a family history of FXS in the US (state of Georgia), AMH was lower in women with ≥ 70 CGG repeats compared to those with < 70 repeats aged 31–40 years (p = 0.015). No association between AMH and FMR1 was found among women over age 40 or ≤ 30 (p > 0.08) [21]. In a study that combined FXS family data from The Netherlands and the US, premutation carriers were found to have lower AMH levels than non-carriers at all ages (multi-level modeling, p < 0.0001) [28].
A recent report with 372 infertile women from all causes, of whom 9 had an intermediate allele and 5 had a premutation, reported no association between AMH and the CGG repeat level after adjusting for age.[3] Among 532 fertile women in the US [13], there was no association between intermediate repeat lengths (defined as 35–54 CGGs) and AMH.
In contrast, one report found a positive rather than inverse association of AMH and CGG repeat. The population consisted of 197 Korean women “at high risk” of diminished ovarian function, either based on low AFC or family history of FXS (n = 7) [2], none of whom carried the premutation. The FMR1 CGG repeats were ≤ 51. Readers are reminded that the CGG repeat length is reported to be slightly lower in Asian populations [20]. Using a multiples of median analysis to control for confounding by age, a positive correlation was found between AMH and the FMR1 CGG repeat length (p = 0.008). No association was seen in women < 35 years.
Our results provide a differing perspective on this topic by the addition of mathematical modeling. With a point of intersection in the linear models around 36 years of age, one could view our results as showing that, among women aged ≤ 36, AMH is higher in women with 35–50 CGG repeats than in women with < 35 repeats. The opposite phenomenon is observed in women over age 36. We believe it is more informative to view the model as a continuum, as this provides a more testable hypothesis for future research.
One key limitation is that this study does not include any women with a premutation CGG repeat length, which may partly explain the difference in our linear and quadratic models. There were also no premutation carriers in the study by Choe et al.[2], where the highest repeat was 51. While Gleicher et al. [6] had 6 women with more than 50 repeats in the paper, their analysis was primarily restricted to the women with ≤ 50 repeats. The results in this publication should be viewed as preliminary, due to the limited sample size in the high normal and intermediate repeat length ranges. Future AMH studies with more women with “high normal”, intermediate zone, and premutation repeat lengths would be very informative. Another limitation is that these data are cross-sectional rather than longitudinal, thus AMH values at different ages represent different women rather than showing the decline in the same women over time. This limitation is also true of four of the prior reports [2,3,6,21]. Other genes may be related to AMH levels (variants in JARID2 in Caucasians and TPRXL and TEME86A in African Americans, for example [22]), and those unmeasured genes may explain our findings or have stronger associations than FMR1. Lastly, our population has a small number of participants, which hinders further analysis. The strengths of this study are the modeling approach that allowed analysis of FMR1 while controlling for age, and the restriction of the sample population to women without a family history of FXS, in contrast to three of the prior reports that included women with a family history of FXS [2,21,28].
There are several implications for fertility clinics and genetic counselors, if future studies support these observations. First, the findings have counseling implications, as the model suggests that women with 35–50 FMR1 CGG repeats may have normal AMH levels for her age up to some unknown age. Second, the results also imply that if a woman with 35–50 CGG repeats is found to have “borderline ovarian reserve”, then she should be referred to a fertility specialist in order to limit any delay to conception because her transition to menopause may be quicker than among other women.
Future longitudinal studies would have direct clinical relevance if a nomogram could be developed (by age, AMH, FMR1, and other genetic markers) to help predict a woman’s fertility window. These data may be of help to women contemplating the option of oocyte cryopreservation or to couples and individuals who are considering when to begin to try to conceive. While longitudinal studies exist and are in process regarding the predictive ability of AMH by age to forecast the final menstrual period [1,10], what is of interest to women of reproductive age who are seeking to become pregnant at a later age is their likelihood of success of conception. Therefore, continued research on the potential impact of the FMR1 gene on ovarian reserve (which includes the diagnostic value of AMH measurements) and fertility is important for providers of fertility care and their patients.
Acknowledgments
We thank the participants in this study, the study co-investigators who are not also co-authors (Dr. Lawrence Silverman, University of Virginia; Dr. Joel Finkelstein, Massachusetts General Hospital). All AMH testing was conducted at the Radioimmunoassay Core of the Reproductive Endocrine Sciences Center, Massachusetts General Hospital under the direction of Dr. Patrick Sluss. We are grateful to Carolina Conceptions for allowing recruitment through their clinic in North Carolina. We also thank the clinical research coordinators at all participating clinics: Parchayi Dalal, Hannah Spencer, Amy Brown, Amanda DeSmit, Angie Morey, Rebecca Briggs, and Janetta Phillips. This work was supported by the Eunice K. Shriver National Center for Child Health and Human Development at the National Institutes of Health (grants HD057485, HD052768 and HD068440 to Lisa Pastore). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Capsule Among 79 women with Diminished Ovarian Reserve, the association between AMH and age was significantly different for women with ≥ 35 FMR1 CGG repeats than for women with < 35 repeats.
References
- 1.Broer SL, Eijkemans MJC, Scheffer GJ, van Rooij IAJ, de Vet A, Themmen APN, et al. Anti-Müllerian Hormone Predicts Menopause: A Long-Term Follow-Up Study in Normoovulatory Women. J Clin Endocrin Metab. 2011;96(8):2532–9. doi: 10.1210/jc.2010-2776. [DOI] [PubMed] [Google Scholar]
- 2.Choe SA, Kim KC, Lee JY, Kim CH, Hwang D, Jee BC. The relationship between the number of CGG repeats and serum level of anti-Mullerian hormone in women without FMR1 premutation. Eur J Obstet Gynecol Reprod Biol. 2013 doi: 10.1016/j.ejogrb.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 3.De Geyter C, M’Rabet N, De Geyter J, Zurcher S, Moffat R, Bosch N, et al. Similar prevalence of expanded CGG repeat lengths in the fragile X mental retardation I gene among infertile women and among women with proven fertility: a prospective study. Genetics in Med. 2013 doi: 10.1038/gim.2013.146. [DOI] [PubMed] [Google Scholar]
- 4.Friese C, Becker G, Nachtigall RD. Rethinking the biological clock: eleventh-hour moms, miracle moms and meanings of age-related infertility. Social Sci Med. 2006;63(6):1550–60. doi: 10.1016/j.socscimed.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 5.Gleicher N, Weghofer A, Barad DH. A pilot study of premature ovarian senescence: I. Correlation of triple CGG repeats on the FMR1 gene to ovarian reserve parameters FSH and anti-Müllerian hormone. Fertil Steril. 2009;91(5):1700–6. doi: 10.1016/j.fertnstert.2008.01.098. [DOI] [PubMed] [Google Scholar]
- 6.Gleicher N, Weghofer A, Oktay K, Barad DH. Correlation of triple repeats on the FMR1 (fragile X) gene to ovarian reserve: A new infertility test? Acta Obstet Gynecol Scand. 2009;88(9):1024–30. doi: 10.1080/00016340903171058. [DOI] [PubMed] [Google Scholar]
- 7.Hadlow N, Longhurst K, McClements A, Natalwala J, Brown SJ, Matson PL. Variation in antimüllerian hormone concentration during the menstrual cycle may change the clinical classification of the ovarian response. Fertil Steril. 2013;99(6). doi:10.1016/j.bbr.2011.03.031 [DOI] [PubMed]
- 8.Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95(1):170–5. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ. Anti-Mullerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrin Metab. 2006;91(10):4057–63. doi: 10.1210/jc.2006-0331. [DOI] [PubMed] [Google Scholar]
- 10.Iino K, Tarakida A, Abe K, Taniguchi R, Higuchi T, Takahashi I, et al. Role of antimullerian hormone as a biomarker of the menopausal transition. Menopause. 2013;20(2):218–22. doi: 10.1097/gme.0b013e3182611574. [DOI] [PubMed] [Google Scholar]
- 11.Kallio S, Puurunen J, Ruokonen A, Vaskivuo T, Piltonen T, Tapanainen JS. Antimullerian hormone levels decrease in women using combined contraception independently of administration route. Fertil Steril.99(5):1305–10 [DOI] [PubMed]
- 12.Karimov CB, Moragianni VA, Cronister A, Srouji S, Petrozza J, Racowsky C, et al. Increased frequency of occult fragile X-associated primary ovarian insufficiency in infertile women with evidence of impaired ovarian function. Hum Reprod. 2011;26(8):2077–83. doi: 10.1093/humrep/der168. [DOI] [PubMed] [Google Scholar]
- 13.Kline JK, Kinney AM, Levin B, Brown SA, Hadd AG, Warburton D. Intermediate CGG repeat length at the FMR1 locus is not associated with hormonal indicators of ovarian age. Menopause. 2014. doi:10.1097/gme.0000000000000139 [DOI] [PMC free article] [PubMed]
- 14.Leader B, Hegde A, Baca Q, Stone K, Lannon B, Seifer DB, et al. High frequency of discordance between antimullerian hormone and follicle-stimulating hormone levels in serum from estradiol-confirmed days 2 to 4 of the menstrual cycle from 5,354 women in U.S. fertility centers. Fertil Steril. 2012;98(4):1037–42. doi: 10.1016/j.fertnstert.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Levi AJ, Raynault MF, Bergh PA, Drews MR, Miller BT, Scott RT., Jr Reproductive outcome in patients with diminished ovarian reserve. Fertil Steril. 2001;76(4):666–9. doi: 10.1016/S0015-0282(01)02017-9. [DOI] [PubMed] [Google Scholar]
- 16.Nelson SM, La Marca A. The journey from the old to the new AMH assay: how to avoid getting lost in the values. Repro Biomed Online. 2011;23(4):411–20. doi: 10.1016/j.rbmo.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Nelson SM, Messow MC, Wallace AM, Fleming R, McConnachie A. Nomogram for the decline in serum antimüllerian hormone: a population study of 9,601 infertility patients. Fertil Steril. 2011;95(2):736–41. doi: 10.1016/j.fertnstert.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Panidis D, Georgopoulos NA, Piouka A, Katsikis I, Saltamavros AD, Decavalas G et al. The impact of oral contraceptives and metformin on anti-Mullerian hormone serum levels in women with polycystic ovary syndrome and biochemical hyperandrogenemia. Gynecol Endocrinol.27(8):587–92 [DOI] [PubMed]
- 19.Pastore LM, Young SL, Baker VM, Karns LB, Williams CD, Silverman LM. Elevated Prevalence of 35–44 FMR1 Trinucleotide Repeats in Women with Diminished Ovarian Reserve. Reprod Sci. 2012;19(11):1226–31. doi: 10.1177/1933719112446074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peprah E. Fragile X Syndrome: The FMR1 CGG Repeat Distribution Among World Populations. Ann Hum Genet. 2011 doi: 10.1111/j.1469-1809.2011.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohr J, Allen EG, Charen K, Giles J, He W, Dominguez C, et al. Anti-Mullerian hormone indicates early ovarian decline in fragile X mental retardation (FMR1) premutation carriers: a preliminary study. Hum Reprod. 2008;23(5):1220–5. doi: 10.1093/humrep/den050. [DOI] [PubMed] [Google Scholar]
- 22.Schuh-Huerta SM, Johnson NA, Rosen MP, Sternfeld B, Cedars MI, Reijo Pera RA. Genetic variants and environmental factors associated with hormonal markers of ovarian reserve in Caucasian and African American women. Hum Reprod. 2012;27(2):594–608. doi: 10.1093/humrep/der391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott RT, Jr, Hofmann GE. Prognostic assessment of ovarian reserve. Fertil Steril. 1995;63(1):1–11. [PubMed] [Google Scholar]
- 24.Scriver J, Baker V, Young S, Behr B, Pastore L. Inter-laboratory validation of the measurement of follicle stimulating hormone (FSH) after various lengths of frozen storage. Reprod Biology Endocrin. 2010;8(1):145. doi: 10.1186/1477-7827-8-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharara FI, Scott JRT, Seifer DB. The detection of diminished ovarian reserve in infertile women. Am J Obstet Gynecol. 1998;179(3):804–12. doi: 10.1016/S0002-9378(98)70087-0. [DOI] [PubMed] [Google Scholar]
- 26.Sherman SL. Premature ovarian failure in the fragile X syndrome. Am J Med Genet. 2000;97:189–94. doi: 10.1002/1096-8628(200023)97:3<189::AID-AJMG1036>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 27.Sowers M, McConnell D, Gast K, Zheng H, Nan B, McCarthy JD. Anti-Müllerian hormone and inhibin B variability during normal menstrual cycles. Fertil Steril. 2010;94(4):1482–6. doi: 10.1016/j.fertnstert.2009.07.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spath MA, Feuth TB, Allen EG, Smits AP, Yntema HG, van Kessel AG, et al. Intra-individual stability over time of standardized anti-Mullerian hormone in FMR1 premutation carriers. Hum Reprod. 2011;26(8):2185–91. doi: 10.1093/humrep/der146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Streuli I, Fraisse T, Pillet C, Ibecheole V, Bischof P, de Ziegler D. Serum antimullerian hormone levels remain stable throughout the menstrual cycle and after oral or vaginal administration of synthetic sex steroids. Fertil Steril.90(2):395–400 [DOI] [PubMed]
- 30.Streuli I, Fraisse T, Ibecheole V, Moix I, Morris MA, de Ziegler D. Intermediate and premutation FMR1 alleles in women with occult primary ovarian insufficiency. Fertil Steril. 2009;92(2):464–70. doi: 10.1016/j.fertnstert.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Taieb J, Olivennes F, Birr AS, Benattar C, Righini C, Frydman R, et al. Comparison of day 3 FSH serum values as determined by six different immunoassays. Hum Reprod. 2002;17(4):926–8. doi: 10.1093/humrep/17.4.926. [DOI] [PubMed] [Google Scholar]
- 32.Therneau T. Survival: A Package for Survival Analysis in S. R package 2.37-2 edition ed2012.
- 33.Visser JA, Themmen AP. Anti-Mullerian hormone and folliculogenesis. Mol Cell Endocrinol. 2005;234(1–2):81–6. doi: 10.1016/j.mce.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10(2):77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]